Abstract
We examined the effects of dietary supplementation of a multicomponent blend of prebiotics and probiotics on health, immune status, metabolism, and performance of newly weaned beef steers during a 35-d receiving period. Eighty newly weaned crossbred steers (12-hour postweaning; 206 ± 12 kg of body weight [BW]) from a single source were stratified by BW into four pens (20 steers per pen) such that each pen had similar BW at the beginning of the experiment. The pens were randomly assigned to receive a corn silage-based diet with no additive (CON; two pens; n = 40 steers) or a basal diet supplemented with SYNB feed additive at an average of 28 g/steer/d (SYNB; two pens; n = 40 steers). The SYNB additive is a blend of live Saccharomyces cerevisiae and the fermentation products of S. cerevisiae, Enterococcus lactis, Bacillus licheniformis, and Bacillus subtilis and was supplemented for the first 21 d only. Percentage of steers treated for bovine respiratory disease (BRD) was calculated for each dietary treatment. Daily dry matter intake (DMI) and meal events (meal frequency and duration) were measured. Weekly BWs were measured to calculate average daily gain (ADG). Blood samples collected on days 0, 14, 21, 28, and 35 were used for ex-vivo tumor necrosis factor alpha (TNF-α) release assay following lipopolysaccharides (LPS) stimulation, plasma metabolome analysis, and mRNA expression analysis of 84 innate and adaptive immune-related genes. Compared with CON, supplemental SYNB increased (P ≤ 0.05) ADG, DMI, and meal events during the first 7 d. At d 21, there was no treatment effect (P > 0.05) on final BW, DMI, ADG, and meal events; however, beef steers fed supplemental SYNB had greater (P = 0.02) meal duration. Over the entire 35-d receiving period, beef steers fed supplemental SYNB had greater (P = 0.01) ADG and feed efficiency, tended to have greater (P = 0.08) meal duration, and had lower percentage (35 vs. 50%) of animals treated for BRD and lower percentage of sick animals treated for BRD more than once (7.15 vs. 45%). Whole blood expression of pro-inflammatory genes was downregulated while that of anti-inflammatory genes was upregulated in beef steers fed supplemental SYNB. Beef steers fed supplemental SYNB had lower (P = 0.03) plasma concentration of TNF-α after LPS stimulation. Six nutrient metabolic pathways associated with health benefits were enriched (false discovery rate ≤ 0.05) in beef steers fed supplemental SYNB. This study demonstrated that dietary supplementation of SYNB during the first 21 d of arrival reduced BRD morbidity, improved the performance, immune, and metabolic status of beef steers over a 35-d receiving period thereby extending the SYNB effect by a further 14 days post supplementation.
Keywords: beef cattle, feed additive, metabolism
INTRODUCTION
The feedlot receiving period of newly weaned beef cattle is characterized by stressors caused by several factors such as weaning, transportation, vaccination, new environment, exposure to pathogens, and diet change (Arthington et al., 2013, Lynch et al., 2019). In addition to these stressors, dry matter intake (DMI) of newly weaned beef cattle is low, resulting in nutrient deficiencies that further increase susceptibility to disease and performance losses (Hutcheson and Cole, 1986; Duff and Galyean, 2007). Hence, nutritional interventions, including the use of microbial feed additives, are often employed during the receiving period as well as during the entire feedlot period to optimize intake, health, immune status, and reduce morbidity of newly weaned beef cattle.
Research studies have demonstrated that dietary supplementation of Saccharomyces cerevisiae-based direct-fed microbial (DFM) can modulate microbial balance in the gastrointestinal tract by stabilizing ruminal pH, promoting ruminal fermentation that ultimately increases total volatile fatty acids (VFAs), improves performance, and stimulates immune function (Newbold et al., 1996; Krehbiel et al., 2003; Adeyemi et al., 2019). However, responses to dietary DFM supplementation are inconsistent across studies due to several factors including differences in species and strains of organisms, dietary inclusion level, diet composition, and animal factors (McAllister et al., 2011; Plaizier et al., 2018). For instance, some studies reported improved growth performance and health of newly weaned beef steers fed S. cerevisiae-based DFM (Armato et al., 2016; Adeyemi et al., 2019); while several other studies reported no effects on performance and health of newly weaned beef steers during the receiving period (Krehbiel et al., 2001; Fink et al. 2014).
The use of fermentation products of microorganisms as prebiotics to improve health and performance of beef cattle has been assessed because these products are known to contain several nutritional metabolites such as B-vitamins, organic acids, enzymes, amino acids, nucleotides, and lipids that can enhance the growth and activities of beneficial microbes in the gut (Tricarico et al., 2007; Deters et al., 2018). Studies have reported positive effects on DMI and growth performance when beef or dairy calves are supplemented with fermentation products of S. cerevisiae (Lesmeister et al., 2004; Harris et al., 2017). However, animal response to these additives is inconsistent, with several studies reporting no effects on overall beef cattle receiving period performance (Deters et al., 2018; Hall et al., 2018; Deters and Hansen, 2019).
In recent years, most microbial products are formulated to contain a blend of one or more microorganisms (probiotics) and their fermentation products (prebiotics) to ensure efficacies and multifactorial responses (McAllister et al., 2011; Ogunade et al., 2020). Inconsistent responses to supplemental microbial products and the development of new DFM strains emphasize the need for more research studies to provide insight into their mechanisms of action. We hypothesized that supplementation of a multicomponent dietary feed additive containing prebiotics and probiotics would improve health and performance by reducing morbidity of newly weaned beef steers during the receiving period. The objective of this study was to evaluate the effects of dietary supplementation of a blend of live S. cerevisiae and the fermentation products of S. cerevisiae, Enterococcus lactis, Bacillus licheniformis, and Bacillus subtilis on growth performance, whole-blood immune gene expression, response to ex-vivo lipopolysaccharides (LPS) challenge, and the plasma metabolome of newly weaned beef steers during a 35-d receiving period.
MATERIALS AND METHODS
Animals, Housing, and Feeding
All animal care and use procedures were in accordance with the guidelines for use of Animals in Agriculture Teaching and Research as approved by the West Virginia University (#2108046615). Eighty (80) newly weaned crossbred steers (12-hour post-weaning; 206 ± 12 kg of body weight [BW]; 180 ± 17 d of age) from a single source were used. The steers were vaccinated at approximately 60 d of age and received the booster shots prior to weaning (approximately 180 d of age). The vaccination protocol consisted of two vaccinations (Alpha-7/MB-1 and Pyramid 5 plus Presponse sq, Boehringer Ingelheim Animal Health, Duluth, GA). The beef steers were transported approximately 150 miles to the research feedlot barn, and immediately weighed, processed, and placed on corn silage-based diet on the day of arrival (d 0). Processing included ear tag placement for unique radiofrequency identification and administration of de-wormers (Valbazen, Zoetis Inc., Kalamazoo, MI). Based on d 0 BW, the steers were stratified by BW into four pens (20 steers per pen) such that each pen had similar BW at the beginning of the experiment. Each pen (size = 14.6 by 46.9 m2) was equipped with three GrowSafe intake nodes (GrowSafe Systems Ltd., Airdrie, Alberta, Canada) to measure individual feed intake. Starting from d 1, the pens were randomly assigned to receive a corn silage-based diet with no additive (CON; two pens; n = 40 steers) or a basal diet supplemented with a SYNB feed additive at an average of 28 g/steer/d (SYNB; two pens; n = 40 steers). The SYNB additive is a blend of live S. cerevisiae and the fermentation products of S. cerevisiae, E. lactis, B. licheniformis, and B. subtilis (Purina Animal Nutrition, Arden Hills, MN). The SYNB additive was supplemented for the first 21 d of the experiment and then removed d 22–35 to identify carry-over effects on inflammatory markers, immune function, calf morbidity, and performance. The basal diet was fed as a total mixed ration (TMR, Table 1) and was formulated following NASEM (2016) recommendations for growing beef cattle. Approximately 125 g of a premix (dried distillers grain with solubles) was formulated to contain 28 g/d of the supplemental additive and was mixed in the TMR at a certain percentage based on previous day’s average intake for each pen (day x intake was used to calculate inclusion rate for day x +1) to provide the required level of the additive for each steer in each pen (average of 28 g of SYNB/hd/d) while a similar quantity of the premix with no additive was added for the CON treatment pens. The CON and SYNB diets were mixed in separate feed trucks to eliminate possibility of cross contamination of diets and the diets were fed ad libitum (to achieve approximately 10% ort) daily at 0900 h.
Table 1.
Ingredient and chemical composition of the basal diet
| Ingredient (%DM) | % of dietary DM |
|---|---|
| Corn silage | 64.0 |
| Mixed grass haya | 20.0 |
| Soybean meal | 8.12 |
| Dehydrated distillers’ grain | 2.58 |
| Soybean hulls | 3.57 |
| Limestone | 0.60 |
| Urea | 0.50 |
| Limestone | 0.50 |
| Vitamin and mineral premixb | 1.63 |
| Nutrient analysis | |
| DM, % | 51.9 |
| CP, % | 16.3 |
| Andf, % | 47.7 |
| ADF, % | 29.6 |
| Ca, % | 0.71 |
| P, % | 0.48 |
| TDN, % | 69.5 |
| NEm, Mcal/kg | 1.62 |
| NEg, Mcal/kg | 1.02 |
Contains a mixture of orchard grass and fescue grass.
Guaranteed analysis: 15% Ca; 7.5% P; 20% salt; 1% Mg; 1% K; 3,600 mg/kg Mn; 12 mg/kg Co; 1,200 mg/kg Cu; 3,600 mg/kg Zn; 27 mg/kg Se; 60 mg/kg I; 660,000 IU/kg vitamin A; 660 IU/kg vitamin E; and 66,000 IU/kg vitamin D.
DM, dry matter; CP, crude protein; Andf, neutral detergent fiber (amylase treated); ADF, acid detergent fiber; EE, ether extract; TDN, total digestible nutrients; NEm, net energy of maintenance; NEg, net energy of gain.
Intake and BW Measurement
Individual feed intake was measured using the GrowSafe intake nodes and a 24-h intake was measured from 0900 h to 0800h the next day. Samples of TMR were collected daily from CON and SYNB diets and were weighed and oven dried at 55 °C for 72 h to determine dry matter content. Subsamples of the dried TMR were composited within treatment, ground using a Wiley mill (Arthur H. Thomas Co., Philadelphia, PA) to pass a 2-mm sieve, and sent to a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY) for analysis for nutritional composition.
BWs of steers were obtained before morning feeding (after 12–14 h of feed and water withdrawal) on d 0, 7, 14, 21, 28, and 35. Average daily gain (ADG) was determined by subtracting the initial weight on day 0 from the final weight on day 35 and then dividing by the duration of the experiment (35). Values of ADG from d 1 to 7, 1 to 21, and 22 to 35 were also calculated. Feed efficiency (gain-to-feed ratio) was calculated for the entire 35 d period.
Meal Events
Meal frequency (AVGFREQ; events/d) and meal duration (AMD; minutes/d) were measured using the GrowSafe system. Meal frequency was defined as the number of meal events recorded each day while AMD was defined as the sum of the durations of meal events recorded each day. The AVGFREQ and AMD data were summarized as the average of each individual steer over a specific period (d 1–7, 1–21, 22–35, and 1–35).
Morbidity
The beef steers were visually examined daily by a licensed veterinarian and treated for bovine respiratory disease (BRD) if needed. For an animal to qualify for treatment, the animal appeared visually sick (e.g., lethargic, coughing, weeping eyes, nasal discharge, drooping head and ears) or had BW loss relative to initial BW and had a rectal temperature greater than 39.5 °C. A suitable medication protocol was followed, and the order of treatment administration consisted of a single subcutaneous injection of Draxxin (Tulathromycin, Pfizer, New York, NY) followed by a single intravenous administration of Banamine (Flunixin meglumine, Merck Animal Health, Summit, NJ). If any animal was non-responsive to earlier treatment, a second or third treatment was given. Any animal that did not respond to the medication after the third treatment was defined as chronic and removed from the study. The percentage of animals treated for sickness during the entire period was calculated for each treatment and no steer was identified as a chronic nonresponder.
Blood Sample Collection
Two sets of blood samples (10 mL each) were taken from each steer before the morning feeding on d 0, 14, 21, 28, and 35. The blood samples were taken from the coccygeal vessels into tubes containing sodium heparin (Vacutainer, Becton Dickinson, Franklin Lakes, NJ). The blood tubes were placed on ice immediately after collection, and a sub-sample of the whole blood (approximately 500 µL) was transferred into RNA-protect tube (cat. no. 76554; Qiagen, Frederick, MD) which contains a reagent that lyses blood cells and stabilizes intracellular RNA. These samples were stored at −80 °C for later RNA extraction. Thereafter, plasma samples were immediately prepared from the remaining samples by centrifugation at 2,500 × g for 20 min at 4 °C and stored at −80 °C until analyses for glucose, non-esterified fatty acids (NEFA), and quantitative metabolome analysis. The second set of whole blood was used for ex-vivo LPS-stimulated whole blood cytokine production.
RNA Extraction and Immune Gene Analysis
Total RNA was extracted from the whole blood samples collected on d 0, 14, 21, 28, and 35 using RNeasy Protect Animal Blood kit (Catalog. No. 73224; Qiagen) following the manufacturer’s instructions. Total RNA concentration was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) with an A260:A280 ratio from 1.8 to 2.0 (Thermo Fisher Scientific, Waltham, MA) and RNA integrity number (> 8.0) was verified using Agilent 2100 bioanalyzer (Agilent Technologies; Santa Clara, CA). After evaluation of RNA concentration and quality, complementary DNA was synthesized through reverse transcription (RT) using the RT2 First Strand Kit (Cat. No. 330401; Qiagen) following the manufacturer’s instructions. The mRNA expression of 84 genes related to innate and adaptive immunity was determined using a RT2 Profiler cow innate and adaptive immune responses Polymerase chain reaction (PCR) Array (PABT-052ZA; Qiagen). The PCR array contained 84 adaptive and innate immune-related genes, five housekeeping genes (β-actin, hypoxanthine phosphoribosyltransferase 1, glyceraldehyde-3-phosphate dehydrogenase, and tyrosine 3-monooxygenase, TATA box-binding protein), three RT, three positive PCR controls, and one genomic DNA control (Supplementary Table S1). Real-time PCR was performed using a QuantStudio 5 Real-Time PCR System (Applied Biosystems, Foster City, CA). The PCR cycling conditions were as follows: 95 °C for 10 min, 40 cycles of denaturation at 95°C for 15 s, and 60°C for 1 min.
Quantitative Metabolome Analysis Using Nuclear Magnetic Resonance Spectroscopy
To assess the metabolic status of the beef steers, metabolome analysis of plasma samples collected on d 0, 14, 21, 28, and 35 were performed using Nuclear Magnetic Resonance (NMR) spectroscopy to quantify a total of 50 metabolites consisting of amino acids, hexoses, organic acids, carnitines, and lipids (Supplementary Table S2). Details of plasma sample preparation and NMR spectral analysis have been published in previous studies (Ogunade et al., 2018). Briefly, a deproteinization step, involving ultrafiltration was initially performed to remove macromolecules such as proteins and lipoproteins (Psychogios et al., 2011). Subsequently, 160 µL of the sample was mixed with 40 µL of a standard buffer solution (54% D2O:46% 250 mM KH2PO4, pH 7.0). The plasma sample (200 µL) was then transferred into 3 mm SampleJet NMR tube for spectral analysis. All 1H-NMR spectra were collected using a 700 MHz Avance III (Bruker) spectrometer equipped with a 5 mm HCN Z-gradient pulsed-field gradient cryoprobe. The 1H-NMR spectra were processed and analyzed using a Bayesil automated analysis software package, which allows for qualitative and quantitative analysis of an NMR spectrum (Ravanbakhsh et al., 2015). Further inspection and verification were performed by an NMR spectroscopist to reduce errors associated with compound identification and quantification.
Glucose and NEFA Measurements
Plasma samples collected on d 0, 14, 21, 28, and 42 were analyzed for NEFA and glucose concentrations in duplicate using commercially available assays, NEFA-C kit (Wako Diagnostics Inc., Richmond, VA), and quantitative colorimetric kit (G7521-1L; Pointe Scientific Inc., Canton, MI), respectively. The intra- and inter-assay coefficients of variation for glucose were 5.15% and 5.32%, respectively while those for NEFA were 6.25% and 8.43%, respectively.
Ex-vivo LPS-Stimulated Whole Blood TNF-α Production
Whole blood samples collected on d 0, 14, 21, 28, and 35 were subjected to an ex vivo cytokine release assay to estimate the innate immunologic response to external stimuli (Damsgaard et al., 2009; Ling et al., 2018). The whole blood samples were stimulated with LPS (Escherichia coli 0111: B4, Sigma Aldrich, St. Louis, MO) within 5 min of collection in 5 mL sterile, pyrogen-free tubes (SuperClear Centrifuge Tubes; Labcon, Petaluma, CA) as described previously (O’Boyle et al., 2006; Ling et al., 2018). Briefly, 3-mL aliquots were stimulated for 3.5 h at 37 °C with 5 µg/mL of LPS or endotoxin-free Dulbecco’s Modified Eagle Medium (Sigma Aldrich) as a negative control. Immediately after incubation, the samples were centrifuged at 1,500 × g for 10 min at 4 °C to harvest the plasma. Plasma samples were immediately stored at −80 °C, and later analyzed in duplicate for TNF-α concentrations using TNF-α Bovine assay kit (Raybiotech, Peachtree Corners, GA) with a detection limit of 0.1 ng/mL. The negative control samples were used to determine the basal TNF-α concentrations. The inter- and intra-assay coefficients of variation were 8.3 and 6.4%, respectively.
Data and Statistical Analysis
All growth performance and meal event data were analyzed as a randomized block design using the GLIMMIX model of SAS (SAS 9.3, SAS Inst. Inc., Cary, NC), using steer as the experimental unit. The model included the fixed effects of treatment and the random effects of block (BW). Parameters such as plasma TNF-α, glucose, and NEFA concentrations were analyzed using repeated measures and tested for the effect of treatment (CON vs. SYNB), day of collection, and the day × treatment interaction. Day 0 data were used as covariates. Appropriate covariance structures were used based on the lowest akaike values (Wang and Goonewardene, 2004). Results were considered significant if P ≤ 0.05. Tendencies were declared when 0.05 > P ≤ 0.10. Cumulative number of steers treated for BRD in each treatment group was calculated and compared between CON and SYNB as a percentage of the total number of animals in that group.
All mRNA expression data were analyzed separately for each day using the Qiagen web-based platform, GeneGlobe (https://geneglobe.qiagen.com). The comparative cycle threshold (Ct) method was used for relative quantification of gene expression (Pfaffl, 2001). Delta-delta-Ct (ΔΔCt) method with normalization of the raw data using the geometric mean of the five housekeeping genes was used to calculate the differences in mRNA expression of the genes between CON and SYNB (Pfaffl, 2001). Stability of the reference genes was confirmed using ΔCt and NormFinder (Andersen et al., 2004; Wan et al., 2017). The expression of genes with absolute fold change (FC) ≥ 2.0 having false discovery rate (FDR)-adjusted (Benjamini and Hochberg, 1995) P-values ≤ 0.05 were differentially expressed.
Plasma metabolome data was used for quantitative enrichment analysis using Metaboanalyst 5.0 software (Pang et al., 2021; https://www.metaboanalyst.ca/) to assess the metabolic pathways that were enriched by supplemental SYNB. Prior to the quantitative enrichment analysis, the metabolome data were log-transformed, pareto-scaled, and normalized using median-scale normalization. First, enrichment analysis of d 0 plasma metabolome data was performed using KEGG (Kyoto Encyclopedia of Genes and Genomes) as the metabolite set library to determine if there were any differentially enriched (FDR ≤ 0.05) pathways prior to feeding supplemental SYNB. Thereafter, metabolome data from d 14, 21, 28, and 35 were combined for quantitative pathway enrichment analysis.
RESULTS AND DISCUSSION
Actual average intake of the supplemental SYNB based on average diet intake (as fed basis) and inclusion rate in the TMR was 29.1 g/steer/d, therefore, average intake was approximately 103.9% of the targeted dose of 28 g/steer/d.
The effects of SYNB supplementation on the growth performance and meal events of the steers are shown in Table 2. Compared with CON, dietary supplementation of SYNB increased ADG (P = 0.05), DMI (P = 0.05), AVGFREQ (P = 0.04), and AMD (P = 0.01) during the first 7 d of the receiving period. Increased DMI, a reflection of increased meal events (AVGFREQ and AMD), during the first few days of arrival of newly weaned beef cattle is essential to alleviating the stress associated with weaning and transportation, and the consequent effects on health and performance of beef cattle. Low nutrient intake during the first few days of the receiving period compromises the immune function and health of beef cattle which makes them more susceptible to infections (Bernhard et al., 2012). The fact that supplemental SYNB increased the DMI and ADG of the beef steers during the first 7 d of arrival is suggestive of improved health. Earlier studies have demonstrated that the benefits of feeding S. cerevisiae-based products in animals are more pronounced under stress conditions and are mediated via improved gastrointestinal health which supports increased nutrient intake and utilization (Salinas-Chavira et al., 2017; Oh et al., 2019; Ogunade et al., 2019). In addition, supplemental SYNB contained fermentation products of S. cerevisiae, E. lactis, B. subtilis, and B. licheniformis such as B-vitamins, amino acids, nucleotides, lipids, and organic acids that can help improve microbial growth by stabilizing the rumen environment or can directly stimulate appetite in stressed cattle (Cruz Ramos et al., 2000; Lauriault et al., 1990). Like these results, several studies have reported improved DMI and ADG of beef cattle during the first few days of feeding supplemental DFM (Elam et al. 2003; Baah et al., 2009; Adeyemi et al., 2019).
Table 2.
Growth performance, intake, health, and feeding behavior of beef steers fed diet supplemented with a multicomponent dietary feed additive containing prebiotics and probiotics during a 35-d receiving period
| CON | SYNB | SEM | P | |
|---|---|---|---|---|
| Initial weight, kg | 206 | 205 | 5.79 | 0.90 |
| Final weight, kg d 21 | 241 | 242 | 1.52 | 0.38 |
| Final weight, kg d 35 | 256b | 263a | 2.01 | 0.01 |
| Day 1–7 | ||||
| ADG, kg/d | 1.52b | 1.98a | 0.24 | 0.05 |
| DMI, kg/d | 3.28 b | 3.54a | 0.13 | 0.05 |
| AMD, min/d | 105b | 125 a | 7.41 | 0.01 |
| AVGFREQ | 15.6b | 17.2a | 0.79 | 0.04 |
| Day 1–21 | ||||
| ADG, kg/d | 1.67 | 1.74 | 0.07 | 0.38 |
| DMI, kg/d | 4.43 | 4.48 | 0.13 | 0.72 |
| AMD, min/d | 108 | 126 | 8.08 | 0.02 |
| AVGFREQ | 16.9 | 17.6 | 0.60 | 0.22 |
| Day 22–35 | ||||
| ADG, kg/d | 1.13b | 1.53 a | 0.11 | 0.01 |
| DMI, kg/d | 6.30 | 6.31 | 0.20 | 0.99 |
| AMD, min/d | 113 | 123 | 9.50 | 0.32 |
| AVGFREQ | 15.3 | 15.3 | 0.70 | 0.96 |
| Day 1–35 | ||||
| ADG, kg/d | 1.45 b | 1.65 a | 0.06 | 0.01 |
| DMI, kg/d | 5.20 | 5.22 | 0.15 | 0.85 |
| AMD, min/d | 110 y | 125 x | 8.42 | 0.08 |
| Feed efficiency, gain:feed ratio | 0.28 b | 0.32 a | 0.01 | 0.01 |
| % of steers treated for BRD | 50.0 | 35.0 | NA | NA |
| % of sick steers treated for BRD more than once | 45.0 | 7.15 | NA | NA |
CON, control; SYNB, a multicomponent microbial feed additive containing prebiotics and probiotics fed at 28 g/ steer/d (Purina Animal Nutrition, Arden Hills, MN); SEM, standard error of mean; ADG, average daily gain; DMI, dry matter intake; AMD, sum of the durations of meal events recorded each day per steer; AVGFREQ, number of meal events recorded each day per steer; NA, not analyzed.
a,bWithin a row, treatment means with different superscripts differ, P ≤ 0.05.
x,yWithin a row, treatment means with different superscripts tend to differ, 0.05 < P ≤ 0.10.
During the first 21 d of the receiving period, there was no treatment effect on final BW (P = 0.38), DMI (P = 0.72), ADG (P = 0.38), and AVGFREQ (P = 0.22); however, beef steers fed supplemental SYNB had greater (P = 0.02) AMD compared to CON (Table 2). During 14 d after removal of SYNB from the diet (d 22–35), beef cattle fed supplemental SYNB (in the first 21 d) had greater (P = 0.01) ADG than steers fed CON, indicating that the positive effects of supplemental SYNB on growth performance of the beef steer were still evident up to 14 d after removing the additive from the diet. Over the entire 35-d receiving period, beef steers fed supplemental SYNB had greater (P = 0.01) ADG and feed efficiency, tended to have greater (P = 0.08) AMD, and had a lower percentage (35 vs. 50%) of animals treated for BRD and lower percentage of BRD retreatment events (7.15 vs. 45%) compared to CON. The observation of fewer SYNB calves exhibiting BRD and fewer sick calves being retreated represents a substantial savings to the cattle industry.
Despite the inconsistency in performance response in ruminants to DFMs across studies, the mechanisms of action by which certain S. cerevisiae based-microbial products improve the health and productivity of ruminants have been proposed as modulating rumen fermentation and metabolism which can directly or indirectly influence host metabolism and immune function (Newbold et al., 1996; McAllister et al., 2011). In a previous study (Ogunade et al., 2020), we evaluated the effects of supplemental SYNB on rumen fermentation and the metabolome of beef cattle and observed an altered ruminal bacterial community, increased total ruminal VFA, and propionate concentrations, and improved energy status of the beef cattle fed a diet supplemented with the additive. Certain strains of S. cerevisiae are known to favor microbial growth and promote the activities of ruminal bacteria that metabolize lactate to propionate (Nagaraja and Titgemeyer, 2007; McAllister et al., 2011). VFAs are the major energy source that supplies up to 70% of the energy requirement for the maintenance and growth of ruminants (Bergman, 1990; Oba and Allen, 2003). The effects of supplemental SYNB on rumen fermentation and energy status reported in our previous study (Ogunade et al., 2020) support the improved growth performance and health of the beef steers observed in this current study. Consistent with our results, a meta-analytic study that examined the effects of S. cerevisiae and its fermentation product reported improved ADG of beef cattle during the receiving period (Wagner et al., 2016).
The effects of SYNB supplementation on plasma glucose and NEFA of the beef steers are shown in Table 3. Compared to CON, beef steers fed supplemental SYNB had greater (P = 0.01) plasma glucose concentration, however, no treatment effect was detected for NEFA concentration (P = 0.28). Several studies have demonstrated that S. cerevisiae-based products increase the relative abundance and activity of lactate-utilizing and cellulolytic bacteria in the rumen which promotes fiber digestion, stabilizes rumen pH, and consequently improves feed utilization (Zhu et al., 2017; Kumprechtova et al., 2019; Ogunade et al., 2020). Such effects modify rumen fermentation towards increased total VFA, acetate, or propionate concentrations in both beef and dairy cattle, thus providing increased availability of substrates for glucose synthesis. The fact that plasma NEFA concentration was unaffected suggests that the diet fed in this study met the energy requirement of the beef steers.
Table 3.
Effects of a multicomponent dietary feed additive containing prebiotics and probiotics on energy status of newly weaned beef steers during a 35-d receiving period
| Item | CON | SYNB | SEM | P | |
|---|---|---|---|---|---|
| Treatment | Treatment × day | ||||
| Glucose, mg/dL | 84.0 | 87.1 | 1.17 | 0.01 | 0.22 |
| NEFA, mEq/L | 0.16 | 0.17 | 0.009 | 0.28 | 0.43 |
CON, control; SYNB, a multicomponent microbial feed additive containing prebiotics and probiotics fed at 28 g/steer/d (Purina Animal Nutrition, Arden Hills, MN).
The effects of SYNB supplementation on mRNA expression of 84 innate and adaptive immune genes in whole blood of the beef steers are presented in Supplementary Table S3. Out of the 84 immune-related genes analyzed, no genes were differentially expressed (FC < (−)2.0 and/or FDR > 0.05) on d 0. The mRNA expression of 2, 2, and 7 genes were differentially expressed (FC ≥ (−)2; FDR ≤ 0.05) on d 14, 21, and 28, respectively; however, no genes were differentially expressed on d 35 (Table 4). On d 14, the mRNA expression of CXCL8 and IRF7 were downregulated (FC ≥ 2; FDR ≤ 0.05) in beef steers fed supplemental SYNB compared to CON. CXCL8, also known as Interleukin-8, is a proinflammatory cytokine and is known to be a key mediator associated with infection where it plays a major role in neutrophil recruitment to the area of inflammation (Ha et al., 2017). In fact, CXCL8 is known to be a reliable inflammatory stress marker for various disease conditions in animals and humans (Hashmi and Zeng, 2006; Gelaleti et al., 2012; Ha et al., 2017). IRF7 is a transcription factor that is activated by multiple pattern-recognition receptors during inflammation and/or pathogenic infections leading to production of pro-inflammatory cytokines (Broz and Monack, 2013). Stressors encountered during feedlot receiving, including vaccination and transportation, can elicit an inflammatory response which has negative impacts on health and performance of beef cattle because more nutrients are prioritized towards fueling the immune cells to fight inflammation (Arthington et al., 2013; Marques et al., 2016). Thus, decreased mRNA expression of CXCL8 and IRF7, which are often considered inflammatory markers, in beef steers fed supplemental SYNB compared to CON, suggests that CON steers were still experiencing inflammatory stress at d 14 but feeding supplemental SYNB abated this stress.
Table 4.
Effects of a multicomponent dietary feed additive containing prebiotics and probiotics on whole-blood immune gene expression in beef steers during a 35-d receiving period
| Gene Name | Fold change | FDR | |
|---|---|---|---|
| Day 14 | |||
| CXCL8 | Interleukin 8 | −2.14 | 0.02 |
| IRF7 | Interferon regulatory factor 7 | −3.09 | 0.01 |
| Day 21 | |||
| iL10 | Interleukin 10 | 2.27 | 0.01 |
| MBL2 | Mannose-binding lectin (protein C) 2, soluble | 18.62 | 0.04 |
| Day 28 | |||
| BOLA-A | Major histocompatibility complex, class I, A-A | 2.01 | 0.01 |
| CCR8 | Chemokine (C-C motif) receptor 8 | −2.24 | 0.01 |
| CD4 | CD4 molecule | 2.08 | 0.01 |
| DDX58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | 2.17 | 0.01 |
| IFNAR1 | Interferon (alpha, beta, and omega) receptor 1 | 2.21 | 0.02 |
| TLR2 | Toll-like receptor 2 | −2.56 | 0.04 |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 | 3.05 | 0.01 |
CON, control; SYNB, a multicomponent microbial feed additive containing prebiotics and probiotics fed at 28 g/steer/d (Purina Animal Nutrition, Arden Hills, MN); FDR, false discovery rate.
Fold change (relative to control) = 2−ΔΔCt = [(CTgene of interest – CTreference genes)SYNB – (CTgene of interest – CTreference genes)CON].
P-values are calculated based on a Student’s t-test.
Only genes with both absolute fold change ≥ 2, relative to CON, and FDR ≤ 0.05 are shown.
No differentially expressed genes were observed on days 0 and 35.
On d 21, the mRNA expression of iL-10 and MBL-2 was upregulated (FC ≥ 2; FDR ≤ 0.05) in beef steers fed supplemental SYNB compared to CON. Interleukin-10 is an anti-inflammatory cytokine that is produced by immune cells to counteract damage caused by excessive inflammation by suppressing the production of inflammatory mediators, thereby maintaining normal tissue homeostasis (Iyer and Cheng, 2012). The MBL-2 gene encodes a mannose-binding lectin, a soluble C-type lectin that is secreted by the liver as part of the acute-phase response and component of the innate immune system (Dommett et al., 2006). The MBL is a pattern recognition molecule, specific for a broad spectrum of ligands including cell wall components (such as mannose) of S. cerevisiae and is known to regulate inflammatory signals mediated by microbial pathogens (Wolf and Underhill, 2018). Earlier studies have shown that MBL binding can inhibit production of LPS-induced inflammatory cytokines, TNF-α, and interferon-gamma (IFN-γ) and promote IL-10 production in natural killer cells (McDonald et al., 2005; Zhou et al., 2019), which explains increased expression of iL-10 in beef steers fed supplemental SYNB in this study. Taken together, increased mRNA expression of iL-10 and MBL-2 in beef steers fed supplemental SYNB suggests that these animals had a better ability than CON to quickly recognize and mount an efficient defense against invading microbial pathogens without undergoing inflammatory stress. Like our results, Deters et al. (2018) observed reduced serum concentration of iL-8, an inflammatory marker, in newly weaned steers fed a S. cerevisiae fermentation product following a vaccination challenge. Similar studies in dairy cows (Li et al., 2016) and mice (Evans et al., 2012) reported reduced blood concentration of inflammatory marker such as IFN-γ in those fed diet supplemented with S. cerevisiae and its fermentation products relative to the control.
On d 28 (7 d after withdrawing the additive from the diet), the mRNA expression of BOLA-A, CD4, DDX58, IFNAR1, and IRAK1 were upregulated (FC ≥ 2; FDR ≤ 0.05) in beef steers fed supplemental SYNB compared to CON. BOLA-A is one of the major histocompatibility complex (MHC) molecules that are involved in fine-tuning both innate and adaptive immune responses. MHC molecules play an essential role in the presentation of foreign antigens, including intracellular pathogen such as a virus, which is a critical step in activation of T cells (ten Broeke et al., 2013). The CD4 gene encodes the integral membrane glycoprotein that plays an essential role in the immune response and serves multiple functions including acting as a co-receptor with T-cell receptor and activation of T-helper cells (Luckheeram et al., 2012). Asp-Glu-Ala-Asp (DEAD) box polypeptide 58 (DDX58) acts as a cytoplasmic sensor of viral nucleic acids and infections, thereby activating a cascade of antiviral responses including the induction of type I interferons (IFNAR1), which explains the increased mRNA expression of interferon receptor 1 gene observed in this study. Type I interferons are a family of cytokines that have both pro- and anti-inflammatory effects (Kole et al., 2013). For instance, IFN-1 can drive the production of anti-inflammatory cytokines, including IL-10 and can inhibit the secretion of IL-1β by blocking inflammasome activation (Guarda et al., 2011). In contrast, mRNA expression of CCR8 and TLR-2 was downregulated in beef steers fed supplemental SYNB. CCR8 is one of the several receptors for chemokines that orchestrate the movement of leukocytes to the sites of infection or inflammation (Zweemer et al., 2014), suggesting that its downregulation was due to reduced inflammation or infection in these animals. Toll-like receptors play a crucial role in the recognition of microbial pathogens (Takeda et al., 2003; Kawai and Akira, 2007) and TLR-2 is known to be the major receptor for molecular patterns of gram-positive bacteria and several other microbial components (Turner, 2003). The mechanism for downregulation of the TLR-2 gene in beef steers fed supplemental SYNB may be partially explained by Xu et al. (2012). Those authors reported attenuated TLR-triggered inflammatory responses via reverse signaling by MHC class I molecules such as BOLA-A, whose mRNA expression was upregulated in this study. These results suggest that supplemental SYNB was still effective at modulating the expression of immune genes of the beef steers 7 days after its withdrawal from diet. Immune gene expression was not (FC < −(2); FDR ≥ 0.05) different on d 35 (14 days after SYNB withdrawal), indicating that SYNB elicited a positive immune response for at least 7 days after withdrawal from the diet but that benefit ended by 14 days after withdrawal. Taken together, our results suggest that beef steers fed supplemental SYNB had better immune mechanism of protection against infections, which explains their lower BRD morbidity, compared to CON steers. It is important to note that unlike several previous studies (Burdick Sanchez et al., 2014; Fink et al., 2014), these animals were not artificially challenged with pathogens or toxins, suggesting that the effects observed in this study represent the response to their natural environmental stressors.
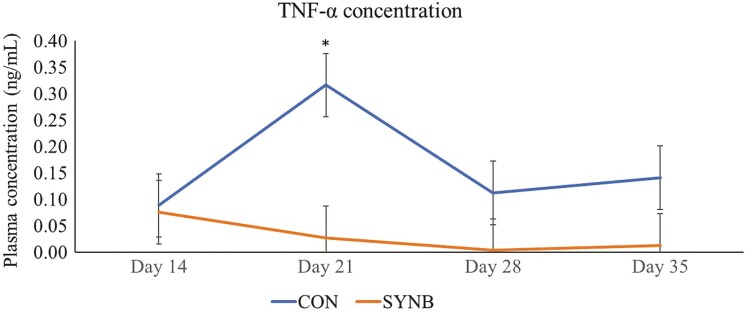
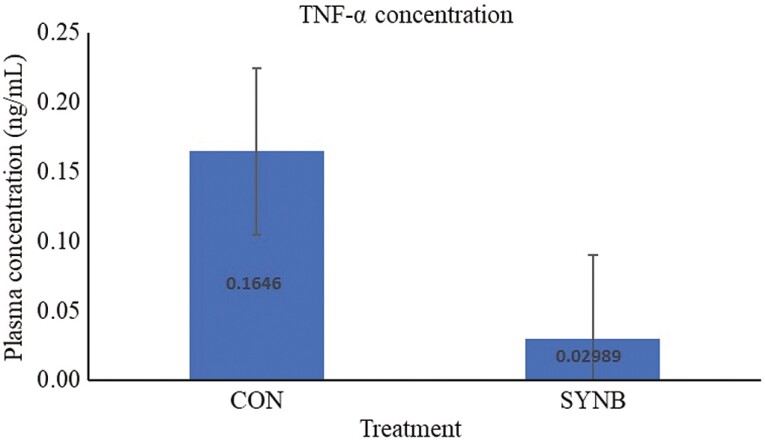
To assess inflammatory response of the animals when experimentally challenged with pathogenic bacteria, we analyzed TNF-α concentration in plasma of the beef steers after ex-vivo whole blood stimulation with LPS. There was no difference in basal TNF- α concentration between treatments (P = 0.63; data not reported). The plasma concentration of TNF-α after LPS stimulation was lower (P = 0.01) only on d 21 in beef steers fed supplemental SYNB, compared to CON (Figure 1). When analyzed over the course of the 35-d receiving period, beef steers fed supplemental SYNB had lower (P = 0.03) plasma concentration of TNF-α after LPS stimulation, relative to CON (Figure 2). Gram-negative bacterial pathogens are the major causative agents of systemic infections and inflammation in animals (Zeng et al., 2017). LPS, the main component of gram-negative bacterial cell wall, is well documented as an inducer of inflammation because it can be easily recognized and bound by the pattern recognition receptor of the immune system which triggers the release of potent chemical mediators such as proinflammatory cytokines, including TNF-α (Kawai and Akira, 2007). Exuberant production of TNF-α during infection can cause fever, inflammation, tissue damage, and sometimes death (Muchamuel et al., 1997). Increased production of TNF-α is known to be inhibited by early and sustained expression of IL-10 and the balance between these two cytokines is essential for maintaining immune homeostasis (Stenvinkel et al., 2005). Therefore, reduced level of TNF-α after stimulation with LPS in plasma of beef steers fed supplemental SYNB may be due to increased mRNA expression of iL-10 and MBL-2 observed in these animals on d 21, which indicates the protective effect of supplemental SYNB against LPS-induced inflammatory stress or tissue damage, suggesting improved immune response to disease. In agreement with our results, Burdick Sanchez et al. (2020) observed attenuated sickness behavior and reduced serum TNF-α from 1 to 2 h post LPS challenge in newly weaned beef steers fed S. cerevisiae fermentation product (Burdick Sanchez et al., 2020). In addition, Carroll et al. (2010) observed reduced inflammatory responses to LPS challenge in beef calves supplemented with live S. cerevisiae.
Figure 1.
Effects of a multicomponent dietary feed additive containing prebiotics and probiotics on plasma concentration of TNF-α following ex-vivo LPS challenge in beef steers on different days during a 35-d receiving period. Values from d 0 were used as independent covariate for each day. Treatment: P = 0.03, SE = 0.06. Treatment × day interaction: P = 0.49, SE = 0.09. Within days: *P ≤ 0.05. CON, control; SYNB, a multicomponent microbial feed additive containing prebiotics and probiotics fed at 28 g/steer/d (Purina Animal Nutrition, Arden Hills, MN).
Figure 2.
Effects of a multicomponent dietary feed additive containing prebiotics and probiotics on plasma concentration of TNF-α following ex-vivo LPS challenge in beef steers during a 35-d receiving period. Treatment: P = 0.03, SE = 0.06.
CON, control; SYNB, a multicomponent microbial feed additive containing prebiotics and probiotics fed at 28 g/ steer/d (Purina Animal Nutrition, Arden Hills, MN).
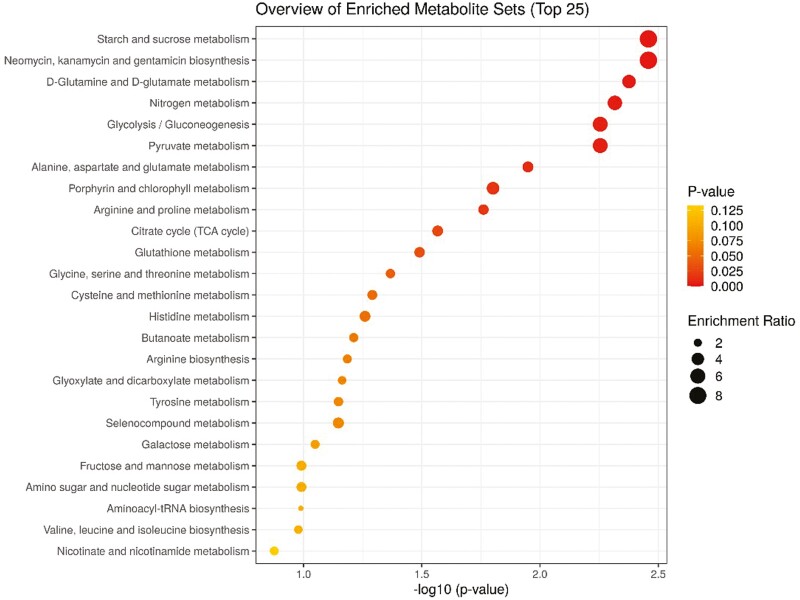
Results of quantitative metabolic pathway enrichment analysis revealed that no differentially enriched pathways were detected on d 0 (Supplementary Table S4) indicating similar host metabolism of the beef steers prior to feeding supplemental SYNB. Six (6) metabolic pathways (starch and sucrose metabolism, neomycin, kanamycin and gentamicin biosynthesis, glutamine and glutamate metabolism, nitrogen metabolism, glycolysis/gluconeogenesis, and pyruvate metabolism) were enriched (FDR ≤ 0.05) in beef steers fed supplemental SYNB compared to CON over the 35-d receiving period (Table 5; Figure 3). Starch and sucrose metabolism, glycolysis, and pyruvate metabolism are pathways that catabolize glucose and other hexoses to produce energy directly as ATP, and indirectly as pyruvate and NADH while gluconeogenesis replenishes glucose from noncarbohydrate metabolites (Wünschiers, 2012). Glucokinase, an enzyme that catalyzes glucose phosphorylation, the first rate-limiting step in glucose metabolism, is also involved in neomycin, kanamycin, and gentamicin biosynthetic pathways. Enrichment of these pathways in beef steers fed supplemental SYNB suggests improved energy metabolism and utilization which supports their better growth performance and health compared to CON. Ruminants have low capacity to synthesize glutamine; however, certain strains of S. cerevisiae possess glutamine synthase that plays a significant role in central nitrogen metabolism (Guillamon et al., 2001), and may be a constituent of the fermentation products contained in supplemental SYNB. This probably explains the enrichment of nitrogen and glutamine/glutamate metabolic pathways observed in this study. Glutamine is considered non-essential amino acid under normal physiological condition; however, immune-regulatory and anti-inflammatory properties of glutamine have been reported during inflammatory conditions such as infection, stress, and injury (Su et al., 2021). It has been demonstrated that glutamine is equally as important as glucose as an oxidative fuel for the maintenance of gut and immune cell function (Newsholme et al., 2003). Though no studies in ruminants are currently available, studies in mice demonstrated that glutamine could modulate a balanced T helper cell polarization which is associated with attenuating inflammation and tissue injury (Hu et al., 2018; Hou et al., 2019). The fact that glutamine/glutamate metabolism was enriched in beef steers fed supplemental SYNB, coupled with the results of the immune gene expression, suggests better health and immune status of these animals compared to CON.
Table 5.
Quantitative pathway enrichment analysis of beef steers fed diet supplemented with a multicomponent dietary feed additive containing prebiotics and probiotics
| Enriched metabolic pathwaya | Enrichment ratio | P | FDR |
|---|---|---|---|
| Starch and sucrose metabolism | 8.48 | 0.0035 | 0.01 |
| Neomycin, kanamycin, and gentamicin biosynthesis | 8.48 | 0.0035 | 0.01 |
| Glutamine and glutamate metabolism | 4.79 | 0.0042 | 0.04 |
| Nitrogen metabolism | 5.58 | 0.0048 | 0.04 |
| Glycolysis/gluconeogenesis | 5.96 | 0.0056 | 0.03 |
| Pyruvate metabolism | 5.96 | 0.0056 | 0.04 |
Enriched pathway relative to control.
Only pathway with false discovery rate (FDR) ≤ 0.05 are shown.
Figure 3.
Quantitative pathway enrichment analysis showing the top 25 enriched metabolic pathways in beef steers fed diet supplemented with a multicomponent dietary feed additive containing prebiotics and probiotics.
CONCLUSION
Feeding SYNB to newly weaned beef steers improved the DMI, growth performance, and meal events during the first 7 d of the receiving period. Over the course of the 35-d receiving period, feeding supplemental SYNB during the first 21 d of arrival reduced BRD morbidity and improved the growth performance and feed efficiency of the beef steers. Although SYNB was removed on day 21, its effects persisted to positively influence health after removal from the diet. These benefits of SYNB can be partially attributed to a reduction in inflammatory stress as evidenced by a reduction in mRNA expression of inflammatory markers with a concomitant increase in mRNA expression of anti-inflammatory markers. Finally, improved nutrient metabolism of SYNB steers evidenced by enrichment of starch and sucrose metabolism, neomycin, kanamycin and gentamicin biosynthesis, glutamine and glutamate metabolism, nitrogen metabolism, glycolysis/gluconeogenesis, and pyruvate metabolism supports immune function and phenotypic performance.
Supplementary Material
Acknowledgments
The study was funded by Purina Animal Nutrition, Arden Hills, MN. Additional funding support was provided by West Virginia University Experimental Station in support of U.S. Department of Agriculture hatch multi-state regional project W-3010 (Scientific Article Number 3433).
Contributor Information
Modoluwamu D Idowu, Division of Animal and Nutritional Science, West Virginia University, Morgantown, WV 26505, USA.
Godstime Taiwo, Division of Animal and Nutritional Science, West Virginia University, Morgantown, WV 26505, USA.
Andres Pech Cervantes, Agricultural Research Station, Fort Valley State University, Fort Valley, GA 31030, USA.
Scott A Bowdridge, Division of Animal and Nutritional Science, West Virginia University, Morgantown, WV 26505, USA.
Ibukun M Ogunade, Division of Animal and Nutritional Science, West Virginia University, Morgantown, WV 26505, USA.
Conflict of interest statement
None declared.
LITERATURE CITED
- Adeyemi, J. A., Harmon D. L., Compart D. M. P., and Ogunade I. M.. . 2019. Effects of a blend of Saccharomyces cerevisiae-based direct-fed microbial and fermentation products in the diet of newly weaned beef steers: Growth performance, whole-blood immune gene expression, serum biochemistry, and plasma metabolome1. J. Anim. Sci. 97:4657–4667. doi: 10.1093/jas/skz308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, C. L., Jensen J. L., and Ørntoft T. F.. . 2004. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Canc. Res. 64:5245–5250. doi: 10.1158/0008-5472. [DOI] [PubMed] [Google Scholar]
- Armato, L., Gianesella M., Morgante M., Fiore E., Rizzo M., Giudice E., and Piccione G.. . 2016. Rumen volatile fatty acids × dietary supplementation with live yeast and yeast cell wall in feedlot beef cattle. Acta Agric. Scand. A Anim. Sci. 66:119–124. doi: 10.1080/09064702.2016.1272628. [DOI] [Google Scholar]
- Arthington, J. D., Cooke R. F., Maddock T. D., Araujo D. B., Moriel P., DiLorenzo N., and Lamb G. C.. . 2013. Effects of vaccination on the acute-phase protein response and measures of performance in growing beef calves. J. Anim. Sci. 91:1831–1837. doi: 10.2527/jas.2012-5724. [DOI] [PubMed] [Google Scholar]
- Baah, J., Y.Wang., and McAllister T. A.. . 2009. Impact of a mixed culture of Lactobacillus casei and L. lactis on in vitro ruminal fermentation and the growth of feedlot steers fed barley-based diets. Can. J. Anim. Sci. 89:2. doi: 10.4141/CJAS08117. [DOI] [Google Scholar]
- Benjamini, Y., and Hochberg Y.. . 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B. Methodol. 57:289––300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bergman, E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Bernhard, B. C., Burdick N. C., Rathmann R. J., Carroll J. A., Finck D. N., Jennings M. A., Young T. R., and Johnson B. J.. . 2012. Chromium supplementation alters both glucose and lipid metabolism in feedlot cattle during the receiving period. J. Anim. Sci. 90:4857–4865. doi: 10.2527/jas.2011-4982. [DOI] [PubMed] [Google Scholar]
- ten Broeke, T., Wubbolts R., and Stoorvogel W.. . 2013. MHC class II antigen presentation by dendritic cells regulated through endosomal sorting. Cold Spr. Harb. Perspect. 5:a016873. doi: 10.1101/cshperspect.a016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz, P., and Monack D. M.. . 2013. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immun. 13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez, N. C., Carroll J. A., Broadway P. R., Edrington T. S., Yoon I., and Belknap C. R.. . 2020. Some aspects of the acute phase immune response to a lipopolysaccharide (LPS) challenge are mitigated by supplementation with a Saccharomyces cerevisiae fermentation product in weaned beef calves. Transl. Anim. Sci. 4:156. doi: 10.1093/tas/txaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick Sanchez, N. C., Young T. R., Carroll J. A., Corley J. R., Rathmann R. J., and Johnson B. J.. . 2014. Yeast cell wall supplementation alters the metabolic responses of crossbred heifers to an endotoxin challenge. Inn. Immun. 20:104–112. doi: 10.1177/1753425913482152. [DOI] [PubMed] [Google Scholar]
- Carroll, J. A., Collier C. T., Hulbert L. E., Corley J. R., Estefan A. G., Fink D. N., and Johnson B. J.. . 2010. Yeast supplementation alters the health status of receiving cattle. J. Anim. Sci. 88:315. doi: 10.15232/S1080-7446(15)30125-X.19717764 [DOI] [Google Scholar]
- Cruz Ramos, H., Hoffmann T., and Marino M.. . 2000. Fermentative metabolism of Bacillus subtilis: Physiology and regulation of gene expression. J. Bacteriol. 182:3072–3080. doi: 10.1128/JB.182.11.3072-3080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgaard, C. T., Lauritzen L., Calder P. C., Kjaer T. M., and Frokiaer H.. . 2009. Whole-blood culture is a valid low-cost method to measure monocytic cytokines—a comparison of cytokine production in cultures of human whole-blood, mononuclear cells, and monocytes. J. Immunol. Methods. 340:95–101. doi: 10.1016/j.jim.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Deters, E. L., and Hansen S. L.. . 2019. Effect of supplementing a Saccharomyces cerevisiae fermentation product during a preconditioning period prior to transit on receiving period performance, nutrient digestibility, and antioxidant defense by beef steers. Transl. Anim. Sci. 3:1227–1237. doi: 10.1093/tas/txz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deters, E. L., Stokes S. R., Genther-Schroeder O. N., and Hansen S. L.. . 2018. Effects of a Saccharomyces cerevisiae fermentation product in receiving diets of newly weaned beef steers. II. Digestibility and response to a vaccination challenge. J. Anim. Sci. 96:3906–3915. doi: 10.1093/jas/sky247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommett, R. M., Klein N., and Turner M. W.. . 2006. Mannose-binding lectin in innate immunity: Past, present and future. Tissue Antigens. 68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff, G. C., and Galyean M. L.. . 2007. Board-invited review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam, N. A., Gleghorn J. F., Rivera J. D., Galyean M. L., Defoor P. J., Brashears M. M., and Younts-Dahl S. M.. . 2003. Effects of live cultures of Lactobacillus acidophilus (strains NP45 and NP51) and Propionibacterium freudenreichii on performance, carcass, and intestinal characteristics, and Escherichia coli strain O157 shedding of finishing beef steers. J. Anim. Sci. 81:2686–2698. doi: 10.2527/2003.81112686x. [DOI] [PubMed] [Google Scholar]
- Evans, M., Reeves S., and Robinson L. E.. . 2012. A dried yeast fermentate prevents and reduces inflammation in two separate experimental immune models. Evid. Based. Complement. Alternat. Med. 2012:973041. doi: 10.1155/2012/973041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, D. N., Ribeiro F. R. B., Burdick N. C., Parr S. L., Carroll J. A., Young T. R., Bernhard B. C., Corley J. R., Estefan A. G., Rathmann R. J., . et al. 2014. Yeast supplementation alters the performance and health status of receiving cattle. Prof. Anim. Sci. 30:333–341. doi: 10.15232/S1080-7446(15)30125-X. [DOI] [Google Scholar]
- Gelaleti, G. B., Jardim B. V., Leonel C., Moschetta M. G., and de Campos Zuccari D. A. P.. . 2012. Interleukin-8 as a prognostic serum marker in canine mammary gland neoplasias. Vet. Immunol. Immunop. 146:106–112. doi: 10.1016/j.vetimm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Guarda, G., Braun M., Staehli F., Tardivel A., Mattmann C., Fo¨rster I., Farlik M., Decker T., Du Pasquier R. A., Romero P., Tschopp J.. . 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Guillamon, J. M., van Riel N. A., Giuseppin M. L., and Verrips C. T.. . 2001. The glutamate synthase (GOGAT) of Saccharomyces cerevisiae plays an important role in central nitrogen metabolism. FEMS Yeast Res. 1:169–175. doi: 10.1111/j.1567-1364.2001.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Ha, H., Debnath B., and Neamati N.. . 2017. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 7:1543–1588. doi: 10.7150/thno.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. B., Laarman A. H., Reynolds M. K., and Smith W. K.. . 2018. Performance of backgrounding steers fed diets containing monensin or a lactobacillus fermentation product. Transl. Anim. Sci. 2:130–133. doi: 10.1093/tas/txy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T. L., Liang Y., Sharon K. P., Sellers M. D., I Y., Scott M. F., Carroll J. A., and Ballou M. A.. . 2017. Influence of Saccharomyces cerevisiae fermentation products, SmartCare in milk replacer and original XPC in calf starter, on the performance and health of preweaned Holstein calves challenged with Salmonella enterica serotype Typhimurium. J. Dairy Sci. 100:7154–7164. doi: 10.3168/jds.2016-12509. [DOI] [PubMed] [Google Scholar]
- Hashmi, S., and Zeng Q. T.. . 2006. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 17:699–706. doi: 10.1097/01.mca.0000236288.94553.b4. [DOI] [PubMed] [Google Scholar]
- Hou, Y. C., Wu J. M., Chen K. Y., Chen P. D., Lee C., Yeh S., and Lin M.. . 2019. Effects of prophylactic administration of glutamine on CD4+ T cell polarisation and kidney injury in mice with polymicrobial sepsis. Brit. J. Nut. 122:657–665. doi: 10.1017/S0007114519000990. [DOI] [PubMed] [Google Scholar]
- Hu, Y. M., Hsiung Y. C., Pai M. H., and Yeh S. L.. . 2018. Glutamine administration in early or late septic phase downregulates lymphocyte PD-1/PD-L1 expression and the inflammatory response in mice with polymicrobial sepsis. J. Parent. Ent. Nut. 42:538–549. doi: 10.1177/0148607117695245. [DOI] [PubMed] [Google Scholar]
- Hutcheson, D. P., and Cole N. A.. . 1986. Management of transit-stress syndrome in cattle: Nutritional and environmental effects. J. Anim. Sci. 62:555–560. doi: 10.2527/jas1986.622555x. [DOI] [Google Scholar]
- Iyer, S. S., and Cheng G.. . 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, T., and Akira S.. . 2007. TLR signaling. Semin. Immunol. 19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kole, A., He J., Rivollier A., Silveira D. D., Kitamura K., Maloy K. J., Kelsall B. L.. . 2013. Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis. J. Immun. 191:2771–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krehbiel, C. R., Barry B. A., Reeves J. M., Gill D. R., Smith R. A., Step D. L., Choat W. T., and Ball R. L.. . Effects of feed additives fed to sale barn-origin calves during the receiving period: animal performance, health, and medical costs. Oklahoma Agricultural Experiment Station. 2001. [accessed January 22, 2021]. Available from http://www.ansi.okstate.edu/research/2001rr/27/27.htm. [Google Scholar]
- Krehbiel, C. R., Rust S. R., Zhanga G., and Gilliland S. E.. . 2003. Bacterial direct fed microbials in ruminant diets: Performance response and mode of action. J. Anim. Sci. 81:120–132. doi: 10.2527/2003.8114_SUPPL_2E120X. [DOI] [Google Scholar]
- Kumprechtova, D., Illek J., Julien C., Homolka P., Jancik F., Auclair E.. . 2019. Effect of live yeast Saccharomyces cerevisiae supplementation on rumen fermentation and metabolic profile of dairy cows in early lactation. J. Anim. Physiol. Anim. Nutr. (Berl). 103:447–455. doi: 10.1111/jpn.13048. [DOI] [PubMed] [Google Scholar]
- Lauriault, L. M., Dougherty C. T., Bradley N. W., and Cornelius P. L.. . 1990. Thiamin supplementation and the ingestive behavior of beef cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 68:1245–1253. doi: 10.2527/1990.6851245x. [DOI] [PubMed] [Google Scholar]
- Lesmeister, K. E., Heinrichs A. J., and Gabler M. T.. . 2004. Effects of supplemental yeast Saccharomyces cerevisiae culture on rumen development, growth characteristics, and blood parameters in neonatal dairy calves. J. Dairy Sci. 87:1832–1839. doi: 10.3168/jds.S0022-0302(04)73340-8. [DOI] [PubMed] [Google Scholar]
- Li, S., I Y., Scott M., Khafipour E., and Plaizier J. C.. . 2016. Impact of Saccharomyces cerevisiae fermentation product and subacute ruminal acidosis on production, inflammation, and fermentation in the rumen and hindgut of dairy cows. Anim. Feed Sci. Technol. 211:50–60. doi: 10.1016/j.Anifeedsci.2015.10.010. [DOI] [Google Scholar]
- Ling, T., Hernandez-Jover M., Sordillo L. M., and Abuelo A.. . 2018. Maternal late-gestation metabolic stress is associated with changes in immune and metabolic responses of dairy calves. J. Dairy Sci. 101:6568–6580. doi: 10.3168/jds.2017-14038. [DOI] [PubMed] [Google Scholar]
- Luckheeram, R. V., Zhou R., Verma A. D., and Xia B.. . 2012. CD4+T Cells: Differentiation and functions. J. Immun. Res. 2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, E., McGee M., and Earley B.. . 2019. Weaning management of beef calves with implications for animal health and welfare. J. App. Anim. Res. 47:167–175. doi: 10.1080/09712119.2019.1594825. [DOI] [Google Scholar]
- Marques, R. S., Cooke R. F., Rodrigues M. C., Cappellozza B. I., Larson C. K., Moriel P., and Bohnert D. W.. . 2016. Effects of organic or inorganic Co, Cu, Mn, and Zn supplementation to late-gestating beef cows on productive and physiological responses of the offspring. J. Anim. Sci. 94:1215–1122. doi: 10.2527/jas.2015-0036. [DOI] [PubMed] [Google Scholar]
- McAllister, T., Beauchemin K. A., Alazzeh A., J B., Teather R., and Stanford K.. . 2011. Review: The use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 91:193–211. doi: 10.4141/cjas10047. [DOI] [Google Scholar]
- McDonald, C., Inohara N., and Nuñez G.. . 2005. Peptidoglycan signaling innate immunity and inflammatory disease. J. Biol. Chem. 280:20177–20180. doi: 10.1074/jbc.R500001200. [DOI] [PubMed] [Google Scholar]
- Muchamuel, T., Menon S., Pisacane P., Howard M. C., and Cockayne D. A.. . 1997. IL-13 protects mice from lipopolysaccharide-induced lethal endotoxemia: Correlation with down-modulation of TNF-alpha, IFN-gamma, and IL-12 production. J. Immunol. 158:2898–2903. [PubMed] [Google Scholar]
- Nagaraja, T. G., and Titgemeyer E. C.. . 2007. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 90:17–38. doi: 10.3168/jds.2006-478. [DOI] [PubMed] [Google Scholar]
- Newbold, C. J., Wallace R. J., and Mcintosh F. M.. . 1996. Mode of action of the yeast Saccharomyces cerevisiae, a feed additive for ruminants. Brit. J. Nut. 76:249–261. doi: 10.1079/bjn19960029. [DOI] [PubMed] [Google Scholar]
- Newsholme, P., Procopio J., Lima M. M., Pithon-Curi T. C., and R C.. . 2003. Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem. Funct. 21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- O’Boyle, N., Corl C. M., Gandy J. C., and Sordillo L. M.. . 2006. Relationship of body condition score and oxidant stress to tumor necrosis factor expression in dairy cattle. Vet. Immunol. Immunopathol. 113:297–304. doi: 10.1016/j.vetimm.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Oba, M., and Allen M. S.. . 2003. Effects of diet fermentability on efficiency of microbial nitrogen production in lactating dairy cows. J. Dairy Sci. 86:195–207. doi: 10.3168/jds.S0022-0302(03)73600-5. [DOI] [PubMed] [Google Scholar]
- Ogunade, I., Jiang Y., Adeyemi J., Oliveira A., D V., and Adesogan A.. . 2018. Biomarker of aflatoxin ingestion: ¹H NMR-based plasma metabolomics of dairy cows fed aflatoxin B1 with or without sequestering agents. Toxins. 10:545. doi: 10.3390/toxins10120545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunade, I. M., McCoun M., Idowu M. D., and Peters S. O.. . 2020. Comparative effects of two multispecies direct-fed microbial products on energy status, nutrient digestibility, and ruminal fermentation, bacterial community, and metabolome of beef steers. J. Anim. Sci. 98:1–9. doi: 10.1093/jas/skaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunade, I. M., Schweickart H., McCoun M., Cannon K., and McManus C.. . 2019. Integrating 16S rRNA sequencing and LC-MS-based metabolomics to evaluate the effects of live yeast on rumen function in beef cattle. Animals. 28:683. doi: 10.3390/ani9010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J., Harper M., Melgar A., Compart D. M. P., and Hristov A. N.. . 2019. Effects of Saccharomyces cerevisiae-based direct-fed microbial and exogenous enzyme products on enteric methane emission and productivity in lactating dairy cows. J. Dai. Sci. 102:6065–6075. doi: 10.3168/jds.2018-15753. [DOI] [PubMed] [Google Scholar]
- Pang, Z., Chong J., Zhou G., Morais D., Chang L., Barrette M., Gauthier C., Jacques P. E., Li S., and Xia J.. . 2021. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucl. Acids Res. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 29:e45–e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaizier, J. C., Danesh Mesgaran M., Derakhshani H., Golder H., Khafipour E., Kleen J. L., Lean I., Loor J., Penner G., and Zebeli Q.. . 2018. Review: Enhancing gastrointestinal health in dairy cows. Animal. 12:s399–s418. doi: 10.1017/S1751731118001921. [DOI] [PubMed] [Google Scholar]
- Psychogios, N., Hau D. D., Peng J., Guo A. C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., and Gautam B.. . 2011. The human serum metabolome. PLoS One 6:e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanbakhsh, S., Liu P., Bjorndahl T. C., Mandal R., Grant J. R., Wilson M., Eisner R., Sinelnikov I., Hu X., and Luchinat C.. . 2015. Accurate, fully automated NMR spectral profiling for metabolomics. PLoS One. 10:e0124219. doi: 10.1371/journal.pone.0124219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Chavira, J., Montano M. F., Torrentera N., and Zinn R. A.. . 2017. Influence of feeding enzymatically hydrolysed yeast cell wall + yeast culture on growth performance of calf-fed Holstein steers. J. Appl. Anim. Res. 1:327–330. doi: 10.1080/09712119.2017.1299742. [DOI] [Google Scholar]
- Stenvinkel, P., Ketteler M., Johnson R. J., Lindholm B., Pecoits-Filho R., Riella M., Heimbürger O., Cederholm T., and Girndt M.. . 2005. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Su, L. H., Ming-Tsan L., Sung-Ling Y., and Chiu-Li Y.. . 2021. Glutamine administration attenuates kidney inflammation in obese mice complicated with polymicrobial sepsis. Mediators Inflamm. 5597118. doi: 10.1155/2021/5597118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, K., Kaisho T., and Akira S.. . 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tricarico, J. M., Abney M. D., Galyean M. L., Rivera J. D., Hanson K. C., McLeod K. R., and Harmon D. L.. . 2007. Effects of a dietary Aspergillus oryzae extract containing alphaamylase activity on performance and carcass characteristics of finishing beef cattle. J. Anim. Sci. 85:802–811. doi: 10.2527/jas.2006-427. [DOI] [PubMed] [Google Scholar]
- Turner, M. W. 2003. The role of mannose-binding lectin in health and disease. Mol. Immunol. 40:423–429. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- Wagner, J. J., Engle T. E., Belknap C. R., and Dorton K. L.. . 2016. Meta-analysis examining the effects of Saccharomyces cerevisiae fermentation products on feedlot performance and carcass traits. Prof. Anim. Sci 32:172–182. doi: 10.15232/pas.2015-01438. [DOI] [Google Scholar]
- Wan, Q., Chen S., Shan Z., Yang Z., Chen L., Zhang C., Yuan S., Hao Q., Zhang X., Qiu D., Chen H., Zhou X.. . 2017. Stability evaluation of reference genes for gene expression analysis by RT-qPCR in soybean under different conditions. PLoS One 12:e0189405. doi: 10.1371/journal.pone.0189405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. A., and Goonewardene Z.. . 2004. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 84:1–11. doi: 10.4141/A03-123. [DOI] [Google Scholar]
- Wolf, A. J., Underhill D. M.. . 2018. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immun. 18:243–254. doi: 10.1038/nri.2017.136. [DOI] [PubMed] [Google Scholar]
- Wünschiers, R., 2012. Carbohydrate Metabolism and Citrate Cycle, In: Michal, G. and Schomburg D., editors. Biochemical pathways: an atlas of biochemistry and molecular biology. 2nd ed. Hoboken (NJ): John Wiley and Sons, Inc.; p. 37–58. [Google Scholar]
- Xu, S., Liu X., Bao Y., Zhu X., Han C., Zhang P., Zhang X., Li W., and Cao X.. . 2012. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat. Immunol. 13:551–559. doi: 10.1038/ni.2283. [DOI] [PubMed] [Google Scholar]
- Zeng, M., Inohara N., and Nuñez G.. . 2017. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J., Mengyao H., Jie L., Yan L., Jialiang L., Liyun Z., Xiao L., Daming Z., and Zhengliang C.. . 2019. Mannan-binding lectin regulates inflammatory cytokine production, proliferation, and cytotoxicity of human peripheral natural killer cells. Mediators Inflamm. 10. doi: 10.1155/2019/6738286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W., Wei Z., Xu N., Yang F., Yoon I., Chung Y., Liu J., and Wang J.. . 2017. Effects of Saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J. Anim. Sci. Biotechnol. 8:36. doi: 10.1186/s40104-017-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweemer, A. J., Toraskar J., Heitman L. H., and Ijzerman A. P.. . 2014. Bias in chemokine receptor signaling. Trends Immunol. 35:243–252. doi: 10.1016/j.it.2014.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.