Abstract
Maillard reaction during food processing contributes to the formation of some unpleasant heat-induced toxicants including advanced glycation end products (AGEs) and 5-hydroxymethylfurfural (HMF), which have been linked to various health risks. The effects of baking factors and recipes, such as baking temperature (130°C–180 °C) and time (8 min–15 min), sucrose levels (0 g–20 g), butter levels (0 g–20 g) and egg liquid levels (0 g–12 g) on the formation of free Nε-(carboxymethyl)lysine (CML), free Nε-(carboxyethyl)lysine (CEL), protein-bound CML, protein-bound CEL, HMF, glyoxal (GO), methylglyoxal (MGO), 3-deoxyglucosone (3-DG) and on the sensory qualities were investigated in butter cookies. The results suggested that the levels of AGEs initially increased and then followed by decrease as baking temperature and time increased, HMF is very sensitive to baking temperature and time and grows sharply. The changes of protein-bound AGEs are lagging behind that of free AGEs. The proportions of sucrose, butter and egg liquid in butter cookies were positively correlated with AGEs, with sucrose greatly promoting on the formation of HMF and 3-DG. In addition, the high level of sucrose and butter in cookies is preferred by panelists, especially in terms of appearance, taste and smell.
Keywords: Advanced glycation end products, 5-Hydroxymethylfurfural, Butter cookies, Sensory qualities
Graphical abstract
Highlights
-
•
AGEs increases and then decreases as baking temperature and time increased.
-
•
HMF is very sensitive to baking temperature and time in butter cookies.
-
•
The changes of protein-bound AGEs are lagging behind that of free AGEs.
-
•
Sucrose greatly promotes the formation of HMF and 3-DG.
-
•
Sucrose and butter promotes release of α-dicarbonyl compounds.
1. Introduction
A typical cookie-making is roughly composed of wheat flour (low gluten), icing sugar, butter, eggs as the main raw materials. The whole process can be roughly classified into mixing, molding, and baking. In particular, baking is a key step in which the dough is transformed into a delicious snack with a light and porous internal structure under the influence of heat (Filipcev et al., 2014). During this high-temperature heating process of butter cookies, Maillard reaction endows the pleasant and desirable color, flavor and taste to butter cookies, but also generates undesirable chemicals such as advanced glycation end products (AGEs) and 5-hydroxymethylfurfural (HMF) (Courel et al., 2009).
Butter cookies are recognized for their high protein level, and high carbohydrate and fat level. These factors lead to thermal reactions including lipid oxidation, caramelization, and Maillard reaction, which have been identified as the main sources of flavor and heat-induced toxicants generation during baking (Paravisini et al., 2019). The presence of eggs and sugar in butter cookies provides amino and carbonyl groups required for Maillard reaction. At elevated temperatures, Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) can be generated from the interaction between glyoxal (GO)/methylglyoxal (MGO) and the epsilon-amino group of lysine (Ahmed et al., 1997; Chuyen, 2006). HMF, which is acyclic aldehyde formed from hexoses by the action of normal acidity, is used as an indicator for the heating of carbohydrate containing foods (Ramírez et al., 2000). HMF is a common product of Maillard reaction and caramelization and is formed from 3-deoxyglucosone (3-DG), the dehydration product derived from 1,2-enolization of glucose and fructose (Degen et al., 2012).
Obviously the AGEs and HMF produced in butter cookies are closely related to the baking factors and recipes, but meanwhile, the taste, appearance, color and other quality indicators will be inevitably affected. Nevertheless, the baking factors such as baking temperature and time, sucrose levels, butter levels, and egg liquid levels have scarcely been simultaneously taken into consideration. This paper aims to probe the relationship between butter cookies baking factors and recipes on the formation of free and protein-bound AGEs and HMF from the perspective of α-dicarbonyl compounds, and we compared the impact of these different factors on the sensory quality of butter cookies. The results from this paper can provide a reference for understanding the baking factors and recipes that affect the formation of AGEs and HMF in butter cookies.
2. Materials and methods
2.1. Chemical and reagents
CML and CEL standards as well as d4-CML and d4-CEL standards were purchased from Toronto Research Chemicals Inc. (North York, Ontario, Canada). HMF, 13C6-HMF, 3-DG, quinoxaline, 2-methylquinoxaline, o-phenylenediamine, 5-methylquinoxaline and formic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ammonia solution (≥25% in H2O), monobasic and dibasic potassium phosphate were of HPLC grade and obtained from Shanghai Aladdin Biochemical Technology Co. (Shanghai, China). Analytical grade chloroform, sodium borohydride, sodium borate, boric acid, sodium hydroxide and hydrochloric acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Methanol (MS grade) was acquired from Merck (Darmstadt, Germany). Oasis HLB or MCX solid phase extraction cartridges (6 cc, 200 mg) were supplied by Waters (Milford, MA, USA). Deionized water was provided by the Watson Group Ltd. (Hongkong, China).
Wheat flour, butter, eggs and sucrose were purchased from local supermarkets in Jiangxi, China.
2.2. Preparation of butter cookies
The preparation method of butter cookies refers to previous research (Hu et al., 2022). The initial cookie recipe consisted of wheat flour (40 g), whole egg (6 g), sucrose (10 g), butter (10 g), and deionized water (2 g). The ingredients were thoroughly mixed by an electronic blender and dough was rolled and placed into a mold to form a circle with a diameter of 5 cm and a thickness of 3 mm. Cookies were baked in a digital oven operated without forced air circulation (CO-2701 model, COUSS Electric Co., Ltd., Zhongshan, China). After baking, the cookie samples were cooled to room temperature, ground and kept at −20 °C until further analysis.
2.2.1. Effect of baking temperature
The butter cookies were baking at 130 °C, 135 °C, 140 °C, 135 °C, 140 °C, 145 °C, 150 °C, 155 °C, 160 °C, 165 °C, 170 °C, 175 °C and 180 °C for 12 min, respectively. (n = 3).
2.2.2. Effect of baking time
The butter cookies were baking at 160 °C for 8 min, 9 min, 10 min, 11min, 12 min, 13 min, 14min, 15min and 16 min, respectively. All experiments were performed in triplicate.
2.2.3. Effect of sucrose levels
The butter cookies were made with different sucrose levels (0 g, 5 g, 10 g, 15 g and 20 g) and were baked at 160 °C for 12 min. Butter cookies containing 0 g of sucrose were treated as the control group. All experiments were performed in triplicate.
2.2.4. Effect of butter levels
The butter cookies were made with different butter levels (0 g, 5 g, 10 g, 15 g and 20 g) and were baked at 160 °C for 12 min. Butter cookies containing 0 g of butter were treated as the control group. All experiments were performed in triplicate.
2.2.5. Effect of egg liquid levels
The butter cookies were made with different egg liquid levels (0 g, 3 g, 6 g, 9 g and 12 g) and were baked at 160 °C for 12 min. Butter cookies containing 0 g of liquid were treated as the control group. All experiments were performed in triplicate.
2.3. Analysis of AGEs, HMF and α-dicarbonyl compounds
2.3.1. Extraction of AGEs, HMF and α-dicarbonyl compounds from butter cookies
The method was consistent with the previous (Hu et al., 2022). 0.5 g of ground cookies powder were added to a 10-mL centrifuge tube and mixed with 10 μL of d4-CML and d4-CEL mixed internal standard solution (10 μg/mL, v/v) and 20 μL of 13C6-HMF internal standard solution (10 μg/mL, v/v) and 2 mL of deionized water. After defatting with twice extractions of 4 mL of chloroform/methanol (2:1, v/v) solution (Folch et al., 1957), the mixtures were vortexed and centrifuged at 4400 g for 10 min to collect the supernatants and residue in the middle layer.
Preprocessing of free CML and CEL, supernatants were subjected to solid phase extraction (SPE) by using an automatic SPE instrument (PrepElite-GVS model, LabTech Co., Ltd., Beijing, China) equipped with Oasis MCX cartridge. The MCX cartridge was preconditioned with 3 mL of methanol followed by 3 mL of deionized water. Then 1 mL of supernatant was loaded onto the cartridge and washed with 1 mL of methanol and 1 mL of deionized water before being eluted with 4 mL of 5% ammonia solution in 80:20 methanol/water (v/v). The eluant was concentrated gently with a stream of nitrogen gas to dryness for the purpose of removing volatile ammonia, and resolved in 1 mL of deionized water, vortexed for 30 s, and then filtered through a 0.22 μm membrane before injection into the LC-MS/MS system.
Preprocessing of protein-bound CML and CEL, the residue was transferred and dried completely with a stream of nitrogen gas in a 30-mL thick-wall pressure-resistant glass tube. Then, 1 mL 2 M NaBH4 (prepared in 0.1 M NaOH) and 2 mL of borate buffer (0.2 M, pH 9.2) were added and left overnight at 4 °C to proceed reduction. After reduction, 4 mL of 12 M HCl was added to the samples, and the pressure-resistant glass tube was flushed with nitrogen, sealed and incubated at 110 °C for 24 h. After then, the cooled acid hydrolysate was filtered through filter paper and diluted with deionized water to obtain a final volume of 25 mL. Finally, 500 μL of the diluted hydrolysate was evaporated to dryness with nitrogen gas (60 °C) and reconstituted in 1 mL of deionized water with the addition of d4-CML, d4-CEL and 13C6-HMF internal standard solutions for the same SPE clean-up procedure as mentioned above.
Preprocessing of HMF, 1 mL of supernatant was passed through an Oasis HLB cartridge preconditioned with 3 mL of methanol followed by 3 mL water. The column was washed with 1 mL of deionized water and finally eluted with 2 mL of methanol. The eluate was collected and filtered through a 0.22 μm membrane before injection into the LC-MS/MS system.
2.3.2. Sample preparation for analysis of α-dicarbonyl compounds in butter cookies
The levels of α-dicarbonyl compounds (GO, MGO, 3-DG) in butter cookies were analyzed according to procedures described in previous research with some modifications (Arribas-Lorenzo and Morales et al., 2010). A 10 μL of 5-MQ (2 mg/mL, internal standard) was added into 0.5 g of ground cookies powder before defatting as described above; then 200 μL of supernatant was mixed with 400 μL of potassium phosphate buffer (0.1 M, pH 7.0) and 400 μL of o-phenylenediamine solution (1%, w/v) in a tube. After vortex mixing for 30 s, the tubes containing the reaction mixture were placed in a thermostatic incubator (SHP-150, Shanghai Sumsung Laboratory Instrument Co., Ltd., Shanghai, China) set at 25 °C and kept in the darkness for 3 h. Finally, the sample solutions were transferred to autosampler vials after passing through a 0.22 μm syringe filter.
2.3.3. LC-MS/MS analysis
The determination of free CML and CEL, protein-bound CML and CEL, HMF and α-dicarbonyl compounds in butter cookies was conducted on an Agilent 1290 high performance liquid chromatography system combined with an Agilent 6460 triple-quadrupole mass spectrometer (Agilent Technologies Inc. Santa Clara, CA, USA).
The analysis of CML, CEL and HMF was performed on a 4 μm Hydro-RP 80 Å column (150 mm × 2 mm, Phenomenex, Torrance, CA, USA) using isocratic mixture of methanol (A) and 0.1% (v/v) formic acid solution (B) at a flow rate of 0.2 mL/min at 30 °C, with 2 μL of injection volume. The gradient profile of the binary pump started at 99% B (0 min), was maintained to 3 min, and was then decreased to 80% of eluent B in 3 min; then increased to 99% of eluent B over 2 min; finally, it was held at 99% B for 2 min for the purpose of equilibrating the column for the next injection. The electrospray ionization (ESI) source was operated in positive mode and selective detection using multiple reaction monitoring (MRM) mode was conducted. Quantitation was achieved by means of the internal standard method; the quantifying and qualifying transition ions were shown in Table 1. The ionization parameters were set as follows: capillary voltage 3.5 KV; drying gas temperature: 350 °C; drying gas flow: 12 L/min; nebulizer: 35 psi; sheath gas temperature: 350 °C; sheath gas flow: 9 L/min; nozzle voltage: 500 V.
Table 1.
Mass spectrometric settings for MRM of the analytes and internal standards.
| Compounds | Precursor ion (m/z) | Product ions (m/z) |
|
|---|---|---|---|
| Quantification ion | Confirmation ion | ||
| CML | 205 | 84 | 130 |
| d4-CML* | 209 | 88 | 134 |
| CEL | 219 | 84 | 130 |
| d4-CEL* | 223 | 88 | 134 |
| HMF | 127 | 109 | 81 |
| 13C6-HMF* | 133 | 115 | 86 |
| QD of GO | 131 | 77 | 51 |
| QD of MGO | 145 | 77 | 92 |
| QD of 3-DG | 253 | 171 | 199 |
| 5-methylquinoxaline* | 145 | 91 | 65 |
Abbreviations: CML, Nε-(carboxymethyl)lysine; CEL, Nε-(carboxyethyl)lysine; HMF, 5-hydroxymethylfurfural; QD, Quinoxaline Derivative. Compounds marked with an asterisk (*) serve as internal standards.
The analysis of quinoxaline derivatives of GO, MGO and 3-DG was carried out on the same instrument and column mentioned above. The mobile phase consisted of 0.1% (v/v) aqueous formic acid solution (A) and methanol (B). The gradient profile of the binary pump was initiated at 60% A and decreased to 30% A from 0 to 4 min, then, maintained at 30% A from 4 to 6 min, and finally went back to 60% A in 2 min, and then equilibrating at 60% A for 2 min. The flow rate was 0.25 mL/min, and the injection volume was 1.0 μL. The ESI source was operated in positive mode and the MS was performed in the MRM mode with the following settings: drying gas temperature: 300 °C; drying gas flow rate: 12 L/min; nebulizer pressure: 35 psi; capillary voltage: 4000 V. Quantitation was achieved by means of the internal standard method, the quantifying and qualifying transition ions were shown in Table 1.
2.4. Sensory evaluation of butter cookies
The sensory panel consisted of ten panelists who were trained with the sensory profile method (Przygodzka et al., 2015). Five sensorial parameters were set up as taste, chewing, odor, color and appearance based on their liking value (V = [0, 5], 5 = like very much, 0 = dislike very much).
2.5. Statistical analysis
Experimental data was reported as mean ± standard deviation. All analytical determinations were carried out in triplicate. The significance of the difference was evaluated with one-way ANOVA (analysis of variance) and Duncan’s test (p < 0.05) by using SPSS Version 22.0. The Pearson correlation analysis was performed at a significance level of p < 0.05.
3. Results and discussion
3.1. Effects of different baking temperature and time on formation of AGEs and HMF in butter cookies
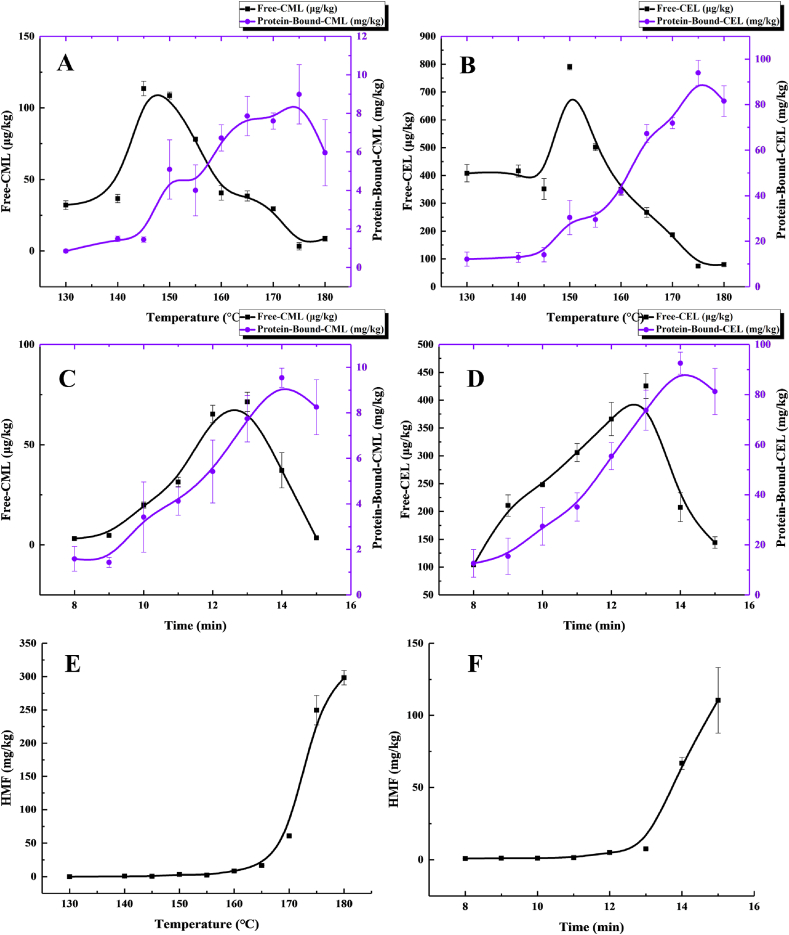
In the present study, effects of different baking temperatures and times on formation of free CML, free CEL, protein-bound CML, protein-bound CML and HMF in the butter cookies were investigated (Fig. 1). All investigated AGEs and HMF could be determined in the butter cookies, and increasing content of free AGEs were observed with higher baking temperatures (130°C–150 °C) and longer baking time (8 min–13 min). The levels of CML and CEL in butter cookies were different, the content of CEL was much higher than the CML. That is most probably due to the different reaction rates of the two dicarbonyl compounds, MGO and GO, lysine at high processing temperatures (Srey et al., 2010). The formation curves of CML and CEL were similar and it was found that the content of free CML (108.57 μg/kg) and CEL (789.51 μg/kg) reached the highest peak when baking temperature rose to 150 °C. As the baking temperature increases, the free CML and CEL gradually decreased until the baking temperature rises to 180 °C, which is clear that AGEs cannot continue to accumulate under high temperature baking conditions. Unlike free AGEs, the amounts of protein-bound AGEs did not decrease immediately until the baking temperature is higher than 175 °C. Similarly, the content of free CML (71.39 μg/kg) and CEL (425.42 μg/kg) reached the highest peak when baking at 13 min. As the baking time continues to extend, the content of free AGEs decreased, while the protein-bound AGEs shows a similar trend after 14 min. Research (Zhang et al., 2011) had shown that CML in free AGEs (free CML) is much more bioavailable and harmful than CML in protein or peptide-bound AGEs (bound CML), since they are more easily released from a food system and absorbed into serum, but low levels of free CML under elevated temperatures in butter cookies seem to avoid this hazard. However, unlike AGEs, with the increase of thermal processing intensity, the content of HMF increased exponentially (Fig. 1 E&F). The level of HMF under 180 °C was almost 36 times higher than that of at 160 °C. As expected, it was confirmed that HMF production from hexose is much more sensitive to the baking temperature and time. Berk (Berk et al., 2019) also found that the formation of HMF showed an increasing trend during roasting at all temperatures in the roasted sesames. HMF is formed as an intermediate from 1,2-enolization and dehydration of glucose or fructose under acidic conditions (Navarro and Morales, 2017). The further dehydration and cyclization of 3-DG is the key step for the formation of HMF. In acidic conditions, HMF is mainly formed at high temperature under dry and pyrolytic conditions; it can arise from fructose and sucrose via the formation of highly reactive fructofuranosyl cations (Locas and Yaylayan, 2008). Therefore, the phenomenon that the formation curves of 3-DG (Fig. 2) and HMF were highly consistent can be explained, and the research result is confirmed.
Fig. 1.
Changes of different baking temperature and time on formation of free AGEs (A&B), protein-bound AGEs (C&D) and HMF(E&F) in the butter cookies; values are expressed as mean ± SD (n = 3) for all treatments.
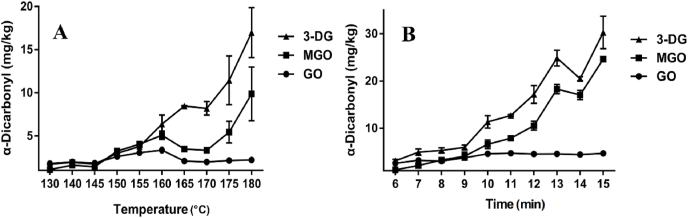
Fig. 2.
Changes of different baking temperature (A) and time (B) on formation of α-dicarbonyl compounds in the butter cookies; values are expressed as mean ± SD (n = 3) for all treatments.
α-Dicarbonyl compounds are important intermediate products originated from dehydration and fragmentation of Amadori or Heyns products, and they may also be formed by either sugar/lipid degradation (Thornalley et al., 1999). GO, MGO and 3-DG are highly reactive α-dicarbonyl compounds capable of stimulating the Maillard reaction towards the formation of AGEs after reaction with amino residues or even other intermediary compounds as HMF by dehydration (Poulsen et al., 2013). The formation of α-dicarbonyl compounds in different baking temperatures and times were studied (Fig. 2). As shown in the figure, most of α-dicarbonyl compounds were positively correlated with baking temperature and time. The formation curves of 3-DG and HMF were similar, which gradually increased with the intensity of the reaction. However, GO and MGO fluctuated under baking temperatures were varied between 130 °C and 160 °C. Reactive dicarbonyls act as intermediate species leading to the formation of AGEs. MGO specially causes the generation of CML and CEL dimer modifying a number of proteins (Singh et al., 2001). It can be seen that the caramelization continues to participate in the whole process and a large amount of HMF was accumulated. The inconsistency of the formation trends of GO and MGO with AGEs indicates that the Maillard reaction would continuously releasing active dicarbonyl compounds, but AGEs might participate in downstream reactions in this dynamic reaction.
3.2. Effects of different recipes on formation of AGEs and HMF in butter cookies
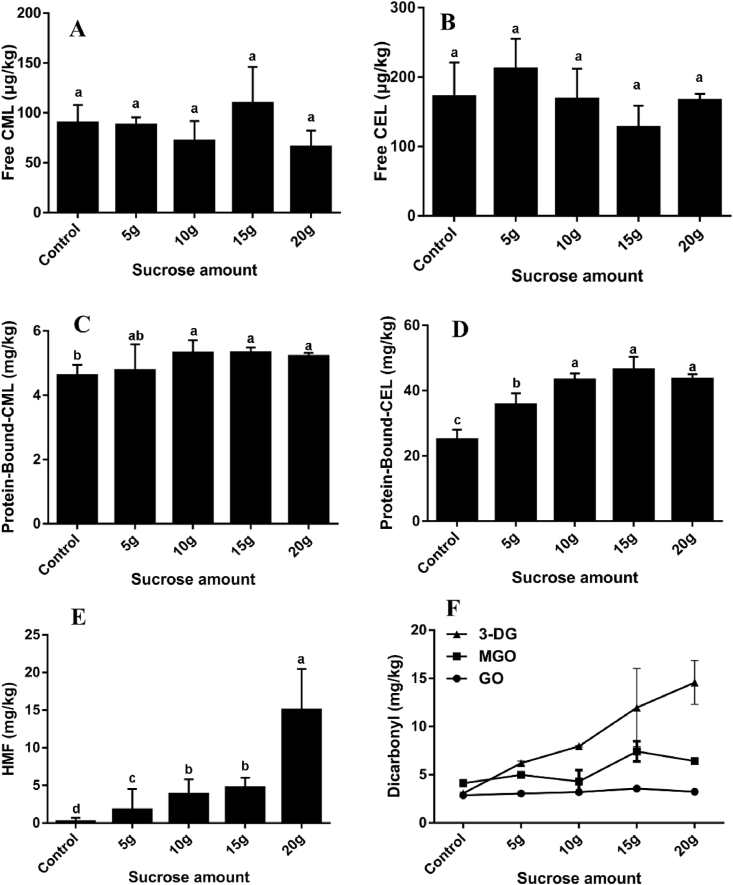
As one of the most indispensable precursors of Maillard reaction, sugar has an extremely important effect on the formation of thermal processing associated hazards in butter cookies. During the baking process of butter cookies, sucrose and starch may be hydrolyzed into reducing sugars (glucose and fructose), or a mixture of maltodextrin and maltose, and the hydrolyzed reducing sugars will be more easily involved in Maillard reaction (Ameur et al., 2007). The levels of AGEs and HMF with different amounts of sucrose in butter cookies were measured and the data were illustrated in Fig. 3. Different ratios of sucrose added in butter cookies did not significantly change the free AGEs, it is most likely due to the low level of free lysine in cookies. But it is worth noting that the protein-bound CML and protein-bound CEL showed a significant growth trend (15.15% and 73.22%) after the addition of sucrose amount was greater than 10 g (p < 0.05). It is worth noting that the content of HMF has been greatly affected by the increase of sucrose, which is similar to the change trend of 3-DG. The accumulation of sucrose level did not directly lead to the accumulation of GO and MGO, and the excess sucrose level apparently did not preferentially participate in the Maillard reaction and was caramelized. Furthermore, GO and MGO could be generated via auto-oxidative degradation of reducing sugars, which directly generated AGEs by reacting with lysine as intermediates in the formation of CML and CEL (Wolf pathway) (Wolff and Dean, 1987). It is clearly shown that the formation of GO, MGO and 3-DG was enhanced under Maillard reaction and caramelization when the proportion of sucrose level in the butter cookie recipe was increased. Above all, the increase of sucrose level in butter cookies directly affects the accumulation of α-dicarbonyl compounds, especially 3-DG, which leads to the simultaneous increase of bound AGEs and HMF.
Fig. 3.
Changes of different sucrose amount on formation of free CML (A), free CEL (B), protein-bound CML (C), protein-bound CEL (D), HMF (E) and α-dicarbonyl compounds (F) in the butter cookies; values are expressed as mean ± SD (n = 3) for all treatments; different letters indicate significant differences (p < 0.05) among different addition level of sucrose.
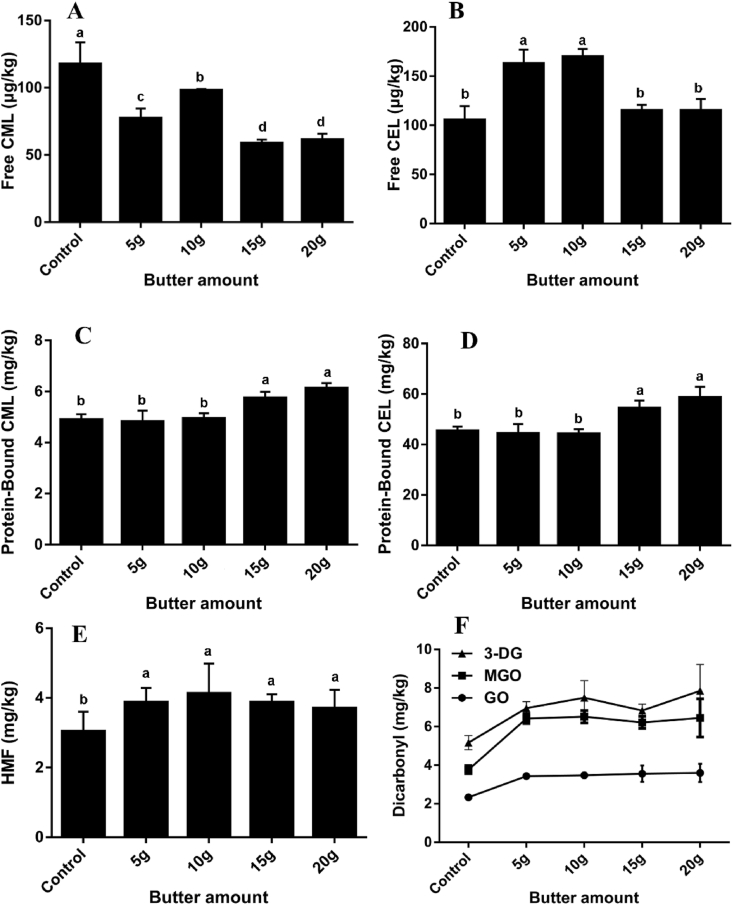
The reaction pathways involved in the formation of AGEs are complex and diverse. They are not only produced by the Maillard reaction pathway, but can also be generated as advanced lipid oxidation end products through the reaction pathway of lipid oxidation (Fu et al., 1996). The levels of AGEs and HMF with different amounts of butter in butter cookies were evaluated and the results were illustrated in Fig. 4. The change of butter ratio in cookies brought some fluctuations to free AGEs, but no obvious regularity was found. And it can be observed that the high level of butter (>15 g) in butter cookies significantly increases the content of protein-bound AGEs (p < 0.05), and the trend of α-dicarbonyl compounds can be seen that the increase in lipid content in butter cookies contributes to the formation of α-dicarbonyl compounds. According to a possible pathway for the formation of AGEs proposed by Fu (Fu et al., 1996), the oxidative degradation of lipids could also generate GO and Jiang (Jiang et al., 2013) also found that short-chain α-dicarbonyl compounds, GO and MGO can also be generated from lipid oxidation reactions involving free radicals. However, the level of HMF did not change significantly (p > 0.05) even though the content of 3-DG increased. This revealed that the formation of HMF is not dependent on lipid level in contrast to previous studies where HMF was affected by sucrose. Furthermore, similar behavior was observed in both dicarbonyls, GO and MGO formation can result from isomerization and subsequent retro-aldolization of sugar or lipid oxidation. In any case, lipids produced a slightly lower boost to AGEs and dicarbonyls than sucrose. Above all, α-dicarbonyl compounds can be produced due to the oxidative degradation of butter, bound AGEs and HMF also increased as in the sucrose group, but HMF was obviously much less affected by butter than sucrose.
Fig. 4.
Changes of different butter amount on formation of free CML (A), free CEL (B), protein-bound CML (C), protein-bound CEL (D), HMF (E) and α-dicarbonyl compounds (F) in the butter cookies; values are expressed as mean ± SD (n = 3) for all treatments; different letters indicate significant differences (p < 0.05) among different addition level of butter.
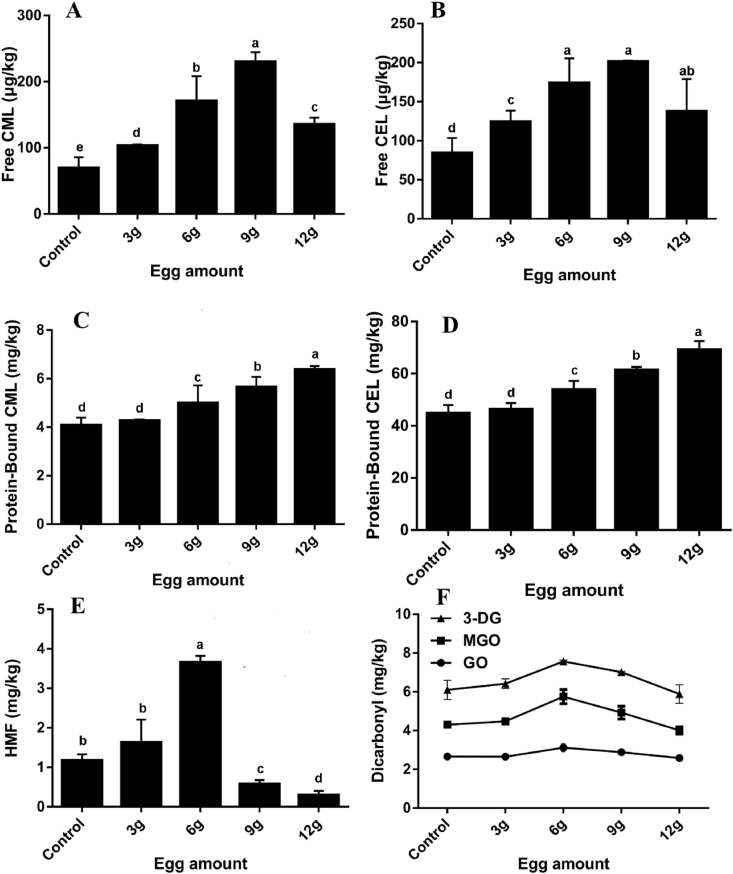
Butter cookies is a kind of complex food system of sugars, proteins (amino acids) and lipids that coexist and react with each other. The amino group in the protein or amino acid reacts with the carbonyl group in the system to generate Schiff base and cyclize into N-glucosylamine, and then 5-HMF is formed through 1, 2-enolization through Amadori rearrangement (Hodge, 1953). Active compounds such as α-dicarbonyl compounds readily react with amino acids and proteins. It can be seen that proteins or amino acids act as the "fuse" in the whole reaction, so that the Maillard reaction can continue the chain reaction. In addition to the protein and amino acids found in flour and butter, the egg white in the egg liquid is also high level in protein. The content of AGEs and HMF with different levels of whole egg liquid in butter cookies were investigated and the results were illustrated in Fig. 5. The cookies with 9 g of egg amount produced the highest amount of free CML (230.00 μg/kg) and free CEL (201.51 μg/kg), cookies with 12 g of egg amount produced the highest amount of protein-bound CML (6.38 mg/kg) and protein-bound CEL (69.19 mg/kg), cookies with 6 g of egg amount produced the highest amount of HMF (3.65 mg/kg). It was concluded from the production trend of free and protein-bound AGEs that the whole egg liquid provides sufficient protein and free amino acids for baking in butter cookies. When the amino group was replenished in a large amount, it was clear that the Maillard reaction dominates during baking. At the same time, when the whole egg level reaches 6 g, the HMF content reaches the extreme value and then drops sharply with the continued increase of egg amount. It was reported that HMF is formed from both the degradation of hexoses and it is also an intermediate in the Mallard reaction (Stadler and Lineback, 2008). The intensification of Maillard reaction promoted the formation of HMF in the initial stage. With the increase of amino group source, HMF obviously participated in the downstream reaction. In the same way, the α-dicarbonyl compounds were also from the excessive production stage to the large consumption stage. In a protein-rich or amino acid-rich food matrix, the Maillard reaction may be more dominant than the caramelization.
Fig. 5.
Changes of different egg liquid amount on formation of free CML (A), free CEL (B), protein-bound CML (C), protein-bound CEL (D), HMF (E) and α-dicarbonyl compounds (F) in the butter cookies; values are expressed as mean ± SD (n = 3) for all treatments; different letters indicate significant differences (p < 0.05) among different addition level of egg liquid.
3.3. Effects of different recipes on sensory qualities of butter cookies
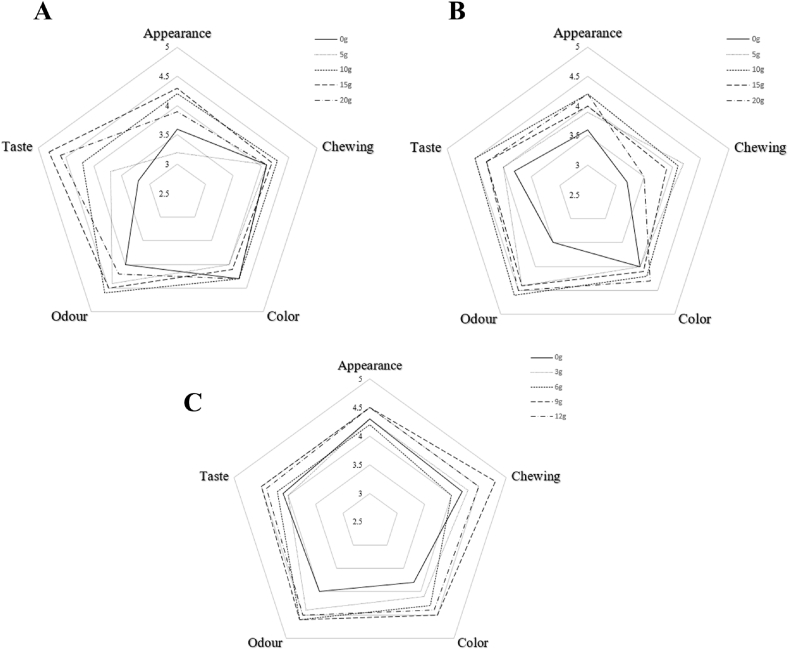
The sensory qualities of different sucrose levels of butter cookies were evaluated and the scores of sensory parameters were presented by a radar plot as shown in Fig. 6. Results suggested addition of sucrose to butter cookies also had an impact on sensory qualities such as score of taste, chewing, odor, color and appearance. The overall evaluation result of 15 g sucrose addition was the best. Generally speaking, butter cookies with a higher sugar level can significantly improve the taste. Obviously, high-content sucrose cookies are more favored by panelists, especially in terms of appearance, taste and odor. Observed from the radar chart Fig. 6B, low-content butter in cookie was obviously unpopular, especially if no butter was added. The overall evaluation result of 10 g butter was the best. Cookies with a higher butter level may have a negative greasy taste for some panelists. The difference from the previous two groups, the sensory results of different egg liquid ratios in butter cookies were not significant, but it is still a positive return to increase the level of egg liquid to improve the odor and taste brought by Maillard reaction.
Fig. 6.
Radar plot of sensory results of cookies. (A) Sucrose group; (B) butter group; (C) egg group.
The Maillard reaction leads to hundreds of molecules, some of these compounds are useful since they are responsible for color and flavor formation or they contribute to texture. Some other Maillard reaction end products can contribute to the off-taste of foods or have potential negative effects on human health. Obviously, sucrose, butter, and egg liquid, as the main raw materials of butter cookies, have a great impact on the sensory qualities of cookies. In general, the popularity of butter cookies is directly proportional to the progression of the Maillard reaction and caramelization, which also poses a challenge for research to control these heat-induced toxicants.
4. Conclusion
In this study, we evaluated the level of free AGEs, protein-bound AGEs, HMF and α-dicarbonyl compounds with different processing conditions and recipes in butter cookies, the effect of different baking conditions and recipes on the sensory properties and quality of butter cookies was studied. Different baking conditions have significant differences in the formation of heat-induced toxicants in butter cookies. The complex and interrelated evolution of chemical parameters in a cookie during baking was described. The increase in temperature and time of baking conditions the degradation of the sugar in cookies. Both sucrose and butter promote the increase of reactive dicarbonyl compounds which directly or indirectly affect the formation of AGEs and HMF in butter cookies. Thus, it is hard to achieve a sync to avoid all heat-induced toxicants being too high while taking into account the flavor. Consumer preference for butter cookies is often based on the pleasant sensory enjoyment of the Maillard reaction due to their high fat and sugar level.
In general, more research and assessment of butter cookie quality are needed in many countries to provide more data and help safeguard the health of humans, such as the selection of raw materials and addition of exogenous additives, which will be the focus of our future investigation to predict AGEs and HMF formation during the thermal processing of butter cookies at the industrial level.
CRediT authorship contribution statement
Huiyu Hu: Data curation, Investigation, Software, Writing – original draft. Yuting Wang: Methodology, Visualization, and. Mingyue Shen: Validation, Writing – review & editing, and. Yousheng Huang: Methodology, Visualization. Chang Li: Validation, Writing – review & editing. Shaoping Nie: Conceptualization. Mingyong Xie: Conceptualization, and.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial supports from the National Key Research and Development Program of China (2017YFC1600405) and the National Natural Science Foundation of China (No: 32060580).
Handling editor: Alejandro G. Marangoni
References
- Ahmed M.U., Brinkmann F.E., Degenhardt T.P., Thorpe S.R., Baynes J.W. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997;324(pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameur L., Mathieu O., Lalanne V., Trystram G., Birlouezaragon I. Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chem. 2007;101(4):1407–1416. doi: 10.1016/j.foodchem.2006.03.049. [DOI] [Google Scholar]
- Arribas-Lorenzo G., Morales F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010;58(5):2966–2972. doi: 10.1021/jf902815p. [DOI] [PubMed] [Google Scholar]
- Berk E., Hamzalıoğlu A., Gökmen V. Investigations on the maillard reaction in sesame (Sesamum indicum L.) seeds induced by roasting. J. Agric. Food Chem. 2019;67(17):4923–4930. doi: 10.1021/acs.jafc.9b01413. [DOI] [PubMed] [Google Scholar]
- Chuyen N.V. Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs. Mol. Nutr. Food Res. 2006;50(12):1140–1149. doi: 10.1002/mnfr.200600144. [DOI] [PubMed] [Google Scholar]
- Courel M., Ait-Ameur L., Capuano E., Fogliano V., J Morales F., Courtois F., Birlouez-Aragon I. Effects of formulation and baking conditions on neo-formed contaminants in model cookies. Czech J. Food Sci. 2009;27(Special Issue 1):S93–S95. doi: 10.17221/954-CJFS. [DOI] [Google Scholar]
- Degen J., Hellwig M., Henle T. 1,2-Dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012;60(28):7071–7079. doi: 10.1021/jf301306g. [DOI] [PubMed] [Google Scholar]
- Filipcev B., Simurina O., Bodroza-Solarov M. Quality of gingernut type biscuits as affected by varying fat content and partial replacement of honey with molasses. J. Food Sci. Technol. 2014;51(11):3163–3171. doi: 10.1007/s13197-012-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane S.G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Fu M., Requena J.R., Jenkins A.J., Lyons T.J., Baynes J.W., Thorpe S.R. The advanced glycation end product, nε-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996;271(17):9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- Hodge J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953;1(15):928–943. doi: 10.1021/jf60015a004. [DOI] [Google Scholar]
- Hu H., Wang Y., Huang Y., Yu Y., Shen M., Li C., Nie S., Xie M. Natural antioxidants and hydrocolloids as a mitigation strategy to inhibit advanced glycation end products (AGEs) and 5-hydroxymethylfurfural (HMF) in butter cookies. Foods. 2022;11(5):657. doi: 10.3390/foods11050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Hengel M., Pan C., Seiber J.N., Shibamoto T. Determination of toxic α-dicarbonyl compounds, glyoxal, methylglyoxal, and diacetyl, released to the headspace of lipid commodities upon heat treatment. J. Agric. Food Chem. 2013;61(5):1067–1071. doi: 10.1021/jf3047303. [DOI] [PubMed] [Google Scholar]
- Locas C.P., Yaylayan V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008;56(15):6717–6723. doi: 10.1021/jf8010245. [DOI] [PubMed] [Google Scholar]
- Navarro M., Morales F.J. Effect of hydroxytyrosol and olive leaf extract on 1,2-dicarbonyl compounds, hydroxymethylfurfural and advanced glycation endproducts in a biscuit model. Food Chem. 2017;217:602–609. doi: 10.1016/j.foodchem.2016.09.039. [DOI] [PubMed] [Google Scholar]
- Paravisini L., Sneddon K.A., Peterson D.G. Comparison of the aroma profiles of intermediate wheatgrass and wheat bread crusts. Molecules. 2019;24(13):2484. doi: 10.3390/molecules24132484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen M.W., Hedegaard R.V., Andersen J.M., de Courten B., Bügel S., Nielsen J., Skibsted L.H., Dragsted L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Przygodzka M., Zieliński H., Ciesarová Z., Kukurová K., Lamparski G. Study on sensory quality, antioxidant properties, and maillard reaction products formation in rye-buckwheat cakes enhanced with selected spices. J. Chem. 2015:1–9. doi: 10.1155/2015/418639. 2015. [DOI] [Google Scholar]
- Ramírez-Jiménez A., Guerra-Hernández E., García-Villanova B. Browning indicators in bread. J. Agric. Food Chem. 2000;48(9):4176–4181. doi: 10.1021/jf9907687. [DOI] [PubMed] [Google Scholar]
- Singh R., Barden A., Mori T., Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Srey C., Hull G.L.J., Connolly L., Elliott C.T., Del Castillo M.D., Ames J.M. Effect of inhibitor compounds onNε-(carboxymethyl)lysine (CML) and nε-(carboxyethyl)lysine (CEL) formation in model foods. J. Agric. Food Chem. 2010;58(22):12036–12041. doi: 10.1021/jf103353e. [DOI] [PubMed] [Google Scholar]
- Stadler R.H., Lineback D.R. John Wiley & Sons, Inc; 2008. Hydroxymethylfurfural (HMF) and Related Compounds; pp. 135–174. [DOI] [Google Scholar]
- Thornalley P.J., Langborg A., Minhas H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999;344(Pt 1):109–116. [PMC free article] [PubMed] [Google Scholar]
- Wolff S.P., Dean R.T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem. J. 1987;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Huang G., Xiao L., Mitchell A.E. Determination of advanced glycation endproducts by LC-MS/MS in raw and roasted almonds (Prunus dulcis) J. Agric. Food Chem. 2011;59(22):12037–12046. doi: 10.1021/jf202515k. [DOI] [PubMed] [Google Scholar]