Summary
The combined use of transcranial magnetic stimulation (TMS), electroencephalogram (EEG), and behavioral performance allows investigation of causal relationships between neural markers and their functional relevance across a number of perceptual and cognitive processes. Here, we present a protocol for combining and applying these techniques on human subjects. We describe correlation approach and causal approach to disentangle the role of different oscillatory parameters, namely alpha frequency and amplitude that control for accuracy and metacognitive abilities, respectively, in a visual detection task.
For complete details on the use and execution of this protocol, please refer to Di Gregorio et al. (2022).
Subject areas: Clinical Protocol, Neuroscience, Cognitive Neuroscience, Behavior
Graphical abstract
Highlights
-
•
EEG-behavior correlations to frame hypotheses on how the brain shapes behavior
-
•
Combined TMS-EEG-behavior to establish causal brain-behavior relationships
-
•
Tune alpha frequency and amplitude to shape perceptual accuracy and metacognition
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The combined use of transcranial magnetic stimulation (TMS), electroencephalogram (EEG), and behavioral performance allows investigation of causal relationships between neural markers and their functional relevance across a number of perceptual and cognitive processes. Here, we present a protocol for combining and applying these techniques on human subjects. We describe correlation approach and causal approach to disentangle the role of different oscillatory parameters, namely alpha frequency and amplitude that control for accuracy and metacognitive abilities, respectively, in a visual detection task.
Before you begin
This study protocol was conducted in accordance with the Declaration of Helsinki and was approved by the bioethics committee of the University of Bologna.
Correlational approach (experiment 1)
Forming hypothesis: Role of alpha oscillations in conscious perception
Alpha oscillatory activity (range 7–13 Hz; Gallotto et al., 2017; Owen, 2013) in the human brain plays an active role in visual perception (Di Gregorio et al., 2022; Klimesch et al., 2007; Palva and Palva, 2007; Samaha and Postle, 2015; VanRullen, 2016). In particular, recent studies have reported that the peak of the individual alpha-frequency predicts the objective perceptual performance with faster alpha oscillations resulting in higher temporal resolution and more accurate perceptual experience (Cecere et al., 2015; Cooke et al., 2019; Mierau et al., 2017; Migliorati et al., 2020; Noguchi, 2022; Ronconi et al., 2022; Samaha and Postle, 2015; Venskus et al., 2021; Venskus and Hughes, 2021; Wutz et al., 2018). Instead, the pre-stimulus alpha-amplitude has been shown to account for a momentary level of cortical excitability (Romei et al., 2008) and to predict subjective confidence in response to visual stimuli (Benwell et al., 2017; Iemi et al., 2017; Samaha et al., 2017). Specifically, lower levels of alpha amplitude seem to account for increased subjective confidence and metacognitive abilities (i.e., the sensitivity of subjective confidence judgments to distinguish between correct and incorrect decisions), without affecting the level of accuracy of the response (Di Gregorio et al., 2022).
Experimental setting preparation
Timing: approximately 3 weeks
-
1.Program the behavioral tasks:
-
a.Stimulus creation (for instance, by using Microsoft PowerPoint, 2016):
-
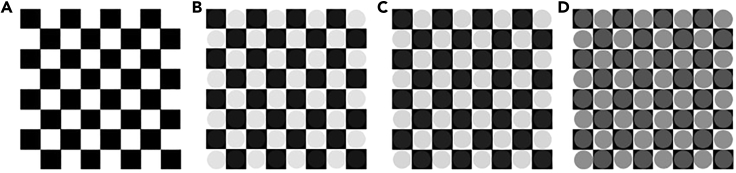
i.Create 8 × 8 black-and-white checkerboards (single square side = 0.5 cm; checkerboard height/width = 4 cm, see Figure 1);
-
ii.Create 64 iso-luminant gray circles (diameter = 0.5 cm) with variations in the RGB parameters (28/227, 32/223, 36/219, 40/215, 44/211, 48/207 and 100/155) superimposed to the checkerboard. Contrast ratios are set between circles and the black-and-white checkerboards obtaining contrasts that can be classified as Low Contrast Stimulus (minimum contrast for circles with RGB = 28/227) or High Contrast Stimulus (maximum contrast for circles with RGB = 100/155);
-
iii.Create two stimuli for every contrast ratio (one for the left hemifield and one for the right hemifield) plus two checkerboards without inner gray circles (i.e., catch stimuli);
-
iv.Save them as images in BMP file format (total number = 16 stimuli).
-
i.
-
b.Program the titration task needed to estimate the individual perceptual threshold in E-prime Software:
-
i.Open E-prime and create 252 trials (divided into 3 blocks) with an equal number of catch stimuli and stimuli containing gray circles with an equal number of stimuli presented on the lower left and lower right quadrant (every contrast is tested twelve times for each single hemifield);
-
ii.Create a first screen containing clear written instructions explaining the task to the participants;
-
iii.Set a screen with a gray background and a black central fixation cross of a jittered duration between 3000 and 5000 ms;
-
iv.Set the stimulus presentation duration to 60 ms. Stimuli are presented only in the lower part of the screen at 4.1°/3.7° eccentricity (horizontal/vertical). The order of appearance of the different stimuli must be randomized;
-
v.Set the spacebar as the only response input allowed;
-
vi.Set a screen with a black central fixation cross of 1500 ms duration.
-
i.
-
c.Program the visual detection task in E-prime Software:
-
i.In the stimuli list insert only two catch stimuli and two target stimuli (those identified during the titration with the contrast values yielding detection thresholds of 50% in the left and right hemifields);
-
ii.Create a first screen containing clear written instructions explaining the task to the participants;
-
iii.Set a screen with a gray background and a black central fixation cross with a duration of 2000 ms;
-
iv.Set a gray screen with a neutral white cue (with the shape of an X created by rotating the fixation cross by 45 degrees) for a time period jittered between 2000 and 3000 ms;
-
v.Set the stimulus appearance with a duration of 60 ms;
-
vi.Randomize stimuli presentation and set an equal probability of stimuli appearing in the lower left and right hemifield and an equal probability of catch or target stimuli;
-
vii.Set a screen with a black central fixation cross of 2000 ms;
-
viii.Create a confidence rating screen asking participants to rate how confident they are about their percept on a 4-point Likert scale (1 = “no confident at all”; 2 = little confident; 3 = “quite confident”; 4 = “highly confident”);
-
ix.Enter the spacebar and the keys 1-2-3-4 of the keyboard as allowed response inputs;
-
x.Set 5 blocks with 60 trials each (total trial number = 300). The 60 trials consist of: 15 repetitions of the target stimulus in the lower left hemifield, 15 repetitions of the target stimulus in the lower right hemifield, 15 repetitions of the catch stimulus in the lower left hemifield, 15 repetitions of the catch stimulus in the lower right hemifield.
-
i.
-
a.
-
2.
Sample size estimation. The sample size can be estimated based on previous literature. Perform a post-hoc power analysis to ensure the correctness of the sample size.
Note: Alternatively, you can perform a power analysis to estimate the number of participants needed based on the desirable power, alpha and effect size.
-
3.Prepare the experimental room:
-
a.Choose an optimal room with the specific aim to carefully record noise-reduced, high quality EEG signal and reduce environmental electromagnetic noises (e.g., Faraday cage).
-
b.Prepare two computers:
-
i.A CRT monitor with high refresh rate (>85 Hz) resolution connected to a computer running E-prime software for stimulus presentation;
-
ii.A computer running an EEG recording software (in our case, we used BrainVision Recorder).
-
i.
-
c.Connect the EEG amplifier system to the computer running the EEG recording software (BrainVision Recorder) via the USB cable and to the computer presenting the visual stimuli on Eprime via a parallel port (in order to receive triggers from E-prime).Note: This set-up depends on the recording system used and the presence of a trigger box.
-
d.Create a new workspace with 64 electrodes (Fp1,Fp2,AF3,AF4,AF7,AF8,F1,F2,F3,F4,F7,F8,FC1,FC2,FC3,FC4,FC5,FC6,FT7,FT8,C1,C2,C3,C4,C5,C6,T7,T8,CP1,CP2,CP3,CP4,CP5,CP6,TP7,TP8,P1,P2,P3,P4,P5,P6,P7,P8,PO3,PO4,PO7,PO8,O1,O2,Fpz,AFz,Fz,FCz,Cz,CPz,Pz,POz,Oz,HEOG,VEOG,M2, reference and ground), set a band-pass filter of 0.01–100 Hz and a sampling rate of 1000 Hz.
-
e.Ensure a distance between the monitor and the participant eyes of 57 cm.Optional: A chin rest is desirable to keep this distance constant throughout the experiment.
-
a.
-
4.
Prepare consent forms and privacy policy.
CRITICAL: Informed consents must clearly state the purposes and carrying out methods of the experiment, the risks and benefits expected from the subject's participation, indicate the person in charge of the study and the intended use of their personal data.
CRITICAL: Informed consent must be presented to and approved by the local ethics committee.
-
5.
Prepare a database in which to collect the anamnestic data of the subjects participating in the experiment.
CRITICAL: The anamnestic data must be separated from the performance data of the participants. Each subject must be associated with an alphanumeric code that does not allow tracing the identity of that person. Files containing sensitive data must be password protected (or treated differently depending on the privacy legislation in your country).
-
6.
Prepare sheets for behavioral data scoring. Prepare a template on Excel with the formulas already set on the basis of the e-prime columns output. This allows you to score faster in the testing phase and to avoid score errors.
-
7.
Prepare data analysis pipelines.
-
8.
For experiment 2 and 3 prepare a screening questionnaire to exclude the presence of contraindications to the use of transcranial magnetic stimulation (TMS) according to Rossi et al. (2011).
Figure 1.
Examples of stimuli of different contrasts
(A) Catch stimulus.
(B) Low contrast stimulus (RGB contrasts: 30/225).
(C) High contrast stimulus (RGB contrasts:40/215).
(D) Maximum contrast stimulus (RGB contrasts:100/155). Adapted from Di Gregorio et al. (2022).
Piloting
Timing: approximately 2 weeks
-
9.Recruit a few subjects and run the experiment to test whether changes in the setting are needed before starting the actual one:
-
a.Check that there are no errors in the task. Ensure that:
-
i.task instructions are clear for participants;
-
ii.the correct number of stimuli is present;
-
iii.the randomization is correct;
-
iv.the stimuli presentation timing is correct;
-
v.the task is not strenuous for the subjects.
-
i.
-
b.Optimize EEG montage and recording:
-
i.check that you have all the necessary materials and that everything works correctly;
-
ii.check that there are no problems with the signal recorded;
-
iii.check that the triggers are present and in the correct number.
-
i.
-
c.Test analysis pipelines for possible errors, bugs and computational feasibility:
-
i.estimates the time required for the analysis of the perceptual threshold;
-
ii.check that the behavioral data scoring sheet is correct and optimize it.
-
i.
-
a.
CRITICAL: Do not use small scale pilot data to estimate effect size in a priori power analysis (Albers and Lakens, 2018).
Causal approach (experiment 2 & 3)
Understanding the entrainment protocol
Oscillatory entrainment by rhythmic, online TMS can be achieved by fine-tuning the frequency of repeated stimulation pulses to target underlying oscillatory activity. Specifically, if we stimulate with one-pulse an area that is spontaneously oscillating at alpha (in our case, visual occipital areas) we will induce an alpha oscillation (Rosanova et al., 2009; Rotenberg et al., 2014). By pacing additional pulses at exactly the same duration of an individual alpha cycle, we would further enhance the synchronization of the induced oscillation with the stimulation train (Thut et al., 2011; see also Romei, et al., 2016 for effects of entrainment observed over motor areas at another frequency band). Importantly, the entrainment of brain oscillations is demonstrated to be more effective when the frequency of stimulation closely matches the natural oscillatory frequency. On the other hand, slight offsets of frequency can drive natural oscillator toward slower or faster pace and, as demonstrated by tACS-EEG studies (Helfrich et al., 2014; Minami and Amano, 2017), thus also inducing changes is the speed of natural rhythms, which may lead to important behavioral modulations (Cecere et al., 2015; Zhang et al., 2019). Therefore, by adjusting the timing between pulses based on individual natural rhythms, we can selectively manipulate the amplitude or the speed of brain rhythms. More specifically, to induce changes in the oscillatory-frequency cycle length, rhythmic-TMS can be applied at a slower or faster pace, relative to a participant’s individual oscillatory-frequency. To selectively modulate oscillatory-amplitude, the frequency of the rhythmic-TMS pulse trains should match the intrinsic individual oscillatory-frequency of the participant, thus enhancing the synchronization of neural firing and phase alignment without influencing the speed of oscillatory activity (e.g., Helfrich et al., 2014).
There are several considerations to be made before running a TMS study:
-
10.
Consider the to-be-stimulated area.
Note: In our case, occipital visual areas have been chosen, as it is well known that visual areas of occipital cortex are involved in our task (identification of changes in a simple peri-threshold stimulus) (Lee et al., 2000).
Note: Previous EEG results (Experiment 1) have confirmed that EEG correlates of perception are indeed present over occipital electrodes (Di Gregorio et al., 2022).
-
11.
Consider the exact timing of stimulation.
CRITICAL: Given the short-lasting effects of the entrainment protocols (Romei, et al., 2016; Thut et al., 2011), that ends a few hundred milliseconds following the stimulation, it is only reasonable to apply stimulation right before stimulus appearance.
Note: In our case, the last stimulation pulse coincided with the presentation of the stimulus (Experiment 2), or with the appearance of the confidence prompt (Experiment 3).
-
12.
Consider the choice of the coil and its exact orientation (see Thut et al., 2011).
CRITICAL: Optimal coil type will depend on the location of the target brain area and the spatial resolution of the to-be-induced magnetic field.
Note: As the visual areas of the occipital cortex are on-surface areas, our coil of choice is a figure-of-eight coil, demonstrated to have highest spatial resolution among other coil shapes. Secondly, the handle of the coil is oriented perpendicular to the medial plane of the subject’s head (latero-medial current direction): an orientation demonstrated to have maximal efficiency for stimulation of visual neurons over occipital areas (Kammer et al., 2001).
-
13.
Consider stimulation intensity.
Note: Most studies stimulating posterior visual pathways are titrated to phosphene threshold of the participant, on average roughly corresponding to 60% of MSO (Maximal Stimulator Output). In this study, given the length of the experimental procedure, we have used a fixed intensity of 60% of the MSO (Bestmann et al., 2007; Cattaneo et al., 2009; Romei et al., 2012; Silvanto and Muggleton, 2008).
-
14.
Consider the most appropriate control conditions.
CRITICAL: Previous studies that implemented entrainment protocols often used different types of sham stimulation as well as arrhythmic stimulation of the same area, that provides same number of pulses of equal intensity, but with jittered timing between them, instead of fixed, frequency-based timing of the experimental conditions (Notbohm and Herrmann, 2016; Thut et al., 2011).
-
15.
Safety considerations.
CRITICAL: Safety risks, such as seizures, headache and hearing problems must be minimized by carefully selecting participants through screening questionnaires and by strictly following current safety and ethical guidelines (Rossi et al., 2011, 2021).
Concurrent TMS-EEG recording
As already mentioned, TMS is based on the principle of electromagnetic induction: thus, in the same manner as it induces an electric field in the brain, it also induced these fields for every electric circuit in the coil vicinity, including peripheral nerves, EEG electrodes and amplifiers. Therefore, an online TMS protocol with concurrent EEG recordings can substantially affect recorded EEG signal in the form of TMS-induced electric artifacts.
In order to minimize their influence, some precautionary steps have to be taken both before, during and after signal recording:
-
16.
Suitable amplifiers (i.e., sample and hold solution, DC amplifiers, low slow-rate amplifiers) should be chosen in order to avoid amplifier saturation.
-
17.
During subject preparation, electrodes should preserve a good contact with the skin and electrode impedance should be kept as low as possible (< 5 kΩ), while sampling rate of the signal during recording is advised to be set to at least 5000 samples per second.
-
18.
During the recording, electrode impedances and coil position and orientation should be kept as stable as possible in order to have reproducible artifacts: a condition necessary for their offline removal (Rotenberg et al., 2014).
-
19.
During post-processing, use pipelines for TMS artifact removal which are already established and successfully implemented (Atluri et al., 2016; Mutanen et al., 2018; Rogasch et al., 2017; Wu et al., 2018) and proven to be efficient and reliable.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Preprocessed Data | Di Gregorio, Trajkovic et al. (2022) | Open Science Framework: https://osf.io/e4bnj/ |
| Experimental models: Organisms/strains | ||
| 92 human subjects (with normal or corrected-to-normal vision and meeting TMS safety criteria; age range: 19–31 years; 49 women) | Di Gregorio, Trajkovic et al. (2022) | N/A |
| Software and algorithms | ||
| Brain Vision recorder software | Brain Products | https://brainvision.com/ |
| E-prime | Psychology Software Tools, Inc. | https://pstnet.com/products/e-prime/ |
| RStudio v1.2.5019 | RStudio, Inc. | https://www.rstudio.com/ |
| SPSS v23 | StatSoft, Inc. | https://www.ibm.com/products/spss-statistics |
| MATLAB, v2018b | MathWorks | https://.mathworks.com/ |
| EEGLAB v13.0.1 toolbox | Delorme and Makeig (2004) | https://sccn.ucsd.edu/eeglab |
| Hierarchical meta-d’ model (HMeta-d) | Fleming (2017) | https://github.com/metacoglab/HMeta-d |
| TESA v1.1.1 toolbox | Rogasch et al. (2017) | https://nigelrogasch.github.io/TESA/ |
| Other | ||
| 64 Ag/AgCl electrode cap for EEG & TMS co-registration | Brain Products | https://brainvision.com/ |
| CRT monitor (100 Hz refresh rate) | N/A | N/A |
| Microsoft PowerPoint | Microsoft | https://www.microsoft.com/ |
| Magstim Rapid Transcranial Magnetic Stimulator | Magstim Company | https://www.magstim.com/ |
| 70 mm figure-of-eight coil | Magstim Company | https://www.magstim.com/ |
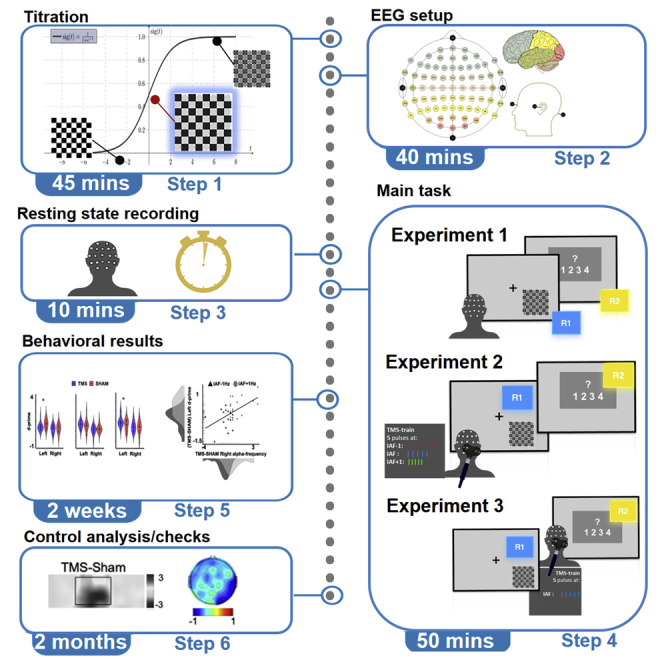
Step-by-step method details
Correlational approach (experiment 1): Titration session
Timing: 45 min
The titration session aims to determine the perceptual threshold of the participant. The threshold is defined as the contrast value corresponding to 50% of correct detection performance for each individual.
-
1.
Welcome the subject and have the informed consent and the privacy policy read, explained, and signed.
-
2.Explain the instructions to the participant:
-
a.Ask the participant to keep the gaze on the central fixation cross for the entire duration of the activity;
-
b.Instruct the participant to answer by pressing the spacebar whenever they perceive gray circles within the checkerboard;
-
c.Instruct the participant to withhold the response whenever they perceive an empty checkerboard;
-
d.Provide the participant with a sample of the stimuli that will be presented;
-
e.Ask the participant to place the right hand in the position to promptly press the spacebar.
-
a.
-
3.
Make sure the instructions are clear to the participant and clarify any questions.
-
4.
Make sure that the participant is in a comfortable position.
-
5.
Check the distance between the participant’s eyes and the screen (57 cm) at the beginning of each block.
-
6.
Make the room light dimmed.
-
7.
Run the task.
Pause point: Encourage participants to take breaks in between blocks.
Note: At the end of each block make sure that the participant has not encountered any problem in carrying out the task.
Correlational approach (experiment 1): Subject preparation for EEG setup
Timing: 30–45 min
This section describes in detail how to perform EEG montage and setup correctly and efficiently, before starting with the EEG registration.
-
8.Cap placement:
-
a.Center the cap at the sagittal plane using the distance between the inion (the bony protrusion at the back of the skull) and nasion (the bony ridge between the eyebrows);
-
b.Center the cap at the coronal plane using the distance between the ears.
-
a.
-
9.Reducing electrode impedance:
-
a.Skin preparation: clean the skin at the electrode-skin contact by scrubbing with alcohol and a cotton swab;
-
b.Apply and distribute the electrode gel with a cotton swab until desired impedance is reached for all of the electrodes (ground, reference and EOG electrodes: < 5 kΩ; remaining electrodes: < 10 kΩ);
-
c.Visually inspect the signal for any other obvious but otherwise undetected problem in the EEG signal.
-
a.
CRITICAL: Regularly check and adjust electrode impedance during the whole experiment.
Correlational approach (experiment 1): Estimating the individual perceptual threshold
Timing: 15 min
This section describes how to establish individual stimuli threshold for every participant, based on the titration session and prior to starting with the main experimental task.
-
10.
Insert the E-prime output scores containing the participant behavioral performance in the prepared scoring sheet.
-
11.Estimate the individual performance for each iso-luminant contrast taking into account also the false alarm rate to calculate a sigmoid function:
-
a.Calculate accuracy percentage separately for each contrast and for the left and right stimulus trials by dividing the number of correct responses for the total number of stimuli with that contrast and multiplying it by 100;
-
b.Calculate false alarms percentage separately for the left and right stimulus trials, dividing the number of false alarms for the total number of catch stimuli of that hemifield and multiplying it by 100;
-
c.Create one column for each side containing the percentage of false alarms changing its number sign in negative and the accuracy percentages.
-
a.
-
12.Calculate the sigmoid function separately for the left and right stimulus trials using the formula:(x = contrast value, c = inflection point of the curve, d = slope of the sigmoid);
-
a.Select the corresponding inflection point as the bias-adjusted threshold (y), and use it for the selection of stimuli that will be presented during the main task.
-
a.
-
13.
Enter the two values identified on the sheet containing participants’ data in order to store this information.
Correlational approach (experiment 1): Main experimental task
Timing: 75 min
This section describes the main experimental procedure for Experiment 1. Participants perform a primary visual detection task followed by a secondary confidence task. For the visual detection task, present only two types of stimuli: catch stimuli (i.e., one empty checkerboard per hemifield) and target stimuli (i.e., one checkerboard containing the gray circles’ contrast value corresponding to the individual perceptual threshold per hemifield). Akin to the titration task, participants press the spacebar whenever they perceive the target stimulus and withhold the response whenever no target stimulus is perceived (i.e., when catch stimuli are presented). In addition, following their primary response, they provide a confidence rating on a Likert scale with 4 levels of confidence regarding their percept.
-
14.
Open the task in E-prime and enter in the list of stimuli to present during task execution, the two values identified as threshold values for the left and right hemifields target stimulus.
-
15.Explain instructions:
-
a.Explain the primary visual detection task;
-
b.Explain that after stimulus presentation the participant will be asked how confident they are about their perception with a value from 1 to 4, ranging from low confidence to high confidence, pressing the corresponding number on the keyboard;
-
c.Before starting the task, instruct the participant to place the right hand in a comfortable position to promptly press the spacebar and the left hand in correspondence to the four numbers necessary to provide the confidence rating;
-
d.Again, ask the participant to maintain the gaze on the fixation cross while reducing the brightness of the room.
-
a.
-
16.EEG recording:
-
a.Check the quality of the signal;
-
b.Instruct the participant about the possible artifacts that can affect the signal and suggest tips that may help maintain artifacts to a minimum throughout the recording;
-
c.Reiterate your request to the participant to stay as relaxed as possible while recording their EEG signal;
-
d.Start the recording.
-
a.
-
17.
Run the E-prime script to launch the stimulus presentation and data collection during the visual detection task.
-
18.
Once started, double-check that your set up is up and running by checking for the effective presence of triggers on the ongoing EEG recording.
Note: Start the EEG recording at least ten seconds before the start of the task and end at least ten seconds after the end of the behavioral task, to allow for the subsequent application of filters during the signal processing.
Correlational approach (experiment 1): Resting state
Timing: 2 min
-
19.
After the end of the task, record a eyes-closed 2 min resting-state EEG.
Correlational approach (experiment 1): Computing task performance
Timing: 1 h for each participant
This step enables the estimation of behavioral performance in the visual detection task and in the confidence task. Specifically, calculate accuracy rate (i.e., the percentage of correct responses) and perceptual sensitivity, (i.e., the d’ sensitivity index). The latter considers both correct responses and false alarms, discounting any potential effect of response bias (Green and Swets, 1966). Moreover, estimate both confidence rate (i.e., the mean of confidence reports) and metacognitive performance of each participant (i.e., the meta d’ measure (Maniscalco and Lau, 2012). Meta d' quantifies the accuracy of confidence ratings to discriminate objectively correct from erroneous responses.
-
20.Behavioral analyses:
-
a.Accuracy rate:
-
i.Sort trials in correct and errors trials. Correct trials consist of correctly detected target trials (i.e., hits) and correctly detected catch trials (i.e., correct rejections). Error trials consist of misses after target trials and false alarms after catch trials;
-
ii.Calculate percentage of correct trials for each participant.
-
i.
-
b.Perceptual sensitivity (d’ score):
-
i.Calculate z-scores of Hit rate (i.e., zH) and false alarms (i.e., zFA);
-
ii.d’ is the difference d’=z(H) – z(FA).
-
i.
-
c.Confidence rate:
-
i.Report for all correct trials the corresponding confidence rating;
-
ii.Calculate the mean of confidence rating for each participant.
-
i.
-
d.Metacognitive performance (meta d’ score):
-
i.Merge low confidence ratings (i.e., confidence responses 1 and 2) and high confidence ratings (confidence responses 3 and 4) trials;
-
ii.Create an array of participants type 2 hit rate (percentage of high confidence correct trials) and type 2 false alarm rate (percentage of high confidence error trials);
-
iii.Calculate the criterion “c”, where c = -.5∗ [z(H) + z(FA)].
-
i.
-
a.
Note: Estimate meta d’ using the function fit_metad_SSE on MatLab.
Correlational approach (experiment 1): Electrophysiological processing and analysis
Timing: 3 h for each participant
This step enables looking at electrophysiological correlates of task performance. Specifically, look at the relationship between individual alpha frequency (IAF) and alpha amplitude for perceptual accuracy and sensitivity and for confidence rating and metacognitive performance.
Note: Perform EEG analyses using EEGLab.
-
21.EEG preprocessing steps:
-
a.Re-reference data to the average of all electrodes;
-
b.Edit channel locations accordingly to a standard template (i.e., standard-10-5-cap385);
-
c.Downsample the data from 1000 Hz to 500 Hz;
-
d.Apply pass-band filter (0.5–30 Hz);
-
e.Data epoching around stimulus onset (-1500 milliseconds–2000 milliseconds);
-
f.Exclude from EEG data artifacts-contaminated epochs using the pop_autorej and pop_jointprobab in EEGLab;
-
g.Exclude EOG epochs with amplitude > 800 μv;
-
h.Correct remaining artifacts with the linear regression procedure using pop_lms_regression function in EEGLab;
-
i.Sort EEG epochs according to the stimulus location (i.e., right visual field RVF and left visual field LVF stimuli);
-
j.Copy and flip EEG data, for RVF-stimuli epochs, from the contralateral (left) electrodes to right-sided electrodes. In this way, all contralateral activity is on one side, which is conventionally defined to be the right;
-
k.Sort EEG epochs accordingly to the behavioral condition (i.e., correct vs error trials; high vs low confidence trials).
-
a.
CRITICAL: Apply the described procedure to all EEG data using the same parameters.
-
22.EEG analysis:
-
a.Calculate pre-stimulus IAF. IAF is defined as the local maximum power within the alpha frequency (7–13 Hz) range:
-
i.Set the baseline between -1500 and -1000 ms;
-
ii.Apply fast Fourier transformation (FFT) (MATLAB function spectopo, frequency resolution: 0.166 Hz) and set the analysis window in the pre-stimulus period (-1000 to 0 ms);
-
iii.Normalize power by z-score decibel (dB=10∗log10[-power/baseline]) transformation at each frequency;
-
iv.Identify in the spectrogram the contralateral electrode with larger power and clear peak in the alpha band;
-
v.Individual alpha-frequency is the local maximum power within the frequency range 7–13 Hz (i.e., alpha peak).
-
i.
-
b.Calculate pre-stimulus alpha-amplitude in the time frequency data:
-
i.Re-epoch data around stimulus onset (-2000 ms–2000 ms);Note: Longer epochs prevent edge artifacts from contaminating time frequency power in the time windows of interest.
-
ii.Extract time-frequency data using a complex sinusoidal wavelet convolution procedure (between 2 and 25 cycles per wavelet, linearly increasing across 50 linear-spaced frequencies from 2.0 Hz to 50.0 Hz) with the newtimef function in EEGLab;
-
iii.Normalize power by decibel (dB=10∗log10[-power/baseline]) transformation at each frequency, using a single trial baseline between -1000 and -500 ms preceding stimulus onset;Note: Use this long baseline window to increase the signal-to-noise ratio during the baseline period.
-
iv.Select the contralateral electrode with larger power in the alpha band;
-
v.Calculate mean alpha (7–13 Hz) amplitude in pre-stimulus-stimulus interval (-500 to 0 ms).
-
i.
-
c.Calculate post-stimulus alpha-amplitude in the time frequency data:
-
i.Time-frequency data are extracted as for step b (see above);
-
ii.Calculate mean alpha (7–13 Hz) amplitude in post-stimulus interval (0–900 ms).Note: Perform all analyses separately for each participant and condition.Note: Use parieto-occipital electrodes in the contralateral hemisphere, along with analogous electrodes in the ipsilateral hemisphere for all of the analyses.
-
i.
-
a.
-
23.Statistical analysis:
-
a.Within participants:
-
i.Compare parameters of alpha activity between the two HEMISPHERES (contralateral vs ipsilateral electrode) and for accuracy (correct vs error trials) and confidence (high vs low confidence trials) in 2 × 2 repeated measures mixed-model ANOVAs;
-
ii.Test differences between conditions by two-tailed t-test (planned comparisons).
-
i.
-
b.Between participants analyses (perceptual sensitivity):
-
i.Divide participants in two numerically equivalent groups using the median split of the d’ scores (high vs low d’);
-
ii.Compare IAF between groups using one-tailed unpaired two-sample t-test.
-
i.
-
c.Between participants analyses (metacognitive performance):
-
i.Divide participants in two numerically equivalent groups using the median split of the meta d’ scores (high vs low meta d’);
-
ii.Compare post-stimulus alpha amplitude between groups using one-tailed unpaired two-sample t-test. The relation between pre-stimulus alpha activity and perception was already reported in previous studies (Samaha et al., 2020; Samaha and Postle, 2015). In particular, alpha-amplitude suppression can predict higher level of subjective confidence in response to visual stimuli (Benwell et al., 2017; Iemi et al., 2017; Samaha et al., 2017) while faster alpha-frequency was related to better objective perceptual accuracy (Samaha and Postle, 2015). In this sense, we expected, in the between participants analyses, that alpha-amplitude suppression accounted for better individual metacognitive performance and faster alpha-frequency accounted for better objective accuracy. Accordingly, we used one-tailed t-test for these analyses.
-
i.
-
a.
CRITICAL: Electrode selection requires visual inspection of the power-spectrum of all parieto-occipital electrodes. A double-blind check is necessary.
Causal approach (experiment 2 & 3): Titration session
Timing: 45 min
Run a titration session before the main experimental session in order to set stimuli contrast ratios corresponding to each individual’s 50% perceptual threshold. Procedure should be the same as in Experiment 1.
-
24.
Run a titration session.
Causal approach (experiment 2 & 3): Subject preparation for EEG setup
Timing: 30–45 min
This section describes EEG montage. Careful and homogenous EEG setup across participants is especially important in this protocol due to online application of rhythmic TMS. In order to minimize the impact of TMS-induced artifact on the EEG signal, here-described steps have to be carefully followed in order to reduce the duration of the artifact and its trial-by trial variability.
-
25.Cap placement: The electrode positions are used as landmarks to localize the stimulation site, thus particular care should be taken when centering the EEG cap:
-
a.Center the cap at the sagittal plane by using the distance between the inion (the bony protrusion at the back of the skull) and nasion (the bony ridge between the eyebrows);
-
b.Center the cap at the coronal plane by using the distance between the earlobes.
-
a.
-
26.Reducing electrode impedance:
-
a.Skin preparation: clean the skin at the electrode-skin contact by scrubbing with alcohol and a cotton swab;
-
b.Apply and distribute electrode gel by a cotton swab until desired impedance is reached for all of the electrodes (ground, reference and EOG electrodes: < 5 kΩ; remaining electrodes: < 10 kΩ).
-
a.
CRITICAL: Regularly check and adjust electrode impedance during the whole experiment.
Causal approach (experiment 2 & 3): Resting-state EEG recording and IAF estimation
Timing: 10 min
This section describes the EEG resting-state recording. For the Experiment 2 and 3, resting-state EEG should be recorded and analyzed at once in order to titrate stimulation parameters for every participant. This step is necessary to determine the exact time lag between the pulses in a 5-pulse train, in order to selectively and efficiently change the speed or amplitude of alpha oscillatory activity of the participant.
-
27.
Resting state eyes-closed recording for 3 min: Have participants comfortably seated in a dimmed-lit room with their eyes closed while recording their EEG activity.
-
28.
Resting state eyes-open recording for 3 min: Have participants comfortably seated in a dimmed-lit room with gaze on a fixation cross on a screen while recording their EEG activity.
Note: Order of eyes-closed and eyes-open resting state recordings should be randomized between subjects.
Pause point: Participants could take a break after finishing the resting-state session.
-
29.Estimation of individual alpha frequency peak from eyes-open resting state:
-
a.Pre-processing of the signal:
-
i.Re-referencing to average;
-
ii.Re-sampling to 500 Hz;
-
iii.Applying a low-pass (0.5 Hz) and high-pass (50 Hz) filter.
-
i.
-
b.Estimating IAF:
-
i.In line with Experiment 1 (showing a local alpha power maxima over O2) and previous studies, calculate alpha-frequency from the O2 electrode, over which rhythmic-TMS is subsequently applied;
-
ii.Apply fast Fourier transformation (FFT), and calculate power spectra. IAF is defined as the local maximum power within the alpha frequency range (7–13 Hz);
-
iii.Double-check the peak visually.
-
i.
-
a.
Note: If the clear peak in the alpha range is not visible during eyes-open resting-state on the O2 electrode, consider adjacent electrodes (PO8 and PO4).
Note: If the clear peak in the alpha range is not visible during eyes-open resting-state on posterior electrodes, consider using eyes-closed resting-state for estimating IAF.
Causal approach (experiment 2 & 3): Conducting main experimental task
Timing: 50 min
This section describes the main experimental procedure for Experiment 2 and 3. Please note that stimuli and tasks in Experiment 2 and 3 are the same as those described for Experiment 1, with the main difference being the active manipulation of alpha activity via the TMS entrainment protocol. Specifically, in Experiment 2, a five-pulses TMS train is delivered over the right occipital cortex (O2) right before stimulus presentation (where the last TMS pulse coincided with onset of the stimulus appearance), and rhythmic-TMS pulse trains could occur at three different frequencies: at the individual alpha-frequency of the participant to manipulate pre-stimulus alpha-amplitude (IAF group); at 1 Hz lower than the individual alpha-frequency (IAF-1 Hz group) to slow-down pre-stimulus alpha-frequency; or at 1 Hz higher than the individual alpha-frequency (IAF+1 Hz group) to speed-up pre-stimulus alpha-frequency. On the other hand, Experiment 3 aimed to selectively enhance post-stimulus alpha-amplitude, prior to the confidence prompt. As such, only one entrainment protocol is applied (i.e., stimulation at the individual alpha-frequency), with the final rhythmic-TMS pulse coinciding with the onset of the confidence prompt.
-
30.TMS setup:
-
a.Apply biphasic stimulation using a Transcranial Magnetic Stimulator via a 70 mm figure-of-eight coil (we used TMS Rapid, Magstim Company, UK) of maximum field strength ∼1.55T.
-
b.Randomizing conditions:
-
i.For Experiment 2: Randomize participants group: IAF-1, IAF and IAF+1 stimulation;
-
ii.For Experiment 2 and 3: Randomize order of ACTIVE vs SHAM stimulation blocks.
-
i.
-
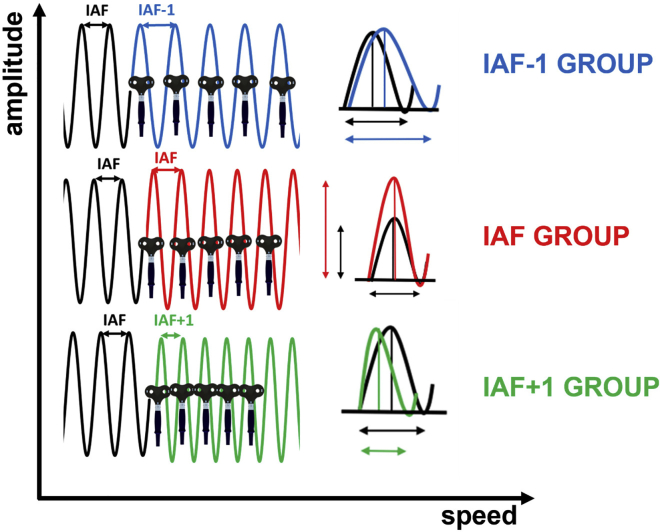
c.Setting up inter-pulse interval in the 5-pulse train (see Figure 2): Use the IAF information previously obtained during the resting state:
-
i.IAF – 1 Hz group: the inter-pulse interval (in ms) is calculated using the following formula: IPI = 1000/ (IAF -1);
-
ii.IAF group: the inter-pulse interval (in ms) is calculated using the following formula: IPI = 1000/ (IAF);
-
iii.IAF + 1 Hz group: the inter-pulse interval (in ms) is calculated using the following formula: IPI = 1000/ (IAF + 1).
-
i.
-
d.Setting up TMS intensity: 60% of the MSO;Optional: Instead of using fixed TMS intensity, you could also titrate the TMS intensity by using phosphene threshold of the participant.
-
e.Placing the coil:
-
i.Place the coil over the O2 electrode of the EEG cap (right occipital site), with the coil surface being tangential to the scalp;Optional: You could use neuro-navigation (instead of EEG landmarks) to locate the area of interest (right occipital V1 area).Note: As systematic differences in visual cortex excitability have not been detected, comparable stimulation effects would be expected after left hemisphere (O1 electrode) entrainment.
-
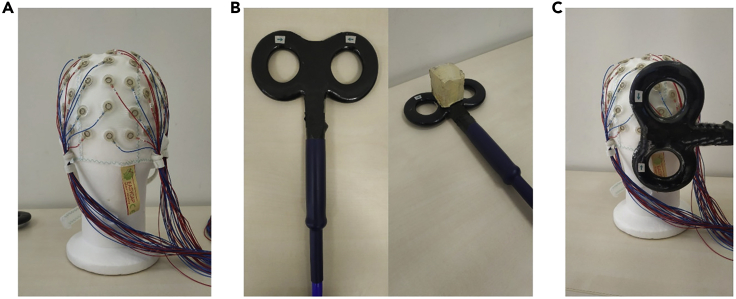
ii.Orient the coil perpendicular to the medial plane of the subjects’ head (latero-medial current direction, see Figure 3).
-
i.
-
a.
-
31.Conducting the main experimental task:
-
a.Three task blocks with ACTIVE TMS, with 60 trials per block (total trial number=180, total number of active TMS pulses: 900);
 Pause point: Participants could take a break after finishing three task blocks.
Pause point: Participants could take a break after finishing three task blocks. -
b.Three task blocks with SHAM TMS, with 60 trials per block (total trial number=180).Note: The presented rhythmic-TMS design is in line with up to-date safety guidelines.Note: steps 10a and 10b should be in randomized order between participants.
 CRITICAL: Make sure that the participant is not experiencing any discomfort or aversive effects during TMS.
CRITICAL: Make sure that the participant is not experiencing any discomfort or aversive effects during TMS. CRITICAL: Make sure that the TMS coils are in the same position and with the same orientation during the main task.
CRITICAL: Make sure that the TMS coils are in the same position and with the same orientation during the main task. CRITICAL: For SHAM stimulation, a modified coil is used that provides enough distance from the scalp to ensure the absence of stimulation, while at the same time maintaining coil position, as well as acoustic sensations (Zanon et al., 2013).
CRITICAL: For SHAM stimulation, a modified coil is used that provides enough distance from the scalp to ensure the absence of stimulation, while at the same time maintaining coil position, as well as acoustic sensations (Zanon et al., 2013).
-
a.
Figure 2.
Entrainment protocols and outcomes: Pre-stimulus alpha activity was fine-tuned relative to individual alpha-frequency using rhythmic five-pulse TMS bursts in which the time lag between pulses was manipulated depending on the group
To induce changes in the alpha-frequency cycle length, rhythmic-TMS was applied at a slower (in blue) or faster pace (in green), relative to a participant’s individual alpha-frequency. To selectively modulate alpha-amplitude, the frequency of the rhythmic-TMS pulse trains was matched to the intrinsic individual alpha-frequency of the participant, thus enhancing the synchronization of neural firing and phase alignment without influencing the speed of alpha activity (in red). In this way, rhythmic-TMS pulse trains could occur at three different frequencies: at the individual alpha-frequency of the participant to manipulate pre-stimulus alpha-amplitude (IAF group, in red); at 1 Hz lower than the individual alpha-frequency (IAF-1 Hz group, in blue) to slow-down pre-stimulus alpha-frequency; or at 1 Hz higher than the individual alpha-frequency (IAF+1 Hz group, in green) to speed-up pre-stimulus alpha-frequency.
Figure 3.
EEG and TMS coil setup
(A) EEG cap: A TMS-compatible 64-channel EEG cap was used.
(B) TMS-coils For active stimulation, a 70 mm figure-of-eight coil was used- For sham stimulation (left), the same type of coil was used, a modified coil was used that provided enough distance from the scalp to ensure the absence of stimulation (right).
(C) Coil position and orientation: Coil should be placed over the O2 electrode of the EEG cap (right occipital site), with the coil surface being tangential to the scalp and the handle oriented perpendicular to the medial plane of the subjects head (latero-medial current direction).
Causal approach (experiment 2 & 3): Computing task performance
Timing: 2 weeks
This step enables looking at behavioral effects of different stimulation protocols, i.e., ultimately testing whether rhythmic stimulation is driving perception in the expected direction in line with a causal brain rhythm-behavior relationship. Specifically, you can look at accuracy, confidence, and metacognition of each participant separately for the two hemifields (stimuli appearing on the left and on the right), and for the two types of within-subject stimulation protocols (ACTIVE vs. SHAM stimulation).
-
32.Behavioral data within participant:
-
a.Calculating perceptual sensitivity (d’ score);
-
b.Calculating confidence mean;
-
c.Estimate metacognitive performance (meta d’ score).
-
a.
-
33.Statistical analysis across participants:
-
a.Experiment 2:
-
i.Compare all scores between the two HEMIFIELDs (left and right) and two STIMULATION types (active rhythmic-TMS and sham) in three GROUPs of participants (IAF±1 Hz, IAF), in 2 × 2 × 3 repeated measures mixed-model ANOVAs;
-
ii.Test differences between conditions by two-tailed t-test (planned comparisons).
-
i.
-
b.Experiment 3:
-
i.Compare all scores for the two HEMIFIELDS (left and right) and between different STIMULATION types (active rhythmic-TMS and sham) in a 2 × 2 repeated measures ANOVA;
-
ii.Test differences between conditions by two-tailed t-test (planned comparisons).
-
i.
-
a.
Causal approach (experiment 2 & 3): Electrophysiological processing and analysis
Timing: 2 months
This step enables looking at electrophysiological effects of different stimulation protocols. Specifically, look at IAF and alpha amplitude of each participant separately for the two hemispheres (right and left hemisphere), and for the two types of within-subject stimulation protocols (ACTIVE vs. SHAM stimulation). This allows to verify whether the rhythmic TMS has the intended effects on brain rhythms (amplitude enhancement, speeding frequency up or down) and hence to verify that it is the TMS-induced changes in brain rhythms mediating the behavioral effects.
-
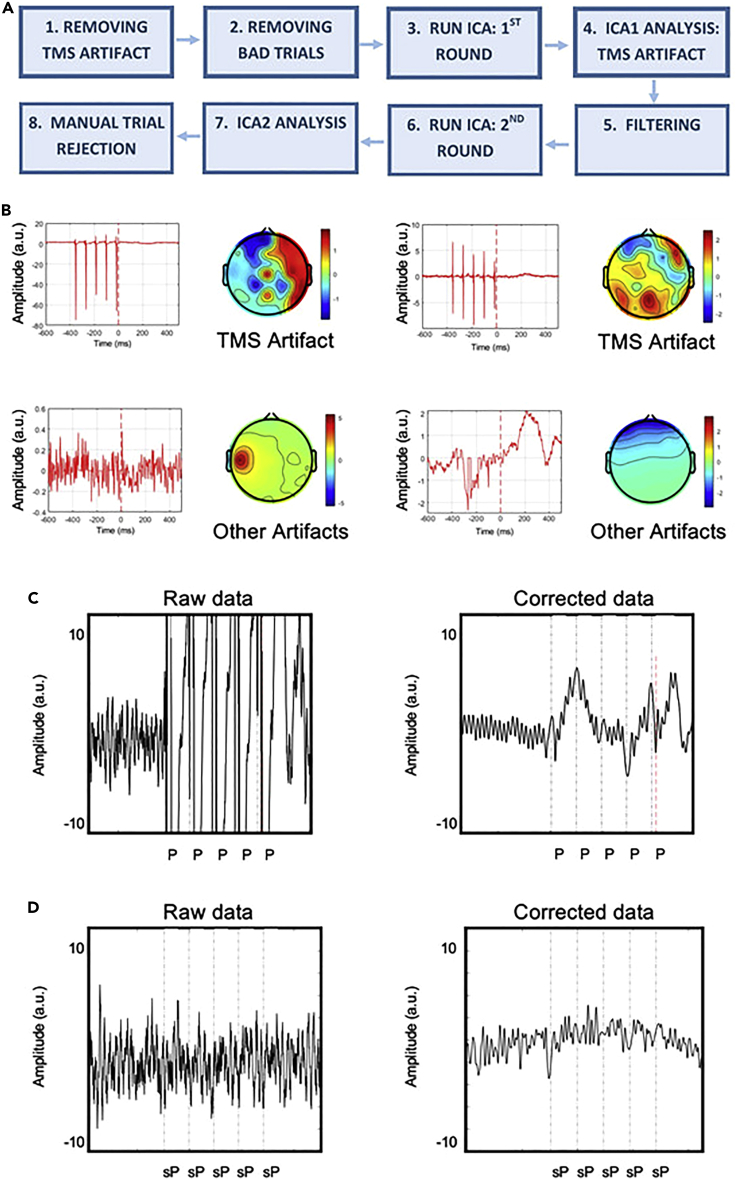
34.EEG preprocessing steps: Removing TMS artifacts (see Figure 4):Note: Identify and remove rhythmic-TMS artifacts using an open-source EEGLab extension, the TMS-EEG signal analyzer (TESA: Rogasch et al., 2017).
-
a.Epoch EEG data around stimulus onset (between -1500 ms and 2500 ms for Experiment 2 and between -1000 ms and 2000 ms for Experiment 3, due to differences in stimulation timing);
-
b.Remove linear trend from the obtained epochs;
-
c.Remove rhythmic-TMS pulse artefact and peaks of rhythmic-TMS-evoked scalp muscle activities (-10 ms +10 ms around TMS pulse);
-
d.Perform cubic interpolation;Note: Interpolating missing data following the removal of the TMS pulse is necessary prior to resampling to avoid sharp steps or transitions in the data, which can lead to ringing artifacts.
-
e.Downsample the data (from 5000 Hz to 1000 Hz);
-
f.Remove again interpolated data;Note: Replacing interpolated data around TMS pulse with constant amplitude data is necessary prior to ICA to improve performance.
-
g.Perform 1st round of Individual Component Analysis (ICA). Specifically, a fastICA algorithm is used (pop_tesa_fastica function): http://research.ics.aalto.fi/ica/fastica/code/dlcode.shtml) to identify individual components representing artifacts, along with automatic component classification (pop_tesa_compselect function), where each component should be subsequently manually checked and reclassified when necessary;Note: This first round of ICA eliminates only components with large amplitude artifacts, such as rhythmic-TMS-evoked scalp muscle artefacts.
-
h.Perform cubic interpolation;
-
i.Apply pass-band (between 1 and 100 Hz) and stop-band (between 48 and 52 Hz) Butterworth filters;
-
j.Remove again interpolated data;
-
k.Perform 2nd round of ICA to remove all other artifacts, such as blinks, eye movement, persistent muscle activity and electrode noise;
-
l.Perform cubic interpolation;
-
m.Re-reference data to the average of all electrodes;
-
n.Visually inspect single trials and remove those containing residual rhythmic-TMS artefact.
 CRITICAL: The described rhythmic-TMS artefact removal procedure should be applied to all EEG data, both for active rhythmic-TMS and sham stimulations.
CRITICAL: The described rhythmic-TMS artefact removal procedure should be applied to all EEG data, both for active rhythmic-TMS and sham stimulations.
-
a.
-
35.EEG analysis:
-
a.Calculate IAF: Alpha-frequency is defined as the local maximum power within the frequency 7–13 Hz range;
-
i.Experiment 2: The analysis window corresponds to the pre-stimulus period (-650 ms to stimulus presentation);
-
ii.Experiment 3: The analysis window corresponds to the period preceding the confidence prompt (850 ms–1500 ms after stimulus presentation, i.e., -650 ms prior to the confidence prompt).
-
i.
-
b.Calculate alpha-amplitude: calculate pre-stimulus alpha-amplitude in the time frequency data (as for Experiment 1).
-
a.
Note: The time window of analyses corresponds to stimulation period for both alpha-frequency and -amplitude.
Note: Use near-stimulation parieto-occipital electrodes in the right hemisphere (PO4,PO8,O2), along with analogous electrodes in the left hemisphere (PO3,PO7,O1) across all the analyses.
-
36.Statistical analysis across participants:
-
a.Experiment 2:
-
i.Compare both alpha power and frequency measures between the two HEMISPHERES (left and right parieto-occipital cluster) and the two STIMULATION types (active and sham rhythmic-TMS) in three GROUPs of participants in 2 × 2 × 3 repeated measures mixed-model ANOVAs;
-
ii.Test differences between conditions by two-tailed t-tests (planned comparisons);
-
iii.Explore the association between rhythmic-TMS-evoked differences in alpha-frequency in the stimulated (right) hemisphere (computed as a difference in alpha-frequency between active rhythmic-TMS and sham stimulation conditions) and differences in perceptual sensitivity in the opposite (left) hemispace (computed as a difference in d’ score between active rhythmic-TMS and sham stimulation conditions) via linear regression.
-
i.
-
b.Experiment 3:
-
i.Compare differences in alpha-amplitude and alpha-frequency again between the two HEMISPHERES (left and right) and between STIMULATION types (active and sham rhythmic-TMS) in a 2 × 2 repeated measures ANOVA;
-
ii.Test differences between conditions by two-tailed t-tests (planned comparisons);
-
iii.Use a linear regression model to determine whether rhythmic-TMS-evoked differences in alpha-amplitude in the stimulated (right) hemisphere (computed as a difference in alpha-amplitude between active and sham rhythmic-TMS conditions) can predict differences in confidence levels in the opposite (left) hemifield (computed as a difference in meta d’ scores between active and sham rhythmic-TMS conditions).
-
i.
-
a.
Figure 4.
TMS artifact correction procedure, related to STAR Methods
(A) EEG data processing workflow and pipeline for Experiments 2 and 3.
(B) Examples of artifact components removed in the first (TMS artifacts, upper row) and second (lower row) ICA analyses.
(C) Effects of artifact rejection procedure on an active TMS condition. One second epoch of one participant before (Raw data) and after (Corrected data) the correction procedure. Dashed lines reflect Pre-stimulus TMS pulses (P).
(D) Effects of artifact rejection procedure on a TMS-artifact free signal. The procedure was applied to ensure that any effect we have observed could not be alternatively explained by a spurious effect of the cleaning protocol adopted. One second epoch of one participant before (Raw data) and after (Corrected data) correction procedure. It can be noticed that the artifact-removal procedure per se does not alter the underlying signal, confirming that the artifact-rejection procedure per se is not accountable for the modulation of the oscillatory activity. Dashed lines reflect simulated pulses (sP). ICA=Independent Components Analyses, A.U.=Arbitrary Units, ms=millisecond. Adapted from Di Gregorio et al. (2022).
Expected outcomes
Correlational approach (experiment 1)
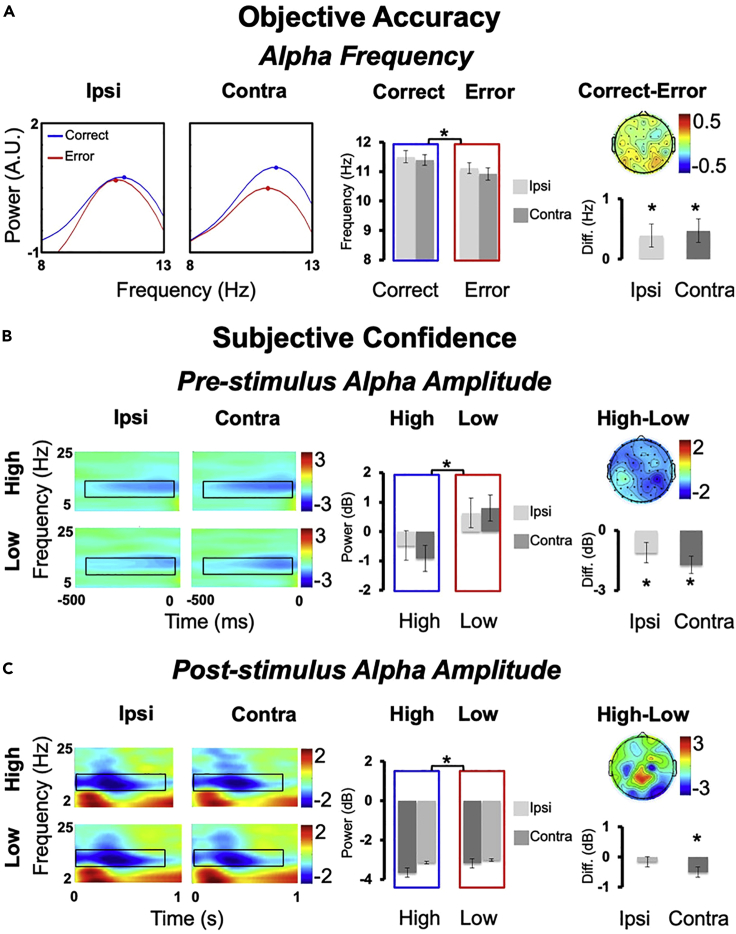
The correlative nature of EEG-behavior studies does not allow mechanistic inferences on how the brain shapes behavior but helps to frame such hypotheses. Namely, our previous results pointed to a functional dissociation of the two oscillatory markers, alpha-frequency and alpha-amplitude, in shaping sensory sampling (Cecere et al., 2015; Cooke et al., 2019) and the subjective readout of this sampling (Limbach and Corballis, 2016), respectively. Therefore, it is expected that the results of the EEG protocol described here implicate alpha-frequency in the level of objective accuracy (see Figure 5). Specifically, higher pre-stimulus alpha-frequency should account for higher accuracy, while playing no role in determining one’s individual perceptual confidence or metacognitive performance. Conversely, alpha-amplitude should be implicated in perceptual decision confidence, but without influencing objective accuracy (see Figure 5). Here, lower pre-stimulus alpha amplitude should relate to higher confidence levels, and lower post-stimulus alpha amplitude should associate with higher metacognition.
Figure 5.
Results experiment 1: Alpha-frequency and -amplitude relate to accuracy and confidence
(A) Objective Accuracy. Averaged alpha-frequency is represented as the z-scored mean power (10∗log10[μv2/Hz]) spectrum in the cue-stimulus time period for the contralateral and the ipsilateral electrodes and for Correct and Error trials within the alpha-band. Bar graphs report correct and error trials and the differences in correct/error responses. Topography represents the difference in Correct-Error (electrodes are flipped to represent contralateral activity in the right-hand side and ipsilateral activity in the left-hand side). Subjective Confidence.
(B and C) Pre-stimulus alpha-amplitude (B) and post-stimulus alpha-amplitude (C) are reported as time-frequency plots. For illustrative purposes we reported data from a cluster of ipsi (P7,PO7,PO3,O1) and contralateral (P8,PO8,PO4,O2) electrodes and for Low and High confident trials. Black boxes denote regions of statistical analyses (alpha-band 7–13 Hz). Bar graphs are reported for Low and High confident trials and for the difference in High-Low. Topography represents the difference in High-Low (electrodes are flipped to have contralateral activity in the right-hand side and ipsilateral activity in the left-hand side). Two-tailed t-test statistical significance is reported (∗p<.05). Error bars represent standard error of the mean. A.U.=arbitrary units; Diff=difference; μv=microvolt; Hz=Hertz; ms=milliseconds; dB=decibel. Adapted from Di Gregorio et al. (2022).
Causal approach (experiment 2 & 3)
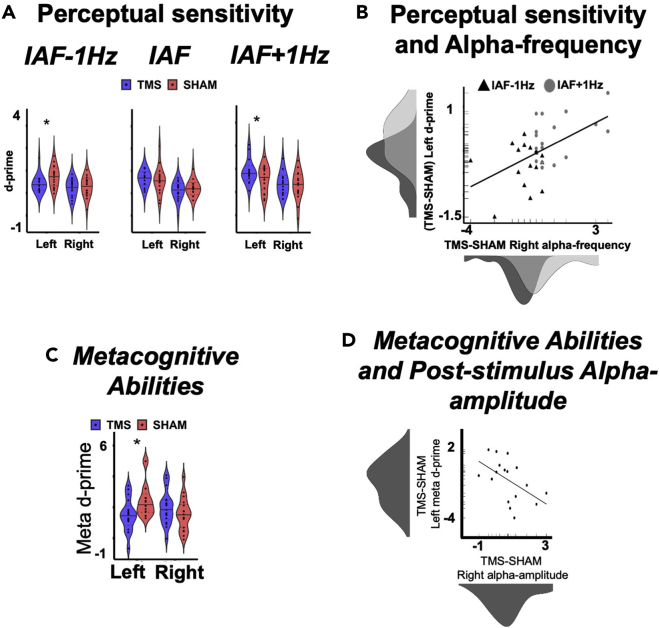
The speed of occipital alpha activity should have a crucial and selective role in modulating perceptual sensitivity. Therefore, it is expected that by applying a rhythmic 5-pulse TMS slower or faster by 1 Hz than IAF right before stimulus presentation should result in slower vs faster pre-stimulus individual alpha frequency in the stimulated hemisphere, relative to sham (Experiment 2). Crucially, induced slower vs faster alpha-pace should shape perceptual sensitivity. Specifically, induced slower IAF should result in lower accuracy in the contralateral hemifield (measured by d’ scores), while induced faster IAF would enhance perception, marked by higher d’ scores. In contrast, applying a rhythmic 5-pulse at exact IAF, should result in higher pre-stimulus alpha-amplitude at the same frequency, possibly influencing confidence ratings (see limitations and troubleshooting).
Post-stimulus changes in alpha amplitude should influence metacognitive performance. Therefore, stimulating at IAF right before the confidence prompt should enhance alpha amplitude in the near-stimulation site, resulting in enhanced metacognition for contralateral stimuli (Experiment 3). For the summary of the expected outcomes, see Figure 6.
Figure 6.
Behavioral results
(A) Experiment 2: Perceptual sensitivity. Results are presented for three groups of participants (IAF±1 Hz and IAF stimulation protocol). Perceptual sensitivity is quantified in d’ scores. Violin plots of d’ are reported for rhythmic-TMS (TMS) and SHAM-control stimulation, and separately for the left and right hemifields. Data are presented as median (full line) ±1 quartile (dashed line).
(B) Experiment 2: Perceptual sensitivity and alpha-frequency. Relationship between TMS-induced differences in alpha-frequency in the stimulated (right) hemisphere (computed as a difference in alpha-frequency between TMS and SHAM stimulation) and differences in accuracy in the opposite (left) hemifield (computed as a difference in d’ score between TMS and SHAM stimulation), across the slower (IAF-1 Hz group, represented as black triangles) and faster rhythmic-TMS groups (IAF+1 Hz group, represented as gray circles). Density distributions of the two variables across the two groups are also presented along the corresponding axes. t-test statistical significance is reported (∗p<.05).
(C) Experiment 3: Metacognitive abilities, quantified via meta-d’ scores. Violin plots of meta d’ for TMS and SHAM-control stimulation, and reported separately for the left and right hemifields. Data are presented as median (full line) ±1 quartile (dashed line).
(D) Experiment 3: Metacognitive abilities and post-stimulus alpha-amplitude. Relationship between rhythmic-TMS-evoked differences in alpha-amplitude in the stimulated (right) hemisphere (computed as a difference in alpha-amplitude between TMS and SHAM stimulation) and differences in metacognition in the opposite (left) hemispace (computed as a difference in meta-d’ score between TMS and SHAM stimulation). Density distributions of the two variables are also presented along the corresponding axes. Two-tailed t-test statistical significance is reported (∗p<.05). A.U.=arbitrary units; μv=microvolt; Hz=Hertz; ms=milliseconds; dB=decibel. Adapted from Di Gregorio et al. (2022).
Limitations
Due to the already increased complexity of the experimental design, please note that we have limited the locus of our stimulation to one area (right occipital cortex), based on previous research and the results of the previously conducted EEG experiment. Thus far, differences in the excitability of the right and left occipital cortex were not found, so we would expect exactly mirrored results for stimulation of the left occipital cortex. Similarly, we would expect a comparable pattern of results for stimulation of parietal areas (Thut et al., 2011), however with possible significant variations of effect size. Future research may detail the level of specificity of the observed effect by stimulation of different nodes of the targeted network.
Secondly, the current experimental design could be further refined, possibly leading to larger behavioral effects. For instance, we would encourage future EEG studies to use source analysis to first identify the target area, and neuro-navigation systems to map it to the individual participant. Likewise, TMS pulse intensity can be individualized by using phosphene threshold, instead of using fixed intensity values. Finally, we estimated IAF values, that were used to set-up stimulation pulse-train parameters, based on resting-state data. Although we did not find within-subject differences between resting-state and pre-stimulus task IAF, it is possible that stimulation based on task-related IAF would be more efficient.
Third, the experimental design of our TMS study (Experiment 2) did not allow us to test the effect of pre-stimulus alpha entrainment on confidence prompt, as entrainment effects are limited to a few hundred milliseconds following stimulation. This is long enough for pre-stimulus TMS entrainment to influence the primary accuracy response, as this was collected immediately after stimulus presentation. The secondary, higher decision confidence response, however, which was associated with pre-stimulus EEG alpha-amplitude, was collected only 1.5–2 s post-stimulus (through the confidence prompt) and hence occurred >1 s after rhythmic-TMS offset, when entrainment effects might not be sufficiently sustained to impact individual response consistently. Currently, we are developing a task where confidence and accuracy response will be collected at the same time-point (right after TMS), thus enabling to estimate pre-stimulus alpha-amplitude changes on subjective perceptual experience.
Finally, although our experiments show that alpha-frequency and –amplitude, and hence sensitivity and confidence, are dissociable entities, these processes work in concert in more ecological situations to maximize the efficiency of our conscious experience. Future research shall address the inter-dependency between sensitivity and confidence, the interaction of these two mechanisms and the understanding of the brain circuits involved.
Troubleshooting
Problem 1
Participants reach ceiling effect (near-maximal performance) or floor effect (chance-performance) during main task (steps 12 and 24).
Potential solution
Most probable cause of this problem is that the stimulus threshold was not calculated well during the titration session. Therefore, make sure that you double-check sigmoid fitting when calculating threshold values and include false alarm rates in the calculation. If the threshold session and the main task are conducted on different days, it is advisable to perform a shorter threshold re-assessment to verify whether participants’ performance was similar to the one measured in the previous session.
Problem 2
EEG signal during recording is disrupted across all electrodes (steps 9 and 26).
Potential solution
Most probable cause of this problem is the loss of acceptable impedance of the reference and/or ground electrodes. In order to avoid losing the meaningful signal during the task, make sure that the initial impedance of ground and reference electrodes is as minimum as possible (<5 kΩ). Likewise, always check the impedances of these electrodes in between experimental blocks.
Problem 3
A clear peak in the alpha range is not visible during eyes-open resting-state on the O2 electrode, that is necessary to be identified in order to set stimulation parameters (step 29).
Potential solution
If a clear peak in the alpha range is not visible during eyes-open resting-state on the O2 electrode, consider adjacent electrodes (PO8 and PO4).
On the other hand, if the clear peak in the alpha range is not visible during eyes-open resting-state across all posterior electrodes, consider using eyes-closed resting-state for estimating IAF.
Problem 4
Inter-pulse interval between the pulses in a 5-pulse TMS train does not seem consistent across trials or some TMS pulse is skipped (step 30).
Potential solution
Before running the experiment, you should always check the timing accuracy of the parallel port/ triggerbox that controls TMS pulses. Please note that the use of parallel port is considered to create less time delays.
Problem 5
Coil gets overheated during stimulation and TMS-pulses cease to be administered (step 31).
Potential solution
During high-intensity, repetitive and rhythmic online stimulation protocols, where the number of pulses is increased, TMS coil will heat up, and, when its temperature goes beyond the threshold, coil will get locked and will not transmit the pulses. Therefore, during piloting, it is important to establish that coil will not overheat during one task block (if it does, you should shorten the block). In between blocks you should always change the coil (you should have at least two coils at your disposal), and make sure that the previously-used one gets cooled down.
Problem 6
EEG signal during TMS/EEG recording gets saturated (step 31).
Potential solution
As already anticipated, one of the main difficulties in TMS-EEG co-registration is the impact that the strong magnetic field gradient produced by TMS has on the EEG amplifiers. During EEG recording, this can cause amplifier saturation, resulting in the absence of brain-signal recording. Therefore, it is advisable to use a DC amplifier, and perform a DC correction of the signal before each block. Likewise, the signal should be carefully observed during recording and DC correction can be also performed online, in case of saturation.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Vincenzo Romei (vincenzo.romei@unibo.it).
Materials availability
See the key resources table for information about resources. This study did not generate new unique reagents.
Acknowledgments
F.D.G. is supported by the Ministero della Salute (SG-2018-12367527); V.R. is supported by the Bial Foundation (204/18).
Author contributions
V.R. conceived the project; V.R., F.D.G., J.T., and G.T. designed the experiment; V.R., F.D.G., E.M., and J.T. Implemented the experiment; J.T. and E.M. conducted the experiment; F.D.G. and J.T. analyzed data; F.D.G., J.T., E.M., and V.R. wrote the first draft of the paper. V.R., F.D.G., E.M., J.T., and G.T. contributed to the final draft of the paper.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jelena Trajkovic, Email: jelena.trajkovic2@unibo.it.
Francesco Di Gregorio, Email: francesco.digregorio@ausl.bologna.it.
Vincenzo Romei, Email: vincenzo.romei@unibo.it.
Data and code availability
The datasets generated during this study have been made publicly available through the Open Science Framework: https://osf.io/e4bnj/. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Albers C., Lakens D. When power analyses based on pilot data are biased: inaccurate effect size estimators and follow-up bias. J. Exp. Soc. Psychol. 2018;74:187–195. doi: 10.1016/j.jesp.2017.09.004. [DOI] [Google Scholar]
- Atluri S., Frehlich M., Mei Y., Garcia Dominguez L., Rogasch N.C., Wong W., Daskalakis Z.J., Farzan F. TMSEEG: a MATLAB-based graphical user interface for processing electrophysiological signals during transcranial magnetic stimulation. Front. Neural Circuits. 2016;10:1–20. doi: 10.3389/fncir.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell C.S.Y., Tagliabue C.F., Veniero D., Cecere R., Savazzi S., Thut G. Pre-stimulus EEG power predicts conscious awareness but not objective visual performance. Eneuro. 2017;4 doi: 10.1523/ENEURO.0182-17.2017. ENEURO.0182-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S., Ruff C.C., Blakemore C., Driver J., Thilo K.V. Spatial attention changes excitability of human visual cortex to direct stimulation. Curr. Biol. 2007;17:134–139. doi: 10.1016/j.cub.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo Z., Silvanto J., Battelli L., Pascual-Leone A. The mental number line modulates visual cortical excitability. Neurosci. Lett. 2009;462:253–256. doi: 10.1016/j.neulet.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Cecere R., Rees G., Romei V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr. Biol. 2015;25:231–235. doi: 10.1016/j.cub.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J., Poch C., Gillmeister H., Costantini M., Romei V. Oscillatory properties of functional connections between sensory areas mediate cross-modal illusory perception. J. Neurosci. 2019;39:5711–5718. doi: 10.1523/JNEUROSCI.3184-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. https://doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Di Gregorio F., Trajkovic J., Roperti C., Marcantoni E., Di Luzio P., Avenanti A., Thut G., Romei V. Tuning alpha rhythms to shape conscious visual perception. Curr. Biol. 2022;32:988–998.e6. doi: 10.1016/j.cub.2022.01.003. [DOI] [PubMed] [Google Scholar]
- Fleming M.S. HMeta-d: hierarchical Bayesian estimation of metacognitive efficiency from confidence ratings. Neurosci. Conscious. 2017;2017:nix007. doi: 10.1093/nc/nix007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallotto S., Sack A.T., Schuhmann T., de Graaf T.A. Oscillatory correlates of visual consciousness. Front. Psychol. 2017;8:1–16. doi: 10.3389/fpsyg.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.G., Swets J.A. John Wiley and Sons, Inc; 1966. Signal Detection Theory and Psychophysics. [Google Scholar]
- Helfrich R.F., Schneider T.R., Rach S., Trautmann-Lengsfeld S.A., Engel A.K., Herrmann C.S. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 2014;24:333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Iemi L., Chaumon M., Crouzet S.M., Busch N.A., Busch X.N.A. Spontaneous neural oscillations bias perception by modulating baseline excitability. J. Neurosci. 2017;37:807–819. doi: 10.1523/JNEUROSCI.1432-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer T., Beck S., Erb M., Grodd W. The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin. Neurophysiol. 2001;112:2015–2021. doi: 10.1016/S1388-2457(01)00673-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lee H.W., Hong S.B., Seo D.W., Tae W.S., Hong S.C. Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology. 2000;54:849–854. doi: 10.1212/WNL.54.4.849. [DOI] [PubMed] [Google Scholar]
- Limbach K., Corballis P.M. Prestimulus alpha power influences response criterion in a detection task. Psychophysiology. 2016;53:1154–1164. doi: 10.1111/psyp.12666. [DOI] [PubMed] [Google Scholar]
- Maniscalco B., Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cogn. 2012;21:422–430. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Mierau A., Klimesch W., Lefebvre J. State-dependent alpha peak frequency shifts: experimental evidence, potential mechanisms and functional implications. Neuroscience. 2017;360:146–154. doi: 10.1016/j.neuroscience.2017.07.037. [DOI] [PubMed] [Google Scholar]
- Migliorati D., Zappasodi F., Perrucci M.G., Donno B., Northoff G., Romei V., Costantini M. Individual alpha frequency predicts perceived visuotactile simultaneity. J. Cogn. Neurosci. 2020;32:1–11. doi: 10.1162/jocn_a_01464. [DOI] [PubMed] [Google Scholar]
- Minami S., Amano K. Illusory jitter perceived at the frequency of alpha oscillations. Curr. Biol. 2017;27:2344–2351.e4. doi: 10.1016/j.cub.2017.06.033. [DOI] [PubMed] [Google Scholar]
- Mutanen T.P., Metsomaa J., Liljander S., Ilmoniemi R.J. Automatic and robust noise suppression in EEG and MEG: the SOUND algorithm. NeuroImage. 2018;166:135–151. doi: 10.1016/j.neuroimage.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Noguchi Y. Individual differences in beta frequency correlate with the audio–visual fusion illusion. Psychophysiology. 2022:e14041. doi: 10.1111/psyp.14041. [DOI] [PubMed] [Google Scholar]
- Notbohm A., Herrmann C.S. Flicker regularity is crucial for entrainment of alpha oscillations. Front. Hum. Neurosci. 2016;10:503. doi: 10.3389/fnhum.2016.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M. Detecting consciousness: a unique role for neuroimaging. Annu. Rev. Psychol. 2013;64:109–133. doi: 10.1146/annurev-psych-113011-143729. [DOI] [PubMed] [Google Scholar]
- Palva S., Palva J.M. New vistas for α-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Rogasch N.C., Sullivan C., Thomson R.H., Rose N.S., Bailey N.W., Fitzgerald P.B., Farzan F., Hernandez-Pavon J.C. NeuroImage. Vol. 147. 2017. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: a review and introduction to the open-source TESA software; pp. 934–951. [DOI] [PubMed] [Google Scholar]
- Romei V., Bauer M., Brooks J.L., Economides M., Penny W., Thut G., Driver J., Bestmann S. Causal evidence that intrinsic beta-frequency is relevant for enhanced signal propagation in the motor system as shown through rhythmic TMS. NeuroImage. 2016;126:120–130. doi: 10.1016/j.neuroimage.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V., Brodbeck V., Michel C., Amedi A., Pascual-Leone A., Thut G. Spontaneous fluctuations in posterior α-band EEG activity reflect variability in excitability of human visual areas. Cereb. Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V., Thut G., Mok R.M., Schyns P.G., Driver J. Causal implication by rhythmic transcranial magnetic stimulation of alpha frequency in feature-based local vs. global attention. Eur. J. Neurosci. 2012;35:968–974. doi: 10.1111/j.1460-9568.2012.08020.x. [DOI] [PubMed] [Google Scholar]
- Ronconi L., Vitale A., Federici A., Mazzoni N., Battaglini L., Molteni M., Casartelli L. Neural dynamics driving audio-visual integration in autism. Cereb. Cortex. 2022:1–14. doi: 10.1093/cercor/bhac083. [DOI] [PubMed] [Google Scholar]
- Rosanova M., Casali A., Bellina V., Resta F., Mariotti M., Massimini M. Natural frequencies of human corticothalamic circuits. J. Neurosci. 2009;29:7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Antal A., Bestmann S., Bikson M., Brewer C., Brockmöller J., Carpenter L.L., Cincotta M., Chen R., Daskalakis J.D., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin. Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Screening questionnaire before TMS: an update. Clin. Neurophysiol. 2011;122:1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rotenberg A., Horvath J.C., Pascual-Leone A. In: Rotenberg A., Horvath J., Pascual-Leone A., editors. Vol. 89. Humana Press; New York, NY: 2014. (Transcranial Magnetic Stimulation. Neuromethods). [DOI] [Google Scholar]
- Samaha J., Iemi L., Haegens S., Busch N.A. Spontaneous brain oscillations and perceptual decision-making. Trends Cogn. Sci. 2020;24:639–653. doi: 10.1016/j.tics.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Samaha J., Iemi L., Postle B.R. Prestimulus alpha-band power biases visual discrimination confidence, but not accuracy. Conscious. Cogn. 2017;54:47–55. doi: 10.1016/j.concog.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha J., Postle B.R. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Curr. Biol. 2015;25:2985–2990. doi: 10.1016/j.cub.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J., Muggleton N.G. A novel approach for enhancing the functional specificity of TMS: revealing the properties of distinct neural populations within the stimulated region. Clin. Neurophysiol. 2008;119:724–726. doi: 10.1016/j.clinph.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Thut G., Veniero D., Romei V., Miniussi C., Schyns P., Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R. Perceptual cycles. Trends Cogn. Sci. 2016;20:723–735. doi: 10.1016/j.tics.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Venskus A., Ferri F., Migliorati D., Spadone S., Costantini M., Hughes G. Temporal binding window and sense of agency are related processes modifiable via occipital tACS. PLoS ONE. 2021;16:e0256987. doi: 10.1371/journal.pone.0256987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venskus A., Hughes G. Individual differences in alpha frequency are associated with the time window of multisensory integration, but not time perception. Neuropsychologia. 2021;159:107919. doi: 10.1016/j.neuropsychologia.2021.107919. [DOI] [PubMed] [Google Scholar]
- Wu W., Keller C.J., Rogasch N.C., Longwell P., Shpigel E., Rolle C.E., Etkin A. ARTIST: a fully automated artifact rejection algorithm for single-pulse TMS-EEG data. Hum. Brain Mapp. 2018;39:1607–1625. doi: 10.1002/hbm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A., Melcher D., Samaha J. Frequency modulation of neural oscillations according to visual task demands. Proc. Natl. Acad. Sci. U.S.A. 2018;115:1346–1351. doi: 10.1073/pnas.1713318115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon M., Battaglini P.P., Jarmolowska J., Pizzolato G., Busan P. Long-range neural activity evoked by premotor cortex stimulation: a TMS/EEG co-registration study. Front. Hum. Neurosci. 2013;7:1–18. doi: 10.3389/fnhum.2013.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Cai P., Luo H., Fang F. The causal role of α-oscillations in feature binding. Proc. Natl. Acad. Sci. U.S.A. 2019;116:17023–17028. doi: 10.1073/pnas.1904160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during this study have been made publicly available through the Open Science Framework: https://osf.io/e4bnj/. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.