Abstract
Aim
Lots of researches have endeavored to elucidate the pathogenetic mechanism of visceral hypersensitivity in order to guide the therapy of diarrhea predominant-irritable bowel syndrome (IBS-D). Transient receptor potential (TRP) channels and their role in visceral nociception have been vastly investigated. We investigated the expression of TRP channels in IBS-D colonic biopsies and its correlation with the severity of the disease.
Methods
Sigmoid biopsies were obtained from 34 IBS-D patients and 28 healthy controls (HCs). IBS-D was diagnosed according to Rome IV criteria. Their clinical parameters were assessed through questionnaires. Expression of TRPV1, TRPV4, TRPA1, TRPM2, and TRPM8 was evaluated with immunohistology staining.
Results
Expression levels of TRPV1, TRPV4, and TRPA1 in the colonic mucosa of IBS-D patients were significantly higher than those in HCs (p < 0.05), while there was no obvious difference of TRPM2 and TRPM8 expression between IBS-D patients and HCs. In addition, the expression levels of TRPV1 and TRPA1, but TRPV4, in the colonic mucosa correlated positively with the severity of diseases (r = 0.6303 and 0.4506, respectively, p < 0.05).
Conclusions
Expression of TRPV1, TRPA1, and TRPV4 in the colonic mucosa was enhanced in IBS-D patients compared with HCs with the former two correlated with the severity of the disease. TRP channels might be promising biomarkers in the diagnosis and estimate of the severity in IBS-D.
1. Introduction
Irritable bowel syndrome (IBS) is a disease manifested by abdominal pain and altered bowel habits, which afflicts 5 to 10% of the population in a relapsing and remitting manner [1]. According to Rome IV, the diagnosis is based on symptom analysis excluding biochemical abnormalities [2]. It is both difficult and money consuming since there is no reliable and recognized biomarker. Thus, discovery of a universally accepted biomarker is in urgent need.
Visceral hypersensitivity is one of the core pathophysiological factors of IBS [3], besides the disordered brain-gut axis [4], intestinal flora disturbance [5], or dysfunction of mucosal immunity [6]. The intestine is equipped with various ion channels and nociceptive receptors to transduce and respond to physiochemical stimuli [7]. Alteration in the expression or functioning would lead to aberrant visceral nociception. Transient receptor potential (TRP) channels are the most extensively studied ion channel family contributing to visceral hypersensitivity.
Of all TRP channels, TRPV1 is the best characterized and most studied. Enhanced expression of TRPV1 was found in the sigmoid of IBS patients [8], and the severity of the disease correlated positively with the content of TRPV1. TRPA1, as demonstrated in various literature, is an important mediator in visceral hypersensitivity [9, 10]. Evidence of its role in IBS is mainly derived from animal models. Previously, we have also ascertained its role in exacerbated mechanonociception after cold exposure in a classical animal model [11]. Unfortunately, clinical studies concerning TRPA1 still lack. It is the same case with TRPV4 [12, 13], TRPM2 [14], and TRPM8 [15, 16], as lots of preclinical studies indicated their involvement in visceral nociception.
In this study, we explored the expression of TRP channels in the colonic mucosa of IBS-D and healthy controls and investigated the relationship between the expression of TRP channels and the severity of diseases. Hopefully, it would offer more evidence of the role of TRP channels in IBS-D and help with the diagnosis and severity evaluation of the disease.
2. Materials and Methods
2.1. Human Biopsies
Thirty-four patients with IBS-D (20 males and 14 females, aged 44.53 ± 2.15 years) and 28 healthy controls (HCs) (12 males and 16 females, aged 47.43 ± 2.66 years) undergoing colonoscopy for colorectal cancer screening were enrolled in the study. IBS-D was diagnosed according to the self-completed ROME IV modular questionnaire. Clinical parameters including weight, height, and patients' abdominal pain severity measured with the visual analogue scale (VAS) [17] were collected prior to bowel preparation. All the involved subjects had no history of abdominal surgery or organic diseases. Three sigmoid mucosal samples were obtained from each subject with standard biopsy forceps and placed in 4% paraformaldehyde for 24 hours. This study has been approved by the Renji Hospital Ethics Committee. All patients and healthy controls provided written informed consent.
2.2. Immunohistochemistry Staining
Fixed colonic mucosal samples were dehydrated, paraffin embedded, and sectioned. Tissue sections of 4 μm thickness were mounted on silicone-coated slides, deparaffinized, and heated in citrate buffer (pH 6) using high-pressure cooking for antigen retrieval. Then, the sections were incubated with primary antibodies of TRPV1 (ab3487, 1 : 50, Abcam, Cambridge, MA, USA), TRPA1 (ACC-037, 1 : 50, Alomone Labs, Jerusalem, Israel), TRPV4 (ACC-034,1 : 50), TRPM2 (ACC-043, 1 : 50), and TRPM8(ACC-049,1 : 200) at 4°C overnight. After being rinsed with phosphate buffer saline (PBS) for three times, they were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) for 30 minutes at room temperature and visualized with a diaminobenzidine-enhanced liquid substrate system (Sigma-Aldrich, St. Louis, MO, USA). For semiquantitation, captured images were analyzed with ImageJ software (National Institutes of Health).
2.3. Statistics
Continuous variables were expressed as mean ± SEM, and statistical analyses were performed using unpaired Student's t-test. Categorical data were expressed as n (%) and analyzed with the chi-square test or Fisher exact tests if appropriate. Correlation between the expression of TRP channels and severity of the disease was assessed by Pearson's correlation coefficient. Based on the previous study about the correlation between TRPV1 levels and pain severity [8], a sample size of at least 22 IBS-D patients was required to reach an effect size of 0.68, with a power of 95% and a 5% level of significance [18]. Data was analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered significant.
3. Results
3.1. Expression of TRPV1 Was Increased in the Colonic Mucosa of IBS-D Patients and Correlated with the Severity of the Disease
To start with, no difference was detected between IBS-D and HC groups with regard to age, gender, or BMI distribution (p > 0.05) (Table 1). The VAS pain scores of patients were normally distributed (Figure 1). Since accumulating evidence has indicated the involvement of TRPV1 in IBS-D, we begun with TRPV1 to verify the disparate expression between HCs and IBS-D. As shown in Figures 2(a) and 2(b), the expression of TRPV1 in the IBS-D group was higher than that in the HC group (p < 0.0001). The correlation analysis between the expression of TRPV1 and severity of disease further revealed that IBS-D patients with higher VAS score tended to exhibit intensified expression of TRPV1 (p < 0.0001, r = 0.6303, Figure 2(c)).
Table 1.
Patient demographics.
| HC | IBS-D | p value | ||
|---|---|---|---|---|
| Case, n | 28 | 34 | ||
| Age, mean ± SEM | 47.43 ± 2.66 | 44.53 ± 2.15 | 0.820 | |
| Gender, n (%) | Female | 16 (57.1%) | 14 (41.2%) | 0.211 |
| Male | 12 (42.9%) | 20 (58.8%) | ||
| BMI, n (%) | Thin | 4 (14.3%) | 3 (8.8%) | 0.921 |
| Normal | 16 (57.1%) | 21 (61.8%) | ||
| Overweight | 7 (25.0%) | 9 (26.5%) | ||
| Obese | 1 (3.6%) | 1 (2.9%) | ||
BMI: body mass index (kg/m2, calculated as weight/height2), thin < 18.5, 18.5 ≤ normal < 24, 24 ≤ overweight < 28, and obese ≥ 28.
Figure 1.
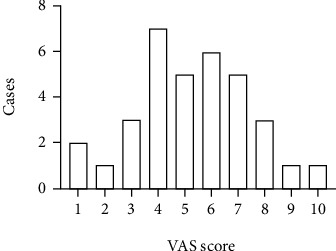
Abdominal pain severity of the IBS-D patients. VAS: visual analogue scale.
Figure 2.
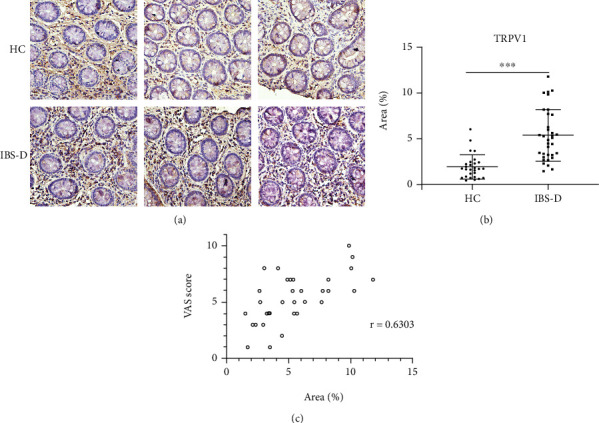
Expression of TRPV1 was increased in the colonic mucosa of IBS-D patients and correlated with the severity of the disease. (a, b) Expression of TRPV1 in the colonic mucosa of IBS-D patients was higher than that of HCs. (c) Expression of TRPV1 in the colonic mucosa correlated with the severity of IBS-D. Scale bar, 50 μM. ∗∗∗p < 0.001.
3.2. TRPA1 and TRPV4 Expression Was Enhanced in the Colonic Mucosa of IBS-D Compared with HC
Since TRPA1 was almost exclusively expressed in TRPV1-positive neurons and they interact with each other under many circumstances [19, 20], we evaluated the expression of TRPA1 in the colonic mucosa. It was shown that expression of TRPA1 was enhanced in the biopsies of the IBS-D group compared with HCs (p = 0.008, Figures 3(a) and 3(b)). Further analysis demonstrated a slightly weaker but significant correlation between the expression of TRPA1 and the severity of disease (p = 0.0075, r = 0.4506, Figure 3(c)). TRPV4 was another TRP channel with enhanced expression in the colonic mucosa of IBS-D patients (Figures 3(d) and 3(e)). However, no correlation between the expression level and severity of the disease was found (p > 0.05).
Figure 3.
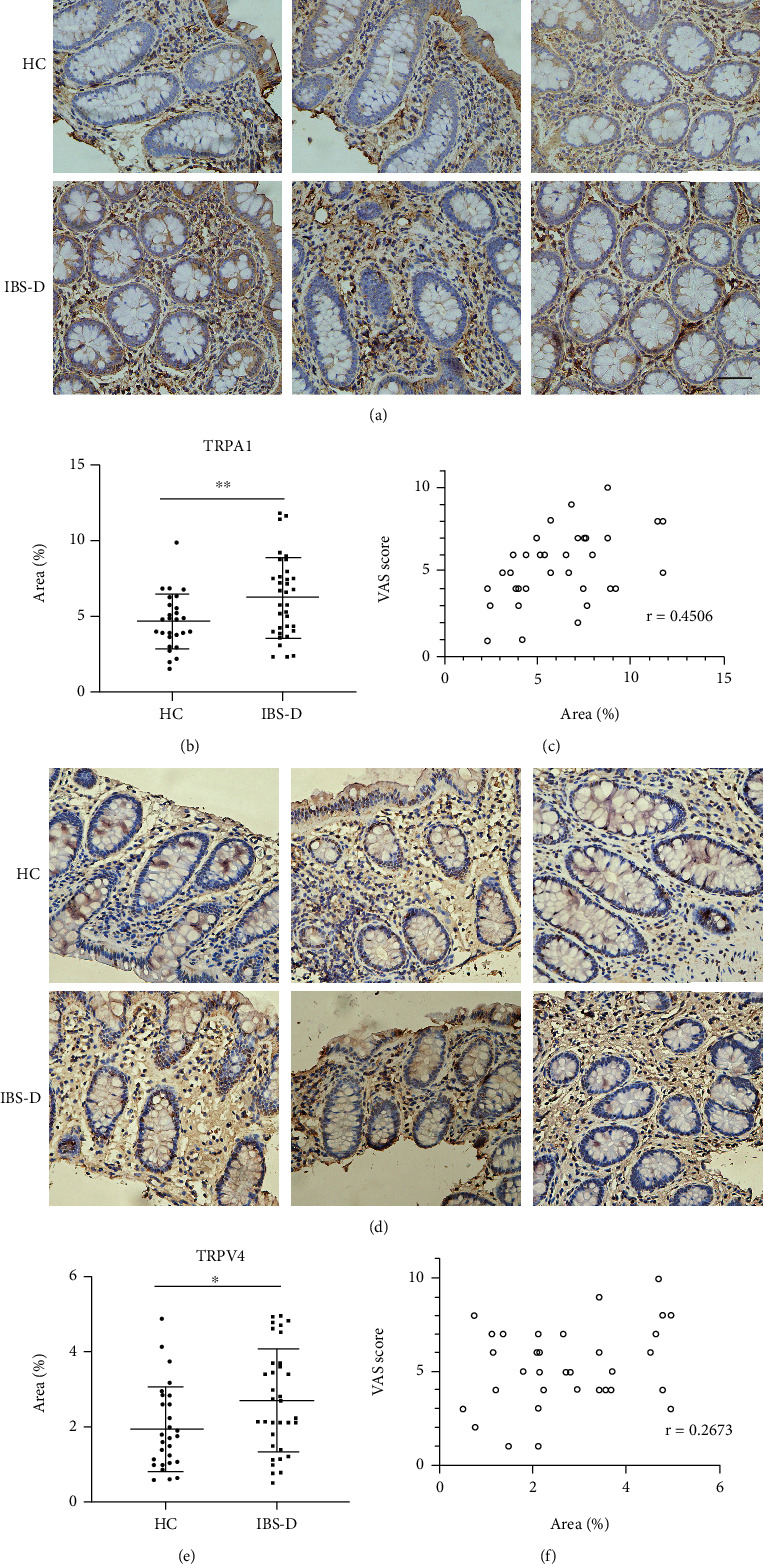
Expression of TRPA1 and TRPV4 was increased in the colonic mucosa of IBS-D patients and TRPA1 content correlated with the severity of the disease. (a, b) The expression of TRPA1 in the colonic mucosa of IBS-D patients was increased compared with HCs. (c) TRPA1 content correlated positively with the disease severity. (d, e) The expression of TRPV4 was enhanced in the colonic biopsies of IBS-D patients. (f) TRPV4 expression did not correlate with the disease severity. Scale bar, 50 μM. ∗p < 0.05, ∗∗p < 0.01.
3.3. TRPM2 and TRPM8 Expression Did Not Differ between IBS-D and HC
Our previous study has found the role of TRPM8 in visceral hypersensitivity [15]. The immunostaining of TRPM8, however, showed no difference between IBS-D and HC (p > 0.05, Figures 4(a) and 4(b)). It was the same case with TRPM2 (p > 0.05, Figures 4(c) and 4(d)).
Figure 4.
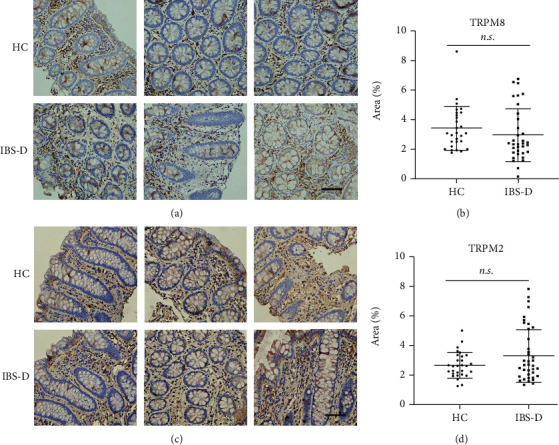
Expression of TRPM2 and TRPM8 did not differ between IBS-D patients and HCs. (a, b) No difference in the expression of TRPM8 was found between IBS-D and HC groups. (c, d) No difference in the expression of TRPM2 was found between IBS-D and HC groups. Scale bar, 50 μM.
4. Discussion
Relief of visceral pain is an unmet need in clinical practice of IBS-D treatment. Mounting evidence indicated the involvement of TRP channels in visceral hypersensitivity, one of the core pathophysiological mechanisms. Except for TRPV1, most studies about TRP channels focused on preclinical animal models and the results varied. Our present study is aimed at comparing the expression of TRP channels in the colonic mucosa of IBS-D patients and HCs and correlation between the expression and the severity of the disease.
The role of TRPV1 in IBS has been extensively investigated [21, 22]. In our study, we verified a similar phenomenon that expression of TRPV1 was enhanced in the colonic mucosa of IBS-D patients compared with HCs and correlated with the severity of the disease [8, 21], further supporting TRPV1 as a valuable target in coping with visceral pain.
Previous researches about the role of TRPA1 in visceral hypersensitivity were mainly based on preclinical studies [23, 24]. In Kun's study, the mRNA level of TRPA1 was increased in the biopsies of active IBD patients rather than in the quiescent state [25], indicating its involvement in acute pain perception. In our study, TRPA1 expression was increased in the colonic mucosa of IBS-D patients and the expression level positively correlated with the severity of the disease. According to some researchers' opinion, TRPA1 was sensitized rather than overexpressed in IBS [26]. The different results may be due to the subtype of IBS patients included in the study. Even though the results concerning TRPA1 were promising, more clinical studies are warranted since the sample size in this study was comparatively small.
Activation of TRPV4 triggers visceral hypersensitivity based on mounting evidence. In one study, it was found that 5,6-EET (an endogenous TRPV4 agonist), but not TRPV1 or the TRPA1 agonist, was increased in the biopsies of IBS patients compared with HCs and the concentration correlated with abdominal pain [27]. According to McGuire et al., human serosal nociceptor mechanosensitivity was attenuated by TRPV4 antagonist, HC067047 [28], which is evidenced by the role of TRPV4 in visceral hypersensitivity. In our study, the expression of TRPV4 in the colonic mucosa was increased in IBS-D groups. However, no relationship was found between the expression level and disease severity.
TRPM2 and TRPM8 were the relatively less-studied TRP channels. We have previously found that either altered distribution or expression of TRPM8 in sensory neurons under different circumstances contributed to visceral hypersensitivity [15]. For TRPM2, its expression was enhanced in the distal colon of a TNBS-colitis rat model and administration of TRPM2 antagonist or TRPM2 deficiency-attenuated visceromotor response to colorectal distension [14]. Unfortunately, in this study, we did not find altered expression of these two TRP channels in IBS-D patients compared with HCs. There were studies suggesting upregulated TRPM8 as a protective factor against inflammatory mediators [16, 29, 30]. With divergent opinion towards the role of TRPM8 and TRPM2, more preclinical and clinical studies are needed to reach a consensus.
The strength of this study is that we compare the expression of TRP channels in IBS-D, a specific subtype of IBS, and HCs, since different subtypes of IBS may have different pathophysiological mechanisms. Besides, we explored the relationship between the expression of TRP channels and the severity of the disease, which may offer insights into the diagnosis and severity measurement of IBS-D. However, the shortcomings of this study are obvious. First, the sample size was rather small. Secondly, a lot of clinical parameters are missing, e.g., food pattern, psychological factors, and stool frequency.
5. Conclusions
In conclusion, TRPV1 is a promising factor in the diagnosis and severity evaluation of IBS-D. It may become an intervenable target in dealing with IBS-D in the future. More preclinical and clinical studies may help verdict the role and relationship between TRP channels and IBS-D.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 82000487 and 81970473).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
S-L C designed the study. L C and Q-Q L performed the experiments and analyzed the data. Q-Q L and S-L C wrote the paper. All authors approved the final manuscript. Li Cheng and Qing-Qing Luo contributed equally to this work.
References
- 1.Sperber A. D., Dumitrascu D., Fukudo S., et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut . 2017;66(6):1075–1082. doi: 10.1136/gutjnl-2015-311240. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F., Lacy B. E., Chang L., et al. Bowel disorders. Gastroenterology . 2016;150(6):1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Akbar A., Walters J. R., Ghosh S. Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Alimentary Pharmacology & Therapeutics . 2009;30(5):423–435. doi: 10.1111/j.1365-2036.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayer E. A., Tillisch K. The brain-gut axis in abdominal pain syndromes. Annual Review of Medicine . 2011;62(1):381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posserud I., Stotzer P. O., Bjornsson E. S., Abrahamsson H., Simren M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut . 2007;56(6):802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aerssens J., Camilleri M., Talloen W., et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology . 2008;6(2):194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brierley S. M., Linden D. R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nature Reviews. Gastroenterology & Hepatology . 2014;11(10):611–627. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- 8.Akbar A., Yiangou Y., Facer P., Walters J. R., Anand P., Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut . 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brierley S. M., Hughes P. A., Page A. J., et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology . 2009;137(6):2084–2095.e3. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brierley S. M., Castro J., Harrington A. M., et al. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. The Journal of Physiology . 2011;589(14):3575–3593. doi: 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Luo Q., Yan X., Li W., Chen S. Vagal transient receptor potential ankyrin 1 mediates stress-exacerbated visceral mechanonociception after antral cold exposure. Journal of Neurogastroenterology and Motility . 2019;25(3):442–460. doi: 10.5056/jnm19014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cenac N., Altier C., Chapman K., Liedtke W., Zamponi G., Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology . 2008;135(3):937–946.e2. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Cenac N., Altier C., Motta J. P., et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut . 2010;59(4):481–488. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K., Takagi K., Kato A., et al. Role of transient receptor potential melastatin 2 (TRPM2) channels in visceral nociception and hypersensitivity. Experimental Neurology . 2016;285:41–50. doi: 10.1016/j.expneurol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Luo Q. Q., Wang B., Chen X., et al. Acute stress induces visceral hypersensitivity via glucocorticoid receptor-mediated membrane insertion of TRPM8: involvement of a non-receptor tyrosine kinase Pyk2. Neurogastroenterology and Motility . 2020;32(10):1514–1528. doi: 10.1111/nmo.13877. [DOI] [PubMed] [Google Scholar]
- 16.Hosoya T., Matsumoto K., Tashima K., et al. TRPM8 has a key role in experimental colitis-induced visceral hyperalgesia in mice. Neurogastroenterology and Motility . 2014;26(8):1112–1121. doi: 10.1111/nmo.12368. [DOI] [PubMed] [Google Scholar]
- 17.Bengtsson M., Ohlsson B., Ulander K. Development and psychometric testing of the visual analogue scale for irritable bowel syndrome (VAS-IBS) BMC Gastroenterology . 2007;7(1):p. 16. doi: 10.1186/1471-230X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondal H., Mondal S. Sample size calculation to data analysis of a correlation study in Microsoft Excel®: a hands-on guide with example. International Journal of Clinical and Experimental Physiology . 2016;3(4):180–189. doi: 10.4103/2348-8832.196896. [DOI] [Google Scholar]
- 19.Spahn V., Stein C., Zollner C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Molecular Pharmacology . 2014;85(2):335–344. doi: 10.1124/mol.113.088997. [DOI] [PubMed] [Google Scholar]
- 20.Story G. M., Peier A. M., Reeve A. J., et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell . 2003;112(6):819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 21.van Wanrooij S. J., Wouters M. M., Van Oudenhove L., et al. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? American Journal of Gastroenterology . 2014;109(1):99–109. doi: 10.1038/ajg.2013.371. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Q., Yang L., Larson S., et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut . 2016;65(5):797–805. doi: 10.1136/gutjnl-2013-306464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christianson J. A., Bielefeldt K., Malin S. A., Davis B. M. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain . 2010;151(2):540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y. B., Yang J., Zuo X. L., Gao L. J., Wang P., Li Y. Q. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochemical Research . 2010;35(5):797–803. doi: 10.1007/s11064-010-0137-z. [DOI] [PubMed] [Google Scholar]
- 25.Kun J., Szitter I., Kemény Á., et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS One . 2014;9(9, article e108164) doi: 10.1371/journal.pone.0108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balemans D., Aguilera-Lizarraga J., Florens M. V., et al. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. American Journal of Physiology. Gastrointestinal and Liver Physiology . 2019;316(3):G338–G349. doi: 10.1152/ajpgi.00116.2018. [DOI] [PubMed] [Google Scholar]
- 27.Cenac N., Bautzova T., Le Faouder P., et al. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology . 2015;149(2):433–444.e7. doi: 10.1053/j.gastro.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 28.McGuire C., Boundouki G., Hockley J. R. F., et al. Ex vivo study of human visceral nociceptors. Gut . 2018;67(1):86–96. doi: 10.1136/gutjnl-2016-311629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran R., Hyun E., Zhao L., et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proceedings of the National Academy of Sciences . 2013;110(18):7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Jong P. R., Takahashi N., Peiris M., et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunology . 2015;8(3):491–504. doi: 10.1038/mi.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


