Abstract
Introduction
Neuroendocrine (NE) transformation has been reported in patients with ALK-rearranged NSCLC after ALK inhibition, but unlike EGFR-mutant NSCLC, the exact mechanism of NE transformation in ALK-rearranged NSCLC is poorly studied.
Methods
We collected the matched pre- and post-transformation samples from a patient with ALK-rearranged lung adenocarcinoma (LUAD) and performed targeted panel sequencing, whole exome sequencing, and bulk RNA sequencing.
Results
Multiple mutations were shared between the pretransformation and post-transformation samples. Neither RB1 nor TP53 mutation was detected, but CDKN2A deletion and CDK4 amplification were found instead. Mismatch repair-associated mutational signature was significantly enriched after transformation. Genes associated with Notch signaling and PI3K/AKT pathway were significantly up-regulated, whereas genes related to lymphocyte activation and NF-kB signaling were down-regulated. Signatures relating to homologous recombination, mismatch repair, and Notch signaling pathways were enriched, which were further validated in The Cancer Genome Atlas cohorts. Macrophages M2 were found to have prominently higher abundance in the tumor immune microenvironment after NE transformation.
Conclusions
The mechanism of NE transformation in ALK-rearranged LUAD may be different from that in EGFR-mutant LUAD.
Keywords: ALK-SYNE1 fusion, Neuroendocrine transformation, CDK4, Notch, M2 macrophage
Introduction
Target therapies have been established as superior treatments in patients with advanced NSCLC with driver oncogenes. Nevertheless, tumor cells evolve under selection pressure from targeted agents and eventually acquire drug resistance. Neuroendocrine (NE) transformation is a kind of resistance mechanism with poor prognosis and lack of effective therapeutic strategies. In published series to date, the molecular characteristics of NE transformation are mainly investigated in EGFR-mutant lung adenocarcinoma (LUAD). Nevertheless, as for ALK-rearranged LUAD, whether it shares the same mechanism or has a unique mechanism is still largely unknown because of a low incidence rate and a paucity of paired pretransformation and post-transformation clinical samples.1,2
Here, we report a patient with metastatic ALK-rearranged LUAD with NE transformation after progressing on the third lines of ALK inhibitors. Further analyses of pretansformation and post-transformation samples from this case were performed at both genomic and transcriptomic levels, providing specific molecular basis of NE transformation in ALK-rearranged LUAD.
Materials and Methods
Patient Information
The patient was treated and evaluated at Guangdong Provincial People’s Hospital. Tissue and liquid samples of this case were collected. Written informed consent of tumor acquisition for research has been obtained before surgery and approved by internal review board from Guangdong Lung Cancer Institute and Guangdong Provincial People’s Hospital (Guangzhou, People’s Republic of China, Institutional Review Board–approved protocol number GDREC2016175H).
Sequencing Analysis
Target panel sequencing, whole exome sequencing (WES), and bulk RNA sequencing were performed in LUAD samples and transformed samples of the case. The details can be found in the Supplementary Materials and Methods.
Results
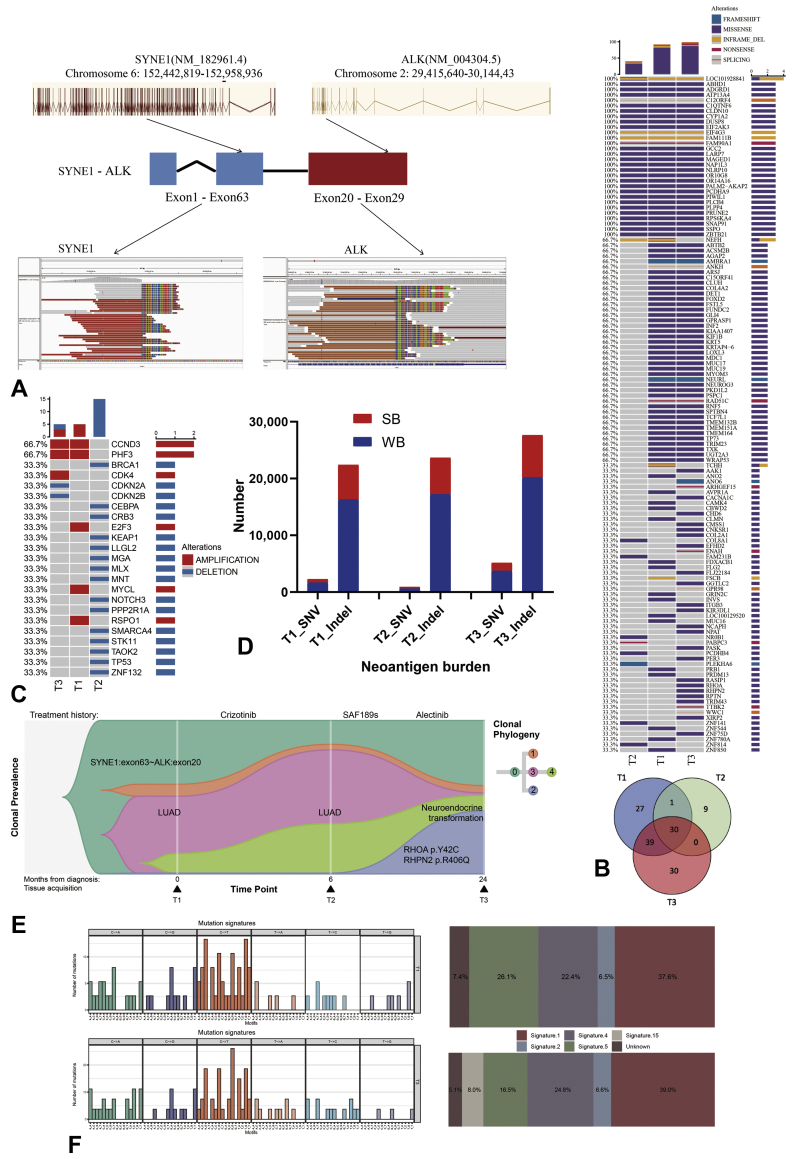
A 58-year-old female never smoker was diagnosed with having stage IVB lung cancer with bilateral lung, bone, and liver metastases in May 2018 (Fig. 1). Biopsy result of the left supraclavicular lymph node revealed adenocarcinoma, and the specimen (T1) was positive for ALK by immunohistochemistry (IHC) (D5F3 clone). Comprehensive genomic profiling using next-generation sequencing (NGS) revealed noncanonical ALK fusion with SYNE1 (Fig. 2A). She received first-line crizotinib and had a partial response lasting only 6 months. Disease progression with lung nodules was identified by computed tomography. She underwent lung biopsy, and the specimen (T2) had the original ALK rearrangement but no crizotinib-resistant mutation. Crizotinib was continued, but new symptoms of dizziness and headache occurred after 2 months, and magnetic resonance imaging result revealed new brain metastases. She refused to change the treatment and continued crizotinib until further disease progression 3 months after. Lumbar puncture was done and cerebrospinal fluid (CSF) was collected at that time. No tumor cell was found in the CSF, but ALK-SYNE1 fusion was detected in CSF cell-free DNA by NGS (Supplementary Fig. 1B). Nevertheless, the screening SAF-189s clinical trial for this patient failed because the patient’s electrocardiogram result revealed QTcF that exceeded 470 msec. She was then treated with SAF-189s in compassionate use of investigational drugs and achieved a progress-free survival of nearly 8 months. After the tumor progressed, she switched to alectinib without rebiopsy. Alectinib resulted in stable disease for approximately 5 months before tumor progression of all known lesions and marked increase of tumor biomarker NSE (Supplementary Fig. 1C). Rebiopsy of the right supraclavicular lymph node revealed SCLC combined with large cell NE carcinoma (approximately 30% and 70%, respectively). ALK protein was retained and programmed death-ligand 1 result was negative by IHC (Supplementary Fig. 1A). The specimen (T3) retained the original ALK rearrangement and harbored new mutations that were not present at the initial sample by NGS. She started on carboplatin/etoposide and continued alectinib. Partial response was achieved intracranially and extracranially, but she became comatose 8 months after. Magnetic resonance imaging result confirmed severe tumor progression in the brain. Lumbar puncture was performed again, and suspected cancer cells were found in the CSF. Further NGS revealed the original ALK rearrangement and some new mutations in the CSF (Supplementary Fig. 1B). Finally, she was switched to lorlatinib for 1 month but her condition deteriorated rapidly. She died eventually nearly 11 months after the detection of NE transformation.
Figure 1.
Clinical history of the case with NE transformation, including treatment details, radiographic and pathologic findings, and plasma and CSF acquisitions. CSF, cerebrospinal fluid; CT, computed tomography; LCNEC, large cell neuroendocrine carcinoma; LUAD, lung adenocarcinoma; MRI, magnetic resonance imaging; NE, neuroendocrine; PET, positron emission tomography.
Figure 2.
Genomic characterization of NE transformation. (A) ALK-SYNE1 fusion was detected by NGS. (B) The mutation landscape defined by WES and the Venn diagram revealing the number of shared mutations in T1, T2, and T3. (C) Copy number variations on cancer genes. (D) Predicted neoantigen burden. (E) Clonal evolution of resistance to ALK inhibitors in the case. (F) Mutational spectra based on 96 trinucleotide contexts and relative contributions of each signature are plotted. HR, homologous recombination; indel, insertion and deletion; MMR, mismatch repair; NE, neuroendocrine; NGS, next-generation sequencing; SB, strong binder; SNV, single nucleotide variant; WB, weak binder; WES, whole exome sequencing.
Genomic Landscape of Pre- and Post-NE Transformation
For in-depth characterization of NE transformation in this patient, we performed WES of specimens T1, T2, and T3. Multiple shared mutations were identified in all specimens, confirming they were clonally related. Nevertheless, T2 shared much less mutations than T1 and T3 possibly owing to interlesional heterogeneity, and similar result was observed in copy number variation (Fig. 2B and C). Neither RB1 nor TP53 mutation was detected before or after transformation, but CDK4 amplification, CDKN2A deletion, and CDKN2B deletion were found in T3 (Fig. 2C), implicating CDK4 might be a potential therapeutic target. In addition, tumor neoantigen burden was notably higher after NE transformation (Fig. 2D). Clonal analysis suggested a founder clone harboring ALK-SYNE1 rearrangement before diagnosis. No acquired ALK mutation but a subclone with RHOA and RHPN2 mutations was developed after sequential ALK tyrosine kinase inhibitor treatments, and parental ALK-SYNE1 clone was still present during transformation (Fig. 2E). There was no obvious change in activation-induced cytidine deaminase (AID)/apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC) hypermutation signature (signature 2), whereas mismatch repair (MMR)-associated mutational signature (signature 15) was significantly enriched after the transformation (Fig. 2F).
Transcriptomic Landscape of Pre- and Post-NE Transformation
We then analyzed gene expression of specimens T1 and T3 using RNA sequencing and determined differentially expressed genes between T1 and T3. Genes associated with Notch signaling and PI3K/AKT pathway were significantly up-regulated in T3, whereas genes related to lymphocyte activation and NF-kB signaling were up-regulated in T1 (Fig. 3A). Gene set enrichment analysis reveals prominent enrichment of signatures relating to the homologous recombination, MMR and Notch signaling pathways in T3 (Fig. 3B), which were validated in The Cancer Genome Atlas cohorts (Supplementary Fig. 1D). In addition, the biological processes enriched in T3 were DNA replication, cell cycle, and neuron projection development. In contrast, T1 was significantly involved in adaptive immune response, lymphocyte activation, and complement activation (Fig. 3C).
Figure 3.
Transcriptomic characterization of NE transformation. (A) A volcano plot of differentially expressed genes between T3 and T1. (B) GSEA of differentially enriched pathways between T3 and T1. (C) Gene Ontology analysis of differentially enriched biological processes between T3 and T1. (D) Differences in the levels of infiltration of the immune cells in T1 and T3. (E) Differences in the levels of infiltration of the immune cells in TCGA cohorts. ∗, <0.05; ∗∗, <0.01; ∗∗∗, <0.001; ∗∗∗∗, <0.0001. FDR, false discovery rate; GSEA, gene set enrichment analysis; LUAD, lung adenocarcinoma; NE, neuroendocrine; NES, Normalized Enrichment Score; NK, natural killer; TCGA, The Cancer Genome Atlas.
Given both differential expression genes and enriched pathways were closely linked to immune response, we further performed immune deconvolution of the transcriptomic data using CIBERSORT.3 T1 possessed higher infiltrated levels of T cells CD8 and NK cells activated, whereas T3 had the most prominent infiltration of macrophages M2 which was further validated in The Cancer Genome Atlas cohorts (Fig. 3D and E), indicating tumor immune microenvironment changed from active to suppressive during the process of transformation.
Discussion
NE transformation has been reported in several patients with ALK-rearranged NSCLC after progression on ALK inhibitors,4, 5, 6 but to our knowledge, this is the first study to take advantage of the matched pretransformation and post-transformation samples to gain insight into the molecular landscape at both DNA and RNA levels in ALK-rearranged lung cancer.
Previous studies revealed that RB1 and TP53 loss is necessary but not sufficient to induce lineage plasticity in EGFR-mutant LUAD.7,8 There are 12 reported ALK-driven cases with NE transformation, three of whom received NGS of RB1 and TP53 and one received IHC analysis of RB1 and TP53.4, 5, 6,9 Nevertheless, only one case has concomitant RB1 and TP53 mutations. In the present case, neither RB1 nor TP53 mutation was detected before or after transformation, but loss of CDKN2A was found instead, which was also observed in Coleman’s case.4 CDKN2A, as a CDK4/6 inhibitor, plays a pivotal role in cell cycle and participates in the RB pathway.10,11 Notably, CDKN2A loss and RB1 loss are mutually exclusive in most cancers, including lung cancer, suggesting the control of CDK4/6 activity and RB status seem to define a single element of the pathway.11 Therefore, CDK4 inhibition might be a potential therapeutic target of NE transformation. The clinical trial evaluating the efficacy of CDK4/6 inhibitors in chemorefractory, Rb wild-type extensive SCLC is still ongoing (NCT04010357). In addition, AID/APOBEC-associated mutational process was hyperactivated during SCLC transformation in EGFR-mutant LUAD.7,12 Nevertheless, MMR-associated mutational signature but not AID/APOBEC mutation signature seems to play a major role in NE transformation for this case.
Consistent with previous publications,8 high expression of genes in cell cycle and DNA repair was found after the transformation. Furthermore, up-regulation of homologous recombination and MMR signaling in post-transformation sample is in agreement with the highly proliferative capacity of SCLC. Notably, it is considered that inhibition of the Notch pathway is a prerequisite for SCLC transformation, as related genes were down-regulated when comparing T-LUAD with control LUAD and comparing T-SCLC with de novo SCLC.8,13 Nevertheless, in this study, Notch signaling was significantly activated on transformation. The above-mentioned discrepancy may be explained by the following possibilities: (1) Notch signaling can be both tumor suppressive and pro-tumorigenic in SCLC14; (2) Notch signaling is in repressed state during the transformation process compared with untransformed LUAD and de novo SCLC, but it is up-regulated at the late stage of the transformation process compared with that in the early stage. M2 macrophages, as an immunosuppressive subtype of tumor-associated macrophages enriched significantly, which at least partially accounted for the immune-suppressive microenvironment after the transformation.
In summary, our case indicated that the mechanism of NE transformation in ALK-rearranged LUAD may be different from that in EGFR-mutant LUAD.
CRediT Authorship Contribution Statement
Jie Huang: Investigation, Data curation, Writing—original draft preparation, Visualization.
Shi-Ling Zhang: Investigation, Data curation, Visualization.
Chaozheng Zhou: Visualization, Formal analysis.
Weiye Huang: Pathological diagnosis, Visualization.
Peng Luo: Formal analysis.
Hua-Jun Chen: Validation.
Jin-Ji Yang: Conceptualization, Methodology, Writing—review and editing.
Acknowledgments
This study was supported by the High-level Hospital Construction Project (grant number DFJH201917 to Dr. Huang); High-level Hospital Construction Project (grant number DFJH201809 to Dr. Yang); the National Natural Science Foundation of People’s Republic of China (grant number 81972164 to Dr. Yang); and the Provincial Natural Science Foundation of Guangdong Province, China (grant number 2019A1515010931 to Dr. Yang). The authors thank all the patients who participated in this study and their families. Besides, the authors thank Nanjing Geneseeq Technology Inc. (Nanjing, People’s Republic of China) for their valuable assistance in data analysis and interpretation.
Footnotes
Drs. Jie Huang and Dr. Shi-Ling Zhang contributed equally to this work.
Disclosure: The authors declare no conflict of interest.
Cite this article as: Huang J, Zhang SL, Zhou C, et al. Genomic and transcriptomic analysis of neuroendocrine transformation in ALK-rearranged lung adenocarcinoma after treatments with sequential ALK inhibitors: a brief report. JTO Clin Res Rep. 2022;3:100338.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100338.
Supplementary Data
Supplementary Fig. 1.
References
- 1.Tabbò F., Reale M.L., Bironzo P., Scagliotti G.V. Resistance to anaplastic lymphoma kinase inhibitors: knowing the enemy is half the battle won. Transl Lung Cancer Res. 2020;9:2545–2556. doi: 10.21037/tlcr-20-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobeika C., Rached G., Eid R., et al. ALK-rearranged adenocarcinoma transformed to small-cell lung cancer: a new entity with specific prognosis and treatment? Per Med. 2018;15:111–115. doi: 10.2217/pme-2017-0069. [DOI] [PubMed] [Google Scholar]
- 3.Chen B., Khodadoust M.S., Liu C.L., Newman A.M., Alizadeh A.A. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–259. doi: 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman N., Wotherspoon A., Yousaf N., Popat S. Transformation to neuroendocrine carcinoma as a resistance mechanism to lorlatinib. Lung Cancer. 2019;134:117–120. doi: 10.1016/j.lungcan.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Ou S.I., Lee T.K., Young L., et al. Dual occurrence of ALK G1202R solvent front mutation and small cell lung cancer transformation as resistance mechanisms to second generation ALK inhibitors without prior exposure to crizotinib. Pitfall of solely relying on liquid re-biopsy? Lung Cancer. 2017;106:110–114. doi: 10.1016/j.lungcan.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y.C., Liao X.H., Wang W.X., et al. Patients harboring ALK rearrangement adenocarcinoma after acquired resistance to crizotinib and transformation to small-cell lung cancer: a case report. Onco Targets Ther. 2017;10:3187–3192. doi: 10.2147/OTT.S139718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offin M., Chan J.M., Tenet M., et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14:1784–1793. doi: 10.1016/j.jtho.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintanal-Villalonga A., Taniguchi H., Zhan Y.A., et al. Multi-omic analysis of lung tumors defines pathways activated in neuroendocrine transformation. Cancer Discov. 2021;11:3028–3047. doi: 10.1158/2159-8290.CD-20-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama K., Katsurada N., Jimbo N., et al. Overexpression of CD 133 and BCL-2 in non-small cell lung cancer with neuroendocrine differentiation after transformation in ALK rearrangement-positive adenocarcinoma. Pathol Int. 2019;69:294–299. doi: 10.1111/pin.12782. [DOI] [PubMed] [Google Scholar]
- 10.Kim N., Song M., Kim S., Seo Y., Kim Y., Yoon S. Differential regulation and synthetic lethality of exclusive RB1 and CDKN2A mutations in lung cancer. Int J Oncol. 2016;48:367–375. doi: 10.3892/ijo.2015.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen E.S., Nambiar R., Rosario S.R., Smiraglia D.J., Goodrich D.W., Witkiewicz A.K. Pan-cancer molecular analysis of the RB tumor suppressor pathway. Commun Biol. 2020;3:158. doi: 10.1038/s42003-020-0873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.K., Lee J., Kim S., et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35:3065–3074. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 13.Sriuranpong V., Borges M.W., Ravi R.K., et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–3205. [PubMed] [Google Scholar]
- 14.Lim J.S., Ibaseta A., Fischer M.M., et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. 2017;545:360–364. doi: 10.1038/nature22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.