Abstract
Background:
Endoscopic third ventriculostomy (ETV) is currently used as a treatment for different types of hydrocephalus. However, the anatomical endoscopic variants of the third ventricle floor (3VF), as well as their surgical implications, have been underrated. The anatomic variations of the 3VF can influence the technique and the success rate of the ETV. The purpose of this article is to describe the anatomical variations of 3VF, assess their incidence, and discuss the implications for ETV.
Methods:
Intraoperative videos of 216 patients who underwent ETV between January 2012 and February 2020 at Hospital Infantil Universitario de San José, Bogotá, Colombia were reviewed. One hundred and eighty patients who met the criteria to demonstrate the type of 3VF were selected.
Results:
3VF types were classified as follows: (1) Thinned, (2) thickened, (3) partially erased, (4) globular or herniated, and (5) narrowed.
Conclusion:
Knowledge of anatomical variations of the 3VF is paramount for ETV and it influences the success rate of the procedure.
Keywords: Cerebrospinal fluid shunt, Endoscopic third ventriculostomy, Hydrocephalus, Neuroendoscopy, Ventriculostomy
INTRODUCTION
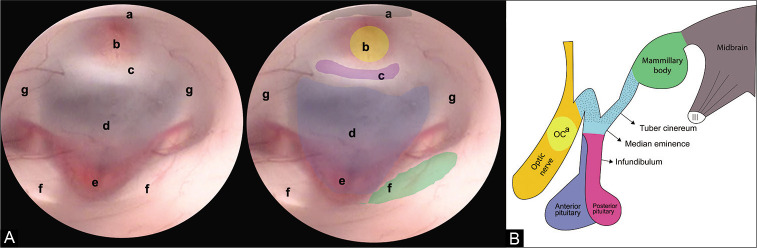
The third ventricle floor (3VF) is a complex anatomical region made up of multiple structures of great importance for endocrinological and executive functions. From rostral to caudal, it involves the supraoptic recess, optic chiasm, infundibulum, tuber cinereum, and mammillary bodies [Figure 1].[17]
Figure 1:
(A) Endoscopic view of the FTV. In this image, the anatomical structures that make up the FTV are recognized, from anterior to posterior we find. (a) Optic chiasm, (b) infundibulum, (c) dorsum sellae, (d) floor of the third ventricle (tuber cinereum), (e) basilar complex, (f) mammillary bodies, and (g) hypothalamic wall. (B) Lateral view of the diencephalon. The structures that make up the floor of the third ventricle can be appreciated. (OCa) Optic chiasm.
First described by Mixter et al. in 1923, the endoscopic third ventriculostomy (ETV) is the technique routinely used for the treatment of obstructive hydrocephalus.[4,5,10,13] ETV was aimed at creating an alternate pathway for the circulation of cerebrospinal fluid (CSF) through a fenestration performed on the 3VF, in which the anatomic knowledge is imperative to successfully perform the procedure. The tuber cinereum is the structure directly related to fenestration, it is composed of nerve fibers that travel from the hypothalamus to the pituitary gland, it is limited anteriorly by the optic chiasm, later by the mammillary bodies, laterally by the hypothalamus, and rests on the basilar artery complex [Figure 1].[1,16,17]
Despite the identification of these anatomical structures, the anatomy of the 3VF is variable. These variations were briefly studied, especially in relation to complications rate and failures of the surgical procedure. In 2016, Sughrue et al. published the first study about the most common variations of the endoscopic anatomy, during ETV, on a series of 50 operated patients.[21] They tried to relate radiological variations dependent on the associated pathology.[3] Even today, we do not know the true implications and other types of variations that may exist and that may have a positive or negative influence on ETV.
On these assumptions, the present image report aims to contribute to the knowledge of endoscopic anatomical variations of the 3VF and its probable incidence and proposes two de novo variants, founded on an extensive series of illustrated cases. The surgical implications of these variations in ETV were further discussed.
MATERIALS AND METHODS
Intraoperative videos of 216 patients undergone ETV between January 2012 and February 2020 at the Hospital Infantil Universitario de San José, Bogotá, Colombia were reviewed. One hundred and eighty patients who met the criteria were chosen to demonstrate the type of 3VF taking into account the quality of the recording. All procedures were performed with Karl Storz ventricular endoscopes (Tuttlingen, Germany).
The videos were reviewed together with the clinical history of each patient, as well as the reports of the surgical procedure, considering their age, sex, history, and whether they required reoperation for the management of hydrocephalus. From each neuroendoscopy video, the 3VF image was captured. From each image obtained, the anatomical structures were identified, labeled, and edited using CorelDRAW Graphics Suite 2020 (Corel TM, Ottawa, Canada).
Beyond a detailed description of the 3VF anatomy [Figure 2], several types of 3VF were detected and classified as follows: (1) Thinned floor [Figure 3A], (2) thickened floor [Figure 3B], (3) partially erased floor [Figure 3C], (4) globose or herniated floor [Figure 3D], and (5) narrow floor [Figure 3E]. The incidence of anatomical variants, independently from the type of 3VF, was also evaluated [Figure 4]. The existence of two additional anatomical variants was proposed [Figure 5].
Figure 2:
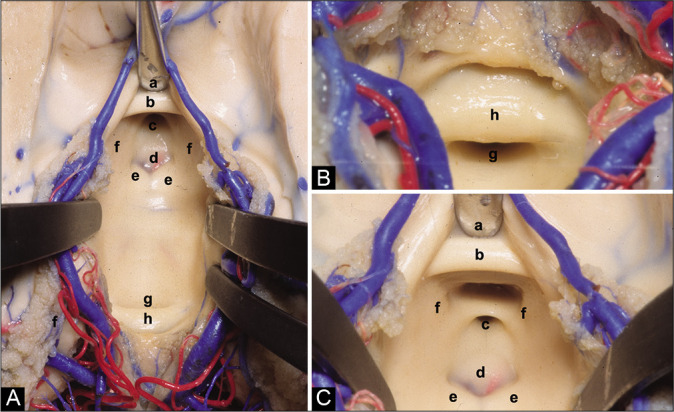
(A) Anatomical superior view of the III ventricle floor. (B) Posterior superior view of the III ventricle floor. (C) Anterior superior view of the III ventricle floor. (a) Chiasmatic recess, (b) anterior commissure, (c) infundibular recess, (d) tuber cinereum, (e) mammillary bodies, (f) medial wall of the hypothalamus, (g) aqueduct, and (h) posterior commissure.
Figure 3:
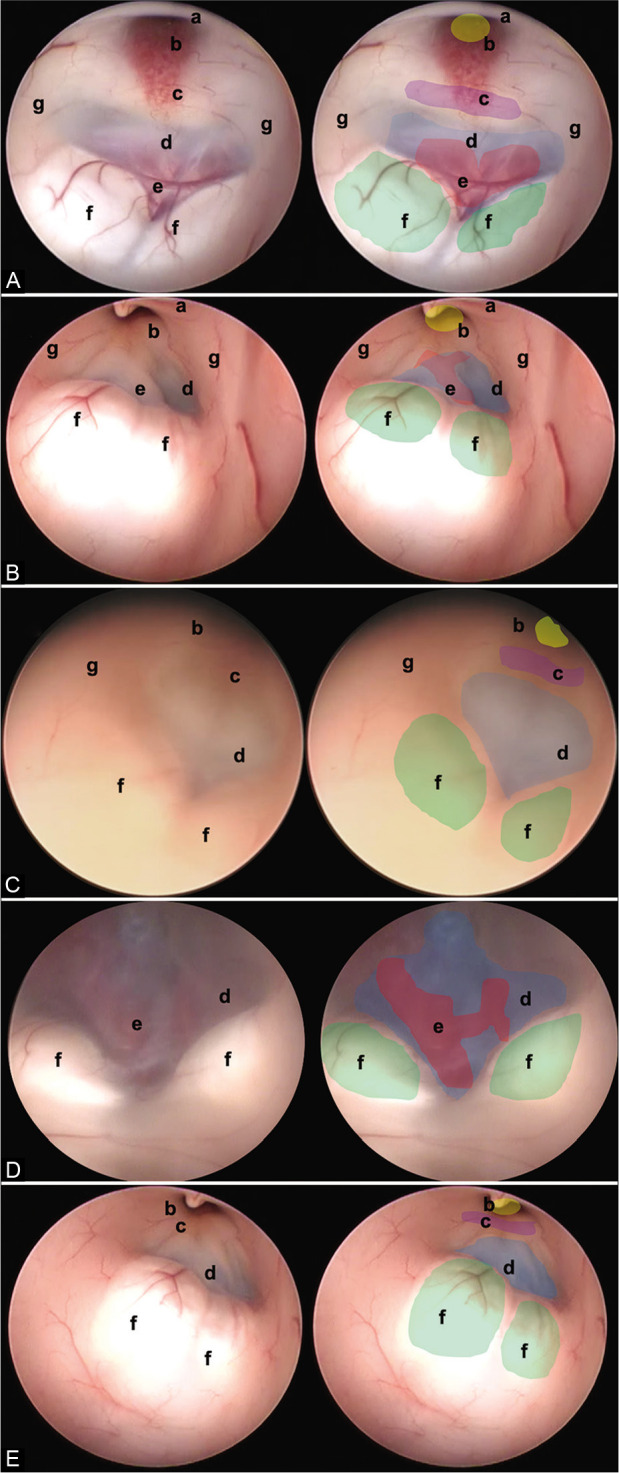
In this figure, it is possible to identify the different endoscopic anatomical variants of the FTV, in each image, a complete view of the ventricle is shown, and next to it is the representation of each of its structures. (A) Thinned floor, (B) thickened floor, (C) cleared floor, (D) globus or herniated floor, and (E) narrow floor. The anatomical structures are found as follows: (a) optic chiasm (black), (b) infundibulum (yellow), (c) dorsum sellae (purple), (d) floor of the third ventricle, (tuber cinereum) (blue), (e) basilar complex (red), (f) mammillary bodies (green), and (g) hypothalamic wall.
Figure 4:
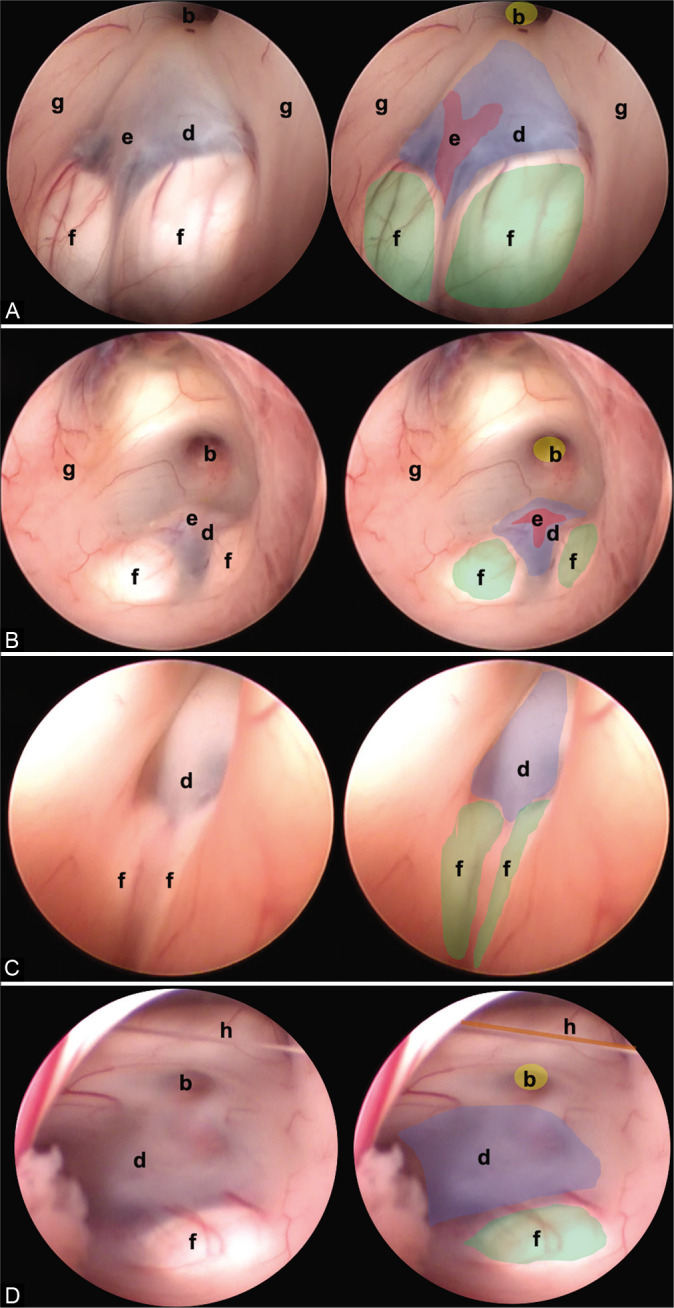
Other anatomic variants independent of the type of TVF can be identified. (A) Separate mammillary bodies, (B) the prominent basilar artery, (C) elongated third ventricle, and (D) interthalamic bands. The anatomical structures are found as follows: (b) infundibulum (yellow), (d) floor of the third ventricle (tuber cinereum) (blue), (e) basilar complex (red), (f) mammillary bodies (green), and (g) hypothalamic wall.
Figure 5:
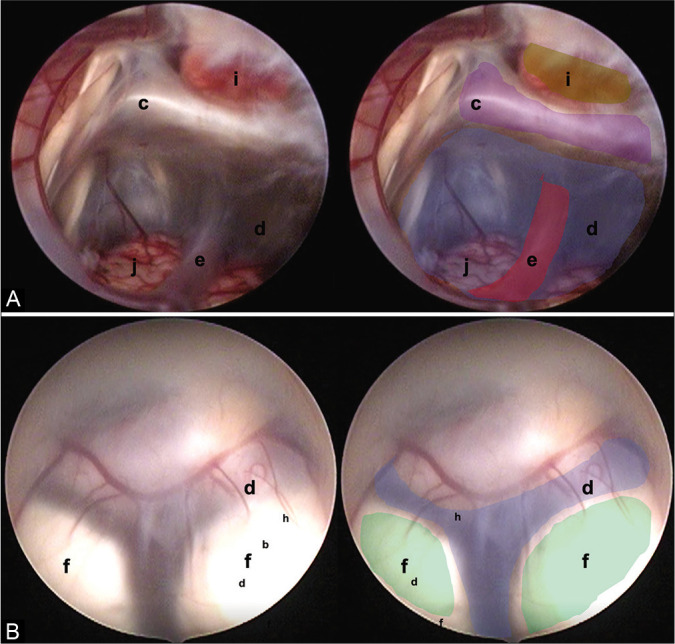
Two endoscopic anatomical variants are presented that we consider are not reported in the medical literature and that can constitute a surgical challenge for the surgeon in charge. (A) Translucent floor, (B) Y-floor. Anatomical structures are found as follows: (c) Dorsum sellae (purple), (d) floor of the third ventricle (tuber cinereum) (blue), (e) basilar complex (red), (f) mammillary bodies (green), (i) pituitary, and (j) brainstem.
The statistical analysis was carried out using the Epi-Info 7.2.3.1 software.
RESULTS
Of the 180 operated patients, 57% were women, and 49% were in the context of acute hydrocephalus. It was observed that 22% of the patients had thinned floors and its association with chronic hydrocephalus was estimated at 37%. The thickened floor represented 53% of our series and a relationship of 47% was estimated with patients previously operated on. The globose floor was only present in 6% of the patients, the partially erased floor in 7% of the patients, and the configuration narrow floor was found in 12% of patients. The reoperation rate was 13%. The number of patients with ventricular bypass systems before 3VF was 56.49%.
DISCUSSION
Understanding the neurosurgical anatomy and its variations are of great importance for neurosurgical practice.[7-9,15] The detailed anatomy and different types of 3VF were previously described.[2,21] In the present image report, we presented the main variations found in our case series.
Anatomic variations of 3VF
Thinned floor
Thinned 3VF is characterized by a thin and transparent membrane and may occur in patients with chronic hydrocephalus.[11] It is believed that due to the constant vector pressure toward the 3VF in obstructive hydrocephalus, the floor decreases in thickness. This allows the visualization of the underlying structures in fenestrations of the 3VF, one could consider a lower risk of vascular lesions or failures in the correct communication of the third ventricle with the cisterns. In our study, it was found that 37% of patients with chronic hydrocephalus had a thinned floor [Figure 3A].
Thickened floor
A 3VF with a thick membrane associated with inflammatory pathologies or long-standing hydrocephalus processes was found.[12] In the series presented, it was found in 18% of patients with chronic hydrocephalus and up to 38% of patients with a history of shunting, so it can be theorized that ependymal inflammatory changes secondary to a foreign body in the ventricle may lead to an increase in the thickness of the 3VF floor. Furthermore, in some series, no relationship has been found between the thickness of the 3VF and reoperations.[14] In our study, 47% of reoperated patients were found to have a thickened 3VF floor. Regarding surgical implications, the time and difficulty of performing a fenestration may increase due to the risk of vascular rupture or incomplete communication and the surgeon must be experienced in the management of this type of variation [Figure 3B].
Partially erased floor
There is only one description of this type of anatomical variation in the literature.[21] The endoscopic characteristics are particular and of great importance for the performance of fenestration. In our series, 12 patients (6.4%) had this variation and 10 (83%) had chronic hydrocephalus, so it can be said that the pathophysiology of the presence of this variation is related to the increased pressure on the 3VF that produces changes in transparency and neural lining. No reoperations were recorded in patients with this variation [Figure 3C].
Globose or herniated floor
This kind of variant of 3VF was the most infrequent in our series and had little relation to the presence of chronic hydrocephalus (7%) although it is described as a cause due to pressure changes in the third ventricle. Some authors show ballooning of the 3VF after perforation of the tuber cinereum[11] or excess Ringer’s lactate irrigation during the procedure.[22] Due to the anatomical layout, fenestration can be difficult, and the surgeon’s expertise is needed to achieve effective communication with the cisterns since relationships are lost on the globose floor, and visualization of the great vessels can be difficult [Figure 3D].
Narrow floor
The intraoperative image shows a variation in which the fenestration space is reduced. The prepontine cistern is the ventral limit of the 3VF and contains the basilar artery. The prepontine interval has been described as an important consideration at the time of surgery.[20,21] In the authors’ experience, a meticulous preoperative evaluation of the images is considered to relate to the anatomy of the basilar artery and avoid accidents at the time of communication, as well as performing the fenestration in the most rostral part of the tuber cinereum and posterior to the dorsum sellae.[20] The relationship between this variant and hydrocephalus is not clear, and in our series, the percentage of presentation is low [Figure 3E].
Variable features of the 3VF
In addition, other variable features of the 3VF were found.
Separate mammillary bodies
Separate mammillary bodies constitute a challenge for the surgeon as they can be mistaken for the tuber cinereum. This may lead to hypothalamic damage at the time of fenestration [Figure 4A].
The prominent basilar artery
The prominent basilar artery occurs in the middle of the tuber cinereum and its herniation, among some communicating vessels, reduces space for fenestration [Figure 4B].[6]
Elongated third ventricle
In this type of variant, the anteroposterior diameter of the ventricular cavity is elongated. This anomaly has been described in patients with hydrocephalus.[19] During the surgical procedure, delicate handling of the endoscope is recommended to avoid damage to the fornix or thalamostriate veins, as well as special care in communication to avoid hypothalamic compromise [Figure 4C].[18]
Interthalamic bands
Dystopic interthalamic communications have been found that do not pose additional difficulty to the procedure. During the review of intraoperative videos, two new variants were found that have not been described in the literature to date [Figure 4D].
Translucent floor: This variant was observed in one patient [Figure 5A] and an almost invisible 3VF was found due to a thin arachnoid layer that was fenestrated without complications.
Y-floor: In this variant, there is an increased intermamillary distance and a small prepontine space, with abnormal vasculature that can lead to vascular accidents if there is insufficient experience [Figure 5B].
CONCLUSION
Neurosurgeons should recognize the variations of 3VF. Many have been described and this study shows the most common and two new and rare ones. Further studies are needed to determine the relationship between the variations and the operative outcome of patients, as well as to predict the effectiveness of endoscopic fenestration.
Footnotes
How to cite this article: Abdala-Vargas NJ, Cifuentes-Lobelo HA, Ordonez-Rubiano E, Patiño-Gomez JG, Villalonga JF, Lucifero AG, et al. Anatomic variations of the floor of the third ventricle: Surgical implications for endoscopic third ventriculostomy. Surg Neurol Int 2022;13:218.
Contributor Information
Nadin J. Abdala-Vargas, Email: abdalaneurosurgery@gmail.com.
Hernando A. Cifuentes-Lobelo, Email: hernando.cifuentes@gmail.com.
Edgar Ordoñez-Rubiano, Email: egordonez@fucsalud.edu.co.
Javier G. Patiño-Gomez, Email: javierpati@gmail.com.
Juan F. Villalonga, Email: jfvillalonga@gmail.com.
Alice Giotta Lucifero, Email: alicelucifero@gmail.com.
Alvaro Campero, Email: alvarocampero@yahoo.com.ar.
Valeria Forlizzi, Email: vforlizzi@fmed.uba.ar.
Matías Baldoncini, Email: drbaldoncinimatias@gmail.com.
Sabino Luzzi, Email: sabino.luzzi@unipv.it.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anik I, Ceylan S, Koc K, Tugasaygi M, Sirin G, Gazioglu N, et al. Microsurgical and endoscopic anatomy of Liliequist’s membrane and the prepontine membranes: Cadaveric study and clinical implications. Acta Neurochir (Wien) 2011;153:1701–11. doi: 10.1007/s00701-011-0978-5. [DOI] [PubMed] [Google Scholar]
- 2.Aydin S, Yilmazlar S, Aker S, Korfali E. Anatomy of the floor of the third ventricle in relation to endoscopic ventriculostomy. Clin Anat. 2009;22:916–24. doi: 10.1002/ca.20867. [DOI] [PubMed] [Google Scholar]
- 3.Corrales M, Torrealba G. The third ventricle. Normal anatomy and changes in some pathological conditions. Neuroradiology. 1976;11:271–7. doi: 10.1007/BF00328385. [DOI] [PubMed] [Google Scholar]
- 4.Demerdash A, Rocque BG, Johnston J, Rozzelle CJ, Yalcin B, Oskouian R, et al. Endoscopic third ventriculostomy: A historical review. Br J Neurosurg. 2017;31:28–32. doi: 10.1080/02688697.2016.1245848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dezena RA. 1st ed. Berlin, Germany: Springer International Publishing; 2017. Atlas of Endoscopic Neurosurgery of the Third Ventricle. [Google Scholar]
- 6.Fabiano AJ, Leonardo J, Grand W. Posterior cerebral artery P1 segment at the stoma during endoscopic third ventriculostomy in adults. J Neurol Neurosurg Psychiatry. 2010;81:374–8. doi: 10.1136/jnnp.2009.177360. [DOI] [PubMed] [Google Scholar]
- 7.Giotta Lucifero A, Baldoncini M, Bruno N, Tartaglia N, Ambrosi A, Marseglia GL, et al. Microsurgical neurovascular anatomy of the brain: The anterior circulation (Part I) Acta Biomed. 2021;92:e2021412. doi: 10.23750/abm.v92iS4.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giotta Lucifero A, Baldoncini M, Bruno N, Tartaglia N, Ambrosi A, Marseglia GL, et al. Microsurgical neurovascular anatomy of the brain: The posterior circulation (Part II) Acta Biomed. 2021;92:e2021413. doi: 10.23750/abm.v92iS4.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giotta Lucifero A, Fernandez-Miranda JC, Nunez M, Bruno N, Tartaglia N, Ambrosi A, et al. The modular concept in skull base surgery: Anatomical basis of the median, Paramedian and lateral corridors. Acta Biomed. 2021;92:e2021411. doi: 10.23750/abm.v92iS4.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzman R, Pendharkar AV, Zerah M, Sainte-Rose C. Use of the NeuroBalloon catheter for endoscopic third ventriculostomy. J Neurosurg Pediatr. 2013;11:302–6. doi: 10.3171/2012.10.PEDS11159. [DOI] [PubMed] [Google Scholar]
- 11.Hellwig D, Grotenhuis JA, Tirakotai W, Riegel T, Schulte DM, Bauer BL, et al. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev. 2005;28:1–34. doi: 10.1007/s10143-004-0365-2. [DOI] [PubMed] [Google Scholar]
- 12.Iaccarino C, Tedeschi E, Rapanà A, Massarelli I, Belfiore G, Quarantelli M, et al. Is the distance between mammillary bodies predictive of a thickened third ventricle floor? J Neurosurg. 2009;110:852–7. doi: 10.3171/2008.4.17539. [DOI] [PubMed] [Google Scholar]
- 13.Jallo GI, Kothbauer KF, Abbott IR. Endoscopic third ventriculostomy. Neurosurg Focus. 2005;19:E11. [PubMed] [Google Scholar]
- 14.Kombogiorgas D, Sgouros S. Assessment of the influence of operative factors in the success of endoscopic third ventriculostomy in children. Childs Nerv Syst. 2006;22:1256–62. doi: 10.1007/s00381-006-0072-0. [DOI] [PubMed] [Google Scholar]
- 15.Luzzi S, Maestro MD, Elia A, Vincitorio F, Perna GD, Zenga F, et al. Morphometric and radiomorphometric study of the correlation between the foramen magnum region and the anterior and posterolateral approaches to ventral intradural lesions. Turk Neurosurg. 2019;29:875–86. doi: 10.5137/1019-5149.JTN.26052-19.2. [DOI] [PubMed] [Google Scholar]
- 16.Resch KD, Perneczky A, Tschabitscher M, Kindel S. Endoscopic anatomy of the ventricles. Acta Neurochir Suppl. 1994;61:57–61. doi: 10.1007/978-3-7091-6908-7_10. [DOI] [PubMed] [Google Scholar]
- 17.Rhoton AL. The lateral and third ventricles. Neurosurgery. 2002;51:S207–71. [PubMed] [Google Scholar]
- 18.Rohde V, Gilsbach JM. Anomalies and variants of the endoscopic anatomy for third ventriculostomy. Minim Invasive Neurosurg. 2000;43:111–7. doi: 10.1055/s-2000-8330. [DOI] [PubMed] [Google Scholar]
- 19.Rohde V, Krombach GA, Struffert T, Gilsbach JM. Virtual MRI endoscopy: Detection of anomalies of the ventricular anatomy and its possible role as a presurgical planning tool for endoscopic third ventriculostomy. Acta Neurochir (Wien) 2001;143:1085–91. doi: 10.1007/s007010100000. [DOI] [PubMed] [Google Scholar]
- 20.Souweidane MM, Morgenstern PF, Kang S, Tsiouris AJ, Roth J. Endoscopic third ventriculostomy in patients with a diminished prepontine interval. J Neurosurg Pediatr. 2010;5:250–4. doi: 10.3171/2009.10.PEDS09187. [DOI] [PubMed] [Google Scholar]
- 21.Sughrue ME, Chiou J, Burks JD, Bonney PA, Teo C. Anatomic variations of the floor of the third ventricle: An endoscopic study. World Neurosurg. 2016;90:211–27. doi: 10.1016/j.wneu.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 22.van Aalst J, Beuls EA, van Nie FA, Vles JS, Cornips EM. Acute distortion of the anatomy of the third ventricle during third ventriculostomy. Report of four cases. J Neurosurg. 2002;96:597–9. doi: 10.3171/jns.2002.96.3.0597. [DOI] [PubMed] [Google Scholar]