Abstract
Dimethylsulfoniopropionate (DMSP) is degraded to dimethylsulfide (DMS) and acrylate by the enzyme DMSP lyase. DMS or acrylate can serve as a carbon source for both free-living and endophytic bacteria in the marine environment. In this study, we report on the mechanism of DMSP-acrylate metabolism by Alcaligenes faecalis M3A. Suspensions of citrate-grown cells expressed a low level of DMSP lyase activity that could be induced to much higher levels in the presence of DMSP, acrylate, and its metabolic product, β-hydroxypropionate. DMSP was degraded outside the cell, resulting in an extracellular accumulation of acrylate, which in suspensions of citrate-grown cells was then metabolized at a low endogenous rate. The inducible nature of acrylate metabolism was evidenced by both an increase in the rate of its degradation over time and the ability of acrylate-grown cells to metabolize this molecule at about an eight times higher rate than citrate-grown cells. Therefore, acrylate induces both its production (from DMSP) and its degradation by an acrylase enzyme. 1H and 13C nuclear magnetic resonance analyses were used to identify the products resulting from [1-13C]acrylate metabolism. The results indicated that A. faecalis first metabolized acrylate to β-hydroxypropionate outside the cell, which was followed by its intracellular accumulation and subsequent induction of DMSP lyase activity. In summary, the mechanism of DMSP degradation to acrylate and the subsequent degradation of acrylate to β-hydroxypropionate in the aerobic β-Proteobacterium A. faecalis has been described.
Dimethylsulfide (DMS), a potential antigreenhouse gas, accounts for as much as 90% of all marine biogenic sulfur emissions to the atmosphere (1) and may play an important role in marine cloud formation and climate regulation (6, 7, 10, 29, 32). The major precursor of DMS, dimethylsulfoniopropionate (DMSP), is an abundant sulfonium compound in marine environments (35, 36) and has been identified in microbial mats, macroalgae, marine phytoplankton, phototrophic prokaryotes, and higher plants, including the salt marsh cordgrass Spartina alterniflora (9, 12, 23, 33, 35, 46). DMSP appears to function as a compatible solute for osmoregulation in marine algae (19, 20, 41) and may also have a similar function in other marine organisms. During the senescence and decay of phytoplankton and cordgrass (30, 38) and during zooplankton grazing on phytoplankton (13, 47), DMSP is released into the water column or sediment where it can be degraded by the endophytic or free-living microbial population (13, 26, 44). The dominant pathway of DMS production in saline environments is through the enzymatic cleavage of DMSP to DMS and acrylate by the enzyme DMSP lyase: (CH3)2S+CH2CH2COO− → (CH3)2S + H2C⩵CHCOO− + H+ (8, 11, 15, 22). DMSP lyase has been purified from several microbial isolates from the marine environment (15, 18, 42) and macroalgal species (17, 31) and was detected in species of smooth cordgrass ascomycetes (4). Both products of the lyase reaction have been identified as potential carbon and energy sources for marine bacteria (25, 40, 44).
The process of acrylate metabolism in marine environments has not been studied to the extent of its precursor, DMSP. In the anaerobic marine isolate Desulfovibrio acrylicus, acrylate generated from DMSP cleavage during growth on lactate was used as an electron acceptor resulting in propionate production (43). Alternatively, Clostridium propionicum isolated from freshwater sediments cleaved DMSP with the resulting acrylate being fermented to propionate and acetate (45). Anoxic marine sediment slurries amended with acrylate metabolized it to acetate and propionate, which were then further degraded (25). Molybdate did not inhibit acrylate metabolism in these sediments, but it did inhibit the disappearance of acetate and propionate, suggesting the involvement of sulfate-reducing bacteria (25).
We recently identified two distinct patterns of DMSP metabolism among the aerobic marine isolates Pseudomonas doudoroffii and Alcaligenes faecalis M3A (49). Although the lyases from these two bacteria displayed similarities in their in vitro Km for DMSP, mass, and N-terminal amino acid sequence (16, 18), the mechanism of DMSP uptake and DMSP lyase induction and location were found to be very different (18, 49). Acrylate served as the inducer of DMSP lyase in A. faecalis, but the metabolic fate of the acrylate resulting from DMSP cleavage has not been determined in aerobic bacteria. In this study, we report the mechanism of DMSP-acrylate metabolism and DMSP lyase induction in A. faecalis M3A and have identified an intermediate, β-hydroxypropionate, which plays an important role in this process.
MATERIALS AND METHODS
Strain identification.
The estuarine isolate used in this study was previously identified as an Alcaligenes-like species designated strain M3A by deSouza and Yoch (15). Fatty acid analysis, determined by using the MIDI program, and 16S rDNA analysis positively identified strain M3A as belonging to A. faecalis (48).
Culturing and cell suspensions.
A. faecalis M3A was grown in 100-ml batch cultures of a basal salt medium containing 40 mM NaCl, 5 mM (NH4)2SO4, 0.8 mM MgSO4, 0.3 mM CaCl2, 89 μM Fe EDTA and the following per liter: 1 ml of Ho-Le trace elements solution (21) and 1 ml of Balch’s vitamin solution (5). The medium was supplemented with either citrate or acrylate (5 mM) as the carbon and energy source, adjusted to pH 6.4, and autoclaved. After cooling, the medium was buffered with 10 mM potassium phosphate from a 1 M stock (pH 6.8) to give a final pH of 7.2. After 18 h of growth, cell suspensions were prepared by centrifuging the culture (17,400 × g for 30 s) and resuspending the pellet in the original volume of 50 mM phosphate buffer (pH 7.45). Acrylate-grown cells were resuspended in half the original volume to compensate for the lower cell yield per mole of substrate than that of citrate-grown cells. The cell suspensions used in these experiments had A600s of ca. 0.52 and 0.26 and protein concentrations of 0.068 and 0.037 mg · ml−1 for citrate- and acrylate-grown cells, respectively.
DMSP and acrylate metabolism.
Cell suspensions (12-ml aliquots in 36-ml glass serum bottles) to which DMSP or acrylate had been added were incubated on an orbital shaker at 100 rpm at ambient temperature. DMSP and acrylate metabolism were measured by acrylate production (from DMSP) and acrylate disappearance from the media, respectively. Acrylate was analyzed by removing 1-ml aliquots from the cell suspension and pelleting the cells by centrifugation (2 min). The supernatant (top 800 μl) was immediately frozen on dry ice and stored at −70°C for later high-pressure liquid chromatography (HPLC) analysis (see below). In parallel cell suspensions, DMS production from DMSP (resulting from DMSP lyase activity) was measured in 1-ml aliquots of cell suspensions in 14.5-ml serum bottles that were capped with Teflon-lined butyl rubber stoppers and crimped with aluminum caps. The headspace gases were analyzed for DMS by gas chromatography (15). To test the effects of the protein synthesis inhibitor, gentamicin (200 μg · ml−1) on DMSP, and acrylate catabolism, the inhibitor was added 30 min prior to the addition of the substrate.
To test DMSP, acrylate, and β-hydroxypropionate as inducers of DMSP lyase, cells were cultured on tryptic soy broth, harvested, resuspended in phosphate buffer (as above), and placed in 14.5-ml bottles (4-ml aliquots). The putative inducers were added over a concentration range of 1 μM to 10 mM. DMSP lyase activity was then tested at 6, 12, and 24 h after the inducers were added by removing 1-ml aliquots, pelleting the cells in a microcentrifuge for 30 s, and resuspending the pellet in phosphate buffer along with excess substrate (2.5 mM DMSP). The rate of DMS production by the cells removed at each time point was assumed to be proportional to the extent of DMSP lyase induction. Data presented in the Results section are representative of at least two replicate experiments.
HPLC analysis.
Supernatants of cell suspensions for HPLC analysis that had been stored at −70°C were thawed, centrifuged for 2 min, and diluted 1:5 with HPLC buffer (2.5% acetonitrile, 0.2% phosphoric acid in double-distilled H2O). Aliquots (150 μl) were analyzed by using a Beckman isocratic liquid chromatograph (model 330) equipped with a Waters μ Bondapack C18 column (3.9 by 300 mm) (Millipore) which was operated at a flow rate of 0.8 ml · min−1. Retention times of acrylate and methylmercaptopropionic acid (MMPA), monitored at 214 nm, were 5.30 and 9.10 min, respectively. Acrylate concentrations were determined from standard curves constructed with known concentrations and diluted 1:5, similar to the unknowns. DMSP, propionate, and β-hydroxypropionate could not be identified by using these HPLC parameters and this detection system.
Purification of endogenous DMSP lyase.
A. faecalis was grown in 5 mM citrate-basal salt medium (10 2-liter batch cultures) on a rotary shaker (100 rpm) at 30°C. The purification steps were the same as those used for the inducible lyase in this organism (15).
Western blot analysis.
Western blots were used to compare the sizes of DMSP lyase purified from either A. faecalis citrate-grown (i.e., endogenous lyase) or A. faecalis acrylate-grown (i.e., induced lyase) cells. A polyclonal DMSP lyase antiserum was raised against a protein shown to be pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and N-terminal amino acid sequencing (16, 18). Samples were subjected to sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis followed by Western blot analysis as described by Sambrook et al. (37).
13C NMR determination of acrylate metabolites.
Three 1-liter cultures of 24-h acrylate-grown cells (A600 ≈ 0.26) were harvested by centrifugation, resuspended in 80 ml of 50 mM phosphate buffer, and placed in a 250-ml Erlenmeyer flask and used for 13C nuclear magnetic resonance (NMR) analysis of acrylate metabolites. Cells were preincubated for 15 min at 100 rpm on a rotary shaker at ambient temperature followed by the addition of 5 mM [1-13C]acrylic acid (Cambridge Isotope Laboratories, Andover, Mass.) from a 150 mM stock solution (pH 7). Cell aliquots (10 ml) were removed at intervals between 10 and 120 min, placed in 10 ml of ice-cold phosphate buffer, and then centrifuged at 17,400 × g for 30 s. The supernatant (extracellular medium) was decanted, frozen on dry ice, and stored at −70°C; these samples were thawed and diluted 1:1 in D2O prior to 1H and 13C NMR analysis. The pellet which contained the intracellular 13C metabolites was washed with 1 ml of ice-cold buffer and then extracted twice for 16 h in 1.5 ml of 95% ethanol at ambient temperatures. The combined ethanol extracts which contained the low Mr organic pool were dried in a rotary evaporator, and the residue was solubilized in 1 ml of D2O and analyzed by NMR.
NMR analysis.
NMR data on acrylate and its metabolic product(s) were collected on a Varian Unity Inova 500. The 1H (500.16 MHz) spectra of the extracellular medium (supernatant) required presaturation of the dominant water resonance. Data were collected by using a 7-kHz window centered on the water resonance (4.63 ppm); 256 transients were collected by using a 45° pulse angle, a 3-s acquisition time, and a 4.5-s interpulse delay. Direct detection of 13C NMR (125.894 MHz) spectra of the intracellular metabolites was obtained by using a 30-kHz window centered at 110 ppm. A total of 512 transients were collected by using a 45° pulse angle, a 1-s acquisition time, and a 2.5-s interpulse delay. Acetone (10 μl) was added to each sample to provide an internal reference (C-1 = 214.97 ppm and C-2 = 29.80 ppm). An indirect detection of 13C spectra was obtained by using the standard gradient-enhanced heteronuclear multiple quantum coherence sequence (HMQC). The magnetization transfer delay was set to 50 ms to optimize detection of spin-spin coupling constants of ≈10 Hz. This allowed for the correlation of protons that were two or three bonds away from the labeled carbon. The 1H dimension was collected with 1,984 complex points over a 4-kHz window that was centered on the water resonance (4.63 ppm). The 13C dimension was collected with 128 free induction decays (fid), over a 120-ppm window that was centered at 160 ppm. Four transients were collected for each fid, and the equilibrium delay was set to 0.9 s. The 13C dimension was externally referenced with acetone by using one of the intracellular metabolite samples from the directly detected experiments. The direct detection of the 13C data was obtained at ambient temperature, while the 1H spectra and gradient-enhanced HMQC results were obtained with the sample temperature regulated at 25°C.
Mass spectroscopy.
Negative ion electrospray mass spectroscopy was used to confirm the identity of the [13C]acrylate metabolite(s). Extracellular supernatant fractions which contained the metabolite were lyophilized to dryness, and the acetone-soluble, organic constituents were extracted with 5 ml of deuterated acetone. This mixture was then centrifuged to pellet any insoluble salts that remained in the acetone extract. To insure this fraction contained the metabolite(s) of interest, it was first analyzed by 1H NMR, after which the acetone was volatilized with a stream of argon. The residue was resuspended in 100% methanol and analyzed by negative ion electrospray mass spectroscopy as described previously (3).
Materials.
β-Hydroxypropionate (hydracrylic acid; Merck reference 4660) was either synthesized from β-hydroxypropionitrile as described by Read (34) or purchased from Chem Service (West Chester, Pa.). All other chemicals or reagents were purchased from Aldrich Chemical Co. Inc., Milwaukee, Wis.
RESULTS
DMSP and acrylate catabolism.
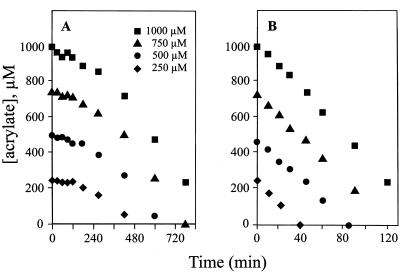
DMSP uptake has been shown to precede its degradation to DMS and acrylate in P. doudoroffii (49) and strain LFR (27), while in A. faecalis M3A, DMSP cleavage appeared to be an extracellular process (49). When DMSP (500 μM) was added to suspensions of citrate-grown cells of strain M3A, there was no lag prior to the appearance of DMS and acrylate in the extracellular medium (Fig. 1A). Both products increased in parallel for the first several hours, with acrylate reaching a maximum concentration of ca. 300 μM in the extracellular medium at 5 h, after which it decreased rapidly until it was depleted from the medium at 9 h. These findings are consistent with DMSP being cleaved outside the cell by an endogenous low level of DMSP lyase activity, which was reported earlier (49). These data also show that acrylate produced during DMSP cleavage first accumulates outside the cell prior to its metabolism. When acrylate was added to a parallel cell suspension, there was a low initial rate of acrylate metabolism followed by a threefold increase in this rate with time (Fig. 1A).
FIG. 1.
Acrylate and DMS production (from DMSP) and acrylate metabolism in suspensions of citrate-grown (A) and acrylate-grown (B) cells. DMSP or acrylate (500 μM) was added to cell suspensions of A. faecalis M3A. Symbols: ▴, DMS from DMSP cleavage; ●, acrylate resulting from DMSP cleavage; ■, added acrylate.
When suspensions of acrylate-grown cells were amended with DMSP (500 μM), it was completely degraded to DMS and acrylate within 10 min (Fig. 1B). Furthermore, the rate of acrylate metabolism was eight to nine times higher than that in suspensions of citrate-grown cells. Acrylate (from either DMSP cleavage or as an acrylate amendment) was metabolized at a linear rate (no lag) and depleted from the extracellular medium within 70 min. While DMSP lyase in A. faecalis was known to be induced by growth on acrylate (15), these data (Fig. 1) indicate that an enhanced rate of acrylate metabolism (acrylase activity) is also induced by exposure of the microbe to this molecule. The kinetics of DMSP lyase and the acrylate-metabolizing mechanism are, however, very different, with the lyase having an eightfold higher rate than the acrylase activity. Since HPLC analysis of the extracellular medium was unable to detect any metabolites produced as a result of acrylate degradation, the fate of the acrylate added to cell suspensions was determined by 1H and 13C NMR analyses (see below).
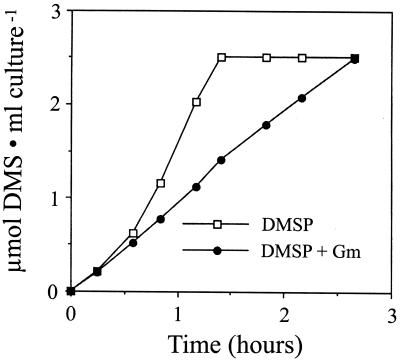
Acrylate metabolism by A. faecalis was further examined by testing the effect of its concentration (50 μM to 1.0 mM) on its rate of utilization by suspensions of both citrate- and acrylate-grown cells. In citrate-grown cells, a lag of ca. 2 h preceded the metabolism of acrylate at all concentrations tested (Fig. 2A), while acrylate-grown cells utilized this compound with no apparent lag and at a much higher rate (Fig. 2B). The rate of acrylate metabolism (disappearance) at each concentration tested was similar, suggesting that the Km for the acrylase was below 50 μM. Although we could detect much lower levels of acrylate, it could not be reproducibly quantitated below 25 μM, and therefore an accurate Km could not be determined. These observations support the premise stated above that acrylate induces the enzyme(s) responsible for its metabolism. Finally, the inducibility of acrylate metabolism was confirmed by showing that the addition of gentamicin to suspensions of citrate-grown cells resulted in acrylate being metabolized at a low endogenous rate, compared to untreated cells (data not shown).
FIG. 2.
Effect of acrylate concentration on its rate of metabolism. Kinetics (disappearance from solution) were measured in suspensions of citrate-grown (A) and acrylate-grown (B) cells. The rate of acrylate metabolism at a concentration of 2.5 mM was the same as that at a concentration of 1.0 mM (data not shown).
MMPA, a DMSP analog that inhibits DMSP lyase activity (16), inhibited the metabolism of both acrylate and DMSP by suspensions of A. faecalis citrate-grown cells (Fig. 3). MMPA therefore competes for enzyme binding sites on the cell surface that are responsible for both DMSP and acrylate degradation. The same results were obtained with acrylate-grown cells (data not shown). MMPA, as an inhibitor of acrylate metabolism (acrylase activity), should prove to be a useful tool for the later study of this enzyme.
FIG. 3.
MMPA inhibition of DMSP and acrylate metabolism in suspensions of citrate-grown cells. MMPA (5 mM) was added to the suspension 30 min before the addition of the substrates (500 μM). Symbols: ■, acrylate; ⧫, acrylate plus MMPA; ●, acrylate from DMSP cleavage; ▴, acrylate from DMSP cleavage plus MMPA.
NMR analysis of [1-13C]acrylate metabolites.
1H and 13C NMR analyses were used to follow the metabolism of acrylate in A. faecalis M3A. To have a basis for comparing the acrylate metabolites, an acrylate standard was first examined by using 1H and 13C NMR. The gradient-enhanced 1H{13C} HMQC spectrum of the [1-13C]acrylate standard showed one 13C resonance (at 175 ppm); this 13C resonance had 1H correlations at 5.55, 5.90, and 6.05 ppm (data not shown). Due to the low natural abundance of 13C (1.1%), it was only possible under these conditions to detect the labeled position (C-1) and protons, two or three bonds removed.
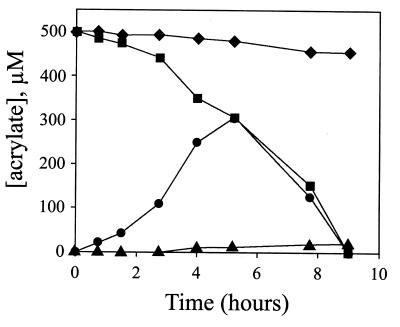
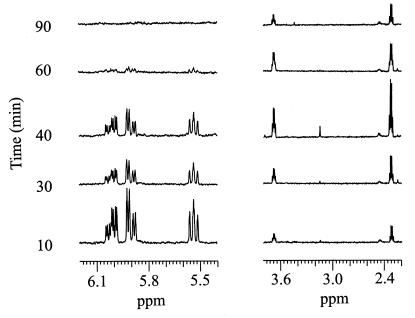
To monitor the time course of acrylate utilization, [1-13C]acrylate was added to suspensions of acrylate-grown cells, and both the extracellular medium (supernatant) and intracellular metabolites (extract from cell pellet) were examined. The 1H NMR spectrum of each extracellular sample taken over the 120-min time course of the experiment is presented in Fig. 4. The first time point (10 min) showed resonances centered at 5.55, 5.90, and 6.05 ppm, indicative of acrylate protons (see above), and two new resonances centered at 2.35 and 3.64 ppm. The decrease in the intensity of the proton resonances of acrylate over time corresponded to an increase in the intensity of the resonances centered at 2.35 and 3.64 ppm, which reached a maximum intensity at 40 min and then decreased rapidly. The 1H NMR spectrum of the last time point (120 min) showed no peaks (data not shown).
FIG. 4.
1H NMR analysis of the extracellular medium following the addition of [1-13C]acrylate to a concentrated cell suspension. Samples were taken at the indicated time intervals; the cells were removed by centrifugation, and the supernatant was assayed directly. The NMR spectra were acquired by using the same conditions and number of scans for each sample so that the peak intensity would represent an approximate concentration of each metabolite relative to each sample. 1H resonances at 5.55, 5.90, and 6.05 ppm and at 2.35 and 3.64 ppm were identified as those belonging to acrylate and β-hydroxypropionate, respectively.
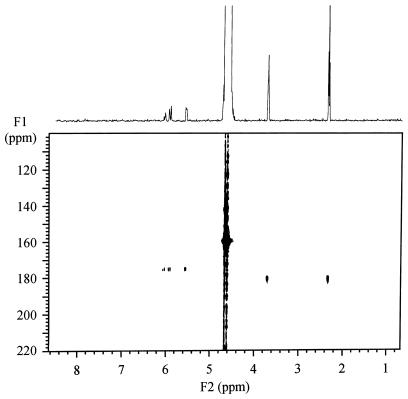
To aid in the identification of the unknown metabolite (resonances centered at 2.35 and 3.64 ppm), a gradient-enhanced 1H{13C} HMQC spectrum of the extracellular medium was obtained after a 40-min exposure of the cell suspension to [1-13C]acrylate (Fig. 5). The spectrum showed two 13C resonances at 175 and 180 ppm that have correlations to 1H resonances at 5.55, 5.80, and 6.05 ppm and at 2.30 and 3.67 ppm, respectively. This indicates that the product of acrylate metabolism consisted of two methylene groups (based on the 1H chemical shifts with similar integral intensities) and a carbonyl group (based on the 13C chemical shift of the C-1 carbon). To help identify the unknown metabolite, several three-carbon molecules, including lactate, propionate, and β-hydroxypropionate, were analyzed by NMR. The β-hydroxypropionate standard had 1H and 13C resonances identical to that of the unknown metabolite (data not shown). The identity of the unknown metabolite in the extracellular medium was confirmed by adding 10 mM β-hydroxypropionate to the 40-min sample, which resulted in an increase in the intensity of the 1H resonances at 2.30 and 3.67 ppm and the 13C resonance at 180 ppm (data not shown). The identity of the unknown metabolite, β-hydroxypropionate, in the extracellular medium was confirmed by using negative ion electrospray mass spectroscopy. The acetone-soluble metabolite extracted from the 40-min lyophilized sample had an m/z of 90. This m/z was the exact molecular weight calculated for β-hydroxypropionate with a 13C-labeled carbonyl group (the β-hydroxypropionate standard that was not 13C labeled gave an m/z of 89). Based on these analyses, the product of acrylate metabolism by strain M3A in the extracellular medium was definitively identified as β-hydroxypropionate. Based on the integral intensity of its NMR signal, β-hydroxypropionate in the extracellular medium was maximum at 40 min and was approximated at a concentration of 3 mM.
FIG. 5.
The gradient-enhanced 1H{13C} HMQC spectrum of the extracellular medium taken 40 min after the addition of 5 mM [1-13C]acrylate to a suspension of A. faecalis cells. The spectrum shows elements of both acrylate and its metabolite, β-hydroxypropionate.
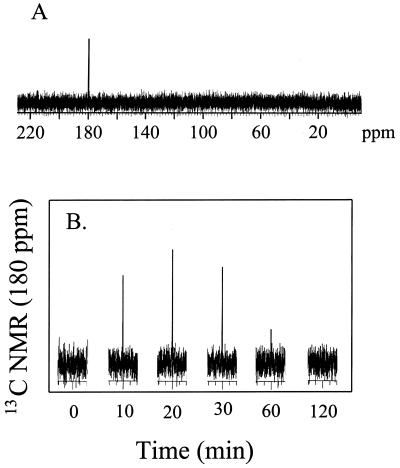
To determine if any of the [1-13C]acrylate or products derived from its metabolism could be found in the cell cytoplasm, cell pellets were ethanol extracted and the dried residue was rehydrated in D2O and examined by 1H and 13C NMR spectroscopy. The 1H NMR spectrum obtained from each of the cell extracts resulted in many overlapping peaks that could not be interpreted. However, 13C NMR analysis of the extracts showed only one resonance at 180 ppm (Fig. 6A). It is assumed that this resonance is from a 13C-labeled metabolite due to its great intensity. Cell extracts prepared over the time course (120 min) of the experiment described above (Fig. 4) showed an increase in the intensity of the resonance at 180 ppm, which reached its maximum at 20 min and thereafter declined (Fig. 6B). When the β-hydroxypropionate standard was added to these samples, the intensity of the resonance centered at 180 ppm increased, which confirmed the identity of the intracellular metabolite as β-hydroxypropionate. The disappearance with time of the resonance centered at 180 ppm and the fact that no other resonances could be identified indicated that the 13C-labeled carbonyl group of β-hydroxypropionate was subsequently decarboxylated.
FIG. 6.
13C NMR spectrum of intracellular [1-13C]acrylate metabolites showing the full spectrum after 20 min (A) and a time course representing the peak intensity centered at 180 ppm, identified as the [13C]carboxyl group of β-hydroxypropionate (B).
DMSP lyase induction.
Previous reports have provided evidence that acrylate is the inducer of DMSP lyase in A. faecalis M3A (15, 49); however, since acrylate does not enter the cell (as shown here), the mechanism of DMSP lyase induction remained unknown. Because β-hydroxypropionate was shown to be a product of acrylate metabolism, DMSP, acrylate, and β-hydroxypropionate were compared as inducers of DMSP lyase in strain M3A over a concentration range of 100 μM to 10 mM, and all served equally well (Table 1). This strongly suggests that β-hydroxypropionate or a molecule derived from its metabolism was the inducer of DMSP lyase.
TABLE 1.
Comparison of β-hydroxypropionate, acrylate, and DMSP as inducers of DMSP lyase in A. faecalis M3A
| Concn of inducer (mM) | % Induction by the following inducera
|
||
|---|---|---|---|
| β-Hydroxypropionate | Acrylate | DMSP | |
| 0.1 | 6 | 6 | 6 |
| 0.5 | 10 | 29 | 21 |
| 1.0 | 36 | 55 | 38 |
| 2.5 | 67 | 63 | 56 |
| 5.0 | 93 | 87 | 82 |
| 10.0 | 100 | 73 | 85 |
The highest level of DMSP lyase activity observed at 6 h was taken to be the 100% value with 10 mM β-hydroxypropionate. The experiment was performed in duplicate, and the variance for all values was less than 10%.
Since acrylate is metabolized to β-hydroxypropionate and is either directly or indirectly the inducer of the lyase in strain M3A, the question arises as to how DMSP is first metabolized to acrylate in cells not induced for DMSP lyase. Figure 7 shows the time course of DMSP degradation (to DMS) in suspensions of uninduced cells. When DMSP was added at time zero, it served as both substrate and inducer of DMSP lyase. The initial low rate of DMS production suggested the presence of an endogenous lyase activity, which was followed by much higher rates (Fig. 7); this biphasic rate suggested that induction of additional DMSP lyase was occurring. The addition of gentamicin (a protein synthesis inhibitor) along with DMSP had no effect on the initial rate of DMS production, confirming the presence of an endogenous lyase activity in this organism (Fig. 7). The initial rate continued until all of the DMSP was consumed. Finally, in comparable experiments with P. doudoroffii and strain JA6 (two marine γ-Proteobacteria that synthesize DMSP lyase), no DMS was produced in the presence of gentamicin, indicating that constitutive DMSP lyase expression is not present in all marine bacterial isolates (2).
FIG. 7.
Endogenous DMSP lyase activity in noninduced cell suspensions (i.e., grown in tryptic soy broth) of A. faecalis M3A. Cells (1 ml) were pretreated for 30 min with 200 μg · ml−1 of gentamicin (Gm). DMSP (2.5 mM) was added to treated and untreated cells, and the headspace gas was assayed for DMS by gas chromatography.
While the endogenous rate of lyase activity may appear high (Fig. 7), it represented less than 10% of what the fully induced cell is capable of synthesizing (2, 15). When the endogenous DMSP lyase from A. faecalis was purified, Western blot analysis showed that it comigrated with the enzyme purified from acrylate-grown cells (i.e., fully induced cells), indicating that the molecular masses (ca. 48 kDa) were indistinguishable. This was also true of their kinetic properties (2). The purification of the endogenous lyase resulted in yields too low to permit N-terminal amino acid sequencing, which could have provided a more definitive comparison.
DISCUSSION
While there is some information about acrylate metabolism in anaerobic bacterial isolates and natural populations in anoxic environments, there are no reports documenting its metabolic fate in aerobic bacteria. This work describes the metabolism of DMSP to β-hydroxypropionate and its subsequent utilization by the cell in a reaction that proceeds as follows: (CH3)2S+CH2CH2CO2− → (CH3)2S + CH2⩵CHCO2− + H+ → HOCH2CH2CO2− → CO2 + X (X represents an unknown metabolite). While there appear to be no previous reports of bacterial metabolism of acrylate to β-hydroxypropionate until this one, there are several reports of this reaction occurring in fungi. β-Hydroxypropionate was produced by the fungus Byssochlamys sp. when grown on media containing high concentrations of acrylate (39). Acrylate was also β-hydroxylated to β-hydroxypropionate by the fungal isolates Geotrichum sp. and Trichoderma sp. from a petrochemical effluent treatment plant (14).
Previous reports have shown DMSP uptake to precede its degradation to DMS and acrylate in both natural populations in oceanic waters (28) and pure cultures of the aerobic marine bacterial isolates, P. doudoroffii and strain LFR (27, 49). These organisms accumulate DMSP intracellularly at concentrations in excess of 70 mM prior to its degradation to DMS and acrylate by internal DMSP lyases (27, 47). In contrast, A. faecalis does not take up or accumulate DMSP in measurable quantities; instead, it metabolizes DMSP to DMS and acrylate outside of the cell by an extracellular DMSP lyase (49). In support of those earlier observations, it was shown that as DMSP was metabolized by a low constitutively expressed level of lyase (indistinguishable from the inducible lyase), acrylate accumulated in the extracellular medium (Fig. 8).
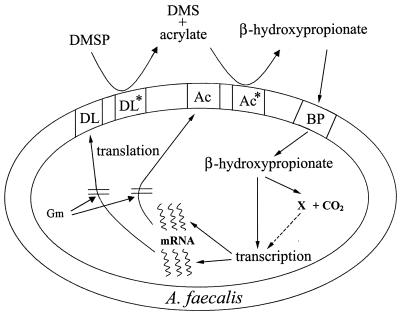
FIG. 8.
A model of DMSP metabolism in A. faecalis M3A. Abbreviations: DL*, endogenous DMSP lyase; Ac*, endogenous acrylate metabolizing enzyme (acrylase); DL and Ac, inducible DMSP lyase and inducible acrylase, respectively; BP, β-hydroxypropionate uptake or binding protein; X, unidentified product of β-hydroxypropionate metabolism; Gm, gentamicin. The dashed line indicates the possibility that the unidentified β-hydroxypropionate metabolite (X) could conceivably be the inducer of DMSP lyase and the acrylate-metabolizing enzyme.
NMR analysis has determined that acrylate, like DMSP, is also metabolized outside the cell by an extracellular enzyme, tentatively called acrylase. This is supported by data that showed the accumulation of β-hydroxypropionate in the supernatant, as acrylate was being metabolized and then disappeared from the external medium (Fig. 4). As β-hydroxypropionate accumulated in the extracellular medium, the 13C resonance at 180 ppm (identified as β-hydroxypropionate) also increased in the cell cytoplasm. Therefore acrylate does not enter the cell, but the product of its metabolism, β-hydroxypropionate, is taken up and accumulates in the cytoplasm, at least transiently (Fig. 6).
DMSP lyase appears to be an inducible enzyme in all species that metabolize DMSP via the cleavage pathway (2). This observation has not been extended to natural populations, however, because extremely low levels of DMSP have been shown to be cleaved with no apparent lag (or induction) period (24, 28). Data from previous reports suggested that acrylate was the inducer of DMSP lyase in A. faecalis. This was supported by the observation that acrylate and its analogs (acrylamide and methacrylate) were also capable of DMSP lyase induction, while DMSP analogs (glycine betaine, dimethylseleniopropionate, and dimethyl glycine) would not induce the lyase (16, 49). When DMSP, acrylate, and β-hydroxypropionate were compared as lyase inducers, all served equally well (Table 1), but acrylate could not be detected within the cell by NMR analysis. Therefore, it is concluded that β-hydroxypropionate is the inducer of DMSP lyase in A. faecalis (Fig. 8 and see below).
An earlier report (16) that dimethylsulfoxide (DMSO) and dimethyldisulfide (DMDS) were inducers of DMSP lyase in A. faecalis has not been reproducible. DMSO reductase yields DMS as one of its reaction products, and it has been determined that DMSO in the interstitial space in the cell pellet released DMS by this reaction, which was mistaken for DMSP cleavage in cell suspensions. Furthermore, we were unaware at that time of the endogenous DMSP lyase activity that also contributed to the immediate release of DMS, leading us to believe that DMSO had induced the lyase. DMDS trapped in the interstitial space of the cell pellet resulted in its release into the gas phase, and because it has nearly the same retention time as that of DMS on our gas chromatograph, it was apparently mistaken for DMS. Using washed cell pellets, the results clearly indicate that neither DMSO nor DMDS are inducers of the lyase in A. faecalis (2).
Figure 8 outlines the mechanism of DMSP-acrylate metabolism, DMSP lyase induction, and β-hydroxypropionate production and metabolism in A. faecalis. The endogenous DMSP lyase in the periplasm of this gram-negative organism is responsible for the initial degradation of DMSP to DMS and acrylate in noninduced cells. Subsequently, an endogenous acrylate-metabolizing enzyme, i.e., acrylase, also external to the cytoplasmic membrane, converts acrylate to β-hydroxypropionate, which is taken up by the cell and metabolized to an unidentified product and CO2. β-Hydroxypropionate serves as the inducer of both additional DMSP lyase and acrylase, but we cannot say at this time if it or a product of its metabolism is the actual inducer. Two potential products of β-hydroxypropionate metabolism are propionate and acetate, but neither of these induce DMSP lyase activity in this organism (2, 16). The production of CO2 from intracellular β-hydroxypropionate metabolism is assumed to account for the loss of the intracellular 13C signal, as the label was in the C-1 position (Fig. 6).
It is possible that the endogenous and inducible DMSP lyases are both the products of the same gene, with the former simply resulting from constitutive readthrough expression; the same is true for the endogenous and inducible acrylase activities.
This report describes a unique mechanism of DMSP-acrylate metabolism in an aerobic gram-negative β-Proteobacterium, which involves a metabolite, β-hydroxypropionate, apparently not yet documented in bacterial metabolism. The ecological significance of this finding has yet to be determined; however, one may speculate on how this influences the overall carbon cycle within the marine microbial loop. Since these reactions take place exterior to the cell membrane, the metabolism of DMSP in natural systems by microbes of this kind may be beneficial even to those microbes not capable of cleaving DMSP. Therefore, microbes that are in close proximity to A. faecalis-like DMSP-cleaving strains may benefit from the acrylate and β-hydroxypropionate released from their DMSP metabolism. This would be significant to those microbes in phytoplankton blooms where DMSP may be found in significant concentrations as the bloom crashes or is heavily grazed by zooplankton (47). Although that scenario is speculative, this report clearly documents a rather complex extracellular mechanism of DMSP metabolism in one DMS-producing salt marsh isolate.
ACKNOWLEDGMENTS
This work was supported in part by grants from the SC Sea Grant Consortium (R-MX-8) and DOE/SCUREF.
REFERENCES
- 1.Andreae M O. The emission of sulfur to the remote atmosphere. In: Galoway J N, Charlson R J, Andreae M O, Rode H, editors. The biogeochemical cycling of sulfur and nitrogen in the remote atmosphere. Boston, Mass: D. Redial Publishing Co.; 1985. pp. 5–25. [Google Scholar]
- 2.Ansede, J. H., and D. C. Yoch. Unpublished data.
- 3.Ansede J H, Pellechia P J, Yoch D C. Selenium biotransformation salt marsh cordgrass Spartina alterniflora: evidence for dimethylselenoniopropionate formation. Environ Sci Technol. 1999;33:2064–2069. [Google Scholar]
- 4.Bacic M K, Newell S Y, Yoch D C. Release of dimethylsulfide from dimethylsulfoniopropionate by plant-associated salt marsh fungi. Appl Environ Microbiol. 1998;64:1484–1489. doi: 10.1128/aem.64.4.1484-1489.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch W E, Fox G E, Magrum L J, Woese C R, Wolfe R S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates T S, Charlson R J, Gammon R H. Evidence for the climatic role of marine biogenic sulfur. Nature. 1987;329:319–321. [Google Scholar]
- 7.Bates T S, Cline J D. The role of the ocean in a regional sulfur cycle. J Geophys Res. 1985;90:9168–9172. [Google Scholar]
- 8.Cantoni G L, Anderson D G. Enzymatic cleavage of dimethylthetin by Polysiphonia lanosa. J Biol Chem. 1956;222:171–177. [PubMed] [Google Scholar]
- 9.Challenger F, Simpson M I. Studies on biological methylation. Part XII. A precursor of the dimethyl sulfide evolved by Polysiphonia fastigata. Dimethyl-2-carboxyethyl-sulfonium hydroxide and its salts. J Chem Soc. 1948;3:1591–1597. doi: 10.1039/jr9480001591. [DOI] [PubMed] [Google Scholar]
- 10.Charlson R J, Lovelock J E, Andreae M O, Warren S G. Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate. Nature. 1987;326:655–661. [Google Scholar]
- 11.Dacey J W H, Blough N V. Hydroxide decomposition of DMSP to form DMS. Geophys Res Lett. 1987;14:1246–1249. [Google Scholar]
- 12.Dacey J W H, King G M, Wakeham S G. Factors controlling emission of dimethylsulfide from salt marshes. Nature. 1987;330:643–645. [Google Scholar]
- 13.Dacey J W H, Wakeham S G. Oceanic dimethyl sulfide: production during zooplankton grazing on phytoplankton. Science. 1986;233:1314–1316. doi: 10.1126/science.233.4770.1314. [DOI] [PubMed] [Google Scholar]
- 14.Dave H, Ramakrishna C, Desai J D. Degradation of acrylic acid by fungi from petrochemical activated sludge. Biotechnol Lett. 1996;18:963–964. [Google Scholar]
- 15.de Souza M P, Yoch D C. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl Environ Microbiol. 1995;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza M P, Yoch D C. Comparative physiology of dimethylsulfide production by dimethylsulfoniopropionate lyase in Pseudomonas doudoroffii and Alcaligenes sp. strain M3A. Appl Environ Microbiol. 1995;61:3986–3991. doi: 10.1128/aem.61.11.3986-3991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Souza M P, Chen Y P, Yoch D C. Dimethylsulfoniopropionate lyase from the marine macroalga Ulva curvata: purification and characterization of the enzyme. Planta. 1996;199:433–438. [Google Scholar]
- 18.de Souza M P, Yoch D C. N-terminal amino acid sequences and comparison of DMSP lyases from Pseudomonas doudoroffii and Alcaligenes strain M3A. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Environmental and biological chemistry on dimethylsulfoniopropionate and related sulfonium compounds. New York, N.Y: Plenum Press; 1996. pp. 293–304. [Google Scholar]
- 19.Dickson D M, WynJones R G, Davenport J. Steady state osmotic adaptation in Ulva lactuca. Planta. 1980;150:158–165. doi: 10.1007/BF00582360. [DOI] [PubMed] [Google Scholar]
- 20.Dickson D M J, Kirst G O. The role of dimethylsulfoniopropionate, glycine betaine and homarine in the osmoacclimation of Platymonas subcordiformis. Planta. 1986;167:536–543. doi: 10.1007/BF00391230. [DOI] [PubMed] [Google Scholar]
- 21.Gherna R, Pienta P, Cote R, editors. 1992 American Type Culture Collection Catalogue of Bacteria and Phages. 18th ed. Rockville, Md: American Type Culture Collection; 1992. [Google Scholar]
- 22.Kadota H, Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- 23.Keller M D, Bellows W K, Guillard R R L. Dimethylsulfide sulfide production in marine phytoplankton. In: Salzman E S, Cooper W J, editors. Biogenic sulfur in the environment. American Chemical Society Symposium Series no. 393. Washington, D.C.: American Chemical Society; 1989. pp. 167–182. [Google Scholar]
- 24.Kiene R P, Service S K. Decomposition of dissolved DMSP and DMS in estuarine waters: dependence on temperature and substrate concentration. Mar Ecol Prog Ser. 1991;76:1–11. [Google Scholar]
- 25.Kiene R P, Taylor B F. Metabolism of acrylate and 3-mercaptopropionate. In: Salzman E S, Cooper W J, editors. Biogenic sulfur in the environment. American Chemical Society Symposium Series no. 393. Washington, D.C.: American Chemical Society; 1989. pp. 222–230. [Google Scholar]
- 26.Kiene R P, Visscher P T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987;53:2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledyard K M, Dacey J W H. Dimethylsulfide production from dimethylsulfoniopropionate by a marine bacterium. Mar Ecol Prog Ser. 1994;110:95–103. [Google Scholar]
- 28.Ledyard K M, Dacey J W H. Kinetics of DMSP-lyase activity in coastal seawater. In: Kiene R P, Visscher P T, Keller M D, Kirst G O, editors. Environmental and biological chemistry on dimethylsulfoniopropionate and related sulfonium compounds. New York, N.Y: Plenum Press; 1996. pp. 325–336. [Google Scholar]
- 29.Legrand M R, Delmas R J, Charlson R J. Climate forcing implications from Vostok ice-core sulfate data. Nature. 1988;334:418–420. [Google Scholar]
- 30.Matrai P, Keller M D. DMS in a large scale coccolithophore bloom in the gulf of Maine. Cont Shelf Res. 1993;13:831–843. [Google Scholar]
- 31.Nishiguchi M K, Goff L J. Isolation, purification, and characterization of DMSP lyase (dimethylpropiothetin dethiomethylase (4.4.1.3)) from the red alga Polysiphonia paniculata. J Phycol. 1995;31:567–574. [Google Scholar]
- 32.Nriagu J O, Holdway D A, Coker R D. Biogenic sulfur and the acidity of rainfall in remote areas of Canada. Science. 1987;237:1189–1192. doi: 10.1126/science.237.4819.1189. [DOI] [PubMed] [Google Scholar]
- 33.Pakulski J D, Kiene R P. Foliar release of dimethylsulfoniopropionate from Spartina alterniflora. Mar Ecol Prog Ser. 1992;81:277–287. [Google Scholar]
- 34.Read R R. β-Hydroxypropionic acid, organic synthesis. 2nd ed. 1941. , collective vol. 1, p. 321–322. John Wiley & Sons, Inc., New York, N.Y. [Google Scholar]
- 35.Reed R H. Measurement and osmotic significance of β-dimethylsulfoniopropionate in marine macroalgae. Mar Biol Lett. 1983;4:173–181. [Google Scholar]
- 36.Salzman E S, Cooper W J, editors. Biogenic sulfur in the environment. American Chemical Society Symposium Series no. 393. Washington, D.C.: American Chemical Society; 1989. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Stefels J, van Boekel W H M. Production of DMS from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp. Mar Ecol Prog Ser. 1993;97:11–18. [Google Scholar]
- 39.Takamizawa K, Horitsu H, Ichikawa T, Kawai K, Suzuki T. β-Hydroxypropionate acid production by Byssochlamys sp. grown on acrylic acid. Appl Microbiol Biotechnol. 1993;40:196–200. [Google Scholar]
- 40.Taylor B T, Gilchrist D C. New routes for aerobic biodegradation of dimethylsulfoniopropionate. Appl Environ Microbiol. 1991;57:3581–3584. doi: 10.1128/aem.57.12.3581-3584.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vairavamurthy A, Andreae M O, Iversen R L. Biosynthesis of dimethyl sulfide and dimethyl propiothetin by Hymenomonas carterae in relation to sulfur source and salinity variations. Limnol Oceanogr. 1985;30:59–70. [Google Scholar]
- 42.van der Maarel M J E C, Aukema W, Hansen T A. Purification and characterization of a dimethylsulfoniopropionate cleaving enzyme from Desulfovibrio acrylicus. FEMS Microbiol Lett. 1996;143:241–245. [Google Scholar]
- 43.van der Maarel M J E C, van Bergeijk S, van Werkhoven A F, Laverman A M, Meijer W G, Stam W T, Hansen T A. Cleavage of dimethylsulfoniopropionate and reduction of acrylate by Desulfovibrio acrylicus sp. nov. Arch Microbiol. 1996;166:109–115. [Google Scholar]
- 44.Visscher P T, Diaz M R, Taylor B F. Enumeration of bacteria which cleave or demethylate dimethylsulfoniopropionate in the Caribbean Sea. Mar Ecol Prog Ser. 1992;89:293–296. [Google Scholar]
- 45.Wagner C, Stadtman E R. Bacterial fermentation of dimethyl-β-propiothetin. Arch Biochem Biophys. 1962;98:331–336. doi: 10.1016/0003-9861(62)90191-1. [DOI] [PubMed] [Google Scholar]
- 46.White R H. Analysis of dimethylsulfonium compounds in marine algae. J Mar Res. 1982;40:529–536. [Google Scholar]
- 47.Wolfe G V, Sherr E B, Sherr B F. Release and consumption of DMSP from Emliana huxleyi during grazing by Oxyrrhis marina. Mar Ecol Prog Ser. 1994;111:111–119. [Google Scholar]
- 48.Yoch, D. C., J. H. Ansede, and M. P. de Souza. Submitted for publication.
- 49.Yoch D C, Ansede J H, Rabinowitz K S. Evidence for intracellular and extracellular dimethylsulfoniopropionate (DMSP) lyases and DMSP uptake sites in two species of marine bacteria. Appl Environ Microbiol. 1997;63:3182–3188. doi: 10.1128/aem.63.8.3182-3188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]