Abstract
Medicinal plants are the primary raw materials used in the production of medicinal products all over the world. As a result, more study on plants with therapeutic potential is required. The tropical tree Ziziphus spina belongs to the Rhamnaceae family. Biological reports and traditional applications including management of diabetes and treatment of malaria, digestive issues, typhoid, liver complaints, weakness, skin infections, urinary disorders, obesity, diarrhoea, and sleeplessness have all been treated with different parts of Z. spina all over the globe. The plant is identified as a rich source of diverse chemical compounds. This study is a comprehensive yet detailed review of Z. spina based on major findings from around the world regarding ethnopharmacology, biological evaluation, and chemical composition. Scopus, Web of Science, BioMed Central, ScienceDirect, PubMed, Springer Link, and Google Scholar were searched to find published articles. From the 186 research articles reviewed, we revealed the leaf extract to be significant against free radicals, microbes, parasites, inflammation-related cases, obesity, and cancer. Chemically, polyphenols/flavonoids were the most reported compounds with a composition of 66 compounds out of the total 193 compounds reported from different parts of the plant. However, the safety and efficacy of Z. spina have not been wholly assessed in humans, and further well-designed clinical trials are needed to corroborate preclinical findings. The mechanism of action of the leaf extract should be examined. The standard dose and safety of the leaf should be established.
1. Introduction
People have resorted to natural sources for cures for various ailments since ancient times [1, 2]. For millennia, people throughout the world have relied on medicinal herbs or plants [3]. Even more impressively, almost 25% of modern drugs are derived from the stem of plants in some way. This shows a robust foundation for plant-derived medicines [4]. Many current medications, nutraceuticals, nutritional supplements, and pharmaceutical products are based on these compounds. Since the development of bacterial and fungal resistance and many other diseases has become an increasing concern, there has been an upsurge in interest in the therapeutic capabilities of traditional medicines. No extensive reviews of Z. spina have been found, according to our literature search. There is only one attempt to review the plants in 2012 [5]. Ethnopharmacology, origin and distribution, taxonomic, morphological, biological evaluation, and chemical composition are examined in this study, which provides a complete overview and up-to-date information on the therapeutic properties of Z. spina with emphasis on its biological activity and chemical composition.
2. Materials and Methods
2.1. Search Criteria
2.1.1. Inclusion criteria
Electronic databases such as ScienceDirect, PubMed, Wiley, Google Scholar, Hindawi, and Springer extracting valuable information from original scientific research papers were used to find articles on Z. spina. These and many more biological evaluations were utilized as key phrases in this research. These included “antifungal and antibacterial”, “anti-inflammatory”, “herbal”, and “anticancer”.
2.1.2. Exclusion criteria
Data from questionable online sources, as well as thesis reports and review publications, were excluded from this investigation (Figure 1).
Figure 1.
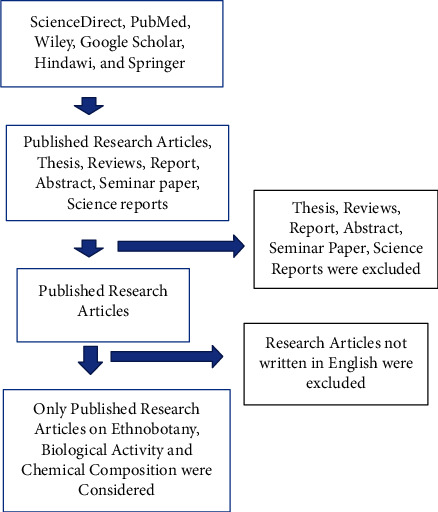
Flowchart of the methodology.
3. Results and Discussion
3.1. Ethnopharmacology
For the majority of human history, people have relied on local flora to heal a broad range of maladies, both those of themselves and their domesticated animals [6]. Depending on the community's culture and religious beliefs, some of the plants were also used for religious rituals [3]. Traditional medicine as a collection of practices and knowledge gathered from the observations and practical experiences of previous generations was described and used for the diagnosis, eradication, and prevention of physical and nonphysical sickness [7]. Ziziphus spina has been traditionally reported in different parts of the world (Figure 2) for the treatment of various ailments [8, 9]. Flowers, leaves, and roots were reported in traditionally treated stomach pain, a disorder in Malawi, Iran, and Sudan [9–11]. In traditional medicine and as a source of nourishment and energy, this species is well known [12]. The extract of the plant is used in the management of dandruff, wounds, and hair loss in Bahrain [13]. In Palestine, the leaves are used in the treatment of skin infections [14]. As a remedy for constipation, people in Turkey rely on the fruit's fiber content [15]. Cough medicine in Nigeria is typically made from the roots [16]. Fruits are used in Sudan to treat diarrhoea, rheumatism, scorpion stings, malaria, and antispasmodics [8]. Decoction is made by boiling leaves and fruits in water for half an hour, and then it should be taken three times a day as an oral supplement to lower cholesterol and cancer risk. Boiling leaves and fruits in water for half an hour produces a typical decoction that is taken three times per day as an oral supplement [12]. All parts of the plants are traditionally used in the treatment and management of various ailments in different parts of the world [9–11].
Figure 2.
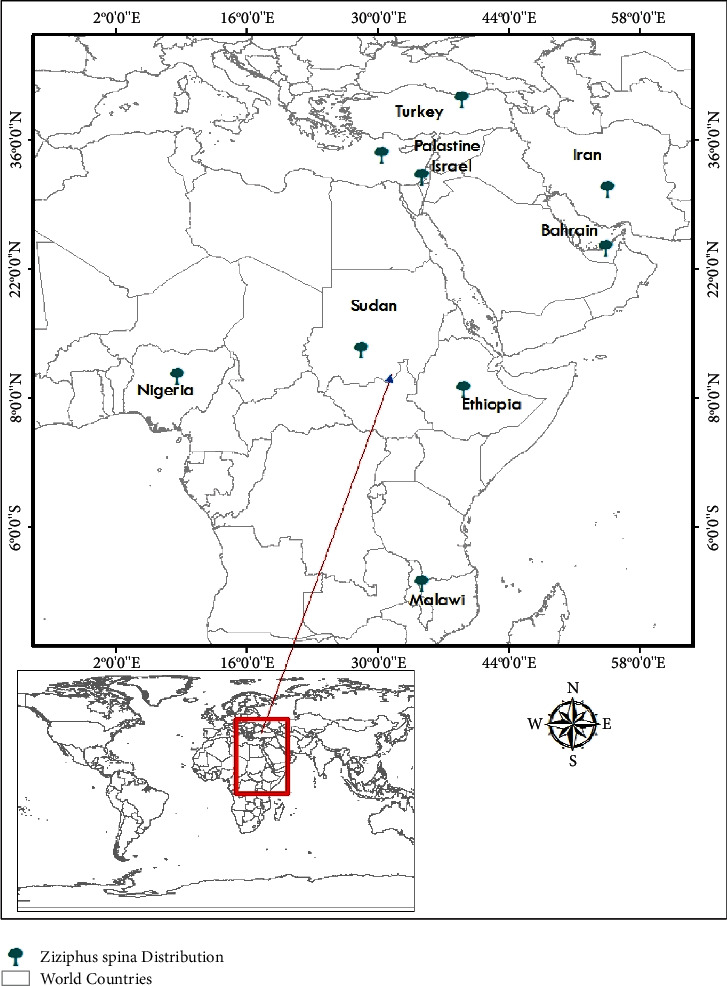
Distribution of Ziziphus spina.
3.1.1. Origin, Taxonomic, and Morphological Description
In southern Sudan, Ethiopia, Northern Lebanon, and Syria, the perennial Z. spina, often known as Christ's thorn, grows [12, 17]. A number of Ziziphus species are naturally suited to dry and hot temperatures, which makes them appropriate for cultivation in tough situations with degraded soil and inadequate water supply [17]. It is a Sudanese-bred tropical evergreen tree. It may be found in every valley and plain in Israel and is generally found at low altitudes [18]. It is a spiky and hardy, tiny shrub or tree with thorns that can withstand heat and dehydration [19]. Although this plant normally matures into a tree, heavy grazing in the latter dry seasons sometimes results in it becoming a shrub instead [19]. More than 170 species of shrubs and small trees are found in warm temperate and subtropical locations of the globe [20]; for example, Ziziphus spina-christi (L.) Desf., synonymous Ziziphus spina-christi var. spina -c hristi, Rhamnus spina -c hristi L., and infraspecific taxa of Ziziphus spina-christi var. aucheri (Boiss.) Qaiser and Nazim. Up to a 45 cm trunk diameter is possible for this tree, which may grow to a height of 5–10 meters [19]. The bark is extensively fissured and yellowish brown or light grey in colour. Round or oval in shape, the crown's thick branches stretch out widely and weep at the ends.
3.2. Biological Evaluation
Alternative medicine is based on the use of medicinal plants, which has led to the development of many novel pharmaceuticals [21]. Increasingly more than 80% of medicine was derived from plants in the nineteenth century, and the scientific revolution led to the development of the pharmaceutical business, where the manufactured pharmaceuticals became more prominent [22]. There is a greater usage of medicinal plants in the treatment of ailments since they are regarded as safe and effective pharmaceuticals, as well as having fewer side effects and costing less than other drugs [23]. Z. spina was subjected to a number of biological evaluations (Table 1).
Table 1.
Biological evaluation of Z. spina.
| S/N | Biological evaluation | Method | Solvents | Plant part | Major findings | Reference |
|---|---|---|---|---|---|---|
| 1 | Antioxidant | Callus extract | Zinc and selenium oxide nanoparticles | On 1-BJ1 normal cells, ZnONPs and SeONPs have promising antioxidant potential | [24] | |
| DPPH and β-carotene-linoleic acid | n-hexane | Fruits | With IC50 values of 5.5 and 4.1 μg/mL, the fruit extract showed antioxidant activity | [25] | ||
| DPPH, FRAP | Ethanol, hexane | Ethanolic extract demonstrates higher inhibitory activity compared with the hexane. The lower the value, the better the plants' ability to scavenge free radicals | [26] | |||
| DPPH, FRAP | Methanolic, ethanolic, and aqueous | Leaves | With IC50 values of 21.4, 24.2, and 54.3 g/mL for methanolic, aqueous, and ethanolic leaf extracts, respectively, the activity demonstrated good antioxidant capacity in terms of radical scavenging activity. The leaf extracts' reducing power was discovered to be concentration dependent | [27] | ||
| In vivo | 70% ethanol | Leaves | Enhanced balance and motor coordination. Short step-through latency was lengthened when the leaf extract was administered to ischemia rats | [28] | ||
| In addition to reducing malondialdehyde levels in the brain and serum, the leaf extract also increased serum and brain antioxidant ability | ||||||
| DPPH | Ethanol | Leaves | With an IC50 of 23.4, leaves had a significant activity against free radicals | [29] | ||
| DPPH | Aqueous and ethanolic | Leaves and bark | The activity of the leaf aqueous and ethanolic extracts was 30 and 91 at a concentration of 0.5 mL, respectively, while that of the aqueous and ethanolic extract of the bark was 44 and 70, respectively | [30] | ||
| In vivo | 70% methanol | Fruits | The findings of this study show that the fruit extract may prevent the development of chronic experimental colitis in rats | [31] | ||
| In vivo | Ethanolic, aqueous | Leaves | The leaf extracts had immunologic and antioxidant effects on rabbits exposed to a 2% H2O2 solution to generate oxidative stress | [32] | ||
| DPPH, FRAP | Seeds | 6–12–24–48 h were all used in the fermentation process. Fermented seed extracts had substantially higher phenolic, vitamin C, and total carotenoid contents and antioxidant activity than unfermented samples at p < 0.05 | [33] | |||
| DPPH | Methanol | Leaves | The leaf extract has an IC50 of 33.91 mg/mL for scavenging activity | [34] | ||
| DPPH | Methanol | Leaves | The leaf extract had a high level of antioxidant activity at 0.086 μg/mL | [35] | ||
| DPPH, ribosomal degradation assay | Aqueous | Essential oil | Scavenging activity of the essential was found with an IC50 value of 53 ± 2 | [36] | ||
| DPPH | Ethyl acetate | Whole plant | The whole plant extract demonstrated significant inhibition capacity at 61 ± 0.04 | [37] | ||
| DPPH | Distilled water, methanol | Leaves | A dose-dependent inhibition was seen in distilled water and methanol leaf extract. However, the proportion of free radical inhibition in the n-butanol fraction was greater than that in the other fractions | [38] | ||
| ABTS, DPPH, FRAP, SRSA, TRPA | Methanol | Fruits | All methods exhibited a strong activity, with chelating methods having the highest at 94% at the concentration of 100 μg/mL | [39] | ||
| Rancimat, DPPH | Leaves | The results show that leaf polyphenols, when added to the test system in varying amounts, have antioxidant activity. Similarly, after only 10 minutes, a scavenging capacity of 40.00 was achieved using four different concentrations of phenolic compounds. After the first 10 minutes of incubation, the scavenging capacity remained the same in all cases | [40] | |||
| DPPH, ABTS, and Fe2+ chelating assays | Aqueous, methanol, ethanol, acetone | Root | DPPH, ABTS, and Fe2+ chelating assays with IC50 values of 0.41 ± 0.01; 0.33 ± 0.14; and 0.24 ± 0.03, respectively | [41] | ||
| Peroxidase, catalase assay | Aqueous, ethanolic | Leaves | Leaf extract exhibited a significant activity against the free radicals | [42] | ||
| In vivo | Leaves | Antioxidants such as T-AOC, GSH-Px, T-SOD, and CAT considerably greater in the blood of rats fed with high leaf diets | [43] | |||
| DPPH, ABTS | Aqueous, methanol | Leaves | When compared with the benchmark of 100 μg/mL, the scavenging activity of the leaf extract was 96%. With an increase in the concentration, there is a simultaneous rise in scavenging activity | [44] | ||
| DPPH | 70% ethanol | Seeds and fruits | The fruits were found to have the highest inhibition of 54.10 at the concentration of 200 μg/mL | [45] | ||
|
| ||||||
| 2 | Anti-inflammatory | In vivo | Ethanol | Root or bark | With a total inhibition of 79.2%. This explains why these plants have long been used as polyherbs to cure ulcers | [46] |
| In vivo | In addition to reducing ulcer size and reducing colitis indicators, pretreatment with the extract (100, 200, and 400 mg/kg/day) at various dosages slowed the progression of inflammation and prevented mucosal damage. In comparison with the reference medicine, mesalazine (MLZ), ZFE (400 mg/kg) therapy reduced inflammatory colonic damage more significantly | [47] | ||||
| In vivo | Leaves | Substantially and dose-dependently reduced sepsis-induced liver and spleen damage, according to our findings. These findings imply that, through eliciting anti-inflammatory and antioxidant effects, they could be used to treat sepsis | [48] | |||
| Protein denaturation | 70% ethanol | Seeds and fruits | Both portions of the plant extract had anti-inflammatory activity that was comparable to that of the common anti-inflammatory medication diclofenac | [45] | ||
| Methanol | Root (ZS-Ag-NPs) | It effectively enhanced mRNA expression levels of vascular endothelial cell growth factor and decreased oxidative stress as well as vascular cell inflammation in adipocyte CM. Obesity progression and metabolic inflammatory pathogenesis associated with age were successfully decreased by ZS-Ag-NPs' molecular mechanical activity | [49] | |||
| Protein denaturation | Methanol, ethanol | Leaves | High activity was recorded with the methanolic extract even at 95 compared with the standard at 20.2%, respectively | [50] | ||
| Ethanolic, aqueous | Leaves | The ethanolic extract demonstrated significant efficacy as well as modest antipyretic effect | [51] | |||
| In vivo | Hexane, chloroform, ethyl acetate, and methanol | Root | In all models, except the tail-flick test, where the activity was not statistically significant, the percentage exhibits some amount of dose-related effect | [52] | ||
| In vivo | Leaves (AgNPs) | Pretreatment with nanoparticles improved histological parameters such as little infiltration and fibrosis, low pleomorphism, and reduced hepatocytes and degeneration | [53] | |||
| In vivo | Leaf | Using extract ointments for burns is beneficial | [54] | |||
| Healing with histological alterations, however, the group treated with leaf extract had the greatest improvement | ||||||
| Ointment had a better cellular response to the inflammatory process than the other group in which re-epithelialization appears early in the healing process | ||||||
|
| ||||||
| 3 | Antibacterial | Ethanol, aqueous | Leaves | [55] | ||
| Agar diffusion | Ethanol, petroleum ether, ethyl acetate, methanol, and aqueous | Leaves, stem bark, fruits | The extract at the concentration of 100 mg/mL was found to have activity against some of the tested strain with methanolic extract having a minimum MIC at 6.25 μg/mL. These findings offer promising preliminary evidence for the use of crude extracts in the treatment of bacterial infections | [56] | ||
| Disc | Ethanol | Leaves | The highest zone of inhibition was found at 15 mm against E. coli | [57] | ||
| Cup-plate agar diffusion | Petroleum ether, chloroform, methanol, and aqueous | Fruits, leaves, seeds, and stems | Methanol extracts from all parts had the highest activity, followed by chloroform and petroleum ether. No activity was recorded from aqueous extracts | [58] | ||
| Ethanolic, aqueous | Leaves | It also stopped the tested strain from growing. However, there was no evidence of analgesic or diuretic action. Surface activity was seen in the aqueous extract of the leaves, with a threshold micelle concentration of 0.25 percent w/v | [51] | |||
| Agar well diffusion | Leaves | With an MIC value of 0.25 mg/mL, Escherichia coli was found to be the most vulnerable bacterium to the extract, while Staphylococcus aureus was discovered to be the most resistant strain with an MIC value of 1.00 mg/mL. To summarize, the plant's leaves might be used in food processing and further investigated for the treatment of microbial illnesses | [59] | |||
| Disc diffusion | Aqueous | Seeds | The extract exhibited substantial action against all tested MDR strains. Besides, its polyphenol component demonstrated a stronger impact. Furthermore, the entire extract MIC varied between 3.125 and 12.5 mg/mL and MBC was 3.125–25 mg/mL against prior strains. While the polyphenol fraction, MIC and MBC were around 0.312–1.25 mg/mL and 0.312–2.5 mg/mL, respectively | [60] | ||
| Aqueous, ethanolic | Leaves | Both extracts have an inhibitory effect on many bacterial species in this investigation. Most effective at 200 mg/mL when they came to killing germs | [61] | |||
| Callus extract | Zinc and selenium oxide nanoparticles | The results showed that both crystals have antibacterial ability against the tested strains, with SeONPs having stronger antimicrobial activity than ZnONPs | [24] | |||
| Agar well | Hexane | Seed oil | At 11, 10, 8, and 8 mm, it was active against the tested strains. The findings of this study have established scientific validity for the use of this seed oil in herbal medicine to treat bacteria-related diseases | [62] | ||
| Disc diffusion | Ethanolic and methanolic | Leaves | Both extracts were discovered to be potent antibacterial agents against all microorganisms tested. At concentrations of 128, 100, 64, and 32 mg/L, inhibitory action was measured. At 128 mg/L, the ethanolic extract exhibited the maximum activity of 21 mm against Salmonella sp., whereas the methanolic extract had the lowest activity of 9 mm against Escherichia coli | [63] | ||
| Disc diffusion | Ethanol | Leave | At doses of 5, 15, and 30 mg/mL, the average diameter of the inhibitory zone was 0 to 17 mm. At 30 mg/mL, it is highly effective against all used bacterial strains. With Staphylococcus aureus, the largest inhibitory zone diameter was 17 mm | [64] | ||
| Agar diffusion | Aqueous and ethanolic | Leaves | The inhibitory zone's diameter in cm varied between 1.5 and 2.3 and 2 and 2.2, respectively | [65] | ||
| Agar well | Ethanol | Stem bark | At p < 0.05, there was a notable increase in activity. Gram-positive bacteria were more vulnerable to this extract than gram-negative bacteria, according to these findings | [66] | ||
| Well-diffusion method | Aqueous | Honey | The microbiological strains were severely hampered. There were no resistant microbial strains, with the highest sensitivity at 36 mm | [67] | ||
| MIC | n-hexane | Fruits | The minimum inhibitory concentrations against the tested microorganisms ranged from 32 to 125 g/mL | [25] | ||
| Agar-well diffusion, MIC, MBC | Methanol | Fruits | Overall, the research found that the fruit extract possessed Gram-negative bacteria that have no antibacterial action, while moderate antibacterial activity was shown against gram-positive bacteria. The discovered actions, however, were not noteworthy compared with antibiotics | [68] | ||
| Well-diffusion method | Aqueous | Aquatic leaves, aquatic stem bark, and combination of leaves + stem bark | All of the tested strains were extremely sensitive to a combination of leaves and stem bark within the inhibition zone of 25–35 mm | [69] | ||
| Disc diffusion | Ethanol, aqueous | Leaves | The ethanolic leaf extract had the highest activity against S. aureus at 18 mm, while the aqueous extract had the lowest activity against B. subtilis at 13 mm | [70] | ||
| Disc diffusion method | Ethanol | Leaves | Within the 8–26 mm range, there was a lot of activity | [71] | ||
| Agar well | AgNO3 aqueous | Leaf | The SNPs had good activity against S. aureus and E. coli, with inhibition zones of 18 and 20 mm, respectively | [72] | ||
| Methanol | Leaves | The extract revealed activity via secondary metabolites such as alkaloids and flavonoids. Significant influences on microbial growth harmed energy metabolism, leading to fat accumulation and protein inhibition | [73] | |||
| Well diffusion method | Methanol | Leaves, fruits, and stems | Leaf extract exhibited higher inhibition zone at p < 0.001 | [74] | ||
| Agar well | Ethanol and methanol | Bark, leaves, fruits, seeds and roots | All of the bacterial strain tested were sensitive to plant extracts. Except for Enterobacter aerogenes, the bark extract was the most effective against all bacteria | [75] | ||
| Well diffusion | Aqueous and ethanol | Leaves and stem bark | The aqueous stem bark extracts had inhibition on the tested strains with the highest inhibition on Klebsiella spp. and E. coli at 20 mm, respectively | [76] | ||
| Disc diffusion | Aqueous | Leaf (AgNPs) | The extract had a good inhibitory impact on all gram-positive and gram-negative bacteria tested, with the maximum activity against P. aeruginosa 16 mm | [77] | ||
| Ethanol | It was found to exhibited activity at 9, 6, 7, 5, and 6 mm against the tested strain | [78] | ||||
| Well diffusion | Aqueous | Leaf (AgNO3) | Maximal inhibitory zones activity of 24, 23, 15, and 17 mm in the extract, respectively | [79] | ||
| Agar disc diffusion | Ethanolic | Seed | These extracts demonstrated inhibitory activity at various stages of germination; the first stage had 22 mm inhibitory activity against S. faecalis and 20 mm against S. aureus, and 15 mm inhibitory activity against P. aeruginosa. The second stage exhibits 15 mm against S. faecalis, 14 mm against P. aeruginosa, and 10 mm against S. aureus | [80] | ||
| Plate agar method | Petroleum ether, chloroform, 80% ethanol, and aqueous | Leaves, fruits, and seeds | A significant activity was recorded from the extract | [81] | ||
| Agar diffusion | Methanolic | Leaves | B. cereus 15, C. perfringens 12, L. monocytogenes 11, S. aureus 10, P. vulgaris 8.5, and V. parahaemolyticus 8 mm, respectively, were tested. The extract had no discernible effect on the remaining strains tested | [82] | ||
| Leaves | With a probability activity value of more than 0.300, PASS analysis revealed that 15 compounds (64.51 percent) have antibacterial potential. The extract inhibited pathogenic bacterial growth in a moderate-to-strong manner, except for V. vulnificus, for which it provided a poor inhibition | [83] | ||||
| Disc diffusion | Ethanol, methanol | Leaves | Both extracts had the lowest antibiofilm impact on the tested strains | [84] | ||
| Disc diffusion | Aqueous, ethanol | Leaves | On all of the studied strains, the MIC shows that the aqueous extract ranges from 12.8 to 8.3 mg/mL, whereas the ethanolic extract ranges from 13.5 to 8.8 mg/mL | [85] | ||
| Agar well diffusion | Aqueous | Leaves | The extracts at 50 μg/mL had no effect on any of the bacterial strains, while the greatest activity was at 100 μg/mL with 9 mm zone of inhibition against Klebsiella oxytoca and Proteus mirabilis, respectively | [86] | ||
| Diffusion assay | Fruits | This lipid fraction was active against the tested bacterial strains. At 2.6 mm, the fatty acid fraction had a lot of activity against it | [87] | |||
| Disc diffusion | Methanol | Leaves, stem | At the concentration of 200 mg/mL recorded an inhibition zones of 16, 14, and 16 mm, respectively | [88] | ||
| Agar well | Aqueous and methanolic | Leaves and seeds | Against five bacterial strains at varied doses of 25, 50, 100, and 200 mg/mL, respectively. At 25 mg/mL, no activity was recorded. The leaves aqueous had the maximum activity at 17.67 mm against Staphylococcus aureus and, similarly, had the lowest activity at 7.33 mm against Pseudomonas aeruginosa | [89] | ||
| Disc diffusion | Ethanol, petroleum ether, ethyl acetate | Leaf, seed, young stem, fruits, and root | It has moderate activity with the minimum inhibition at 6 and maximum at 10 mm, respectively | [90] | ||
| Disc diffusion | Aqueous and ethanolic | Leaves and bark | Ethanolic bark inhibits higher inhibition with at 22 for Escherichia coli and 15 mm for Staphylococcus aureus, respectively | [30] | ||
| MIC, MBC | Ethanol | Leaves | Have minimum inhibitory zone activity against bacteria, enteropathogenic E. coli at concentrations of 50% and minimum bactericidal activity at concentrations of 75% at 106 CFU/mL | [91] | ||
| Agar well | Ethanol | Leaves | At a dosage of ≥0.25 g/mL, it has antibacterial action and inhibits the bacteria P. acne | [29] | ||
| Disc diffusion | Ethanol, methanolic | Leaves | The maximum inhibitory activity for S. aureus and B. cereus were 18 and 14 mm for methanolic extract and 15 mm for ethanolic extract against S. aureus and P. mirabilis, respectively | [92] | ||
| Agar cup | Ethanolic | Leaves | MIC and MBC were 20 mg and 40 mg mL−1, respectively | [93] | ||
| Well method | ||||||
| Agar well | Aqueous, ethanol | Leaves | The greatest rate of inhibition diameter against Staphylococcus aureus, 18 mm in concentration 500 mg mL. No activity was recorded against the tested strains from aqueous extract | [94] | ||
| Agar plate diffusion and broth dilution | Aqueous | Stem bark | The extract showed efficacy against all organisms tested. Except for S. pyogenes, which had an MIC of 25 mg/mL, the MIC was 12.5 mg/mL against all species | [95] | ||
| Agar well | Methanol and ethanol | Leaves | The activity of 2.5 percent NaOCl against E. faecalis was the strongest, followed by hydroalcoholic and methanolic extracts. Until research like this identifies a better alternative, NaOCl is an effective irrigant in root treatment | [96] | ||
| In vitro, in vivo | Aqueous cold water and ethanol | Leaves | Significantly inhibited the growth of the tested strains at 95% | [97] | ||
| Agar well | Aqueous ethanol | Bark | The maximum inhibition was recorded at 22 mm against E. coli | [98] | ||
| Microtiter plate | Hot and aqueous | Leaves | With the concentration of 50 mg/mL, the results demonstrated the ability of the extract to prevent biofilm formation | [99] | ||
| Disc diffusion | Leaves | Any inhibition below 6 mm was considered as no activity with 800 μg/disc. The following study found no activity against the tested strains | [100] | |||
| Agar well | Aqueous, ethanolic | Leaves | Ethanolic leaf extract exhibited the highest activity against S. aureus with an inhibition zone of 20 mm | [101] | ||
| Cup-plate agar diffusion | Petroleum ether, ethyl acetate, ethanol, methanol, and distilled water | Stem bark | Studies revealed that the methanolic extract at 100 mg/mL exhibited the highest activity of 25 mm. The extract reduced the development of all bacteria, with most extracts showing antimicrobial action on multiple levels | [102] | ||
| Disc | Honey | Demonstrate a significant activity against the tested strain | [103] | |||
| Aqueous | Leaves | S. aureus and S. haemolyticus biofilm formation was prevented by a hot extract tested against the biofilm formation. The results showed that the two antibiotics and the plant extract could both prevent the biofilm formation | [104] | |||
| Agar well | Aqueous and ethanolic | Leaves | The aqueous extracts demonstrated a significant inhibition zone of 5.6 mm against Streptococcus pyogenes at a dosage of 100 mg/mL, with the least inhibition around S. aureus. The ethanolic extract, at a dosage of 100 mg/mL, has the biggest inhibitory zone against Klebsiella pneumonia, measuring 6.6 mm. Our findings imply that is a good alternative antibacterial agent against a variety of pathogenic bacteria | [42] | ||
| Pour-plate method | Ziziphus honey | In most dilutions, the extract was higher after 120 hours of incubation for each of the tested strains. After 120 hours, the microbial count was reduced by 3–7.5 logs compared with the control | [105] | |||
| Well diffusion methods | Aqueous | Fruits | AgNPs' inhibitory activity against all investigated human pathogenic microorganisms rose with fruit ripening progress from mature fruit to unripe fruit to immature fruit | [106] | ||
| Cup-plate agar diffusion | Ethyl acetate | Whole plant | At the bacterial concentration of 1 mg/mL, the extract recorded the highest inhibition of 22 mm against S. aureus | [37] | ||
| Agar plate | Aqueous | Pulp | Study revealed increased sensitivity to E. coli and P. aeruginosa. MIC of 6.25 mg/mL. For S. pyogenes, MBC of 12.5 mg/mL | [107] | ||
| Micro broth dilution | Aqueous | Essential oil | Inhibited the development of Penicillium digitatum and Klebsiella pneumonia at doses of 128 and 512 g/mL, respectively | [36] | ||
| Microdilution method | Hot water and ethanol | Leaves | The ethanolic extract has the highest inhibition at 4 ± 1.03 | [108] | ||
|
| ||||||
| 4 | Antifungal | Cup-plate agar diffusion | Petroleum ether, chloroform, methanol, and aqueous | Fruits, leaves, seeds, and stems | No activity recorded | [58] |
| Ethanol | Leaves | In comparison with the control, cells treated with 150 μL/mL of the extract had an average sterol content approximately three times higher | [109] | |||
| Cup-plate agar diffusion | Petroleum ether, ethyl acetate, ethanol, methanol, and distilled water | Stem bark | Exhibited strong activity at concentration 100 mg/mL with an inhibition zone of 20 mm. These discoveries can be used to aid in the treatment of fungal illnesses | [102] | ||
| Agar well diffusion | Ethanol and methanol | Leaves | According to the findings, the ethanolic extract possesses antifungal characteristics and can be utilized to treat fungal infections. More research is needed to evaluate the effectiveness of this plant in treating candidiasis patients | [110] | ||
| Micro broth dilution | Aqueous | Essential oil | In a dosage of 64 μg/mL, 99.9% of Aspergillus niger does not grow | [36] | ||
| Agar well | Ethanol | Stem bark | Fungal strains with significant activity at p < 0.05 ranged from 3.90 to 32.3 g/mL | [66] | ||
| Well diffusion | Aqueous | Honey | The microbiological strains were severely hampered. There were no resistant microbial strains, with the highest sensitivity at 36 mm | [67] | ||
| Agar well | Aqueous, ethanolic | Leaves | At a dosage of 500 mg/mL, both ethanolic and aqueous extracts inhibited Candida albicans with inhibition diameters of 32 mm and 18 mm, respectively | [94] | ||
| The study revealed increased sensitivity to the tested strain, indicating the antifungal activity of the extract | [107] | |||||
| Agar plate diffusion and broth dilution | Aqueous | Stem bark | There was no inhibition at low doses, and it was susceptible above 600 mg/mL, implying that the extract has antifungal potential | [95] | ||
| Agar well | Aqueous and ethanolic | Bark | The maximum inhibition was recorded at 17 mm | [98] | ||
| Callus extract | Zinc and selenium oxide nanoparticles | Both crystals have antifungal activity against the tested strain, with SeONPs having greater antifungal activity than ZnONPs, according to the findings | [24] | |||
| Agar cup well | Ethanolic | Leaves | No activity against the tested fungal strain | [93] | ||
| Agar dilution | Aqueous | Leaves | The extracts were found to have action against Fusarium, Helminthosporium, Alternaria, and Rhizoctonia species, as well as inhibiting Alternaria and Fusarium sporogenesis | [111] | ||
| In vivo | Ethanol | Leaves | A shampoo containing the plant extract was then developed and tested on 80 people with dandruff for a period of four weeks. With the Sidr shampoo formulation, 86% of the participants reported significant improvement in their dandruff symptoms | [112] | ||
| Plate agar method | Petroleum ether, chloroform, 80% ethanol, and aqueous | Leaves, fruits, and seeds | A significant activity was recorded from the extract | [81] | ||
| Extracts were found to have antifungal efficacy against all fungi examined. These findings suggested that the extracts could be used as a substitute for chemical additives in the treatment of fungal diseases in plants | [113] | |||||
| Ethanol | Leaves | When treated with the extract at a dosage of 20%, it failed to generate spores | [114] | |||
| 96-well plates | Ethanol (80%, v/v) | Fruits (unripe and ripe) | The minimum inhibitory concentration 90 values for ripe and unripe fruits were 25 and 0.1 g/mL, respectively | [115] | ||
| Agar diffusion | Methanolic | Leaves | No activity | [82] | ||
|
| ||||||
| 5 | Antidiarrhoeal | In vivo | Stem bark | The extract was shown to protect rats against castor oil-induced diarrhoea as well as reduce intraluminal fluid collection and gastrointestinal transit, according to the results. In mice, the intraperitoneal and oral LD50 values were 3465 and 1200 mg/kg, respectively | [116] | |
|
| ||||||
| 6 | Antiparasitic | In vivo | 70% ethanol | Leaves | Endothelial contraction was dose-dependent and substantial (p < 0.0001) in both intact and denuded aortas. A comparable reaction to the leaf extract at a concentration of 5 mg/mL was seen with KCl (50 mM). Aortic endothelium intact and denuded aorta contractions were decreased by 66.7% (mean + SEM) and 71.67% (mean + SEM) when verapamil was applied, respectively | [117] |
| Aqueous | Leaves | At concentrations of 6, 25, 12.5, 25, 50, 100, and 200 mg/mL, the drug effective against Egyptian species of schistosomes | [118] | |||
| In vivo | 70% methanol | Leaves | As a result, the extract was shown to have antiapoptotic, antifibrotic, antioxidant, and protective properties against S. mansoni-induced liver lesions in this investigation. The extract's antifibrinogenic and nuclear factor erythroid 2-related factor 2 (Nrf2) advantages against S. mansoni were due to the enhancement of these two pathways | [47] | ||
| Aqueous | Leaves | The findings indicated that the extract at concentrations of 500, 250, and 125 μg/mL killed 100% of Egyptian Schistosoma strains of adult worms and schistosomula of S. haematobium within 6 to 12 hours of incubation. As a result, these medicinal plant extracts might be utilized to treat schistosomiasis in a safe and effective manner | [119] | |||
| In vivo | 70% methanol | Leaves | In the 3rd, 4th, and 5th groups, oocyst shedding was dramatically reduced to roughly 10.7 × 103, 28.3 × 103, and 23.8 × 103 oocysts/g faeces, respectively | [120] | ||
| In vitro/in vivo | Aqueous and methanolic | Leaves | On day 21 after treatment, the extract exhibited a decrease in egg count percent (EPG) in faeces of 61.5 and 78.7% at dosages of 100 mg/kg and 400 mg/kg. EPG of faeces decreased by 24.4, 73.1, and 85.1% at 100, 400, and 800 mg/kg dosages, respectively | [121] | ||
| Methanolic | Leaves | The extract significantly reduced the viability of L. major amastigotes at p < 0.001, whereas it induced NO production and release, apoptosis, and plasma membrane permeability in macrophage cells with no evidence of harm | [122] | |||
| MTT assay | Methanolic, aqueous | Aqueous extracts had IC50 values of 60 and 80 μg/mL, respectively, for methanol and water. Promastigotes of L. major were severely affected by all plant extracts | [123] | |||
| In vivo | 70% methanol | Leaves | E. papillata infection enhanced the generated jejunal damage. Furthermore, treatment of infected mice with 100 and 300 mg ZLE/kg increased the number of goblet cells in the jejunal villi substantially | [120] | ||
|
| ||||||
| 7 | Antiviral | Plate agar method | Petroleum ether, chloroform, 80% ethanol, and aqueous | Leaves, fruits, and seeds | A significant activity was recorded from the extract | [81] |
| In vivo | Leaves | The leaf extract was effectively used to treat rashes on a 50-year-old man | [124] | |||
| In vivo | Ethanol | Leaves | Significantly reduced the growth of the rashes compared with the control group | [125] | ||
|
| ||||||
| 8 | Antimalarial | In vivo | 70% methanol | Leaves | The liver and spleen histopathology examinations revealed severe histological abnormalities. The histopathological appearance of the liver and spleen in treated animals improved significantly. Treatment resulted in a significant return of oxidative indicators to normal levels, according to biochemical analyses | [126] |
| In vivo | Leaves | To sum up, the findings suggest that the extract's antiplasmodial and antioxidant properties might help reduce the devastation caused by P. berghei-induced cerebral malaria | [127] | |||
| In vivo | 70% methanol | Leaf | Had a considerable impact on liver function enzymes as well as histological images of the liver. It is possible to infer that the extracts protect against Plasmodium infection, as shown by considerable improvements in hepatic oxidative indicators | [128] | ||
|
| ||||||
| 9 | Antidiabetic | Alpha amylase and glucosidase assay | Methanol, ethanol | Leaves | The enzyme exhibited strong activity with methanolic extract against alpha-amylase and glucosidase at 8.9 and 39.12 μg/mL, respectively | [50] |
| In vivo | Methanol | Leaves | At concentrations of 100 μg/mL, the extracts were shown to inhibit alpha-amylase and glucosidase by 54 and 43%, respectively | [129] | ||
| In vivo | Ethanol | Leaves | Compared with the control group, diabetic rats had significantly lower glucose levels and significantly higher blood insulin levels. When compared with diabetic control and nondiabetic control rats, the treatment group demonstrated a significant reduction in triglycerides, indicating that it had a hypolipidemic impact | [130] | ||
| In vivo | Aqueous and methanolic | Leaves | Following therapy with 500 mg/kg, the highest activity at 25.59 and 39.48% after 7 and 15 days, respectively, was discovered at p > 0.001. Similarly, the 500 mg/kg therapy had the greatest (p > 0.001) hypoglycaemic effect at 29.07 and 35.56% after 7 and 15 days, respectively | [44] | ||
|
| ||||||
| 10 | Antiobesity | In vivo | Leaves | Improved liver and kidney function and lowered lipid peroxidation in hypercholesterolemic male rats treated with the extract. As a result of its high concentration of phenolic chemicals, the antihyperlipidemic effects of this extract might be linked to inhibition of oxidative stress | [131] | |
| In vivo | Aqueous | Seed | Biochemical and histological changes were reversed with the extract in G3 therapy. The extract lowered hypercholesterolemia, inhibited oxidative stress, and restored biochemical and histological characteristics that had been changed | [132] | ||
|
| ||||||
| 11 | Antianxiety | In vivo | Leaf | Administering the extract after HgCl2 exposure stopped mercury build-up in the cortical slices. As a result, the levels of malondialdehyde were reduced, as were those of nitrite and nitrate production and nitrite and nitrate creation enzymes. Glutathione levels were also boosted, as were those linked to the antioxidant enzymes glutathione reductase and glutathione peroxidase. Might be used to reduce the damage to neurons caused by HgCl2 poisoning | [133] | |
| Rotarod testing was utilized to assess motor coordination. The raised plus maze's open-arm duration was dramatically lengthened when Z. spina extract (200 mg/kg) was administered. A lower proportion of entrances into the closed arms and less time spent in the closed arms were both lowered by the extract. Motor coordination and balance were unaffected by the concentration of Z. spina extract in the study. Scopolamine-induced anxiety is greatly reduced by Z. spina hydroalcoholic extract | [134] | |||||
| In vivo | Ethanol | Leaves | The leaf extract significantly reduces the expression of the indicators studied compared with the induction group. The extract may protect the male against pentylenetetrazole-induced harm | [135] | ||
|
| ||||||
| 12 | Growth promoter | In vivo | Leaves | The findings of this study could have supported the use of low doses of a 20 g SL/kg diet as natural growth promoters without affecting rabbit performance | [136] | |
| R2 had significantly greater end weight and total growth (p < 0.05) than R1, but not significantly higher than R3. R2's daily increase, on the other hand, was much greater than R3's. As a result, feed additives of ZSCL (15 g/kg DM) are strongly suggested in the feeding practices of developing lambs | [137] | |||||
| In vivo | Leaves | Consumption level of 10%, the findings revealed that the treatment had a significant impact on broiler chicken output and mortality (p < 0.05). In the care of broiler chicks, the extract may be used as an alternative to synthetic nutrients | [138] | |||
|
| ||||||
| 13 | Insecticidal | Aqueous, methanol, ethanolic, and acetonic | Root | The extract has the strongest insecticidal potential when tested against Lasioderma serricorne. The ethanolic and acetonic extracts had LC95 values of 3.17 and 4.86 μg/insect, respectively. The current study adds to the usefulness of Z. spina as a source of epicatechin, which can be employed as a bio-antioxidant and a bioinsecticide | [41] | |
|
| ||||||
| 14 | Anticancer | MTT assay | Methanol | Leaves | The IC50 of the extract for the RD cell line was 154.44 μg/mL. The extract demonstrated a possible antiproliferative effect against the RD cell | [34] |
| MTT assay | 70% ethanol | Leaves | On MCF7 cells, extracts had a cytotoxic impact. In MCF7 cells, 1 mg/mL dramatically boosted the expression of the Bax and Bcl-2 genes | [139] | ||
| MTT | Aqueous | Leaves (silver nanoparticles) | Polysaccharides had an IC50 value of 1.5 mg/mL, but the IC50 value for polysaccharide-coated AgNPs was 0.705 mg/mL | [140] | ||
| MTT | Ethanol, ethanol-aqueous, aqueous | Leaves | After forty-eight hours of administration, the ethanolic fraction had the lowest IC50 value of 0.02 mg/mL and triggered cell cycle arrest at the G1/S phase as well as apoptosis | [141] | ||
| In vivo | Methanolic | Leaves | Extract treatment of DENA-induced hepatocarcinoma alleviated all except cholangioma-induced abnormalities. Finally, ZSCL (300 mg/kg BM) showed a significant therapeutic effect against DENA-induced hepatocellular cancer by focusing on oxidative stress and oncogenes | [142] | ||
| MTT assay | Hexane | Leaves | According to the findings of this study, the leaf extract contains chemicals with anticancer action, making it a promising target for further research to identify new anticancer drugs | [143] | ||
|
| ||||||
| 15 | Toxicity | In vivo | Aqueous | Stem bark | For the liver enzymes ALT, AST, ALK, serum protein, and albumin, biochemical examination revealed no significant difference between the different concentrations treated at 200, 400, and 800 mg/kg correspondingly and the control group. Furthermore, there was no significant variation in serum electrolytes (p > 0.05) | [95] |
| Prophage F116 induction | Ethanol | Leaves | When compared with control, the employed concentrations at 5, 15, and 30 mg/mL did not reveal an increase in prophage induction. The mutagenic index revealed that the spontaneous release of phage from the lysogenic strains resulted in no increase in pfu/mL. As a result, the plant extracts evaluated have no genotoxic potential | [64] | ||
| In vivo | Ethanol | Root, bark | In both the short- and long-term studies, all rats survived at a limit dose of 3000 mg/kg. There was no mortality, although the rats in all groups showed evidence of sleepiness for about 1 to 2 hours | [46] | ||
| In vivo | Aqueous-methanol | Seeds | Lymphocytes, platelets, direct and total bilirubin, albumin, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, serum Ca2+, creatinine, urea, and organ-body weight ratios were all significantly elevated at p < 0.05 by the extract. At 600 and 1000 mg/kg BW, the extract induced hepatic vascular congestion and fibrosis but had no effect on the kidney's histoarchitecture. There are hepatotoxic and nephrotoxic properties in the extract, thusly folklore medicine calls for caution when using this herb | [144] | ||
| MTT assay | Ethanol | Stem bark | In HCT-116 and MCH-7 cell lines at a concentration of 50–400 g, the control cells had a high rate of proliferation, which was taken as 100% | [66] | ||
| 1-BJ1 normal cells | Callus extract | Zinc and selenium oxide nanoparticles | Low toxicity was recorded. The particles show potential antibacterial and antioxidant actions and will be used to combat resistant microorganisms | [24] | ||
| Fl-cells | Ethanol | Showed no toxicity | [78] | |||
| In vivo | Hydroalcoholic | Leaves | No serious side effect was observed | [125] | ||
| In vivo | Leaves | No changes in liver or kidney function were found after the juice was given to the animals. It was found that a dose of BHT (200 parts per million) significantly increased the enzyme activity and serum levels of all three products. | [40] | |||
| In vivo | Methanolic | Seeds | Phagocytic index values were the lowest in snails exposed to LC50 of the extracts. Results demonstrated no mortality in Daphnia magna individuals during the first 12 h of the trial | [145] | ||
| In vivo | Aqueous | Leaf (AgNPs) | When compared with MeHg intoxication, AgNPs/MeHg caused a far larger increase in lipid peroxides as a marker of oxidative stress, and of course, compared with healthy control animals | [77] | ||
| In vivo | Ethanol | Leaves | The animal model received daily oral dosages of 50 to 200 mg/kg for 28 days. Biochemical tests comparing the extract's toxicity to that of a control group revealed no differences. However, oral administration of the extract at dosages of 100 and 200 mg/kg resulted in minor histologically detrimental effects on liver and kidney tissues | [112] | ||
| In vivo | Aqueous | Fruits | All biochemical indicators and histological images of the liver, kidney, and testis improved significantly in animals treated with the extract alone or in combination with AF. The extract may have a powerful function in protecting against aflatoxicosis | [146] | ||
| In vivo | Ethanol | Leaves | 14-day course of oral 400 mg/kg extract dose. Lactate dehydrogenase and total bilirubin levels in rats' blood were substantially elevated (p < 0.05) after oral administration of the extract, but no other liver enzymes were affected. Increased insulin levels, reduced triglyceride levels, and no significant changes in the other lipid profile components were seen when therapy was administered | [147] | ||
| In vivo | Aqueous | Pulp | There was no significant difference between the treatment group and the control group in terms of liver enzymes such as ALT, AST, ALK, serum protein, and serum albumin, according to the results of the biochemical study | [107] | ||
| Aqueous, ethanol | Leaves | Both kinds of extracts in concentrations of 500 mg/mL showed no cytotoxicity towards red blood cells | [94] | |||
| Aqueous | Leaves | Extract did not exhibit any morphological alterations from the control at MNTD values of 250, 350, and 300 μL/mL, respectively, in a cytotoxicity experiment | [118] | |||
| In vivo | Leaf | In addition, after HgCl2 poisoning, a shift in apoptotic proteins in favor of proapoptotic proteins was identified. However, combining the extract with HgCl2 considerably reduced the molecular, biochemical, and histological changes caused by HgCl2 intoxication. Our results imply that the extract might be utilized to reduce the effects of HgCl2 exposure on reproduction | [148] | |||
| In vivo | Ethanol | Leaves | The leaf extract has no harmful impact on the liver when administered at dosages below 1500 mg/kg BW. In conclusion, the hazardous dosage of leaf extract in white Wistar rats is over 4000 mg/kg BW | [149] | ||
Note. MIC: minimum inhibitory concentration, MBC: minimum bacterial concentration, DPPH: 2,2-diphenyl-1-picrylhydrazyl, FRAP: ferric reducing antioxidant power (FRAP) assay.
3.2.1. Antioxidants
Antioxidant plant-based medicine formulations are used to prevent and cure complicated illnesses such as atherosclerosis, stroke, diabetes, Alzheimer's disease, and other neurological disorders [150]. In the human body and food system, free radical reactions occur. In the form of reactive oxygen and nitrogen species, free radicals are a natural element of physiology. The hunt for antioxidants from natural sources has got a lot of attention, and researchers are working hard to find chemicals that may replace synthetic antioxidants [151]. The potential capability of Z. spina was evaluated using animal models, DPPH and β-carotene-linoleic acid, FRAP, ribosomal degradation test, SRSA, TRPA, ABTS, Rancimat, and many more procedures (Table 1). Antioxidant potentials were found in all assessment techniques (Table 1). The activity revealed high antioxidant ability in terms of radical scavenging activity, with IC50 values of 21.4, 24.2, and 54.3 g/mL for methanolic, aqueous, and ethanolic extracts, respectively. The reducing power of the extracts was revealed to be concentration dependant [27]. The plant displayed antioxidant activity with IC50 values of 5.5 and 4.1 g/mL [25]. The extracts demonstrated immunologic and antioxidant effects on rabbits subjected to a 2 percent H2O2 solution to induce oxidative stress, according to our results [32]. The IC50 value for scavenging activity was determined to be 53 [36]. The plant extract's naturally occurring antioxidants may be synthesized into nutraceutical that can help prevent oxidative damage in the body.
3.2.2. Anti-Inflammatory
Physical trauma, noxious chemicals, and microbial infections may all produce inflammation, which is the body's natural reaction to protecting itself from further damage. A host of infections, irritants, and damaged tissues are dealt with during this procedure [1, 6]. Many medications are available to combat inflammation, but long-term usage may result in side effects such as nausea, vomiting, bone marrow depression, and fluid or salt retention [6]. A new supply of structurally essential compounds from plants has been discovered by traditional medicine, which means that it is always expanding its horizons [3]. It is well known that plants are rich in chemical compounds. Compositional diversity in plants has gone largely unexploited, and novel lead chemicals for the treatment and management of inflammation might be found. The crude extract was tested in a variety of solvents and shown to be efficient in treating a variety of inflammation-related diseases, as indicated in Table 1, with a total inhibition of 79.2%. This explains why these species have traditionally been used as polyherbs to treat ulcers [46]. High activity was recorded with the methanolic extract even at 95 compared with the standard at 20.2%, respectively [50]. There is a dose-related impact in all models except for tail-flick, which has no statistically significant activity [52]. In terms of histological changes, the group treated with leaf extract had the highest improvement (ointment), whereas the other group, which had early re-epithelialization, had a greater cellular response to the inflammatory process. Burn wounds are frequent in both rich and developing countries; however, in poor countries, burns represent a serious public health issue due to the high frequency of severe sequelae. Burn wound healing is a complicated process that requires little assistance but still produces discomfort, and the wounds are susceptible to infection and other consequences [152]. Burn healing efficiency of Z. spina extract was assessed in the rat model (Table 1). To sum it up, ointment from the plant leaf was found to have good promise for speeding up the healing of burn wounds (Table 1). In vitro and in vivo studies show that Z. spina may be used to treat and control inflammation.
3.2.3. Antibacterial
Because of their unique qualities, plant extracts have recently got a lot of interest in terms of producing antibacterial agents. Because there is a rising interest in environmental protection, alternative synthesis processes that are ecologically benign and do not require harmful ingredients are needed. Due to activities such as inappropriate and careless administration of medicines in the clinic, bacterial strains have evolved resistant to a broad spectrum of antibiotics, resulting in the creation of multidrug-resistant microorganisms [153]. The preliminary examination of Z. spina against several Gram-positive and Gram-negative bacteria revealed that it was extremely significant. The extract was shown to exhibit action against some of the tested strains at a concentration of 100 mg/mL, with a minimum MIC of 6.25 g/mL for the methanolic extract. These results provide early evidence that crude extracts may be used to treat bacterial infections [56]. Both crystals had antibacterial activity against the tested strains, with SeONPs having greater antimicrobial activity than ZnONPs, according to the findings [24]. The ethanolic extract had the maximum activity against S. aureus, with an activity of 18 mm, while the aqueous extract had the lowest activity against B. subtilis, with an activity of 13 mm [70]. All examined strains were inhibited by the aqueous stem bark extracts, with the maximum inhibition against Klebsiella spp. and E. coli at 20 mm and 20 mm, respectively [76]. Because of the current exploratory findings, Z. spina extract might be a valuable source for the identification and development of novel antibacterial active compounds. The plant may be used to make effective antibiotics against bacterial infections.
3.2.4. Antifungal
Plant extracts and natural goods are gaining popularity since they do not pose a health risk or pollute the environment [111]. The lack of viable treatment choices, as well as pathogen cross-resistance to the earlier medications fluconazole and itraconazole, has required the search for novel antifungal agents from a variety of sources, including medicinal plants [110]. The following study found the extract exhibited the growth of fungal strains (Table 1). Several research projects have highlighted the antifungal properties of the species (Table 1). It was sensitive at 600 mg/mL and was not inhibited at low dosages, showing that the extract possesses antifungal activity [95]. At a concentration of 128 mg/mL, there was no evidence of any action against the Candida species [82]. At a dosage of 100 mg/mL, it showed considerable action with a 20 mm inhibitory zone. These findings might help in the treatment of fungal infections [102]. At a dosage of 500 mg/mL, both ethanolic and aqueous extracts inhibited Candida albicans with inhibition diameters of 32 mm and 18 mm, respectively [94]. As a result, it has been reasonable to conclude that the rise in extract concentration has antifungal action is due to the compounds' synergistic impact, which increases the contact area and extract access to the fungal strain.
3.2.5. Antidiarrhoeal Effects
Diarrhoea can be described as an adult's daily bowel movement that surpasses 200 g and contains between 60 and 95% of water. Diarrhoea caused by an infectious agent is the leading cause of newborn mortality in underdeveloped countries [154]. Children under the age of two have been found to have the greatest mortality rates, with a mortality rate of 20 fatalities per 1000 people [154]. Diarrhoea is responsible for more illnesses and deaths in children than any other disease combined in some regions of the world [155]. The World Health Organization has established a Diarrhoeal Disease Control (DDC) program to address the issues of diarrhoea in poor countries. This program involves investigations of traditional medicinal practices [155]. According to the findings, the extract of Z. spina protected rats from castor oil-induced diarrhoea and reduced intraluminal fluid collection and gastrointestinal transit. The LD50 values for intraperitoneal and oral administration in mice were 3465 and 1200 mg/kg, respectively (Table 1). The findings revealed that the extract may include physiologically active components that are antidiarrhoeal, which could explain its traditional use for gastrointestinal disorders.
3.2.6. Antiparasitic
A major public health problem is parasitic infections, which can cause morbidity and even death in their victims. The use of chemical drugs to combat parasites is effective, but there are drawbacks, such as drug resistance, drug residues, and undesirable side effects. Alternative remedies need to be studied [156]. The leaf extract is effective against Egyptian species of schistosomes at concentrations of 6, 25, 12.5, 25, 50, 100, and 200 mg/mL [118]. The extract of the leaves dramatically reduced the viability of leishmanial parasites at p > 0.001, while inducing NO generation and release, apoptosis, and plasma membrane permeability in macrophage cells without causing injury (Table 1). Methanol and aqueous extracts had IC50 values of 60 and 80 g/mL, respectively. The extracts had a devastating effect on the parasites (Table 1). Traditional usage of the leaf extract for antiparasitic ailments may have been based on the discovery of physiologically active components in the leaves.
3.2.7. Antiviral
Since the Stone Age, medicinal plants have played an important role in addressing human health difficulties. They help the human body by acting as restorative, defensive, and supporting agents. Because antiviral medications are frequently ineffective in treating viral infections, there is a growing demand for new antiviral agents that can combat viral resistance. New and better antiviral medicines are needed to combat viral infections. Antiviral medications currently on the market are frequently ineffective in treating viral infections because of the issue of viral resistance [157]. A 50-year-old guy was successfully treated with the leaf extract for rashes (Table 1). When compared with the control group, there was a significant reduction in the growth of the rashes [125]. There is a growing demand for the discovery of novel antiviral substances. Only one research was shown to substantially suppress the development of the virus, according to the study (Table 1). Because of this, the study recommended more research into viral disorders.
3.2.8. Antimalarial
Malaria has long been seen as a public health threat around the globe. More than 3.2 billion individuals are in danger of contacting malaria parasites, according to estimates [3]. The histology investigations of the liver and spleen indicated serious abnormalities. Improved histopathology results were shown in animals that had been treated. Biochemical tests showed a considerable recovery to normal levels of oxidative markers after treatment [126]. Liver function enzymes and histological pictures of the liver were significantly impacted. Because of the significant improvements in hepatic oxidative markers, it has been reasonable to assume that the extracts provide protection against Plasmodium infection [128].
3.2.9. Antidiabetic
Diabetes mellitus is the most common endocrine illness in the world, affecting an estimated 200 million individuals. In 2030, the population is expected to reach 366 million [158]. The highest levels of activity at 25.59 and 39.48%, respectively, at p > 0.001 were seen after 7 and 15 days of treatment with 500 mg/kg. At 29.07 and 35.56% after 7 and 15 days, the 500 mg/kg treatment provided the highest hypoglycaemic impact [44]. The extract inhibited alpha-amylase and glucosidase by 54 and 43%, respectively, at doses of 100 g/mL [129]. Diabetic rats exhibited much lower glucose levels and significantly higher blood insulin levels than the control group. The treatment group showed a significant reduction in triglycerides when compared with diabetic control and nondiabetic control rats, showing that it had a hypolipidemic effect (Table 1). The enzyme had high activity against alpha-amylase and glucosidase in methanolic extract, with 8.9 and 39.12 g/mL, respectively [50]. All preliminary research on the plant extract's ability to inhibit alpha-glucosidase and alpha-amylase showed the species has been shown to be a promising antidiabetic source in both in vitro and in vivo studies (Table 1). Diabetes-related disorders may benefit from the plant extract's preventive and therapeutic properties. Clinical trials are important because the investigations on this plant were done in vitro and in vivo.
3.2.10. Antiobesity
At pandemic levels, obesity is a key factor in the worldwide burden of chronic disease and disability. There are currently over one billion overweight adults in the globe, with at least 300 million of those individuals being classified as clinically obese [159]. Obesity management and therapy necessitates further research on medicinal plants, given the present conditions. In hypercholesterolemic male rats, the extract improved liver and kidney functions and reduced lipid peroxidation. The antihyperlipidemic actions of this extract may be connected with a suppression of oxidative stress because of its high content of phenolic compounds (Table 1).
3.2.11. Antianxiety
Anxiety is a medical condition that affects both our mental and physical health, and it has a variety of characteristics, including cognitive, emotional, behavioural, and somatic. About one-eighth of the global population is affected by anxiety, making it an essential research topic [160]. A number of studies show Z. spina to have a significant effect on anxiety (Table 1). Compared with the induction group, the data show that the extract considerably suppresses the expression of the markers investigated. The extract may help protect males from the harmful effects of pentylenetetrazole (Table 1). Administering the extract after HgCl2 exposure stopped mercury build-up in the cortical slices. As a result, the levels of malondialdehyde were reduced, as were those of nitrite and nitrate production and nitrite and nitrate creation enzymes. Glutathione levels were also boosted, as were those linked to the antioxidant enzymes glutathione reductase and glutathione peroxidase. Hence, the extract might be used to reduce the damage to neurons caused by HgCl2 poisoning. [133].
3.2.12. Anticancer
Cancer is a disease in which cells divide improperly and uncontrolled. In 2012, around 14 million new cancer cases were reported worldwide, with 8.2 million cancer-related deaths [161]. The development and spread of the contemporary healthcare system has been supported by medicinal plants. As their acceptability and acknowledgment spread over the world, medicinal plants remain the only path ahead. According to the findings of this investigation, the leaf extract contains compounds that have anticancer properties, making it a promising target for future research to create novel anticancer medications (Table 1). If extensive scientific research is conducted, the leaf extract of Z. spina will aid in the development of novel anticancer drugs. There are certain drawbacks to utilizing natural alternatives to pharmaceutical medications. They can be quite poisonous if they are not properly selected and prepared.
3.2.13. Toxicity
The increased interest in using plant extracts to treat human and animal diseases contributes to the current state of knowledge regarding the use of plant products in medicine. There are certain drawbacks to utilizing natural alternatives to pharmaceutical medications. They can be quite poisonous if they are not properly selected and prepared. Because of this, it is essential to determine the plant extracts' safety. Many studies have proven that medicinal plants contain a wide array of compounds that have a positive biological effect [21, 151]. These components are only beneficial if they are confirmed to be nontoxic or have minimal toxicity. Quite a number of studies have been carried out on the toxicity of Z. spina parts (Table 1) both in vivo and in vitro. In both the short- and long-term experiments, all rats survived at a limit dose of 3000 mg/kg of the root extract. There was no mortality; however, the rats in all groups showed symptoms of tiredness for about 1 to 2 hours (Table 1). Prophage induction did not increase at concentrations of 5, 15, or 30 mg/mL of the leaf extract compared with the control. The pfu/mL did not rise due to the phage's spontaneous release from lysogenic strains, according to the mutagenic index. As a result, no genotoxic potential was found in the plant extracts tested [64]. The leaf extract was discovered to contain a toxic level of 4050 mg/kg BW. When fed at doses below 1500 mg/kg BW, the leaf extract has no toxic effects on the liver (Table 1). Excessive use of the leaf extract may have toxicological consequences, according to the findings of this study; hence, it is recommended that only modest amounts be used.
3.3. Chemical Composition
Generally, plants have been documented overtime as medicine and/or lead compound sources [162]. Although chemical and synthetic methods have made medicines easier to obtain, the incorporation of plants as sources of medicine or lead compounds in drug discovery has led to the introduction of new and promising lead compounds possessing eccentric biological activities on various diseases [162]. The remarkable biological activity and the traditional medical applications of Z. spina have prompted a lot of investigations into its chemical composition. A total of 431 compounds were reportedly isolated from its genus (Ziziphus) with alkaloids and flavonoids being reported as the major classes of compounds [163]. In Z. spina, saponins, fatty acids, and phenolics in addition to alkaloids and flavonoids have been reported from various parts. This section of this review provides a comprehensive analysis of the phytochemical composition reported from different parts of Z. spina. Different parts of Z. spina have been reported in several studies to contain diverse classes of phytochemicals. The leaves obtained from Indonesia [29, 83] as well as other parts of the world [70, 85], were reported to have shown the presence of flavonoids, alkaloids, saponins, tannins, steroids, and triterpenes. The presence of monosaccharides, reducing sugars, pentose, ketosis, deoxy sugars, and indole alkaloids were reported in the leaves collected from the Plateau state, Nigeria [122, 164]. The detection of glycosides in the aerial parts and cardiac glycoside in the leaves was also reported [85]. Preliminary phytochemical screening of the fruit, pulp, seeds, and almonds of Z. spina collected from Settat and Khouribga cities in Morocco revealed the presence of alkaloids, saponins, triterpenes, quinones, and steroids in the fruit. Alkaloid was reportedly absent in the seed and almonds, while steroid was reportedly absent in the pulp [165]. In contrast to these reports, alkaloids and cardiac glycosides were reportedly absent in the leaves collected from Niger state, Nigeria [93]. Difference in geographical location has been noted to influence the biosynthesis and accumulation of secondary metabolites in plants. In the aerial parts collected in Tabuk, Saudi Arabia [122], and fruits and seeds collected in Qena city, Egypt [75], saponin was reportedly absent. Studies on the bark, fruit, root, seed, and leaf extracts were reported to have shown the presence of steroids, flavonoids, tannins, and alkaloids, but absence of triterpenes [75]. In the same study, phlobatannin was reportedly detected only in the bark extract of the plant. Cyclopeptide alkaloids basically are compounds that are polyamidic in structure with 13-/14- or 15-member ring structure having a side chain that is either basic or neutral in terms of characteristics based on the absence or presence of a terminal nitrogen [163]. These cyclopeptide alkaloids have been reported to be widely distributed in the family Rhamnaceae, particularly the genus Ziziphus [166]. Tuenter et al. [167] reported the isolation of two integerrine-type cyclopeptide alkaloids—nummularine-E and nummularine-D—and three amphibine-B 5(14)-type cyclopeptide alkaloids coupled with β-hydroxy-proline moiety—spinanine-B, spinanine-C, and amphibine-D—from the stem bark of dichloromethane fraction of Z. spina. In another study, a 14-membered cyclopeptide alkaloid, spinanine-A, belonging to amphibian F type was also isolated from the stem bark [168]. From the leaf (80% methanol) extract, the isolation of a new cyclopeptide alkaloid named 4(13) nummularine-C was reported [2]. Many other cyclopeptide alkaloids have been isolated and characterized from different parts of Z. spina, and they are as presented in Table 2 and Figure 3. The high content of saponins in the leaves of Z. spina that has found commercial use in the making of shampoo and detergents [172] has attracted lots of attention to the phytochemistry of this plant. Using 1D, 2D, HRESIMS, and GC-MS (identification of sugar moieties), identification and characterization of dammarane-type saponins, jujuboside B1, 22α-acetoxy christinin A, christinin A1, christinin A2, lotoside III, and 15-acetoxy lotoside IV were reported from n-butanol fraction of the leaves [172]. Ziziphine-F, jubarine-A, and amphibine-H have been reportedly isolated from the stem bark [168]. A novel triterpenic acid, 13-dehydrobetulin, isolated from chloroform fraction, stem ethanolic extract [20], and 3 new dammarane triterpenoids, sidrigenin, konarigenin, and siconigenin, isolated from the leaves [172] have all been reported in Z. spina. Other triterpenic acids that have been reported from Z. spina are summarized in Table 2 and Figure 3. The UHPLC/PDA/ESI-MS analytical technique was used to identify and characterize four new O-flavonoids—myricetin-3-O-(6-rhamnosyl)-hexoside, kaempferol-3-O-(2,6-diharmnosyl)-hexoside, kaempferol-3-O-rhamnoside, and quercetin-3-O-[(2-hexonyl)-6-rhamnosyl]-hexoside—and six new acyl flavonoids—quercetin-3-O-p-coumaroyl (2,6-dirhamnosyl)-hexoside, 6′-caffeoyl 3′,5′-di-C-glucopyranosylphloretin, kaempferol-3-O-(4-O-p-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-galactoside, quercetin-3-O-(4-O-p-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-glucoside, kaempferol-3-O-(4-O-p-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-glucoside, and quercetin 3-O-[4-carboxy-3-hydroxy-3-methylbutanoyl]-(⟶6)-hexoside—isolated from the leaf methanol extract of Ziziphus spina-christi [2]. One new polyphenol, quercetin 3-O-(4-O-trans-p-coumaroyl)-α-L-rhamnopyranosyl-(1⟶2)-[α-L-rhamnopyranosyl-(1⟶6)]-β-D-galactopyranoside, was reportedly isolated from n-butanol fraction of the leaves [172]. LC-MS-ESI analysis of the root ethanol extract revealed the presence of epicatechin [41]. Spectra data from HPLC and UV-visible analysis revealed the presence of catechin, gallic acid, ellagic acid, chlorogenic acid, rutin, isoquercitrin, quercetin, and kaempferol in the methanolic extract of the fruit [31]. Moreover, from the fruit were isolated and characterized different flavonoids carrying different sugar moieties. These flavonoids include quercetin 3-O-robinobioside, quercetin 3-O-β-D-xylosyl-(1⟶2)-α-L-rhamnoside, quercetin 3-O-β-D-xylosyl-(1⟶2)-α-L-rhamnoside-4`-O-α-L-rhamnoside, quercetin 3-O-β-D-galactoside, and quercetin 3-O-β-D-glucoside [173]. A summary of other polyphenolic compounds isolated/detected in Z. spina are presented in Table 2 and Figure 3. Using head space solid-phase microextraction (HS-SPME) and GC-MS methods, identification of α-pinene, β-pinene, β-myrcene, β-phellandrene, L-menthone, carane, trans-caryophyllene, and bicyclogermacrene was achieved as the major constituents of the essential oils of Ziziphus spina-christi [180]. Odeh et al. [181] reported the presence of benzaldehyde, phenylacetyldehyde, phenylethylalcohol, benzene, acetonitrile, 2-ethyl hexanoic acid, octanoic acid, 2-methoxy-4-(1-propanol)-6-acetate phenol, nonanoic acid, decanoic acid, 1-hydroxy-2,4,6-trimethylbenzene, and 5-hydroxymethyl-2-furan carbonyldehyde in honey obtained from Z. spina using HS-SPME-GC-MS methods. In the same study, phenylacetonitrile and 5-hydroxymethyl-2-furan carbonyldehyde were identified as potential markers of honey based on the premise that the different ratios of components present in honey could be utilized as a characteristic to differentiate their floral origins. Said et al. [182] reported the presence of various volatile compounds in the fruits including hexanoic acid, octanoic acid, dodecanoic acid, tetradecanoic acid methyl ester, tetradecanoic acid, hexadecenoic acid methyl ester, oleic acid methyl ester, and oleic acid ethyl ester as the major constituents. GC-MS analysis of stem bark diethyl ether extract after ethyl acetate was reported to have shown the presence of butyl hydroxy toluene bicyclo(4,4,0)dec-2-ene-4-ol, 2-methyl-9-(prop-1-en-3-ol-2-yl), dotriacontane, phytol, and 14-a-H-pregna as the major constituents [66]. Table 2 shows the major constituents of volatile compounds reported from Z. spina. Other compounds such as dodecaacetyl prodelphinidin B3, acetyl betulinic acid, sitosterol-tetraacetyl-β-D-glucoside, pentaacetyl glucose, and octaacetyl sucrose [174] have all been reported from the leaves. NMR (1D and 2D) and GC-MS were used for the identification and characterization of two cyclic amino acids, namely, 4-hydroxymethyl-1-methyl pyrrolidine-2-carboxylic acid and 4-hydroxymethyl-4-hydroxymehtyl-1-methylpyrolidine-2-carboxylic acid, from the seeds of Z. spina. Quantitative phytochemical analysis on different parts of Z. spina has been studied. It is noteworthy that different parts of Z. spina accumulated different constituents with variations in their quantities. Table 3 presents the quantitative phytochemical analysis reported on Z. spina.
Table 2.
Compounds reported from Z. spina.
| Compounds | Technique(s) | Quantity (%/μg/g DW) | Plant part (s) | References |
|---|---|---|---|---|
| Cyclopeptides | ||||
| Mauritine F | UHPLC-PDA-ESI-MS | Leaves | [2] | |
| Sanjonine F | ||||
| Sanjonine B | ||||
| Lotusanine A/frangulanine | ||||
| Jubanine C | ||||
| Adouetine Z | ||||
| Scutianine A | ||||
| Oxyphyline A | UHPLC-PDA-ESI-MS | Leaves | [2] | |
| HPLC-DAD-MS and HPLC-PDA-(HRMS)-SPE-NMR | Stem bark | [167] | ||
| Spinanine-A | MS, UV, IR | Stem bark | [169] | |
| Mauritine A | MS, IR, PMR, co-TLC, and optical rotation | Bark | [170] | |
| Mauritine C | ||||
| Amphibine F | ||||
| Amphibine E | ||||
| Amphibine A | ||||
| Saponins | ||||
| Christinin A | MS, IR, and NMR | Leaves | [171] | |
| Christinin C | ||||
| Christinin D | ||||
| Christinin B | UHPLC-PDA-ESI-MS/MS, IR, and NMR | Leaves | [2, 171] | |
| Christinin A/C | ||||
| Christinin A2 | NMR and HRESIMS/UHPLC-PDA-ESI-MS | Leaves | [2, 172] | |
| Jujubogenin-3-O-(di-deoxyhexosyl)-hexoside | UHPLC-PDA-ESI-MS | Leaves | [2] | |
| Jujubasaponin II/III isomer | ||||
| Polyphenols | ||||
| 3′,5′-di-C-β-glucosylphloretine | NMR and HRESIMS | Leaves | [172] | |
| HPLC–PDA–MS and NMR | Fruits | [173] | ||
| Hexaacetyl (+)-gallocatechin | NMR | Leaves | [174] | |
| Hexaacetyl (-)-epigallocatechin | ||||
| Kaempferol 3-O-robinobioside | NMR and HRESIMS | Leaves | [172] | |
| HPLC-PDA-MS and NMR | Fruits | [173] | ||
| Rutin | HPLC/LC-MS/MS/UV and NMR | Leaves | [29, 48, 175] | |
| Spinosin | HPLC | Leaves | [48] | |
| Ellagic acid | ||||
| Isoquercetrin | ||||
| Apigenin | ||||
| Kaempferol | ||||
| Kaempferol 3-O-rutinoside | NMR and HRESIMS | Leaves | [172] | |
| HPLC-PDA-MS and NMR | Fruits | [173] | ||
| Gallocatechin | HPLC | Leaves | [48] | |
| Epigallocatechin | NMR and HRESIMS | [172] | ||
| Quercetin 3-O-α-arabinosyl-(1⟶2)-α-rhamnoside | NMR, HRESIMS/HPLC-PDA-MS, and NMR | Leaves | [172] | |
| Fruits | [173] | |||
| Quercetin 3-O-β-xylopyranosyl-(1 ⟶ 2)-α-rhamnopyranoside 4ʹ-O-α-rhamnopyranoside | NMR and HRESIMS | Leaves | [172] | |
| Quercetin 3-O-α-rhamnopyranosyl-(1 ⟶ 6)-α-rhamnopyranosyl-(1 ⟶ 2)-β-galactopyranoside | ||||
| Prodelphinidin | ||||
| Quercetin 3-O-robinobioside | HPLC-PDA-MS and NMR | Fruits | [173] | |
| Quercetin 3-O-β-D-xylosyl-(1 ⟶ 2)-a-L-rhamnoside | ||||
| Quercetin 3-O-β-D-galactoside | ||||
| Quercetin 3-O-β-D-glucoside | ||||
| Quercetin 3-O-β-D-xylosyl-(1 ⟶ 2)-a-L-rhamnoside-4ʹ-O-a-L-rhamnoside | ||||
| Naringenin-6,8-di-C-hexoside | UHPLC-PDA-ESI-MS | Leaves | [2] | |
| Quercetin-3-O-[(2-hexosyl)-6-rhamnosyl]-hexoside | ||||
| (Epi)catechin-di-C-hexoside | ||||
| Quercetin-3-O-robinoside | ||||
| Bayarin | ||||
| Quercetin-3-O-hexoside | ||||
| Quercetin-3-O-(2-pentosyl-rhamnoside)-4′-O-rhamnoside | ||||
| Quercetin-3-O-(4-O-p-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-galactoside | ||||
| Quercetin 3-O-rutinoside | UHPLC-PDA-ESI-MS, HPLC-PDA-MS, and NMR | Leaves | [2] | |
| Fruits | [173] | |||
| Quercetin 3-xylosyl-(1⟶2) rhamnoside-4′-rhamnoside | UV and NMR | Leaves | [175] | |
| Quercitrin | ||||
| Gallic acid | HPLC | Leaves | [48] | |
| HPLC-DAD | 5.09 ± 1.23 | Pulp | [176] | |
| 13.38 ± 1.66 | Seed | |||
| 3.00 ± 0.84 | Almond | |||
| Catechin | HPLC | Leaves | [48] | |
| HPLC-DAD | 1.28 ± 1.66 | Pulp | [176] | |
| 10.98 ± 2.78 | Seed | |||
| Procyanidin B2 | HPLC-DAD | 63.22 ± 10.21 | Pulp | [176] |
| 425.44 ± 11.35 | Seed | |||
| Chlorogenic acid | HPLC | Leaves | [48] | |
| HPLC-DAD | 33.80 ± 2.66 | Pulp | [176] | |
| 15.0 ± 4.88 | Seed | |||
| 8.0 ± 1.77 | Almond | |||
| Cyanidin-3-galactosidase | HPLC-DAD | 36.77 ± 4.12 | Pulp | [176] |
| 131.78 ± 12.78 | Seed | |||
| 22.85 ± 2.60 | Almond | |||
| Caffeic acid | HPLC | Leaves | [48] | |
| HPLC-DAD | 52.19 ± 17.02 | Pulp | [176] | |
| 576.33 ± 23.19 | Seed | |||
| 2.78 ± 0.92 | Almond | |||
| Anthocyanin | HPLC-DAD | 1.27 ± 0.78 | Pulp | [176] |
| 586.09 ± 34.77 | Seed | |||
| Epicatechin | HPLC | Leaves | [48] | |
| HPLC-DAD | 11.33 ± 1.56 | Pulp | [176] | |
| 73.66 ± 12.66 | Seed | |||
| Cyanidin-3-rutinoside | HPLC-DAD | 10.27 ± 0.80 | Pulp | [176] |
| 43.88 ± 15.03 | Seed | |||
| p-Hydrobenzoic acid | HPLC-DAD | 113.45 ± 11.30 | Seed | [176] |
| Vanillic acid | 13.79 ± 1.09 | |||
| Syringic acid | HPLC | Leaves | [48] | |
| HPLC-DAD | 269.55 ± 22.89 | Pulp | [176] | |
| 210.04 ± 28.66 | Seed | |||
| Ferulic acid | HPLC-DAD | 125.22 ± 11.67 | Pulp | [176] |
| Sinapic acid | HPLC-DAD | 171.88 ± 31.02 | Pulp | [176] |
| 119.78 ± 10.55 | Seed | |||
| 185.67 ± 12.67 | Almond | |||
| Naringin | HPLC-DAD | 1.23 ± 0.12 | Pulp | [176] |
| 2.78 ± 0.78 | Seed | |||
| Rosmarinic acid | HPLC-DAD | 222.18 ± 34.89 | Pulp | [176] |
| 560.08 ± 35.28 | Seed | |||
| Hyperin | UV and NMR | Leaves | [175] | |
| HPLC-DAD | 1.18 ± 0.19 | Pulp | [176] | |
| 2.11 ± 0.73 | Seed | |||
| Avicularoside | HPLC-DAD | 19.23 ± 1.37 | Seed | [176] |
| Resveratrol | HPLC-DAD | 19.33 ± 4.86 | Pulp | [176] |
| 22.60 ± 2.82 | Seed | |||
| Quercetin | HPLC/NMR and HRESIMS | Leaves | [48, 172] | |
| HPLC-DAD | 25.08 ± 1.83 | Pulp | [176] | |
| 31.78 ± 6.88 | Seed | |||
| Volatile compounds | ||||
| α-Sitosterol | GC-MS | 5.63 | Leaves | [119] |
| 5-Phenylundecane | GC-MS | 10.88 | Fruits | [25] |
| 4-Phenylundecane | 5.97 | |||
| 3-Phenylundecane | 5.41 | |||
| 2-Phenylundecane | 8.39 | |||
| 6-Phenyldodecane | 14.90 | |||
| 4-Phenyldodecane | 5.51 | |||
| 3-Phenyldodecane | 4.67 | |||
| 2-Phenyldodecane | 5.41 | |||
| 6-Phenyltridecane | 11.38 | |||
| 2-Phenyltridecane | 4.06 | |||
| Phytol | GC-MS | 16.17 | Leaves | [142] |
| Palmitic acid | 24.66 | |||
| Oleic acid, omega 9 | 11.12 | |||
| Palmitoleic acid, methyl ester | GC-MS | 4.84 | Leaves | [142] |
| 7-Octadecenoic acid, methyl ester | GC-MS, IR | 19.63 | Seeds | [177] |
| Methyl stearate | 28.11 | |||
| Cis-11-eicosenoic acid, methyl ester | 16.97 | |||
| Eicosanoic acid, methyl ester | 4.97 | |||
| Docosanoic acid, methyl ester | 10.76 | |||
| 8.60 | ||||
| Geranyl acetone | GC and GC-MS | 14.0 | Leaves | [178] |
| Methyl hexadecanoate | 10.0 | |||
| Methyl octadecenoate | 9.9 | |||
| Farnesyl acetone | 9.9 | |||
| Hexadecanol | 9.7 | |||
| Ethyl octadecenoate | 8.0 | |||
| Ethyl hexadecanoate | 4.3 | |||
| Trihydroxy-octadecadienoic acid | UHPLC-PDA-ESI-MS | Leaves | [2] | |
| Dihydroxydodecadienoic acid | ||||
| Trihydroxy-octadecenoic acid | ||||
| Amino-hexadecanediol | ||||
| Amino-methyl; heptadecantriol | ||||
| 2-Amino-1,3-octadecanediol | ||||
| Octadecatetraenoic acid | ||||
| Triterpenic acid | ||||
| Oleanonic acid/bitulonic acid | UHPLC-PDA-ESI-MS | Leaves | [2] | |
| Ceanothic acid | ||||
| Ceanothic acid isomer | ||||
| 3-o-z-p-coumaroylalphitolic acid/3-o-z-p-coumaroylmaslinic acid | ||||
| Betulinic acid | ||||
| Alphitolic acid/maslinic acid | ||||
| Zizyberanalic acid/pomonic acid | ||||
| Other compounds | ||||
| Phloretin 3′/5′ di-c-galactoside | LC/ESI/MS | Fruits | [179] | |
| Phloretin 3′-c-glucoside 5′-c-galactoside | ||||
| Cis-5-o-p-coumaroylquinic acid | ||||
| Diosmetin 3′-c-galactoside 7-o-rutinoside | ||||
| Diosmetin 3′-o-glucoside 7-o-rutinoside | ||||
| Trans-5-o-caffeoylquinic acid | ||||
| Trans-5-o-p-coumaroylquinic acid |
Figure 3.
Some of the major compounds from Z. spina parts. (a) Kaempferol-3-O-rhamnoside. (b) Jujuboside B1. (c) Myricetin-3-O-(6-rhamnosyl) hexoside. (d) Quercetin-3-O-[(2-hexonyl)-6-rhamnosyl]-hexoside. (e) Quercetin-3-O-p-coumaroyl (2,6-dirhamnosyl)-hexoside. (f) Kaempferol-3-O-(4-O-(p)-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-galactoside. (g) Quercetin-3-O-(4-O-p-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-glucoside. (h) Kaempferol-3-O-(4-O-p-coumaroyl)-2-rhamnosyl-[6-rhamnosyl]-glucoside. (i) Quercetin 3-O-[4-carboxy-3-hydroxy-3-methylbutanoyl]-(6)-hexoside.
Table 3.
Quantitative phytochemical data of Z. spina.
| Plant part | Extract/fraction | Class of compounds | Quantity (%/mg/g/μg/mL/mg/100 g) | References |
|---|---|---|---|---|
| Leaves | Methanol extract | Polyphenols (GAE) | 74.6–58.7 | [31] |
| Flavonoids (QE) | 64.2–47.3 | |||
| Leaves | Methanol extract | Quercetin | 1.39–3.66 | [183] |
| Saponin | 1.80–2.6 | |||
| Leaves | Methanol extract | Total flavonoids (RE) | 710 ± 28.5 | [142] |
| Total phenolic content (GAE) | 500 ± 10.5 | |||
| Roots | Aqueous extract | Total phenolic content (GAE) | 59.89 ± 0.42 | [41] |
| Methanol extract | Total flavonoid content (QE) | 9.01 ± 0.01 | ||
| Ethanol extract | Catechin content (CE) | 12.28 ± 1.07 | ||
| Acetone extract | Total phenolic content (GAE) | 59.98 ± 0.1 | ||
| Total flavonoid content (QE) | 9.45 ± 0.03 | |||
| Catechin content (CE) | 19.6 ± 0.19 | |||
| Total phenolic content (GAE) | 60.47 ± 0.04 | |||
| Total flavonoid content (QE) | 12.04 ± 0.04 | |||
| Catechin content (CE) | 23.98 ± 0.38 | |||
| Total phenolic content (GAE) | 60.26 ± 0.16 | |||
| Total flavonoid content (QE) | 11.64 ± 0.02 | |||
| Catechin content (CE) | 17.14 ± 2.01 | |||
| Aerial part | Methanol extract | Phenolics (GAE) | 51.33 ± 0.4 | [122] |
| Flavonoids (QE) | 14.78 ± 0.36 | |||
| Tannins (CE) | 21.6 ± 1.51 | |||
| Honey | Total phenolic (CE) | 98.46 ± 2.24 | [184] | |
| Pulp | Aqueous extract | Total phenolic content (GAE) | 30.97 ± 0.67 | [176] |
| Total flavonoid content (RE) | 5.05 ± 1.09 | |||
| Seed | Total phenolic content (GAE) | 74.46 ± 2.12 | ||
| Total flavonoid content (RE) | 19.84 ± 1.56 | |||
| Almond | Total phenolic content (GAE) | 31.05 ± 1.30 | ||
| Total flavonoid content (RE) | 8.65 ± 0.78 | |||
| Honey | Total phenolic content (GAE) | 20.15 ± 2.79–21.98 ± 0.70 | [185] | |
| Leaves | 80% methanol extract | Total phenolic content (GAE) | 87.3 ± 4.7 | [186] |
| Total flavonoid content (QE) | 9.6 ± 1.1 |
Note. GAE = gallic acid equivalent, QE = quercetin equivalent, RE = rutin equivalent, CE = catechin equivalent.
4. Conclusion and Future Research
This review attempts to summarize the key findings of numerous research groups involved in the hunt for naturally occurring active principles from Z. spina against a variety of human diseases, the objective of which has been to get new effective medications. In this review, the ethnobotanical, pharmacological, and chemical content of the abundantly diversified species have been emphasized. The study found traditionally the plant parts especially the leaves were used for the treatment of diabetes, malaria, digestive issues, typhoid, liver complaints, weakness, skin infections, urinary disorders, obesity, diarrhoea, and sleeplessness. Preclinical investigations have already been conducted on a variety of biological activities. The leaves were found to have significant biological activity, and this is due to the presence of high contents of polyphenol compounds. There is a lot of room for fresh scientific and developmental research. The evidence reported in this analysis provides a foundation for future investigations that are desperately required. Detailed examinations of the following topics are among the top objectives for future research: (1) identification of species based on micromorphology and anatomy in each region of the world, (2) mechanism of action of the isolated compounds, (3) clinical trial, (4) the standard dose and safety of the leaf be established, and (5) formulation of herbal medicine from the leaf.
Data Availability
The data are available within the manuscript.
Conflicts of Interest
The authors do not have any potential conflicts of interest to declare.
Authors' Contributions
All the authors contributed equally to data search, analysis of the retrieved data, and drafting the manuscript.
References
- 1.Mahmoud Dogara A. Review of ethnopharmacology, morpho-anatomy, biological evaluation and chemical composition of Syzygium polyanthum (wight) walp. Plant Science Today . 2021;9(1):167–177. doi: 10.14719/pst.1386. [DOI] [Google Scholar]
- 2.Sakna S. T., Mocan A., Sultani H. N., El-Fiky N. M., Wessjohann L. A., Farag M. A. Metabolites profiling of Ziziphus leaf taxa via UHPLC/PDA/ESI-MS in relation to their biological activities. Food Chemistry . 2019;293:233–246. doi: 10.1016/j.foodchem.2019.04.097. [DOI] [PubMed] [Google Scholar]
- 3.Dogara A. M., Labaran I., Yunusa A. Ethnobotany of medicinal plants with antimalarial potential in Northern Nigeria. Ethnobotany Research and Applications . 2020;19:1–8. doi: 10.32859/era.19.32.1-8. [DOI] [Google Scholar]
- 4.Anand U., Tudu C. K., Nandy S., et al. Ethnodermatological use of medicinal plants in India: from ayurvedic formulations to clinical perspectives–a review. Journal of Ethnopharmacology . 2022;284 doi: 10.1016/j.jep.2021.114744.114744 [DOI] [PubMed] [Google Scholar]
- 5.Asgarpanah J., Haghighat E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. African Journal of Pharmacy and Pharmacology . 2012;6(31):2332–2339. doi: 10.5897/AJPP12.509. [DOI] [Google Scholar]
- 6.Mahmoud A. D., Dogara A. M., Abba A. Ethnomedicinal survey of plants used for management of inflammatory diseases in Ringim local government, Jigawa state, Nigeria. Ethnobotany Research and Applications . 2021;22:1–27. doi: 10.32859/era.22.47.1-27. [DOI] [Google Scholar]
- 7.Awang N. A., Ali A. M., Abdulrahman M. D., Mat N. Edible bitter mushroom from Besut, Malaysia. Journal of Agrobiotechnology . 2018;9(2):70–79. [Google Scholar]
- 8.El-Kamali H., El-Khalifa K. Folk medicinal plants of riverside forests of the Southern Blue Nile district, Sudan. Fitoterapia . 1999;70(5):493–497. doi: 10.1016/S0367-326X(99)00073-8. [DOI] [Google Scholar]
- 9.Madani I., Ibrahim D. A. A., Hamdeen H. M. Abundance and ethnomedicinal use of tree and shrub species in Azaza and Mokla forests in the Blue Nile state, Sudan. EPRA International Journal of Multidisciplinary Research (IJMR) . 2021;7(7):167–173. doi: 10.36713/epra7634. [DOI] [Google Scholar]
- 10.Delfan B., Bahmani M., Hassanzadazar H., et al. Ethnobotany study of effective medicinal plants on gastric problems in Lorestan province, West of Iran. Journal of Chemical and Pharmaceutical Research . 2015;2:483–492. [Google Scholar]
- 11.Musa M. S., Abdelrasool F. E., Elsheikh E. A., Ahmed L. A., Mahmoud A. L. E., Yagi S. M. Ethnobotanical study of medicinal plants in the blue Nile state, south-eastern Sudan. Journal of Medicinal Plants Research . 2011;5(17):4287–4297. [Google Scholar]
- 12.Said O., Khalil K., Fulder S., Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the golan heights and the west bank region. Journal of Ethnopharmacology . 2002;83(3):251–265. doi: 10.1016/S0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 13.Alalwan T. A., Alkhuzai J. A., Jameel Z., Mandeel Q. A. Quantitative ethnobotanical study of some medicinal plants used by herbalists in Bahrain. Journal of Herbal Medicine . 2019;17-18 doi: 10.1016/j.hermed.2019.100278.100278 [DOI] [Google Scholar]
- 14.Abou A. M. An ethnobotanical use of plants in the middle area, Gaza Strip, Palestine. Advances in Environmental Biology . 2011;5(11):3681–3688. [Google Scholar]
- 15.Tetik F., Civelek S., Cakilcioglu U. Traditional uses of some medicinal plants in Malatya (Turkey) Journal of Ethnopharmacology . 2013;146(1):331–346. doi: 10.1016/j.jep.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 16.Adamu H. M., Abayeh O., Agho M., et al. An ethnobotanical survey of Bauchi State herbal plants and their antimicrobial activity. Journal of Ethnopharmacology . 2005;99(1):1–4. doi: 10.1016/j.jep.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Asatryan A., Tel-Zur N. Intraspecific and interspecific crossability in three Ziziphus species (Rhamnaceae) Genetic Resources and Crop Evolution . 2014;61(1):215–233. doi: 10.1007/s10722-013-0027-8. [DOI] [Google Scholar]
- 18.Dafni A., Levy S., Lev E. The ethnobotany of Christ’s thorn jujube (Ziziphus spina-christi) in Israel. Journal of Ethnobiology and Ethnomedicine . 2005;1(1):8–11. doi: 10.1186/1746-4269-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saied A. S., Gebauer J., Hammer K., Buerkert A. Ziziphus spina-christi (L.) Willd: a multipurpose fruit tree. Genetic Resources and Crop Evolution . 2008;55(7):929–937. doi: 10.1007/s10722-007-9299-1. [DOI] [Google Scholar]
- 20.Soliman S., Hamoda A. M., El-Shorbagi A. N. A., El-Keblawy A. A. Novel betulin derivative is responsible for the anticancer folk use of Ziziphus spina-christi from the hot environmental habitat of UAE. Journal of Ethnopharmacology . 2019;231:403–408. doi: 10.1016/j.jep.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Abdulrahman M. Antioxidant, alpha glucosidase and antibacterial evaluation of Syzygium mytifolium (Roxb.) Walp. Plant Science Today . 2021;8(2):410–415. doi: 10.14719/pst.2021.8.2.1113. [DOI] [Google Scholar]
- 22.Ullah R., Alqahtani A. S., Noman O. M., Alqahtani A. M., Ibenmoussa S., Bourhia M. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi Journal of Biological Sciences . 2020;27(10):2706–2718. doi: 10.1016/j.sjbs.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odhav B., Thangaraj K., Khumalo N., Baijnath H. Screening of African traditional vegetables for their alpha-amylase inhibitory effect. Journal of Medicinal Plants Research . 2013;4(14):1502–1507. [Google Scholar]
- 24.lashin I., Hassan S. A., Hassan S. A. M., Hashem A. H. Green biosynthesis of zinc and selenium oxide nanoparticles using callus extract of Ziziphus spina-christi: characterization, antimicrobial, and antioxidant activity. Biomass Conversion and Biorefinery . 2021;1–14 doi: 10.1007/s13399-021-01873-4. [DOI] [Google Scholar]
- 25.El-Hefny M., Mohamed A. A., Salem M. Z. M., Abd El-Kareem M. S. M., Ali H. M. Chemical composition, antioxidant capacity and antibacterial activity against some potato bacterial pathogens of fruit extracts from Phytolacca dioica and Ziziphus spina-christi grown in Egypt. Scientia Horticulturae . 2018;233:225–232. doi: 10.1016/j.scienta.2018.01.046. [DOI] [Google Scholar]
- 26.Adeyemo S. Studies on in-vitro antioxidant and free radical scavenging potential and phytochemical screening of leaves of Ziziphus mauritiana L. and Ziziphus spina-christi L. compared with ascorbic acid. Journal of Medical Genetics . 2011;3(2):28–34. [Google Scholar]
- 27.Khaleel S. M., Jaran A. S., Haddadin M. S. Evaluation of total phenolic content and antioxidant activity of three leaf extracts of Ziziphus spina-christi (Sedr) grown in Jordan. British Journal of Medicine and Medical Research . 2016;14(6):1–8. doi: 10.9734/bjmmr/2016/24935. [DOI] [Google Scholar]
- 28.Setorki M., Hooshmandi Z. Neuroprotective effect of Ziziphus spina-christi on brain injury induced by transient global cerebral ischemia and reperfusion in rat. Bangladesh Journal of Pharmacology . 2017;12(1):69–76. doi: 10.3329/bjp.v12i1.29964. [DOI] [Google Scholar]
- 29.Aziza H., Sumarlin L. O., Nur Azizah Y. Formulation, antioxidant and antibacteria activities of peel-off gel mask, enriched with Bidara leaf (Ziziphus spina-christi L.) extract. International Journal of Geometry . 2020;18(68):66–72. doi: 10.21660/2020.68.5656. [DOI] [Google Scholar]
- 30.Hussein M. B., Hamad M. N. M. Phytochemical screening, antimicrobial and antioxidant activity of Ziziphus spina-christi (L.)(Rhamnaceae) leaves and bark extracts. MAR Microbiology . 2021;2(2):1–9. [Google Scholar]
- 31.Almeer R. S., El-Khadragy M. F., Abdelhabib S., Abdel Moneim A. E. Ziziphus spina-christi leaf extract ameliorates schistosomiasis liver granuloma, fibrosis, and oxidative stress through downregulation of fibrinogenic signaling in mice. PLoS One . 2018a;13(10) doi: 10.1371/journal.pone.0204923.e0204923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Ali J. T., Jewad A. M. Effect of the alcoholic or the aqueous extracts of Ziziphus spina-christi leaves on the white blood cells and the oxidative stress status in local rabbits. Journal of International Pharmaceutical Research . 2019;46(5):548–554. [Google Scholar]
- 33.Hassan A. B., Al Maiman S. A., Mohammed M. A., et al. Effect of natural fermentation on the chemical composition, mineral content, phytochemical compounds, and antioxidant activity of Ziziphus spina-christi (L) Processes . 2021;9(7):1228–1311. doi: 10.3390/pr9071228. [DOI] [Google Scholar]
- 34.Abu-Raghif A. R., Jasim G. A., Hanoon M. M. Anti-proliferative activity of Zizyphus spina christi leaves methanol extract against rhabdomyosarcoma (rd) cell line. International Journal of Pharmacy and Pharmaceutical Sciences . 2017;9(2):279–282. doi: 10.22159/ijpps.2017v9i2.15013. [DOI] [Google Scholar]
- 35.Elaloui M., Ghazghazi H., Ennajah A., et al. Phenolic profile, antioxidant capacity of five Ziziphus spina-christi (L.) Willd provenances and their allelopathic effects on Trigonella foenum-graecum L. and Lens culinaris L. seeds. Natural Product Research . 2017;31(10):1209–1213. doi: 10.1080/14786419.2016.1226830. [DOI] [PubMed] [Google Scholar]
- 36.Papari Moghadam Fard M., Ketabchi S., Farjam M. H. Chemical composition, antimicrobial and antioxidant potential of essential oil of Ziziphus spina-christi var. aucheri grown wild in Iran. Journal of Medicinal Plants and By Products . 2020;9:67–71. doi: 10.22092/JMPB.2020.121752. [DOI] [Google Scholar]
- 37.Hamadnalla H. M., Mohamed Ali M. A. A., Ahmed A. A. Anti-microbial and anti-oxidant activities of Ziziphus spina christi, Sudanese medicinal plant. Journal of Biotechnology & Bioinformatics Research . 2020;2(2):1–3. doi: 10.47363/jbbr/2020(2)108. [DOI] [Google Scholar]
- 38.Ogbiko C., Okoh E., Abubakar H. Phytochemical screening, in vitro antioxidant activity and polyphenolic content of the leaves of Nigerian Ziziphus spina–christi (L.) Willd. International Journal of Science for Global Sustainability . 2020;6(4):24–32. [Google Scholar]
- 39.Singh V., Guizani N., Mohamed Es M., Shafiur Ra M., Selvaraju S. In vitro antioxidant activities of Ziziphus spina-christi fruits (red date) grown in Oman. Biotechnology . 2012;11(4):209–216. doi: 10.3923/biotech.2012.209.216. [DOI] [Google Scholar]
- 40.AL-Marzooq M. A. Phenolic compounds of Napek leave (Zizyphus spina-christi L.) as natural antioxidants. Journal of Food and Nutrition Sciences . 2014;2(5):207–214. doi: 10.11648/j.jfns.20140205.11. [DOI] [Google Scholar]
- 41.Elaloui M., Hamdi S. H., Ghazghazi H., et al. Characterization of epicathechin contents in the Ziziphus spina-christi L. root extracts using LC-MS analyses and their insecticidal potential. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology . 2020;155(4):685–690. doi: 10.1080/11263504.2020.1779837. [DOI] [Google Scholar]
- 42.Badr S. F., El-Sherif N. A., Ii S., El-Deeb M., Al-Shinqiti F. Y. M. Evaluation of genetic variation, antioxidant and antibacterial activities of two Sidr varieties in Medina. Pakistan Journal of Pharmaceutical Sciences . 2020;33(1):61–69. doi: 10.36721/PJPS.2020.33.1.REG.061-069.1. [DOI] [PubMed] [Google Scholar]
- 43.Abduljawad S. H. Digestive fermentation, antioxidant status, and haemato-biochemical indices of growing rabbits fed on diets supplemented with Ziziphus spina-christi leaf. Journal of Nutrition and Metabolism . 2020;2020:6. doi: 10.1155/2020/9046862.9046862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Ghamdi A. A., Shahat A. A. Antioxidant, hypoglycemic and anti-diabetic activities of Ziziphus spina-christi (L) Willd (Rhamnacae) leaf extract. Tropical Journal of Pharmaceutical Research . 2018;16(11):2601–2610. doi: 10.4314/tjpr.v16i11.5. [DOI] [Google Scholar]
- 45.Alhakmani F., Khan S. A., Ahmad A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pacific Journal of Tropical Biomedicine . 2014;4(2):S656–S660. doi: 10.12980/APJTB.4.2014APJTB-2014-0273. [DOI] [Google Scholar]
- 46.Ugwah M. O., Etuk E. U., Bello S. O., Aliero A. A., Ugwah-Oguejiofor C. J. Comparative studies of anti-ulcerogenic activities of three Nigerian medicinal plants: a preliminary evaluation. Research Journal of Medicinal Plant . 2013;7(9):490–495. [Google Scholar]
- 47.Almeer R. S., Mahmoud S. M., Amin H. K., Abdel Moneim A. E. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food and Chemical Toxicology . 2018;115:49–62. doi: 10.1016/j.fct.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Dkhil M. A., Al-Quraishy S., Moneim A. E. A. Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via anti-oxidant and anti-inflammatory effects. Inflammopharmacology . 2018;26(3):779–791. doi: 10.1007/s10787-017-0439-8. [DOI] [PubMed] [Google Scholar]
- 49.Yagoub A. E. A., Alshammari G. M., Subash-Babu P., Mohammed M. A. A., Alhosain A. I. Synthesis of Ziziphus spina-christi (Jujube) root methanol extract loaded functionalized silver nanoparticle (ZS-Ag-NPs); physiochemical characterization and effect of ZS-Ag-NPs on adipocyte maturation, adipokine and vascular smooth muscle cell interaction. Nanomaterials . 2021;11(10) doi: 10.3390/nano11102563.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khaleel S. M. Anti-oz-glucosidase, anti-o-amylase and anti-inflammatory effects of leaf extracts of Ziziphus spina-christi (sedr) grown in Jordan. Research Journal of Biological Sciences . 2018;13(1):1–7. [Google Scholar]
- 51.Tanira M. O. M., Ageel A. M., Tariq M., Mohsin A., Shah A. H. Evaluation of some pharmacological, microbiological and physical properties of Zizyphus spina-christi. International Journal of Crude Drug Research . 1988;26(1):56–60. doi: 10.3109/13880208809053889. [DOI] [Google Scholar]
- 52.Adzu B., Haruna A. K. Studies on the use of Zizyphus spina-christi against pain in rats and mice. African Journal of Biotechnology . 2007;6(11):1317–1324. [Google Scholar]
- 53.Alajmi R. A., Al-Megrin W. A., Metwally D., et al. Anti-Toxoplasma activity of silver nanoparticles green synthesized with phoenix dactylifera and Ziziphus spina-christi extracts which inhibits inflammation through liver regulation of cytokines in Balb/c mice. Bioscience Reports . 2019;39(5):1–30. doi: 10.1042/BSR20190379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.ANaeem L. Comparative study between Nigella sativa and Ziziphus spina-christi effectiveness on skin a superficial burn healing in rabbits. Basrah Journal of Veterinary Research . 2015;14(1):311–319. doi: 10.33762/bvetr.2015.102448. [DOI] [Google Scholar]
- 55.Dangoggo S., Hassan L., Sadiq I., Manga S. Phytochemical analysis and antibacterial screening of leaves of Diospyros mespiliformis and Ziziphus spina-christi. Journal of Chemical Engineering . 2012;1(1):31–37. [Google Scholar]
- 56.El-Kamali H. H., Mahjoub S. A. T. Antibacterial activity of Francoeuria crispa, Pulicaria undulata, Ziziphus spina-christi and Cucurbita pepo against seven standard pathogenic bacteria. Ethnobotanical Leaflets . 2009;13(6):722–733. [Google Scholar]
- 57.Kredy H. M. Antibacterial activity of saponins extract from sider (Ziziphus spina-christi) University of Thi-Qar Journal . 2010;1(6):1–7. [Google Scholar]
- 58.Ali A. B., Almagboul A. Z., Mohammed O. M. Antimicrobial activity of fruits, leaves, seeds and stems extracts of Ziziphus spina-christi. Arabian Journal of Medicinal and Aromatic Plants . 2015;1(2):94–107. doi: 10.48347/IMIST.PRSM/ajmap-v1i2.4325. [DOI] [Google Scholar]
- 59.Ogbiko C., Adiya Z., Adamu S., Isah A., Ibrahim I. Phytochemical, nutritional and antimicrobial properties of the leaves of Nigerian Ziziphus Spina–christi (l.) Willd. International Journal of Pharmaceutical Research and Allied Sciences . 2021;18(1):3445–3452. [Google Scholar]
- 60.Sofy A. R., Aboseidah A. A., El-Morsi E. S., Azmy H. A., Hmed A. A. Evaluation of antibacterial and antibiofilm activity of new antimicrobials as an urgent need to counteract stubborn multidrug-resistant bacteria. Journal of Pure and Applied Microbiology . 2020;14(1):595–608. doi: 10.22207/JPAM.14.1.62. [DOI] [Google Scholar]
- 61.Hamad A. I. The effect of aqueous and alcoholic extract of Ziziphus spina-christi on some bacterial isolates causing gingivitis. Tikrit Journal for Dental Sciences . 2018;6(2):58–63. [Google Scholar]
- 62.Bukar A., Kyari M., Gwaski P., Gudusu M., Kuburi F., Abadam Y. Evaluation of phytochemical and potential antibacterial activity of Ziziphus spina-christi L. against some medically important pathogenic bacteria obtained from University of Maiduguri Teaching Hospital, Maiduguri, Borno State–Nigeria. Journal of Pharmacognosy and Phytochemistry . 2015;3(5):98–101. [Google Scholar]
- 63.Ksa A. Q. Antibacterial activity of sider (Ziziphus spina-christi), leaves extract against selected pathogenic bacteria. European Journal of Pharmaceutical and Medical Research . 2016;3(5):138–144. [Google Scholar]
- 64.Hassan A. A., Abd-Elaziz G. O. Genotoxicity and antimicrobial activity of Myrtus communis L., Ziziphus spina-christi (L.) Willd and Cassia angustifolia Vahl extracts. Bangladesh Journal of Botany . 2020;49(3):557–566. doi: 10.3329/bjb.v49i3.49640. [DOI] [Google Scholar]
- 65.Salih R., Gibreel H., Ahmed D., Hammad Z., Ahmed A. Antimicrobial effect of aqueous and ethanolic leaves extracts of Ziziphus species against animal bacterial pathogens. Curr Trends Forest Res . 2019;3(1036):2638–0013. [Google Scholar]
- 66.Ads E. N., Rajendrasozhan S., Hassan S. I., Sharawy S. M. S., Humaidi J. R. Phytochemical, antimicrobial and cytotoxic evaluation of Ziziphus spina-christi (L.) stem bark. Biomedical Research . 2017;28(15):6646–6653. [Google Scholar]
- 67.Owayss A. A., Elbanna K., Iqbal J., et al. In vitro antimicrobial activities of Saudi honeys originating from Ziziphus spina-christi L. and Acacia gerrardii Benth. trees. Food Sciences and Nutrition . 2020;8(1):390–401. doi: 10.1002/fsn3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdallah E. M. Antibacterial activity of fruit methanol extract of Ziziphus spina-christi from Sudan. International Journal of Current Microbiology and Applied Sciences . 2017;6(5):38–44. doi: 10.20546/ijcmas.2017.605.005. [DOI] [Google Scholar]
- 69.Jebur M. H., Hind N. K. K., Hamza H. J., Alkaim A. F. The activity of aquatic extract of Ziziphus spina-christi against bacteria, an in vitro study. International Journal of Psychosocial Rehabilitation . 2020;24(5):1821–1827. doi: 10.37200/IJPR/V24I5/PR201854. [DOI] [Google Scholar]
- 70.Mohammed B. S., Awatif A. M. Preliminary phytochemical screening and antibacterial activity of ethanolic and aqueous extracts of Sudanese medicinal plant Ziziphus spina-christi L leaves. AJMAP . 2018;4(1):36–44. doi: 10.48347/IMIST.PRSM/ajmap-v4i1.11377. [DOI] [Google Scholar]
- 71.Moghadam M. S., Maleki S., Darabpour E., Motamedi H., Seyyed Nejad S. M. Antibacterial activity of eight Iranian plant extracts against methicillin and cefixime restistant Staphylococcous aureus strains. Asian Pacific Journal of Tropical Medicine . 2010;3(4):262–265. doi: 10.1016/S1995-7645(10)60063-6. [DOI] [Google Scholar]
- 72.Bamsaoud S. F., Basuliman M. M., Bin-Hameed E. A., Balakhm S. M., Alkalali A. S. The effect of volume and concentration of AgNO3 aqueous solutions on silver nanoparticles synthesized using Ziziphus Spina–Christi leaf extract and their antibacterial activity. Journal of Physics: Conference Series . 2021;1900(1) doi: 10.1088/1742-6596/1900/1/012005.012005 [DOI] [Google Scholar]
- 73.Taghipour M. T., Nameni R., Taghipour M., Ghorat F. Phytochemical analysis and antimicrobial activity of Ziziphus spina-christi and Tamarix aphylla leaves’ extracts as effective treatment for Coronavirus disease 2019 (COVID-19) Thrita . 2020;9(2) doi: 10.5812/thrita.107776. [DOI] [Google Scholar]
- 74.Mathew M., Sghaireen M. G. Study on antibacterial activity of dental cements with extracts of Ziziphus spina-christi on Streptococcus mutans: an in vitro study. Journal of International Oral Health . 2020;12(6):568–572. doi: 10.4103/jioh.jioh_104_20. [DOI] [Google Scholar]
- 75.Temerk H., Salem W., Sayed W., Hassan F. Antibacterial effect of phytochemial extracts from Ziziphus-spina christi against some pathogenic bacteria. Egyptian Journal of Botany . 2017;57(3):595–604. doi: 10.21608/EJBO.2017.665.1035. [DOI] [Google Scholar]
- 76.Korji S. H. Inhibition of nitrate reductase production from gramnegative bacteria using Ziziphus spina-christi extract and comparing with some antibiotics. The Iraqi Journal of Agricultural Sciences . 2012;43(2):144–150. [Google Scholar]
- 77.El-Ansary A., Warsy A., Daghestani M., et al. Characterization, antibacterial, and neurotoxic effect of green synthesized nanosilver using Ziziphus spina Christi aqueous leaf extract collected from Riyadh, Saudi Arabia. Materials Research Express . 2018;5(2) doi: 10.1088/2053-1591/aaaf5f.025033 [DOI] [Google Scholar]
- 78.Ali N. A., Jülich W. D., Kusnick C., Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. Journal of Ethnopharmacology . 2001;74(2):173–179. doi: 10.1016/S0378-8741(00)00364-0. [DOI] [PubMed] [Google Scholar]
- 79.Halawani E. M. Rapid biosynthesis method and characterization of silver nanoparticles using Zizyphus spina christi leaf extract and their antibacterial efficacy in therapeutic application. Journal of Biomaterials and Nanobiotechnology . 2017;8(1):22–35. doi: 10.4236/jbnb.2017.81002. [DOI] [Google Scholar]
- 80.Nkafamiya I., Shagal M., Haruna M. Potential of Ziziphus spina-christi seed ethanolic extract on inhibition of microbial growth. Academia Journal of Biotechnology . 2013;1(4):53–56. [Google Scholar]
- 81.Shahat A. A., Pieters L., Apers S., et al. Chemical and biological investigations on Zizyphus spina-christi L. Phytotherapy Research . 2001;15(7):593–597. doi: 10.1002/ptr.883. [DOI] [PubMed] [Google Scholar]
- 82.Gheith I., El-Mahmoudy A. Assessment of the antimicrobial potential of the hydro-methanolic extract of Sidr (Ziziphus spina-christi) plant against selected pathogens in vitro. Life Science Journal . 2018;15(9):27–34. doi: 10.7537/marslsj150918.03. [DOI] [Google Scholar]
- 83.Sarjito P. L., Purnamayati L., Riyadi P. H., Desrina P. S. B., Prayitno S. B. Phytochemical analysis and antibacterial activities of sidr leaf extract (Ziziphus spina-christi) against pathogenic bacteria in aquaculture. Pertanika Journal of Tropical Agricultural Science . 2021;44(4):845–864. doi: 10.47836/pjtas.44.4.08. [DOI] [Google Scholar]
- 84.Monadi M., Motamedi H., Sanei N. The effects of ethanol and methanol extracts of Ziziphus spina-christi, Peganum harmala, Salvia officinalis, and Querqus brantii on the growth and biofilm formation by Staphylococcus aureus in vitro. Qom University of Medical Sciences Journal . 2021;15(7):482–489. doi: 10.32598/qums.15.7.2224.1. [DOI] [Google Scholar]
- 85.Alhassan K. A., Indabawa A. S., Shah M. M. Phytochemical analysis, proximate composition and antibacterial activities of Ziziphus species (Z. jujube and Z. spina-christi) Journal of Applied and Advanced Research . 2019;4(1):42–46. doi: 10.21839/jaar.2019.v4i1.262. [DOI] [Google Scholar]
- 86.Al-Kaabi H. K. J., Hmood B. A., Jebur L. S., Abdullah S. M., AL-Qraghi S. A. A. Determination of Ziziphus spina-christi leaves extracts antibacterial activity against some pathogenic bacteria. Al-Kufa University Journal for Biology . 2021;13(1):14–20. [Google Scholar]
- 87.Nazif N. M. Phytoconstituents of Zizyphus spina-christi L. fruits and their antimicrobial activity. Food Chemistry . 2002;76(1):77–81. doi: 10.1016/S0308-8146(01)00243-6. [DOI] [Google Scholar]
- 88.Kalayou S., Haileselassie M., Gebre-egziabher G., et al. In–vitro antimicrobial activity screening of some ethnoveterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray Region, Ethiopia. Asian Pacific Journal of Tropical Biomedicine . 2012;2(7):516–522. doi: 10.1016/s2221-1691(12)60088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Bayatti K. K., Aziz F. M., Abdalah M. E. A study of antibacterial activity of Cidar (Zizyphus spinachristi L.) on bacterial pathogens isolated from skin infections. Al Mustansiriyah Journal of Pharmaceutical Sciences . 2011;9(1):13–20. doi: 10.32947/ajps.v9i1.268. [DOI] [Google Scholar]
- 90.Ali-Shtayeh M., Yaghmour R. M. R., Faidi Y., Salem K., Al-Nuri M. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. Journal of Ethnopharmacology . 1998;60(3):265–271. doi: 10.1016/S0378-8741(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 91.Mulyani S., Adriani M., Wirjatmadi B. Antibacterial activity of extract ethanol Bidara leaves (Ziziphus spina-Christi L) on enteropathogenic coli. Indian Journal of Forensic Medicine & Toxicology . 2021;15(1):1589–1595. [Google Scholar]
- 92.Motamedi H., Seyyednejad S. M., Hasannejad Z., Dehghani F. Comparative study on the effects of Ziziphus spina-christi alcoholic extracts on growth and structural integrity of bacterial pathogens. Iranian Journal of Pharmaceutical Sciences . 2014;10(2):1–10. [Google Scholar]
- 93.Abalaka M., Daniyan S., Mann A. Evaluation of the antimicrobial activities of two Ziziphus species (Ziziphus mauritiana L. and Ziziphus spina-christi L.) on some microbial pathogens. African Journal of Pharmacy and Pharmacology . 2010;4(4):135–139. doi: 10.5897/AJPP.9000150. [DOI] [Google Scholar]
- 94.Neama J., Abu-Mejdad N., Jaber A. Evaluation the antimicrobial activity of aqueous and alcoholic extracts of leaves Ziziphus spina-christi (L) Desf. Basrah Journal of Science . 2007;25(1):1–15. [Google Scholar]
- 95.Mohammed G., Yesufu H., Abdulrahman F., et al. Antimicrobial and toxicological screening of the aqueous stem bark extract of Zizyphus spina-christi (Linnaeus Desf) Journal of Microbiology and Biotechnology . 2012;2(2):337–342. [Google Scholar]
- 96.Saeidi A., Hamidi M. R., Davoodabadi A., Mahmoudi E., Khafri S., Nikouee Rad Z. Comparison of antibacterial effect of methanolic and hydro-alcoholic Ziziphus spina-christi extract with 2.5% sodium hypochlorite on enterococcus faecalis: an in vitro study. Caspian Journal of Dental Research . 2018;7(1):37–42. [Google Scholar]
- 97.Journal B. S. Study the effect of aqueous cold water and alcoholic extracts of Ziziphus spina christi against bacteria isolated from conjunctivitis in vitro and in vivo. Baghdad Science Journal . 2015;12(1):14–19. doi: 10.21123/bsj.12.1.14-19. [DOI] [Google Scholar]
- 98.Alomari A. A., Fadlelmula A. A., Abdalla M. O. M. Evaluation of the antibacterial and antifungal activities and phytochemical screening of bark extract of Ziziphus Spina Christi L (ZSC) in Albaha area. Journal of Biological Physics . 2016;7(1):77–86. [Google Scholar]
- 99.Al-Mousawi A. H., Al-Kaabi S. J. Effect of Ziziphus spina Christy extract in biofilm formation of methicillin resistance Staphylococcus aureus and Staphylococcus haemolyticus. Pakistan Journal of Biotechnology . 2017;14(4):791–795. [Google Scholar]
- 100.Alzoreky N., Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. International Journal of Food Microbiology . 2003;80(3):223–230. doi: 10.1016/S0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 101.Mathkoor M. M., Abd- ulamer Oda N., Omran Z. S. Comparative study antibacterial activity of some medicinal plants extracts (leaves and peel) against some multi-drug resistant bacteria from clinical isolates. International Journal of Drug Delivery Technology . 2019;9(3):41–46. doi: 10.25258/ijddt.v9i3.25. [DOI] [Google Scholar]
- 102.Makhawi A. M., Mustafa M. I., Uagoub H. A. Phytochemical screening and antimicrobial activity of Ziziphus spina-christi stem barks. bioRxiv . 2020;2020 doi: 10.1101/2020.02.24.963157. [DOI] [Google Scholar]
- 103.Abdallah E. M., Hamed A. I. Screening for antibacterial activity of two jujube honey samples collected from Saudi Arabia. Journal of Apitherapy . 2019;5(1):6–9. doi: 10.5455/ja.20190120035814. [DOI] [Google Scholar]
- 104.Al-Mousawi A. H., Al-Kaabi S. J. Effect of Ziziphus spina christy on biofilm formation of methicillin resistance Staphylococcus aureus and Staphylococcus haemolyticus. Plant Archives . 2018;18(1):1033–1038. [Google Scholar]
- 105.Ekhtelat M., Ravaji K., Parvari M. Effect of Iranian Ziziphus honey on growth of some foodborne pathogens. Journal of Natural Science, Biology and Medicine . 2016;7(1):54–57. doi: 10.4103/0976-9668.175069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mostafa M., Al-Emam A., Sayed M., et al. Silver nanoparticles from Ziziphus spina-christi (L.) Desf. fruits extract and their antimicrobial activity. Bangladesh Journal of Botany . 2020;49(4):1127–1134. doi: 10.3329/bjb.v49i4.52629. [DOI] [Google Scholar]
- 107.Tom G. M., Yesufu H. B., Abdulrahman F. I. Antimicrobial screening and effect of the pulp extracts of Zizyphus spina-christi (Linnaeus Desf) on some biochemical parameters in rats. Journal of Pharmacy & Bioresources . 2011;6(2) doi: 10.4314/jpb.v6i2.63329. [DOI] [Google Scholar]
- 108.Ebid A. I. Anti-bacterial activity of folk medicinal plant extracts of Saudi Arabia on isolated bacteria. Journal of Applied Life Sciences International . 2015;3(1):49–54. doi: 10.9734/jalsi/2015/16395. [DOI] [Google Scholar]
- 109.Al-Ali S., Al-Judaibi A. Biochemical and molecular effects of phoenix dactylifera and Ziziphus spina-christi extracts on Candida albicans. Journal of Biosciences and Medicines . 2019;7(3):29–43. doi: 10.4236/jbm.2019.73004. [DOI] [Google Scholar]
- 110.Salimi A., Watt H., Harasymowycz P. Antifungal activity of topical microemulsion containing Ziziphus spina-christi L. for the treatment of fungal vaginitis. Eye and Vision (London, England) . 2021;8(1):43–50. doi: 10.1186/s40662-021-00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alotibi F. O., Ashour E. H., Al-Basher G. Evaluation of the antifungal activity of Rumex vesicarius L. and Ziziphus spina-christi (L) Desf. aqueous extracts and assessment of the morphological changes induced to certain myco-phytopathogens. Saudi Journal of Biological Sciences . 2020;27(10):2818–2828. doi: 10.1016/j.sjbs.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alzomor A. K., Al-Madhagi W. M., Sallam N. M. N., Mojamel H., Alawar M. M., Al-Hetari A. G. Subacute toxicity study and clinical trials for Zizyphus spina-christi leaves extract as an anti-dandruff shampoo. Thai Journal of Pharmaceutical Sciences . 2021;45(2):126–136. [Google Scholar]
- 113.Hadizadeh I., Peivastega B., Kolahi M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pakistan Journal of Biological Sciences . 2008;12(1):58–63. doi: 10.3923/pjbs.2009.58.63. [DOI] [PubMed] [Google Scholar]
- 114.Abu-Taleb A. M., El-Deeb K., Al-Otibi F. O. Assessment of antifungal activity of Rumex vesicarius L. and Ziziphus spina-christi (L.) Willd. extracts against two phytopathogenic fungi. African Journal of Microbiology Research . 2011;5(9):1001–1011. doi: 10.5897/AJMR10.826. [DOI] [Google Scholar]
- 115.Mardani M., Badiee P., Gharibnavaz M., Jassebi A., Jafarian H., Ghassemi F. Comparison of anti-Candida activities of the ancient plants Lawsonia inermis and Ziziphus spina christi with antifungal drugs in Candida species isolated from oral cavity. Journal of Conservative Dentistry . 2018;21(4):359–362. doi: 10.4103/JCD.JCD_291_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adzu B., Amos S., Amizan M., Gamaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Tropica . 2003;87(2):245–250. doi: 10.1016/S0001-706X(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 117.Godini A., Kazem M., Naseri G., Badavi M. The effect of Zizyphus spina-christi leaf extract on the isolated rat aorta. Journal of Pakistan Medical Association . 2009;59(8):537–539. [PubMed] [Google Scholar]
- 118.Abou El-Nour M. F. Evaluation of molluscicidal, miracicidal and cercaricidal activities of crude aqueous extracts of Origanum majorana, Ziziphus spina-christi and Salvia fruticosa on Schistosoma mansoni and Schistosoma haematobium. Egyptian Journal of Aquatic Biology and Fisheries . 2021;25(2):913–933. doi: 10.21608/EJABF.2021.173661. [DOI] [Google Scholar]
- 119.Fadladdin Y. A. J. Antischistosomal activity of Origanum majorana, Ziziphus spina-christi, and Salvia fruticosa plant extracts on Hamster Infected with Schistosoma haematobium. BioMed Research International . 2021;2021:15. doi: 10.1155/2021/5545331.5545331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alzahrani F., Al-Shaebi E. M., Dkhil M. A., Al-Quraishy S. In vivo anti-eimeria and in vitro anthelmintic activity of Ziziphus spina-christi leaf extracts. Pakistan Journal of Zoology . 2016;48(2):409–413. [Google Scholar]
- 121.Intisar A. O., Goreish I., Shaddad S. A., Elamin T. H. Eltayeb I anthelmintic activity of Zizyphus spina-christi leaves. JFPI . 2015;4(3):94–99. [Google Scholar]
- 122.Albalawi A. E. Antileishmanial activity of Ziziphus spina-christi leaves extract and its possible cellular mechanisms. Microorganisms . 2021;9(10):2113–2211. doi: 10.3390/microorganisms9102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Albakhit S., Doudi M. The evaluation of methanolic and aqueous extracts effect of Zizyphus spina-Christi against Leishmania major (MHOM/IR/75/ER) promastigotes using MTT assay. Iranian Journal of Microbiology . 2016;10(3):54–60. [Google Scholar]
- 124.Alzahrani A. M., Alzahrani A. A., Alsharm A. A. The use of Ziziphus spina-christi extract in treating erlotinib (Tarceva®) associated rash: a case report. Case Reports in Oncology . 2019;12(3):909–912. doi: 10.1159/000504696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shakiba R., Nilforoushzadeh M. A., Hashem-Dabaghian F., et al. Effect of cedar (Ziziphus spina-christi) topical solution in mild to moderate acne vulgaris: a randomized clinical study. Journal of Dermatological Treatment . 2021;32(2):197–202. doi: 10.1080/09546634.2019.1692125. [DOI] [PubMed] [Google Scholar]
- 126.Hafiz T. A., Mubaraki M. A. The potential role of Ziziphus spina-christi leaf extracts against Plasmodium berghei-induced liver and spleen injury. Biomedical Research (India) . 2016;27:1027–1032. [Google Scholar]
- 127.Mubaraki M. A., Hafiz T. A., Al-Quraishy S., Dkhil M. A. Oxidative stress and genes regulation of cerebral malaria upon Zizyphus spina-christi treatment in a murine model. Microbial Pathogenesis . 2017;107:69–74. doi: 10.1016/j.micpath.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 128.Hafiz T. A., Mubaraki M. A., Diab M. S., Dkhil M. A., Al-Quraishy S. Ameliorative role of Ziziphus spina-christi leaf extracts against hepatic injury induced by Plasmodium chabaudi infected erythrocytes. Saudi Journal of Biological Sciences . 2019;26(3):490–494. doi: 10.1016/j.sjbs.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Ghamdi A. A. M., El-Zohri M., Shahat A. A. Hepatoprotective, nephroprotective, anti-amylase, and antiglucosidase effects of Ziziphus spina-christi (L.) against carbon tetrachloride-induced toxicity in rats. Tropical Journal of Pharmaceutical Research . 2021;18(4):781–790. doi: 10.4314/tjpr.v18i4.15. [DOI] [Google Scholar]
- 130.Khaleel S. M., Almuhur R. A., Al-Deeb T. M., Jaran A. S., Al-Jamal A. A. Antidiabetic and hypolipidemic effects of ethanolic leaf extract of Ziziphus spina-christi on normal and streptozotocin-induced diabetic rats. EurAsian Journal of BioSciences . 2020;14(2):5865–5870. [Google Scholar]
- 131.El Rabey H. A., Attia E. S., Al-Seeni M. N., et al. The hypolipidemic and antioxidant activity of Christ’s thorn (Ziziphus spina-christi) leaves powder in hypercholesterolemic male rats. Life Science Journal . 2014;11(10):1010–1021. [Google Scholar]
- 132.Al-Sieni A. I., El Rabey H. A., Al-Seeni M. N. The aqueous extract of Christ’s thorn (Ziziphus spina-christi) seed modulates hyperlipidemia in hypercholesterolemic male rat. Biomedical Research . 2020;31(3):71–78. [Google Scholar]
- 133.Almeer R. S., Alkahtani S., Alarifi S., Moneim A. E. A., Abdi S., Albasher G. Ziziphus spina-christi leaf extract mitigates mercuric chloride-induced cortical damage in rats. Combinatorial Chemistry & High Throughput Screening . 2021;25(1):103–113. doi: 10.2174/1386207323666201204124412. [DOI] [PubMed] [Google Scholar]
- 134.Setorki M. Effect of hydro-alcoholic extract of Ziziphus spina-christi against scopolamine-induced anxiety in rats. Bangladesh Journal of Pharmacology . 2016;11(2):421–427. doi: 10.3329/bjp.v11i2.26505. [DOI] [Google Scholar]
- 135.Al-Humaidhi A. M., Abd A. H., Ghazi H. F. Hydro-alcoholic extract of Ziziphus spina christi attenuates pentylenetetrazole-induced kindling in male mice. Systematic Reviews in Pharmacy . 2021;12(2):314–317. [Google Scholar]
- 136.Basyony M., Elsheikh H., Abdel Salam H., Mohamed K., Zedan A. Utilization of Ziziphus spina-christi leaves as a natural growth promoter in rabbit’s rations. Egyptian Journal of Rabbit Science . 2017;27(2):427–446. doi: 10.21608/EJRS.2017.46666. [DOI] [Google Scholar]
- 137.El-Sheikh H., Sayed H. A., Mohamed K. I., Idris A., Zeden A. H. Comparative efficacy of Ziziphus spina-christi leaves or monensin on growing lambs performance. Egyptian Journal of Sheep Goats Sciences . 2018;13(1):1–14. doi: 10.21608/EJSGS.2018.26302. [DOI] [Google Scholar]
- 138.Yusup F. H., Frasiska N., Rahayu N. Addition of bidara leaves (Ziziphus spina–christi L.) in drinking water on production and mortality of broiler chickens. IOP Conference Series: Earth and Environmental Science . 2021;902(1) doi: 10.1088/1755-1315/902/1/012045.012045 [DOI] [Google Scholar]
- 139.Ghaffari K., Ahmadi R., Saberi B., Moulavi P. Anti-proliferative effects of Ziziphus spina-christi and Phlomis russeliana leaf extracts on HEK293 and MCF-7 cell lines and evaluation of Bax and Bcl-2 genes expression level in MCF-7 cells. Asian Pacific Journal of Cancer Prevention . 2021;22(S1):81–87. doi: 10.31557/apjcp.2021.22.s1.81. [DOI] [PubMed] [Google Scholar]
- 140.Thawini H. K., Al-Shawi A. A. AAA polysaccharide of Ziziphus spina-Christi L. and it’s silver nanoparticles induced reactive oxygen species and late apoptosis in liver cancer cells. Nanomedicine Research Journal . 2021;6(3):237–247. doi: 10.22034/NMRJ.2021.03.004. [DOI] [Google Scholar]
- 141.Farmani F., Moein M., Amanzadeh A., Kandelous H. M., Ehsanpour Z., Salimi M. Antiproliferative evaluation and apoptosis induction in MCF-7 cells by Ziziphus spina christi leaf extracts. Asian Pacific Journal of Cancer Prevention . 2016;17(1):315–321. doi: 10.7314/APJCP.2016.17.1.315. [DOI] [PubMed] [Google Scholar]
- 142.El-Din M. S., Taha A. M., Sayed A. A. A., Salem A. M. Ziziphus spina-christi leaves methanolic extract alleviates diethylnitrosamine-induced hepatocellular carcinoma in rats. Biochemistry and Cell Biology . 2019;97(4):437–445. doi: 10.1139/bcb-2018-0318. [DOI] [PubMed] [Google Scholar]
- 143.Jafarian A., Zolfaghari B., Shirani K. Cytotoxicity of different extracts of arial parts of Ziziphus spina-christi on Hela and MDA-MB-468 tumor cells. Advanced Biomedical Research . 2014;3:38–45. doi: 10.4103/2277-9175.125727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Owolarafe T. A., Salau A. K., Salawu K. Phytochemical screening and toxicity study of aqueous-methanol extract of Ziziphus spina-christi seeds in Wistar albino rats. Comparative Clinical Pathology . 2020;29(1):267–274. doi: 10.1007/s00580-019-03043-5. [DOI] [Google Scholar]
- 145.Ibrahim A. M., Sayed S. S. M. Assessment of the molluscicidal activity of the methanolic seed extracts of Ziziphus spina-christi and Carica papaya on immunological and molecular aspects of Biomphalaria alexandrina snails. Aquaculture Research . 2021;52(5):2014–2024. doi: 10.1111/are.15050. [DOI] [Google Scholar]
- 146.Abdel-Wahhab M. A., Omara E. A., Abdel-Galil M. M., et al. Zizyphus spina-christi extract protects against aflatoxin B1-intitiated hepatic carcinogenicity. African Journal of Traditional, Complementary and Alternative Medicines . 2007;4(3):248–256. [PMC free article] [PubMed] [Google Scholar]
- 147.Khaleel S. M., Almuhur R. A., Al-Deeb T. M., Jaran A. S. Biochemical changes in the liver, kidney and serum of rats exposed to ethanolic leaf extract of Ziziphus spina-christi. Jordan Journal of Biological Sciences . 2021;14(4):763–767. doi: 10.54319/jjbs/140417. [DOI] [Google Scholar]
- 148.Almeer R. S., Albasher G., Kassab R. B., et al. Ziziphus spina-christi leaf extract attenuates mercury chloride-induced testicular dysfunction in rats. Environmental Science & Pollution Research . 2020;27(3):3401–3412. doi: 10.1007/s11356-019-07237-w. [DOI] [PubMed] [Google Scholar]
- 149.Dhuha N. S., Putri H. E. Acute toxicity ethanol extract of bidara leaves (Ziziphus spina-christi L.) against liver and kidney function of white rats. Eureka Herba Indonesia . 2020;1(1):24–31. doi: 10.37275/ehi.v1i1.3. [DOI] [Google Scholar]
- 150.Lema A. A., Mahmod N. H., Khandaker M. M., Abdulrahman M. D. Roselle anthocyanin stability profile and its potential role in post-harvest deterioration: a review. Plant Science Today . 2022;9(1):119–131. [Google Scholar]
- 151.Abdulrahman M. D., Hasan N. N., Khandaker M. M., Ali A. M., Mat N. In vitro biological investigations on Syzygium polyanthum cultivars. International Journal of Agriculture and Biology . 2019;22(6):1399–1406. doi: 10.17957/IJAB/15.1214. [DOI] [Google Scholar]
- 152.Pirbalouti A. G., Yousefi M., Nazari H., Karimi I., Koohpayeh A. Evaluation of burn healing properties of Arnebia euchroma and Malva sylvestris. EJBio . 2009;5(3):62–66. [Google Scholar]
- 153.Macedo N. S., dos Santos C. R. B., Pereira R. L. S., et al. Phytochemical prospection, evaluation of antibacterial activity and toxicity of extracts of Libidibia ferrea (Mart. ex Tul.) LP Queiroz. Arabian Journal of Chemistry . 2022;15(2):103632–103639. doi: 10.1016/j.arabjc.2021.103632. [DOI] [Google Scholar]
- 154.Shoba F. G., Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. Journal of Ethnopharmacology . 2001;76(1):73–76. doi: 10.1016/S0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 155.Abdullahi A. L., Agho M. O., Amos S., Gamaniel K. S., Wambebe C. Antidiarrhoeal activity of the aqueous extract of Terminalia avicennoides roots. Phytotherapy Research . 2001;15(5):431–434. doi: 10.1002/ptr.860. [DOI] [PubMed] [Google Scholar]
- 156.Amirmohammadi M., Khajoenia S., Bahmani M., Rafieian-Kopaei M., Eftekhari Z., Qorbani M. In vivo evaluation of antiparasitic effects of Artemisia abrotanum and Salvia officinalis extracts on Syphacia obvelata, Aspiculoris tetrapetra and Hymenolepis nana parasites. Asian Pacific Journal of Tropical Disease . 2014;4:S250–S254. doi: 10.1016/S2222-1808(14)60449-7. [DOI] [Google Scholar]
- 157.Vijayan P., Raghu C., Ashok G., Dhanaraj S. A., Suresh B. Antiviral activity of medicinal plants of Nilgiris. Indian Journal of Medical Research . 2004;120:24–29. [PubMed] [Google Scholar]
- 158.Abubakar U. S., Abdullahi S., Ayuba V., Kaigama S., Halidu U. S., Ayuba M. K. Medicinal plants used for the management of diabetes mellitus in Zaria, Kaduna state, Nigeria. Journal of Pharmacy & Pharmacognosy Research . 2017;5(3):156–164. [Google Scholar]
- 159.Yun J. W. Possible anti-obesity therapeutics from nature–a review. Phytochemistry . 2010;71(14-15):1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 160.Grundmann O., Nakajima J. I., Seo S., Butterweck V. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. Journal of Ethnopharmacology . 2007;110(3):406–411. doi: 10.1016/j.jep.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 161.Dogara A., Labaran I., Hamad S. W., Lema A. A., Jakada B. H. Traditional medicinal plants used for the treatment of cancer in Mubi, Adamawa State, Nigeria. Al-Qadisiyah Journal Of Pure Science . 2021;26(4):258–268. doi: 10.29350/qjps.2021.26.4.1423. [DOI] [Google Scholar]
- 162.Balunas M. J., Kinghorn A. D. Drug discovery from medicinal plants. Life Sciences . 2005;78(5):431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 163.El Maaiden E., El Kharrassi Y., Qarah N. A. S., Essamadi A. K., Moustaid K., Nasser B. Genus Ziziphus: a comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. Journal of Ethnopharmacology . 2020;259 doi: 10.1016/j.jep.2020.112950.112950 [DOI] [PubMed] [Google Scholar]
- 164.Ibrahim J. A., Egharevba H. O., Nnamdi R. A., Kunle O. F. Comparative pharmacognostic and phytochemical analysis of Ziziphus spina-christi (L.) Desf. and Ziziphus abyssinica hochst. Ex A. Rich. Int Journal of Pharmacognosy and Phytochemistry . 2015;7(6):1160–1166. [Google Scholar]
- 165.El Maaiden E., El Kharrassi Y., Moustaid K., Essamadi A. K., Nasser B. Comparative study of phytochemical profile between Ziziphus spina christi and Ziziphus lotus from Morocco. Journal of Food Measurement and Characterization . 2018;13(1):121–130. doi: 10.1007/s11694-018-9925-y. [DOI] [Google Scholar]
- 166.El-Seedi H. R., Zahra M. H., Goransson U., Verpoorte R. Cyclopeptide alkaloids. Phytochemistry Reviews . 2006;6(1):143–165. doi: 10.1007/s11101-006-9029-x. [DOI] [Google Scholar]
- 167.Tuenter E., Foubert K., Staerk D., Apers S., Pieters L. Isolation and structure elucidation of cyclopeptide alkaloids from Ziziphus nummularia and Ziziphus spina-christi by HPLC-DAD-MS and HPLC-PDA-(HRMS)-SPE-NMR. Phytochemistry . 2017;138:163–169. doi: 10.1016/j.phytochem.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 168.Abdel-galil F. M., El-jissry M. A. Cyclopeptide alkaloids from Zizyphus spina-christi. Phytochemistry . 1991;30(4):1348–1349. doi: 10.1016/S0031-9422(00)95238-5. [DOI] [Google Scholar]
- 169.Gournelis D. C., Laskaris G. G., Verpoorte R. Cyclopeptide alkaloids. Natural Product Reports . 1997;14(1):75–82. doi: 10.1039/np9971400075. [DOI] [PubMed] [Google Scholar]
- 170.Tschesche R., Khokhar I., Spilles C., Von Radloff M. Peptide alkaloids from Ziziphus spina christi. Phytochemistry . 1974;13(8) doi: 10.1016/0031-9422(74)80352-3.1633 [DOI] [Google Scholar]
- 171.Mahran G. E. D. H., Glombitza K. W., Mirhom Y., Hartmann R., Michel C. Novel saponins from Zizyphus spina-christi growing in Egypt. Planta Medica . 1996;62(2):163–165. doi: 10.1055/s-2006-957842. [DOI] [PubMed] [Google Scholar]
- 172.Bozicevic A., De Mieri M., Di Benedetto A., Gafner F., Hamburger M. Dammarane-type saponins from leaves of Ziziphus spina-christi. Phytochemistry . 2017;138:134–144. doi: 10.1016/j.phytochem.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 173.Pawlowska A. M., Camangi F., Bader A., Braca A. Flavonoids of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (rhamnaceae) fruits. Food Chemistry . 2009;112(4):858–862. doi: 10.1016/j.foodchem.2008.06.053. [DOI] [Google Scholar]
- 174.Weinges K., Schick H. Dodecaacetylprodelphinidin b3 from the dried leaves of Ziziphus spina-christi. Phytochemistry . 1995;38(2):505–507. doi: 10.1016/0031-9422(94)00574-d. [DOI] [Google Scholar]
- 175.Nawwar M. A. M., Ishak M. S., Michael H. N., Buddrust J. Leaf flavonoids of Ziziphus spina-christi. Phytochemistry . 1984;23(9):2110–2111. doi: 10.1016/S0031-9422(00)84999-7. [DOI] [Google Scholar]
- 176.El Maaiden E., El Kharrassi Y., Qarah N. A. S., Essamadi A. K., Moustaid K., Nasser B. Chemical composition and evaluation of protective effect of Ziziphus spina-christi L. against iron-induced oxidative DNA damage in Tetrahymena pyriformis. Journal of Food Measurement and Characterization . 2021;15(5):3884–3892. doi: 10.1007/s11694-021-00975-x. [DOI] [Google Scholar]
- 177.Abubaker M. A., Mohammed A. A. A., Farahb A. A. M., Zhang J. Phytochemical screening by using GC-MS and FTIR spectrum analysis of fixed oil from Sudanese Ziziphus spina Christi seeds. Eurasian Chem. Commun . 2021;3(4):244–256. doi: 10.22034/ecc.2021.273055.1137. [DOI] [Google Scholar]
- 178.Ghannadi A., Tavakoli N., Mehri-Ardestani M. Volatile constituents of the leaves of Ziziphus spina-christi(L.) willd. From Bushehr, Iran. Journal of Essential Oil Research . 2003;15(3):191–192. doi: 10.1080/10412905.2003.9712109. [DOI] [Google Scholar]
- 179.Elsadig Karar M. G. Phenolic profile and in vitro assessment of cytotoxicity and antibacterial activity of Ziziphus spina-christi leaf extracts. Medicinal Chemistry . 2016;6(3):143–156. doi: 10.4172/2161-0444.1000339. [DOI] [Google Scholar]
- 180.Bahmani M., Jalilian A., Salimikia I., Shahsavari S., Abbasi N. Phytochemical screening of two Ilam native plants Ziziphus nummularia (Burm.f.) Wight & Arn. and Ziziphus spina-christi (Mill.) Georgi using HS-SPME and GC-MS spectroscopy. Plant Science Today . 2020;7(2):275–280. doi: 10.14719/pst.2020.7.2.714. [DOI] [Google Scholar]
- 181.Odeh I., Abu-Lafi S., Al-Najjar I. Determination of unifloral honey volatiles from Centaurea iberica and Zizyphus spinachristi by solid-phase microextraction and gas chromatography-mass spectrometry. Acta Chromatographica . 2014;26(3):485–493. doi: 10.1556/AChrom.26.2014.3.7. [DOI] [Google Scholar]
- 182.Said A., Fawzy G., tabl E. S. A. A., Tzakou O. Volatile constituents ofZizyphus jujubaAerial parts andZizyphus spina-christiiFruits from Egypt. Journal of Essential Oil Bearing Plants . 2010;13(2):170–174. doi: 10.1080/0972060x.2010.10643807. [DOI] [Google Scholar]
- 183.Baghazadeh-Daryaii L., Sharifi-Sirchi G. R., Samsampoor D. Morphological, phytochemical and genetic diversity of Ziziphus spina–christi (L) des. In south and southeastern of Iran. Journal of Applied Research on Medicinal and Aromatic Plants . 2017;7:99–107. doi: 10.1016/j.jarmap.2017.06.006. [DOI] [Google Scholar]
- 184.Al-Mamary M., Al-Meeri A., Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutrition Research . 2002;22(9):1041–1047. doi: 10.1016/S0271-5317(02)00406-2. [DOI] [Google Scholar]
- 185.Idris Y. M. A., Mariod A. A., Hamad S. I. Physicochemical properties, phenolic contents and antioxidant activity of Sudanese honey. International Journal of Food Properties . 2011;14(2):450–458. doi: 10.1080/10942910903243673. [DOI] [Google Scholar]
- 186.Ramadan S. S., Almeer R. S., Alkahtani S., Alarifi S., Albasher G., Abdel Moneim A. E. Ziziphus spina-christi leaf extract attenuates mercuric chloride-induced liver injury in male rats via inhibition of oxidative damage. Environmental Science and Pollution Research . 2021;28(14):17482–17494. doi: 10.1007/s11356-020-12160-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available within the manuscript.


