Abstract
Introduction
NSCLC is a solid tumor with a growing number of actionable biomarkers that may inform treatment. Current guidelines recommend a broad, panel-based approach be taken to identify actionable markers. This retrospective study used a deidentified electronic health records database in the United States to evaluate utilization of various testing modalities.
Methods
Data from all adult patients diagnosed with having advanced/metastatic nonsquamous NSCLC between January 2015 and March 2021 were eligible if there was evidence of systemic therapy within 90 days of diagnosis.
Results
Records from a total of 17,513 patients (91.6% from community-based practices) were eligible with 83,064 genomic biomarker tests recorded from 2015 to 2021. The proportion of patients who received biomarker testing by next-generation sequencing (NGS)-based methods ranged from 28.3% in 2015 to 68.1% in 2020. The proportion of biomarker testing methods with inconclusive or unsuccessful results ranged from 3.4% for NGS to 9.7% for fluorescence in situ hybridization. The median time to receive results ranged from 4.0 days for polymerase chain reaction-based tests to 10.0 days for immunohistochemistry- and NGS-based tests. Median time to receive results was 8 days for academic and 9 days for community practices.
Conclusions
These real-world data suggest increased adoption of NGS-based testing, yet nearly one-third of all patients with advanced/metastatic nonsquamous NSCLC still did not receive broad-based genomic testing by 2020.
Keywords: Biomarker testing, Non–small cell, Real-world evidence, Next-generation sequencing
Introduction
More than 235,000 Americans will be diagnosed with lung cancer each year.1 Most of these diagnoses will be at the metastatic stage, where cancer has spread and the prognosis is poor; only 6.3% of patients with metastatic disease are expected to live for 5 years or more after the diagnosis.1,2 Most lung cancer diagnoses are NSCLC, and of these, approximately 75% are of nonsquamous histology (adenocarcinoma, large cell carcinoma, or carcinoma not otherwise specified).3 Fortunately, survival rates have been increasing for patients diagnosed with having NSCLC, likely in part due to the regulatory approval and increased utilization of novel targeted therapies for patients with actionable biomarkers and checkpoint inhibitors that were found to have improvements in survival outcomes.4 More than half of patients diagnosed with having nonsquamous NSCLC harbor an alteration associated with an approved targeted therapy.5,6 Most recently, RET fusions and KRAS G12C mutations have been added to the growing list of alterations with available targeted therapies in NSCLC.7,8 With the increasing number of actionable biomarkers (i.e., biomarkers associated with an available targeted therapy), national treatment guidelines in NSCLC have for several years strongly recommended that all patients with advanced/metastatic nonsquamous NSCLC undergo biomarker testing by a broad, panel-based assay, usually performed by next-generation sequencing (NGS), when clinically feasible.9,10
Recent publications have importantly noted that relatively little data are available about biomarker testing in clinical practice outside of clinical trial settings, in part due to the lag with which these data have begun to be collected within electronic health record (EHR) systems.11, 12, 13 There is emerging work that has begun to explore diagnostic testing in real-world settings, but gaps remain in the knowledge of current practices in biomarker testing and its utilization across community and academic practice settings.5,14,15 This study, therefore, was designed to evaluate the utilization of single-gene and panel-based biomarker testing (by NGS) among patients with nonsquamous advanced or metastatic NSCLC in community and academic practice settings across the United States to quantify the rates of testing in current practice to inform the development of guidelines or initiatives to address gaps in the receipt of biomarker testing.
Materials and Methods
Database
This study used the nationwide Flatiron Health EHR-derived deidentified database (Flatiron Health, Inc.). This EHR-derived database consisted of deidentified structured and unstructured longitudinal patient-level data, curated by means of technology-enabled abstraction.16,17 Biomarker data are included from human abstraction of unstructured data, including pathology reports, biomarker test reports, and physician notes. During the study period, data in the EHR originated from approximately 280 cancer clinics (approximately 800 sites of care) in the United States. This deidentified database is refreshed monthly and includes both structured and unstructured data elements, such as patient demographics (sex, race/ethnicity, birth year, and state of residence), type of cancer facility visited (community versus academic), clinical diagnoses, laboratory data, selected genomic biomarker tests (e.g., EGFR, ROS1, ALK, and BRAF), programmed death-ligand 1 (PD-L1) expression level, biomarker test methods and results, medications ordered or administered, line of therapy (derived), month and year of death, and other characteristics, including cancer stage at diagnosis, tumor histology, and performance status.
The advanced NSCLC database was used for this study, which is limited to patients who have a confirmed diagnosis of advanced or metastatic NSCLC. This cohort is therefore limited to patients initially diagnosed with having stage IIIB, IIIC, IVA, or IVB disease and patients diagnosed with early stage disease who subsequently developed recurrent or progressive disease. Follow-up data were available to March 2021 at the time of analysis. The data that support the findings of this study originated from Flatiron Health, Inc. These deidentified data may be made available on request and are subject to a license agreement with Flatiron Health <DataAccess@flatiron.com>. Institutional Review Board approval of the study protocol for data collection from the real-world cohort used in this study was obtained before study conduct and included a waiver of informed consent (WCG Institutional Review Board, protocol approval number 420180044).
Eligibility Criteria
Adult patients 18 years of age or older were included in this study with advanced or metastatic NSCLC who had evidence of receiving systemic anticancer therapy within 90 days after this diagnosis. Patients with squamous-only histology were excluded; patients with tumors of all other histologies (nonsquamous, large cell, NSCLC not otherwise specified) were included. Patients who initiated first-line systemic therapy before January 1, 2015, or who had no evidence of receiving systemic therapy within 90 days of index diagnosis (the date of the first observation of the stage IIIB to IV diagnosis or first observation of progression after early stage disease) were also excluded to ensure a contemporaneous cohort.
Statistical Analysis
Descriptive statistics, such as number (N), mean (SD), median, minimum, and maximum for continuous variables, and n (%), were calculated for the outcomes of biomarker tests received, timing of test order, time to receive results, and methodologies used. Date fields included recorded date of test ordered and recorded date of biomarker test result received. The date of the test ordered was evaluated in the context of dates of receipt of systemic anticancer therapy to evaluate if it is before initiation of therapy. Missing data were listed as a separate category and included in statistical testing; no imputation was made for these variables. Owing to the fact that biomarker testing rates were expected to change over the time period of this study (January 1, 2015–March 31, 2021), a descriptive analysis by year of index diagnosis date was conducted for receipt of at least one test for biomarkers recorded in the database. This study was limited to EGFR, ROS1, ALK, and BRAF, which were the only genomic biomarkers directing therapy selection that were available in the Flatiron Health database at the time of analysis. Although PD-L1 expression level is recorded in the database, this study was rather focused on genomic testing and the role of NGS-based methods and did not focus on the role of PD-L1 testing.
Results were explored between community and academic practice settings in the database to identify potential differences by site of care. The database contains a singular variable for practice setting, which reflects if the primary site of care is an academic cancer center or a community-based oncology practice. Results were further evaluated by method of testing, which is recorded in the database as polymerase chain reaction, fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), NGS, other sequencing, or unknown. No further details about the methods or exact panels used are available in this database. Time to receive results was calculated by using the two date fields: the date the specimen was received in the laboratory and the date the result was available to the provider. Biomarker testing was also evaluated between those conducted within the institution (in-house testing) or sent to an external commercial laboratory. The Flatiron Health database applies an algorithm to determine start and stop dates of lines of therapy. These line of therapy rules were applied to evaluate the timing of genomic biomarker testing relative to the care trajectory of patients included in the study cohort. Owing to the descriptive nature of this study, no sample size requirements were applied to the analysis. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Inc.).
Results
Data from a total of 17,513 patients with nonsquamous NSCLC were included in this analysis after applying study eligibility criteria (Table 1). Mean patient age was 68.2 years, 51.0% were male, and 68.7% White race. A small proportion of patients (n = 217, 1.2%) were diagnosed with having advanced/metastatic disease in 2014, before the initiation of therapy in 2015, and only 131 patients (0.8% of the study cohort) were available for analysis who initiated treatment in 2021.
Table 1.
Cohort of Patients With Advanced/Metastatic Nonsquamous NSCLC
| Characteristics | N = 17,513 |
|---|---|
| Year of advanced/metastatic diagnosis, n (%) | |
| 2014 | 217 (1.2) |
| 2015 | 2771 (15.8) |
| 2016 | 2969 (17.0) |
| 2017 | 3076 (17.6) |
| 2018 | 3049 (17.4) |
| 2019 | 2894 (16.5) |
| 2020 | 2406 (13.7) |
| 2021 | 131 (0.8) |
| Mean (SD) age, y | 68.17 (9.5) |
| Sex, n (%) | |
| Female | 8586 (49.0) |
| Male | 8927 (51.0) |
| Body mass index, n (%) | |
| Underweight | 1074 (6.1) |
| Normal | 6846 (39.1) |
| Overweight | 5275 (30.1) |
| Obese | 3697 (21.1) |
| Missing/unknown | 621 (3.6) |
| Practice setting, n (%) | |
| Academic | 1474 (8.4) |
| Community | 16,039 (91.6) |
| U.S. geographic region, n (%) | |
| Northeast | 3145 (18.0) |
| Midwest | 2632 (15.0) |
| South | 7319 (41.8) |
| West | 2626 (15.0) |
| Unknown | 1791 (10.2) |
| Race, n (%) | |
| White | 12,038 (68.7) |
| Black or African American | 1604 (9.2) |
| Asian | 353 (2.0) |
| Other | 1751 (10.0) |
| Unknown/missing | 1767 (10.1) |
| Hispanic ethnicity, n (%) | 591 (3.4) |
| ECOG performance status, n (%) | |
| 0 | 3983 (22.7) |
| 1 | 5765 (32.9) |
| 2 | 2108 (12.0) |
| 3 | 465 (2.7) |
| 4 | 22 (0.1) |
| Unknown/missing | 5170 (29.5) |
| Cancer stage at initial diagnosis | |
| Stage 0 | 1 (0.0) |
| Stage I | 1309 (7.5) |
| Stage II | 728 (4.2) |
| Stage III | 3150 (18.0) |
| Stage IV | 12,009 (68.6) |
| Unknown/missing | 316 (1.8) |
| Biomarker testing throughout study period, n (%) | |
| Any biomarker test | 15,635 (89.3) |
| Next-generation sequencing | 8321 (47.5) |
| No evidence of biomarker testing | 1878 (10.7) |
ECOG, Eastern Cooperative Oncology Group.
Testing Patterns
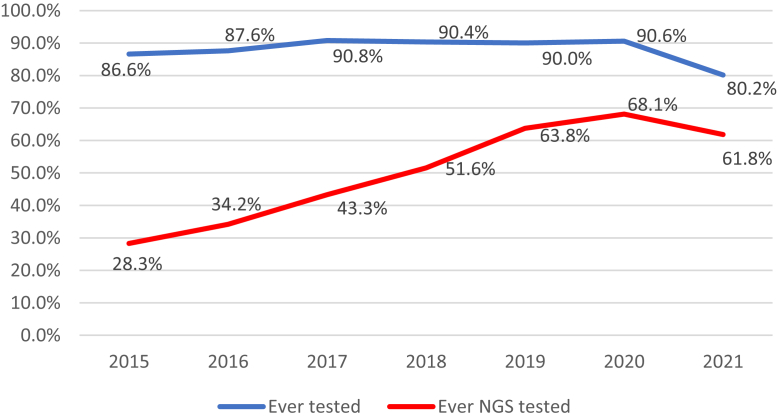
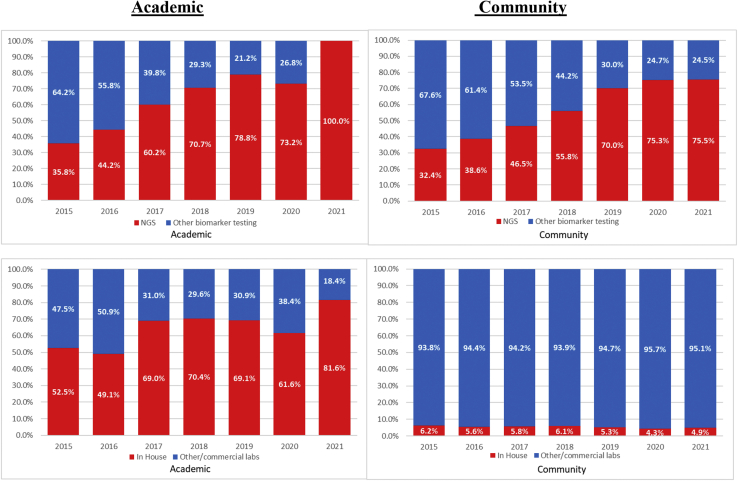
Most patients, 15,635 (89.3%), had evidence of at least one biomarker test in the database, and 1878 (10.7%) had no evidence of any biomarker testing ever performed. A total of 8321 (47.5%) had evidence of biomarker testing using NGS-based methods (with or without other biomarker tests). The proportion of patients undergoing biomarker testing where the testing was done using NGS methods rose from 28.3% in 2015 to 68.1% in 2020 (Fig. 1). As noted, data are yet incomplete for 2021 as they continue to be collected. Among patients who received biomarker testing, the proportion of patients who received at least one NGS-based test had somewhat similar patterns between academic and community practice settings (Fig. 2). More than 70% of all biomarker tests were NGS based in academic practice settings by 2018; community practices reached this level of testing on the following year.
Figure 1.
Proportion of patients who received biomarker testing by year of advanced/metastatic diagnosis. Follow-up data are limited in 2021 and data should be interpreted with caution. NGS, next-generation sequencing.
Figure 2.
Proportion of biomarker tests by academic and community practice settings (top: NGS versus other biomarker tests; bottom: in-house versus other/commercial laboratories). NGS, next-generation sequencing.
The use of in-house versus other/commercial laboratories for biomarker testing was notably different by practice setting (Fig. 2). In academic centers, the proportion of biomarker tests that were performed in-house exceeded 60% in 2017 and each year thereafter, whereas in community practices, the proportion of biomarker tests performed in-house never exceeded 8% in any year during the study period.
A total of 14,820 patients (84.6%) received at least one biomarker test before the initiation of first-line therapy. The most frequently tested biomarkers at any time included EGFR and ALK; 86.3% and 84.4% of the patients had at least one EGFR and/or ALK test, respectively. The timing of test order by biomarker is summarized in Table 2. Among patients who received testing, most biomarker tests were conducted before the initiation of first-line therapy.
Table 2.
Timing of Biomarker Test Order by Biomarker
| First Biomarker Test of Any Type Observed |
First EGFR Test Observed |
First ALK Test Observed |
First BRAF Test Observed |
First ROS1 Test Observed |
First KRAS Test Observed |
|
|---|---|---|---|---|---|---|
| Timing of Biomarker Test | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| No biomarker test observed | 1989 (11.4) | 2408 (13.7) | 2728 (15.6) | 7364 (42.0) | 4990 (28.5) | 7810 (44.6) |
| At least one biomarker test observed | 15,524 (88.6) | 15,105 (86.3) | 14,785 (84.4) | 10,149 (58.0) | 12,523 (71.5) | 9703 (55.4) |
| Before first-line therapya | 14,820 (95.5) | 14,289 (94.6) | 13,950 (94.4) | 8882 (87.5) | 11,488 (91.7) | 8380 (86.4) |
| From start of first-line therapy to before start of second linea | 350 (2.3) | 401 (2.7) | 403 (2.7) | 520 (5.1) | 447 (3.6) | 532 (5.5) |
| From start of second-line therapy to before start of third linea | 46 (0.3) | 51 (0.3) | 60 (0.4) | 157 (1.6) | 101 (0.8) | 145 (1.5) |
| From start of third-line therapy to before start of fourth linea | 11 (0.1) | 12 (0.1) | 20 (0.1) | 57 (0.6) | 42 (0.3) | 56 (0.6) |
| From start of fourth-line therapy to before start of fifth linea | 5 (0.0) | 6 (0.0) | 9 (0.1) | 32 (0.3) | 21 (0.2) | 27 (0.3) |
| From start of fifth-line therapy to before start of sixth linea | 1 (0.0) | 1 (0.0) | 1 (0.0) | 10 (0.1) | 5 (0.0) | 9 (0.1) |
| On or after start of sixth-line therapya | 291 (1.9) | 345 (2.3) | 342 (2.3) | 491 (4.8) | 419 (3.4) | 554 (5.7) |
The denominator is all patients receiving at least one biomarker test (in bold) for each column.
Time to Receive Results
There were 83,064 biomarker tests recorded among all eligible patients with nonsquamous NSCLC in this study cohort. Time to receive results from the time the specimen was received in the laboratory that performed the test is summarized in Table 3. Although the average and median times to receive results across all test modalities and practice settings were less than 15 days, the variability was high. The proportion of tests that were considered unsuccessful or inconclusive are presented by testing modality and biomarker in Table 4. A total of 9.7% of FISH tests were unsuccessful or inconclusive, whereas 3.4% of tests were inconclusive using NGS-based methods. Polymerase chain reaction (4.3% unsuccessful/inconclusive) and IHC (6.2% unsuccessful/inconclusive) tests were also observed. Although some modality/biomarker combinations were observed in less than 30 tests and may not represent reliable findings (data not found), unsuccessful results were observed most frequently for IHC and FISH for ROS1 (13.2% and 10.5%, respectively). The time to receive results was comparable between academic and community-based practices (median 8 and 9 days, respectively). Other, unknown or missing methods for biomarker testing represented 13,921 (16.6%) of recorded biomarker tests in the database.
Table 3.
Days From Specimen Received to Test Result Available to Provider
| Biomarker Test Type or Setting | Number of Tests | Mean (SD), d | Median (IQR), d |
|---|---|---|---|
| Test modality | |||
| All tests | 73,065 | 10.4 (11.5) | 9.0 (6.0–13.0) |
| FISH | 14,505 | 9.8 (13.6) | 7.0 (5.0–12.0) |
| IHC | 1076 | 11.7 (12.8) | 10.0 (7.0–14.0) |
| NGS | 40,288 | 11.5 (9.4) | 10.0 (7.0–13.0) |
| PCR | 9006 | 5.9 (8.4) | 4.0 (1.0–7.0) |
| Laboratory | |||
| In-house NGS | 3271 | 12.9 (9.3) | 11.0 (8.0–16.0) |
| All other NGS | 37,017 | 11.3 (9.4) | 10.0 (7.0–3.0) |
| Practice setting | |||
| Academic | 4734 | 11.4 (15.0) | 8.0 (6.0–14.0) |
| Community | 68,331 | 10.3 (11.2) | 9.0 (6.0–13.0) |
FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; SD, standard deviation; IQR, interquartile range; NGS, next-generation sequencing; PCR, polymerase chain reaction.
Table 4.
Proportion of Tests With Inconclusive Results by Testing Modality and Biomarker
| Test Modality | Biomarker | Number of Tests Observed in the Database | Unsuccessful/Inconclusive Results, n (%) |
|---|---|---|---|
| FISH | Any biomarker | 16,725 | 1616 (9.7) |
| IHC | Any biomarker | 1298 | 80 (6.2) |
| NGS | Any biomarker | 42,237 | 1423 (3.4) |
| PCR | Any biomarker | 9664 | 412 (4.3) |
| Any test modality | ALK | 20,507 | 1411 (6.9) |
| Any test modality | BRAF | 13,237 | 607 (4.6) |
| Any test modality | EGFR | 20,783 | 1204 (5.8) |
| Any test modality | KRAS | 12,459 | 497 (4.0) |
| Any test modality | ROS1 | 16,859 | 1277 (7.6) |
| FISH | ALK | 9354 | 839 (9.0) |
| FISH | BRAF | 21 | 4 (19.1) |
| FISH | EGFR | 6 | 0 (0.0) |
| FISH | ROS1 | 7344 | 773 (10.5) |
| IHC | ALK | 853 | 37 (4.3) |
| IHC | BRAF | 134 | 7 (5.2) |
| IHC | EGFR | 107 | 9 (8.4) |
| IHC | ROS1 | 204 | 27 (13.2) |
| NGS | ALK | 7077 | 175 (2.5) |
| NGS | BRAF | 9163 | 338 (3.7) |
| NGS | EGFR | 9862 | 366 (3.7) |
| NGS | KRAS | 9385 | 344 (3.7) |
| NGS | ROS1 | 6750 | 200 (3.0) |
| PCR | ALK | 950 | 52 (5.5) |
| PCR | BRAF | 1815 | 66 (3.6) |
| PCR | EGFR | 4534 | 213 (4.7) |
| PCR | KRAS | 1675 | 51 (3.0) |
| PCR | ROS1 | 690 | 30 (4.4) |
FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; IQR, interquartile range; NGS, next-generation sequencing; PCR, polymerase chain reaction.
Discussion
This contemporary real-world database of more than 14,000 patients and more than 83,000 biomarker tests recorded through March 2021 provides a robust body of real-world evidence to evaluate biomarker testing in the United States. The findings from this study reveal that despite the increased adoption of NGS-based testing over time since 2015, nearly one-third of patients with advanced or metastatic NSCLC still did not receive comprehensive biomarker testing by 2020. Although evaluating the use of NGS does not guarantee a full analysis of all relative biomarkers in NSCLC, NGS rates are used as a surrogate for panel-based, multiplexed testing that is directionally aligned with the recommended approach. The National Comprehensive Cancer Network guidelines have included the recommendation for broad molecular profiling since version 4.2016 and more clearly recommended broad panel-based testing since 2020.18 The lack of broader use of NGS-based testing represents an opportunity to improve concordance with clinical guidelines for patients and to guide the appropriate use of targeted therapies in both academic and community-based practice settings, where appropriate for individual patient care and where delivery of accurate results can be obtained in a timely manner. The role of biomarker testing is particularly valuable to identify therapy that can optimize patient outcomes, as several studies have revealed that patients who receive guideline-concordant targeted therapy experience improved survival outcomes.6,19 This study evaluated the timing of tests being ordered by providers and did not explicitly look at the time point in the care trajectory that the result was received, other than evaluating the time from order to test result. Therefore, the timing of testing may not fully reflect the time at which providers had access to test results.
The observed trends in NGS-based testing were similar among academic and community-based practices; however, these data suggest that the academic practices adopted broad-based testing with these methods earlier than the community practices. Of note, further details about “NGS” are not provided; this category likely includes a broad range of various panels, but all are expected to include the biomarkers recorded in the EHR system and that are part of this study. This observation should be confirmed in other databases due to both the nonspecificity of the practice setting variable and the fact that less than 10% of all patients were cared for in academic settings in the database. As a result of these limitations, this finding is not generalizable to all academic practices.
Despite the limitation of the low number of academic practices in the database, a notable finding included the use of in-house versus commercial laboratories for biomarker and NGS-based testing. Not surprisingly, most patients cared for in the academic setting had the testing conducted within laboratories at the institution. Community practices do not always have pathology laboratories with in-house equipment, as observed by the most biomarker tests sent to external commercial laboratories. The time to conduct the testing and return results to the oncologist did not seem to differ between the laboratories used for testing in this study; however, lack of specificity in date fields entered should be noted. It is impossible to know who received the test result as the variable is limited to the date entered within the field “result date.” Both reported a median of 8 to 9 days, but the variability is high. In addition, the time to prepare and send the sample to the laboratory conducting the test was not accounted for, so it cannot be used as a true proxy for overall turnaround time. Future research should incorporate the various steps in the process starting at the time of specimen collection, as there may be delays that were not observed in this study related to the storage and shipment of specimens to the laboratories that are not accounted for. There also did not seem to be variation between academic and community-based practices in any of the other variables evaluated in this study related to diagnostic testing among patients with nonsquamous NSCLC.
Inconclusive or unsuccessful test results are another factor evaluated in this study that could affect quality patient care. NGS-based methods had the highest success rate (approximately 97% success rate), whereas FISH had the lowest (approximately 90% of tests were successful). The data do not have additional details to allow for further examination of the reasons for unsuccessful testing. The factors that could lead to these findings could be clerical, methodological, related to the reagents, or could be caused by the quality or quantity of the sample provided to the laboratory.20 The findings related to failure/success rates are consistent with that observed in prior research.21
This study is a singular, yet very large U.S. database that encompasses hundreds of clinical sites across the United States. Although not nationally representative, the findings in this study are highly consistent with an emerging body of evidence revealing that these gaps in testing are consistent across the broader population of patients with advanced or metastatic NSCLC. A recent study conducted by the U.S. Oncology Network found that in their practice sites, only 44% of patients were receiving NGS-based testing by 2020.22 Another single community-based practice study found that 59% of patients received NGS-based testing.15 Although the findings from this study suggest that NGS-based testing may be higher among the clinical practices in the Flatiron Health network, it is clear that underutilization of recommended testing is a national problem.
These data are observational, and as such, gaps and errors in recording may occur as real-world data are not collected for research purposes. Importantly, some actionable biomarkers in NSCLC, such as RET, NTRK, and MET, were not recorded in the Flatiron Health database at the time of this study. Therefore, these could not be evaluated. If NGS-based testing was conducted, it is very probable that the test was observed related to other biomarkers, but if single-gene tests were conducted for these biomarkers, it is highly likely that these were missed in the database. It is also not possible to fully elucidate the reasons for testing or not testing in this database. To best understand the reasons for testing, more comprehensive solutions should be pursued, such as a combination of physician and patient surveys, including investigation into real-world databases that contain variables that encompass social determinants of health. Early evidence suggests that race may continue to play a role in access to equal care, and additional work is needed to continue to reduce the health care disparities that persist in the health care system.14
In conclusion, despite the limitations of these data, this study reveals that biomarker testing is not conducted for nearly one-third of all patients diagnosed with having advanced or metastatic nonsquamous NSCLC in the United States. There remain significant opportunities to improve broad-based genomic testing for actionable biomarkers for these patients; these findings suggest that additional efforts are needed to improve testing not only to comply with treatment guidelines but to ensure that each patient receives optimal care after diagnosis, particularly with respect to appropriate targeted therapies. Prior work revealing that patients with actionable biomarkers who receive guideline-concordant targeted therapy experience improved survival outcomes,6,19 combined with the evidence from this study revealing similar time to obtain results, provides a growing body of evidence that supports the feasibility of optimal treatment decision making before initiation of first-line therapy.
CRediT Authorship Contribution Statement
Lisa M. Hess: Conceptualized the study, Designed the study, Drafted the manuscript.
Diane Haldane, Yimei Han: Analyzed the data.
Lisa M. Hess, Peter M. Krein, Diane Haldane, Yimei Han, Anthony N. Sireci: Interpreted the data, Contributed to the content, Made substantive contributions by providing scientific content.
Acknowledgments
This was an unfunded research project supported by employee time and database access from Eli Lilly and Company.
Footnotes
Disclosure: Drs. Hess, Han, and Haldane are employees of Eli Lilly and Company. Drs. Krein and Sireci are employees of LOXO Oncology, a wholly-owned subsidiary of Eli Lilly and Company.
An earlier version of this work was presented at the Virtual Association of Molecular Pathology (AMP) 2020 Annual Meeting and Expo; the abstract was subsequently published in the Journal of Molecular Diagnostics November 2020 online supplement. https://www.jmdjournal.org/issue/S1525-1578(20)X0003-1.
Cite this article as: Hess LM, Krein PM, Haldane D, Han Y, Sireci AN. Biomarker testing for patients with advanced/metastatic nonsquamous NSCLC in the United States of America, 2015 to 2021. JTO Clin Res Rep. 2022;3:100336.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Noone A.M., Krapcho M., et al. National Cancer Institute; Bethesda, MD: April 2020. SEER cancer statistics review, 1975-2017.https://seer.cancer.gov/csr/1975_2017/ Accessed May 30, 2022. [Google Scholar]
- 3.Duma N., Santana-Davila R., Molina J.R. Non–small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Bar J., Urban D., Amit U., et al. Long-term survival of patients with metastatic non-small-cell lung cancer over five decades. J Oncol. 2021;2021:7836264. doi: 10.1155/2021/7836264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadler E., Pavilack M., Clark J., Espirito J., Fernandes A. Biomarker testing rates in patients with advanced non-small cell lung cancer treated in the community. J Cancer Ther. 2019;10:971–984. [Google Scholar]
- 6.Zhu Y., Han Y., Bhandari N.R., Hess L. CGE21-031: genomic biomarker testing, treatments, and survival outcomes among patients with advanced or metastatic NSCLC in the US: a retrospective cohort study. J Natl Compr Canc Network. 2021;19:CGE21-031 [Google Scholar]
- 7.Blair H.A. Sotorasib: first approval. Drugs. 2021;81:1573–1579. doi: 10.1007/s40265-021-01574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford D., Larkins E., Mushti S.L., et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021;27:2130–2135. doi: 10.1158/1078-0432.CCR-20-3558. [DOI] [PubMed] [Google Scholar]
- 9.NCCN. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. version 5.2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed June 30, 2021.
- 10.NCCN. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. version 3.2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed May 30, 2022. [DOI] [PubMed]
- 11.van de Ven M., Koffijberg H., Retèl V., et al. Real-world utilization of biomarker testing for patients with advanced non–small cell lung cancer in a tertiary referral center and referring hospitals. J Mol Diagn. 2021;23:484–494. doi: 10.1016/j.jmoldx.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Pennell N.A., Arcila M.E., Gandara D.R., West H. Biomarker testing for patients with advanced non–small cell lung cancer: real-world issues and tough choices. Am Soc Clin Oncol Educ Book. 2019;39:531–542. doi: 10.1200/EDBK_237863. [DOI] [PubMed] [Google Scholar]
- 13.Nadler E., Arondekar B., Aguilar K.M., et al. Treatment patterns and clinical outcomes in patients with advanced non-small cell lung cancer initiating first-line treatment in the US community oncology setting: a real-world retrospective observational study. J Cancer Res Clin Oncol. 2021;147:671–690. doi: 10.1007/s00432-020-03414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruno D.S., Hess L.M., Li X., et al. Racial disparities in biomarker testing and clinical trial enrollment in non-small cell lung cancer (NSCLC) J Clin Oncol. 2021;39(suppl 15) 9005–9005. [Google Scholar]
- 15.Sireci A., Krein P.M., Hess L.M., et al. Biomarker testing patterns in patients with stage IV non-small cell lung cancer (NSCLC) in U.S. community-based oncology practice setting. J Clin Oncl. 2021;39(suppl 28) doi: 10.1016/j.cllc.2023.03.002. 300–300. [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Long L., Moon S., et al. 2020. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv.https://www.medrxiv.org/content/10.1101/2020.03.16.20037143v2 Accessed February 10, 2022. [Google Scholar]
- 17.Birnbaum B., Nussbaum N., Seidl-Rathkopf K., et al. 2020. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv.https://arxiv.org/ftp/arxiv/papers/2001/2001.09765.pdf Accessed February 10, 2022. [Google Scholar]
- 18.Ettinger D.S., Wood D.E., Aisner D.L., et al. NCCN guidelines insights: non–small cell lung cancer, version 2.2021. J Natl Compr Canc Network. 2021;19:254–266. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 19.Singal G., Miller P.G., Agarwala V., et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non–small cell lung cancer using a clinicogenomic database. JAMA. 2019;321:1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keppens C., Schuuring E., Dequeker E.M.C. Causes behind error rates for predictive biomarker testing: the utility of sending post-EQA surveys. Virchows Arch. 2021;478:995–1006. doi: 10.1007/s00428-020-02966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderLaan P.A., Rangachari D., Majid A., et al. Tumor biomarker testing in non-small-cell lung cancer: a decade of change. Lung Cancer. 2018;116:90–95. doi: 10.1016/j.lungcan.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert N.J., Nwokeji E.D., Espirito J.L., et al. Biomarker tissue journey among patients (pts) with untreated metastatic non-small cell lung cancer (mNSCLC) in the U.S. oncology network community practices. J Clin Oncol. 2021;39(suppl 15) 9004–9004. [Google Scholar]