Abstract
Background and Objectives:
Candida species are antifungal-resistant opportunistic infections that spread through contaminated medical staff hands and hospital surfaces creating a nosocomial infection risk. Iodine′s antibacterial properties are well established; however, its antifungal properties remain unknown. The objective of this study was to investigate the antifungal effects of lugol on cell viability and oxidative stress on Candida albicans and Candida glabrata strains.
Materials and Methods:
MTT reduction test and sensitivity growth assay were used to determine viability and minimal inhibitory concentration, colorimetric tests were used to analyzing lipoperoxidation and antioxidant status in C. albicans, parental C. glabrata, C. glabrata lacking catalase gene (cta1) and superoxide dismutase 1 and 2 double mutants (sod1Δ sod2Δ) strains exposure to lugol were used.
Results:
In both C. albicans and C. glabrata wild types lugol treatment decreased cellular viability in a dose-dependent manner at 30 mm. The cytotoxic lugol effect was characterized by the increase of oxidative stress and the reduction of superoxide dismutase and catalase enzyme activities. C. glabrata strains lacking catalase (cta1) and superoxide dismutase 1 and 2 double mutants (sod1Δ sod2Δ) were less resistant to lugol than parental C. glabrata strains.
Conclusion:
In Candida strains iodine lugol solution has antifungal properties, producing cytotoxicity and oxidative stress. Superoxide dismutase 1 and 2 activities are involved in resistance of Candida to iodine.
Keywords: Iodine, Oxidative stress, Antioxidants, Candida, Antifungal agents
INTRODUCTION
In hospitalized patients who are receiving invasive operations, antibiotic therapy, or another medicines that impair the immune system, nosocomial infections are a risk factor for morbidity and death (1–3). In immunocompromised patients, the most common sources of infection include contact with healthcare staff, as well as exposure to bacteria and fungi-contaminated surfaces and medical equipment (4). According to the World Health Organization (WHO), nosocomial infections are from 5–10% of all hospitalizations in North America and Europe, whereas in Latin America, Asia, and Africa are more than 40% (5). C. albicans is the most common pathogenic fungus in humans, accounting for half of all cases and is followed by C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, C. lusitaniae, C. guilliermondii and most recently C. auris (6–9).
Azoles are the most commonly prescribed treatments for fungal infections, but their widespread use has resulted in resistance, particularly in Candida species such as C. albicans and C. glabrata (10). Candida species have antioxidant mechanisms to avoid phagocytes (11). For example, C. glabrata has a high tolerance to H2O2 and only requires one catalase Cta1 gene, which is regulated by the transcriptional factors Yap1 and Skn7 (12, 13). Furthermore, superoxide dismutases 1 and 2 (SOD1 and SOD2, respectively) are the first line of defense against oxidative stress caused by O2 (14). The role of SODs in C. glabrata has been previously reported, and they contributes to the C. glabrata resistance mechanism (12, 13, 15, 16).
On the other hand, proper surface disinfection in hospitals is crucial for reducing pathogen contamination and the frequency of nosocomial infections in various areas of the hospital (17). Because prolonged use of disinfectants leads to resistance, multiple disinfectants should be used depending on the pathogen, the product developed, and the material in contact with the disinfectant on the surface (18). In humans, iodine is an important element with antioxidant and antiproliferative characteristics, which helps to maintain normal tissue physiology (19). On the other hand, iodine has broad-spectrum bactericidal activity, and causes DNA denaturation and oxidative stress (20). In hypothyroidism rats, iodine supplementation causes oxidative stress (21). The purpose of this study was to analyze how iodine as lugol (a mix of elemental iodine and potassium iodine) affected yeast viability, oxidative stress and antioxidant activity in C. albicans and C. glabrata yeast strains.
MATERIALS AND METHODS
Strains and media.
The following strains were used: C. albicans SC 5314 (ATCC MYA-2876), C. glabrata CBS 138 (ATCC-2001) and two mutant C. glabrata strains; Cta1Δ lacking the catalase enzyme(CTA1) and Sod1Δ sod2Δ double mutant lacking superoxide dismutase enzyme CuZnSOD (SOD1) and the MnSOD (SOD2) were previously reported (11, 12, 16). All strains were generously donated by Alejandro de las Peñas and Irene Castaño from IPICYT, México. The yeast strains were grown in standard yeast media YPD broth containing 10 g/L yeast extract, 20 g/L peptone, 2% glucose were supplied by Fisher. YPD-MB agar (YPD containing 0.01% methylene blue and 2.0% agar) was used for growth sensitivity assays and YPD without 2.0% agar for viability MTT assay. All plates and liquid cultures were incubated at 30°C for 48 hours.
Viability MTT assay.
Lugol's cytotoxic effect was determinate by MTT assays, aliquots of 1.0 mL with 1×106 CFUs/ml of each strain were pipetted into eppendorf tubes with 1–100 mM of lugol in YPD medium or vehicle. The suspensions were incubated at 30°C for 6–48 h. After incubation, 50 μL of 5 mg mL−1 MTT was added and incubated at 30°C for 2 h. The reaction was stopped with 100 μL of DMSO and then, the absorbance was measured at 570 nm against yeast-free control.
Methylene blue disk diffusion and growth assays.
Modified Kirby-Bauer disc diffusion test (22) was used to evaluate chronic effect. In brief, 1 × 106 CFUs/ml of each yeast suspension was prepared in phosphate-buffered saline (PBS on YPD supplemented with 0.5 μg/ml methylene blue (MB). Disc filters of 8mm of diameter were impregnated with 0.1, 1, 5, 10, 30 and 50 mM of lugol and then placed on YPD-MB agar plates previously inoculated with 1 × 106 CFU/ml of each strain of Candida evaluated. The plates were incubated at 30°C for 24 h. The presence of inhibition zones or halo (precipitate of methylene blue) was measurement by the diameter of the inhibition zone around each filter using a Motic AE31 inverted microscope. Gap distance of the inhibition zone was measured using Motic Imagine Plus software 2013. To assess the acute effect of Lugol, growth assay was carried out as described previously (23, 24). All strains were diluted with constant shaking in fresh YPD broth. Each strain was exposed to different concentrations of lugol (0, 0.1, 1, 5, 10, 30, 50 and 100 mM) and after treatment, YPD broth with lugol was removed by centrifugation. The cultures were suspended in distilled water and their OD600 were adjusted to 0.5 and 10-fold. Serial dilutions were made in 96-well plates and 5 μl of each dilution was spotted onto YPD agar plates and photographed in a GelDoc documentation system (BioRad).
Determination of the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC).
The YDP fungal suspension was used to inoculate the tubes in the test group. The test groups were prepared with 1 ml of YPD medium containing 10 μl of dimethyl sulfoxide (DMSO) 10%, the final concentration never exceeded 2%, and 200 μl of each strain of yeast suspension previously adjusted. Lugol, fluconazole, and amphotericin were respectively added to tubes containing culture medium in the test groups, then adding a sufficient amount of glucose 2% to a final volume of 1 ml and serial dilutions and concentration gradient is established as follows: Lugol 50 to 0.1 mmol, fluconazole 7.5 × 10−6 to 1 × 10−5 mmol and amphotericin B of 3.3 10−6 to 5 × 10− 7. The test tubes were incubated at 30°C on an orbital shaker for 24 hours, and the MICs were determined. The MIC was defined as the lowest concentration of the lugol at which the microorganism did not demonstrate visible growth. The growth of microorganisms is evaluated counting with hemocytometer for each repetition. To determine the minimum fungicidal concentration (MFC), aliquots (20 μl) of broth were taken from each negative after reading MIC tube, and cultured in the YPD plates and incubated at 30°C for 24 h. The MFC was defined as the lowest concentration of the lugol at which the microorganism was completely killed. Each test was performed in triplicate. Fluconazole and amphotericin B were used as positive controls antifungal. For each strain tested, the growth conditions and medium sterility were checked in two control tubes. The safety of DMSO was checked with the highest tested concentration. All experiments were performed in triplicate.
Oxidative stress determination.
Oxidative stress was measured by conjugated dienes in 0.5 mL of sample, which was mixed with 7 mL of chloroform/methanol (2:1 v/v) and shaken for 1 min, then, the samples were centrifuged at 3,500 rpm for 10 min. The chloroform phase obtained was warmed, and the residue was reconstituted with 1 mL of hexane and its absorbance was read and measured against a hexane blank at 233 nm in a microplate reader (Spectramax Plus; Molecular Devices, Sunnyvale, CA). Results were expressed in absorbance units. TBARS was measured after treatment with lugol. Yeast strain cell suspension was centrifuged (12,000 g, 5 min), pellet was re-suspended in lysis buffer (2% Triton-x100, 1% SDS, 100 mM NaCl, 10 mM TRIS-HCl, 1 mM EDTA pH 8.0). Following centrifugation (12,000×g, 5 min), mix with 0.4 % of tiobarbituric acid, 20% acetic acid pH 3.0 were added to each sample, all samples were warmed in a thermoblot to at 95°C during 45 minutes and rapidly were cooled in ice and 1.2% of KCl was added. The samples were centrifuged and the supernatant was read and measured at 532–600 nm in a microplate reader (Spectramax Plus; Molecular Devices, Sunnyvale, CA). The results were expressed in absorbance units per 0.1 ml of sample nmoles/mg protein.
Superoxide dismutase and catalase activities.
Proteins extracts from Candida strains treated with lugol were isolated by homogenizing the cells with zirconia beads in PBS supplemented with protease inhibitors (Sigma FAST). Protein content was determined previously by the Bradford assay (25), followed manufacturer instructions (BIO-RAD). Total superoxide dismutase activity was assayed according to McCord (26). The assay was performed in a total volume of 1ml containing 50mmol l−1 glycine buffer (pH 10), 60mmol l−1 epinephrine, and the enzyme. Epinephrine was added, and adrenochrome formation was recorded at 480nm in an ultraviolet-visible (UV-Vis) spectrophotometer during 4 min. One unit of SOD activity was equivalent to the amount of enzyme required to inhibit epinephrine oxidation by 50% under the experimental conditions. The assays were performed in triplicate. For catalase activity, we used the method of Aebi (27), H2O2 solution (10mM), enzyme extract, and 50mM phosphate buffer (pH 7) were pipetted into a cuvette. The reduction of H2O2 was followed at a wavelength of 240nm for 4 min against a blank containing 50mM phosphate buffer and enzyme extract. Catalase activity was expressed in ΔE min−1 (mg protein)−1. The assays were performed in triplicate.
Statistical analysis.
The data presented in all the figures are mean ± standard deviation (SD) of independent experiments (n =5 by triplicate). Data from different experiments were normalized to the control value before being combined for statistical analyses. Differences between several groups were determined using one-way ANOVA followed by the Student Newman Keuls test using GraphPad software (GraphPad Software Inc., San Diego, CA). T test was used for comparison between two groups: p < 0.05 was considered significant.
RESULTS
Chronic and acute effect of lugol on growth of C. albicans and C. glabrata.
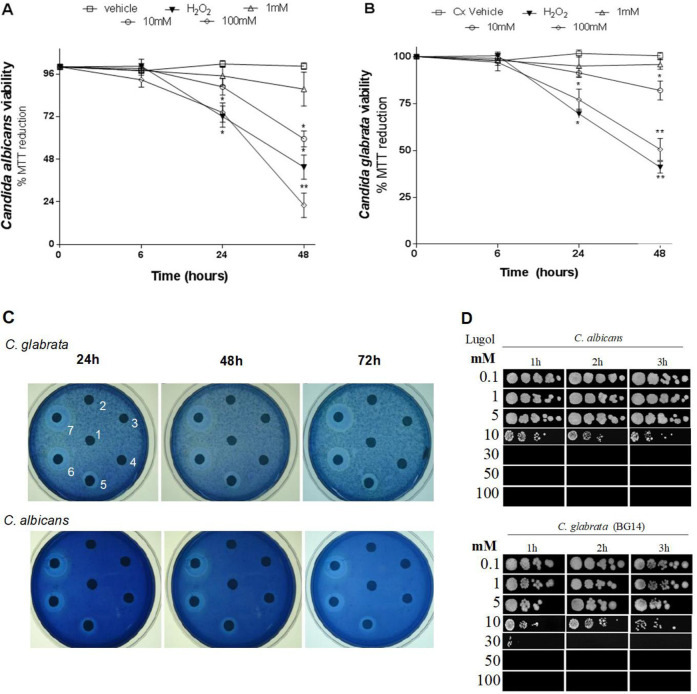
MTT and MB tests were used to determine the chronic effect of lugol exposure (long-term) on C. albicans and C. glabrata growth. Candida strains were subjected for 6 to 48 h to lugol concentrations ranging from 0.1 to 100 mm. After 48 h of chronic lugol exposure, MTT test revealed that C. albicans and C. glabrata strains were substantially suppressed in a dosage and time dependent manner, with 70 and 50% yeast viability in both C. albicans and C. glabrata (Fig. 1A and B). Furthermore, since 30 mm of lugol at 24 h of treatment, the MB disk diffusion experiment reveals a dose-dependent growth inhibition against C. albicans and C. glabrata (Fig. 1C). The acute lugol effect has similar results than chronic lugol effect stated above, therefore C. albicans and C. glabrata strains both demonstrated a reduction in viability to lugol after 1 hour of treatment (Fig. 1D).
Fig. 1.
C. albicans and C. glabrata yeast viability test after exposure to lugol. Saturated C. albicans and C. glabrata parental strain (BG14) yeasts cultures were diluted into fresh YPD broth and grown to reach a OD600 = 0.5 after seven doublings at 30°C, each culture was then divided and exposed to different concentrations of lugol solution at 0.1, 1, 5, 10, 30, 50 and 100 mm for 6 to 48 hours to analyze the chronic effect. A), and B), MTT viability tests; C), MB disk diffusion assay, in which lugol concentration were 0.1mM in the center disk of petri dishes and then clockwise at 12 o’clock; D), acute lugol effect, after of 1 to 3 hours of lugol treatments, the cultures were suspended in distilled water and their OD600 was adjusted to 0.5, following the cultures were logarithmically diluted and each dilution was spotted onto YPD agar plates.
Minimal inhibitory and fungicidal lugol concentration.
The data in the Table 1 demonstrate that lugol has a potent antifungal property. After of 24 h of treatment, the MIC50 and MIC90 doses against C. albicans were from 3 to 23 mmol and 21 to 39 mmol against C. glabrata, respectively.
Table 1.
Lugol's minimum inhibitory concentrations (MIC50 and MIC90 ) for Candida strains.
| Lugol & antifungal drugs | Range (mmol) | MIC50 24 h (mmol) | MIC90 24 h (mmol) | |
|---|---|---|---|---|
| C. albicans (n=6) | Lugol | 0.1–50 | 13 | 22.3 |
| Fluconazole amphotericin | 7.5 × 10−6 to 1 × 10−5 | 2.38 × 10−7 | 4.56 × 10−7 | |
| 3.3 × 10−6 to 5 × 10−7 | 1.83 × 10−6 | 3.49 × 10−6 | ||
| C. glabrata (n=7) | Lugol | 0–50 | 21 | 39.17 |
| Fluconazole amphotericin | 7.5 × 10−6 to 1 × 10−5 | 8.3 × 10−6 | 1.51 × 10−6 | |
| 3.3 × 10−6 to 5 × 10−7 | 2.3 × 10−3 | 4.1 × 10−3 |
All data were calculated from five independent experiments.
Lugol susceptibility of C. glabrata in the absence of catalase and superoxide dismutase 1 and 2 genes.
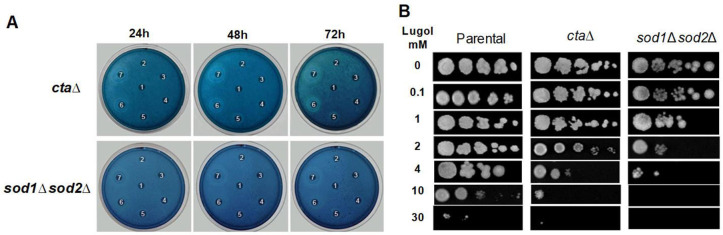
C. glabrata possesses one catalase cta and two superoxide dismutase Sod 1 and sod2 genes to defend against oxidative stress, according to previous research (11, 12). The sensitivity of C. glabrata sod1Δ sod2Δ double mutant and ctaΔ strains to lugol concentrations were investigated and the sod1Δ sod2Δ mutant and ctaΔ were more susceptible to lugol treatment in chronic effect as the parental C. glabrata strain (Fig. 1C and 2A). The acute effect, on the other hand, reveals that only the sod1Δ sod2Δ double mutant was more susceptible to lugol exposure since 1 mm compared to parental and ctaΔ C. glabrata strains (Fig. 2A and 2C).
Fig. 2.
Candida glabrata ctaΔ and sod1Δ sod2Δ mutant strains yeast viability after exposure to lugol. After seven doublings at 30°C, a saturated culture of C. glabrata parental strains was diluted into fresh YPD broth and grown to an OD600 = 0.5. Each culture was divided and exposed to different concentrations of lugol solution at 0.1, 1, 5, 10, 30, 50 and 100 mm for 24 to 72 hours to examine the chronic effect. A), MB disk diffusion test, where the lugol solution in the middle disk of petri dishes and then clockwise at 12 o’clock; B), After of 1 to 3 hours of 0 to 30mM lugol treatments, the cultures were withdrawn, suspender in distilled water, and their OD600 were adjusted to 0.5. The cultures were logarithmically diluted and each dilution was spotted onto YPD agar plates.
Lugol induce oxidative stress and increase of catalase and superoxide dismutase’s activities in C. albicans and C. glabrata strains.
Our findings show that ctaΔ and Sod1Δ Sod2Δ C. glabrata mutants are more susceptible to acute lugol treatment administration than parental C. glabrata, implying that iodine causes oxidative stress. Conjugated dienes and TBARS were measured to confirm these findings. Table 2 shows that 10 mm of lugol exposure significantly increased conjugated dienes and TBARS levels in both C. albicans and C. glabrata strains compared to control. Similarly, treated C. glabrata and C. albicans strains exposed to 10 mM of lugol showed significant increases in catalase and superoxide enzymatic activities (Table 2).
Table 2.
C. albicans, C. glabrata, C. glabrata ctaΔ and C. glabrata sod1Δ sod2Δ levels o of antioxidant and oxidant after exposure to lugol.
| Biochemical parameters | C. albicans | C. albicans/ Lugol 10 mM | C. glabrata | C. glabrata/lugol mM |
|---|---|---|---|---|
| Conjugated dienes (A/3353 nmmg protein)−1 | 0.0391 ± 0.01 | 0.0917 ± 0.022* | 0.139 ± 0.028 | 0.193 ± 0.035* |
| 353 nm | ||||
| TBARS (nmol MDA/mg protein)−1 | 0.0089 ± 0.0012 | 0.0183 ± 0.003* | 0.011 ± 0.003 | 0.022 ± 0.005* |
| Superoxide dismutase (U/mg protein−1) | 139.15 ± 6.13 | 238.91 ± 11.148** | 165.32 ±8.72 | 221.11 ± 9.01** |
| Catalase (ΔE min−1/mg protein−1) | 1.22 ± 0.6 | 1.41 ± 0.8* | 1.62 ± 0.99 | 2.15 ± 0.4** |
All data represent means ± SD calculated from five independent experiments.
p<0.05,
p<0.01
DISCUSSION
The antimicotic action of lugol against Candida species such as C. albicans and C. glabrata was demonstrated in this study. Our findings are consistent with prior findings using iodopovidones to prevent C. albicans biofilm development (28).
Our initial findings demonstrated that iodine solution in the form of lugol inhibits Candida strain development in a dose-dependent manner in both the acute (from 1 mM) and chronic periods (from 100 mM). These findings are consistent with earlier studies of antimicrobial and disinfectant effects of iodine against Gram-positive bacteria, Gram-negative bacteria, spores and protozoa (29, 30). The antibacterial activity of iodopovidones appears to be mediated by an oxidative influence on fatty acids and amino acids in the bacterial cell membrane, as well as cytosolic enzymes involved in the respiratory chain, causing denaturalized biomolecules and loss of cell function (28).
In this study we use lugol, because two polymers, polyvinylpyrrolidone or N-Vinyl-2-pyrrolidone are used in the production of iodopovidone complex, these are excreted in urine after exposure in both humans and experimental animals, and it has been reported that inhalation of low concentrations of N-vinyl-2-pyrrolidone causes nasal cavity inflammation, atrophy of olfactory epithelium and hyperplasia of respiratory basal cells, also N-Vinyl-2-pyrrolidone is an irritant to skin and mucous membranes, causes hepatotoxicity in rats and mice. In humans and experimental animals, polyvinylpyrrolidone accumulates in the vacuoles of cells in various organs, and is associated with pulmonary fibrosis and pneumonia (31–33).
In algae and cancerous breast cells, molecular iodine required a facilitated diffusion pathway in order to pass the cell membrane (34, 35). Once inside, our finding demostrated that lugol induce oxidative stress, increasing conjugated dienes at levels of 30–50 mm and apoptosis (36). Due to the importance of conjugated dienes in the lipoperoxidation process, lugol is most likely acting as a strong free radical, causing cell membrane damage and tiol group reduction (37, 38). For example, elevated levels of lipoperoxides have been described in animal models fed with high-iodine diet, suggesting that these latter may be acting as a mechanism to counteract the damage caused by oxidtive stress (38). Iodine can also interact with the carbon double bonds in celular membranes of certain polyunsaturated fatty acids, causing physical changes such as immobilization and damage owing to intracellular material loss (38).
However, the enzymatic activity of SOD and CAT reduced in all strains after lugol exposure at 1.0 mm, whereas dosages of 30 to 50 mm resulted in a decrease in both enzymes, which could be attributed to a decrease in Candida strain proliferation. According to our findings, lugol has an oxidative effect on SOD and CAT, especially on the S-H group of cysteine, causing disulfide alterations and, as a result, a loss in protein integrity (38, 39). Some amonio acids hydrogen chains can be blocked by iodine derivatives, resulting in structutal alterations (38). Our findings also revealed that the mutant strain Sod1Δ Sod2Δ is more sensitive to lugol treatments than the other strains, implying that lugol-induced oxidative stress is most likely mediated by superoxide anions (O), which have been found in mitochondria from FTRL thyroid cells treated with iodine excess (40).
CONCLUSION
For first time, we demonstrated that lugol exhibits antifungal effects against Candida yeasts, particularly C. albicans and C. glabrata strains, with the mutant strains Sod1Δ and Sod2Δ being the most susceptible to lugol. The antifungal lugol mechanism involves an increase in oxidative stress and a decrease in antioxidant activity of both SOD and CAT enzymes. To fully comprehend the chemical activity of iodine as lugol solution against yeast, more research is required.
ACKNOWLEDGEMENTS
Sergio Cuellar-Rufino, a Biomedical Science PhD was supported by graduate fellowships from CONA-CyT 297563. Aranthxa Salazar-Luna was supported by 46925 fellowships from Universidad Veracruzana and Omar Arroyo-Xochihua by Public Health Institute fellowships. The authors also wish to thank Irene Xochihua Rosas for her contribution in proofreading.
REFERENCES
- 1.Mehta Y, Gupta A, Todi S, Myatra S, Samaddar DP, Patil V, et al. Guidelines for prevention of hospital acquired infections. Indian J Crit Care Med 2014; 18:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rencber S, Karavana SY, Yilmaz FF, Erac B, Nenni M, Ozbal S, et al. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. Int J Nanomedicine 2016; 11:2641–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roques C, Al Mousa H, Duse A, Gallagher R, Koburger T, Lingaas E, et al. Consensus statement: patient safety, healthcare-associated infections and hospital environmental surfaces. Future Microbiol 2015; 10:1629–1634. [DOI] [PubMed] [Google Scholar]
- 4.García-Cruz CP, Najera AMJ, Arroyo-Helguera OE. Fungal and bacterial contamination on indoor surfaces of a hospital in Mexico. Jundishapur J Microbiol 2012; 5:460–464. [Google Scholar]
- 5.World Health Organization Prevention of hospital-acquired infections: A practical guide. (2002) https://apps.who.int/iris/bitstream/handle/10665/67350/WHO_CDS_CSR_EPH_2002.12.pdf
- 6.Durnas B, Wnorowska U, Pogoda K, Deptula P, Watek M, Piktel E, et al. Candidacidal activity of selected ceragenins and human cathelicidin ll-37 in experimental settings mimicking infection sites. PLoS One 2016; 11(6):e0157242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arendrup MC. Candida and candidaemia. susceptibility and epidemiology. Dan Med J 2013; 60:B4698. [PubMed] [Google Scholar]
- 8.Stojanovic P, Stojanovic N, Stojanovic-Radic Z, Arsic Arsenijevic V, Otasevic S, Randjelovic P, et al. Surveillance and characterization of Candida bloodstream infections in a Serbian tertiary care hospital. J Infect Dev Ctries 2016; 10:643–656. [DOI] [PubMed] [Google Scholar]
- 9.Pathirana RU, Friedman J, Norris HL, Salvatori O, Mc-Call AD, Kay J, et al. Fluconazole-Resistant Candida auris is susceptible to salivary histatin 5 killing and to intrinsic host defenses. Antimicrob Agents Chemother 2018; 62(2):e01872–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Xiao M, Watts MR, Wang H, Fan X, Kong F, et al. Development of fluconazole resistance in a series of Candida parapsilosis isolates from a persistent candidemia patient with prolonged antifungal therapy. BMC Infect Dis 2015; 15:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briones-Martin-Del-Campo M, Orta-Zavalza E, Canas-Villamar I, Gutierrez-Escobedo G, Juarez-Cepeda J, Robledo-Marquez K, et al. The superoxide dismutases of Candida glabrata protect against oxidative damage and are required for lysine biosynthesis, DNA integrity and chronological life survival. Microbiology (Reading) 2015; 161:300–310. [DOI] [PubMed] [Google Scholar]
- 12.Cuellar-Cruz M, Briones-Martin-del-Campo M, Canas-Villamar I, Montalvo-Arredondo J, Riego-Ruiz L, Castano I, et al. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryot Cell 2008; 7:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roetzer A, Klopf E, Gratz N, Marcet-Houben M, Hiller E, Rupp S, et al. Regulation of Candida glabrata oxidative stress resistance is adapted to host environment. FEBS Lett 2011; 585:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem 2011;11:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briones-Martin-Del-Campo M, Orta-Zavalza E, Juarez-Cepeda J, Gutierrez-Escobedo G, Canas-Villamar I, Castano I, et al. The oxidative stress response of the opportunistic fungal pathogen Candida glabrata. Rev Iberoam Micol 2014; 31:67–71. [DOI] [PubMed] [Google Scholar]
- 16.Cuellar-Cruz M, Castano I, Arroyo-Helguera O, De Las Penas A. Oxidative stress response to menadione and cumene hydroperoxide in the opportunistic fungal pathogen Candida glabrata. Mem Inst Oswaldo Cruz 2009; 104:649–654. [DOI] [PubMed] [Google Scholar]
- 17.Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL, Umscheid CA. Cleaning hospital room surfaces to prevent health care–associated infections: a technical brief. Ann Intern Med 2015; 163:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tortola GJ, Funke BR, Case CL. (2007). Introducción a la microbiología. 9nd ed. Médica Panamericana. Buenos aires, Argentina [Google Scholar]
- 19.Vidal ZE, Rufino SC, Tlaxcalteco EH, Trejo CH, Campos RM, Meza MN, et al. Oxidative stress increased in pregnant women with iodine deficiency. Biol Trace Elem Res 2014; 157:211–217. [DOI] [PubMed] [Google Scholar]
- 20.Tonoyan L, Boyd A, Fleming GTA, Friel R, Gately CM, Mc Cay PH, et al. In vitro comparative cytotoxicity study of a novel biocidal iodo-thiocyanate complex. Toxicol In Vitro 2018;50:264–273. [DOI] [PubMed] [Google Scholar]
- 21.Hussein Ael-A, Abbas AM, El Wakil GA, Elsamanoudy AZ, El Aziz AA. Effect of chronic excess iodine intake on thyroid function and oxidative stress in hypothyroid rats. Can J Physiol Pharmacol 2012; 90:617–625. [DOI] [PubMed] [Google Scholar]
- 22.Hombach M, Maurer FP, Pfiffner T, Böttger EC, Furrer R. Standardization of operator-dependent variables affecting precision and accuracy of the disk diffusion method for antibiotic susceptibility testing. J Clin Microbiol 2015;53:3864–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castano I, Pan SJ, Zupancic M, Hennequin C, Dujon B, Cormack BP. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol Microbiol 2005; 55:1246–1258. [DOI] [PubMed] [Google Scholar]
- 24.De Las Penas A, Pan SJ, Castano I, Alder J, Cregg R, Cormack BP. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev 2003; 17:2245–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campanha NH, Pavarina AC, Brunetti IL, Vergani CE, Machado AL, Spolidorio DM. Candida albicans inactivation and cell membrane integrity damage by microwave irradiation. Mycoses 2007;50:140–147. [DOI] [PubMed] [Google Scholar]
- 26.McCord JM. Analysis of superoxide dismutase activity. Curr Protoc Toxicol 2001; Chapter 7:Unit 7.3. [DOI] [PubMed] [Google Scholar]
- 27.Linares CE, Giacomelli SR, Altenhofen D, Alves SH, Morsch VM, Schetinger MR. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Rev Soc Bras Med Trop 2013;46:752–758. [DOI] [PubMed] [Google Scholar]
- 28.Hoekstra MJ, Westgate SJ, Mueller S. Povidone-iodine ointment demonstrates in vitro efficacy against biofilm formation. Int Wound J 2017; 14:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaggi G, Monstrey S, Van Landuyt K, Hamdi M, Blondeel P. The role of iodine in antisepsis and wound management: a reappraisal. Acta Chir Belg 2003; 103:241–247. [DOI] [PubMed] [Google Scholar]
- 30.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg 2017; 44:260–268. [DOI] [PubMed] [Google Scholar]
- 31.Kaneda Y, Tsutsumi Y, Yoshioka Y, Kamada H, Yamamoto Y, Kodaira H, et al. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004;25:3259–3266. [DOI] [PubMed] [Google Scholar]
- 32.Oesch F, Honarvar N, Fabian E, Berger FI, Landsiedel R. N-vinyl compounds: studies on metabolism, genotoxicity, carcinogenicity. Arch Toxicol 2021;95:3143–3159. [DOI] [PubMed] [Google Scholar]
- 33.Oesch F, Fruth D, Hengstler JG, Fabian E, Berger FI, Landsiedel R. Enigmatic mechanism of the N-vinylpyrrolidone hepatocarcinogenicity in the rat. Arch Toxicol 2021;95:3717–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Küpper FC, Carrano CJ. Key aspects of the iodine metabolism in brown algae: a brief critical review. Metallomics 2019;11:756–764. [DOI] [PubMed] [Google Scholar]
- 35.Arroyo-Helguera O, Anguiano B, Delgado G, Aceves C. Uptake and antiproliferative effect of molecular iodine in the MCF-7 breast cancer cell line. Endocr Relat Cancer 2006; 13:1147–1158. [DOI] [PubMed] [Google Scholar]
- 36.Arroyo-Helguera O, Rojas E, Delgado G, Aceves C. Signaling pathways involved in the antiproliferative effect of molecular iodine in normal and tumoral breast cells: evidence that 6-iodolactone mediates apoptotic effects. Endocr Relat Cancer 2008; 15:1003–1011. [DOI] [PubMed] [Google Scholar]
- 37.Joanta AE, Filip A, Clichici S, Andrei S, Daicoviciu D. Iodide excess exerts oxidative stress in some target tissues of the thyroid hormones. Acta Physiol Hung 2006; 93:347–359. [DOI] [PubMed] [Google Scholar]
- 38.Kitagawa E, Akama K, Iwahashi H. Effects of iodine on global gene expression in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 2005; 69:2285–2293. [DOI] [PubMed] [Google Scholar]
- 39.Heseltine P. (2001). Disinfection, Sterilization, and Preservation. In: Infection control & hospital epidemiology. Ed, Block SS.. Lippincott Williams & Wilkins, 5th Ed. Philadelphia, pp. 109–109. [Google Scholar]
- 40.Yao X, Li M, He J, Zhang G, Wang M, Ma J, et al. Effect of early acute high concentrations of iodide exposure on mitochondrial superoxide production in FRTL cells. Free Radic Biol Med 2012; 52:1343–1352. [DOI] [PubMed] [Google Scholar]