Summary
Background
COVID-19 has affected many healthcare workers (HCWs) globally. We performed state-wide SARS-CoV-2 genomic epidemiological investigations to identify HCW transmission dynamics and provide recommendations to optimise healthcare system preparedness for future outbreaks.
Methods
Genome sequencing was attempted on all COVID-19 cases in Victoria, Australia. We combined genomic and epidemiologic data to investigate the source of HCW infections across multiple healthcare facilities (HCFs) in the state. Phylogenetic analysis and fine-scale hierarchical clustering were performed for the entire dataset including community and healthcare cases. Facilities provided standardised epidemiological data and putative transmission links.
Findings
Between March-October 2020, approximately 1,240 HCW COVID-19 infection cases were identified; 765 are included here, requested for hospital investigations. Genomic sequencing was successful for 612 (80%) cases. Thirty-six investigations were undertaken across 12 HCFs. Genomic analysis revealed that multiple introductions of COVID-19 into facilities (31/36) were more common than single introductions (5/36). Major contributors to HCW acquisitions included mobility of staff and patients between wards and facilities, and characteristics and behaviours of patients that generated numerous secondary infections. Key limitations at the HCF level were identified.
Interpretation
Genomic epidemiological analyses enhanced understanding of HCW infections, revealing unsuspected clusters and transmission networks. Combined analysis of all HCWs and patients in a HCF should be conducted, supported by high rates of sequencing coverage for all cases in the population. Established systems for integrated genomic epidemiological investigations in healthcare settings will improve HCW safety in future pandemics.
Funding
The Victorian Government, the National Health and Medical Research Council Australia, and the Medical Research Future Fund.
Keywords: Covid-19, Healthcare workers, Genomic epidemiology, Pandemic preparedness
Research in context.
Evidence before the study
Since the beginning of the COVID-19 pandemic in December 2019, there have been 212 million people infected and more than 4.4 million deaths worldwide as of August 24th, 2021. Genomic analysis has emerged as an important tool for tracking the spread of the virus in the community and healthcare settings. Due to the low prevalence of COVID-19 in Australia at the time of the study, samples from all cases underwent genomic sequencing, allowing for rapid tracking of spread as well as timely public health responses. We searched PubMed, medRxiv, and bioRxiv for primary research studies published in English between Jan 1, 2020, and March 20, 2021, using combinations of “SARS-CoV-2”, “genomics”, “genomic epidemiology”, “healthcare worker”, and “nosocomial”. We identified eight articles that used genomic epidemiology to track COVID-19 outbreaks in healthcare workers at the time the study was done, including United Kingdom (n=5), Portugal, the Netherlands and Australia; only one study describes real-time genomic epidemiological investigations. We did not identify any studies describing city-wide healthcare facility analyses or guidelines for genomic epidemiological analyses.
Added value of the study
Here we present the first report, to our knowledge, applying genomic epidemiological investigations of COVID-19 healthcare associated infections across multiple healthcare networks of a major city. We developed a set of minimum and enhanced metadata and a workflow to facilitate investigations across multidisciplinary groups of healthcare infection control staff, laboratory staff and genomic epidemiologists to optimise the utility of HCW investigations. We worked with healthcare facilities to identify transmission networks and inform infection control procedures. Over seven months we sequenced over 2000 genomes from HCWs and patients with COVID-19 infections in healthcare settings. We determined the minimum number of genomic introductions to HCFs and uncovered previously unlinked ward outbreaks of hospital-acquired infections. Cryptic transmission events were identified within complex transmission networks that had previously been unanticipated by infection control teams. We provide insight into how sequencing laboratories can work together with healthcare infection control staff to provide actionable results with public health implications during an active pandemic.
Implications of all the available evidence
Our study highlights the ways that rapid genomic sequencing and genomic epidemiological analysis can contribute to healthcare associated infection investigations through added evidence for or against transmission events. Genomic epidemiological investigations can assist to identify complex transmission networks within HCFs and across healthcare networks and direct infection control interventions to areas of need. The metadata and workflows for genomic epidemiological investigations suggested here provide an actionable framework that will assist with future pandemic preparedness with applications to infectious diseases wider than SARS-CoV-2 alone.
Alt-text: Unlabelled box
Introduction
The COVID-19 pandemic has resulted in the hospitalisation of large numbers of patients with severe disease, particularly in older age groups.1, 2, 3, 4, 5 Healthcare workers (HCWs) on the frontline have acquired COVID-19 in many different settings, often despite adequate availability and choice of appropriate personal protective equipment (PPE).6, 7, 8, 9, 10, 11 To optimise the safety of HCWs and patients, it is critical for hospital infection control teams and, more broadly, healthcare systems to understand the drivers of infections in HCWs, through systematic investigations of the circumstances around these putative transmissions in healthcare settings. Internationally, genomics of SARS-CoV-2 has been a powerful tool for understanding transmission links and outbreaks.12, 13, 14, 15, 16, 17, 18, 19 Whilst the investigation of HCW infections has traditionally been achieved through epidemiologic assessments, combined genomic and epidemiologic analyses have now emerged as the new standard-of care for these investigations.20,21
The state of Victoria, Australia (population ∼6.7 million)22 experienced two waves of COVID-19 in 2020. Comprehensive prospective genomic sequencing of SARS-CoV-2-positive samples was undertaken by the public health genomic reference laboratory (the Microbiological Diagnostic Unit – Public Health Laboratory (MDU-PHL)), with samples sequenced from 75% of cases. The first wave (March – April 2020) was largely a polyclonal outbreak, characterised by multiple introductions from overseas travellers with limited onwards transmission in the population, and very limited transmission to HCWs.10,23 The second wave (July – October 2020) in Victoria was largely a clonal outbreak, centred in Melbourne, Victoria, originating from a breach in the hotel quarantine system for returned travellers.12 This second wave resulted in outbreaks occurring across many healthcare facilities (HCF) and aged care facilities (ACF).12 Globally, HCWs are at increased risk of infection with coronavirus disease (COVID-19).6,24 Multiple studies document nosocomial transmission and infection in HCWs, tailored infection control investigations and responses.16, 17, 18, 19, 20, 21,25, 26, 27, 28 Whole genome sequencing can contribute high resolution data to describe and investigate such transmission networks.
Here we describe the process and findings of investigations of HCW infections in multiple HCFs across our state as part of an urgent pragmatic response to COVID-19. We hypothesised that an integrated genomic epidemiological analysis of COVID-19 HCW infections, applied in real-time at the hospital coalface and interpreted in the broader context of all healthcare and community infections, would enhance understanding of the source of HCW infections and identify common transmission risks. Our results aim to provide a framework for workflows and metadata required to maximise HCF preparedness to investigate COVID-19 HCW infections, and optimise staff safety for future outbreaks.
Methods
Setting and data sources
This project was undertaken in the state of Victoria, Australia (population ∼6.7 million),22 where the healthcare network includes eleven major metropolitan health services. During the time of these investigations, all samples positive for SARS-CoV-2 by RT-PCR were requested to be forwarded to MDU Public Health Laboratory for genomic sequencing.12,23,29 Prospective sequencing was conducted on all samples received at MDU-PHL, with samples sequenced from approximately 75% of cases.12
The genomic epidemiology team at MDU-PHL assisted all HCFs requesting genomic investigations of COVID-19 outbreaks in HCWs (and often including patients) in their facilities; a qualitative thematic review of the results of these investigations are presented here. Investigations were conducted to inform operational improvements at each healthcare facility, including infection prevention and control, with each healthcare facility providing the epidemiological data to inform the genomic epidemiological investigation. Investigations were an iterative process developed through collaboration with healthcare facilities, refined to a standard workflow and list of required and desirable metadata (Box 1). Some of these investigations were conducted in near to real time whilst others were requested retrospectively once capacity was available at the HCF to perform the epidemiological assessment. For this study, HCWs were defined as any staff, students or volunteers working in a hospital or paramedic setting, excluding community residential aged care facilities (RACFs).
BOX 1.
Genomic epidemiological investigations.
| Part 1 – Establishing basic genomic and epidemiologic data |
| 1. Establish HCF transmission hypotheses for investigation |
| 2. Collect case list and metadata (demographic & case information)a. |
| 3. Identify missing data, follow up on sample and sequencing availability. |
| 4. Build phylogenetic tress with suitable context isolates (temporal & geographic). |
| 5. Match metadata to available genomic data. |
| 6. Discuss genomic clustering with HCF. |
| a. Optional stopping point |
| Part 2 – Integrating case information |
| 7. Overlay detailed epidemiological metadata (date of diagnosis, and patient/staff role) |
| 8. Discuss with HCF the concordance between epidemiological data and phylogenetic data. |
| Part 3 – Integrating exposure and location data |
| 9. Overlay detailed epidemiological location data & exposure data (known exposure events) |
| 10. Refine genomic clustering with detailed epidemiological metadata. |
| 11. Final written report. |
| Optimal metadata to include: |
| Individual level metadata |
| 1. Demographic data |
| a. Name |
| b. Date of birth |
| c. Lab / UR number |
| 2. Case information |
| a. Date of diagnosis, Date of onset, Date of collection |
| b. Role - HCW (with or without patient contact; specific role) / Patient / Visitor |
| 3. Location data |
| a. Patient admission date, ward and bed number and movement details |
| b. Staff shift dates, primary and secondary locations (where available) |
| c. Furlough |
| 4. Exposure data |
| a. Known COVID positive contacts with dates of contact |
| b. PPE breach or other known high-risk events – positive cases, contact level |
| c. Staff links to other HCF or ACF |
| d. Travel History international and local |
| e. Contact with other staff outside the workplace e.g. car-pooling or social events Staff living with / links to other HCW ACW |
| f. Residence in or exposure to community “hotspot” (a location of intense community transmission) |
| Facility level metadata |
| a. PPE donning and doffing procedures /locations |
| b. Staff facilities, e.g. shared team rooms |
| c. Facility links to other HCF or ACF |
a Consider local legislation and policies governing permissions required to collect individual HCW and patient data.
Genomic data and bioinformatic analysis
Detailed methods are described in Supplementary Methods and elsewhere12,23; briefly, extracted RNA from SARS-CoV-2 RT-PCR positive samples underwent tiled amplicon PCR using either ARTIC version 1 or 3 primers,30 following published protocols.31 Reads were aligned to the reference genome (Wuhan Hu-1; GenBank MN908947.3) and consensus sequences generated. Quality control (QC) metrics on consensus sequences were slightly modified from the previous publications due to the highly-clonal nature of the ‘second wave’ of SARS-CoV-2 in Victoria; QC parameters for this paper included requiring ≥65% genome recovered, ≤35 single nucleotide polymorphisms (SNPs) from the reference genome, and ≤300 ambiguous bases (note that these parameters have evolved over the course of the pandemic, and are not necessarily recommended for current practice). A single sequence was selected from each patient for phylogenetic analysis. Genomic clusters were defined as two or more related sequences using a complete-linkage hierarchical clustering algorithm of pairwise genetic distances derived from a maximum likelihood phylogenetic tree. Genomic clustering was used to identify plausible genomic links between cases, which were further interpreted together with epidemiological data. The number of community cases present in each genomic cluster was also taken into account, in that clusters with a higher proportion of community cases required stronger epidemiologic evidence of exposure to be designated likely healthcare transmission.
Combined genomic and epidemiologic analysis
Genomic epidemiological analyses were performed in three stages (Box 1). Beginning with a line list from HCFs identifying HCW and patients with sufficient identifiers to match to available lab and genomic data. Stage one linked cases with samples and grouped cases by genomic cluster, identifying the minimum number of genomic introductions likely to have taken place, and formed the foundation for all further investigations. Stage two expanded the investigation by including the case information such as date of sample collection, symptom onset and diagnosis for each individual. The results of this step allowed for focusing of further epidemiological investigations. Stage three provided in-depth epidemiological investigation of genomic clusters by combining epidemiological location and exposure data.
Results of each analysis were reported to the facilities as an iterative process, with collaborative meetings cases included in the analysis were reviewed, then the genomic data were presented. Facilities were given the opportunity to review and add any epidemiological data to assist with the analysis and to put forward any specific queries based on their epidemiological analysis. The analyses were then refined based on the outcomes of the meetings and compiled into a final report (Figure 1).
Figure 1.
Process of genomic epidemiological analysis. Genomic epidemiological investigations are highly iterative and must be able to accommodate input of additional information at each stage of the analysis. New cases and metadata can become available at various stages of the analysis while bioinformatic techniques change rapidly in line with global advances.
For a subset of healthcare facilities where facility-wide analyses were performed, likely HCWs acquisition sources were designated by combining genomic and epidemiological data (healthcare-acquired, community-acquired or unclear). Estimates of total numbers of HCW infections acquired at work were subsequently compiled for these facilities.
Qualitative thematic analysis of findings
To provide a high-level overview of the common themes emerging from these investigations across multiple different facilities, we reviewed the findings from each investigation, compiling feedback from healthcare facilities including whether the genomic results were concordant with their epidemiologic hypotheses, and likely factors contributing to the transmission of SARS-CoV-2 in their facilities.
Statistical analysis
Statistical analyses (generating summary statistics) were performed using R version 4.0.2.
Ethics
Ethical approval was received from the University of Melbourne Human Research Ethics Committee (study number 1954615.4).
Role of the funding source
The Microbiological Diagnostic Unit Public Health Laboratory receives funding from the State Government of Victoria. This work was funded by the National Health & Medical Research Council (NHMRC) through the Medical Research Future Fund (MRFF) – Coronavirus Research Response: 2020 Tracking COVID-19 in Australia using Genomics Grant Opportunity (MRF9200006). NLS was supported by an Australian Government Research Training Program (RPT) scholarship (GNT1093468). BPH was supported by an NHMRC Investigator Grant (GNT1196103). Funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Results
Between March and October, 2020, MDU-PHL were approached by 12 HCFs to assist with genomic epidemiological investigations as part of urgent pragmatic responses into HCW COVID-19 cases. Investigations ranged in scope from individual suspected transmission events to ward- or facility-level investigations. MDU-PHL assisted with 36 investigations, with 9/12 facilities requesting more than one investigation. The majority of investigations were undertaken in large public university hospitals, with a small number of private facilities, including a total of 21 campuses and more than 9900 beds,32 as well as the metropolitan paramedic service. A total of 765 HCWs and 1,273 patients were investigated, with sequencing available for 80% (612) of these HCWs and 80.8% (1,028) of these patients (data summarised in Table 1 and Supplementary Figure 1, Table 1). This represents 62% of the total number of HCW infections notified across the state (n=1240).33 Transmissions were identified from patients to HCWs, HCWs to patients, and between HCWs, although directionality was often difficult to assess. Sensitivity analysis of the genomic clustering used in 2020 was re-assessed with best practice analysis criteria from April 2022, including refined masking and tree building algorithms and a ≥90% genome coverage threshold for sequences. 82 (5%) samples were excluded from the analysis with <90% genome available. The resulting clustering had a concordance of 93.2% with clusters observed in the original analyses conducted in 2020 (Supplemental methods). With 95% of sequences retained in the repeated analysis, and a very high correlation of the clustering observed between the original analyses and repeated analysis, we conclude that the initial genomic interpretations were robust, it should be noted that the inferences on possible transmission made during 2020 were assessed based on sample quality, phylogenetic and epidemiological data.
Table 1.
Summary of the 36 genomic epidemiological investigations.
| HCW | Patients | |
|---|---|---|
| Total - N | 765 | 1273 |
| Samples received at MDU-PHL – N (%) | 674 (88.1) | 1144 (89.9) |
| Sequences available – N (%) | 612 (80.0) | 1028 (80.0) |
| Number per investigation – Median (Range) | 6 (1-237) | 4 (1-395) |
| Characteristics | ||
| No. of HCF | 12 | |
| No. of campuses | 21 | |
| Total no. of beds | >9900 (median 159.3, range 14 – 704) | |
| Public acute care | 14 | |
| Public subacute care | 6 | |
| Large private hospital | 1 | |
| Paramedic Services | Multiple locations |
For the five healthcare networks where we performed analyses across the whole institution (all campuses) with detailed epidemiological data available, an estimated 59% to 80% of HCW infections were deemed to be likely acquired at the HCF.
Genomic results often, but not always, had high concordance with epidemiologic investigations
Genomic analysis provides an estimation of the minimum number of introductions to a facility through the number of genomic clusters present. The median number of introductions in these analyses was 3 per facility (IQR 2 - 8, range 1 – 35) and the median number of HCWs per genomic cluster was 1 (IQR 1-3, range 1 – 104). These analyses found that 31/36 (86.1%) investigations included cases resulting from multiple introductions, while 5/36 (13.9%) investigations involved a single introduction. Investigations with multiple introductions had a median of 6 HCWs (IQR 1 – 17, range 1 - 237) and 7 patients (IQR 3 – 39 range 1 - 395) while investigations with single introductions had a median of 1 HCW (IQR 1 – 6, range 1 – 7) and 2 patients (IQR 1 – 36, range 1 – 56). Thirteen of these analyses were instances of investigations into single staff members and their contacts; three of these could not be resolved as sequence data for the case or contacts was unavailable. While it is more likely to have multiple genomic introductions when there are high case numbers present at a facility, we found that low case numbers did not always result in fewer genomic introductions.
In these investigations, we largely observed high levels of concordance between epidemiological hypotheses (healthcare acquired infection or not) and genomic data where transmission had occurred, with some notable exceptions. Facility A, a multi-campus facility, epidemiologically identified multiple individual outbreaks within their campuses. The combined genomic epidemiological analysis found transmission events that were not detected by epidemiologic investigations, and that most of the individual outbreaks and unlinked cases were linked back to a single introduction or source (Figure 2, A). Conversely, Facility B experienced a large outbreak at one campus; genomics identified three concurrent outbreaks from separate genomic clusters at a time of high community prevalence (Figure 2, B). In both cases, genomic data significantly altered the understanding of transmission in the facilities, leading to changes in infection control practices. For example, at Facility A, upon reviewing the epidemiology in the light of the genomic data, it become clear that some epidemiological links were missed, highlighting the need to strengthen contact tracing applications and resources for this facility.
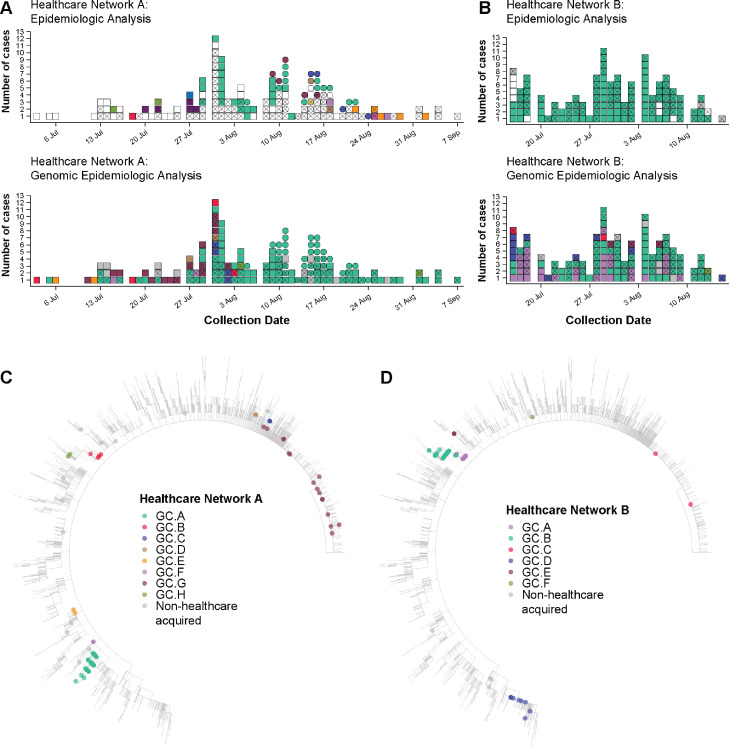
Figure 2.
Comparison of clustering (identifying cases of HCW and patient infections that are likely to be related) by epidemiology and genomics analyses at two facilities. Colour indicates cluster (epidemiological cluster for epidemiologic analyses and genomic cluster for genomic epidemiological analyses); white indicates unknown cluster/acquisition; grey indicates non-healthcare acquired infection; X indicates HCW case; squares and circles in panel A indicate two different campuses of the healthcare network. Panel A. Epidemiological analysis of COVID-19 cases at Facility A (two separate campuses) identified 12 epidemiologic clusters of likely transmission and 88 cases with no known acquisition source. Genomic epidemiologic analysis for the same network showed that the vast majority of cases were linked within eight genomic clusters, including one dominant cluster (lighter green), and only 12 cases not genomically linked to the HCF. Panel B. Epidemiological analysis of cases at Facility B identified 114 HCW cases likely acquired at the facility, all thought to be part of a single epidemiologic cluster, and nine HCW cases not thought to be healthcare acquired. Genomic epidemologic analysis indicated multiple introductions, rather than a single introduction, with six different genomic clusters co-occurring, and only six cases not genomically-linked to the HCF. Panels C and D. Maximum likelihood phylogenetic trees of Australian SARS-CoV-2 samples from wave 2, July–October 2020. Colour indicates cluster; genomic clustering is independent for each analysis; grey indicates non-healthcare acquired infection. Samples identified as part of genomic cluster GC.G (Panel C) and GC.C (Panel D) are not considered HCF-acquired without strong epidemiological evidence due to the high prevalence of this cluster in the wider community. Some larger clusters contain cases from different healthcare networks, which is not necessarily indicative of transmission between the networks (correlated with epidemiologic evidence).
Mobility of HCWs and patients often implicated in hospital transmissions
A common theme from HCFs was that many infections resulted from the mobility of staff or patients. Movement of staff and patients between wards and campuses while pre-symptomatic or asymptomatic was implicated in dissemination of COVID-19 between facilities within hospital networks (4/8 facilities where multiple campuses were investigated). At Facility C a single patient was found to have seeded cases in two wards due to transport while asymptomatic. Their movement between general and rehabilitation wards resulted in spread to 5 naïve patients and 15 HCWs. Identification of spread due to patient mobility led to one HCF to introduce asymptomatic testing for any patient moving from acute to subacute wards during periods of high community transmission.
Patient features or behaviours contributing to COVID-19 transmission to HCWs
In the course of these investigations, elderly patients with altered mental states were found to exhibit behaviours that contributed to the spread of COVID-19 within at least four HCFs. Patients suffering from delirium or dementia were often highly mobile (wandering behaviours) and exhibiting aerosol-generating behaviours (coughing, shouting or singing). Due to the nature of these patients and their increased need of HCW support, direct contact was often implicated in the transmission. In these cases, combined genomic and epidemiological data showed that one or more patients, admitted from a single ACF at the same time, were found to be the likely acquisition source for staff that contracted COVID-19 working on a ward for COVID-19 positive patients with dementia or delirium.
Limiting the scope of investigations may lead to erroneous conclusions
Investigations limited to a single ward were found to have limited utility when performed at large facilities with high numbers of positive cases. These investigations often found cases without any known transmission source, transmission, with multiple outbreaks deemed separate by epidemiological investigations, subsequently identified as single outbreaks by genomics. For example, investigation of a ward-based outbreak at Facility C identified eight genomically-linked cases. An expanded investigation, including all HCWs and patients at the facility in a similar time, identified an additional 10 cases were part of the same genomic transmission network as the first ward, indicating that unidentified transmission had likely occurred from the first ward analysed, and providing opportunities for further targeted epidemiologic investigations (Figure 3).
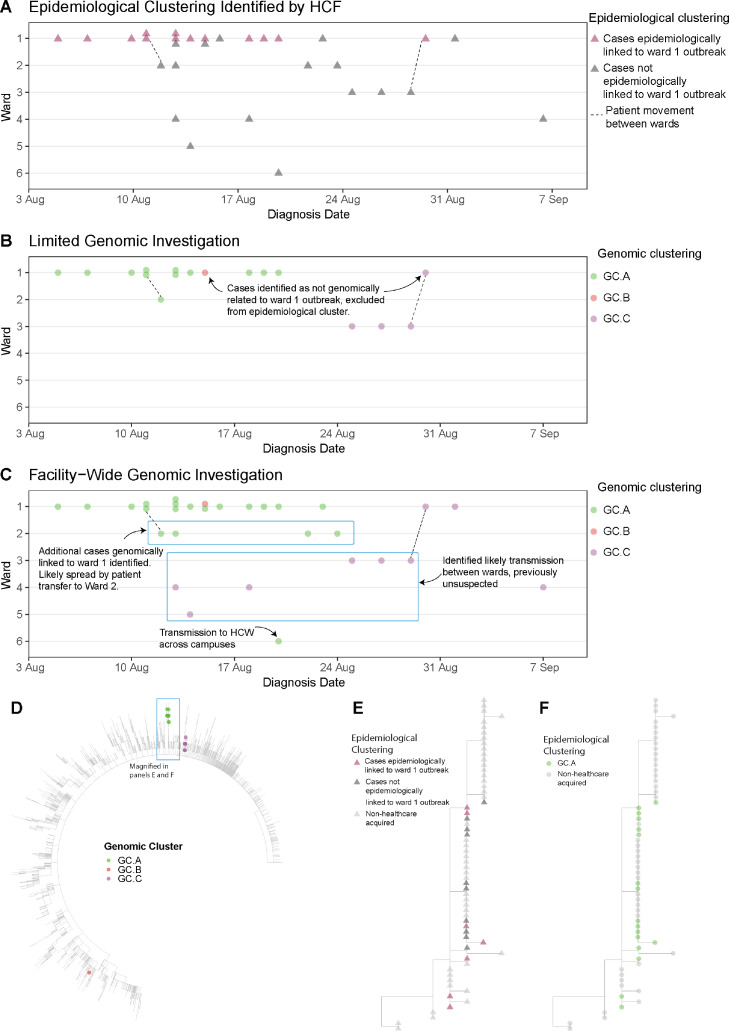
Figure 3.
Comparison of clustering (identifying cases of HCW and patient infections that are likely to be related) at Facility C using three different models: epidemiological clustering (Panel A), limited genomic investigation (cases in a single ward selected by HCF, panel B), and facility-wide genomic infections (Panel C). Each panel shows the distribution of cases (triangles in panel A, circles in panels B and C) across six different wards (Wards 1-6) over a six-week time period in 2020. In panel A, thirteen cases were identified by the HCF as a likely epidemiologic cluster (pink triangles). These cases, with the addition three cases from adjacent ward (Ward 3) were submitted for a limited genomic investigation (Panel B); cases (circles) are coloured by genomic cluster. This showed that most of the cases submitted were part of the same genomic cluster, but two of the Ward 1 cases were not linked (one case from GC.B, and one case from GC.C, which was linked to two other cases on Ward 3). Panel C shows a broader facility-wide genomic investigation that was undertaken to investigate cases on other wards; all HCW and patient cases were included in the facility-wide investigation. This genomic analysis found the main outbreak from Ward 1 was larger than first identified, linking outbreaks in adjacent wards to the Ward 1 outbreak, with cryptic transmission between wards resulting in spread, including transmission to another hospital campus. Unexpected links were also identified for GC.C, with cases spread over four wards. These genomic links were used to direct further investigations to identify causes of transmission and introduce mitigation strategies. Panel D shows a maximum likelihood phylogenetic tree of Facility C cases with Australian SARS-CoV-2 samples from wave 2, July – October 2020. Colour indicates cluster; genomic clustering labels are independent from previously presented analyses (labels simplified for ease of communication). Panel E shows a sub-section of the tree of panel D, with nodes coloured by epidemiologic clustering identified by the HCF, as in panel A; cases thought likely part of epidemiologic cluster (pink triangles), not epidemiologically linked (dark grey triangles) or not associated with the HCF (light grey triangles). Panel F shows same sub-tree as panel E, but coloured by genomic cluster; GC.A indicates the genomic cluster originally identified in ward 1 as in panels B and C; light grey circles indicate samples not associated with the HCF.
Similarly, limitations were found when examining investigations without contemporaneous genomic data from community cases. Lack of sufficient community cases for context can lead to inaccurate interpretation of transmission events. Initial investigations performed for Facility D without community context indicated likely transmission between HCWs in a work setting. The same data, when interpreted with community context, indicated that transmission was more likely to have occurred in a social setting external to the workplace, and confirmed by further epidemiologic investigations (Figure 4).
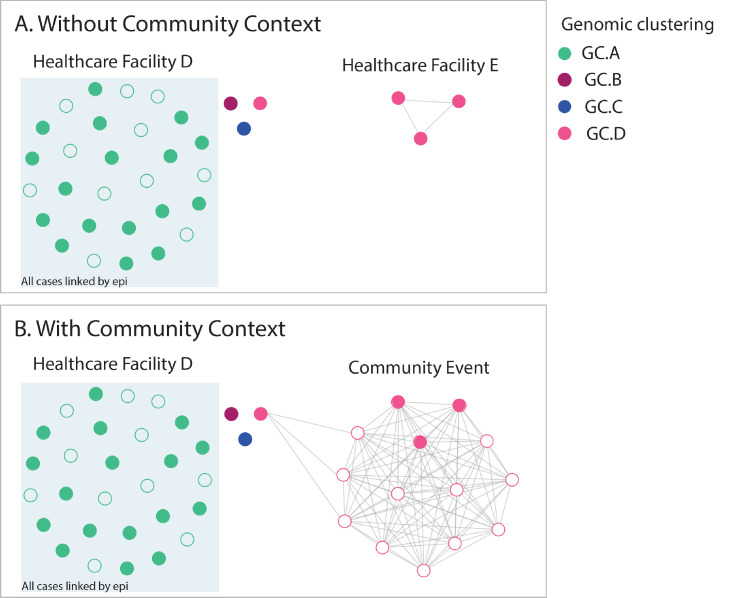
Figure 4.
Comparison of genomic epidemiological analyses analysed with and without genomic data for community cases. Filled circles indicate HCWs, unfilled circles indicate non HCWs, colour indicates genomic cluster. Panel A shows analysis of cases from facility D (mostly linked by epidemiology and genomics with dominant genomic cluster GC.A (green), and three additional HCW cases from different genomic clusters (genomic clusters GC.B, GC.C and GC.D), plus three cases at facility E (related to each other) from genomic cluster GC.D. In isolation, this suggests possible cryptic transmission between the two healthcare facilities. Addition of community sequences into the analysis (Panel B) demonstrated that the HCWs at both facility D and facility E likely acquired infection from a social event in the community that was attended by these cases.
Key learnings for genomic investigations of HCW infections
The collaborative meetings with HCFs provided an opportunity to educate clinicians about the utility and limitations of genomic analyses, share initial findings from the genomic analysis, add additional relevant epidemiological data to assist with interpretation, gauge the understanding of the genomic results and clarify further where necessary. They also provided an opportunity for additional epidemiological data that may have been missed during data collection, such as data on social links between cases e.g., staff often socialised together after working hours or lived in shared housing with other HCWs that maybe from the same or other HCFs, which is difficult to capture in standard line lists shared as part of the early investigation process. Anecdotally, one HCF identified that 50% of their HCWs lived with other HCWs.
Discussion
The COVID-19 pandemic has reinforced the need to optimise HCF systems to protect both patients and HCWs from infectious diseases threats.34 Here we detail pragmatic real-time genomic epidemiological investigations undertaken by a reference public health genomics laboratory of COVID-19 infections in HCWs across multiple facilities in Victoria, Australia and define a framework for this type of activity in future. Through an iterative, collaborative process with 12 HCFs, we performed 36 investigations for 765 HCWs, representing 62% of the total number of HCW infections notified for the state. Underpinning these analyses was efficient case ascertainment and a very high proportion of positive cases sequenced, including samples from HCWs and patients as well as the community, due to aggressive testing and contact tracing strategies. Several of these investigations were conducted in near-to-real-time which allowed facilities to rapidly change infection prevention protocols to limit further spread. A clear strength of the investigative process in this study was establishing a forum of laboratory and clinical experts to initiate, discuss and progress investigations which facilitate the integration of genomic results with infection prevention and control methods.
Whilst epidemiologic outbreak investigations conducted by IPC teams identify many likely cases of transmission and contributing factors, this study highlights cases where integrated genomic and epidemiologic analysis adds greater resolution to outbreak investigations, enhancing (but not replacing) traditional methods. We emphasise here important commonalities that were seen across the facilities investigated and the importance of understanding SARS-CoV-2 transmission for future outbreak prevention. We found that the physical movement of individuals as well as aerosol generating behaviours were contributing factors to transmission within the facilities we investigated, and genomic analyses were able to identify links here that were not suggested by epidemiologic investigations. While this pattern of staff and patient movement is likely ubiquitous to HCFs and has been seen to contribute to the spread of COVID-19 elsewhere,20,21,35 it highlights the importance of investigating all positive cases of HCWs and patients within a facility. We noted instances such as at Facility A, where the genomic data refuted the findings of the epidemiologic data, interpretation of the two data sets together would significantly change the infection control response. Similar scenarios were found by Meijer et al.36
In settings where capacity to perform these analyses are limited, informative results can be obtained from stage one described in the methods and Demographic data outlined in Box 1. Where feasible these results can be expanded by combining genomic data with baseline epidemiological data such as those outlined in Case information in Box 1. While genomic analyses can be informative with basic epidemiological data, the rich detail added by comprehensive epidemiological data can significantly improve their utility. Rapid and effective data capture and management was a significant challenge for most facilities during the epidemic, delaying and limiting infection control investigations; implementation of sustainable continuous data collection processes within HCFs should be a priority for future epidemic preparedness, allowing earlier initiation of epidemiological and genomic investigations.
Based on our experiences, we propose a set of minimum and enhanced metadata and a workflow to optimise the utility of HCW investigations (Box 1), recognising that expansion and resourcing for such systems can vary between facilities. Wherever possible, integration with existing data systems should be leveraged, such as data from employee databases. Metadata should be collected in standardised templates, and captured in a secure version-controlled database (e.g. REDCap). This maintains data integrity during staff turnover or when surge capacity is called for in response to events. The World Health Organisation (WHO) has outlined the minimum metadata to ensure that genomic sequencing of SARS-CoV-2 samples will be of most use.37 From our experiences here, we propose that these metadata should ideally be expanded when performing genomic epidemiological analysis. To allow for rapid utilisation of data when the need arises, prior consideration should be given to the governance framework for the use and integration of the data into other systems, such as disclosure to public health laboratories during investigations, and its relationship to data captured by other public health organisations.
Limitations of the study include the highly clonal nature of cases in Victoria at this time, with >95% of cases from the second wave being seeded for a single transmission event. This limited the ability to resolve some transmission networks, particularly early in the outbreak, and may erroneously suggest single introductions of a cluster when there may have been multiple introductions from a genomic cluster from the community. Distinguishing community from healthcare acquisition can also be more challenging with clonal outbreaks, hence it is important for IPC teams to liaise closely with their sequencing laboratory and genomic epidemiologists to ensure adequate genomic diversity is present to accurately infer potential healthcare transmissions. At this time in Victoria, strict public health restrictions and aggressive contact tracing efforts were in place, meaning that potential community exposures for HCWs were well-defined, and were taken into account during the genomic epidemiologic investigations. This emphasises the importance of high-quality epidemiologic data to assist with interpretation of genomic data when performing these analyses. Alternative analytical methods for highly-clonal datasets, such as examining minor allele frequencies and using advanced phylodynamic tools that incorporate some epidemiologic data, could also be considered in healthcare settings where bioinformatic resources and data governance allow.
Our investigations were also limited by HCW and patient cases that were not able to be sequenced although numbers were relatively small, and the proportion of cases successfully sequenced was greater than most other jurisdictions. Similar processes could easily be applied to other healthcare systems where genomics is less commonly available; in particular, focussed sequencing of hospitalised cases and HCWs could achieve very similar results, albeit with a small chance of false-positive genomic links due to multiple introductions of the same genomic cluster from the community.
The results from each facility have shown that there were multiple contributors to COVID-19 infections in HCWs in Victoria in 2020, and that while there were common factors contributing to transmission across different facilities, each outbreak was in fact a unique combination of contributors and had to be assessed individually. Through our experience working with multiple HCFs, we found that it was essential to investigate all positive HCW and patient cases in a facility along with detailed epidemiological data, wherever feasible. Collaborative and interactive exploration of the combined data uncovered further epidemiological links, maximising the impact of the analyses for the HCF, and providing the greatest opportunities for HCFs to optimise the safety of HCWs and patients in the future.
Contributors
AEW, NLS, PA, BPH conceived manuscript concept. AEW, NLS, PA, analysed and interpreted data, and were major contributors in writing the manuscript. CRL, SJ, MW, CC, DJB, performed data analysis and management. KH, TS performed bioinformatic analysis. MS, SAB, managed sample testing and sequencing of samples. CM, MK, RS, CM, JCK, PB, PGK, AC, SG, NM, KS, DAW, performed local epidemiological analysis at health care facilities. BPH, PA, TS edited the manuscript. All authors read and approved the final manuscript.
Declaration of interests
All authors declare no competing interests and confirm that authors or their institutions have not received any payments or services in the past 36 months from a third party that could be perceived to influence, or give the appearance of potentially influencing, the submitted work
Data availability
All consensus sequences and Illumina sequencing reads are available at https://github.com/MDU-PHL/COVID19-paper, a subset of samples listed under paper 1 and paper 2. Sequence accession numbers can be found in Supplementary Table 1 (note: some cases were included in investigations at more than one facility).
Acknowledgments
We gratefully acknowledge the large number of staff at Victorian healthcare facilities and diagnostic laboratories who collected data and undertook diagnostic testing for COVID-19 in Victoria. We would particularly like to acknowledge the considerable efforts of infection prevention and control staff at affected facilities. We would especially acknowledge the contribution of Terri Butcher and Adrian Tramontana (Western Health), Andrew Stewardson, Allen Cheng, Amanda Dennison, Anton Peleg, and Denis Spelman (Alfred Health), Claire Gordon (Austin Health), Kylie Hall and Alexandra Bonello (Eastern Health), Bradley Gardiner and Suman Majumdar (Epworth), Tony Korman (Monash Health), Victoria Madigan, Barbara Brozic and Madelaine Flynn (Northern Hospital), Susan Gonelli (Peninsula Health), Katherine Bond, Vivian Leung, Chris Bailie, Laura Piu, and Kirsty Buising (Royal Melbourne Hospital), Leilani Knapp, Mary-Jo Waters, Yves Lorenzo and Samantha Palmby (St Vincent's Hospital).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100487.
Appendix. Supplementary materials
References
- 1.Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36(3):287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vahey GM, McDonald E, Marshall K, Martin SW, Chun H, Herlihy R, Tate JE, Kawasaki B, Midgley CM, Alden N, Killerby ME. Risk factors for hospitalization among persons with COVID-19—Colorado. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0256917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang G, Xie L, Chen Z, Hao S, Fu C, Wu Q, Liu X, Li S. Clinical risk factors for mortality of hospitalized patients with COVID-19: systematic review and meta-analysis. Ann Palliat Med. 2021;10(3):2723–2735. doi: 10.21037/apm-20-1278. [DOI] [PubMed] [Google Scholar]
- 4.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PG, Fu H, Dighe A. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanei M, Keyvani H, Haghdoost A, Abolghasemi H, Janbabaei G, Jamshidi HR, Ghazale AH, Saadat SH, Fesharaki MG, Raei M. The risk factors and related hospitalizations for cases with positive and negative COVID-19 tests: a case-control study. Int Immunopharmacol. 2021;98 doi: 10.1016/j.intimp.2021.107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S, Machado F, Kwizera A, et al. COVID-19: a heavy toll on health-care workers. Lancet Respiratory Med. 2021;9(3):226–228. doi: 10.1016/S2213-2600(21)00068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quigley AL, Stone H, Nguyen PY, Chughtai AA, MacIntyre CR. Estimating the burden of COVID-19 on the Australian healthcare workers and health system during the first six months of the pandemic. Int J Nurs Stud. 2021;114 doi: 10.1016/j.ijnurstu.2020.103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whyler NCA, Sherry NL, Lane CR, et al. Viral genomics to inform infection control response in occupational COVID-19 transmission. Clin Infect Dis. 2020;73(7):ciaa1385. doi: 10.1093/cid/ciaa1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapolla P, Mingoli A, Lee R. Deaths from COVID-19 in healthcare workers in Italy—What can we learn? Infection Control Hospital Epidemiol. 2021;42(3):364–365. doi: 10.1017/ice.2020.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane CR, Sherry NL, Porter AF, et al. Genomics-informed responses in the elimination of COVID-19 in Victoria, Australia: an observational, genomic epidemiological study. Lancet Public Health. 2021;6(8):e547–e556. doi: 10.1016/S2468-2667(21)00133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson P, Sherry NL, Howden BP. Surveillance for SARS-CoV-2 variants of concern in the Australian context. Med J Aust. 2021;214(11):500–502.e1. doi: 10.5694/mja2.51105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geoghegan JL, Ren X, Storey M, et al. Genomic epidemiology reveals transmission patterns and dynamics of SARS-CoV-2 in Aotearoa New Zealand. Nat Commun. 2020 Dec;11(1):1–7. doi: 10.1038/s41467-020-20235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Giallonardo F, Duchene S, Puglia I, et al. Genomic epidemiology of the first wave of SARS-CoV-2 in Italy. Viruses. 2020;12(12):1438. doi: 10.3390/v12121438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myhrman S, Olausson J, Ringlander J, Gustavsson L, Jakobsson HE, Sansone M, Westin J. Unexpected details regarding nosocomial transmission revealed by whole-genome sequencing of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) Infect Control Hospital Epidemiol. 2021 doi: 10.1017/ice.2021.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellingford JM, George R, McDermott JH, Ahmad S, Edgerley JJ, Gokhale D, Newman WG, Ball S, Machin N, Black GC. Genomic and healthcare dynamics of nosocomial SARS-CoV-2 transmission. Elife. 2021;10:e65453. doi: 10.7554/eLife.65453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Løvestad AH, Jørgensen SB, Handal N, Ambur OH, Aamot HV. Investigation of intra-hospital SARS-CoV-2 transmission using nanopore whole-genome sequencing. J Hosp Infect. 2021;111:107–116. doi: 10.1016/j.jhin.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis RV, Billam H, Clarke M, Yates C, Tsoleridis T, Berry L, Mahida N, Irving WL, Moore C, Holmes N, Ball JK. The impact of real-time whole-genome sequencing in controlling healthcare-associated SARS-CoV-2 outbreaks. J Infect Dis. 2022;225(1):10–18. doi: 10.1093/infdis/jiab483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucey M, Macori G, Mullane N, et al. Whole-genome sequencing to track severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in nosocomial outbreaks. Clin Infect Dis. 2021;72(11):e727–e735. doi: 10.1093/cid/ciaa1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meredith LW, Hamilton WL, al Warne Bet. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20(11):1263–1272. doi: 10.1016/S1473-3099(20)30562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National, state and territory population, December 2020 | Australian Bureau of Statistics [Internet]. [cited 2021 Jun 21]. Available from: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release
- 23.Seemann T, Lane CR, Sherry NL, et al. Tracking the COVID-19 pandemic in Australia using genomics. Nat Commun. 2020;11(1):1–9. doi: 10.1038/s41467-020-18314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poletti P, Tirani M, Cereda D, et al. Seroprevalence of and risk factors associated with SARS-CoV-2 infection in health care workers during the early COVID-19 pandemic in Italy. JAMA Network Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa-Martínez CL, Schwierzeck V, Mellmann A, Hennies M, Kampmeier S. Healthcare-associated sars-cov-2 transmission—experiences from a german university hospital. Microorganisms. 2020;8(9):1378. doi: 10.3390/microorganisms8091378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gholami M, Fawad I, Shadan S, Rowaiee R, Ghanem H, Omer A, Ho HS. COVID-19 and healthcare workers: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:335–346. doi: 10.1016/j.ijid.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumley SF, Constantinides B, Sanderson N, Rodger G, Street TL, Swann J, Chau KK, O'Donnell D, Warren F, Hoosdally S, Prevention OI. Epidemiological data and genome sequencing reveals that nosocomial transmission of SARS-CoV-2 is underestimated and mostly mediated by a small number of highly infectious individuals. J Infect. 2021;83(4):473–482. doi: 10.1016/j.jinf.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans S, Agnew E, Vynnycky E, et al. The impact of testing and infection prevention and control strategies on within-hospital transmission dynamics of COVID-19 in English hospitals. Philosophical Transactions of the Royal Society B. 2021;376(1829) doi: 10.1098/rstb.2020.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caly L, Druce J, Roberts J, et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med J Aust. 2020;212(10):459–462. doi: 10.5694/mja2.50569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.artic-ncov2019/primer_schemes/nCoV-2019/V3 at master · artic-network/artic-ncov2019 · GitHub [Internet]. [cited 2021 May 11]. Available from: https://github.com/artic-network/artic-ncov2019/tree/master/primer_schemes/nCoV-2019/V3
- 31.nCoV-2019 sequencing protocol [Internet]. [cited 2021 May 11]. Available from: https://www.protocols.io/view/ncov-2019-sequencing-protocol-bbmuik6w?version_warning=no
- 32.Hospital resources 2017–18: Australian hospital statistics, Hospitals and average available beds - Australian Institute of Health and Welfare [Internet]. [cited 2021 Jun 23]. Available from: https://www.aihw.gov.au/reports/hospitals/hospital-resources-2017-18-ahs/contents/hospitals-and-average-available-beds
- 33.Victorian healthcare worker (clinical and non-clinical) COVID-19 data | Coronavirus Victoria [Internet]. [cited 2021 Jun 21]. Available from: https://www.coronavirus.vic.gov.au/healthcare-worker-covid-19-data
- 34.Abbas M, Nunes TR, Martischang R, et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrobial Resist Infect Control. 2021;10(1):1–3. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng VC, Fung KS, Siu GK, et al. Nosocomial outbreak of coronavirus disease 2019 by possible airborne transmission leading to a superspreading event. Clin Infect Dis. 2021;73(6):e1356–e1364. doi: 10.1093/cid/ciab313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer SE, Harel N, Ben-Ami R, et al. Unraveling a nosocomial outbreak of COVID-19: the role of whole genome sequence analysis. InOpen Forum Infectious Diseases 2021. [DOI] [PMC free article] [PubMed]
- 37.WHO. Genomic sequencing of SARS-CoV-2: a guide to implementation for maximum impact on public health [Internet]. [cited 2021 Mar 26]. Available from: https://www.who.int/publications/i/item/9789240018440
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All consensus sequences and Illumina sequencing reads are available at https://github.com/MDU-PHL/COVID19-paper, a subset of samples listed under paper 1 and paper 2. Sequence accession numbers can be found in Supplementary Table 1 (note: some cases were included in investigations at more than one facility).