Summary
Here, we present a protocol for flow cytometry analysis of endothelial cells (ECs) and CD8+ T cells in murine tumor models, at baseline and after cancer immunotherapy with anti-PD-1/anti-CTLA-4 antibodies. We provide gating strategies for identification of specific cell subsets including ECs from tumor-associated high endothelial venules (TA-HEVs), stem-like, and terminally exhausted CD8+ T cells. This protocol represents a valuable tool for the analysis of rare subsets of tumor ECs and CD8+ T cells with critical roles in antitumor immunity.
For complete details on the use and execution of this protocol, please refer to Asrir et al. (2022).
Subject areas: Cell Biology, Cell isolation, Single Cell, Flow Cytometry/Mass Cytometry, Cancer, Health Sciences, Immunology, Model Organisms
Graphical abstract

Highlights
-
•
Protocol for simultaneous flow cytometry analysis of murine tumor ECs and CD8+ T cells
-
•
Easy, rapid, and effective procedures for tumor dissociation and single-cell isolation
-
•
Identification of TA-HEV endothelial cells (TA-HECs) and subsets of exhausted CD8+ T cells
-
•
Applicable to various murine syngeneic tumor models
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we present a protocol for flow cytometry analysis of endothelial cells (ECs) and CD8+ T cells in murine tumor models, at baseline and after cancer immunotherapy with anti-PD-1/anti-CTLA-4 antibodies. We provide gating strategies for identification of specific cell subsets including ECs from tumor-associated high endothelial venules (TA-HEVs), stem-like, and terminally exhausted CD8+ T cells. This protocol represents a valuable tool for the analysis of rare subsets of tumor ECs and CD8+ T cells with critical roles in antitumor immunity.
Before you begin
Institutional permissions
This protocol requires tumor tissue derived from murine tumor models. Ethical approvals are required prior to starting this procedure. All mice were handled according to institutional guidelines under protocols approved by the French Ministry of Research and the FRBT (C2EA-01) animal care committee (Projects APAFIS#00664.02, APAFIS#12256-2017112021529157v3, APAFIS#12297-2017112313506769v2, APAFIS#23416-2019122019025727v3).
Murine tumor models
Timing: 12 days
Murine tumor models used in this protocol are based on subcutaneous injection of tumor cells in the flank or mammary fat pad of syngeneic mice. Various tumor cell lines were used and validated for this protocol including methylcholanthrene (MCA) chemical carcinogen-induced tumor-derived MCAprogressor (MCAprog) 9609 and MCAregressor (MCAreg) 1969 fibrosarcoma cells, MMTV-PyMT mammary carcinoma-derived VO-PyMT cells, and colon carcinoma-derived CT26 cells (troubleshooting 3).
Note: To allow for sufficient development of the immune system, we recommend using 6–10-week-old mice. Ensure that the appropriate mouse strain is used for implantation of syngeneic tumor cells (e.g., MCAprog tumor cells in C57BL/6J mice and CT26 tumor cells in BALB/cByJ mice).
For detailed procedures on tumor models, including cell culture of tumor cell lines, subcutaneous injection of tumor cells and tumor growth monitoring, refer to Asrir et al. (2022).
Anti-PD-1 and anti-CTLA-4 treatment
Timing: 9 days
This section describes immune checkpoint blockade (ICB) with combined anti-PD-1 and anti-CTLA-4 antibodies in tumor-bearing mice.
Mice are injected intraperitoneally with 0.2 mg of anti-PD-1 (clone RMP1-14) and anti-CTLA-4 (clone 9D9) or Isotype control. Treatment is initiated 3 days after tumor cell inoculation and repeated every 3 days until day 12 (endpoint).
During treatment, tumor volume and appearance should be carefully monitored to ensure that ethical requirements for animal welfare are respected.
Note: Therapeutic response to anti-CTLA-4 antibodies is generally dose-dependent, but note that using high doses could induce toxicities in mice.
Design flow cytometry panels
Timing: 0.5–1 h, should be done prior to the day of the protocol
-
1.
Use two separate panels for easy and effective analysis of tumor-derived endothelial cells (ECs) and CD8+ T cells. Validated panels for identification of tumor endothelial cells and tumor-infiltrating CD8+ T cells are detailed in Tables 1 and 2.
Note: Before starting, make sure that your flow cytometer has the correct configuration to detect all parameters included in your panels. The detailed configuration used in this protocol can be found in the materials and equipment section.
Table 1.
Panel for flow cytometry analysis of tumor endothelial cells
| Marker | Fluorophore | Final dilution | Volume for 10 samples |
|---|---|---|---|
| Extracellular markers | |||
| CD45 | PerCP | 1:1,000 | 1 μL |
| CD31 | Pacific Blue | 1:300 | 3,3 μL |
| MECA-79 | Alexa Fluor 488 | 1:150 | 6,6 μL |
| CD62P | Alexa Fluor 647 | 1:250 | 4 μL |
| CD62E | BV605 | 1:250 | 4 μL |
| Total volume of antibodies | 18.9 μL | ||
| Volume of Staining buffer for 10 samples | 981.1 μL | ||
Table 2.
Panel for flow cytometry analysis of tumor-infiltrating CD8+ T cells
| Marker | Fluorophore | Final dilution | Volume for 10 samples |
|---|---|---|---|
| Extracellular markers | |||
| CD45 | PerCP | 1:1,000 | 1 μL |
| CD8a | Pacific Blue | 1:600 | 1,6 μL |
| CD44 | APC-Cy7 | 1:300 | 3,3 μL |
| PD-1 | PE-Cy7 | 1:300 | 3.3 μL |
| SLAMF6 | PE | 1:300 | 3.3 μL |
| TIM3 | BV711 | 1:200 | 5 μL |
| Total volume of antibodies | 17,5 μL | ||
| Volume of Staining buffer for 10 samples | 982,5 μL | ||
| Intracellular markers | |||
| TCF-1 | Alexa Fluor 488 | 1:150 | 6,6 μL |
| TOX | eFluor 660 | 1:150 | 6,6 μL |
| Total volume of antibodies | 13.2 μL | ||
| Volume of Permeabilization buffer for 10 samples | 986.8 μL | ||
Prepare reagents and buffers
Timing: 0.5–1 h, should be done prior to the day of the protocol
-
2.
Prepare reagents and buffers as described in the materials and equipment section.
Prepare materials
Timing: 0.5–1 h, should be done prior to the day of the protocol
-
3.Prepare materials as indicated below.
-
a.Prepare tumor collection tubes by adding 1 mL Sample buffer in 1.5 mL microtubes.
-
b.Prepare tumor digestion tubes by adding 1 mL Sample buffer in 15 mL conical centrifuge tubes.
-
c.Prepare tubes for single-cell isolation by adding 12 mL FACS buffer in 50 mL conical centrifuge tubes, and place 70 μM cell strainers on top of pre-filled 50 mL tubes.
-
d.Prepare tubes for flow cytometry acquisition by adding 100 μL FACS buffer in 1 mL cluster tubes.
-
a.
Note: All prepared tubes should be kept at 4°C until the day of the protocol.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD62P-Alexa Fluor 647 (Clone RB40.34) (dilution 1:250) | BD Biosciences | Cat# 563674; RRID: AB_2738366 |
| Anti-Mouse CD62E-BV605 (Clone 10E9.6) (dilution 1:250) | BD Biosciences | Cat 745251; RRID: AB_2742835 |
| Anti-Mouse CD8a-Pacific Blue (Clone 53-6.7) (dilution 1:600) | BioLegend | Cat# 100725; RRID: AB_493425 |
| Anti-Mouse CD31/PECAM1-Pacific Blue (Clone 390) (dilution 1:300) | BioLegend | Cat# 102422; RRID: AB_10613457 |
| Anti-Mouse CD44-APC-Cy7 (Clone IM7) (dilution 1:300) | BioLegend | Cat# 103027; RRID: AB_830784 |
| Anti-Mouse CD45-PerCP (Clone 30-F11) (dilution 1:500) | BioLegend | Cat# 103130; RRID: AB_893339 |
| Anti-Mouse SLAMF6-PE (Clone 330-A5) (dilution 1:300) | BioLegend | Cat# 134605; RRID: AB_1659258 |
| Anti-Mouse TIM3-BV711 (Clone RMT3-23) (dilution 1:200) | BioLegend | Cat# 119727; RRID: AB_2716208 |
| Anti-Mouse PD-1-PeCy7 (Clone 29F.1A12) (dilution 1:300) | BioLegend | Cat# 135215; RRID: AB_10696422 |
| Purified anti-mouse CD16/32 Antibody (Clone 93) (dilution 1:200) | BioLegend | Cat# 101302; RRID: AB_312801 |
| InVivoMAb anti-Mouse CTLA-4 (Clone 9D9) | Bio X Cell | Cat# BE0164; RRID: AB_10949609 |
| InVivoMAb anti-Mouse PD-1 (Clone RMP1-14) | Bio X Cell | Cat# BE0146; RRID: AB_10949053 |
| InVivoMAb mouse IgG2b isotype control (Clone MPC-11) | Bio X Cell | Cat# BE0086; RRID: AB_1107791 |
| InVivoMAb rat IgG2a isotype control (Clone 2A3) | Bio X Cell | Cat# BE0089; RRID: AB_1107769 |
| Anti-Human/Mouse TCF1- Alexa Fluor 488 (Clone C63D9) (dilution 1:150) | Cell Signaling Technology | Cat# 6444S; RRID: AB_2797627 |
| Anti-Human/Mouse High Endothelial Venule Marker-Alexa Fluor 488 (Clone MECA-79) (dilution 1:150) | eBioscience | Cat# 53-6036-80; RRID: AB_10805867 |
| Anti-Mouse TOX-eFluor 660 (Clone TXRX10), eBioscience™ (dilution 1:150) | Invitrogen | Cat# 53-6502-80; RRID: AB_2574264 |
| Anti-Mouse Ki67-eFluor 660 (Clone SoIA15), eBioscience™ (dilution 1:150) | Invitrogen | Cat# 50-5698-82; RRID: AB_2574235 |
| Chemicals, peptides, and recombinant proteins | ||
| DPBS w/o Calcium w/o Magnesium | Dutscher | Cat# L0615-C10LS |
| DPBS, calcium, magnesium | Gibco | Cat# 14040091 |
| Fetal Bovine Serum, qualified, Brazil | Gibco | Cat# 10270106 |
| Fixable Viability Dye eFluor 506 | Invitrogen | Cat# 65-0866-14 |
| Collagenase VIII | Sigma | Cat# 74104 |
| DNase I | Sigma | Cat# 74104 |
| EDTA acid disodium salt dihydrate | Sigma | Cat# E4884 |
| Critical commercial assays | ||
| Foxp3/Transcription factor staining buffer set | Invitrogen | Cat# 00-5523-00 |
| Experimental models: Cell lines | ||
| Mouse: colon carcinoma-derived CT26 cells | ATCC | Cat# ATCC CRL-2638 |
| Mouse: methylcholanthrene (MCA)-induced fibrosarcoma-derived MCAprog cells (9609) | Laboratory of Robert Schreiber (Washington University School of Medicine, Saint-Louis, USA) | N/A |
| Mouse: methylcholanthrene (MCA)-induced fibrosarcoma-derived MCAreg cells (1969) | Laboratory of Robert Schreiber (Washington University School of Medicine, Saint-Louis, USA) | N/A |
| Mouse: MMTV-PyMT mammary carcinoma-derived VO-PyMT cells | Laboratory of Zena Werb (University of California, San Francisco, USA) | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J (6–10-week-old female) | Charles River | Cat# 027 |
| Mouse: BALB/cByJ (6–10-week-old female) | Charles River | Cat# 028 |
| Mouse: FVB (6–10-week-old female) | Charles River | Cat# 207 |
| Software and algorithms | ||
| DiVa V8.0.1 | BD Biosciences | https://www.bdbiosciences.com/en-us/products/software/instrument-software/bd-facsdiva-software |
| FlowJo 10.6.1 | BD | https://www.flowjo.com/solutions/flowjo/downloads |
| Other | ||
| Corning® 70 μm Cell Strainer | Corning | Cat# 431751 |
| Single-use 2.5 mL polypropylene syringe (TERUMO) | Dutscher | Cat# 050004B |
| Thermo Scientific™ Abgene™ Blank and Alphanumeric Storage Tubes (cluster tubes) | Fisher Scientific | Cat# AB-0672 |
| Fisherbrand™ 1.5 mL Premium Microcentrifuge Tubes | Fisher Scientific | Cat# 11926955 |
| Falcon™ 15 mL Conical Centrifuge Tubes | Fisher Scientific | Cat# 10773501 |
| Falcon™ 50 mL Conical Centrifuge Tubes | Fisher Scientific | Cat# 10788561 |
| Nunc™ 96-Well Polystyrene Conical Bottom Plates | Thermo Scientific | Cat# 249570 |
Materials and equipment
Antibodies
We recommend using the antibodies listed in the key resources table (troubleshooting 4). Working dilutions are provided in the antibody panels detailed in Tables 1 and 2, but antibody titration should be performed if using tumor models distinct from those presented in this protocol. Dilute antibodies in a buffer containing Fc block (purified anti-CD16/32 antibody).
Note: Blocking cells with purified anti-CD16/32 antibody (Fc block) reduce non-specific binding of your antigen-specific antibodies, and thus ameliorate the quality of your flow cytometry analysis.
Antibody cocktails must be kept at 4°C in the dark and should be prepared only the day of the protocol (avoid preparation days before).
Buffers
Sample buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| DPBS with calcium and magnesium | n/a | 98 mL |
| FBS | 2% | 2 mL |
The buffer can be stored at 4°C for 1 week.
CRITICAL: Ensure that DPBS with calcium and magnesium is used for enzymatic digestion.
FACS buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| DPBS without calcium and magnesium | n/a | 485 mL |
| FBS | 2% | 10 mL |
| EDTA (0,2 M) | 2 mM | 5 mL |
The buffer can be stored at 4°C for 1 week.
Staining buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| FACS buffer | n/a | 4975 μL |
| Purified anti-CD16/32 antibody (Fc block) | 1:200 | 25 μL |
The buffer can be stored at 4°C for 24 h.
100× collagenase VIII stock
Dissolve 500 mg Collagenase VIII in 5 mL DPBS with calcium and magnesium to make a 100 mg/mL stock. Store at −20°C in 500 μL single-use aliquots for up to 6 months.
100× DNase I stock
Dissolve 100 mg DNase I in 10 mL 1× DPBS with calcium and magnesium to make a 10 mg/mL stock. Store at −20°C in 500 μL single-use aliquots for up to 6 months.
EDTA (0,2 M) stock
To make 1 L 0,2 M EDTA, dissolve 74.45 g EDTA (acid disodium salt dehydrate) in 1 L of water and adjust the buffer’s pH to 8 by adding 5 M NaOH. Store at 18°C–22°C for up to 6 months.
70% ethanol
To make 500 mL 70% ethanol, mix 350 mL of absolute ethanol with 150 mL of water. Store at 18°C–22°C.
Fixable Viability Dye
Fixable Viability Dye should be stored at −80°C in 5 μL aliquots for up to 6 months. For staining of tumor-derived cells, use Fixable Viability Dye diluted 1:1000 in DPBS without magnesium and calcium.
Flow cytometer and data analysis
Samples were acquired on a BD LSRFortessa flow cytometer using BD FACSDiva software (BD Biosciences). The panels used in this protocol were designed for use on the BD LSRFortessa flow cytometer with the following configuration: 3 channels for the violet laser 405 nm (Pacific Blue, eFluor 506, Brilliant Violet 711), 2 channels for the blue 488 nm laser (Alexa Fluor 488 and PerCP), 2 channels for the red 640 nm laser (APC or Alexa Fluor 647, and APC-Cy7) and 2 channels for the yellow-green 561 nm laser (PE and PE-Cy7).
Data were analyzed with FlowJo 10.6.1 (BD).
Step-by-step method details
Sample collection
Timing: 5 min/mouse
This part details how to resect subcutaneous tumors from tumor-bearing mice.
-
1.
Euthanize tumor-bearing mice by CO2 inhalation or other approved method.
-
2.
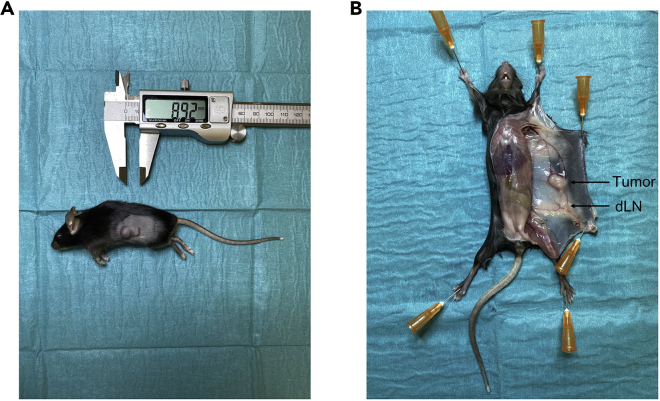
Subcutaneous tumors grow on the shaved flanks of mice. Tumor length and width can be measured using calipers (Figure 1A).
-
3.
Place the animal on its back on a dissection mat, pin the paws on the mat and wet the fur with 70% ethanol.
-
4.
Make an incision in the abdominal area above the urethral opening, without penetrating the abdominal wall, and extend the incision until the neck with a pair of scissors.
-
5.
On the tumor-bearing side of the mouse, extent the incision to hind and forelegs and pin the loose skin on the mat. The flank subcutaneous tumor and the draining inguinal lymph node (dLN) should be seen (Figure 1B).
-
6.
Using a pair of scissors and forceps, clean up the tumor area by removing fat and take off the draining inguinal lymph node to avoid contamination during tumor resection.
-
7.
Using a scalpel, delicately harvest the tumor and ensure that skin is not collected during the procedure.
-
8.
Weigh the tumor and transfer it in a collection tube pre-filled with 1 mL Sample buffer.
-
9.
Place the tube on ice and work on the next mouse.
Note: From sample collection to sample processing and staining, maintain tissue and cells on ice.
Figure 1.
Tumor resection
(A) Subcutaneous tumor 10 days after inoculation of tumor cells (MCAprog).
(B) Visualization of the tumor and the draining lymph node (dLN) following initial dissection.
Sample processing
Timing: 1.5–2 h
This part details how to prepare single-cell suspensions from resected murine tumors (Figure 2A).
-
10.
Using a pair of scissors, mince the tumor into approximately 1–2 mm2 fragments inside the collection tube (Figure 2B).
-
11.
Transfer tumor fragments in a digestion tube pre-filled with 1 mL Sample buffer.
-
12.
Rinse twice the collection tube with 0.5 mL Sample buffer to obtain a final digestion volume of 3 mL (Figure 2C). Place the tube on ice while working on the next samples.
-
13.
Once all samples have been processed as in steps 10–12, prepare a digestion mix with collagenase VIII (final concentration 1 mg/mL) and DNase I (final concentration 0.1 mg/mL). An example of digestion mix for 10 tumors is provided below.
Digestion mix (10 samples)
| Reagent | Final concentration | Amount/sample | Total for 10 samples |
|---|---|---|---|
| Collagenase VIII (100 mg/mL) | 1 mg/mL | 30 μL | 300 μL |
| DNase I (10 mg/mL) | 0.1 mg/mL | 30 μL | 300 μL |
-
14.
Add the required volume of digestion mix in each digestion tubes (2 × 30 μL = 60 μL), mix well and incubate the tubes on a shaker at 180 rpm for 30 min at 37°C (Figure 2D).
CRITICAL: Enzyme concentration and timing of digestion are critical parameters for optimal tumor digestion. The purpose is to achieve sufficient tissue dissociation while maximizing cell viability and preserving cell surface antigen expression. We recommend optimizing enzyme concentration and timing of digestion if using tumor models distinct from those presented in this protocol. Note that the quality of collagenase is also an important parameter for optimal digestion (troubleshooting 1) (troubleshooting 2).
Note: For a given tumor model, the size of tumors could vary depending on the initial number of tumor cells injected and the endpoint of the experiment. The digestion volume stated here (3 mL) is working well for tumors up to 0,5–0,6 g. For bigger tumors, digestion volume should be increased, but the concentration of the enzymes should not be modified.
-
15.
Following 30 min incubation, the buffer in the digestion tube turns cloudy because of cell dissociation, and only small remaining tumor fragments are observed (Figure 2E). Transfer the contents of each digestion tube to individual 50 mL tubes pre-filled with 12 mL FACS buffer and overlaid with a 70 μM cell strainer.
-
16.
Carefully disaggregate the remaining tumor fragments on the 70 μM strainer with a 2,5 mL syringe plunger (Figure 2F).
-
17.
Rinse the cell strainer and syringe plunger with 10 mL of FACS buffer to flush the cells through the filter (Figure 2G). The 50 mL tube now contains 25 mL of tumor single-cell suspension (Figure 2H).
CRITICAL: Perform steps 15–17 on ice immediately after the 30 min incubation to rapidly stop the enzymatic digestion.
-
18.
Once all samples have been processed as in steps 15–17, place the 50 mL tubes in a centrifuge and spin at 500 × g for 5 min at 4°C.
-
19.
Discard the supernatant and resuspend the pellet with 1 mL FACS buffer.
-
20.
Count total cell number in each sample using a hemocytometer or automated cell counter.
-
21.
Transfer equal numbers of cells per sample to a 96-well plate (Figure 2I). For simultaneous analysis of endothelial cells and CD8+ T cells from a single tumor, prepare two distinct wells with tumor-derived cells from the same sample.
CRITICAL: For analysis of rare cell subsets (e.g., TA-HECs), use a sufficient number of tumor-derived cells for flow cytometry analysis (4–8 × 106 cells).
Note: Determining the total number of cells in your samples is required to calculate total numbers of given cell populations from your flow cytometry analysis.
Note: Use remaining cells for controls (e.g., fluorescence minus one (FMO) and unstained controls).
Figure 2.
Tumor digestion
(A) Resected tumor (MCAprog) in the collection tube.
(B) Minced tumor.
(C) Tumor fragments in the digestion tube before enzymatic digestion.
(D) Digestion tubes placed on a shaker for enzymatic digestion in a warm room.
(E) Tumor cell suspension and remaining fragments after enzymatic digestion.
(F) Filtration of the tumor cell suspension and mechanic dissociation of remaining fragments with a syringe plunger in the 50 mL tube overlaid with a cell strainer.
(G) Flushing of the cell strainer and syringe plunger.
(H) Visualization of the 25 mL of tumor single-cell suspension after entire processing.
(I) Tumor-derived cells in the 96-well plate prepared for flow cytometry analysis.
Cell staining
Timing: 1.5–2 h
This part details how to stain tumor-derived cells for flow cytometry analysis. If intracellular staining is required, proceed first to extracellular staining followed by cell fixation and intracellular staining. For optimal analysis of your flow cytometry data, we recommend preparing FMO and unstained controls. All the following steps need to be performed in the dark.
-
22.
Centrifuge the 96-well plate containing cells at 500 × g for 5 min at 4°C.
-
23.
Discard the supernatant and resuspend cells in 100 μL of Fixable Viability Dye diluted 1:1000 in DPBS without magnesium and calcium, and incubate the cells at 4°C for 10 min.
Note: Discriminating live and dead cells significantly ameliorates the quality of flow cytometry analysis since non-specific binding of antibodies to dead cells is common.
-
24.
Wash with 100 μL FACS buffer per well and centrifuge the 96-well plate at 500 × g for 5 min at 4°C.
-
25.
Discard the supernatant and resuspend cells in 100 μL of antibody cocktails (extracellular staining) prepared in the Staining buffer as indicated in Tables 1 and 2, and incubate the cells at 4°C for 30 min. When performing simultaneous analysis of endothelial cells and CD8+ T cells, add the specific antibody cocktail in the wells reserved for endothelial and CD8+ T cell staining.
-
26.Wash with 100 μL FACS buffer per well and centrifuge the 96-well plate at 500 × g for 5 min at 4°C.
-
a.If intracellular staining is not performed (e.g., flow cytometry analysis of tumor endothelial cells), discard the supernatant, resuspend cells in 150 μL FACS buffer and transfer the cells in 1 mL cluster tubes pre-filled with 100 μL FACS buffer. These samples are now ready for acquisition on the flow cytometer.
-
b.If intracellular staining is performed (e.g., flow cytometry analysis of tumor-infiltrating CD8+ T cells), fix cells in 100 μL Fixation/Permeabilization solution (dilute 1:3 Fixation/Permeabilization concentrate in Fixation/Permeabilization diluent from the Foxp3/transcription factor staining kit) and incubate the cells at 18°C–22°C for 20 min.
-
a.
Pause point: Fixed cells can be stored at 4°C for up to 18 hours. Wash with 100 μL FACS buffer and centrifuge the 96-well plate at 700 × g for 5 min at 4°C. Discard the supernatant and resuspend cells in 200 μL FACS buffer. The next steps can be performed on the following day.
-
27.
Wash with 100 μL Permeabilization buffer (dilute 1:9 Permeabilization buffer 10× from the Foxp3/transcription factor staining kit in distilled water) and centrifuge the 96-well plate at 700 × g for 5 min at 4°C.
Note: Following cell fixation, centrifugation speed can be increased at 700 × g to ensure optimal cell pelleting.
-
28.
Discard the supernatant and resuspend cells in 100 μL of antibody cocktails (intracellular staining) prepared in the Permeabilization buffer as indicated in the Table 2, and incubate the cells at 4°C for 0,5–1 h (troubleshooting 5).
-
29.
Wash with 100 μL FACS buffer and centrifuge the 96-well plate at 500 × g for 5 min at 4°C.
-
30.
Discard the supernatant, resuspend cells in 150 μL FACS buffer and transfer the cells in 1 mL cluster tubes pre-filled with 100 μL FACS buffer. The samples are now ready for acquisition on the flow cytometer.
Acquisition on the flow cytometer
Timing: 1.5–2 h
This part details the acquisition of samples on the flow cytometer. The following steps were designed for acquisition on a BD LSRFortessa flow cytometer using BD FACSDiva software (BD Biosciences).
-
31.
For auto-compensation settings, prepare unstained control and single-stained controls for every fluorochrome used in your panel.
Note: We recommend using mouse-derived cells (e.g., splenocytes), but compensation beads could also be used. For the viability dye fluorochrome, using heat-killed cells stained with Fixable Viability Dye is a suitable option.
-
32.
Once controls are prepared, go to your flow cytometry facility and open a new experiment on BD FACSDiva software.
-
33.
Use unstained and single-stained controls to set appropriate PMT voltages and calculate auto-compensation.
-
34.
Define the gating strategy.
Note: Refer to data analysis section for additional information on the gating strategy.
-
35.
Acquire a sufficient number of cells per sample while watching the flow rate (8000 events/s or less).
CRITICAL: For optimal analysis of rare tumor-associated endothelial cell subsets (e.g., TA-HECs), we recommend acquisition of 1 × 106 cells. For tumor-infiltrating CD8+ T cells, 0.2–0.5 × 106 cells are sufficient.
Data analysis
Timing: 1 h
This part details guidelines for analyzing tumor-derived endothelial cells and CD8+ T cells in your flow cytometry data. Data presented in Figures 3, 4, 5, and 6 were obtained in the MCAprog tumor model.
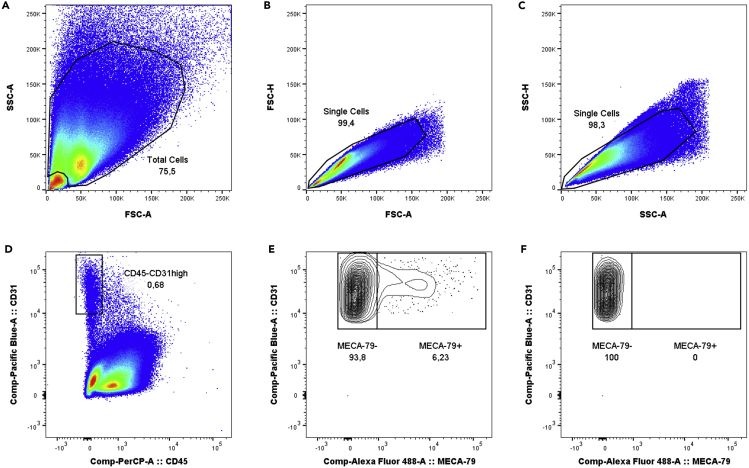
Figure 3.
Gating strategy for analysis of tumor endothelial cells
(A) Forward and sideward scatter gating to exclude debris.
(B) Single cell gating.
(C) Single cell gating.
(D) CD45-CD31high cell gating to identify total tumor-derived endothelial cells.
(E) Identification of MECA-79- tumor-associated endothelial cells (TA-ECs) and MECA-79+ tumor-associated HEV endothelial cells (TA-HECs).
(F) FMO MECA-79 control.
Figure 4.
Analysis of CD62P and CD62E expression in MECA-79+ TA-HECs
(A) Histogram showing CD62P expression in MECA-79+ TA-HECs.
(B) Histogram showing CD62E expression in MECA-79+ TA-HECs.
Figure 5.
Gating strategy for analysis of tumor-infiltrating CD8+ T cells
(A) Single cell gating.
(B) Forward and sideward scatter gating to identify lymphoid cells.
(C) Live CD45+ cell gating.
(D) CD8+ cell gating.
(E) CD44+ cell gating.
(F) PD-1+ cell gating.
(G) Identification of SLAMF6+TIM3- stem-like and SLAMF6-TIM3+ terminally exhausted CD8+ T cells.
(H) FMO SLAMF6 control.
(I) FMO TIM3 control.
Figure 6.
Analysis of TCF-1 and TOX expression in tumor-infiltrating exhausted CD8+ T cells
(A) Histograms showing TCF-1 expression in SLAMF6+TIM3- stem-like (SLAMF6+) and SLAMF6-TIM3+ terminally exhausted (TIM3+) CD8+ T cells.
(B) Histograms showing TOX expression in SLAMF6+TIM3- stem-like (SLAMF6+) and SLAMF6-TIM3+ terminally exhausted (TIM3+) CD8+ T cells.
Among non-hematopoietic CD45- cells, tumor-derived endothelial cells are identified as CD45-CD31high cells. Among the total population of tumor-derived endothelial cells, tumor-associated high endothelial cells (TA-HECs) are identified based on staining with MECA-79, an HEV-specific antibody (Blanchard and Girard, 2021; Girard et al., 2012). The gating strategy is presented in Figure 3. MECA-79+ TA-HECs can be further characterized with additional markers of inflamed endothelium such as CD62P (P-selectin) and CD62E (E-selectin), as shown in Figure 4.
Among hematopoietic CD45+ cells, tumor-infiltrating CD8+ T cells are identified using the CD8a marker. Among the total population of tumor-infiltrating CD8+ T cells, antigen-experienced exhausted CD8+ T cells are identified as CD44+PD-1+ cells. Two subsets of exhausted CD8+ T cells can be further identified using the markers SLAMF6 and TIM3. SLAMF6+TIM3- are defined as stem-like CD8+ T cells and SLAMF6-TIM3+ are defined as terminally exhausted CD8+ T cells (Miller et al., 2019; Siddiqui et al., 2019). The gating strategy is presented in Figure 5. SLAMF6+TIM3- stem-like and SLAMF6-TIM3+ terminally exhausted CD8+ T cells can be further characterized with additional markers important for T cell exhaustion such as TCF-1 and TOX transcription factors (Khan et al., 2019; Miller et al., 2019; Scott et al., 2019; Siddiqui et al., 2019), as shown in Figure 6.
Expected outcomes
From the digestion of intermediate-stage tumors, you should obtain approximatively 40–60 × 106 total cells with cell viability of 60%–70%. However, it should be noted that these parameters greatly vary depending on the tumor model.
In the MCAprog tumor model, the percentage of tumor-derived endothelial cells generally ranges from 0.6 to 1.2% of total cells. In non-treated tumors, the subset of TA-HECs represents 2%–6% of total tumor-derived endothelial cells, but the frequency of TA-HECs depends on the endpoint chosen for analysis of the tumor microenvironment. The percentage of tumor-infiltrating CD8+ T cells usually ranges from 5 to 10% of total hematopoietic CD45+ cells. Among the subset of CD44+PD-1+ exhausted CD8+ T cells, the distribution between SLAMF6+TIM3- stem-like and SLAMF6-TIM3+ terminally exhausted CD8+ T cells is also highly influenced by the tumor stage.
ICB with combined anti-PD-1 and anti-CTLA-4 antibodies has a profound impact on tumor-associated endothelial cells (TA-ECs): it reduces MECA-79- TA-ECs while preserving MECA-79+ TA-HECs, resulting in increased frequency of MECA-79+ TA-HECs (Figure 7). ICB also increases the proportion of tumor-infiltrating CD8+ T cells (Figures 8A and 8E). Among CD44+PD-1+ exhausted CD8+ T cells, several effects can be observed. ICB massively expands PD-1int cells, resulting in drastic reduction of the frequency of PD-1high exhausted CD8+ T cells (Figures 8B and 8F). Regarding specific subsets of exhausted CD8+ T cells, both SLAMF6+TIM3- stem-like and SLAMF6-TIM3+ terminally exhausted cells are increased (Figures 8C and 8G), the latter being predominant as previously reported (Miller et al., 2019). Finally, increased proportion of proliferating SLAMF6+TIM3- stem-like CD8+ T cells is observed following ICB, as revealed by Ki67 staining (Figures 8D and 8H).
Figure 7.
Impact of immune checkpoint blockade on tumor-associated endothelial cells
(A–D) Representative images of flow cytometry analysis of total tumor-derived endothelial cells (A and C) and MECA-79+ TA-HECs (B and D) in the MCAprog tumor model following treatment with Isotype control (Iso) or combined anti-PD-1 and anti-CTLA-4 antibodies (ICB).
Figure 8.
Impact of immune checkpoint blockade on tumor-infiltrating CD8+ T cells
(A–H) Representative images of flow cytometry analysis of tumor-infiltrating CD8+ T cells (A and E), PD-1+ CD8+ T cells (B and F), SLAMF6+TIM3- stem-like and SLAMF6-TIM3+ terminally exhausted CD8+ T cells (C and G) and Ki67high SLAMF6+TIM3- stem-like CD8+ T cells (D and H) in the MCAprog tumor model following treatment with Isotype control (Iso) or combined anti-PD-1 and anti-CTLA-4 antibodies (ICB).
Quantification and statistical analysis
For complete and relevant flow cytometry analysis of tumor-derived cell populations, we recommend analyzing and presenting data in a number of different consistent ways (e.g., total numbers, numbers per mg of tumor, percentages and ratios of cell subsets) to do not miss some effects or trends because of inappropriate analysis and interpretations.
Percentages and absolute numbers of cell populations are calculated with FlowJo software. Total numbers, numbers per mg of tumor and ratios of cell subsets can be calculated with a spreadsheet (e.g., Microsoft Excel) using the total number of cells in the tumor and the tumor weight. For instance, to obtain the total number of CD8+ T cells in a tumor, multiply the number of CD8+ T cells identified in this sample with FlowJo analysis by the total number of tumor cells counted during tumor processing. Divide this number by the total number of cells acquired for this sample. To then obtain the number of CD8+ T cells per mg of tumor, divide the total number of CD8+ T cells calculated by the tumor weight.
Limitations
This protocol was optimized for flow cytometry analysis of endothelial cells and CD8+ T cells from murine transplantable tumor models. Tissue dissociation and antibody panels were designed for this type of tumor model, working with other types of tumor will likely require optimization.
While this protocol is of particular value for easy, rapid and effective flow cytometry analysis of murine tumor-derived endothelial cells and CD8+ T cells, deep characterization of these cell populations may be limited. Pre-enrichment with CD45 magnetic beads (to isolate the CD45- fraction) and CD8 magnetic beads (to isolate the CD8+ fraction) is recommended for deeper characterization of endothelial cells and CD8+ T cells, respectively.
Flow cytometry is instrumental for robust analysis and quantification of tumor-derived cell populations at single-cell resolution, but spatial information (e.g., cell-cell interactions or cell organization in specific tissue niches) is absent. Although separate analysis of specific tumor regions could provide some spatial information, we recommend performing histologic analysis on tumor sections in addition to flow cytometry analysis when investigating tumor immunology. We also recommend the use of dynamic techniques such as intravital microscopy and multiphoton in vivo imaging to better define the functionality of different subsets of ECs and T cells in tumors (Asrir et al., 2022).
This protocol focuses on the analysis of tumor-associated endothelial cells and CD8+ T cells. Although ECs lining tumor blood vessels (particularly TA-HEVs) and CD8+ T cells play critical roles in cancer immunity and immunotherapy (Asrir et al., 2022; Blanchard and Girard, 2021), several other stromal and immune cells (e.g., fibroblasts, lymphatics, myeloid cells, CD4+ T cells and B cells) are also important for antitumor immune responses. This protocol could provide a framework for analysis of such cells, but optimization is likely to be required.
Troubleshooting
Problem 1
Poor cell viability.
Potential solution
Enzymatic digestion is a critical step determining the viability of your samples (refer to steps 14–17). Enzyme concentration and timing of digestion are important parameters; using too much enzymes or digesting too long will drastically reduce the overall viability of your cells. Moreover, following incubation at 37°C, samples must be rapidly placed on ice and diluted in FACS buffer to stop enzyme activity. Note that the quality of your enzymes is also important: use DNase I and collagenase VIII with high quality and prepare aliquots to avoid freeze-thaw cycles.
Other general recommendations to increase cell viability in your samples: ensure that tumor tissue and cells are stored in FBS-containing buffer when waiting and during the different steps, procedures have to be carried out on ice and centrifugations should be realized at 4°C with a centrifugation speed not exceeding 500 × g (for unfixed cells).
Problem 2
Undetectable, insufficient or inappropriate signals for cell surface markers.
Potential solution
Ensure that proper controls are set up during your flow cytometry analysis (refer to cell staining section): isotype controls to detect antibody non-specific binding, and unstained and FMO controls to appropriately gate positive cell populations. Additionally, titration of your antibodies, and doing a blocking step (with Fc-block) before or during cell staining, will reduce non-specific binding.
Once again, the enzymatic digestion is a critical step since enzymes can cleave cell surface markers (steps 14–17). Enzyme concentration and timing of digestion should be finely optimized. Alternatively, you can change the enzyme used for digestion (e.g., Liberase or collagenase D instead of collagenase VIII).
Finally, it could be useful to concurrently analyze lymphoid tissues when working on tumors. For instance, lymph node HEVs and CD8+ T cells can be used as controls for staining.
Problem 3
Absence of TA-HEVs.
Potential solution
Several parameters can influence the presence or amount of TA-HEVs in tumors (refer to murine tumor models section). First, the type of model used; some tumor models spontaneously develop TA-HEVs whereas some do not (e.g., non-immunogenic cold tumors). Second, cancer treatments can affect the amount of TA-HEVs. For instance, lymphocyte-based cancer immunotherapies generally increase the proportion of TA-HEVs.
We also recommend performing concurrent HEV staining on lymph node samples to control the efficacy of the MECA-79 staining.
Problem 4
Absence of PD-1 signal on tumor-infiltrating CD8+ T cells during ICB with combined anti-PD-1 and anti-CTLA-4 antibodies.
Potential solution
In vivo blockade with anti-PD-1 antibody could interfere with PD-1 staining (refer to anti-PD-1 and anti-CTLA-4 treatment section). It is critical to use compatible antibody clones to detect PD-1-expressing cells despite the presence of anti-PD-1 antibodies in vivo. We recommend the following antibody clones: RMP1-14 for PD-1 in vivo blockade and 29F.1A12 for ex vivo PD-1 staining. This anti-PD-1 antibody combination has been validated in various murine tumor models (Asrir et al., 2022; Francis et al., 2020; Polesso et al., 2021).
Problem 5
No detection of intracellular staining.
Potential solution
Ensure that cells are incubated with antibodies targeting intracellular markers in Permeabilization buffer and not FACS buffer (refer to steps 26–30). For challenging intracellular targets, we recommend incubating cells with antibodies at 18°C–22°C for 1 h. Alternatively, homemade fixation/permeabilization solutions or fixation/permeabilization kits from other manufacturers can be tested.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jean-Philippe Girard (jean-philippe.girard@ipbs.fr).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by grants from Fondation ARC pour la Recherche sur le Cancer (SL220110603471, PGA 120150202411, and PGA1 RF20180206911 to J.-P.G.), Institut National du Cancer (INCa_2013-098 and INCa_2017-155 to J.-P.G.), Agence Nationale de la Recherche (ANR-12-BSV1-0006-01 to J.-P.G.), IDEX UNITI (ATS 2014 to J.-P.G.), and Laboratoire d’Excellence Toulouse Cancer (LABEX TOUCAN). C.T., J.C., R.L., L.B., and E.V. were supported by fellowships from LABEX TOUCAN, Fondation ARC (DOC20170505820), French Ministry of Research, and Fondation pour la Recherche Médicale (FRM FDT20160435636, ECO201806006827, ECO202006011469, and FDT202106012889).
We thank L. Martinet for initial observations and members of the Girard laboratory for helpful discussions. We are grateful to Genotoul core facilities, ANEXPLO-IPBS, and Cytometry TRI-IPBS. We acknowledge the help of E. Näser for cytometry. We thank R. Schreiber and Z. Werb for providing tumor cell lines. The graphical abstract was created with BioRender.com.
Author contributions
J.-P.G. conceived the study, supervised the work, and analyzed data. L.B., E.V., A.A., C.T., J.C., R.L., D.T., S.B., K.V., F.L., E.B., and N.O. contributed to the establishment of murine tumor models, immune checkpoint blockade treatment, and tumor digestion procedures. L.B., A.A., C.T., and J.C. developed the staining panels. L.B., E.V., and D.T. performed sample collection, processing, staining, and flow cytometry analysis of data presented in the manuscript. L.B. and E.V. designed the figures. L.B. wrote the manuscript. E.V., N.O., and J.-P.G. edited the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Lucas Blanchard, Email: lucas.blanchard@ipbs.fr.
Jean-Philippe Girard, Email: jean-philippe.girard@ipbs.fr.
Data and code availability
This study did not generate/analyze datasets/code.
References
- Asrir A., Tardiveau C., Coudert J., Laffont R., Blanchard L., Bellard E., Veerman K., Bettini S., Lafouresse F., Vina E., et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell. 2022;40:318–334.e9. doi: 10.1016/j.ccell.2022.01.002. [DOI] [PubMed] [Google Scholar]
- Blanchard L., Girard J.-P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis. 2021;24:719–753. doi: 10.1007/s10456-021-09792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D.M., Manspeaker M.P., Schudel A., Sestito L.F., O’Melia M.J., Kissick H.T., Pollack B.P., Waller E.K., Thomas S.N. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci. Transl. Med. 2020;12:eaay3575. doi: 10.1126/scitranslmed.aay3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J.-P., Moussion C., Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- Khan O., Giles J.R., McDonald S., Manne S., Ngiow S.F., Patel K.P., Werner M.T., Huang A.C., Alexander K.A., Wu J.E., et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.C., Sen D.R., Al Abosy R., Bi K., Virkud Y.V., LaFleur M.W., Yates K.B., Lako A., Felt K., Naik G.S., et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesso F., Munks M.W., Rott K.H., Smart S., Hill A.B., Moran A.E. PD-1-specific "Blocking" antibodies that deplete PD-1+ T cells present an inconvenient variable in preclinical immunotherapy experiments. Eur. J. Immunol. 2021;51:1473–1481. doi: 10.1002/eji.202048960. [DOI] [PubMed] [Google Scholar]
- Siddiqui I., Schaeuble K., Chennupati V., Fuertes Marraco S.A., Calderon-Copete S., Pais Ferreira D., Carmona S.J., Scarpellino L., Gfeller D., Pradervand S., et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50:195–211.e10. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- Scott A.C., Dündar F., Zumbo P., Chandran S.S., Klebanoff C.A., Shakiba M., Trivedi P., Menocal L., Appleby H., Camara S., et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.