Abstract
Hydrogen sulfide (H2S) has been identified as an important gaseous signal molecule in plants. Here, we investigated the effects of H2S on postharvest senescence and antioxidant metabolism of Lingwu Long Jujube (Ziziphus jujuba cv. Mill) fruits (LLJF). Fumigation of Jujube fruits with H2S released from 0.4 mm NaHS could significantly prolong the postharvest shelf life of jujube fruits, reduce the decay rate of fruit, the weight loss of fruit, and inhibit the fruit loss, hardness, color, soluble solids, and titratable acidity. Compared with the control group, exogenous H2S fumigation significantly decreased the loss of chlorophyll, carotenoids, soluble protein, ascorbic acid, phenols, and flavonoids in jujube fruits during post-harvest storage. At the same time, H2S could significantly delay the accumulation of malondialdehyde (MDA), hydrogen peroxide (H2O2) and superoxide anion (O2∙−) and promote catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD) activity, and inhibit polyphenol oxidase (PPO) activity. To summarize, H2S can effectively alleviate postharvest senescence and decay of jujube fruits by regulating the ROS accumulation and antioxidant enzymes, and prolong the storage period of postharvest.
Keywords: Hydrogen sulfide, Lingwu long jujube fruits, Post-harvest senescence, Quality maintenance, Antioxidant enzymes, Active oxygen
Graphical abstract
Highlights
-
•
H2S treatment could significantly prolong the postharvest shelf life of jujube fruits.
-
•
H2S could significantly delay the accumulation of MDA, H2O2 and O2∙− during storage of jujube fruits.
-
•
H2S treatment promote CAT, SOD, APX, POD activity, and inhibit PPO activity during storage of jujube fruits.
-
•
Provides a new method for storage of post-harvest jujube fruits.
1. Introduction
Lingwu Long Jujube (Ziziphus jujuba cv. Mill) is a specialty of Lingwu city in Ningxia and an important economic fruit. It has been cultivated in Lingwu city for more than 1300 years, and is recognized as the best fresh jujube variety. The fruit is rich in nutrients, especially the highest content of vitamin C. (Dai et al., 2017; Han et al., 2019). It has high medicinal value, and has been used as a traditional folk medicine for more than 4000 years, e.g., it plays an important role in anti-oxidation, anti-allergic, anti-inflammatory, anti-cancer, anti-obesity, and gastrointestinal protection, etc (Xu et al., 2020; Rashwan et al., 2020; Feng et al., 2019). However, Lingwu Long Jujube fruits (LLJF) are easy to lose water after harvesting, which always result in fruit shrinkage, softening, decay, mildew, etc. The gradual decline in nutritional value and economic value has greatly affected the post-harvest storage and sales of jujube fruits (Liu et al., 2018), and severely restricted the development of the fresh food industry of jujube fruits in China (Liu et al., 2018).
Hydrogen sulfide (H2S) is colorless, flammable, and has a strong smell of rotten eggs. H2S is the 3rd endogenous gaseous signal besides carbon monoxide (CO) and nitric oxide (NO), and is known to play multi-faceted roles in the regulation of plants and animals processes (Wang, 2012; Ali et al., 2019). In recent years, many reports have shown that H2S plays a key role in adventitious root formation (Lin et al., 2012), stomatal movement and photosynthesis (Duan et al., 2015), and seed germination (Zhang et al., 2008) during plant growth and development. H2S, as a gaseous regulator or signaling molecules, can also affect the harvested vegetables, such as bananas (Ge et al., 2018), persimmons (Niazi et al., 2021), kiwi fruit (Zhang et al., 2013a, Zhang et al., 2013b), cabbage (Al Ubeed et al., 2018), broccoli (Li et al., 2014a, Li et al., 2014b), etc., by regulating the active oxygen system, slowing down respiration and energy metabolism to delay the aging of fruits and vegetables (Huo et al., 2018).
Although, the recent research focuses on the fresh-keeping technology of jujube mainly on the aspects of modified atmosphere preservation (Xu et al., 2020), low-temperature preservation (Yu et al., 2021), chemical preservation (Li et al., 2014a, Li et al., 2014b), and film preservation (Zhang et al., 2014). Therefore, research on the new LLJF postharvest storage and preservation technology is of great significance to promote the jujube fresh food industry. To date, there is no study on the application of H2S in the postharvest storage of LLJF.
We speculated that H2S might be also involved in the regulation of physiological and biochemical characters of LLJF during postharvest storage. In present research, the effects of H2S signal on the postharvest storage of LLJF were investigated, including of physicochemical properties (decay rate, hardness, color, and titratable acidity, etc.), the natural antioxidants, and the bioactivities of antioxidant related enzymes. The obtained results can provide a theoretical basis to extend the storage period of post-harvested LLJF.
2. Materials and methods
2.1. Treatments of fruits
LLJFs were collected from the farm in Lingwu county, Ningxia province (China) during Mid-September, 2021. The physically damaged LLJF were removed, and the fruits of regular shape, uniform size, and color, and disease-free were kept for further treating, they were soaked in 75% alcohol for 2 min for disinfection and then natural air-drying. Solution of NaHS·3H2O (Sigma, Shanghai, China) was used as H2S donor according to our previous study (Ni et al., 2016). NaHS solutions with concentrations of 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, and1.1 mM were prepared in sealed containers for further testing on daily basis. Each group of LLJFs were treated with different concentration of H2S (0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, and 1.1 mM) in the sealed containers at 25 °C in three replicates. During treatment, their photographs were taken and the phenotypic changes were documented such as the appearance of mildew and softening of the fresh LLJFs. Simultaneously, the rates of the rotten fruit and the quality of the fruit were also observed.
2.2. Appearance evaluation
Fruit decay rate was investigated every other day using the following formula, fruit decay rate (%) = rotten fruit number/total fruit number × 100% (Hu et al., 2019).
The rate of weight loss was determined using the weighing method: the weight of LLJFs was measured every 48 h, and the rate of weight reduction was calculated using the following formula (Zhang et al., 2020):
| Weight loss rate (%) = [(a-b)/a] × 100% |
The hardness was measured with a texture analyzer (TMS-PRO physical property analyzer, FTC, USA) (Zhang et al., 2020). A cylindrical probe with a diameter (2 mm) was used for hardness measurement. The settings were as follows: The test speed was 30 mm/min; the downward pressure deformation was 40%; the trigger force was 0.15 N; with the average of 10 capsules in each test.
The a*, b*, L*, C*, and H* values of peel and pulp color were measured on symmetrical two sides of the equatorial region of each fruit using a calibrated colorimeter (HP-200 colorimeter) in reference to the following method (Prabhakar et al., 2022). Briefly, L* indicates brightness, ranging from 0 (black) to 100 (white); a* value indicate a red (+) to green (−) bias in substances colored; b* value indicates yellow (+) to blue (−) bias; saturation C*= (a*2+ b*2)1/2; tonal angle H* = arctan(b*/a*); total changes in color are indicated with ΔE, the color difference value ΔE represents the difference in color (L, a, b) at Nth day of storage of fruit compared to that at 0th day of storage (L*, a*, b*) (Zhang et al., 2021; Chen et al., 2014). ΔE was calculated as follows:
Total 10 LLJFs were investigated each time using the average values.
Total soluble solids (TSS) were measured using a digital refractometer (Tongfang Inc., Shanghai, China) with values being expressed as % (Jiang et al., 2004).
The titratable acidity (TA) of the LLJFs (three replicates of each treatment) was assayed using titration with NaOH (0.1 mM) at pH 8.3 (Francisca et al., 2016).
2.3. Assay of chlorophyll and carotenoid content
Chlorophyll and carotenoid contents of LLFJs were determined as follows (Nath et al., 2011; Etzbach et al., 2020). An accurate amount of LLJFs was homogenized and incubated on ice in an flask containing 10 mL of an absolute ethanol: acetone solution: water volume ratio of 4.5 : 4.5: 1 (measure 45 mL of acetone, 45 mL of absolute ethanol and distilled water to make the volume to 100 mL, and mix well) as an extraction solvent. And then, the extractions were kept in darkness for 48 h at 4 °C. After centrifugation, the values of OD663 nm, OD 645 nm, and OD 440 nm of supernatant were assayed, respectively (Lichtenthaler and Wellburn, 1983). The contents of chlorophyll and carotenoid (mg. g−1) were calculated as follows:
| Carotenoid = A440 V/W; Chla = (12.72·A663-2.59·A645)V/W; |
| Chlb = (22.88·A645-4.67·A663)V/W; |
| Chl = Chla + Chlb |
2.4. Assay of ascorbic acid (Vc), total phenols, flavonoids, and soluble protein
Ascorbic acid (Vc) in samples were determined according to the method by Moo-Huchin et al. (2014). LLJF samples (2.0 ± 0.05 g) were ground using 20 g/L oxalic acid, diluted to the volume of 100 mL, extracted for 10 min, and filtered for filtrate collection. Further, the 10 mL of the filtrate was poured in a 100 mL Erlenmeyer flask and titrated with 2,6- dichlorophenol-indophenol until a pink color appeared and remained unchanged for 15 s.
The total phenolics and flavonoid in LLJFs were determined followed the descriptions by previous reports (Pirie and Mullins 1976; Zhishen et al., 1999). 0.2 g of LLJFs sample was mixed with 2 ml pre-cooled HCl-methanol solution (1%), and grind on ice; and then, the mixture was diluted the volume to 10 ml with HCl-methanol solution (1%), and incubated 4 °C for 20 min under dark. After filtration, the values of OD280nm and OD325nm of filtrates were measured, respectively. Based on the standard curve with gallic acid and rutin, the content of total phenols and flavonoids of samples were calculated and expressed as mg·g−1 FW (Pirie and Mullins 1976; Zhishen et al., 1999).
For determination of soluble protein contents LLJFs samples (1.0 ± 0.05 g) were obtained as a homogenate and centrifuged (12,000 rpm for 20 min) at 4 °C followed by mixing of supernatant (1 mL) with Coomassie Brilliant Blue (5 mL) (Bradford, 1976). The values of OD595 nm of different samples were recorded, and the soluble protein contents were calculated and expressed as mg·g−1 FW.
2.5. Assay of MDA, H2O2 and O2∙- content in samples
The MDA, H2O2, and O2∙- contents were determined according to the manufacturer's instructions using kits (Suzhou Comin Biotechnology Co., Ltd. Suzhou, China). For MDA assay, 1.0 g samples were grinded with 5.0 ml TCA solution (100 g/L); After centrifugal at 10,000 g for 20 min at 4 °C, the supernatants were obtained and used for measurement using kit A003-1 (Suzhou Comin Biotechnology Co., Ltd. Suzhou, China). H2O2 and O2∙- content of samples were measured using H2O2 -1-Y kit and SA-1-G kit (Suzhou Comin Biotechnology Co., Ltd. Suzhou, China), respectively. All treatments were repeated three times.
2.6. Determination of CAT, SOD, APX, POD, and PPO activities
The activities of CAT, SOD, APX, POD and PPO were determined using respective assay kit (Suzhou Comin Biotechnology Co., Ltd. Suzhou, China) following the manufacturer's instructions. All treatments were repeated three times. CAT activity of samples was assayed based on method of ammonium vanadate-molybdate using CAT-1-W kit (Suzhou Comin Biotechnology Co., Ltd. Suzhou, China). SOD, APX, POD and PPO activity of samples were assed using SOD-1-W kit (WST-1 method), APX-1-W kit, BC0090 kit, PPO-1-Y kit (Suzhou Comin Biotechnology Co., Ltd. Suzhou, China), respectively.
2.7. Statistical analysis
The One-way or Two-way analysis of variance (ANOVA) to data were used for significant differences analysis using SPSS Statistics software at p<0.05 (SPSS version 20.0, Armonk, NY, USA).
3. Results
3.1. Effects of H2S on the appearance and decay rate of jujube fruit during storage
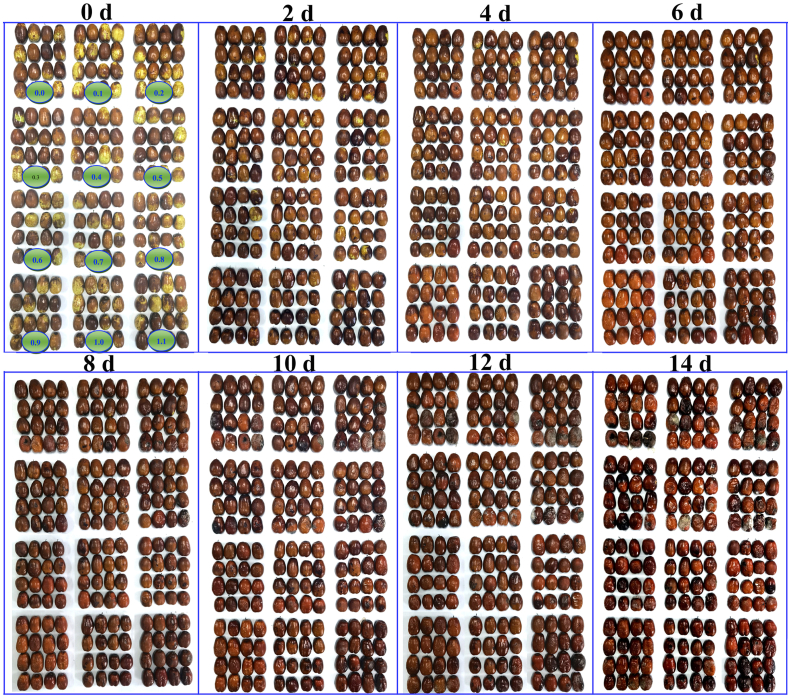
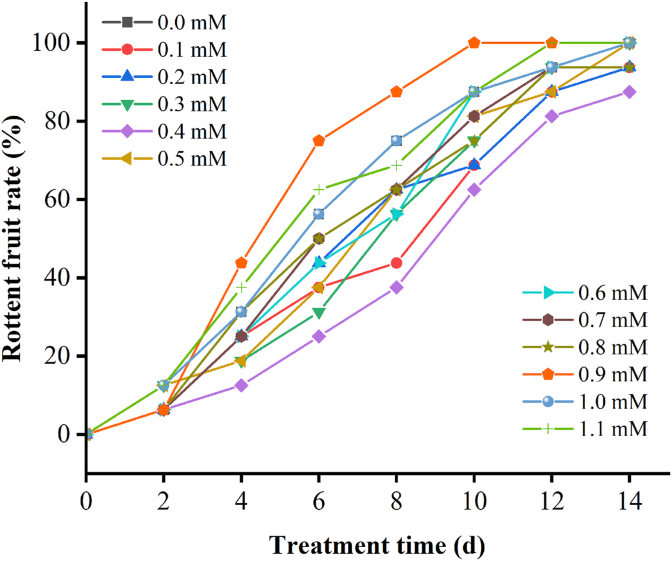
LLJFs were fumigated with H2S (0.1 mM–1.1 mM) and water treatment as control (Fig. 1.) Treatment under 0.4 mM hydrogen sulfide (H2S) delays the senescence and rotting of jujube fruits in a dose-dependent manner. As the storage time prolonged, the decay rate of higher concentration of NaHS treatment was increased. Particularly, 0.4 mM NaHS showed the remarkable inhibitory effects on the decay, moldy growth, softening, and shrinkage of Jujube fruits. Therefore, this concentration was used for the subsequent experiments throughout the study (Fig. 2).
Fig. 1.
The photographs of LLJFs after H2S treatment. Jujube fruit was fumigated daily with various concentrations of aqueous NaHS (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, and 1.1 mM). The preservation temperature was 25 °C.
Fig. 2.
The rotten rate of LLJF after H2Streatment during preservation. Every day, jujube fruits were fumigated with various concentrations of aqueous NaHS (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0 and 1.1 mM). The preservation temperature was 25 °C.
3.2. Effects of H2S on weight loss, hardness, color difference, and titratable acidity in fruit
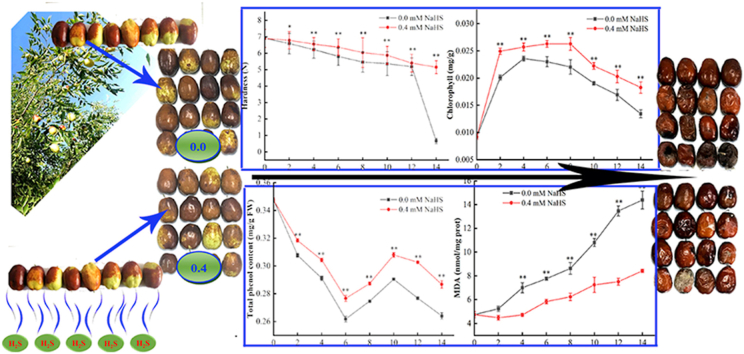
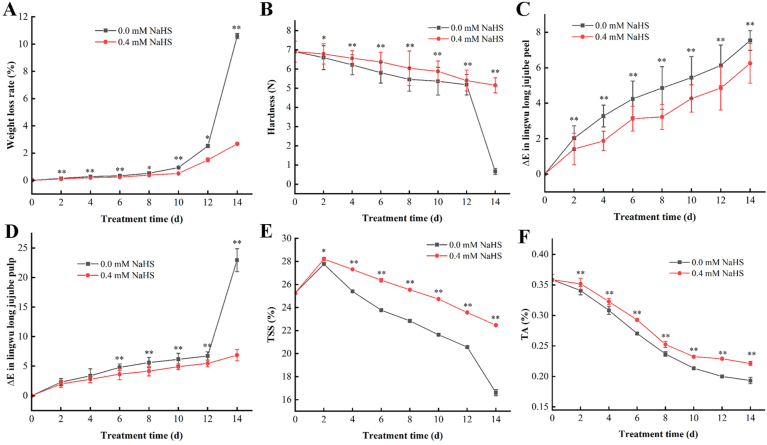
After harvest, the weight loss rate of jujube fruit increased linearly with the storage time owing to water transpiration (Fig. 3 A). Furthermore, the weight loss in control was higher significant than those of the H2S treated groups during storage (p < 0.05).
Fig. 3.
Effects of H2S on weight loss rate (A), hardness (B), peel color difference (C), pulp color difference (D), total soluble solids (TSS) (E) and titratable acidity (F) of Lingwu Long Jujube fruits. Jujube fruits were fumigated with 0.4 mM H2S donor NaHS aqueous solution with water as the control groups for 0–14 d. Data are presented as means ± SD (n = 3 replicates for A, E, F), n = 10 replicates for (B, C, D). The symbols ∗ and ∗∗ in figure and the following ones stand for a significant difference between control and 0.4 mM NaHS treatment at p < 0.05 and p < 0.01, respectively. FW = fresh weight. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Hardness is an important parameter reflecting the degree of fruit ripeness and softening. The hardness (Fig. 3B) of jujube fruit declines with storage time; however, the loss in hardness is delayed in H2S treated jujube fruit compared to the control group. From the 2nd day of the treatment, the hardness of H2S treated groups were higher than that of the control, and from the 4th day of H2S treatment, a significant difference (p < 0.05) was observed. It shows that treatment under 0.4 mM H2S obviously delay the senescence and softening process of jujube fruit during post-harvest storage.
The color change of the skin and pulp of jujube fruit during storage was depicted in Fig. 3. The color difference(ΔE) result indicates that the color difference between H2S treated and control group grew with the storage time and that the color difference between the skin (Fig. 3C) and pulp (Fig. 3D) of jujube fruit treated with H2S slowly changed. H2S can retard fruit ripening and senescence by altering the pigment color difference.
The soluble solids (TSS) content in fruit increased at first and then decreased with storage time (Fig. 3E). Throughout the storage period, the soluble solids content in treated group was continuously greater than that of the control with significant difference (p < 0.05) from 4th day to 14th day. Titratable acidity (Fig. 3F) in the control and H2S treated jujube fruit gradually dropped with the prolonged storage period. On the contrary, titratable acidity in NaHS treatment was in decreasing trend yet significantly higher than that of untreated group (p < 0.05) during storage period (Fig. 3E).
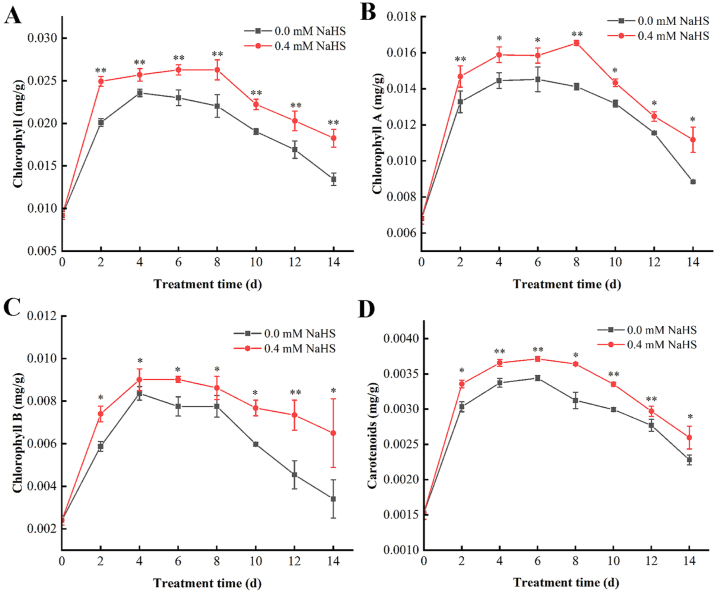
3.3. Effects of H2S on the chlorophyll and carotenoid contents in jujube fruit
The total chlorophyll, chlorophyll a, chlorophyll b, and carotenoids (Fig. 4A–D) of LLJFs showed a rising trend at first and then decrease with the extension of the storage period. The content of total chlorophyll and chlorophyll a in the treatment group reached the maximum on the 8th day, while the control group raised to the highest content on the 4th day, the chlorophyll b content of the treatment group and the control group reached the highest on the 4th day, and the carotenoid content reached the highest on the 6th day, and then revealed a downward trend during post-harvest storage in both un-treated and H2S treated groups, whereas H2S could restore relatively higher level during storage period. The above results indicate that the H2S treatment can delay the degradation of chlorophyll and carotenoids in jujube fruits, maintain the fresh color of the fruit, and delay the senescence of the fruit after harvest.
Fig. 4.
Effects of hydrogen sulfide on the contents of total chlorophyll (A), chlorophyll a (B), chlorophyll b (C) and carotenoids (D) in Lingwu Long Jujube fruits. Jujube fruits were fumigated with 0.4 mM H2S donor NaHS aqueous solution with water as the control groups for 0–14 d. Data are presented as means ± SD (n = 3). FW = fresh weight.
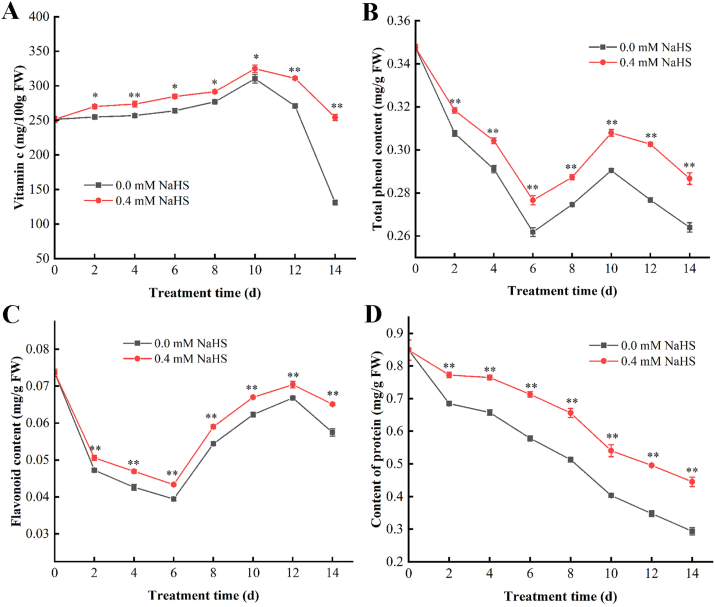
3.4. Effects of hydrogen sulfide on the contents of Vc, total phenols, flavonoids, and soluble protein in jujube fruit
The ascorbic acid in two groups increased firstly and then decreased with the storage, and reached the highest on the day 11 with 336.49 mg/100 g and 330.26 mg/100 g, respectively (Fig. 5A). The reason is that ascorbic acid is gradually consumed during fruit ripening after harvest, the ascorbic acid content of the H2S treated groups were dramatically higher than that of the un-treated group (p<0.05) during storage.
Fig. 5.
Effect of hydrogen sulfide on the content of ascorbic acid (A), total phenols (B), flavonoids (C) and soluble protein (D) in Lingwu Long Jujube fruits. Jujube fruits were fumigated with 0.4 mM H2S donor NaHS aqueous solution for 0–14 days, and distilled water was used as the control group. Data are expressed as mean ± SD (n = 3). FW = fresh weight.
The total phenol content of the H2S treated groups were always higher than that of the control group throughout the storage period (Fig. 5B). And during the storage process, the total phenol content was first decreased, then increased, and then decreased. From day 6 to day 10 of storage, the total phenol content slowly recovered, while the rising trend of the control group was comparatively lower. The similar trend was noticed for the alterations in flavonoid content (Fig. 5C). H2S treatment could considerably alleviate the decline and maintain the elevated levels of phenolics and flavonoid throughout the storage period.
As shown in Fig. 5D, the soluble protein content decreased during the post-harvest storage; H2S treatment could alleviate the decreasing of soluble protein content significantly (p<0.05). The above results demonstrated that H2S treatment could prevent the protein degradation in jujube fruit during storage.
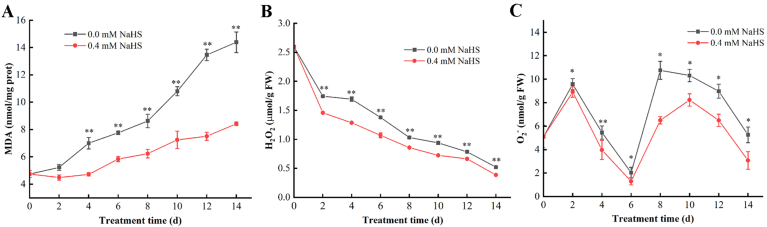
3.5. Effects of H2S treatment on the MDA, H2O2 and O2∙- in jujube fruits
The MDA content of jujube fruits increased throughout the storage in both of groups (Fig. 6A). MDA content in fruits of the un-treated group increased rapidly, while the increasing trend was relatively slow in the treatment group. The MDA content in treatment groups were consistently higher than that of the un-treated group (from day 4 to day 14) (p<0.05). As an index of lipid peroxidation (Li et al., 2019), H2S treatment significantly reduced MDA accumulation suggesting the role of H2S in alleviating the lipid peroxidation.
Fig. 6.
Effects of H2S on the content of malondialdehyde (MDA) (A), hydrogen peroxide (H2O2) (B) and superoxide anion (O2∙−) (C) in Lingwu Long Jujube fruits. Jujube fruits was fumigated with 0.4 mM H2S donor NaHS aqueous solution for 0–14 days, and distilled water was used as the control group. Data are expressed as mean ± SD (n = 3). FW = fresh weight.
Fig. 6B illustrated the influence of H2S on H2O2 of LLJFs after post-harvest. Throughout the storage period, the H2O2 content of the H2S treated groups were always lower than that of the un-treated group (p<0.05). H2S treatment could slow down the accumulation of H2O2 content. Fig. 6C illustrated the influence of H2S on the O2∙− content of post-harvest LLJFs. During the storage period, the content of O2∙− increased at first, then decreased followed by further alterations; however, the treatment group always showed the lower content than the control group. The H2S treatment group can delay the appearance of the accumulation peak of O2∙− content. The above results indicated that H2S treatment significantly prevented the synthesis of H2O2 and O2∙− during storage, and hence delayed the post-harvest senescence process.
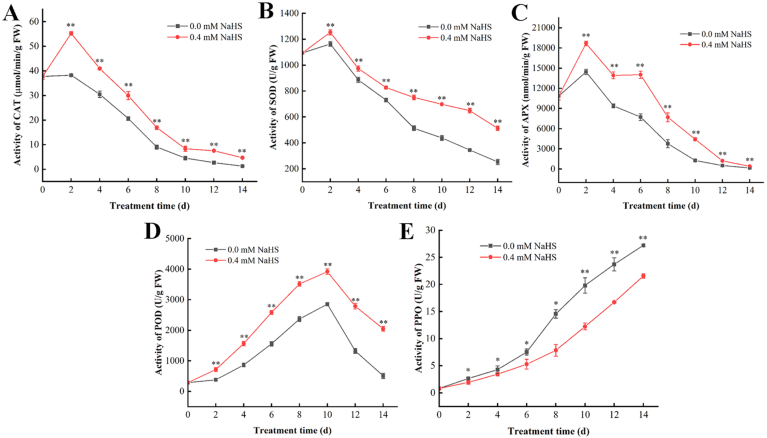
3.6. Effects of H2S on the activities of CAT, SOD, APX, POD and PPO in jujube fruit
Fig. 7A illustrated the influence of H2S on the CAT activity of jujube fruits following harvest. H2S treatment could rapidly increase the CAT activity on day 2 followed by a gradual decrease. Throughout the storage period, the CAT activity of the H2S groups were constantly higher than that of the un-treated group (p<0.05). H2S considerably improved the CAT activity in jujube fruits throughout the storage period (Fig. 7A).
Fig. 7.
Effects of H2S on the activities of catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), polyphenol oxidase (PPO) activity in Lingwu Long Jujube fruits. Jujube fruits was fumigated with 0.4 mM H2S donor NaHS aqueous solution for 0–14 days, and distilled water was used as the control group. Data are expressed as mean ± SD (n = 3). FW = fresh weight.
SOD is an important antioxidant enzyme in the antioxidant system of fruits. Its main function is to protect the cell membrane system from the damage of ROS, thereby delay the fruit ripening. The SOD activity of jujube fruits in the treatment group was consistently more significant than that in the control group (p<0.05) (Fig. 7B), which indicated that H2S treatment could delay the decline of SOD activity.
Fig. 7C illustrated the influence of H2S on the APX activity of jujube fruits following harvest. H2S treatment could rapidly increase the APX activity of Jujube fruits on day 2 followed by a gradually decreasing. Throughout the storage period, the APX activity of jujube fruits in the treatment group was consistently more significant (p<0.05).
Throughout the storage period, both the control and treatment groups demonstrated an initial increase followed by a further decrease. POD activity reached the maximum value on the day 10 of storage, and then showed a downward trend (Fig. 7D). The POD activity of the H2S group showed comparatively higher level from the day 2 of storage (p<0.05).
The PPO activity in jujube fruits showed an upward trend throughout the storage (Fig. 7E). The PPO activity of the H2S treatment groups were comparatively lower than that of un-treated samples (p<0.05).
Above results of indicated that H2S could reduce the ROS accumulation, increase the activity of antioxidant enzymes, and inhibit the activity of polyphenol oxidase, thereby alleviated the postharvest senescence of fruits, and maintained the jujube fruit quality.
4. Discussion
LLJFs have bright color, thin skin, thick flesh, and rich nutrient content (Feng et al., 2019). However, it is very easy to lose water and shrink after harvest, leading to metabolic disorders, and it is prone to softening, mildew and other phenomena, the fruits became darker, and the color difference was gradually increased with the extension of storage time, which seriously affect its nutritional and economic values (Zhou et al., 2019; Chen et al., 2014). Our study revealed that H2S treatment could effectively reduce the weight loss in jujube fruits and maintain the higher firmness. H2S fumigation slowed the degradation of TSS and TA in jujube fruits indicating its role in delaying fruit maturation. Color is also a significant indicator of the fruit's quality and worth as a commodity, H2S fumigation significantly reduced the color intensity change (ΔE) of the skin and pulp of jujube fruits during storage. Our results were consistent with the reports by Hu et al. (2012) and Yao et al. (2020), who reported that strawberry fruits fumigated with different concentrations of H2S had significantly lowered rot index and fruit hardness compared with the control group, and H2S treatment can also slow the change of tomato and strawberry color and delay the ripening of the fruits during post-harvest storage.
Fruit ripening and senescence are always accompanied by chlorophyll degradation (Fu et al., 2017; Hörtensteiner, 2006). In our study, H2S treatment could significantly prevent the chlorophyll and carotenoid degradation in jujube fruits, which was consistent with the previous findings suggesting that H2S treatment could significantly prevent the chlorophyll and carotenoid degradation in Fresh-cut Kiwifruit (Zhang et al., 2013a, Zhang et al., 2013b).
Ascorbic acid, phenols, flavonoids, and soluble proteins are important nutrients in jujube fruits (Ni et al., 2016; Shukanta et al., 2020). In our study, H2S treatment could significantly prevent the content of ascorbic acid, phenols, flavonoids, and soluble proteins degradation in jujube fruits, which was consistent with the previous findings suggesting that H2S treatment could significantly prevent the phenols, flavonoids, and soluble proteins degradation in Fresh-cut Kiwifruit and eggplant fruit and hawthorn fruit (Zhang et al., 2013a, Zhang et al., 2013b; Barzegar et al., 2021; Aghdam et al., 2018).
Environmental stress and tissue aging can lead to the ROS accumulation in fruit and vegetable tissues, inducing a large number of free radicals, damaging biological cell membranes, and producing a large number of membrane peroxidation products such as malondialdehyde (Li et al., 2019). ROS are the primary mediators of oxidative damage in plants, and they mainly consist of H2O2 and O2∙−. Accumulation of a certain amount can cause oxidative damage to cell components and promote fruit senescence and spoilage (Zhang et al., 2013a, Zhang et al., 2013b). We found that H2S could effectively lower the H2O2 accumulation and O2∙− content via significantly enhancing the activities of CAT, SOD, POD, and APX in Lingwu Long Jujube (Ziziphus jujuba cv. Mill) fruit. In our study, H2S treatment could comparatively restore the higher levels of ascorbic acid, flavonoid, and phenolics in jujube fruits. The flavonoids content in Chinese jujube treated with composite coating was relatively higher indicating that the composite coating can delay the decomposition of flavonoids (Yu et al., 2021).
PPO is the main enzyme that causes enzymatic browning. The ripening and senescence of fruit tissue is often accompanied by the increase of PPO activity, which eventually leads to the browning of fruit tissue, which changes the color of the fruit and affects its storage quality (Li et al., 2014a, Li et al., 2014b). We found that H2S donor treatment could inhibit the rapid rise of PPO activity in the fruit, thereby alleviating the degree of browning of the fruit during storage.
This was consistent with the H2S treatment that could effectively inhibit the postharvest rot of fruits and vegetables (Fu et al., 2017), and the changes in physiological and biochemical indicators during maturation and senescence of plants such as fresh cut flowers (Zhang et al., 2011; Zhang et al., 2021), fresh cut pears (Hu et al., 2017), and mulberries (Hu et al., 2014).
5. Conclusions
To summarize, we demonstrated that exogenous H2S can effectively delay the degradation of jujube fruits following harvest and preserves their hardness, color, nutrients, and natural antioxidants. H2S could inhibit the buildup of ROS such as H2O2 and O2∙− and the lipid peroxidation products MDA and PPO activity. Moreover, it could also enhance the antioxidant enzymes CAT, SOD, APX and POD activities which ultimately prolonged the senescence of post-harvested jujube fruits.
Funding
This research was funded by National Natural Science Foundation of China (32160588), the National Natural Science Foundation of Ningxia Province (2022AAC03280), Innovation Team for Genetic Improvement of Economic Forests of Ningxia Province (2022QCXTD04), the Youth talent cultivation project of North Minzu University (2021KYQD27, FWNX14), Key research and development projects in Ningxia (2021BEF02013).
CRediT authorship contribution statement
Yan-Mei Lv: Methodology, Validation, Investigation, Data curation, Writing – original draft, preparation, All authors have read and agreed to the published version of the manuscript. Elam Elnur: Methodology, Investigation, Data curation, All authors have read and agreed to the published version of the manuscript. Wei Wang: Software, All authors have read and agreed to the published version of the manuscript. Kiran Thakur: Validation, Writing – review & editing, All authors have read and agreed to the published version of the manuscript. Juan Du: Validation, All authors have read and agreed to the published version of the manuscript. Hong-Nian Li: Validation, All authors have read and agreed to the published version of the manuscript. Wen-Ping Ma: Formal analysis, All authors have read and agreed to the published version of the manuscript. Ya-Qin Liu: Resources, All authors have read and agreed to the published version of the manuscript. Zhi-Jing Ni: Conceptualization, Project administration, Funding acquisition, All authors have read and agreed to the published version of the manuscript. Zhao-Jun Wei: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Alejandro G. Marangoni
Contributor Information
Yan-Mei Lv, Email: 20207273@stu.nun.edu.cn.
Elam Elnur, Email: e18309578133@163.com.
Wei Wang, Email: wwang@nun.edu.cn.
Kiran Thakur, Email: kumarikiran@hfut.edu.cn.
Juan Du, Email: 20217528@stu.nun.edu.cn.
Hong-Nian Li, Email: li981101@163.com.
Wen-Ping Ma, Email: 2008025@nun.edu.cn.
Ya-Qin Liu, Email: liuyaqin650622@163.com.
Zhi-Jing Ni, Email: lovebear@vip.163.com.
Zhao-Jun Wei, Email: zjwei@hfut.edu.cn.
References
- Aghdam M.S., Mahmoudi R., Razavi F., Rabiei V., Soleimani A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci. Hortic. 2018;238:264–271. [Google Scholar]
- Al Ubeed H.M.S., Wills R.B.H., Bowyer M.C., Golding J.B. Comparison of hydrogen sulphide with 1-methylcyclopropene (1-MCP) to inhibit senescence of the leafy vegetable, pak choy. Postharvest Biol. Technol. 2018;137:129–133. [Google Scholar]
- Ali S., Nawaz A., Ejaz S., Haider S.T., Alam M.W., Javed H.U. Effects of hydrogen sulfide on postharvest physiology of fruits and vegetables: an overview. Sci. Hortic. 2019;243:290–299. [Google Scholar]
- Barzegar T., Najafi R., Razavi F., Ghahremani Z. Hydrogen sulfide and phenylalanine alleviate chilling injury in eggplant fruits during cold storage by enhancing antioxidant activities and membrane stability. J. Food Process. Preserv. 2021;45 [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen Qi, Bi J., Zhou Y., Liu X., Wu X., Chen R. Multi-objective optimization of spray drying of jujube (Zizyphus jujuba Miller) powder using response surface methodology. Food Bioprocess Technol. 2014;7:1807–1818. [Google Scholar]
- Dai Y., Wang Y., Xue J., Liu X., Liu B., Guo X. Research of segmentation method on image of Lingwu long Jujubes based on a new extraction model of hue. IEEE Sensor. J. 2017;17:6029–6036. [Google Scholar]
- Duan B., Ma Y., Jiang M., Yang F., Ni L., Lu W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2015;75:33–44. [Google Scholar]
- Etzbach L., Meinert M., Faber T., Klein C., Schieber A., Weber F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr. Res. Food Sci. 2020;3:73–81. doi: 10.1016/j.crfs.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Wang B., Zhao A., Wei L., Shao Y., Wang Y., Cao B., Zhang F. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2019;277:238–245. doi: 10.1016/j.foodchem.2018.10.104. [DOI] [PubMed] [Google Scholar]
- Francisca H., Luis N., Francisco B., Aneta W., Ángel A C., Pilar L. Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. J. Sci. Food Agric. 2016;96:2682–2691. doi: 10.1002/jsfa.7386. [DOI] [PubMed] [Google Scholar]
- Fu L., Hu K., Hu L., Li Y., Hu L., Yan H., Liu Y., Zhang H. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS One. 2017;9 doi: 10.1371/journal.pone.0104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y., Hu K., Wang S., Hu L., Chen X., Li Y., Yang Y., Yang F., Zhang H. Correction: hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M., Kan J., Wang Y. Ellipsoid fitting using variable sample consensus and two-ellipsoid-bounding-counting for locating Lingwu long Jujubes in a natural environment. IEEE Access. 2019;7:164374–164385. [Google Scholar]
- Hörtensteiner S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006;57:55–77. doi: 10.1146/annurev.arplant.57.032905.105212. [DOI] [PubMed] [Google Scholar]
- Hu H., Shen W., Li P. Effects of hydrogen sulphide on quality and antioxidant capacity of mulberry fruit. Int. J. Food Sci. Technol. 2014;49:399–409. [Google Scholar]
- Hu K., Wang Q., Hu L., Gao S., Wu J., Li Y., Zheng J., Han Y., Liu Y., Zhang H. Hydrogen sulfide prolongs postharvest storage of fresh-cut pears (Pyrus pyrifolia) by alleviation of oxidative damage and inhibition of fungal growth. PLoS One. 2017;9 doi: 10.1371/journal.pone.0085524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Hu S., Wu J., Li Y., Zheng J., Wei Z., Liu J., Wang H., Liu Y., Zhang H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012;60:8684–8693. doi: 10.1021/jf300728h. [DOI] [PubMed] [Google Scholar]
- Hu M., Zhu Y., Liu G., Gao Z., Li M., Su Z., Zhang Z. Inhibition on anthracnose and induction of defense response by nitric oxide in pitaya fruit. Sci. Hortic. 2019;245:224–230. [Google Scholar]
- Huo J., Huang D., Zhang J., Fang H., Wang B., Wang C., Liao W. Hydrogen sulfide: a gaseous molecule in postharvest freshness. Front. Plant Sci. 2018 doi: 10.3389/fpls.2018.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Sheng Q., Jiang Y., Zhou X. Effects of 1‐methylcyclopropene and gibberellic acid on ripening of Chinese jujube (Zizyphus jujuba M) in relation to quality. J. Sci. Food Agric. 2004;84:31–35. [Google Scholar]
- Li B., Ding Y., Tang X., Wang G., Wu S., Li X., Huang X., Qu T., Chen J., Tang X. Effect of L-arginine on maintaining storage quality of the white button mushroom (Agaricus bisporus) Food Bioprocess Technol. 2019;12:563–574. [Google Scholar]
- Li L., Ban Z., Li X., Xue T. Effect of 1-methylcyclopropene and calcium chloride treatments on quality maintenance of 'Lingwu Long' jujube fruit. J. Food Sci. Technol. 2014;51:700–707. doi: 10.1007/s13197-011-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Hu K., Hu L., Li Y., Jiang A., Xiao F., Han Y., Liu Y., Zhang H. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J. Agric. Food Chem. 2014;62:1119–1129. doi: 10.1021/jf4047122. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H.K., Wellburn A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemistry. 1983;11:591–592. [Google Scholar]
- Lin Y., Li M., Cui W., Lu W., Shen W. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J. Plant Growth Regul. 2012;31:519–528. [Google Scholar]
- Liu X., Wang T., Chen L., Li L., Wang Y., Li X., Xing Y. Transcriptomic and gene expression changes in response to postharvest surface pitting in ‘Lingwu Long’ jujube fruit. Hortic. Environ. Biote. 2018;59:59–70. [Google Scholar]
- Moo-Huchin V.M., Estrada-Mota I., Estrada-León R., Cuevas-Glory L., Ortiz-Vázquez E., Vargas M.L.V., Betancur-Ancona D., Sauri-Duch E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014;152:508–515. doi: 10.1016/j.foodchem.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Nath A., Bagchi B., Misra L.K., Deka B.C. Changes in post-harvest phytochemical qualities of broccoli florets during ambient and refrigerated storage. Food Chem. 2011;127:1510–1514. [Google Scholar]
- Ni Z., Hu K., Song C., Ma R., Li Z., Zheng J., Fu L., Wei Z., Zhang H. Hydrogen sulfide alleviates postharvest senescence of grape by modulating the antioxidant defenses. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/4715651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi Z., Razavi F., Khademi O., Aghdam M.S. Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 2021;285 [Google Scholar]
- Pirie A., Mullins M.G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol. 1976;58:468–472. doi: 10.1104/pp.58.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar H., Bock C.H., Kerr W.L., Kong F. Pecan color change during storage: kinetics and modeling of the processes. Curr. Res. Food Sci. 2022;5:261–271. doi: 10.1016/j.crfs.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashwan A.K., Karim N., Shishir M.R.I., Bao T., Lu Y., Chen W. Jujube fruit: a potential nutritious fruit for the development of functional food products. J. Funct.Foods. 2020;75:163–174. [Google Scholar]
- Shukanta S., Hena B.H., Shamima N., Rifat S. Effects of drought stress on pigment and protein contents and antioxidant enzyme activities in five varieties of rice (Oryza sativa L.) Bangladesh J. Bot. 2020;49:997–1002. [Google Scholar]
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- Xu M., Pan Y., Leng J., Li H., Li X. Effect of different high oxygen treatments on preservation of seedless long jujube in low temperature storage. J. Food Process. Preserv. 2020;44 [Google Scholar]
- Yao G.F., Li C., Sun K.K., Tang J., Huang Z.Q., Yang F., Huang G.G., Hu L.Y., Jin P., Hu K.D., Zhang H. Hydrogen sulfide maintained the good appearance and nutrition in post-harvest tomato fruits by antagonizing the effect of ethylene. Front. Plant Sci. 2020;11:584. doi: 10.3389/fpls.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Guo W., Liu Y., Sang Y., Yang W., Guo M., Cheng S., Chen G. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao) Sci. Hortic. 2021;288 [Google Scholar]
- Zhang H., Hu L., Hu K., He Y., Wang S., Luo J. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 2008;50:1518–1529. doi: 10.1111/j.1744-7909.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- Zhang H., Hu S., Zhang Z., Hu L., Jiang C., Wei Z., Liu J., Wang H., Jiang S. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011;60:251–257. [Google Scholar]
- Zhang H., Ma Z., Wang J., Wang P., Lu D., Deng S., Lei H., Gao Y., Tao Y. Treatment with exogenous salicylic acid maintains quality, increases bioactive compounds, and enhances the antioxidant capacity of fresh goji (Lycium barbarum L.) fruit during storage. LWT. 2021;140 [Google Scholar]
- Zhang H., Wang H.L., Lv K., Hu K.D., Hu L.Y., Gao S.P., Li Y.H., Han Y., Liu Y.S. Hydrogen sulfide delays postharvest senescence and plays an antioxidative role in fresh-cut kiwifruit. Hortscience. 2013;48:1385–1392. [Google Scholar]
- Zhang H., Zhao L., Fan C., Wang P., Cui M., Liu L., Yang H., Wang J. Impact of methyl salicylate on storage quality, ethylene action, and protein profiling of ‘Zaosu’ pear Pyrus bretschneideri) Sci. Hortic. 2020;264 [Google Scholar]
- Zhang S., Yu Y., Xiao C., Wang X., Lei Y. Effect of ultraviolet irradiation combined with chitosan coating on preservation of jujube under ambient temperature. LWT. 2014;57:749–754. [Google Scholar]
- Zhang Z., Huber D.J., Rao J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013;76:58–64. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- Zhou H., Jia J., Kong D., Zhang Z., Song S., Li Y., Pang X. Genome-wide identification and analysis of the DREB genes and their expression profiles under abiotic stresses in Chinese jujube (Ziziphus jujuba Mill.) J. Forestry. Res. 2019;30:1277–1287. [Google Scholar]