Abstract
In photodynamic therapy, intermittent irradiation modes that incorporate an interval between pulses are believed to decrease the effect of hypoxia by permitting an interval of re-oxygenation. The effect of the irradiation intermittency factor (the ratio of the irradiation pulse time to the total irradiation time) on singlet oxygen formation and inflammatory cytokine production was examined using azulene as a photosensitizer. Effects of difference intermittency factor on singlet oxygen formation and inflammatory cytokine were examined. Azulene solutions (1/10 μM) were irradiated with a 638-nm 500 mW diode laser in fractionation (intermittency factor of 5 or 9) or continuous mode using 50 mW/cm2 at 4 or 8 J/cm2. Singlet oxygen measurement was performed using a dimethyl anthracene probe. Peripheral blood mononuclear cells (PBMC) were stimulated by 10 ng/ml rhTNF-α for 6 h, before addition of 1 and 10 μM azulene solutions and irradiation. PGE2 measurement was undertaken using a human PGE2 ELISA kit. Kruskal-Wallis with Dunn Bonferroni test was used for statistical analyses at p < 0.05.Irradiation of 1 μM azulene+4 J/cm2+intermittency factor of 9 increased singlet oxygen 3-fold (p < 0.0001). Irradiation of 10 μM azulene at either 4 J/cm2+intermittency of 9 or 8 J/cm2+intermittency factor of 5 reduced PGE2 expression in PBMCs to non-inflamed levels. Thus, at 50 mW/cm2, 10 μM azulene-mediated photodynamic therapy with a high intermittency factor and a low energy density generated sufficient singlet oxygen to suppress PGE2 in Inflamed PBMCs.
Keywords: Azulene, Inflammation, Intermittency factor, Photodynamic therapy, Pulse mode, Singlet oxygen
Graphical abstract
Irradiating azulene with a 638-nm diode laser at low power density (50 mW/cm2) in pulse mode resulted in formation of higher levels of singlet oxygen than under continuous mode due to the break in irradiation time allowing for the replenishment of oxygen substrates from the environment. Treating rhTNF-α-induced human peripheral blood mononuclear cells with 10 μM azulene pulse irradiated with either 4 J/cm2 using an intermittency factor of 9 or 8 J/cm2 using an intermittency factor of 5 tended to reduce levels of PGE2.
Highlights
-
•
Different intermittency factors can stimulate ROS formation differently.
-
•
Relative high intermittency factor with azulene induces high ROS formation.
-
•
Relative high intermittency factor with low energy density inhibits PGE2 production.
-
•
Azulene-based photodynamic therapy suppresses inflammation.
1. Introduction
It is well-accepted that oxygen is a crucial factor for many activities in the living organism. The specific biochemical and biophysical reactions induced by using light at an appropriate wavelength to stimulate an agent called a photosensitizer in the presence of oxygen can lead to target tissue/organ alteration/modification while maintaining the integrity of adjacent tissues/organs. This emerging therapy is known as photodynamic therapy. In photodynamic therapy (PDT), oxygen is one of three main components [1] as the source of reactive oxygen species (ROS), which are the molecules that exert the therapeutic effect in target cells/tissues. If the oxygen level in the cells/tissues is low, generally known as hypo-oxygenation, that will cause an inadequate quantum yield of ROS, which will prevent effective PDT [2,3]. Hence, the concept of oxygen resupply is emerging as a key factor in PDT as oxygen replenishment in target cells/tissues may enhance tumor cell destruction or hyperactive immune cell suppression. The specific oxygen consumption rate in the relationship of light fluency and photosensitizer concentration is well-documented [4,5]. Typically, when irradiation is applied continuously to target cells/tissues, oxygen rapidly disappears. Three main strategies have been employed to prevent premature hypoxic conditions. Irradiation-related methods include prolonging irradiation time with low irradiance light [6], and alternating non-irradiation sessions with irradiation sessions, namely pulse modes [7,8], Photosensitizer-related methods include using oxygen vehicles like hyperbaric oxygen [9], combining with other therapies such as in chemo-PDT [10], using hypoxia-independent PDT by incorporating a motif like a chloromethyl group in the photosensitizer so that this molecule can function even in hypoxic conditions [11], or by using a compound that can be activated without O2 [12], and using an hypoxia-reducible compound such as azobenzene that sensitively and efficiently generates ROS in hypoxic conditions [13]. One tissue microenvironment modification method is to change H2O2 to oxygen in the tissue. This can be accomplished by the addition of MnO2 [14], and catalase [15]. Among these strategies, modification of the irradiation method is the most simple, reasonable, and practical solution.
In pulse mode irradiation, the ratio between the pulse repetition period and the total pulse duration is known as the intermittency factor/index [7]. An intermittency factor of five, for example, adds a 4 s period of non-irradiation for each irradiation pulse of 1 s. These periods of non-irradiation facilitate the replenishment of cellular and tissue oxygen3 avoiding the reduced therapeutic effects caused by hypoxic conditions. Previously, longer discontinuous irradiation sessions alternated with short irradiation sessions were pivotal for oxygen to resupply [3]. Based primarily on a theoretical model equation and an in vitro model assumption, a higher intermittency factor could generate significantly higher amounts of singlet oxygen [7]. Notably, at the same total energy density, a continuous wave resulted in cell necrosis, while pulse mode tended to induce cell apoptosis [7]. An efficient pulse intermittency should be in the hundred to several hundred milliseconds range, based on a previous study that used a nanosecond pulsed 670 nm Nd:YAG laser (with peak fluence rate of 1 mW/cm2 at a frequency of 30 Hz) to irradiate a photosensitizer named 13,17-bis[1-carboxypropionyl]carbamoyl-ethyl-3-ethenyl-8-ethoxyiminoethylidene-7-hydroxy-2,7,12,18-tetra methylporphyrin sodium (PAD-S31) and induce more cytotoxicity in mouse renal carcinoma cells (Renca) than continuous mode irradiation [16]. Additionally, using pulse mode of light (ultraviolet light at a wavelength 365 nm, 30 mW/cm2 1 s on/1 s off or intermittency factor 1, cumulative energy density of 7.2 J/cm2) could prevent development of keratoconus for 12 months in human subjects [17]. To our knowledge, there are no gold-standard recommendations regarding the optimum intermittency factor for effective induction of ROS formation either in vitro or in vivo.
Currently, photodynamic therapy is used for three purposes: anti-tumor, anti-pathogenic microorganisms, and immunomodulation (including anti-inflammation). Medical and dental treatments using PDT aim to preserve the genetically normal cells/tissues to facilitate easy and rapid restoration and recovery of physiological functions. For cancer treatment, we expect PDT to attenuate cancer cells by generating large amounts of ROS, which leads to tumor cell death via four modes: necrosis, apoptosis, necroptosis, and autophagy [18,19]. The nature of many common oral diseases, however, is inflammation orchestrated by hyper-reactivity of immune cells [20]. Thus, it is preferable to generate sublethal levels of ROS in PDT for the control or suppression of hyper-stimulated inflammatory cells. A previous study showed that ROS in the range of 120–150 μM (of H2O2 equivalents) or 2–5 μmol of H2O2 equivalent/107 cells affected transient cell adaptation, which subsequently led to quiescence [21].
Azulene, the simplest terpenoid found in chamomile and peppermint, is a blue hydrophobic photosensitizer with potent anti-inflammatory potential [22]. Low concentrations of azulene irradiated with 4.2 J/cm2 using a 625 nm LED could stimulate the formation of a significant amount of singlet oxygen in vitro [23]. Preliminary results from our lab have demonstrated that azulene irradiated with a 635 nm red diode laser (40 J/cm2, power density 1,515 mW/cm2) under fractionation mode reduced PGE2 levels in inflamed human PBMCs without any effect on normal cells [24]. However, the power density or fluence rate in this study was relatively high. We postulated that there was limited time for oxygen to replenish under the conditions of this photodynamic reaction, thus the reduction in inflammatory cytokines that we observed was small. Based on Klimenko et al. in 2016, a power density in the range of 10–100 mW/cm2 could exert a superior effect on singlet oxygen formation [7].
T lymphocytes, thymus-derived immune cells, play an important role not only in the immune system but also in chronic intraoral inflammatory diseases, including periodontal diseases [25] and oral lichen planus [26]. Furthermore, disease progression and chronicity often involve the interplay of multiple cell types rather than a single cell type. Peripheral blood mononuclear cells (PBMCs) are a mixed population composed mainly of T and B cells and have been well-accepted as a model for the study of T-cell mediated inflammatory diseases [27]. Commonly used systemic anti-inflammatory agents have drawbacks in long term usage, for instance, low drug compliance and liver toxicity. Fortunately, azulene exerts no such disadvantages. One key mechanism to treat T-cell mediated inflammatory diseases is the suppression or exhaustion of this cell type. It has been well-documented that tumor necrosis factor-alpha (TNF-α) is one of the most potent and main etiologic molecules in common oral diseases like oral lichen planus [20,26]. Thus, an inflammatory disease can be mimicked by stimulating immune cells via this molecule. A reduction in the amount of an effector molecule, such as prostaglandin E2, is one acceptable indicator for inflammatory condition monitoring [28]. The present study investigated the effect of different intermittency factors/indices on PDT using azulene in inflamed human peripheral blood mononuclear cells.
2. Materials and methods
The present study was approved with approval number HE621453 by Khon Kaen University Ethics Committee for Human Research (KKUEC). We performed all reactions in dark conditions and at normal room temperature.
2.1. Azulene preparation
Azulene powder (Sigma Aldrich, Steinheim, Germany) was dissolved in 99% v/v ethanol (the amount of ethanol was <12.5 μl/ml in the final solution), and double distilled water to yield final concentrations of 1, and 10 μM. The solution was kept in the dark until use.
2.2. Irradiation procedure
Irradiation sessions were performed in our novel, arbitrary, semi-automatic irradiation machine (Fig. 1). The light source was a 4 lightbulb-diode laser (638 nm, 0.5 W, diameter 5.6 mm) with a lens (ML501P73, Mitsubishi laser diodes, Tokyo, Japan), peak power at light source = 500 mW, average power density at light source = 2,029 mW/cm2. The average power density at bottom of well was set as 50 mW/cm2, and total energy densities were 4 and 8 J/cm2. The distance from each light bulb to the bottom of each well was 7 mm. The intermittency factors used were 1 (continuous mode), 5, and 9 and were set by fixing the light-on time at 200 msec. The intermittency factor 5 and 9 light-off times were 800 and 1600 msec, respectively. The total irradiation time to achieve an energy density of 4 J/cm2 was 80 s in continuous mode, 400 s for intermittency factor 5, and 720 s for intermittency factor 9. The total irradiation times to achieve an energy density of 8 J/cm2 in continuous mode, and for intermittency factors 5 and 9 were 160, 800, and 1440 s, respectively.
Fig. 1.
The 638 nm-diode laser irradiation apparatus. The irradiation box is composed of a black acrylic box with an irradiation chamber. Parts of machine composed of 1) electric circuit with laser light bulbs, 2) monitor connected with electric circuit, 3) parameter adjustment button, 4) machine reset hole, 5) operation indicating light, 6) light distraction plate, 7) light projection slit, 8) culture plate holder. This machine is connected with normal AC supply.
2.3. Singlet oxygen measurement by fluorescence probe method
Ten microliters of 1 mM 9,10- dimethyl anthracene was added to 100 μL of each azulene concentration and distilled water to 1000 μL (the final concentration of 9,10-dimethyl anthracene was 10 μM)). Then 100 μL of each sample was subjected to irradiation. The negative control was distilled water, while the positive control was 10 μM (final concentration) erythrosine. Immediately after irradiation, singlet oxygen was detected by optical density measurement at excitation/emission wavelengths of 375/436 nm, respectively using a Varioskan Flash plate reader (Varioskan®, Thermofisher Scientific, Waltham, MA, USA). The fluorescence intensities are converted to relative singlet oxygen using following equation:
| Relative singlet oxygen amount = 100 - (100% - [FI_DMA]t/[FI_DMA]t = 0) |
Where [FI_DMA]t = Fluorescence intensity of DMA at time = t
| [FI_DMA]t = 0 = Fluorescence intensity of DMA at time = 0 (baseline) |
2.4. Anti-inflammatory assay in PBMCs
Buffy coats from anonymous healthy human subjects were courtesy of the Central Blood Bank, Srinakarin Hospital, Khon Kaen, Thailand. Upon arrival, Lymphoprep solution (STEMCELL Technologies Singapore Pte Ltd, Singapore) was used to extract peripheral blood mononuclear cells from the buffy coats as previously described.23,28 Cells with ≥95% viability were used. Purified PBMCs (1 × 105 cells/well) were plated into each well of a black 96-well plate, stimulated with 10 μL of rhTNF-α (Thermofisher, Waltham, MA, USA) at a final concentration of 10 ng/ml for 6 h before irradiation in either continuous or fractionation modes. The negative control was inflamed cells cultured in RPMI-1640 medium and the positive control was cells pre-treated with 50 μg/mL of indomethacin for 24 h before stimulation with rh-TNF-α. Immediately after irradiation was completed, 50 μL of each sample was tested using a PGE2 ELISA kit (Thermofisher, Waltham, MA, USA) following the manufacturers' instructions. Absorbance at excitation and emission wavelengths of 405 and 420 nm, respectively, was measured using the Varioskan Flash microplate reader. Duplicate experiments with two repetitions (total n = 4) were conducted.
2.5. Statistical analysis
The measured optical densities (relative singlet oxygen, relative PGE2 amount) are expressed as mean and standard error of the mean. We used the Shapiro-Wilk test for normal distribution of the data. Due to the non-normal distribution of the data, the Kruskal-Wallis with Dunn Bonferroni test (SPSS version 20 for Window, Chicago, USA) was utilized for all statistical analyses with significance at p-value < 0.05. Correlation between singlet oxygen and PGE2 was evaluated by Pearson’s correlation test.
3. Results
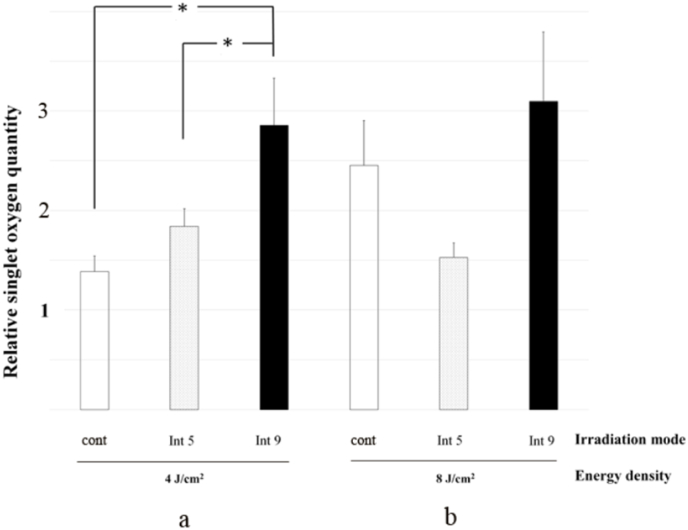
The effects of different modes of irradiation on singlet oxygen formation are shown in Fig. 2, Fig. 3. In general, fractionation modes tended to induce the formation of more singlet oxygen than continuous mode, regardless of energy density, intermittency factor and concentration of azulene. When irradiating 1 μM azulene at the low energy density (4 J/cm2, Fig. 2a), the amount of singlet oxygen formed by fractional irradiation was 3 times higher than continuous mode (0.62 ± 0.09) at intermittency factor 9 (1.78 ± 1.39 p < 0.0001) and 1.7 times higher than continuous mode at intermittency factor 5 (1.07 ± 0.35, p < 0.0001). There was no statistically significant difference between intermittencies of 5 and 9 in singlet oxygen formation at the low energy density setting. At the high energy density (8 J/cm2, Fig. 2b), fractional irradiation of 1 μM azulene yielded more singlet oxygen than continuous mode at intermittency factor 5, but there was no difference at intermittency factor 9. At intermittency factor 5, irradiation of 1 μM azulene at 8 J/cm2 generated twice the amount of singlet oxygen as continuous mode (1.22 ± 0.66 vs 0.61 ± 0.14, p < 0.0001) and 1.7 times more than at intermittency factor 9 (0.7 ± 0.18, p = 0.005). For 10 μM azulene, irradiation induced larger increases in singlet oxygen than seen for the corresponding 1 μM azulene results except the group with intermittency factor 5 at 8 J/cm2 (Fig. 2, Fig. 3, all p < 0.05). With these increases in singlet oxygen levels, the significant difference in the amount of singlet oxygen generated in 10 μM azulene solutions was at the low 4 J/cm2 power density between fractional irradiation intermittency factor 9 and continuous mode (2.86 ± 1.64 vs. 1.39 ± 0.53, p = 0.007) and between intermittency factor 9 and 5 (2.86 ± 1.64 vs. 1.84 ± 0.62, p = 0.039). Nevertheless, in 10 μM azulene solutions, singlet oxygen levels tended to be higher at higher intermittencies.
Fig. 2.
Relative singlet oxygen quantity in 1 μM azulene-mediated PDT using a power density of 50 mW/cm2, a) energy density of 4 or b) energy density of 8 J/cm2 from a 638 nm red laser and fractionation mode of irradiation (intermittency factor of 5 and 9 and continuous) and using 9,10-dimethylanthracene (DMA) as a singlet oxygen specific fluorescence probe. Positive control = continuous mode, negative control = non irradiation group. n = 12 * = significant difference at p-value < 0.05, a = significant difference from positive control at the same mode of light at p-value < 0.05 (Cont = continuous mode, Int = intermittency factor).
Fig. 3.
Relative singlet oxygen quantity in 10 μM azulene-mediated PDT using a power density of 50 mW/cm2, a) energy density of 4 or b) energy density of 8 J/cm2 from a 638 nm red laser and fractionation mode of irradiation (intermittency factor of 5 and 9 and continuous) and using 9,10-dimethylanthracene (DMA) as a singlet oxygen specific fluorescence probe. Positive control = continuous mode, negative control = non irradiation group. n = 12 * = significant difference at p-value < 0.05, a = significant difference from positive control at the same mode of light at p-value < 0.05 (Cont = continuous mode, Int = intermittency factor).
Fig. 4a shows the effect of 1 μM azulene, which produced relatively high singlet oxygen, on PGE2 levels in peripheral blood mononuclear cells. Irradiated 1 μM azulene tended to increase PGE2 levels at all energy densities and intermittency factors (range 0.57 ± 0.43 to 2.84 ± 1.06 ng/ml). All 1 μM azulene treated groups had higher PGE2 levels compared to the non-inflamed positive control group (0.038 ± 0.03 ng/ml, all p < 0.05), and all groups except energy density 4+intermittency 9 produced more PGE2 than the inflamed negative control group (0.282 ± 0.171 ng/ml, p < 0.05). The PGE2 concentrations after exposure to 10 μM azulene-mediated photodynamic therapy are shown in Fig. 4b. The irradiated 10 μM azulene induced a smaller increase in the amount of PGE2 in inflamed peripheral blood mononuclear cells (range 0.038 ± 0.03 to 2.02 ± 1.37 ng/ml) compared to 1 μM azulene (range 0.57 ± 0.43 to 2.84 ± 1.06 ng/ml) showing a reduction in PGE2 of 28%–93%. Among the 10 μM azulene treated peripheral blood mononuclear cells, an energy density of 8 J/cm2 + intermittency of 9 resulted in the highest amount of PGE2 (2.02 ± 1.37 ng/ml) followed by an energy density 4 J/cm2+intermittency factor 5 (0.65 ± 0.25 ng/ml). These PGE2 levels were significantly higher than the non-inflamed group (p = 0.021 and 0.02, respectively). Interestingly, treatment of inflamed peripheral blood mononuclear cells with 10 μM azulene irradiated at either an energy density of 4 J/cm2+intermittency of 9 or an energy density of 8 J/cm2+intermittency factor of 5, reduced the amount of PGE2 to non-inflamed levels (0.04 ± 0.02 and 0.06 ± 0.03 ng/ml, respectively), corresponding to an 86% and 79% reduction in the inflamed level, respectively. Although the amount of PGE2 generated in these two conditions was less than one-tenth of their 1 μM azulene counterparts, there was no relationship between singlet oxygen generation and PGE2 quantity.
Fig. 4.
PGE2 quantity after a) 1 μM b) 10 μM azulene-mediated PDT using a power density of 50 mW/cm2, energy density of 4 or 8 J/cm2 from a 638 nm red laser and fractionation mode of irradiation (intermittency factor of 5 and 9 and continuous) treated rhTNFα-induced inflammatory peripheral blood mononuclear cells and competitive human ELISA kit for detection. Positive control = peripheral blood mononuclear cells pretreated with 50 μg/mL of indomethacin, Negative control = rhTNFα-induced inflammatory peripheral blood mononuclear cells. a = significant difference from Positive control at p-value < 0.05, b = significant difference from Negative control at p-value < 0.05, dot line = basal level PGE2 in non-inflamed cells, a = significant different from (Int = intermittency factor, +ve con = positive control, -ve con = negative control, n = 4).
Fig. 5 illustrates the relationship between singlet oxygen and PGE2 at different intermittency factors. At both intermittency factor, a negative correlation was seen. At intermittency factor 5 a nearly strong negative correlation was seen (correlation coefficient −0.67, 95% CI = −0.9 to −0.15), while at intermittency factor 9, a low-to-moderate negative correlation was observed (correlation coefficient −0.51, 95% CI = −0.84 to 0.09).
Fig. 5.
Correlation between singlet oxygen and PGE2 in (a) intermittency factor 5, (b) intermittency factor 9, experiments were performed in triplicate fashion and duplicate for singlet oxygen and PGE2, respectively. Error bar = standard deviation of singlet oxygen amount.
4. Discussion
The precise biochemical and biophysical reactions induced during photodynamic therapy result in a specific effect that is limited to the targeted organ. In the present study, the incorporation of an optimum concentration of azulene, a novel photosensitizer, with the appropriate light energy based on the optimum intermittency factor may lead to a quantum yield of reactive oxygen species including singlet oxygen that can, in turn, suppress hyperactive cell activities.
The present study investigated the anti-inflammatory efficacy of pulse mode irradiation in azulene-mediated red laser photodynamic therapy in inflamed human PBMCs using different intermittency factors at low energy densities. We also examined whether this effect correlated with singlet oxygen formation as a previous report demonstrated that singlet oxygen was the ultimate product from azulene-mediated PDT [23]. Based on previous real-time monitoring of singlet oxygen formation in PDT, oxygen depletion can occur rapidly due to the conversion of oxygen (O2) to singlet oxygen (1O2) or superoxide (O2•-) [29,30], which then contributes to premature photodynamic reaction termination or cessation. Hence, the environmental oxygen supply plays a vital role in the facilitation of successful photodynamic therapy. Various methods have been developed to measure the oxygen consumption of photodynamic reactions [29,31,32] and to monitor clinical success. One straightforward and promising strategy to overcome hypo-oxygenation is fractionation of the light to allow environmental oxygen to resupply. This strategy is simple, has no adverse effects and has been shown to have benefits in PDT cancer treatment, both in vitro [33] and in vivo [34]. To our knowledge, the present study is the first study demonstrating the benefits of light fractionation in PDT with azulene on inflammatory cell suppression.
The aim of anti-cancer PDT or antimicrobial PDT is to produce as much ROS as possible to destroy target cells. To accomplish this, it uses high azulene concentrations (50 μM) that cause phototoxicity [35]. In the present study, the aim was to use low concentrations of azulene for immunosuppressive PDT that works by producing sufficient ROS that exhausts or quiesce localized hyperactive immune/inflamed cells [21] while preserving cell viability. Azulene-mediated anti-inflammatory PDT has potential advantages over a standard anti-inflammatory drug treatments due to the relatively rapid absorption of azulene applied locally into cells with no hepatic burden. A recent study reported that PDT caused a transient increase in immunosuppression of regulatory T (Treg) cells [36]. Therefore, in the present study we examined whether PDT induces immunosuppression of human PBMCs upon production of a significant amount of singlet oxygen.
Accumulating evidence has shown that discontinuous sessions of irradiation promote oxygen replenishment in PDT and produce a higher quantum yield [7] presumably by facilitating more time for oxygen to react with azulene. In the current study, we found that the formation of singlet oxygen was higher at 4 J/cm2 than 8 J/cm2. This could be due to low rates (fluence or fluence rate) of light delivery facilitating oxygen conservation [37,38] and high rates providing excess light energy that depletes oxygen. Also, in higher azulene concentrations, the tendency to lower singlet oxygen yields was possibly due to remaining un-reacted azulene acting as an oxygen scavenger [39]. Theoretically, the relative oxygen amount should change proportionally according to the irradiation dose, but in our hands the relative oxygen formation at the higher concentration of azulene was not proportional to the irradiation dose. Thus, other measurements such as singlet oxygen sensor green and electron paramagnetic resonance spectroscopy should be used to confirm this.
Regarding the diminution of inflammatory cytokines in azulene-mediated PDT, irradiation with a long intermittency factor combined with low energy density or irradiation with a short intermittency factor combined with high energy density both decreased levels of PGE2 in TNF-α-inflamed PBMCs. This could arise from the synergistic effect of ROS and TNF-α to suppress NF-κB [40], which would result in reduced inflammation and cell function. Also, because the NF-κB binding site is located on the COX-2 promoter site [41], inhibition of NF-κB in severe oxidative stress conditions would also inhibit COX-2. We used indomethacin, the non-steroidal anti-inflammatory drug, as a positive control in this study due to its ability to reduce PGE2 by inhibiting COX-1 and COX-2 enzymes [40]. To clarify whether azulene-mediated PDT reduces inflammation via COX-1 or COX-2 or both, a COX-2 specific inhibitor like celecoxib may be used. We did not include continuous mode in the PGE2 study because Damrongrungruang et al. [24] recently reported that PGE2 was not reduced by continuous mode irradiation at 4 J/cm2 with a similar intermittency factor to that used to the present study. Previously, our group used a very high power density, thus the reaction time was relatively short [24]. In the present study, we reduced the power density from 1,515 mW/cm2 to 50 mW/cm2 and increased the intermittency factors for better facilitation of the re-oxygenation process. These changes produced a dramatic PGE2 reduction. Azulene concentration also played a role in PGE2 reduction. This could be due to the anti-inflammatory effects of azulene39 at the higher 10 μM concentration or the inadequate supply of photosensitizer molecules at the lower 1 μM concentration to generate sufficient singlet oxygen to suppress inflammation. Notably, irradiation of both concentrations of azulene at intermittency factor 5 + 8 J/cm2 or intermittency factor 9 + 4 J/cm2 reduced PGE2 levels. Thus, the optimum conditions to generate total ROS in the range of 120–150 μM (of H2O2 equivalents) [21] for effective PGE2 suppression were intermittency factor 5 + 8 J/cm2 and intermittency factor 9 + 4 J/cm2.
As mentioned earlier, upon selective optical illumination, azulene produced mainly singlet oxygen. This is in line with a previous report demonstrated that only type II ROS or singlet oxygen but not type I ROS from photodynamic reaction of azulene derivative could not be detected in both electron paramagnetic spectroscopy and fluorescence method [28]. This entity is relatively safe when compared with the main type I photosensitizer product hydroxyl radical, which is more toxic [42] and more difficult to eliminate [43]. Singlet oxygen is reduced more rapidly by photobleaching and reacting with nearby molecules to produce oxygenated products such as endoperoxide and dioxetanes [5]. Thus, the consumption of oxygen by azulene during PDT is probably higher than for type I photosensitizers like curcumin [44]. This has led to the recommendation to use pulse modes of irradiation with singlet oxygen-producing photosensitizers. The amount of singlet oxygen that can be safely produced by azulene in PDT is limited because the absorption peak that yields the highest molar extinction coefficient in the triplet stage is in the UV range at 340 nm [45]. Nevertheless, it has been demonstrated that the molar extinction coefficient of azulene and its derivatives could be enhanced in the presence of H+ [45]. In the present study, the addition of ethanol as a source of H+ might yield an adequate molar extinction coefficient for azulene to exert an efficient anti-inflammatory PDT reaction. Importantly, azulene itself possesses anti-inflammatory properties [22], but in the present study, there was an inadequate amount of azulene remaining after the PDT reaction for inhibition of inflammatory cytokines.
Regarding the negative correlation between singlet oxygen and PGE2 in both intermittency factor, this emphasizes the fact that certain large amount of singlet oxygen might plausibly suppress overall cell function including inflammatory cascade. Singlet oxygen, however, is not the only molecule that plays a role in inflammation, other molecules especially reactive nitrogen species (RNS) also promote inflammation [46]. Previous reports have shown that azulene and derivatives are capable of producing novel nitrogen species [47]. While these molecules are produced at different levels in different reactions, the summative end products can drive the system into inflammation. Direct and accurate measurement of RNS could help explain the findings in the present study.
Another concern is the association between glutathione (GSH)/sulphur compounds and singlet oxygen. GSH plays a role in intracellular oxidative stress reduction and inflammatory suppression by scavenging ROS. As sulphur containing molecules such as thiocyanate (SCN−) react readily and selectively with singlet oxygen [48], these molecules may contribute to the overall intracellular inflammatory condition. Increased intracellular amounts of the oxidized form of glutathione (GSSG) compared to the reduced form (GSH) indicate lower inflammation and stress [49]. The 655 nm red LED light with low power density setting was demonstrated to increase the GSSG/GSH ratio [50] and reduce inflammation. Measuring the ratio of GSSG to GSH generated from different intermittency factors could help clarify the influence of different intermittency factors on inflammatory stage of PBMCs in PDT.
A potential limitation of the present study was that inflammation stimulation depended only on TNF-α, when inflammation depends on a variety of molecules to initiate and maintain the inflammatory process. Thus, other molecules, such as IL-1β and IL-6 might also be used in combination for closer biomimicry of inflammatory reactions in humans. Applying long term incubation of cells with inflammatory cytokines should clarify any anti-inflammatory effect and improve the generalizability of azulene-mediated photodynamic therapy. To examine the relationship between the amount of ROS and cell fate, especially the determination of transient short/long term cell quiescence, novel methods using a singlet oxygen explicit dosimeter5 or a multispectral singlet oxygen dosimeter [28] with a conversion equation to generate H2O2 equivalents should be utilized. Additionally, it is difficult to create oxygen depletion conditions in vitro using only a probe and photosensitizer solution, thus the oxygen available in the solvent, especially water, may also play some role in singlet oxygen formation. Oxygen consumption in the present PDT reaction was very low and occurred along with 9,10-dimethylanthracene oxidation and azulene photobleaching, thus the difference in singlet oxygen formation seen in pulse mode may not only be influenced by re-oxygenation. Further investigation concerning photobleaching is pivotal.
The molecular mechanism of possible azulene toxicities is an important factor to be considered. Previously, it was demonstrated that azulene damaged cells by causing DNA cleavage [51], but the required azulene dose to cause such a serious sequala is in the range of 3–4 g (23–32 mmol)/kg body weight [22], which is substantially higher than the doses used in the present study. Small amounts of azulene, such as in the present study, may pass through the nuclear membrane without exerting any mutagenic effect.
Azulene is a smaller terpenoid with very high bioavailability and a relatively short half life [22]. Hence azulene might be degraded prior to reaching the inflammatory origin due to thermolabile effects and oxidation-induced degradation and volatilization [52]. To prolong bioavailability, nanoencapsulation of terpenoids with essential oils has been utilized [53] and this has resulted in improved bioavailability and controlled release of terpenoids, including azulene, as well as improved transportation of active ingredients through biological barriers. The relatively weak anti-inflammatory effect of azulene has lead to attempts to develop azulene derivatives with higher anti-inflammatory activity. For example, two thia-azulene derivatives that were developed by incorporating benzene rings into azulene’s structure with substitution of a sulphur atom for a carbon atom demonstrated dramatically high potency for TNF-alpha inhibition [54,55].
The photostability of the photosensitizer is another property associated with the efficacy of photodynamic therapy. It has been demonstrated that azulene reacts and degrades very quickly in the presence of UV light [56]. However, substitution of the 5-member ring of azulene structure with an indole group (or NH substitution at position 1) and carboxamide dramatically increased photostability [57]. We postulate that this derivative might show the significant reduction of inflammation when receiving light energy as used in the present study.
Although the main purpose of the photodynamic therapy concept is to suppress hyperactive peripheral blood mononuclear cells based on the amount of ROS produced, combining this photosensitizer with an anti-oxidant molecule, for instance melatonin, immediately after irradiation might help sustain inflammatory suppression. Melatonin can also improve the sleep-wake cycle [58], which would be beneficial in elderly individuals. Interestingly, recent evidence indicates that azulene can also improve sleeping [59]. Therefore, an azulene-melatonin combination might be a candidate regimen suitable for geriatric patients who suffer from chronic inflammation and insomnia.
In the case of severe inflammation, it is not only cell quiescence that should be targeted, but also cell damage via apoptosis. The singlet oxygen generated by mitochondria-targeted photosensitizers such as azulene could induce mitochondria membrane toxicity [60] via mitochondria membrane potential collapse [61]. Singlet oxygen is not only localized in mitochondria, but can also interact with lysosomes [62] resulting in lysosomal disruption leading to cell apoptosis [63] and better anti-inflammatory photodynamic therapy efficiency. Crosstalk between ROS (especially singlet oxygen) and inflammation via inflammatory cytokines, for example PGE2 suppression, could induce a vicious cycle that may suppress or impair inflamed cells.
It is generally accepted that red light can adequately penetrate tissue and suppress molecules to the level of blood vessels. Although red light was relatively poorly absorbed by azulene in the present study, it proved adequate for the suppression of PGE2 formation. The effectiveness of red-light irradiation of azulene for anti-inflammatory PDT should be examined in an in vivo system incorporating blood flow to determine the effect of intravascular reoxygenation. Using higher energy densities at various intermittency factors with photostable derivatives of azulene should be examined for better understanding of their effects on other proinflammatory cytokines such as IL-1, IL-6 as well as on anti-inflammatory cytokines such as IL-10.
5. Conclusion
Photodynamic therapy using red diode laser and azulene at a low power density (50 mW/cm2) generated an optimum amount of singlet oxygen and suppressed PGE2 production in inflamed human peripheral blood mononuclear cells at an energy density of 4 J/cm2 combined with a relatively long intermittency factor (9) and at an energy density of 8 J/cm2 combined with a relatively short intermittency factor (5). These effects might relate in part to reoxygenation in an intermittency light.
Ethical considerations
The present study was complied with ethical standards with Khon Kaen University Ethical Committee Approval number HE621453.
Authorships
TD was the initiator, supervisor, and administrator of the project. SP, NS, and CS contributed with the study design, and validation, AT prepared original initial and literature search, KM, AK, and CR performed machine assembly and data analysis. WW performed data and statistical analysis. All authors have participated in the literature search, data interpretation, and writing of the final paper. All authors have approved of the final version of this paper.
Funding
This work was supported by grants from the Melatonin Research Program, Research and Academic Services, Khon Kaen University, the National Science, Research, and Innovation Fund (NSRF) and the 30th Anniversary Fund of Faculty of Dentistry, Khon Kaen University [2/2562].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank from Mr. Piboon Ngaonee and Mrs. Porada Petsuk of the Cytogenetic Unit in the Faculty of Dentistry, Khon Kaen University, Thailand for technical support. The authors thank Dr. Glenn Borlace, Faculty of Pharmaceutical Sciences, Khon Kaen University for English language assistance.
Contributor Information
Teerasak Damrongrungruang, Email: dteera@kku.ac.th, teerasak11@hotmail.com.
Sujaree Phiphitaporn, Email: peangor.sujaree@gmail.com.
Nuttakul Salacheep, Email: noonntk@gmail.com.
Chonlada Sritragool, Email: chonladaniing@hotmail.co.th.
Aroon Teerakapong, Email: arotee@kku.ac.th.
Kittipitch Meesawat, Email: mkittiphong@kku.ac.th.
Anan Kruesubthaworn, Email: anankr@kku.ac.th.
Chaiyapong Ruangsuwan, Email: chaiyapg@kku.ac.th.
Wilawan Weera-archakul, Email: wilwee@kku.ac.th.
References
- 1.Allison R.R., Motaa H.C., Bagnatob V.S., Sibata C.H. Bio-nanotechnology and photodynamic therapy—state of the art review. Photodiagnosis Photodyn. Ther. 2008;5:19–28. doi: 10.1016/j.pdpdt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Wang F., Liu C., Wang Z., Kang L., Huang Y., Dong K., Ren J., Qu X. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano. 2018;12:651–661. doi: 10.1021/acsnano.7b07746. [DOI] [PubMed] [Google Scholar]
- 3.Larue L., Myrzakhmetov B., Ben-Mihoub A., Moussaron A., Thomas N., Arnoux P., Baros F., Vanderesse R., Acherar S., Frochot C C. Fighting hypoxia to improve PDT. Pharmaceuticals. 2019;12:163. doi: 10.3390/ph12040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K.K., Mitra S., Foster T.H. A comprehensive mathematical model of microscopic dose deposition in photodynamic therapy Med. Phys. Nor. 2007;34:282–293. doi: 10.1118/1.2401041. [DOI] [PubMed] [Google Scholar]
- 5.Kim M.M., Ghogare A.A., Greer A., Zhu T.C. On the in-vivo photochemical rate parameters for PDT reactive oxygen species modelling. Phys. Med. Biol. 2017;62 doi: 10.1088/1361-6560/62/5/R1. R1–R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshadri M., Bellnier D.A., Vaughan L.A., Spernyak J.A., Mazurchuk R., Foster T.H., Henderson B.W. Light delivery over extended time periods enhances the effectiveness of photodynamic therapy. Clin. Cancer Res. 2008;14:2796–2805. doi: 10.1158/1078-0432.CCR-07-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klimenko V.V., Knyazev N.A., Moiseenko F.V., Rusanov A.A., Bogdanov, Dubina M.V. Pulse mode of laser photodynamic treatment induced cell apoptosis. Photodiagnosis Photodyn. Ther. 2016;13:101–107. doi: 10.1016/j.pdpdt.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 8.de Bruijn H.S., Brooks S., van der Ploeg-van den Heuvel A., ten Hagen T.L.M., de Haas E.R.M., Robinson D.J. Light fractionation significantly increases the efficacy of photodynamic therapy using BF-200 ALA in normal mouse skin. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mei L.H., Yang G., Fang F. Hyperbaric oxygen combined with 5-aminolevulinic acid photodynamic therapy inhibited human squamous cell proliferation. Biol. Pharm. Bull. 2019;42:394–400. doi: 10.1248/bpb.b18-00611. https://doi: 10.1248/bpb.b18-00611 [DOI] [PubMed] [Google Scholar]
- 10.He H., Zhu R., Sun W., Cai K., Chen Y., Yin L. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale. 2018;10:2856–2865. doi: 10.1039/c7nr07677k. [DOI] [PubMed] [Google Scholar]
- 11.Tian N., Sun W., Guo X., Lu J., Li C., Hou Y., Wang X., Zhou Q. Mitochondria targeted and NADH triggered photodynamic activity of chloromethyl modified Ru(ii) complexes under hypoxic conditions. Chem. Commun. 2019;55:2676–2679. doi: 10.1039/c8cc09186b. [DOI] [PubMed] [Google Scholar]
- 12.Babii O., Afonin S., Garmanchuk L.V., Nikulina V.V., Nikolaienko T.V., Storozhuk O.V., Shelest D.V., Dasyukevich O.I., Ostapchenko L.I., Iurchenko V., Zozulya S., Ulrich A.S., Komarov I.V. Direct photocontrol of peptidomimetics: an alternative to oxygen-dependent photodynamic cancer therapy. Angew Chem. Int. Ed. Engl. 2016;55:5493–5496. doi: 10.1002/anie.201600506. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X., Wu M., Li J., Lan S., Zeng Y., Liu X., Liu J. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemotherapy. ACS Appl. Mater. Interfaces. 2018;10:21909–21919. doi: 10.1021/acsami.8b06491. [DOI] [PubMed] [Google Scholar]
- 14.Lin T.S., Zhao X., Zhao S., Yu H., Cao W., Chen W., Wei H., Guo H. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics. 2018;8:990–1004. doi: 10.7150/thno.22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H.R., Chao Y., Liu J., Zhu W., Wang G., Xu L., Liu Z. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials. 2018;181:310–317. doi: 10.1016/j.biomaterials.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Kawauchi S., Morimoto Y., Sato S., Arai T., Seguchi K., Asanuma H., Kikuchi M. Differences between cytotoxicity in photodynamic therapy using a pulsed laser and a continuous wave laser: study of oxygen consumption and photobleaching. Laser Med. Sci. 2004;18:179–183. doi: 10.1007/s10103-004-0288-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim C.Y., Kim M.K. Effect of the retention ring-assisted continuous application of riboflavin in pulsed-light accelerated corneal collagen cross-linking on the progression of keratoconus. BMC Ophthalmol. 2019;19:72. doi: 10.1186/s12886-019-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frame F.M., Savoie H., Bryden F., Giuntini F., Mann V.M., Simms M.S., Boyle R.W., Maitland M.J. Mechanisms of growth inhibition of primary prostate epithelial cells following gamma irradiation or photodynamic therapy include senescence, necrosis, and autophagy, but not apoptosis. Cancer Med. 2016;5:61–73. doi: 10.1002/cam4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawczyk-Krupka A., Bugaj A.M., Latos W., Wawrzyniec K., Oleś P., Mertas A., Czuba Z., Król W., Sieroń-Stołtny K., Sieroń A. ALA-mediated photodynamic effect on apoptosis induction and secretion of macrophage migration inhibitory factor (MIF) and of monocyte chemotactic protein (MCP-1) by colon cancer cells in normoxia and in hypoxia-like conditions in vitro. Photodiagnosis Photodyn. Ther. 2015;12:27–35. doi: 10.1016/j.pdpdt.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Carrozzo M., Porter S., Mercadante V., Fedele S. Oral lichen planus: a disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontology. 2000;80:105–125. doi: 10.1111/prd.12260. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Pryor W.A., Houk K.N., Foote C.S., Fukuto J.M., Ignarro L.J., Squadrito G.L., Davies K.J. Free radical biology and medicine: it's a gas, man. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 22.Anderson F.A. Final report on the safety assessment of azulene. Int. J. Toxicol. 1999;18:27–32. doi: 10.1177/109158189901800304. [DOI] [Google Scholar]
- 23.Damrongrungruang T., Kitchindaopat N., Thanasothon P., Theeranut K., Tippayawat P., Ruangsuwan C., Suwannee B. Effects of photodynamic therapy with azulene on peripheral blood mononuclear cell viability and singlet oxygen formation. Photodiagnosis Photodyn. Ther. 2018;24:318–323. doi: 10.1016/j.pdpdt.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Damrongrungruang T., Rattanayatikul S., Sontikan N., Wuttirak B., Teerakapong A., Kaewrawang A. Effect of different irradiation modes of azulene-mediated photodynamic therapy on singlet oxygen and PGE2 formation. Photochem. Photobiol. 2019;97:427–434. doi: 10.1111/php.13346. [DOI] [PubMed] [Google Scholar]
- 25.Figueredo C.M., Lira-Junior R., Love R.M. T and B cells in periodontal disease: new functions in a complex scenario. Int. Mol. Sci. 2019;20:E3949. doi: 10.3390/ijms20163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutafchieva M.Z., Draganova-Filipova M.N., Zagorchev P.I., Tomov G.T. Oral lichen planus - known and unknown: a review. Folia Med. 2018;60:528–535. doi: 10.2478/folmed-2018-0017. [DOI] [PubMed] [Google Scholar]
- 27.Lu J., Fang K., Wang S., Xiong L., Zhang C., Liu Z., Guan X., Zheng R., Wang G., Zheng J., Wang F. Anti-inflammatory effect of columbianetin on lipopolysaccharide-stimulated human peripheral blood mononuclear cells, Mediators. Inflamm. 2018;2018 doi: 10.1155/2018/9191743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phutim-Makhalathon A., Teerakapong A., Tippayawat P., Morales N.P., Morkmued S., Puasiri S., Priprem A., Damrongrungruang T. Anti-inflammatory effect of photodynamic therapy using guaiazulene and red lasers on peripheral blood mononuclear cells. Photodiagnosis Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101747. [DOI] [PubMed] [Google Scholar]
- 29.Moritz T.J., Zhao Y., Hinds M.F., Gunn J.R., Shell J.R., Pogue B.W., Davis S.J. Multispectral singlet oxygen and photosensitizer luminescence dosimeter for continuous photodynamic therapy dose assessment during treatment. J. Biomed. Opt. 2020;25:1–13. doi: 10.1117/1.JBO.25.6.063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weston M.A., Patterson M.S. Measurement of intracellular oxygen concentration during photodynamic therapy in vitro. Photochem. Photobiol. 2014;90:878–888. doi: 10.1111/php.12256. [DOI] [PubMed] [Google Scholar]
- 31.Pogue B.W., Elliott J.T., Kanick S.C., Davis S.C., Samkoe K.S., Maytin E.V., Pereira S.P., Hasan T. Revisiting photodynamic therapy dosimetry: reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2006;61 doi: 10.1088/0031-9155/61/7/R57. R57–R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B., Lin L., Lin H., Wilson B.C. Photosensitized singlet oxygen generation and detection: recent advances and future perspectives in cancer photodynamic therapy. J. Biophot. 2016;9:1314–1325. doi: 10.1002/jbio.201600055. [DOI] [PubMed] [Google Scholar]
- 33.Sacková V., Kuliková L., Mikes J., Kleban J., Fedorocko P. Hypericin-mediated photocytotoxic effect on HT-29 adenocarcinoma cells is reduced by light fractionation with longer dark pause between two unequal light doses. Photochem. Photobiol. 2005;81:1411–1416. doi: 10.1562/2005-05-05-RA-514. [DOI] [PubMed] [Google Scholar]
- 34.de Souza A.L., Marra K., Gunn J., Samkoe K.S., Kanick S.C., Davis S.C., Chapman M.S., Maytin E.V., Hasan T., Pogue B.W. Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer. Br. J. Cancer. 2016;115:805–813. doi: 10.1038/bjc.2016.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Yan J., Wang S., Cohly H., Fu P.P., Hwang H.M., Yu H. Phototoxicity and DNA damage induced by the cosmetic ingredient chemical azulene in human Jurkat T-cells. Mutat. Res. 2004;562:143–150. doi: 10.1016/j.mrgentox.2004.06.002. https://doi: 10.1016/j.mrgentox.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 36.Falk-Mahapatra R., Gollnick S.O. Photodynamic therapy and immunity: an update. Photochem. Photobiol. 2020;96:550–559. doi: 10.1111/php.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson B.W., Busch T.M., Vaughan L.A., Frawley N.P., Babich D., Sosa T.A., Zollo J.D., Dee A.S., Cooper M.T., Bellnier D.A., Greco W.R., Oseroff A.R. Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res. 2000;60:525–529. [PubMed] [Google Scholar]
- 38.Zilberstein J., Bromberg A., Frantz A., Rosenbach-Belkin V., Kritzmann A., Pfefermann R., Salomon Y., Scherz A. Light-dependent oxygen consumption in bacteriochlorophyll-serine-treated melanoma tumors: on-line determination using a tissue-inserted oxygen microsensor. Photochem. Photobiol. 1997;65:1012–1019. doi: 10.1111/j.1751-1097.1997.tb07962.x. [DOI] [PubMed] [Google Scholar]
- 39.Wakui N., Ando S., Kobayashi A., Sasaki N., Yamamura M., Machida Y., Sakurai S. Measurement of antioxidant power of mouthwashes indicated in stomatitis. Asian J. Pharmaceut. Clin. Res. 2015;8:93–96. [Google Scholar]
- 40.Broekgaarden M., Weijer R., van Gulik T.M., Hamblin M.R., Heger M. Tumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015;34:643–690. doi: 10.1007/s10555-015-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanabe T., Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostag. Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 42.T. Mu, H. Sun, M. Zhang, C. Wang, Sweet Potato Processing Technology, first ed., Academic Press, USA.
- 43.Vatansever F., de Melo W.C., Avci P., Vecchio D., Sadasivam M., Gupta A., Chandran R., Karimi M., Parizotto N.A., Yin R., Tegos G.P., Hamblin M.R. Antimicrobial strategies centered around reactive oxygen species--bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitawee D., Teerakapong A., Morales N.P., Jitprasertwong P., Hormdee D. Photodynamic therapy of Curcuma longa extract stimulated with blue light against Aggregatibacter actinomycetemcomitans. Photodiagnosis Photodyn. Ther. 2018;22:101–105. doi: 10.1016/j.pdpdt.2018.03.001. 2018. [DOI] [PubMed] [Google Scholar]
- 45.Koch M., Blacque O., Venkatesan K. Impact of 2,6-connectivity in azulene: optical properties and stimuli responsive behavior. J. Mater. Chem. C. 2013;1:7400–7408. doi: 10.1039/C3TC31610F. [DOI] [Google Scholar]
- 46.Grimm E.A., Sikora A.G., Ekmekcioglu S. Molecular pathways: inflammation-associated nitric-oxide production as a cancer-supporting redox mechanism and a potential therapeutic target. Clin. Cancer Res. 2013;19:5557–5563. doi: 10.1158/1078-0432.CCR-12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoji T., Okujima T., Ito S. Development of heterocycle-substituted and fused azulenes in the last decade (2010–2020) Int. J. Mol. Sci. 2020;21:7087. doi: 10.3390/ijms21197087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamblin M.R., Abrahamse H. Inorganic salts and antimicrobial photodynamic therapy: mechanistic conundrums? Molecules. 2018;23:3190. doi: 10.3390/molecules23123190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toldi D., Gyugos M., Darkó É., Szalai G., Gulyás Z., Gierczik K., Székely A., Boldizsár Á., Galiba G., Müller M., Simon-Sarkadi L., Kocsy G. Light intensity and spectrum affect metabolism of glutathione and amino acids at transcriptional level. PLoS One. 2019;14 doi: 10.1371/journal.pone.0227271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parekh K.A., Jiao Y., Yu H. Light-induced damages by cosmetic and skin care products. J. Miss. Acad. Sci. 2002;47:24. [Google Scholar]
- 52.Turek C., Stintzing F.C. Stability of essential oils: a review. Compr. Rev. Food Sci. F. 2013;12:40–53. doi: 10.1111/1541-4337.12006. [DOI] [Google Scholar]
- 53.de Matos S.P., Teixeira H.F., de Lima Á.A.N., Veiga-Junior V.F., Koester L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: a review. Biomolecules. 2019;9:138. doi: 10.3390/biom9040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozimec Landek I., Pešić D., Merćep M., Stanić B., Mesić M. Synthesis and anti-inflammatory activity of 8H-1-thia-8-aza-dibenzo[e,h]azulenes. J. Heterocycl. Chem. 2011;48:856–863. doi: 10.1002/jhet.605. [DOI] [Google Scholar]
- 55.Ozimec Landek I., Pešić D., Novak P., Stanić B., Nujić K., Merćep M., Mesić M. 2,8-Dithia-dibenzo[e,h]azulenes and their 8-oxa analogs. synthesis and anti-inflammatory activity. Heterocycles. 2009;78:2489–2507. doi: 10.3987/COM-09-11755. [DOI] [Google Scholar]
- 56.Selco J.I., Brooks T., Chang M., Trieu M.T., McDonald J.K., McManus S.P. Solution photochemistry of azulene. J. Org. Chem. 1994;59:429–433. doi: 10.1021/jo00081a024. [DOI] [Google Scholar]
- 57.Leino T.O., Sieger P., Yli-Kauhaluoma J., Wallén E.A.A., Kley J.T. The azulene scaffold from a medicinal chemist's perspective: physicochemical and in vitro parameters relevant for drug discovery. Eur. J. Med. Chem. 2022;237 doi: 10.1016/j.ejmech.2022.114374. [DOI] [PubMed] [Google Scholar]
- 58.Xie Z., Chen F., Li W.A., Geng X., Li C., Meng X., Feng Y., Liu W., Yu F. A review of sleep disorders and melatonin. Neurol. Res. 2017;39:559–565. doi: 10.1080/01616412.2017.1315864. [DOI] [PubMed] [Google Scholar]
- 59.Bakun P., Czarczynska-Goslinska B., Goslinski T., Lijewski S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021;30:834–846. doi: 10.1007/s00044-021-02701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahalingam S.M., Ordaz J.D., Low P.S. Targeting of a photosensitizer to the mitochondrion enhances the potency of photodynamic therapy. ACS Omega. 2018;3:6066–6074. doi: 10.1021/acsomega.8b00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmadian E., Eftekhari A., Fard J.K., Babaei H., Nayebi A.M., Mohammadnejad D., Eghbal M.A. In vitro and in vivo evaluation of the mechanisms of citalopram-induced hepatotoxicity. Arch Pharm. Res. (Seoul) 2017;40:1296–1313. doi: 10.1007/s12272-016-0766-0. [DOI] [PubMed] [Google Scholar]
- 62.Li M., Tian R., Fan J., Du J., Long S., Peng X. A lysosome-Targeted BODIPY as potential NIR photosensitizer for photodynamic therapy. Dyes Pigments. 2017;147:99–105. doi: 10.1016/j.dyepig.2017.07.048. [DOI] [Google Scholar]
- 63.Gunaydin G., Gedik M.E., Ayan S. Photodynamic therapy-current limitations and novel approaches. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.691697. [DOI] [PMC free article] [PubMed] [Google Scholar]