Abstract
For the hyperthermophilic and barophilic methanarchaeon Methanococcus jannaschii, we have developed a medium and protocols for reactor-scale cultivation that improved the final cell yield per liter from ∼0.5 to ∼7.5 g of packed wet cells (∼1.8 g dry cell mass) under autotrophic growth conditions and to ∼8.5 g of packed wet cells (∼2 g dry cell mass) with yeast extract (2 g liter−1) and tryptone (2 g liter−1) as medium supplements. For growth in a sealed bottle it was necessary to add Se to the medium, and a level of 2 μM for added Se gave the highest final cell yield. In a reactor M. jannaschii grew without added Se in the medium; it is plausible that the cells received Se as a contaminant from the reactor vessel and the H2S supply. But, for the optimal performance of a reactor culture, an addition of Se to a final concentration of 50 to 100 μM was needed. Also, cell growth in a reactor culture was inhibited at much higher Se concentrations. These observations and the data from previous work with methanogen cell extracts (B. C. McBride and R. S. Wolfe, Biochemistry 10:4312–4317, 1971) suggested that from a continuously sparged reactor culture Se was lost in the exhaust gas as volatile selenides, and this loss raised the apparent required level of and tolerance for Se. In spite of having a proteinaceous cell wall, M. jannaschii withstood an impeller tip speed of 235.5 cms−1, which was optimal for achieving high cell density and also was the higher limit for the tolerated shear rate. The organism secreted one or more acidic compounds, which lowered pH in cultures without pH control; this secretion continued even after cessation of growth.
Methanococcus jannaschii (13) is a hyperthermophilic (optimal growth temperature, 85°C) and barophilic (18, 19) methanarchaeon isolated from the surface material collected from the base of a “white smoker” submarine hydrothermal vent (13). Although this organism was the first archaeon for which the sequence of the entire genome was determined (6), the biochemical studies have been limited due to the inability of investigators to successfully mass culture the organism to high cell densities. The entire genome sequence for another methanarchaeon, Methanobacterium thermoautotrophicum ΔH, has also been determined (26, 36). The availability of whole genome sequences provides excellent opportunities for thorough comparative biochemical studies. The sequencing of the genome of M. jannaschii has generated interest in studying this organism; the requests for cultures, cell pastes, and protocols for growing this organisms have greatly increased (our own experience) (4, 11).
Previous to the present study, the typical maximum cell yield for M. jannaschii in reactor-scale cultures was ∼0.5 g of packed wet cells per liter of culture (University of Illinois Fermentor Facility records) (27, 32). The published information on the growth of M. jannaschii is very limited and almost entirely pertains to small-scale (5- to 200-ml) cultures (13, 18, 19, 24). In addition, none of these studies sought to optimize the cell yield. A rare study that involved the cultivation of M. jannaschii in a reactor offers no details of the reactor and reports a very slowly growing culture under limited gas supply and at 65°C that reaches an optical density at 660 nm (OD660) of 0.8 to 1.0 in 5 days (9). We describe here the media recipes and protocols for mass culture of this organism in a 16-liter constantly stirred tank reactor to high cell densities and provide guidelines for scaling up such cultures to higher volumes.
MATERIALS AND METHODS
Organism and media.
M. jannaschii JAL-1 (13) was obtained from David R. Boone (Oregon Collection of Methanogens, Portland, Oreg.) and maintained in 25 ml of medium 1 in a 160-ml serum bottle as described below.
A number of media for maximizing the growth rate and cell yield at the bottle and reactor scale were examined. Medium 1 was developed in this work, and it contained the following components at the indicated concentrations (units of millimolar): K2HPO4, 0.32; KH2PO4, 0.41; KCl, 13.4; NaCl, 430; NaHCO3, 10; CaCl2 · 2H2O, 2.5; MgCl2 · 6H2O, 38; NH4Cl, 22; Fe(NH4)2(SO4)2 · 6H2O, 0.031; Na3-nitrilotriacetate, 0.32; Na2SeO4, 0.002 (varied for specific experiments; 0.05 to 0.1 mM for high growth rate and cell yield in a reactor culture); Na2WO4 · 2H2O, 0.010; Na2MoO4 · 2H2O, 0.010; Na2S · 9H2O, 2.0 mM (or a continuous supply of H2S at the reactor scale); and 10 ml of a 100-fold-concentrated trace metal solution per liter (7), where we replaced FeCl2 · 4H2O with an equimolar amount of FeCl3 · 6H2O. Medium 2 was the defined growth medium of Jones et al. (13), except the vitamins were omitted. For certain experiments we replaced the NaHCO3 in medium 1 with MES (4-morpholineethanesulfonic acid) (50 mM), and here the pH of the medium was adjusted to 6 before boiling (for small-scale preparation) or before autoclaving (for reactor scale). Whenever the addition of vitamins is mentioned, 10 ml of a 100-fold-concentrated vitamin solution described by Wolin et al. (34) was included in each liter of medium. Other variations have been included in the Results. Yeast extract and tryptone were from Difco Laboratories (Detroit, Mich.). All other medium components were of reagent grade from standard suppliers. All gases were of technical grade (S. J. Smith Welding Supply, Davenport, Iowa).
Small-scale cultivation protocol.
To study growth of M. jannaschii in tubes or bottles and to prepare inocula for reactors, the techniques of Balch and Wolfe (2) were used, with the following modifications. Two types of culture bottles were used: one was a 160-ml serum bottle (catalog no. 223748; Wheaton Science Products, Millville, N.J.) which contained 25 ml of medium and which was sealed with a solid rubber stopper (catalog no. 2048-11800; Bellco Glass Inc., Vineland, N.J.) (2) and the other was a 530-ml serum bottle (catalog no. 223952; Wheaton Science Products) (7) which contained 150 ml of medium and which was sealed with a no. 1 black rubber stopper that had one-third of its bottom cut off (7); the rubber stoppers were crimped in place. Unless otherwise mentioned (see below), for the preparation of medium, all components (including vitamins, yeast extract, and tryptone, wherever indicated) except MgCl2 · 6H2O, CaCl2 · 2H2O, and NH4Cl (and also excluding Fe(NH4)2(SO4)2 · 6H2O and Na3-nitrilotriacetate for medium 1) were dissolved in distilled deionized water. This solution was made anaerobic by boiling (2) under a stream of N2-CO2 (80:20 [vol/vol]). After the anaerobic solution was cooled under a N2-CO2 atmosphere and transferred inside an anaerobic bag (2), MgCl2 · 6H2O, CaCl2 · 2H2O, and NH4Cl were added as solids to it to desired final concentrations. This procedure prevented precipitation of Mg2+ and loss of NH3 during boiling due to a rise in pH. Such a precaution was not needed for a medium with MES as the buffer, since here the pH did not rise during boiling (ΔpKa/°C for MES, −0.009) (19), and therefore MgCl2 · 6H2O, CaCl2 · 2H2O, and NH4Cl were included in the medium before boiling. Further steps were carried out as detailed previously (2), except the headspace gas of the bottles with anaerobic medium was exchanged with H2-CO2 (80:20 [vol/vol]; 0.15 to 0.2 × 105 Pa before the bottles were autoclaved. Before inoculation of the sterile medium, sodium sulfide was added from an anaerobic sterile stock to a final concentration of 2 mM. Also, for medium 1, at this stage Fe(NH4)2(SO4)2 · 6H2O and Na3-nitrilotriacetate were added from a sterile anaerobic stock solution. For growth experiments the cultures were initiated with an inoculum (2 ml of mid-logarithmic phase culture per 100 ml of medium) that had been grown by two sequential transfers in the intended test medium. The inoculated cultures were pressurized to 1.7 × 105 Pa with H2-CO2 (80:20 [vol/vol] and shaken at 85°C and 200 rpm in a gyratory shaker (model 3527X Orbit Environ-Shaker; Lab-Line Instruments, Inc., Melrose Park, Ill.). For growth of inocula each bottle was removed from the shaker every 2 h and the headspace was repressurized with H2-CO2. For growth experiments, the atmosphere in each bottle was repressurized every hour, except every third hour the atmosphere was flushed out for 30 s by allowing 2 liters of gas to escape through a 22-gauge needle prior to pressurization. To minimize the temperature drop in a culture during this rejuvenation process, an insulating sleeve made out of soft cellulose was put on the bottle; even then the culture temperature often dropped to as low as 78°C. For studying the effect of Se concentration on growth, the serum bottles were soaked overnight in 1 M H2SO4 and then washed extensively with distilled deionized water before use; the rubber stoppers were washed with distilled deionized water. Here, medium 1 was prepared without Na2SeO4, but it was added to the sterile medium to a desired final concentration from a sterile anaerobic stock.
Reactor and accessories.
The reactor-scale experiments were carried out in a 16-liter (12-liter working volume) stainless steel constantly stirred tank reactor (model Microgen; New Brunswick Scientific Company, New Brunswick, N.J.) with four vertical baffles and three six-bladed Rushton type turbine impellers (diameter, 7.5 cm) (see Fig. 1 of reference 8). The bottommost impeller was situated 5 cm above the vessel bottom and 1.3 cm above the single-hole sparger. The distance between two consecutive impellers was 10.2 cm. The top impeller was 7 cm below the liquid surface (when the vessel content was not being stirred). The vessel was fitted with a gel-filled sterilizable pH probe and the same type of redox probe (Broadly James Corp., Irvine, Calif.) for the measurements of culture pH and redox potential, respectively, in situ. For making anaerobic and sterile additions (manual or automatic) to the culture two of the addition ports on the head plate of the vessel were fitted with rubber stoppers (no. 5 1/2 black rubber stopper) as detailed in Fig. 1 (20). All manual additions of sterile and anaerobic solutions to the culture were performed through one of these two rubber stoppers by using either a sterile syringe fitted with a 22-gauge needle (Fig. 1) or a double-needle (22-gauge) system of Baresi and Wolfe (3) (Fig. 1). Each automatic addition (NaOH solution or water) was via a 20-gauge needle inserted through the other rubber stopper and connected to a supply line by using a 1.6-mm polypropylene male Luer fitting (catalog no. E-30504-00; Cole Palmer Instrument Co., Vernon Hills, Ill.). For automatic control of pH, a pH controller (model M1055-1000; New Brunswick Scientific Co.), a reservoir (a 530-ml sealed serum bottle fitted with a 21-gauge needle through its stopper) of sterile and anaerobic solution of 5 N NaOH and a peristaltic pump (a Masterflex LS variable-speed modular drive and an LS size 13 pump head; Barnant Co., Barrington, Ill.) were used. The flow path for the NaOH solution, including the pumping section, was made up of a Masterflex size 13 Norprene A60G tube (internal diameter, 0.8 mm; Norton Performance Plastic Loop, Akron, Ohio). A similar system was used for the continuous addition of sterile anaerobic water to the reactor, and here a 1-liter culture bottle, described by Balch and Wolfe (2), fitted with a 21-gauge needle through its rubber stopper served as the reservoir. The inoculum was transferred to the sterile medium from a 160-ml serum bottle by use of the double-needle system shown in Fig. 1 (3). To obtain consistent results we recommend that the detailed procedures presented in the legend for Fig. 1 be used. Unless otherwise mentioned, all gases were supplied to the culture at the bottom of the vessel through a single-hole sparger situated directly below the agitator shaft. The N2, CO2, and H2 streams were made oxygen free by passage through a common heated bed of copper turnings (2); the flow of hydrogen ensured continuous regeneration of oxidized copper. The flow rates of gases were measured and controlled by using rotameters (Cole Palmer Instrument Co.), and the reported values correspond to a pressure of 1 atm or 1.01 × 105 Pa. Heating and cooling of reactor contents were accomplished by using flows of steam (2.8 × 105 Pa) and water (22°C and 2.8 × 105 Pa), respectively, through the hollow baffles. The cells were harvested by use of a Sharples centrifuge (type AS-14), frozen quickly in liquid nitrogen, and stored at −70°C.
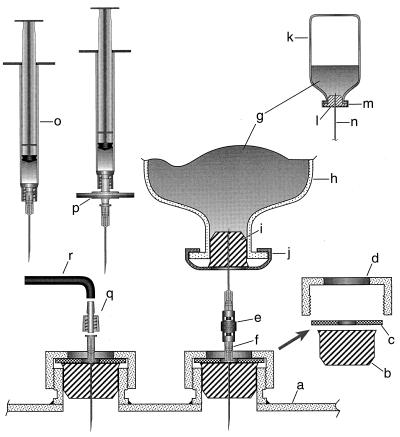
FIG. 1.
Modified ports on the reactor head plate and accessories for manual and continuous addition of anaerobic sterile liquid to the reactor culture. This figure is not to scale. The head plate shown is a schematic of a part of the model Microgen fermentor (New Brunswick Scientific Company). Items: a, fermentor head plate; b, no. 5 1/2 black rubber stopper; c, stainless steel washer; d, stainless steel collet nut; e, double-male LUER-LOK adapter (catalog no. 3114; Becton Dickinson and Co., Rutherford, N.J.); f, 21-gauge sterile needle; g, inoculum or sterile anaerobic liquid; h, 530-ml serum bottle (catalog no. 223952; Wheaton Science Products); i, no. 1 black rubber stopper; j, 30-mm-diameter aluminum crimp with center disk removed (catalog no. 224187; Wheaton Science Products); k, 160-ml serum bottles (catalog no. 223748; Wheaton Science Products); l, lipped solid-rubber stopper (catalog no. 2048-11800; Bellco Glass Inc.) (2); m, 20-mm-diameter aluminum crimp with center disk removed (catalog no. 224183; Wheaton Science Products); n, a part of double-male LUER-LOK adapter with needles (see items e and f); o, sterile syringe containing sterile and anaerobic liquid and fitted with a sterile needle (21 or 22 gauge); p, sterile syringe filter (0.2 μm) fitted with a sterile needle (21 or 22 gauge) and mounted onto a syringe for addition of small volumes of aerobic liquid; q, 1.6-mm-diameter polypropylene male Luer fitting (catalog no. E-30504-00; Cole Palmer); r, Masterflex size 13 Norprene A60G tube (internal diameter, 0.8 mm; Norton Performance Plastic Loop). Manual additions were as follows. After sterilizing the rubber surface by flaming it with a propane torch, liquids were added either from a syringe or from a bottle; the atmosphere in each bottle was pressurized to 2 × 105 Pa with N2-CO2 (80:20 [vol/vol]) for a chemical solution or with H2-CO2 (80:20 [vol/vol]) for a culture to be used as an inoculum. Automated additions were as follows. A sterile and anaerobic solution of NaOH from a pressurized 530-ml sealed serum bottle fitted with a 21-gauge needle through its stopper or sterile anaerobic water from a 1-liter bottle (2) fitted with a 21-gauge needle was pumped into the vessel (see Materials and Methods and item q). This figure includes certain pieces of information from references 3 and 20).
Reactor-scale cultivation protocol.
For the preparation of medium 1 or 2, all components except MgCl2 · 6H2O, CaCl2 · 2H2O, and NH4Cl (and also excluding Fe(NH4)2(SO4)2 · 6H2O and Na3-nitrilotriacetate for medium 1) were dissolved in the vessel in water and the vessel content was sterilized at 121°C for 40 min. The sterilized medium was cooled to 30°C under nitrogen (supplied as an overlay from the top of the vessel), and the pH controller was reset to the pH for a sample of the sterilized medium, determined outside of the vessel. Then the flows of hydrogen (2,500 ml min−1) and CO2 (700 ml min−1) were initiated and the nitrogen flow was discontinued. As a result the medium pH dropped rapidly to 6 to 6.2. At this stage an anaerobic sterile solution of MgCl2 · 6H2O, CaCl2 · 2H2O, and NH4Cl from a sealed 530-ml bottle was added to the medium by use of the double-needle system (3); this solution had been made anaerobic by evacuation of the headspace and had been placed under N2 (0.15 to 0.2 × 105 Pa) before autoclaving and was pressurized to 1.7 × 105 Pa after poststerilization cooling. The same protocol was used to add yeast extract and tryptone. For medium 1, at this time an aerobic solution (∼20 ml) of Fe(NH4)2(SO4)2 · 6H2O (final concentration, 0.0122 g liter−1 or 31 μM) and Na3-nitrilotriacetate (final concentration, 0.083 g liter−1 or 0.32 mM) was added to the medium through a 0.2-μm sterile syringe filter (Fig. 1). After the redox potential reading for the medium stabilized (typically at −160 mV after ∼30 min of gassing), a continuous flow of a gas mixture of N2 and H2S (90:10 [vol/vol]; henceforth referred to as H2S) was initiated at a flow rate of 50 ml min−1. After the medium was gassed with H2S for 20 min, the redox potential reading stabilized at about −330 mV. At this stage the agitation speed and gassing rates were adjusted to desired values (see Results and Discussion), and the medium was inoculated with 25 ml of a mid-log-phase culture (OD600, 0.6 to 0.8) from a 160-ml culture bottle by use of the double-needle system (3). Further additions and manipulations of culture conditions were as indicated in the Results section. Throughout the cultivation period the vessel was maintained at a positive pressure of 1.25 × 105 Pa and the culture temperature was maintained at 85°C. Whenever needed, foaming in the culture was suppressed by addition of a 0.2-ml anaerobic and sterile aqueous solution (50%) of Sigma Antifoam 289 (Sigma Chemical Co., St. Louis, Mo.). The protocols for medium with MES as the buffer were essentially the same as detailed above, except the pH of the medium was adjusted to 6.0 with NaOH before sterilization and, for the reason mentioned above (see “Small-scale cultivation protocol”) MgCl2 · 6H2O, CaCl2 · 2H2O, and NH4Cl could be included in the medium before sterilization.
Analytical methods.
The OD of a culture was monitored at 600 nm by use of a model DU 640 UV-visible spectrophotometer (Beckman Instruments, Inc., Fullerton, Calif.) with a cuvette having a 1-cm light path. The protein content of a cell pellet was determined as follows. At a desired time a sample of the culture was withdrawn and centrifuged at 14,000 × g to obtain a cell pellet; this pellet was resuspended in water (1 ml of water per ml of culture centrifuged) and stored at −20°C for processing at a later time. Each sample of resuspended cell pellet was thawed, mixed with 34 μl of 1.5 M NaOH (final concentration, ∼50 mM), and incubated at 80°C for 20 min to maximize the release of proteins from the cells; these optimized digestion conditions were established through experimentation (data not shown). Each digest was then cooled to room temperature, mixed with 34 μl of 1.5 M HCl, and assayed for protein content as described by Bradford (5) with the dye reagent from Bio-Rad Laboratories (Richmond, Calif.); serum albumin served as the standard. The data from our early experiments showed that each of the growth patterns obtained from the values of OD600 was parallel to the corresponding profile for the pellet protein content per milliliter of culture. Hence, all growth patterns reported in this work were determined by measuring the values of OD600. For determining the dry-cell content of a culture, the cell pellet from a known volume of culture (collected by centrifugation at 14,000 × g) was resuspended in a minimum volume of water and dried (initially overnight at 95°C and then at 105°C) to constant weight. The sulfide concentration in the culture liquid was determined by the methylene blue method of Pachmayr (21) as detailed by Trüper and Schlegel (28).
The kinetic data in Fig. 3A were analyzed by using the KinetAsyst program, version 1.01 (Intellikinetics, State College, Pa.). The values for two types of growth rates have been reported here: the specific growth rate (μ; hours−1) for the logarithmic growth phase and the linear growth rate (change in OD600 units per hour) for the linear growth phase. Each rate or OD600 value reported in Fig. 3 is an average of data from duplicate experiments. The data set in Fig. 2 is from one of the two experiments performed under identical conditions.
FIG. 3.
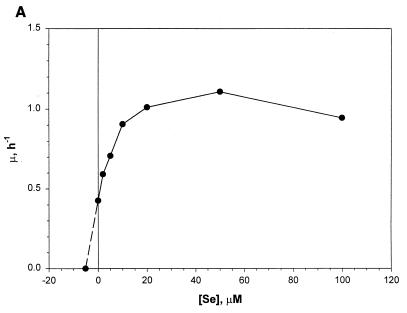
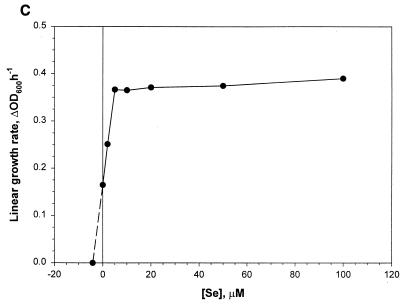
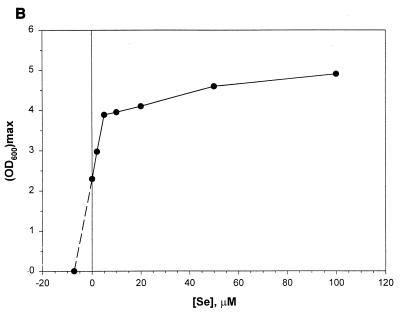
Effects of added Se levels on the growth characteristics of M. jannaschii in reactor cultures with medium 1. Se was added as Na2SeO4. Each data point represents an average of values derived from two independent experiments. (A) Specific growth rate versus Se concentration ([Se]). Each μ value was calculated from the culture turbidity data in the logarithmic growth phase. (B) Observed maximum culture turbidity (in centimeters−1) versus [Se]. (C) Linear growth rate versus [Se]. This growth rate was calculated from the culture turbidity data in the linear growth phase that occurred at an OD600 of >0.7 to 1.0. For other details, see the legend of Fig. 2.
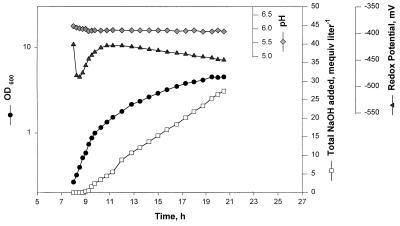
FIG. 2.
Autotrophic growth of M. jannaschii in a constantly stirred tank reactor in medium 1 containing 50 μM Na2SeO4. The culture volume was 12 liters. The gas flow rates (in milliters per minute with respect to 1.01 × 105 Pa) were as follows: H2, 19,200; CO2, 4,800; H2S, 215. The vessel pressure was maintained at 1.25 × 105 Pa, and an impeller rotational speed of 600 rpm (tip speed, 235.5 cms−1) was used. The culture pH was maintained at 6 ± 0.5 by automatic addition of 5 N NaOH solution. Sterile anaerobic water was added continuously at a rate of 200 ml h−1 to compensate for evaporative loss of water.
RESULTS AND DISCUSSION
Growth in serum bottles.
For a 150-ml culture in a 530-ml serum bottle, the values for maximum specific growth rates in medium 1 and in other (9, 13, 24) media were in the range of 1 to 1.5 h−1 and were comparable to those observed by Jones et al. (13); here the final concentration of Se in medium 1 was 2 μM. The values for the final culture turbidities and cell yields in these media were also comparable to each other, except that those for medium 1 (OD600, ∼1.7; ∼0.65 g dry cell weight liter−1) were about twofold higher than those for others. Certain observations at the reactor scale (see below) prompted us to study the effect of added-Se level in medium on the growth of M. jannaschii at the serum bottle scale, and we chose medium 1 for this purpose. We found that M. jannaschii was dependent on added Se for growth. In the 0 to 100 μM range, a Se concentration of 2 μM offered the highest cell yield, and the levels higher than 10 μM were inhibitory. Our data show that a series of 530-ml bottles each containing 150 ml of medium can be used for generating gram quantities of cells. This system also allows withdrawal of samples for monitoring growth and for analysis of culture liquid without significantly changing the growth conditions and provides sufficient cell mass at the mid-logarithmic and late logarithmic stages for enzyme and cofactor level measurements.
Growth cycle, medium composition, impeller speed, shear, foaming, and water loss in a reactor.
To allow close monitoring of the logarithmic and late logarithmic stages, we inoculated the sterile medium late in the evening and allowed the cultivation to proceed at 200 rpm overnight with the following gas flow rates (in milliliters per minute): H2, 2,500; CO2, 700; N2-H2S (90:10 [vol/vol]), 20. At this agitation rate, which limited the gas transfer to the liquid, the organism grew slowly and was ready in the morning (∼8 h after inoculation) at an OD600 of ∼0.2 (with each medium tested) for immediate rapid logarithmic growth when the higher agitation (600 rpm) and gassing rates (H2, 19,200 ml min−1; CO2, 4,800 ml min−1; H2S, 215 ml min−1) were imposed (Fig. 2). All reactor-scale data presented here were collected by following this protocol. It was also possible to extend the initial slow growth stage up to 12 to 16 h without significantly shortening the vigorous logarithmic growth stage (data not shown). The option of initiating the agitation and gassing at optimal rates immediately after inoculation of the medium proved impractical. This was because such a growth cycle often included a lag period of ∼4 h and, as seen in Fig. 2, after the onset of the logarithmic growth phase a reactor culture of M. jannaschii took ∼12 h to reach the late logarithmic or stationary stage. Also, as the stationary phase began, cells started to lyse.
We compared the available medium recipes (13, 24) for performance in reactor-scale cultures in terms of growth rates, cell yield, and reproducibility. Of these, in our hands the medium recipe of Rajagopal and Daniels (24) gave the best result. Numerous experiments using several variations of this medium (data not shown) led to the development of medium 1, in which growth occurred at high rates and to high cell densities with relatively high reproducibility. For example, in comparison to values obtained with medium 2 containing 2.65 μM Se (13), in medium 1 (with 2 μM added Se) the specific growth rate in the logarithmic growth phase was the same, but the maximum OD600 and the growth rate in the linear phase were, respectively, about 1.3- and 2.2-fold higher. Replacement of bicarbonate in medium 1 with MES did not alter the growth rate in the logarithmic growth phase and reduced the final cell yield by only 10% but lowered the linear growth rate by 10-fold. MES is also more expensive than sodium bicarbonate. However, for studies dealing with the effect of CO2 (aqueous) or HCO3− on the physiology of M. jannaschii, MES (pKa at 85°C, 5.6) (23) could be used as the buffer in the medium.
M. jannaschii derives all the needed energy and the reducing power needed for cell material biosynthesis from the oxidation of H2. On the other hand H2 is a very sparingly soluble gas; Henry’s constant for H2 (in units of atmospheres per mole fraction of solute dissolved in solution) at 80°C is 7.55 × 104 and at 90°C is 7.51 × 104 (16). Thus, the rate of H2 dissolution is expected to limit this organism’s growth rate. With medium 1, an increase in impeller rotational speed from 400 to 600 rpm improved the value of the maximum specific growth rate twofold, although the value of the final cell density improved only marginally. The increase in impeller rotational speed from 400 to 600 rpm corresponded to a change in the value of the impeller Reynolds number or NRe from 3.75 × 104 to 5.6 × 104 and to a 1.5-fold increase in shear rate. The NRe values were calculated from the relationship NRe = (rotations per second) × (impeller diameter in centimeters)2 × (density of culture liquid in grams per cubic centimeter)/viscosity of culture liquid in grams per centimeter per second and by assuming the density and viscosity of culture liquid to be 1 g cm−3 and 0.01 g cm−1 s−1, respectively. The relative shear rates were judged from the values of impeller tip speed, which are given by π × impeller diameter × impeller rotational speed. Neither the growth rate nor the final cell density values at an impeller speed of 800 rpm (NRe, 7.5 × 104) were different from those at 600 rpm. Rather, at 800 rpm the culture, after reaching an OD600 of 0.75, foamed almost continuously in spite of frequent antifoam additions; this effect was most likely caused by shear-induced cell lysis. But it should be noted that, in spite of having only a proteinaceous cell envelope (15), this organism tolerated a fairly high shear rate (600 rpm for an impeller with a diameter of 7.5 cm; a tip speed of 235.5 cms−1), which is routinely used for reactor cultivation of M. thermoautotrophicum (8, 20), an organism with a rather rigid cell wall composed of pseudomurein, a type of peptidoglycan (15). Our observation is in contrast to the reported fragility of Methanococcus vannielii (12), which is why this organism has been cultivated in a 400-liter reactor without mechanical mixing. A sensitivity to higher agitation rates has also been seen with Methanothermus fervidus (22), even though the organism’s cell envelope compositions (15) suggest a capacity to withstand high shear; a very likely explanation for this observation is the inability of the reactor system in use to prevent oxygen contamination (22). Our observations suggest that other methanogens with seemingly weaker cell envelopes should be tested for shear sensitivity while developing a method for mass culture with sparingly soluble gases as substrates. Each of the values of NRe reported here corresponded to a turbulent mixing regime (25). Our results could form the basis for scale-up of M. jannaschii culture to higher operating volumes. It is customary to scale-up gas transfer rate-limited microbial cultures based on the optimized value for power input per unit volume or mass transfer coefficient at the smaller scale (29). However, the moderate shear sensitivity of M. jannaschii, as observed by us, suggested that a scale-up effort should use the shear rate as the first basis to determine the mixing rate at the higher scale. For this purpose an impeller tip speed of 235.5 cm s−1 (corresponding to an impeller rotational speed of 600 rpm in our system) could be used. It is expected that such a scale-up method would give for the larger scale a H2 transfer rate lower than the one that was in effect for the reactor used in this study. For this reason, after the suitability of the use of the tip speed of 235.5 cm s−1 at the larger scale is established, attempts should be made to optimize the power input as well as the gassing rates.
Occasionally and unpredictably, foaming occurred in reactor cultures even at an impeller speed of 600 rpm, after the culture turbidity (OD600) reached 0.5 to 0.8. We used Sigma Antifoam 289 for suppressing foam formation. For a culture volume of 12 liters, a 0.2-ml 50% aqueous anaerobic and sterile solution of the antifoam was used for each addition. Such additions at about 2- to 4-h intervals did not alter the growth rates and final cell yields.
Our reactor was operated without a condenser on the exhaust gas line. The consequence was a substantial evaporative loss of water in this thermophilic culture; at a total gas flow rate of 24,215 ml min−1 the water loss rate was ∼200 ml h−1. To compensate for this loss, we instituted a continuous addition of sterile anaerobic water at a rate of ∼200 ml h−1.
Formation of acids in reactor cultures.
It was necessary to add NaOH to maintain the pH at 6 ± 0.5. In the absence of pH control, the culture pH dropped (to a value as low as 3.5 for media 1 and 2) after the OD600 of the culture reached a value of about 0.7 and the growth rate and final cell yield were reduced (data not shown). Intermittent manual addition of base and addition of base via a control system with a set point of 6 ± 0.5 were found to be equally effective. With 10 mM NaHCO3 and a partial pressure of 0.445 × 105 Pa for CO2 (H2/CO2 ratio [vol/vol], 80:20; total pressure, 1.25 × 105 Pa), the experimentally determined value for the pH of medium 1 at 85°C was 6.1. The corresponding calculated value, based on the available solubility and dissociation constant data (16, 30), was 6.9. However, this calculation did not take into account the effect of salts at high concentrations on the values for the constants, as well as the effects of salts and elevated temperatures on the probe. Nevertheless, both the experimentally determined and the calculated values for the pH of uninoculated medium were much higher than those of cultures grown without pH control. It is possible that M. jannaschii excreted certain acidic compounds (with low values for their pKas) in amounts that, in the absence of pH control, overwhelmed the buffering capacity of the medium. In each culture raised with pH control, the NaOH consumption paralleled cell growth; Fig. 2 shows an example where, by the end of growth, the culture consumed as much as 16 meq of base per g of dry cell produced. The base consumption continued even after the cessation of growth. It has been shown that Methanosarcina species excrete acetate when they are grown on H2-CO2, methanol, or trimethylamine (31, 35). At this point neither the nature of the excreted acidic compound(s) nor its metabolic origin nor its effect on the physiology of M. jannaschii is known. We are currently pursuing this topic.
Selenium.
Jones et al. (13) reported that the addition of selenium in medium significantly stimulates the growth of M. jannaschii. A previous careful study has established an absolute requirement of Se for the growth of Methanococcus voltae on H2-CO2 and has shown that for this organism Se at a final concentration of 10 μM increases growth rate by 50% and culture turbidity by 2.7-fold over those obtained with unsupplemented medium presumably containing Se as a contaminant (33). For Methanococcus maripaludis an added-Se level of 10 μM enhances the growth rate by 20% over the control (14), and for Methanococcus vannielii a level of 1 μM substantially improves both growth rate and cell yield on formate at large scale (12). Also, these organisms possess several Se-containing enzymes that are linked to their energy metabolism (10). Since our medium development work started from the medium recipes of Jones et al. (13) and Rajagopal and Daniels (24), where the added-Se levels were 2.4 and 2 μM, respectively, in our early attempts to cultivate this methanococcus in a reactor we added Na2SeO4 to the medium at a level of 2 μM. These experiments offered relatively high growth rates (∼1 h−1) and cell densities (OD600, ∼3.5; ∼6 g of wet cell paste liter of culture−1; ∼1.34 g dry cell mass liter of culture−1) compared to past accomplishments (see Introduction). However, after several months of success we could no longer reproduce these observations, especially with respect to the growth rate (data not shown). This switch coincided with a change of the H2S supply tank. This problem was alleviated when the medium was supplemented with additional Na2SeO4. Although a detailed study using H2S tanks from several sources is needed to obtain a clear explanation of these observations, it is plausible that our earlier success in growing M. jannaschii at high growth rates and to high cell densities in medium 1 with 2 μM added Se was due to a continuous supply of H2Se to the culture as a contaminant in the H2S gas. Whitman et al. (33) have indicated that most methanogen cultures probably receive Se as a contaminant in sodium sulfide that is used as a medium reductant (2). Therefore, we studied the effect of added Se on the growth rate and final cell yield in bottle- and reactor-scale cultures of M. jannaschii. The data for the bottle cultures have been presented above and are discussed below. Figure 2 shows the parameter profiles in a reactor culture with added Se at a concentration of 50 μM. Figure 3 presents data from an analysis of such cultures at various Se levels. Similar to what is seen in Fig. 2, each culture showed a transition from a logarithmic to a linear growth phase at a culture OD600 of 0.7 to 1. The corresponding specific growth rate (μ), calculated from the culture turbidity data in the logarithmic growth phase, showed an apparent hyperbolic saturation kinetics with respect to the added-Se level in medium (Fig. 3A). An extrapolation of the data in Fig. 3A showed that the calculated value of Se level for μ = 0 was −5.2 μM. In other words, if the extrapolated section of the plot were to reflect a real culture property, the contaminating level of Se in a medium without added Se would be 5.2 μM. Since an inspection of the plot in Fig. 3A showed a sign of inhibition of logarithmic growth at high Se concentrations, attempts were made to fit the data to the standard substrate inhibition model μ = μmax[Se]/{Ks + [Se] + ([Se]2/Ki)} where [Se] is the Se concentration, Ks is the substrate affinity constant (in micromolar units), and Ki is the inhibition constant (in micromolar units). For this purpose the [Se] values were corrected for the contaminating Se by using the above-calculated concentration value of 5.2 μM. But the simulation showed that the data did not fit well to the above-mentioned substrate inhibition model and thus suggested a more complex behavior (see below). The observed maximum culture density also showed a hyperbolic saturation kinetics with respect to Se concentration (Fig. 3B), and from this set of data the calculated concentration value for Se appearing as a contaminant was 7.3 μM. The response of the linear growth rate to the Se concentration is shown in Fig. 3C. Up to a concentration of 5 μM, the growth rate was dependent on the Se level, but further addition of Se did not influence the growth rate. Here the calculated level of contaminating Se was 4.2 μM. At an added Se concentration of 100 μM, the autotrophically grown cultures approached a maximum OD600 of 4.9 and produced up to 7.5 g of wet cell paste or 1.8 g of dry cells per liter.
Compared to a bottle-scale culture (see above), a reactor culture required a much higher concentration of Se for optimal growth (Fig. 3B) and tolerated a higher level of Se (Fig. 3A). One possible explanation for this difference was that a portion of the added Se was lost from the reactor, whereas in a sealed bottle most of it was retained; flushing out the bottle headspace would lead to some loss. It has been shown before that SeO42− inhibits methane formation from methylcobalamin in cell extracts of Methanobacterium and that the extracts can reduce selenate to hydrogen selenide and produce methylated selenide (17). Thus, in a continuously sparged reactor culture of M. jannaschii, Se could be lost in the exhaust gas as volatile selenides, and this loss would raise the apparent required level of and tolerance for Se; such as an effect could also account for the fact that the data shown in Fig. 3A did not fit a standard substrate inhibition model. In that case, the concentrations presented in the previous paragraph and in Fig. 3 would correspond to the added amount of SeO4−2 but not to the actual concentrations in a developing culture (cells plus fluid). However, these values would remain useful for the mass culture of the organism.
Other growth parameters.
Jones et al. (13) observed that organic compounds such as yeast extract, Trypticase, vitamins, acetate, and formate are neither required for nor stimulate the growth of M. jannaschii. But, in our hands, supplementation of medium 1 containing 20 μM Se with yeast extract (2 g liter−1), tryptone (2 g liter−1), and vitamins (34) improved the final cell yield by as much as 1.3-fold and yielded up to 8.5 g of wet cell paste liter of culture−1 or 2 g dry cell mass liter of culture−1; the specific and linear growth rates improved by ∼1.5- and ∼1.25-fold, respectively. Of these supplements, when added individually, yeast extract and tryptone, but not the vitamin mixture, provided substantial enhancement of the final cell yield (data not shown). It would be interesting to know whether the improvements in cell yields by the heterotrophic supplements were due to some sort of limitation on the cell material biosynthesis imposed by the growth conditions employed. The data from our preliminary experiments at an added Se concentration of 2 μM and low gas flow rates provided some clues. For most growth studies with methanogens an 80:20 (vol/vol) mixture of H2 and CO2 is used, and this ratio corresponds to the stoichiometry in the methanogenesis reaction (4H2 + CO2→CH4 + 2H2O). We observed that for M. jannaschii cultures raised at H2 and CO2 flow rates of, respectively, 5,500 and 1,410 ml min−1 (H2/CO2 ratio, 79.6:20.4) the cell yield was approximately threefold higher than that with H2 and CO2 flow rates of 5,500 and 450 ml min−1 (H2/CO2 ratio, 92.4:7.6), respectively. When cultures were examined at the latter gas flow rates, it was found that the inclusion of tryptone (2 g liter−1)-yeast extract (2 g liter−1)-vitamin into the medium provided up to 1.8-fold enhancement in cell yield, whereas no such enhancement was observed at the earlier flow rates. The exact reason for the observed enhancement in cell yield by complex supplements under CO2-limiting conditions is currently unknown. Neither do we know whether there are other environmental conditions that would invoke such a dependence on an exogenous source of nutrients for vigorous growth. Experiments on these aspects are in progress.
The transition of a reactor culture from a logarithmic to a linear growth phase at a culture OD600 of 0.7 to 1 indicated that at this point the reactor or the medium or both were no longer able to fully meet the demands of growing cells and that the growth rate was determined by the rate of supply of one or more limiting nutrients. The results from our preliminary investigations suggested that the conditions used for obtaining data in Fig. 2 and 3 did not represent a gas transfer-limited situation. However, in these cultures the sulfide level remained below 0.1 mM (the detection limit for the assay method used) (21, 28) throughout the cultivation period, and this low value was due to the lower solubility of sulfide in an acidic medium; pKa for H2S/HS− is 7.04 (1). Also, it was noted that the growth phase change coincided with a rise in culture redox potential (Fig. 2); the reason and implication of this change are not known.
It has been reported previously that for M. jannaschii both growth rate and methanogenesis rate increase with an increase in pressure up to 750 atm or 7.60 × 107 Pa (18, 19), and this observation reflects the situation in this organism’s natural habitat (13). It is not known whether the final cell density is influenced by the higher pressures; we carried out our work at the working pressures of 1.7 × 105 Pa at the bottle scale and of 1.25 × 105 Pa at the reactor scale, and most laboratories would be able to use the protocols optimized at such pressures.
In summary, with a reactor of the type used here, the best values of the autotrophic growth rate (∼1 h−1) and final cell yield (approaching 7.5 g of packed wet cells and 1.8 g of dry cells per liter of culture) for M. jannaschii were obtained under the following conditions: growth medium, medium 1 with 100 μM Se; impeller rotational speed, 600 rpm (impeller tip speed, 235.5 cms−1); flow rates for H2, CO2, and 10% H2S (in N2), respectively, 19,200, 4,800, and 215 ml min−1; and a controlled culture pH of 6 ± 0.5. Supplementation of growth medium with yeast extract (2 g liter−1) and tryptone (2 g liter−1) improved the growth rate and cell yield substantially. The described protocols will also be very useful in designing experiments for studying the physiology of M. jannaschii as well as in examining other medium recipes for further improvements in growth rates and cell yield.
ACKNOWLEDGMENTS
We thank Vipool J. Patel and Cynthia L. Kreder for excellent technical assistance. We thank Kevin R. Sowers, William B. Whitman, H. Hippe, and D. R. Boone for communicating unpublished observations.
This work was supported by the Department of Energy grant DE-FG02-87ER13651. All reactor culture experiments were conducted in the Department of Microbiology Fermentor Facility, University of Illinois at Urbana-Champaign.
REFERENCES
- 1.Appleby C A. Inhibitors of respiratory enzymes, photosynthesis and phosphorylation; uncoupling reagents. In: Dawson R M C, Elliott D C, Elliott W H, Jones K M, editors. Data for biochemical research. 2nd ed. London, United Kingdom: Oxford University Press; 1969. pp. 380–387. [Google Scholar]
- 2.Balch W E, Wolfe R S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baresi L, Wolfe R S. Levels of coenzyme F420, coenzyme M, hydrogenase, and methylcoenzyme M methylreductase in acetate-grown Methanosarcina. Appl Environ Microbiol. 1981;41:388–391. doi: 10.1128/aem.41.2.388-391.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone, D. R. Personal communication.
- 5.Bradford M M. A rapid and sensitive approach for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kaine B P, Borodovsky M, Klenk H-P, Frasher C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 7.Daniels L, Belay N, Rajagopal B S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol. 1986;51:703–709. doi: 10.1128/aem.51.4.703-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels L, Belay N, Mukhopadhyay B. Considerations for the use and large-scale growth of methanogenic bacteria. In: Scott C D, editor. Proceedings of the 6th Symposium on Biotechnology in Fuels and Chemicals. Biotechnology and bioengineering symposium no. 14. New York, N.Y: John Wiley and Sons, Inc.; 1984. pp. 199–213. [Google Scholar]
- 9.Ferrante G, Richards J C, Sprott G D. Structures of polar lipids from the thermophilic, deep-sea archaeobacterium Methanococcus jannaschii. Biochem Cell Biol. 1990;68:274–283. doi: 10.1139/o90-038. [DOI] [PubMed] [Google Scholar]
- 10.Grahame D A, Stadtman T C. Redox enzymes of methanogens: physiochemical properties of selected, purified oxidoreductases. In: Ferry J G, editor. Methanogenesis: ecology, physiology, biochemistry and genetics. New York, N.Y: Chapman and Hall; 1993. pp. 335–359. [Google Scholar]
- 11.Hippe, H. Personal communication.
- 12.Jones J B, Stadtman T C. Methanococcus vannielii: culture and effects of selenium and tungsten on growth. J Bacteriol. 1977;130:1404–1406. doi: 10.1128/jb.130.3.1404-1406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones W J, Leigh J A, Mayer F, Woese C R, Wolfe R S. Methanococcus jannaschii sp. nov., an extreme thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol. 1983;136:254–261. [Google Scholar]
- 14.Jones W J, Paynter M J B, Gupta R. Characterization of Methanococcus maripaludis sp. nov., a new methanogen isolated from salt marsh sediment. Arch Microbiol. 1983;135:91–97. [Google Scholar]
- 15.Kandler O, Konig H. Cell envelopes of archaebacteria. In: Woese C R, Wolfe R S, editors. The bacteria. Vol. 7. New York, N.Y: Academic Press, Inc.; 1985. pp. 413–457. [Google Scholar]
- 16.Liley P E, Reid R C, Buck E. Physical and chemical data, section 3. In: Perry R H, Green D W, Maloney J O, editors. Perry’s chemical engineers’ Handbook. 6th ed. New York, N.Y: McGraw-Hill, Inc.; 1984. pp. 1–291. [Google Scholar]
- 17.McBride B C, Wolfe R S. Biosynthesis of dimethylarsine by Methanobacterium. Biochemistry. 1971;10:4312–4317. doi: 10.1021/bi00799a024. [DOI] [PubMed] [Google Scholar]
- 18.Miller J F, Almond E L, Shah N N, Ludlow J M, Zollweg J A, Streett W B, Zinder S H, Clark D S. High-pressure-temperature bioreactor for studying pressure-temperature relationships in bacterial growth and productivity. Biotech Bioeng. 1988;31:407–413. doi: 10.1002/bit.260310503. [DOI] [PubMed] [Google Scholar]
- 19.Miller J F, Shah N N, Nelson C M, Ludlow J M, Clark D S. Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschii. Appl Environ Microbiol. 1988;54:3039–3042. doi: 10.1128/aem.54.12.3039-3042.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay B. Fermentor growth of Methanobacterium thermoautotrophicum strain Marburg in relation to agitation and gas transfer, and purification of N5,N10-methenyl-H4MPT cyclohydrolase from Methanobacterium thermoautotrophicum strain Marburg. M.S. thesis. Iowa City: University of Iowa; 1987. [Google Scholar]
- 21.Pachmayr F. Vorkommen und bestimmung von schwefelverbindungen in mineral-wasser. Ph.D. Thesis. Munich, Germany: University of Munich; 1960. [Google Scholar]
- 22.Pepper C B, Monbouquette H G. Issues in the culture of the extreme thermophilic methanogen Methanothermus fervidus. Biotech Bioeng. 1993;41:970–978. doi: 10.1002/bit.260411008. [DOI] [PubMed] [Google Scholar]
- 23.Purwantini E, Mukhopadhyay B, Spencer R W, Daniels L. Effect of temperature on the spectral properties of coenzyme F420 and related compounds. Anal Biochem. 1992;205:342–350. doi: 10.1016/0003-2697(92)90446-e. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopal B S, Daniels L. Investigations of mercaptans, organic sulfides, and inorganic sulfur compounds as sulfur sources for the growth of methanogenic bacteria. Curr Microbiol. 1986;14:139–144. [Google Scholar]
- 25.Rushton J H, Costich E W, Everett H J. Power characteristics of mixing impellers. Part 2. Chem Eng Prog. 1950;46:467–476. [Google Scholar]
- 26.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Hershel S, Patwell D, Prabhakar S, McDougall S, Shimar G, Goyal A, Pietrokovski S, Church G M, Daniels C J, Nolling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowers, K. R. Personal communication.
- 28.Trüper H G, Schlegel H G. Sulphur metabolism in Thiorodacae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- 29.Wang D I C, Cooney C L, Demain A L, Dunnil P, Humphrey A E, Lilly M D. Fermentation and enzyme technology. New York, N.Y: Wiley-Interscience; 1979. [Google Scholar]
- 30.Weast R C, Astle M J, Beyer W H. CRC handbook of chemistry and physics. 66th ed. Boca Raton, Fla: CRC Press; 1985. [Google Scholar]
- 31.Westermann P, Ahring B K, Mah R A. Acetate production by methanogenic bacteria. Appl Environ Microbiol. 1989;55:2257–2261. doi: 10.1128/aem.55.9.2257-2261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitman, W. B. Personal communication.
- 33.Whitman W B, Ankwanda E, Wolfe R S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1983;156:19–29. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolin E A, Wolin M J, Wolfe R S. Formation of methane by bacterial extracts. J Biol Chem. 1963;238:2882–2886. [PubMed] [Google Scholar]
- 35.Yamaguchi M, Minami K. Production of acetic acid from methanol by thermophilic Methanosarcina sp.: acetate production as an index in abnormal methane fermentation. J Ferment Bioeng. 1998;86:239–242. [Google Scholar]
- 36.Zeikus J G, Wolfe R S. Methanobacterium thermoautotrophicus sp. nov., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972;109:707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]