Abstract
In order to exploit the ability of anaerobic bacteria to degrade certain contaminants for bioremediation of polluted subsurface environments, we need to understand the mechanisms by which such bacteria partition between aqueous and solid phases, as well as the environmental conditions that influence partitioning. We studied four strictly anaerobic bacteria, Desulfomonile tiedjei, Syntrophomonas wolfei, Syntrophobacter wolinii, and Desulfovibrio sp. strain G11, which theoretically together can constitute a tetrachloroethylene- and trichloroethylene-dechlorinating consortium. Adhesion of these organisms was evaluated by microscopic determination of the numbers of cells that attached to glass coverslips exposed to cell suspensions under anaerobic conditions. We studied the effects of the growth phase of the organisms on adhesion, as well as the influence of electrostatic and hydrophobic properties of the substratum. Results indicate that S. wolfei adheres in considerably higher numbers to glass surfaces than the other three organisms. Starvation greatly decreases adhesion of S. wolfei and Desulfovibrio sp. strain G11 but seems to have less of an effect on the adhesion of the other bacteria. The presence of Fe3+ on the substratum, which would be electropositive, significantly increased the adhesion of S. wolfei, whereas the presence of silicon hydrophobic groups decreased the numbers of attached cells of all species. Measurements of transport of cells through hydrophobic-interaction and electrostatic-interaction columns indicated that all four species had negatively charged cell surfaces and that D. tiedjei and Desulfovibrio sp. strain G11 possessed some hydrophobic cell surface properties. These findings are an early step toward understanding the dynamic attachment of anaerobic bacteria in anoxic environments.
In polluted subsurface systems, the movement and degradation of contaminants cause changes in the environmental conditions under which subsurface microorganisms live. Numerous studies have shown that environmental factors, such as nutrient availability (23), ionic strength, and dissolved solutes (14–18), influence the attachment of bacteria to solid surfaces. Similarly, changes in the concentration and composition of contaminants are expected to influence attachment of biodegradative bacteria in polluted subsurface environments. Understanding the significance of environmental conditions and the mechanisms by which biodegradative microorganisms partition between the aqueous and solid phases is a critical requirement for the design and evaluation of in situ bioremediation efforts. Whereas the relationship between bacterial adhesion and transport has been studied intensively with aerobic bacteria (3, 6, 14–16, 22, 26, 33), much less is known about the adhesive characteristics and transport of anaerobic bacteria. So far, the adhesion of anaerobic bacteria has been studied primarily in anaerobic bioreactors (25) and in the formation of dental plaque (20). From a bioremediation point of view, however, it is the anaerobic bacteria that have the potential for biological cleanup of subsurface systems polluted with chlorinated hydrocarbons, such as tetrachloroethylene (PCE) and trichloroethylene (TCE) (5, 8, 9). These solvents are among the most commonly reported contaminants at DOE facilities and waste sites (28), and in situ bioremediation is an attractive solution for dealing with these large volumes of contaminated soils and groundwater.
This study is part of a larger, multidisciplinary project that studies the transport of both bacteria and contaminants through the anaerobic subsurface environment. The present work represents the initial characterization of the adhesion of four anaerobic microorganisms, Desulfomonile tiedjei, Syntrophomonas wolfei, Syntrophobacter wolinii, and Desulfovibrio sp. strain G11. These four species were selected as model organisms, representative of actual anaerobic subsurface microorganisms, because together they can constitute a hypothetical PCE- and TCE-dechlorinating consortium. D. tiedjei was chosen as the PCE- and TCE-dechlorinating organism, because it can use these chlorinated compounds as terminal electron acceptors (4, 10). However, because D. tiedjei cannot use PCE or TCE as a carbon source, it depends on other subsurface microorganisms for a growth substrate. S. wolfei and S. wolinii were chosen because they can provide D. tiedjei with a carbon source, such as formate, necessary for energy and growth. These syntrophic fatty acid-oxidizing organisms can produce formate when growing syntrophically with an H2-consuming organism such as Desulfovibrio (1, 2, 24). Desulfovibrio sp. strain G11 was selected as a model organism not only because it can function as an H2 consumer in syntrophic cultures with fatty acid oxidizers but also because it uses sulfate, as well as formate, and may thus compete with D. tiedjei for these compounds. Competition for electron acceptor and carbon sources is most likely a common phenomenon in the subsurface environment.
In this study, we investigated the effects of certain physiological and environmental conditions, as well as the influence of specific physicochemical characteristics of the substratum, on the adhesion of pure cultures of these organisms to solid surfaces. The results are an early step toward a better understanding of the dynamic attachment and detachment of anaerobic bacteria, and thereby their transport through the subsurface, and illustrate the diversity in strategies employed by bacteria to attach to solid surfaces.
MATERIALS AND METHODS
Organisms and culture conditions.
All organisms used in this study were obtained from D. R. Boone, Director of the Subsurface Microbial Culture Collection, at the Oregon Graduate Institute, Beaverton. D. tiedjei and S. wolinii were cultured in a bicarbonate-buffered medium described elsewhere (2). S. wolfei LYB (2) and Desulfovibrio sp. strain G11 were cultured in a derivative of this medium that has decreased concentrations of Trypticase peptone and yeast extract (0.5 g per liter) and increased sodium sulfide concentration (0.5 g per liter) and that does not contain mercaptoethanesulfonate. For Desulfovibrio sp. strain G11, 5 mM Na2SO4 was added to the medium, serving as the electron acceptor. Growth media were supplemented with a vitamin solution (34). For D. tiedjei, niacinamide (Sigma, St. Louis, Mo.) and 1,4-naphthoquinone (Aldrich, Milwaukee, Wis.) were also added to final concentrations of 500 and 200 μg/liter, respectively (7). Growth substrates used for the different organisms were pyruvate (D. tiedjei), formate (Desulfovibrio sp. strain G11), fumarate (S. wolinii), and crotonate (S. wolfei), all at a concentration of 20 mM. Cultures were grown in volumes of 50 ml in 160-ml serum bottles under a CO2-N2 (3:7) atmosphere, without shaking, at 37°C. Because of the slow growth rates of most of these organisms and the typically low cell yield of 50-ml batch cultures, an experimental method that minimized the volume of suspended cells needed for adequate exposure of test surfaces to cell suspensions was designed (see below).
Preparation of surfaces.
The surfaces used in these experiments were small circular coverslips (12 mm in diameter; Fisher Scientific, Pittsburgh, Pa.). After acid washing for 12 to 24 h in 1:1 concentrated HCl-HNO3, the surfaces were rinsed in sterile deionized water and air dried. Hydrophobic surfaces were obtained by dipping clean coverslips into a siliconizing agent (Sigmacote; Sigma). Electropositively charged surfaces were obtained by coating clean coverslips with amorphous iron, following a modification of the procedure described by Scheidegger et al. (30). In short, clean coverslips were incubated in a 0.24 M solution of FeCl3 · 6H2O at pH 7.5 for 12 h under gentle agitation, washed several times in distilled water followed by 0.1 mM NaCl, and air dried. The resulting surfaces had a yellow color which was sufficiently optically transparent to allow light microscopy. The surfaces were held in rubber disks which were cut to size from flanged, slotted butyl rubber serum bottle stoppers (Fisher Scientific). Before use, the disks were kept in water for 24 h to leach any organic compounds and sterilized in 95% ethanol. These disks were designed to fit into the small test vials described below and held four surfaces each.
Anaerobic adhesion assays.
Cells were harvested by centrifugation (8 min at 9,000 × g) either during the logarithmic phase of growth or a few days after stationary phase had started. To maintain anaerobic conditions, culture bottles and centrifuge tubes were only opened inside an anaerobic chamber (Coy, Grass Lake, Mich.). Plastic centrifuge tubes (Nalge Nunc, Milwaukee, Wis.; type Oak Ridge), assay vials, and test surfaces were stored inside the anaerobic chamber for at least a week before use to ensure desorption of oxygen. Cells were washed and resuspended in either culture medium or a dilute mineral salt nutrient solution (MSNS) (26) reduced with sodium sulfide (0.5 g per liter). The cell density of the washed cell suspension was determined microscopically with a counting chamber (Weber Scientific International, Lancing, England) and then adjusted to a density of 0.3 × 109 to 109 cells/ml, unless otherwise noted. Rubber disks holding four test surfaces were inserted into the test vials (Fisherbrand shell vials; 15 by 45 mm; Fisher Scientific), and 5 ml of cell suspension was used to completely fill each vial. The vials were closed with neoprene rubber stoppers (Bellco Glass, Vineland, N.J.). A needle inserted in the stopper prevented any bubbles from being caught under the stopper during closing. The closed vials were attached to a shaker-rotator (Barnstead/Thermolyne, Dubuque, Iowa) and continuously rotated throughout the incubation to prevent settling of the cells by gravitation. Incubation was typically for 3 h, unless otherwise noted. After incubation, the vials were removed from the anaerobic chamber and opened, and formalin was added to a final concentration of 5% (vol/vol) to fix the cells. The surfaces were then rinsed with 25 ml of water to remove loosely attached bacteria. During rinsing, the surfaces were prevented from being exposed to the air-water interface by running rinse water through the vials by means of inlet and outlet ports in a tightly fitting rubber stopper connected with tubing to a peristaltic pump (12). After being rinsed, the surfaces were air dried and stained for 5 s in a crystal violet solution (Hucker formula) for easy microscopic observation. Staining is unnecessary for D. tiedjei cells because of their relatively large size (up to 10 μm). The surfaces were fixed to microscope slides with clear nail polish. Attached cells were counted microscopically under oil immersion (microscope model CH30; Olympus, Lake Success, N.Y.). A minimum of 10 areas of 100 μm2 were counted for each surface. All of the experiments were repeated at least twice.
Aerobic adhesion assays.
To determine the effect of aerobic conditions on the adhesion of the organisms, anaerobic cell suspensions were prepared as described above. Then part of the suspension was removed from the anaerobic chamber and transferred to test vials (Fisherbrand shell vials; 24 by 85 mm; Fisher Scientific). A minimum of 6 ml of cell suspension was needed to completely submerge the larger test surfaces (18 mm in diameter) used for aerobic assays. With sterile needles, air was bubbled through the suspensions throughout the incubation. As control experiments, anaerobic adhesion assays were performed simultaneously with the remainder of the suspension.
Anaerobic detachment assays.
Cells were allowed to attach to surfaces for 3 h, as described above. After the attachment period, the cells in the control vials were fixed with formalin and loosely attached cells were removed by rinsing with MSNS, as described above. All other surfaces were rinsed with reduced MSNS and left in this dilute salt solution inside the anaerobic chamber for up to 4 h. During the detachment period, the closed vials were rotated, as described above. At time intervals ranging from 8 to 30 min, cells which remained attached to the surfaces were fixed with formalin, after which the surfaces were rinsed once more to remove any loosely attached cells still present. During the detachment experiments, the vials were not removed from the anaerobic chamber.
Characterization of cell surface properties. (i) HIC.
Relative cell surface hydrophobicity was determined by hydrophobic interaction chromatography (HIC) following an adaptation (21) of the method first described by Smyth et al. (31). Glass wool-plugged Pasteur pipettes were rinsed with 5 ml of 95% ethanol before the resin (1 ml) was applied. Octyl Sepharose CL-4B was used as the hydrophobic resin, with Sepharose CL-4B as the control (Sigma). The columns were equilibrated with 5 ml of sterile 4 M NaCl buffered with 10 mM phosphate buffer at pH 7.4. Anaerobic assays were performed in an anaerobic chamber with oxygen-free, sterile solutions. Columns to be used for anaerobic determinations were placed in the anaerobic chamber for at least 1 week before use. Aliquots of 200 μl of concentrated, washed bacterial cells (approximate density, 0.5 × 1010 to 1010 cells/ml) were added to the tops of the columns, allowed to drain into the column bed, and equilibrated for 15 min. The columns were then eluted with 4 M NaCl buffered with 10 mM phosphate buffer. Five fractions of 1.5 ml were collected, and the optical densities at 590 nm (OD590) of the fractions were measured spectrophotometrically (Spectronic 710; Milton Roy Co.). The number of cells present in each fraction was calculated from the OD590 value, with calibration curves prepared for each bacterial species. For these calibration curves, the cell concentrations of suspensions of various OD were determined microscopically with a counting chamber (Weber Scientific International). All assays were performed in duplicate. Hydrophobic interactions were expressed as the percentage of retention to the octyl Sepharose column relative to the control column (Sepharose CL-4B).
(ii) EIC.
Relative surface charge was assayed by an adaptation (21) of the method described by Pedersen (27). DEAE-Sepharose CL-6B was used as an anion-exchange resin, Carboxymethyl Sepharose CL-6B was used as a cation-exchange resin, and Sepharose CL-6B was used as a control (Sigma). EIC columns were prepared as described above for HIC columns and equilibrated with 5 ml of sterile 0.2 M phosphate buffer at pH 7.4. Application of cells, elution, and analysis of the eluted fractions were performed as described for HIC. Electrostatic interactions were expressed as the percentage of retention to the positively (DEAE) or the negatively (carboxymethyl) charged resin relative to that for the control column (Sepharose CL-6B; Sigma).
(iii) Cell surface hydrophobicity.
The hydrophobicities of the surfaces of D. tiedjei cells at different times during growth were determined by the bacterial adhesion to hydrocarbons (BATH) method (29). Anaerobic cultures were sampled during growth, and the cells were washed in 10 mM phosphate buffer to remove the pink color of the resazurin and then resuspended to the original volume. The OD590 of the suspension was measured before and after mixing it with hexadecane (1:5 [vol/vol]; Aldrich). The relative hydrophobicity of the cells was expressed as the percent decrease in OD of the aqueous cell suspension after the partitioning of the cells into the organic phase.
(iv) Flagella.
Flagella on cells of Desulfovibrio sp. strain G11 and S. wolfei were visualized for light microscopy with the SpotTest flagellum stain (Difco, Detroit, Mich.).
RESULTS
Comparison of different organisms.
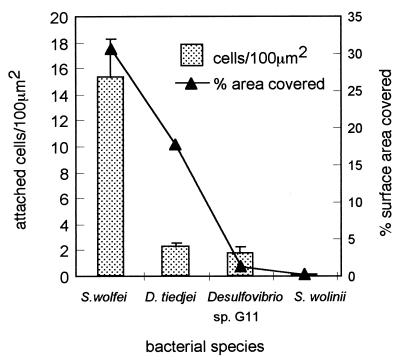
The four anaerobic organisms tested in this study adhered to clean glass surfaces to different degrees, with S. wolfei adhering in the largest numbers (Fig. 1). In S. wolfei suspensions of 109 cells/ml, typically 15.3 ± 3 cells attached per 100 μm2. With an estimated average cell size of 1 by 2 μm, and thus a “footprint” of 2 μm2, this number of cells covers approximately 30.5% of the surface. The adhesion potential of this organism was already apparent during culture; a large proportion of the cells were firmly attached to the walls of the culture bottles and could not be removed by vigorous shaking, only by swabbing. Determination of the numbers of cells removed by swabbing the inside of the culture bottles indicated that they accounted for approximately 30% of the total number of cells. Even though D. tiedjei cells attach in much lower numbers to glass surfaces (typically 2.2 ± 0.33 cells per 100 μm2 in suspensions of 109 cells/ml), these cells cover up to 18% of the surface because of their large average size of approximately 8 μm2. Whereas S. wolfei cells attach to surfaces in a rather uniform manner, D. tiedjei cells were often seen to attach in groups, with cells lying across each other. Both Desulfovibrio sp. strain G11 cells and S. wolinii cells adhere only in low numbers, and these small cells cover only a small percentage of the surface. When adhesion assays were performed under static conditions, settling played a major role in the adhesion kinetics of D. tiedjei. When surfaces were placed even at the slightest angle, the side facing up would accumulate large numbers of attached cells, whereas the side facing down would accumulate few cells. Settling did not appear to play a major role in the adhesion of the other organisms tested. All data presented here were obtained from adhesion assays in which settling of cells was prevented by continuous rotation of the assay vials.
FIG. 1.
Adhesion of anaerobic bacteria to solid surfaces. Clean glass surfaces were exposed to anaerobic suspensions of bacterial cells in culture medium (density, 109 cells/ml) for 3 h. Attached cells were fixed with formalin and counted microscopically as described in Materials and Methods. The approximate percentages of the surface area covered by the different numbers of attached bacteria were calculated from the average cell sizes: S. wolfei, 2 μm2 (1 by 2 μm); D. tiedjei, 8 μm2 (1 by 8 μm); Desulfovibrio sp. strain G11, 0.75 μm2 (0.5 by 1.5 μm2); and S. wolinii, 3 μm2 (1 by 3 μm). Standard deviations are indicated by error bars.
Effect of substratum properties.
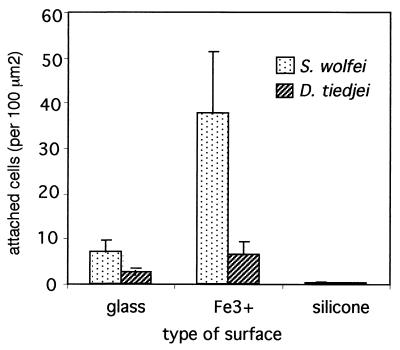
The presence of electropositive determinants on the substratum, in the form of Fe3+, was shown to affect the adhesion of S. wolfei and D. tiedjei cells. The effect was particularly striking for S. wolfei (Fig. 2). When S. wolfei cells were used at concentrations of 109 cells per ml or higher, the bacteria accumulated to such high numbers that they almost entirely covered Fe3+-coated surfaces with a uniform monolayer of cells, which corresponded to approximately 65 ± 5 cells per 100 μm2 (data not shown). Hydrophobic determinants, on the other hand, greatly decreased adhesion of S. wolfei cells, resulting in only 0.2 cells per 100 μm2 when the surfaces were exposed to 5 × 108 cells per ml. A similar trend was observed with cells of D. tiedjei. The presence of electropositive groups on the substratum resulted in an increase in the number of attached D. tiedjei cells, though not to the extent observed with S. wolfei. Adhesion of S. wolinii and Desulfovibrio sp. strain G11 was not significantly affected by the presence of iron on the surfaces (data not shown). Adhesion of these two organisms to silicone-coated surfaces was not tested because of their overall low potential for adhesion to regular glass surfaces (Fig. 1).
FIG. 2.
Effect of substratum properties on adhesion of S. wolfei and D. tiedjei cells. Glass surfaces, untreated or coated with Fe3+ or silicone, were exposed to anaerobic suspensions of actively growing cells in a mineral salts solution (density, 3.5 × 108 to 5 × 108 cells/ml) for 3 h. Attached cells were fixed with formalin and counted microscopically as described in Materials and Methods. Standard deviations are indicated by error bars.
Effect of physiological condition of the cells.
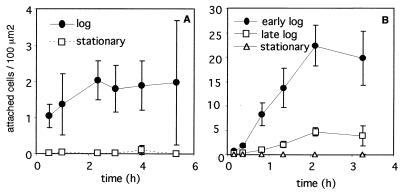
The growth phase of some of the tested anaerobes had a significant effect on their ability to adhere to solid surfaces. Desulfovibrio sp. strain G11, which adheres only in small numbers to solid surfaces when it is in log phase, does not adhere at all when the cells are in stationary phase (Fig. 3). Microscopic observations demonstrated that log-phase cells were highly motile, whereas stationary-phase cells were nonmotile. Desulfovibrio sp. strain G11 cells did not continue to accumulate on the surface over time after an initial attachment period of 2 h but appeared to reach a saturation level of attached cells (Fig. 3A). The density of the cell suspension did not greatly affect the number of cells which attached over time, and even at cell densities as low as 3 × 108 cells/ml, the average number of adhered G11 cells was 1 ± 0.5 per 100 μm2 (data not shown).
FIG. 3.
Effect of the growth phase of cells on adhesion to solid surfaces. Clean glass surfaces were exposed to anaerobic suspensions of Desulfovibrio sp. strain G11 cells (A) (density, 1.2 × 109 cells/ml) or S. wolfei cells (B) (density, 5 × 108 cells/ml) at different phases of batch growth for 3.3 to 5.3 h. The log-phase cells were supplied with 20 mM formate (A) or crotonate (B). Attached cells were fixed with formalin and counted microscopically as described in Materials and Methods. Standard deviations are indicated by error bars.
Adhesion of S. wolfei cells, which, among the organisms tested, adhere in the largest numbers to solid surfaces, was greatly affected by the growth phase of the cells. It could be shown that the ability of these organisms to adhere to solid surfaces decreased with culture age and became negligible once growth substrates were depleted (Fig. 3B). Similar to G11 cells, S. wolfei cells continued to accumulate on the surface over time, until the number of cells attached to the surface reached a maximum level after two or more hours. Active S. wolfei cells adhered to the surfaces whether a carbon source for growth was present or absent, and the presence of a growth substrate appeared to slightly increase the numbers of attached cells (data not shown). S. wolfei cells possess several laterally inserted flagella but exhibit only sluggish twitching motility (24). Flagella could be visualized by specific staining of both log-phase and stationary-phase cells (data not shown). The growth phase of D. tiedjei and S. wolinii cells did not significantly affect the adhesion of these organisms to solid surfaces (data not shown).
The addition of sodium azide to suspensions of actively growing S. wolfei cells almost completely inhibited the ability of these cells to adhere to solid surfaces. This metabolic inhibitor did not have a significant effect, however, on the adhesion of D. tiedjei and Desulfovibrio sp. strain G11 cells (data not shown).
Effect of exposure to aerobic conditions.
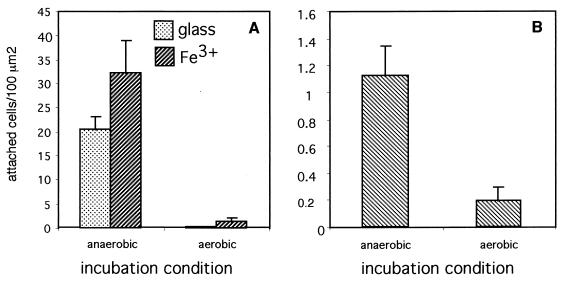
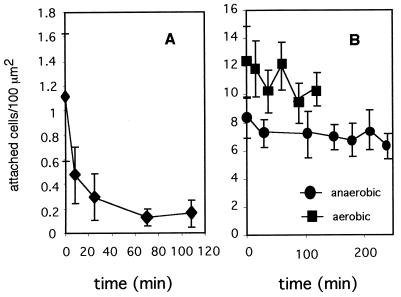
Aerobic conditions greatly decreased the ability of S. wolfei cells to adhere to solid surfaces, resulting in few attached cells (Fig. 4A). Exposure to air also had a negative effect on the adhesion of Desulfovibrio sp. strain G11 cells (Fig. 4B) but did not significantly affect the adhesion of the other test species.
FIG. 4.
Effect of aeration on the adhesion of anaerobic bacteria. Clean glass surfaces were exposed to suspensions of log-phase S. wolfei (A) or Desulfovibrio sp. strain G11 (B) cells in dilute mineral medium (density, 109 cells/ml) for 3 h under aerobic or anaerobic conditions. Attached cells were fixed with formalin and counted microscopically as described in Materials and Methods. Standard deviations are indicated by error bars.
Detachment assays.
To investigate whether attached cells could detach from surfaces, coverslips with attached cells were placed in cell-free anaerobic mineral medium for different time intervals. Attached cells of the different species were shown to detach from solid surfaces at different rates. Desulfovibrio sp. strain G11 cells detached rapidly. More than 60% of the attached cells detached after an 8-min incubation in cell-free medium, and within 1 h, 85% of the initially attached cells had detached (Fig. 5A). S. wolfei cells detached at a much slower rate (Fig. 5B), and on average, only 17% of the cells detached during an incubation period of up to 44 h (data not shown). As mentioned above, these cells adhered tightly to the culture bottles during growth and remained attached to the glass of the bottles even in stationary phase. Aerobic conditions during the detachment phase did not result in altered detachment rates (Fig. 5B). D. tiedjei cells also detached slowly, and even after 2 h, most cells remained attached to the glass surfaces (data not shown).
FIG. 5.
Detachment of anaerobic bacteria. Clean glass surfaces were exposed to anaerobic suspensions of log-phase Desulfovibrio sp. strain G11 (A) or S. wolfei (B) cells in dilute mineral medium (density, 5 × 108 to 1 × 109 cells/ml) for 3 h. After the attachment period, the control surfaces were fixed with formalin. All other surfaces were rinsed with reduced mineral medium and left in this solution for various periods of time, under anaerobic (A; B, circles) and aerobic conditions (B, squares). At chosen time points, the remaining attached cells were fixed with formalin and counted microscopically as described in Materials and Methods. Standard deviations are indicated by error bars.
Cell surface characteristics.
Results of EIC under both aerobic and anaerobic conditions indicated that the cell surfaces of all four bacteria were negatively charged (Table 1). Most cells applied to anion-exchange columns were retained. An exception were the cells of S. wolinii, which initially completely blocked the eluent flow through the columns by settling as a film on top of the column material. When this film was pierced with the tip of a Pasteur pipette, eluent flow continued, and a large percentage of the cells were eluted (log-phase cells under aerobic conditions were all eluted). The growth phase of the cells did not affect the EIC results. The black metal sulfide precipitates that form during growth of Desulfovibrio sp. strain G11 cells were also retained by anion-exchange columns. However, the retention of this material did not affect the OD590, which was used to calculate the number of eluted cells.
TABLE 1.
Cell surface characterization by HIC and EIC
| Organism | % Cells, at indicated condition and growth phase, retained by:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrophobic resina
|
Anion-exchange resinb
|
Cation-exchange resinb
|
||||||||||
| Anaerobic
|
Aerobic
|
Anaerobic
|
Aerobic
|
Anaerobic
|
Aerobic
|
|||||||
| Log | Stationary | Log | Stationary | Log | Stationary | Log | Stationary | Log | Stationary | Log | Stationary | |
| D. tiedjei | 71 | 37 | 48 | 74 | 96–100 | 96 | 100 | 100 | 3 | 0 | 0 | 0 |
| Desulfovibrio sp. strain G11 | 48 | 37 | 25 | 33 | 96 | 100 | 100 | 100 | 5 | 0 | 0 | 3 |
| S. wolinii | 14 | 0 | 3 | 4 | 45 | 35 | 0 | 45 | 2 | 16 | 0 | 0 |
| S. wolfei | 13 | 0 | 0 | 0 | 100 | 100 | 100 | 76 | 0 | 0 | 0 | 0 |
As determined by HIC.
As determined by EIC.
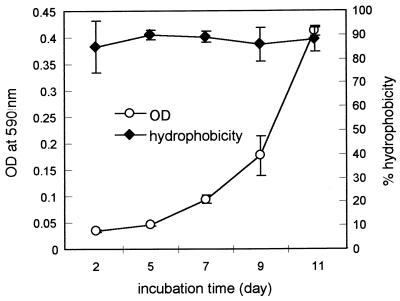
Results of HIC showed that cells of D. tiedjei and Desulfovibrio sp. strain G11 have hydrophobic surface properties. Up to 48% of the G11 cells were retained by hydrophobic resin, and this percentage was relatively unaffected by the growth phase of the cells or the assay conditions. D. tiedjei cells appeared to be even more hydrophobic, depending on the physiological condition and the presence or absence of air. The hydrophobic nature of D. tiedjei cells was corroborated by the BATH test, another commonly used assay to determine bacterial cell surface hydrophobicity (29). During all phases of growth, from lag to stationary phase, 85 to 90% of D. tiedjei cells partitioned into hexadecane (Fig. 6).
FIG. 6.
Cell surface hydrophobicity of D. tiedjei cells during growth measured by the BATH assay. Samples from growing cultures of D. tiedjei were tested for cell surface hydrophobicity by the BATH assay (29). Standard deviations are indicated by error bars.
DISCUSSION
Years of research on bacterial attachment have shown that there is an enormous diversity and flexibility in the mechanisms and strategies with which microorganisms attach to solid surfaces (13). Though most of this research has been performed on aerobic microorganisms, the four anaerobic bacterial species investigated in our study were also found to exhibit different adhesion characteristics.
Bacterial attachment to solid surfaces in aqueous systems is a complex interaction between the bacterium, the solid substratum, and the liquid phase. Factors known to affect bacterial adhesion include biological features, such as the presence of particular surface proteins, extracellular polymers, appendages such as flagella and fimbriae, the degree of cell surface hydrophobicity and electrostatic charge, motility, cell size, and the overall physiological status of the cell (3, 6, 16, 22, 23, 33). Physicochemical aspects of the substratum and the liquid phase include properties such as surface free energy, hydrophobicity, electrostatic charge, ionic strength, and presence of metabolizable carbon sources (14, 15, 17, 18). Each of these factors can vary with changes in environmental conditions (13).
Appendages such as flagella may play a role in adhesion of some species (13). DeFlaun et al. (6) generated a nonmotile Tn5 transposon mutant of Pseudomonas fluorescens without flagella that had a >50%-reduced ability to adhere to sand columns, compared to the wild type. S. wolfei possesses, like P. fluorescens, several flagella. Based on our results, it seems unlikely that the pronounced adhesion ability of S. wolfei is caused, in part, by flagella, since stationary-phase S. wolfei cells do not adhere but still possess their flagella. Gannon et al. (16) studied the transport characteristics of a number of bacteria and were not able to relate retention in soil columns to the presence of flagella or motility. Theoretically, motility could play a role in bacterial attachment, because it may either increase the force with which a bacterium encounters the surface or simply increase the statistical probability of a bacterium encountering the surface (11, 13). Indeed, we observed that the polarly flagellated Desulfovibrio sp. strain G11 cells are most adhesive during log phase, when they are highly motile, and do not adhere in stationary phase when they are nonmotile. Still, the relationship between motility and adhesion is not always clear-cut. For example, motile bacteria have been shown to penetrate farther through saturated sediment columns than their cold-induced nonmotile counterparts, suggesting that motile cells attach less readily (22). Flagella do not always confer motility to cells (16). S. wolfei cells, for example, are at best sluggishly motile, in spite of their multiple flagella. However, for this organism, inhibition of the metabolism with sodium azide all but inhibits attachment. Apparently, active processes other than motility, such as polymer production, are required for adhesion of S. wolfei cells.
Electrostratic and hydrophobic interactions between bacterial cells and solid surfaces may also affect adhesion (13, 21). Makin and Beveridge (21) were able to show that cell surface hydrophobicity was the primary mediator of adhesion of Pseudomonas aeruginosa strains to hydrophobic surfaces, whereas for hydrophilic cells, surface charge played a major role. Like most microorganisms, the species used in our study were all negatively charged. It was therefore expected that they would preferentially adhere to surfaces with a positive charge. Though the adhesion of S. wolfei cells was indeed significantly increased on Fe3+-coated surfaces, adhesion of the other species was less or not at all affected. Based on cell surface hydrophobicity measurements (by both HIC and BATH testing) we expected increased adhesion of Desulfovibrio sp. strain G11 and D. tiedjei cells on hydrophobic surfaces. However, silicone-coated surfaces inhibited adhesion of all the strains, including the relatively hydrophobic D. tiedjei cells. Such discrepancies are difficult to explain, but gross measurement of surface properties such as charge and hydrophobicity does not always consistently correlate with attachment or transport through porous media (16, 19, 23).
Adhesion to solid surfaces exerts a major influence on the transport of microbial cells through porous media. Although it is not clear why attachment would lead to preferential retention of cells of certain shapes and sizes, some reports suggest that cell attachment to solid surfaces may indeed be greater for elongated cells than for spherical cells. Fontes et al. (14) found that small coccoid cells had a much higher recovery rate in column effluents than larger, rod-shaped cells. Similarly, when comparing the transport characteristics of 19 bacterial isolates through soil columns, Gannon et al. (16) found that bacterial retention was statistically related to cell size only and not to other cell properties such as electrostatic charge, cell surface hydrophobicity, capsules, and flagella. Bacteria shorter than 1 μm usually had high recovery rates. On the other hand, Camper et al. (3) were not able to statistically correlate cell size with recovery in column effluents. Weiss et al. (33) showed that cell shape, quantified as the ratio of cell width to cell length, and not simply cell size affects the transport of bacterial cells through porous media. Nearly spherical cell shapes were the least retained in their sand column studies. The bacterial species used in the present study differ greatly in size and shape, ranging from vibrio-shaped Desulfovibrio cells smaller than 1 μm to the large rods of D. tiedjei, which can reach sizes of up to 10 μm. We observed that suspended cells of D. tiedjei settle relatively quickly under static conditions, probably due to their size. The cell shapes and sizes of the test organisms may be expected to significantly affect their transport through porous media.
The physiological status of a microorganism clearly has a significant effect on its ability to adhere to surfaces. However, the actual effect may differ from species to species. Motility, for example, is often reduced in stationary-phase cells. In our study, stationary-phase Desulfovibrio sp. strain G11 cells were no longer motile and did not adhere. Cell surface hydrophobicity may change when cells enter stationary phase (19), or it may remain unchanged, as was the case with most of our species tested. Though it has been hypothesized that increased adhesion ability in the starved state would be a survival tactic (19), we have observed that stationary-phase cells of S. wolfei and Desulfovibrio sp. strain G11 were less adhesive than actively growing cells. Similar observations have been made by other authors. Cells of the anaerobic organism Citrobacter amalonaticus, for example, showed a rapid increase in attached numbers upon addition of a metabolizable substrate (25). Upon prolonged starvation, other processes that may affect adhesion, including cell size reduction and alterations in the lipid composition of the cell membrane, are known to occur in bacterial cells (19, 32) and could be related to alterations in attachment.
Although the presence of air is lethal or inhibitory to the physiology of anaerobes, it does not necessarily affect their attachment abilities. For example, aeration severely inhibited S. wolfei adhesion but had no effect on adhesion of D. tiedjei. Thus, there are clearly differences in (i) the extent to which cell surface properties are altered by exposure to air and (ii) the involvement of active metabolic processes in the attachment mechanisms. The significance of air in the modification of attachment properties may be relevant to the application of bioremediation technologies.
The fact that the adhesive characteristics of the model organisms tested differ greatly has consequences for the development of mathematical models for the transport of anaerobic bacteria and for the design of in situ bioremediation scenarios (26). For example, bioremediation efforts for PCE- and/or TCE-contaminated environments would rely on organisms such as D. tiedjei, which can dechlorinate chlorinated compounds. In order to accomplish dechlorination reactions, D. tiedjei cells depend on the presence of syntrophic partners such as fatty acid oxidizer S. wolfei and H2-consuming Desulfovibrio species. Our results have shown that D. tiedjei does not attach in large numbers and that environmental conditions do not significantly affect its adhesion to solid surfaces. Physiological conditions, influenced by the nutritional status of the organism, do not affect its adhesion greatly either. However, once attached to a surface, D. tiedjei cells remain attached even when subjected to conditions lacking nutrients. Therefore, theoretically, changes in the environmental conditions caused by the movement of a PCE or TCE contaminant plume may not have much of an effect on the number of attached D. tiedjei cells at a given location. On the other hand, the syntrophic fatty acid oxidizer S. wolfei, unlike D. tiedjei, does adhere in large numbers to solid surfaces. However, attachment only occurs when cells are actively growing and not when they are starved for nutrients. Attached cells, however, appear to remain attached for long periods of time, even when subjected to starvation conditions. Of course, the presence of chlorinated compounds in the groundwater may affect bacterial adhesion. However, preliminary results from our laboratory could not indicate an effect of TCE at concentrations up to 10 ppm. Soil particles with electropositive charges would be especially strong retainers of S. wolfei cells. Thus, in theory, nonattached, starved S. wolfei cells could be expected to attach when groundwater movement supplies them with nutrients, whereas attached, starved cells may yield free-living daughter cells, which may then attach locally or further downstream. Active, attached Desulfovibrio cells, on the other hand, would easily be detached by the movement of groundwater. The multidisciplinary approach used in the project of which this study is a part will allow the verification of the results of this study at larger scales. The transport of these organisms through soil columns and large flow cells containing heterogeneous porous media and the effects of environmental and physiological conditions on transport are being tested before predictive models will be constructed.
For successful and complete dechlorination processes to occur, the members of the dechlorinating consortia should most likely establish close associations (2, 9, 10, 24). It remains to be determined whether the pure-culture microorganisms tested in this study establish such associations on solid surfaces and whether the adhesion of one species will enhance or inhibit the attachment of another species. Interactive attachment of bacteria in the anaerobic environments of dental plaque (20) and methanogenic bioreactors (25) has been described. In dental plaque, the first bacterial colonizers attach to clean tooth surfaces and then provide binding sites for subsequent colonization by other species via specific bridging and coaggregation mechanisms (20). Similarly, the methanogen Methanosarcina barkeri was shown not to adhere to glass surfaces unless C. amalonaticus had formed an initial biofilm on these surfaces (25). Our laboratory is currently investigating if and how the bacteria described in this study form physically attached consortia on solid surfaces.
ACKNOWLEDGMENTS
This work was sponsored by the U.S. Department of Energy, grant no. DE-FG07-97ER62355.
We thank E. M. Murphy, T. R. Ginn, and D. R. Boone for their collaboration and for helpful discussions.
REFERENCES
- 1.Boone D R, Bryant M P. Proprionate-degrading bacterium Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol. 1980;40:626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boone D R, Johnson R L, Liu Y. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 uptake. Appl Environ Microbiol. 1989;55:1735–1741. doi: 10.1128/aem.55.7.1735-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camper A K, Hayes J T, Sturman P J, Jones W L, Cunningham A B. Effects of motility and adsorption rate coefficient on transport of bacteria through saturated porous media. Appl Environ Microbiol. 1993;59:3455–3462. doi: 10.1128/aem.59.10.3455-3462.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole J R, Fathepure B Z, Tiedje J M. Tetrachlorethene and 3-chlorobenzoate dechlorination activities are co-induced in Desulfomonile tiedjei. Biodegradation. 1995;6:167–172. doi: 10.1007/BF00695347. [DOI] [PubMed] [Google Scholar]
- 5.de Bruin W O, Kotterman M J J, Posthumus M A, Schraa G, Zehnder A J B. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992;58:1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFlaun M, Tanzer A S, McAteer A L, Marshall B, Levy S. Development of an adhesion assay and characterization of an adhesion-deficient mutant of Pseudomonas fluorescens. Appl Environ Microbiol. 1990;56:112–119. doi: 10.1128/aem.56.1.112-119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeWeerd K, Mandelco L, Thauer R S, Woese C R, Suflita J M. Desulfomonile tiedjei gen. nov. and sp. nov., a novel anaerobic dehalogenating, sulfate-reducing bacterium. Arch Microbiol. 1990;154:23–30. [Google Scholar]
- 8.DiStefano T D, Gossett J M, Zinder S H. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol. 1991;57:2287–2292. doi: 10.1128/aem.57.8.2287-2292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Fantroussi S, Naveau H, Agathos S N. Anaerobic dechlorinating bacteria. Biotechnol Prog. 1998;14:167–188. doi: 10.1021/bp980011k. [DOI] [PubMed] [Google Scholar]
- 10.Fathepure B Z, Nengu J P, Boyd S A. Anaerobic bacteria that dechlorinate perchloroethene. Appl Environ Microbiol. 1987;53:2671–2674. doi: 10.1128/aem.53.11.2671-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher M. The question of passive versus active attachment mechanisms in non-specific bacterial adhesion. In: Berkely R C W, Lynch J M, Melling J, Rutter P R, Vincent B, editors. Microbial adhesion to surfaces. Chichester, United Kingdom: Ellis Horwood Limited; 1980. [Google Scholar]
- 12.Fletcher M. Methods for studying adhesion and attachment to surfaces. Methods Microbiol. 1990;22:251–283. [Google Scholar]
- 13.Fletcher M. Bacterial attachment in aquatic environments: a diversity of surfaces and adhesion strategies. In: Fletcher M, editor. Bacterial adhesion: molecular and ecological diversity. New York, N.Y: Wiley-Liss, Inc.; 1996. [Google Scholar]
- 14.Fontes D E, Mills A L, Hornberger G M, Sherman J S. Physical and chemical factors influencing transport of microorganisms in porous media. Appl Environ Microbiol. 1991;57:2473–2481. doi: 10.1128/aem.57.9.2473-2481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gannon J, Tan Y, Baveye P, Alexander M. Effect of sodium chloride on transport of bacteria in a saturated aquifer material. Appl Environ Microbiol. 1991;57:2497–2501. doi: 10.1128/aem.57.9.2497-2501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gannon J T, Manilal V B, Alexander M. Relationship between cell surface properties and transport of bacteria through soil. Appl Environ Microbiol. 1991;57:190–193. doi: 10.1128/aem.57.1.190-193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon A S, Millero F J. Electrolyte effects on attachment of an estuarine bacterium. Appl Environ Microbiol. 1984;47:495–499. doi: 10.1128/aem.47.3.495-499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewett D G, Hilbert T A, Logan B E, Arnold R G, Bales R C. Bacterial transport in laboratory columns and filters: influence of ionic strength and pH on collision efficiency. Water Res. 1995;29:1673–1680. [Google Scholar]
- 19.Kjelleberg S, Hermansson M. Starvation-induced effect on bacterial surface characteristics. Appl Environ Microbiol. 1984;48:497–503. doi: 10.1128/aem.48.3.497-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolenbrander P E. Oral microbiology and coaggregation. In: Shapiro J A, Dworkin M, editors. Bacteria as multicellular organisms. New York, N.Y: Oxford University Press, Inc.; 1997. pp. 245–269. [Google Scholar]
- 21.Makin S A, Beveridge T J. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology. 1996;142:299–307. doi: 10.1099/13500872-142-2-299. [DOI] [PubMed] [Google Scholar]
- 22.McCalou D R, Bales R C, Arnold R G. Effect of temperature-controlled motility on transport of bacteria and microspheres through saturated sediment. Water Res. 1995;31:271–280. [Google Scholar]
- 23.McEldowney S, Fletcher M. Effect of growth conditions and surface characteristics of aquatic bacteria on their attachment to solid surfaces. J Gen Microbiol. 1986;132:513–523. [Google Scholar]
- 24.McInerney M J, Bryant M P, Hespell R B, Costerton J W. Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol. 1981;41:1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier-Schneiders M, Busch C, Diekert G. The attachment of bacterial cells to surfaces under anaerobic conditions. Appl Microbiol Biotechnol. 1993;38:667–673. [Google Scholar]
- 26.Murphy E M, Ginn T R, Chilakapati A, Resch C T, Phillips J L, Wietsma T M, Spadoni C M. The influence of physical heterogeneity on microbial degradation and distribution in porous media. Water Res. 1997;33:1087–1103. [Google Scholar]
- 27.Pedersen K. Electrostatic interaction chromatography, a method for assaying the relative surface charges of bacteria. FEMS Microbiol Lett. 1980;12:365–367. [Google Scholar]
- 28.Riley R G, Zachara J M, Wobber F J. Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research. DOE/ER-0547T. Washington, D.C.: Office of Energy Research, U.S. Department of Energy; 1992. [Google Scholar]
- 29.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 30.Scheidegger A M, Borkovec M, Sticher H. Coating of silica sand with goethite: preparation and analytical identification. Geoderma. 1993;58:43–65. [Google Scholar]
- 31.Smyth C J, Johnsson P, Olsson E, Söderlind O, Rosengren J, Hjerten S, Waldstrom T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978;22:462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner U, Egli T. Dynamics of microbial growth and cell composition in batch culture. FEMS Microbiol Rev. 1990;75:19–44. doi: 10.1111/j.1574-6968.1990.tb04084.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss T H, Mills A L, Hornberger G M, Herman J. Effect of bacterial cell shape on transport of bacteria in porous media. Environ Sci Technol. 1995;29:1737–1740. doi: 10.1021/es00007a007. [DOI] [PubMed] [Google Scholar]
- 34.Wolin E A, Wolin M J, Wolfe R S. Formation of methane by bacterial extracts. J Biol Chem. 1963;238:2882–2886. [PubMed] [Google Scholar]