Abstract
Fungal infections with non-Aspergillus species are increasingly reported even among immunocompetent individuals. We report a case of bilateral injection abscesses by Scedosporium apiospermum in an immunocompetent patient. This rare fungus was isolated and identified by culture from the surgical tissue and was confirmed by Vitek MS and sequencing of the internal transcribed spaces region of rDNA. The patient is being treated with Voriconazole for the past 3 months with no recurrence of the abscesses.
Keywords: Scedosporium, GRAPHUM, Injection, Abscess, Sequencing, MALDI-TOF
1. Introduction
Fungi are an important yet frequently overlooked cause of morbidity and mortality in humans. While Aspergillus fumigatus is the most important and well-documented mold pathogen of humans, non-Aspergillus molds such as Fusarium, Scedosporium, Lomentospora and Mucoromycetes species are increasingly reported as agents of disseminated diseases. These “Big five mold killers of humans” are now firmly established as pathogens not only in immunosuppressed but also in immunocompetent individuals [1].
Scedosporium apiospermum or its teleomorph (sexual stage) Pseudallescheria boydii, is a ubiquitous saprophytic filamentous fungus present in soil, sewage and polluted waters especially in temperate regions [[2], [3], [4], [5]].
The nomenclature of the genus Scedosporium/Pseudallescheria has undergone numerous changes over the last decade following the introduction of molecular phylogenetics in 2005. With the establishment of the “One Fungus = One Name” rule in fungal taxonomy which allows only a single name per fungus [6], the genus name “Scedosporium” was retained in place of “Pseudallescheria. [3,7]. S. apiospermum is considered a species complex, with 5 species within the complex [7].
In the clinical setting, the most commonly isolated species are S.boydii and S. apiospermum [5], causing serious disseminated infections in the immunosuppressed host. However, in immunocompetent patients, the lesions are more indolent (cutaneous and subcutaneous) and are generally caused by penetrating injuries, that aid in the entry of the fungus into the deeper tissues [2]. The lesion grows slowly with well-defined margins, remaining localized for long periods [2] and seen as a chronic progressive granulomatous infection. For a deep-seated lesion, as in the present case, swelling may be the only symptom, or it may be accompanied by pain and tenderness [2].
In this case report, we describe an unusual case of injection abscesses due to the Graphium type of S. apiospermum, that was probably implanted in the subcutaneous tissue through the contaminated needles used during multiple intra-muscular analgesic injections. This uncommon fungus was isolated and identified by culture from the resected surgical tissue and was confirmed by Vitek MS and sequencing of the internal transcribed spaces region of ribosomal DNA. The patient was successfully treated with Voriconazole and there was no recurrence of the abscesses at a follow-up of 3 months.
2. Case report
A 64 - year - old lady presented to the General Surgery outpatient services of Kamineni General Hospital, Hyderabad, Telangana, India, (day 0) with complaints of mildly painful bilateral loin swellings of 3 months duration.
She was apparently asymptomatic 3 months prior (day-90), when she developed a swelling on the right loin which was gradually increasing in size and associated with pain radiating to the right lower limb. One month later (day-60), she noticed a similar swelling in the left loin with pain radiating to left lower limb. She did not complain of any fever.
The patient informed that three months prior (day-180), to the development of the swelling on the right side, she was administered multiple intramuscular analgesics in both the loins for a severe low back ache, on different days, at a local clinic in her village, in Nalgonda district, Telangana, India, by Registered Medical Practitioners (RMPs). She is a known type II diabetic on Insulin and a known hypertensive on oral antihypertensives.
On physical examination, the patient was afebrile, conscious, coherent, vitals were normal (BP 130/80 mmHg, pulse rate 80/minute, respiratory rate was 20/minute). Cardiovascular, respiratory, central and peripheral nervous systems were normal. Abdomen was soft, non-tender, no guarding rigidity, no masses were felt.
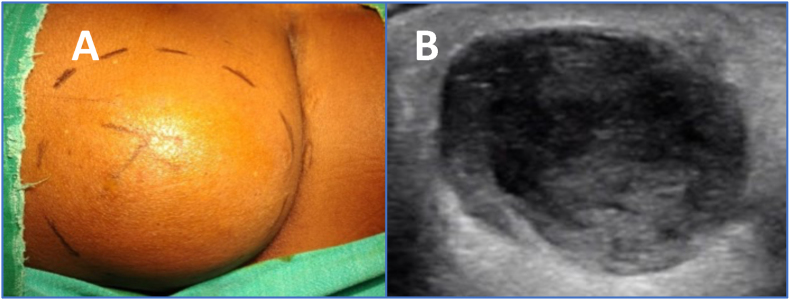
On examination of the loins, the right-side swelling (Fig. 1A) was larger than that on the left side. A mildly tender 10 × 6 cms swelling was noticed in the right loin region. The skin over the swelling was normal, non-erythematous and intact with no discharging sinuses and there was no rise in temperature over the swelling. The skin was not adherent and could be lifted off the swelling. There was no loss of sensation. A small swelling, 4 × 3 cms, was noticed in the left loin region with similar features as on the right side. An ultra-sonography of the swellings showed well defined anechoic collection with central hyperechoic contents, suggestive of an abscess (Fig. 1B).
Fig. 1.
A. Right loin swelling. B. Ultrasonography of Right loin swelling showing well definedanechoic collection with central hyperechoic contents, suggestive of an abscess.
The provisional clinical diagnosis was bilateral epidermoid cysts or subcutaneous injection abscesses.
An ultrasound guided aspiration of the right swelling was performed on day 0.
Histology of the aspirate suggested a suppurative inflammation with extensive necrotic debris on Hematoxylin & Eosin stain (H&E).
The Microbiology laboratory reported isolation of a rapidly growing hyaline mold. A repeat aspirate was requested to rule out any contaminating fungus. No bacteria, including Mycobacteria, were isolated from the aspirate. A week later (day+7), a repeat aspiration was done from both the loin swellings and submitted for cultures. The specimen were processed separately for bacterial, Mycobacterial and fungal growth, as described later. A rapidly growing hyaline mold, that was similar to the earlier one, was once again isolated.
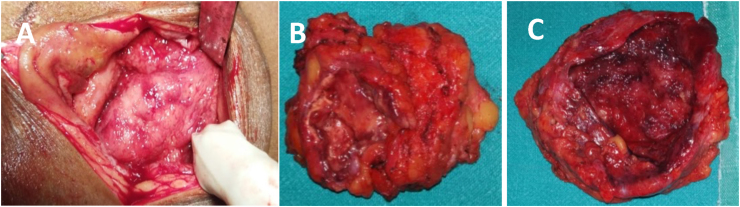
After a thorough preoperative evaluation including cardiac fitness, an informed consent was taken from the patient for surgery under spinal anesthesia (day+10). An incision and drainage of the abscesses with evacuation of the pus was performed on day 10 under spinal anesthesia. Intra operatively,100 ml of purulent fluid and 60 ml of serous fluid was drained from the right and left abscesses respectively. The thick fibrotic walls of both the abscesses were resected in toto (Fig. 2 A & B). A thorough antiseptic wash was given to remove residual pus. Drains were placed in both the cavities and primary closure of the cavities was done. Cefotaxime (200 mg thrice daily orally for 5 days) was started. On the 2nd postoperative day (day+12), the drains were removed and on the 5th postoperative day (day+15), the patient was without pain. At discharge (day+17) she was prescribed empiric antifungal therapy antifungal therapy with fluconazole (150mg) once daily awaiting culture and histology results.
Fig. 2.
A. Intraoperative appearance of the abscess. B. Gross appearance of the resected abscess.C. Thick fibrotic wall of the abscess.
At a one and a half month follow up (day+45), she was in good health and the
surgical wound was well healed. By this time the fungal isolate was identified as S. apiospermum and as per the recommended treatment guidelines, the antifungal therapy was changed to Voriconazole 200 mg twice daily [5] and
Has been continuing over the past 3 months (day+135).
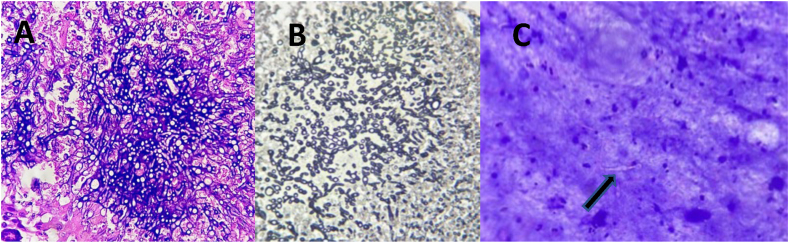
Histology of multiple sections from both the specimen including the abscess walls, on H&E (Fig. 3 A) and Gomori Methenamine Silver stain (GMS) (Fig. 3 B), showed multiple foci of necrosis with embedded, slender, elongated, irregularly branching, septate fungal hyphae. No definite granulomas were seen. Review of the initial cytology smears from the pre-operative aspirated pus (Fig. 3 C) showed few fungal filaments embedded and obscured by necrotic debris. The final histopathology report was a bilateral fungal granulomatous inflammation.
Fig. 3.
A. Hematoxylin – Eosin stain of surgical tissue showing necrotic focus with slender, elongated, irregularly branching septate hyphae along with neutrophils & giant cells (10x10 magnification). B.Gomori Methenamine Silver stain of surgical tissue showing short, septate, acute angle branching hyphaeand few foci of conidia (10x10 magnification). C. Hematoxylin – Eosin stain of preoperative aspirate showing few foci of fungal filaments embedded and obscured by necrotic debris. (shown with arrow)(10x40 magnification).
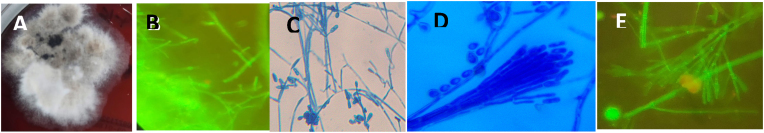
A direct Gram’s stain of the initial pre-operative aspirate showed moderate number of inflammatory cells. No bacteria or fungal elements were observed. Culture of the fluid on 7% sheep blood (COS) and Chrome agar (CPS ID, bioMerieux, Marcy L’Etoile, France) plates after 2 days, showed a rapidly spreading, effuse white fungal mold was observed, which later turned olivaceous to black with a white margin (Fig. 4 A) in 10 days. No bacteria, including Non-tuberculous Mycobacteria, were isolated.
Fig. 4.
A. Rapidly spreading effuse, Cottony white fungal colony with aerial mycelium on 7% Sheep Bloodagar. B. Fluorescence staining of tissue specimen showing thin, septate branching hyphae with apple green fluorescence (10x40× magnification). C. Lacto Phenol Cotton Blue mount (10x40× magnification) shows septate hyphae (2–4 μm in diameter), with simple, long or short conidiophores bearing conidia singly or in small groups. D. Graphium type of asexual synanamorph conidiation. (10x40× magnification). E.Fluorescence stain of mature colony showing Synnemata with apple green fluorescence, bearing conidiasingly and in small clusters (10x40× magnification. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A direct fluorescence staining of the surgical tissue specimen, using an in-house fluorescence stain [8], showed thin, septate branching hyphae with apple green fluorescence (Fig. 4 B). Culture of the repeat pre-operative aspirate and the surgical specimen on Sabouraud’s dextrose agar (SDA), Potato dextrose agar (PDA) (incubated at 28 °C) and Brain Heart Infusion (BHI) agar (Hi-Media, India) (incubated at 37 °C), showed profuse growth of the fungus with similar colony morphology, as described earlier.
Slide cultures of the mold, mounted with Lacto Phenol Cotton Blue (LPCB) stain, showed septate hyphae (2–4 μm in diameter), with simple, long and short conidiophores bearing hyaline, one-celled, smooth, sub-globose to oval or clavate (annelloconidia) conidia, singly or in small groups (Fig. 4 C). The fungal isolate was presumptively identified as Scedosporium species.
The mature fungal colony, after about 10 days, showed the Graphium type of asexual synanamorph conidiation (Fig. 4 D) with simple, long and short, dark erect conidiophores, cemented together forming the characteristic Synnemata, bearing conidia singly or in small clusters at the apex. A fluorescence staining of the mature colony also showed apple green, fluorescent Synnemata (Fig. 4. E). The sexual state of the fungus could not be induced [9]. Based on the microscopic features and the characteristic Synnemata, the fungus was identified as Graphium type of S. apiospermum.
The identification of the fungal isolate was further confirmed as S. apiospermum by the Vitek-MS MALDI-TOF (bioMerieux, Marcy L’Etoile, France).
A definitive speciation of the fungal isolate was performed by Sanger sequencing using 3500DX genetic analyzer targeting the Internal Transcribed Spacer (ITS) regions using universal pan fungal primer ITS4 and ITS5 regions of the fungal ribosomal DNA (rDNA) gene. Identification was congruent to that obtained by rDNA ITS region sequencing demonstrating 96.77% homology to the published S. apiospermum sequences (GenBank Accession number KF417734; https://blast.ncbi.nlm.nih.gov/Blast.cgi).
The final diagnosis offered to the patient was bilateral injection abscesses caused by Graphium type of Scedosporium apiospermum. The patient showed a good clinical response to voriconazole, with no side effects or evidence of relapse at a 3 month (day+235) follow up.
The isolate was deposited with an assigned strain number 107056, in the National Culture Collection of Pathogenic Fungi (NCCPF) (Mycology Division), Postgraduate Institute of Medical Education & Research, Chandigarh, India.
3. Discussion
There have been numerous reports of infections due to S. apiospermum, in immunocompetent individuals, including mycetoma, osteomyelitis, discitis and arthritis [2,10]. Though, injection abscess(es) due to several other fungi are well documented, including a recent report of bilateral chromoblastomycotic abscess [11], to the best of our knowledge, no case of injection abscess(es) caused by S. apiospermum has been reported to date. As with other subcutaneous mycoses, Scedosporium species gain entrance to the host environment through penetrating transcutaneous trauma, including puncture wounds (such as from thorns, wood splinters, or speculated seeds), abrasions, or any contact with sharp objects such as agricultural tools [2]. As per the history narrated by our patient, the abscesses developed after about 3 months of frequent intramuscular analgesic injections administered on her loins at different times. The possible use of contaminated injection needles at the rural clinic, probably facilitated inoculation and implantation of the fungus in the tissues. The lesions grew slowly as deep-seated abscesses with well-defined margins, remaining localized in the loins. Swelling may be the only symptom, or it may be accompanied by pain and tenderness [2,12], as was seen in the present case. The 2019 updated definitions for invasive fungal disease (IFD) by the European Organization for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium (EORTC/MSGERC), emphasize the visualization of fungal hyphae and/or culture of the fungus from an affected site as a criterion for proven fungal infection [5,13]. In the present case, a complete removal of the abscesses was performed, and the fungal hyphae could be visualized on the tissue sections. On histology, the close resemblance of the hyphae of Scedosporium in the tissue sections may be mistaken for those of Aspergillus and Fusarium species [14,15]. Several unique features such as irregular branching off to the side at a 60–70° angle, which is different from the 45° angle seen with Aspergillus spp or intravascular, intratissue conidiation and pyriform adventitious conidia, located terminally or laterally on the hyphae, can be useful identifiers of Scedosporium mycoses [5]. The occasional presence of branches bridging two parallel hyphae to form an H-shaped pattern is considered to be highly suggestive of Scedosporium [5]. The fungal pathogen, S. apiospermum, could be easily isolated, but the species identification was a challenge. The microscopic features of the Synnemata in mature cultures [5,7,9,16] were the key to a definitive identification as Graphium type of S. apiospermum. According to the EORTC/MSGERC guidelines [5], isolation and identification of Scedosporium species from the infected tissue specimen is critical for initiating and guiding targeted antifungal therapy against this highly resistant pathogen [7,13,15]. Identifying rare fungal pathogens such as Scedosporium by newer diagnostic, non-culture-based molecular techniques is therefore recommended [7,12,15] for a confirmatory diagnosis. These new non-culture methods are now considered as the next generation diagnostic tools for fungal identification in the routine mycology laboratory [7]. rDNA ITS sequence-based analysis is the current gold standard for fungal identification, to identify the main species of Scedosporium.
MALDI-TOF MS offers good potential for fast, and relatively economical identification of Scedosporium species and has become the first-line identification of these filamentous fungi with an accuracy comparable to that of DNA sequencing. The Vitek® MS v3.0 (bioMerieux, Marcy L’Etoile, France) database was approved by the US Food and Drug Administration (FDA) for identification of molds. However, only S. apiospermum and S. boydii within the S. apiospermum complex, are available in its database [7].Among the antifungal agents, the triazoles and polyenes have varying levels of activity against Scedosporium species. The azoles (such as itraconazole and voriconazole) typically have the lowest mean inhibitory concentration (MIC) [15]. Most international guidelines recommend voriconazole as first-line therapy [13,17] the present patient is being successfully managed with oral Voriconazole 200 mg twice daily over the past 3 months. In conclusion, the present case is probably the first one of an injection abscess(es) caused by GRAPHUM type of S. apiospermum in an immunocompetent patient. Since the invasive fungal diseases are on the rise, a high degree of clinical suspicion and awareness about the rare fungal pathogens is essential for a timely diagnosis and appropriate antifungal therapy. This can be facilitated by a complete surgical resection of the lesion(s) with collection of an adequate, deep-seated tissue specimen [18]. With reference to the iatrogenic infections such as an injection abscess, there is a need for awareness, both among the health care providers and public, regarding the use of good infection prevention practices to protect patients from being harmed by avoidable infections. Principles of safe injection practices with strict use of aseptic measures including sterile needles, blades, during invasive procedures or injections at any hospital/clinic, especially in remote and rural areas, must be implemented. The RMP, who administered the injections to this patient, was alerted and requested to use safe injection practices. No further interventions could be done as it was a private clinic.
Authors’ contributions
-
a.
Dr. Sravanti Kanumuri is the treating surgeon. She operated and managed the patient.
-
b.
Dr. Ashwini Maddi is the Radiologist. She performed and reported on the ultra-sonogram of the abscesses.
-
c.
Dr. Pavani Marapaka is the histopathologist. She reviewed and interpreted all the histopathology smears.
-
d.
Dr. Pravalika Bhimasani is the Microbiology Postgraduate. She processed and maintained the fungal isolate.
-
e.
Dr. Lakshmi Vemu is the Clinical Microbiologist. She identified the fungus and compiled the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare no conflict of interest. All authors have read and agreed to the final version of the manuscript.
Acknowledgements
The authors sincerely thank
1. Dr. Vijay Yeldandi, and Dr.Vishwanath, Suvarna Swasthya Research Centre, Hyderabad, India for identification of the fungal isolate on the Vitek MS - MALDI TOF in his laboratory.
2. Dr. Arunaloke Chakraborty, Dr. R. Shivaprakash and team at National Culture Collection of Pathogenic Fungi (NCCPF) (Mycology Division), Postgraduate Institute of Medical Education & Research, Chandigarh, India, for performing the genetic sequencing of the isolate and arranging for the deposit of the isolate in the NCCPF repository.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mmcr.2022.05.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Thornton C.R. Detection of the 'big five' mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes. Adv. Appl. Microbiol. 2020;110:1–61. doi: 10.1016/bs.aambs.2019.10.003. Epub 2019 Nov 20. PMID: 32386603. [DOI] [PubMed] [Google Scholar]
- 2.Cortez K.J., Roilides E., Quiroz-Telles F., et al. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 2008;21(1):1157–1197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez-Garcia A., Pellon A., Rementeria A., etal Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med. Mycol. 2018;56(suppl_1):102–125. doi: 10.1093/mmy/myx113. PMID: 29538735. [DOI] [PubMed] [Google Scholar]
- 4.Rynga D., Capoor M.R., Varshney S., Naik M., Gupta V. Scedosporium apiospermum an emerging pathogen in India: case series and review of literature. Indian J. Pathol. Microbiol. 2017;60:550–555. doi: 10.4103/IJPM.IJPM_742_16. [DOI] [PubMed] [Google Scholar]
- 5.Hoenigl M., Slamanton-Garcia J., Walsh T.J., Nucci M., Neoh C., Jenks J.D., Lackner M., Sprute R., Alhatmi R.A., Bassetti M., et al. Global guideline for the diagnosis and management of rare mold infections: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(20)30784-2. Published Online, [DOI] [PubMed] [Google Scholar]
- 6.Hawksworth D.L., Crous P.W., Redhead S.A., Reynolds D.R., Samson R.A., Seifert K.A., Taylor J.W., Wingfield M.J., Abaci O., Aime C., et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2:105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S.C.-A., Halliday C.L., Hoenigl M., Cornely O.A., Meyer W. Scedosporium and Lomentospora infections: contemporary Microbiological tools for the diagnosis of invasive disease. J.Fungi. 2021;7:23. doi: 10.3390/jof7010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirani K.S., Chandrika V.S. Efficacy of in-house fluorescent stain for fungus. Indian J. Pathol. Microbiol. 2017;60:57–60. doi: 10.4103/0377-4929.200049. [DOI] [PubMed] [Google Scholar]
- 9.Walsh T.J., Hayden Randall T. Larone's Medically Important Fungi - a Guide to Identification. sixth ed. ASM press; Washington DC, USA: 2018. Davise larone. Scedosporium spp. complex; pp. 222–224. [Google Scholar]
- 10.Stripeli Fotini, Pasparakis D., Velegraki Aristea, Lebessi E., Arsenis G., Kafetzis D., Tsolia M. Scedosporium apiospermum skeletal infection in an immunocompetent child. Med. Mycol. 2009;47(4):441–444. doi: 10.1080/13693780802695470. [DOI] [PubMed] [Google Scholar]
- 11.Veerpandiyan A., Sekaran B.S.S., Rahamathullah H.M., Veerapandiyan A. A rare etiology for injection related gluteal abscess. J Infect Dis Epidemiol. 2020;6:174. doi: 10.23937/2474-3658/1510174. [DOI] [Google Scholar]
- 12.Tóth E.J., Nagy G.R., Homa M., Ábrók M., Kiss I.E., Nagy G., Bata-Csörgő Z., Kemény L., Urbán E., Vágvölgyi C., Papp T. Recurrent Scedosporium apiospermum mycetoma successfully treated by surgical excision and terbinafine treatment: a case report and review of the literature. Ann. Clin. Microbiol. Antimicrob. 2017;16(1):31. doi: 10.1186/s12941-017-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly J.P., Chen S.C.-A., Kauffman C.A., Stienbach W.J., Baddley J.W., Verweij P.E., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and update of the consensus definitions for invasive fungal disease from the European organization for research and treatment of cancer and the mycoses Study group education and research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y., Gohara A.F., Mrak R.E., Muldrew K.L. Misidentification of Scedosporium boydii infection as aspergillosis in a patient with chronic renal failure. Case Rep Infect Dis. 2020;2020 doi: 10.1155/2020/9727513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy M.W., Katragkou A., Iosifidis E., Roilides E., Walsh T.J. Recent advances in the treatment of Scedosporiosis and fusariosis. J Fungi. 2018;4(2):73. doi: 10.3390/jof4020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarro Josep, Kantarcioglu A. Serda, Horré Regine, Luis Rodriguez-Tudela Juan, Estrella Manuel Cuenca, Juan Berenguer, Sybren De Hoog G. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 2006;44(4):295–327. doi: 10.1080/13693780600752507. [DOI] [PubMed] [Google Scholar]
- 17.McKenna E.B., Dao Harry, Estep Jerry D., Huttenbach Yve T., Hemmige Vagish. Utilization of voriconazole drug monitoring in the treatment of cutaneous Scedosporium apiospermum infection. Medical Mycology Case Reports. 2018;22:52–54. doi: 10.1016/j.mmcr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manikandan P.S., Narendran V., Vijayakumar R., Shobana C.S. Keratomycosis caused by GRAPHUM eumorphum (GRAPHUM state of Scedosporium apiospermum) J. Clin. Diagn. Res. 2015;9(4):3–4. doi: 10.7860/JCDR/2015/12089.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.