Abstract
Nanomedicine offered hope for improving the treatment of cancer but the survival benefits of the clinically approved nanomedicines are modest in many cases when compared to conventional chemotherapy. Metronomic therapy, defined as the frequent, low dose administration of chemotherapeutics – is being tested in clinical trials as an alternative to the conventional maximum tolerated dose (MTD) chemotherapy schedule. Although metronomic chemotherapy has not been clinically approved yet, it has shown better survival than MTD in many preclinical studies. When beneficial, metronomic therapy seems to be associated with normalization of the tumor microenvironment including improvements in tumor perfusion, tissue oxygenation and drug delivery as well as activation of the immune system. Recent preclinical studies suggest that nanomedicines can cause similar changes in the tumor microenvironment. Here, by employing a mathematical framework, we show that both approaches can serve as normalization strategies to enhance treatment. Furthermore, employing murine breast and fibrosarcoma tumor models as well as ultrasound shear wave elastography and contrast-enhanced ultrasound, we provide evidence that the approved nanomedicine Doxil can induce normalization in a dose-dependent manner by improving tumor perfusion as a result of tissue softening. Finally, we show that pretreatment with a normalizing dose of Doxil can improve the efficacy of immune checkpoint inhibition.
Keywords: Drug delivery, Tumor perfusion, Immune checkpoint inhibition, Shear wave elastography, Contrast enhanced ultrasound
Graphical abstract
Metronomic nanomedicine normalizes the tumor microenvironment to restore vessel function and enhance immunostimulation and efficacy of immune checkpoint inhibitor (ICI).
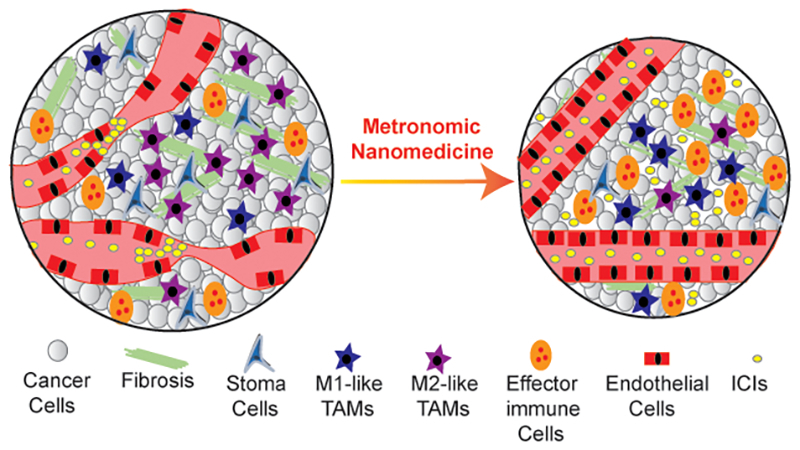
1. Introduction
Nanoparticle formulations are considered to be advantageous compared to conventional chemotherapy owing to their preferential accumulation in tumor tissues as a result of the enhanced permeability and retention (EPR) effect and their prolonged circulation in the blood [1,2]. Preferential nanoparticle delivery to tumors is associated with less severe adverse effects than conventional chemotherapy, although both therapies might lead to similar survival in cancer patients. Modest survival benefits of clinically approved nanoparticles are in large part attributed to the fact that the abnormal tumor microenvironment poses physiological barriers that can significantly decrease the delivery of nanoparticle formulations throughout tumors [1,[3], [4], [5], [6]]. Nanoparticle delivery could be improved with the careful design of their parameters, such as the size, shape and/or charge of the nanoparticle, its drug release kinetics and for targeted particles their binding affinity to cancer cells [2]. Recently, with the interest of cancer therapies shifted towards the activation of antitumor immunity, the use of nanomedicines has been extended to improve the efficacy of cancer immunotherapy [[7], [8], [9]].
A metronomic approach, using a lower and more frequent dose schedule, has emerged as an alternative to the standard maximum tolerated dose (MTD) chemotherapy schedule [[10], [11], [12]]. This approach has been shown to yield better survival than MTD in several preclinical tumor models, and is currently being evaluated in multiple clinical trials [10,[12], [13], [14], [15], [16], [17], [18], [19]]. Metronomic chemotherapy has the potential to normalize the tumor microenvironment, improve blood vessel function and perfusion, and increase the cytotoxic activity of immune effector cells turning the immunosuppressive tumor microenvironment to an immunostimulatory one [12,[20], [21], [22], [23], [24], [25]]. This chemotherapy-induced normalization results in increased death of both cancer cells and stem-cell like cancer cells [26,27]. Despite its pleiotropic effects, however, metronomic schedule of cytotoxic drugs has not received FDA-approval yet. One challenge with metronomic therapy is that stimulation of the immune response is not only dose and schedule dependent, but also depends on the chemotherapeutic agent employed. Some chemotherapeutic agents seem to increase immune-response, while others do the opposite [28,29]. For the chemotherapeutics that increase immune-response and induce immunogenic cell death, metronomic administration can improve the efficacy of immune checkpoint inhibition [[30], [31], [32]].
Interestingly, in recent studies, CRLX101 nanoparticles, which are composed of cyclodextrin-containing polymer conjugated to the highly potent cytotoxic drug camptothecin, have shown to improve therapeutic effects on both cancer cells and stem-cell like cancer cells, alleviate hypoxia and improve perfusion [[33], [34], [35], [36]] similar to the normalization effects of metronomic chemotherapy. Even though CRLX101 failed in clinical trials, similarities between nanomedicine and metronomic therapy suggest that these two approaches can be compared using the same unified theoretical framework of inducing normalization of tumor microenvironment to enhance therapy (Table 1). Interestingly, apart from CRLX101, a few other nanoparticle formulations, which have not reached clinical testing yet, have also shown normalization effects similar to metronomic therapy in preclinical studies [29,37,38]. Of note is the metronomic administration of nanoparticles containing oxaliplatin that leads to increased antitumor efficacy compared to free oxaliplatin metronomic treatment [39,40]. A key to the effectiveness of both approaches compared to MTD is that they can potentially maintain effective drug levels in the blood for long duration. Nanomedicines achieve this by the prolonged blood circulation time of the nanoparticles and the controlled release of the drug, whereas in metronomic therapy effective drug levels are maintained with the more frequent administration of the drug (Fig. 1).
Table 1.
Similarities, differences and lessons from the use of nanomedicines and metronomic chemotherapy to treat solid tumors.
| Similarities |
Differences |
Recent lessons |
|---|---|---|
| Both nanomedicine and metronomic chemotherapy can: | Nanomedicines differ from metronomic chemotherapy in that: | The biggest lesson from nanomedicine and metronomic chemotherapy today is that: |
| i) maintain effective drug levels in the blood for long duration | i) nanomedicines can exhibit improved accumulation to tumor tissue (EPR effect) and target to cancer cells | i) with the use of nanomedicines the accumulation of drug is not as high as initially hoped for [4] |
| ii) induce vascular normalization | ii) nanomedicines are likely to have less adverse effects on normal tissues, except the liver | |
| iii) improve tumor perfusion and oxygenation | iii) nanomedicines can exhibit limited penetration into tumor tissue owing to their large size | ii) certain chemotherapeutic agents can actually increase immune-response, while others do the opposite [28] |
| iv) improve drug delivery and treatment efficacy | iv) nanomedicines require less frequent dosing | |
| v) potential to make the tumor microenvironment more immune-supportive | v) a few nanoparticles have been approved for clinical use, but metronomic therapy is not yet approved |
Fig. 1.
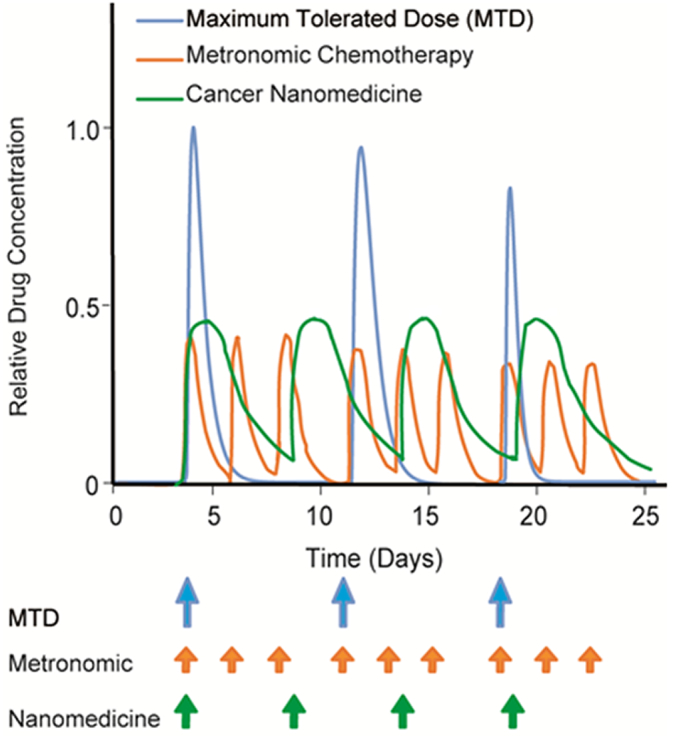
Schematic of intratumoral drug concentration as a function of time for MTD, metronomic chemotherapy and nanomedicine based on model simulations. MTD treatment causes increased peak concentration of the drug to the tumor but the less frequent dose schedule results in longer drug free periods. Metronomic scheduling of chemotherapy and nanomedicines ensure effective drug levels in the blood for longer periods. Concentration values are normalized to that of MTD. Dose schedule was based on our previous work [41].
Apart from similarities, nanomedicine and metronomic therapy also have certain differences, as summarized in Table 1. To build on the lessons learned from successes and failures of each approach, we recently developed a mathematical framework for tumor growth and response to drug administration accounting for cancer cells, stem-cell like cancer cells and immune cells as well as the tumor vasculature and their interactions [41,42]. The model also accounted for the delivery and killing potential of cytotoxic drugs based on their dose and schedule. By comparing multiple sets of experimental data with our mathematical model, we concluded that for metronomic therapy to be effective, it should induce functional normalization of tumor blood vessels, which results in improved tumor perfusion. Improved perfusion increases tissue oxygenation – converting the immunosuppressive tumor microenvironment to immunostimulatory, and improves delivery of drugs resulting in better therapeutic outcomes. Previous studies have developed mathematical models to show the antitumor and antiangiogenic effects of metronomic chemotherapy compared to MTD protocol, but none of them accounts for the use of nanomedicine to induce metronomic effects [[43], [44], [45], [46], [47], [48], [49]].
Here, we hypothesize that nanoparticle formulations, given the controlled release of their payload and long blood circulation time, can trigger the same cascade of activities as metronomic therapy. To test our hypothesis, we tailored our mathematical framework to model nanoparticles and analyzed the experimental data on the effect of the nanoparticles on the growth of mouse models of breast cancers. Additionally, we show that improved perfusion caused by nanoparticle treatment resulted in an increased population of effector immune cells (NK cells and CD8+ T cells) and in skewing Tumor Associated Macrophages (TAMs) polarization away from the immunosuppressive M2-like phenotype to the M1-like phenotype. To validate model predictions, we performed in vivo experiments in murine breast and fibrosarcoma tumor models, and employed the clinically approved nanoparticle formulation Doxil (PEGylated liposomal doxorubicin). Doxil has an elimination half-life 20–30 h [50] and thus, we hypothesized that to produce similar to metronomic therapy effects, effective drug levels should be maintained. To this end, we employed three different dose schedules providing the same amount of Doxil on a weekly basis: every day, every other day and every 6 days. During Doxil treatment we monitored changes in tumor stiffness and perfusion using ultrasound shear wave elastography (SWE) and dynamic contrast enhanced ultrasound (DCEUS), respectively [51]. We found that low doses of Doxil every day or every second day can effectively reduce tumor stiffness and improve perfusion thus, inducing normalization of the tumor microenvironment. A normalizing dose of Doxil could also enhance the efficacy of immune checkpoint inhibition. Finally, to identify optimal nanoparticle design, we generated mathematical model simulations by varying the size of the particles, the drug release kinetics and the binding affinity to cancer cells.
2. Materials and methods
A detailed description of all materials and methods employed in the study are presented in the Supplementary Information. Here, we provide a summary of them.
2.1. Mathematical model
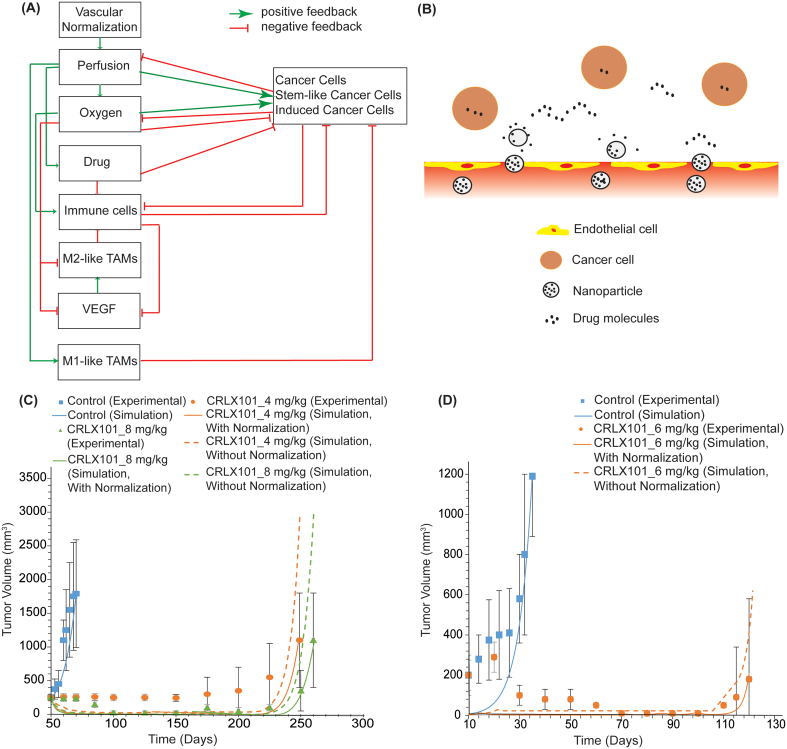
The mathematical framework for tumor growth and response to therapy was described and validated in previous work and its basic components are presented in Fig. 2A [41,42]. Here, the model has been extended to account for nanoparticle transport taking explicitly into consideration nanoparticle size, drug release rate and binding affinity to cancer cells (details in Supplementary Information). Briefly, the model accounts for three populations of cancer cells: i) the non-stem cancer cells, ii) the stem-cell like cancer cells which are relatively resistant to drugs and hypoxia, and iii) cancer cells that are induced by chemotherapy to acquire a more stem-like phenotype [52]. The model also incorporates immune cells (natural killer (NK) cells, conventional CD4+ T-cells, CD8+ T-cells and regulatory CD4+ T-cells (Treg)), Tumor Associated Macrophages (M1-like and M2-like TAMs), the tumor vasculature (i.e., endothelial cells and pericytes), various proangiogenic molecules, including angiopoietins (Ang1 and Ang2), platelet-derived growth factor (PDGF), vascular derived growth factor (VEGF) and CXCL12, chemotherapy delivery and tissue oxygenation (details in Supplementary Information). Here, we adapted the model to account for delivery of nanoparticles and release of the drug (Fig. 2B) and specified the model parameters to match the properties reported for CRLX101 (Supplementary Table S1).
Fig. 2.
(A) Schematic of mathematical model components and their interactions. Vascular normalization improves tumor perfusion and thus, oxygen supply and drug delivery. Improved oxygenation activates immune cells, causes TAMs polarization away from the immunosuppressive M2-like phenotype to the M1-like phenotype but also increases the proliferation of cancer cells but decreases the proliferation of stem-like cancer cells and VEGF concentration. Conventional CD4+T cells decrease levels of VEGF, while overexpression of VEGF increases levels of M2-like TAMs. M1-like TAMs have a tumoricidal effect on cancer cells. M2-like TAMs inactivate immune effector cells. Cancer cells consume oxygen and reduce the number and efficacy of immune cells. Improved drug delivery will increase both cancer cell and immune cell killing. Cancer cell killing decompresses tumor vessels further improving vessel functionality. (B) Schematic and equations for nanoparticle delivery to tumors without binding. Model validation using (C) experimental data by Pham et al. [34] and (D) experimental data by Conley et al. [33].
In our theoretical framework, vascular normalization was modeled as a restoration of vascular function driven by reduced vascular leakiness (i.e., vessel wall pores) of the tumor blood vessels rather than explicitly including the parallel effects of remodeling the structure of the vascular network. In mouse breast tumor models, vessel normalization has been shown to decrease the pores of the interendothelial openings from 400 nm to 150 nm, which determines the size of nanoparticles that can enter the tumor interstitial space [53]. Reduction of vessel wall pores can restrict extravasation of large nanoparticles from the vascular to the interstitial space of the tumor, and, at the same time can reestablish a pressure gradient across the tumor vessel wall that enhances delivery of small particles. As a result, vascular normalization can improve significantly the intratumoral delivery of nanoparticles as large as 40 nm in diameter, and thus the killing of cancer cells [[53], [54], [55]]. Cancer cell killing, in turn, reduces the total cell volume decompressing tumor blood vessels, improving perfusion and tissue oxygenation and further enhancing drug delivery [[56], [57], [58]]. Vessel decompression is also induced as a result of tumor softening and alleviation of intratumoral mechanical forces [59]. Therefore, normalization of the entire tumor microenvironment could be achieved promoting chemo- as well as nano-therapy.
2.2. Animal tumor models and treatment protocols
Tumor models: A syngeneic orthotopic model of murine mammary tumors was generated by implantation of 5 × 104 4T1 cancer cells in 40 μL of serum-free medium into the third mammary fat pad of 6–8 week-old BALB/c female mice. A syngeneic fibrosarcoma model was generated by implantation of 2.5 × 105 MCA205 cells in 50 μL of serum-free medium into the flank of 6–8 week old C57BL/6 male mice. Treatment Protocol: When tumors reached an average size of 150 mm3 for 4T1 and 200 mm3 for MCA205 tumors, treatment with Doxil was initiated. We employed three different treatment schedules to investigate the effect of Doxil on inducing normalization by reducing tumor stiffness and improving perfusion: i) 1 mg/kg (daily for six days), ii) 2 mg/kg (every other day) and iii) 6 mg/kg (once a week). All treatments were administered intravenously (i.v.) and consisted of two cycles. For the first cycle, we intended to observe whether Doxil can induce tumor normalization when it is administered in a dose schedule that ensures continuous presence of the drug in the blood, following our rationale as demonstrated in Fig. 1. Doxil has an elimination half-life 20–30 h which appears to be constant between mouse and human [50], therefore two dose schedules within this range were employed (i.e., every day and every other day) and also a third higher and less frequent dose (once a week) was employed acting as a negative control. For the second cycle of treatment, apart from Doxil, we incorporated in the study an immune checkpoint inhibitor (ICI) aPDL1 (clone B7-H1, BioXcell) 10 mg/kg intraperitoneally (i.p.) every 3 days (totally 3 doses) alone or in combination with the three treatment protocols of Doxil. Given the increasing interest in nano-immunotherapy, as already demonstrated in triple negative breast cancer with the combination of nanoparticle albumin-bound paclitaxel and the immune checkpoint inhibitor (ICI) antibody, atezolizumab [7,60,61], our purpose was to investigate whether pretreatment with a normalizing dose of nanomedicine can optimize nano-immunotherapy. Tumor stiffness and perfusion monitoring: We monitored the elastic modulus and tumor perfusion with ultrasound shear wave elastography (SWE) and dynamic contrast-enhanced ultrasound (DCEUS), respectively. We performed the ultrasound measurements in three time points: before the initiation of any treatment, before the initiation of the second cycle of Doxil and the first dose of aPDL1 and a day after the end of all treatments.
Interstitial fluid pressure and growth-induced stress: Prior to tumors removal, we measured the interstitial fluid pressure (IFP) using the wick-in-needle technique [62] and following tumors excision, we measured the residual growth-induced stress of the tumors, performing the tumor opening experiment [63].
Tumor dimensions were measured during the in vivo phase of the experiment every second day with a digital caliper and tumor volume was calculated from the volume of ellipsoid, measuring the two dimensions (x,y) and assuming the third dimension to be equal to .
Ultrasound Shear Wave Elastography (SWE). SWE was employed on a Philips EPIQ Elite ultrasound system using a linear array transducer (eL18-4), according to previous research [51]. The method generates shear wave velocity via an acoustic push pulse, creating a color mapped elastogram where red indicates hard and blue soft tissue. A confidence display was also used as a reference of the highest shear wave quality of the user-defined region of interest (ROI). The average value of the tumor region was automatically generated by the system under default scanner settings and expressed in kPa. The settings that were used are: frequency, 10 MHz; power, 52%; B-mode gain, 22 dB; dynamic range, 62 dB. SWE was performed prior to Doxil treatment on day 11, prior to aPDL1 treatment on day 18 and prior to tumor removal on Day 25 for both cancer cell lines.
Dynamic contrast-enhanced ultrasound (DCEUS). Tumor associated vascular perfusion was assessed with DCEUS after bolus injection of contrast agents (8 μL of sulphur hexafluoride microbubbles encapsulated by a phospholipid shell with a mean diameter of 2.5 μm, retrorbital administration). Ultrasound scanning of tumor was performed using the linear array transducer L12-5. Contrast first harmonic signals were received at 8 MHz with a mechanical index of 0.06. For all subjects the depth of the focus was set to 3 cm allowing measurements of the full depth of the tumor. Gain was set at 90% for each recording. Focus was optimized and standardized for each subject when finding the tumor area using B-mode imaging. Real-time power modulation imaging was started after flashing imaging with a high mechanical index to destroy the microbubbles in tumor tissue to peak contrast intensity to allow visualization of bubble replenishment [64,65]. Image analysis was performed offline using an ultrasound quantification and analysis software (QLAB, Phillips Medical Systems) [66]. From the produced time intensity curves, we used as measures of perfusion the Mean Transit Time (MTT) and the time required to reach the peak intensity-rise time (RT), which are related to blood flow [64,67]. We also present the values of the peak intensity and the area under the curve, which are related to blood volume (Supplementary Fig. S1) [64]. Prior to each ultrasound application, mice were anesthetized by i.p. injection of Avertin (200 mg/kg) and ultrasound gel was applied to the imaging region to prevent any pressure of the transducer on the underlying tissue.
Details on the cancer cell lines and drugs employed, the development of tumor models and the measurements of the interstitial fluid pressure and growth-induced stress can be found in the Supplementary Information.
3. Results and discussion
3.1. Comparison of mathematical model predictions with experimental data
To test our hypothesis that nanoparticles have normalization effects similar to metronomic therapy, we compared model predictions of tumor growth to experimental data for CRLX101 particles in mouse breast tumor models from two independent studies [33,34]. The only model parameter whose value was specified to the experimental data was the parameter, k1, that describes the dependence of cancer cell proliferation on oxygen concentration (Supplementary Table S2). The value of k1 was determined so that the predicted final tumor volume matched the experimental value of control/untreated group and it was kept the same for comparison of model predictions against the experimental data from all groups of the same study (Fig. 2C and D). In the first study [34], the treatment started at day 50 post-implantation with CRLX101 at doses of 4 mg/kg and 8 mg/kg administered weekly for a period of five months. Subsequently, tumor growth was monitored and mice were removed from the study when tumors exceeded 1000 mm3. Treatment with CRLX101 was found to significantly delay tumor growth in a dose-dependent manner. Our model predictions were in very good agreement with the experimental data of tumor growth for both doses when vascular normalization (i.e., reduction in vessel leakiness) was considered (Fig. 2C), whereas it significantly over-predicted final tumor volume when vascular normalization and the subsequent enhancement in tissue oxygenation, drug delivery and immune response were not taken into account.
Similarly, in the second study [33], when tumors reached an average of 200 mm3, mice were administered weekly with CRLX101 (6 mg/kg) for a period of six weeks and subsequently they were removed from the study when tumor volume exceeded 1000 mm3. Apart from the improvement in overall survival, these authors found that this treatment increased tumor oxygenation and killing of stem-cell like cancer cells. Our model accurately predicted the progression and final volume of the treated mice when accounting for vascular normalization, while overpredicting the tumor volume by a factor of three when normalization was ignored (Fig. 2D). Therefore, the results of both studies can be well explained when nanoparticle treatment works via similar processes as metronomic therapy, revealing a new insight into the same treatment.
Model predictions for tumor vascular density and vessel diameter, tissue oxygenation, drug delivery as well as the populations of cancer, immune cells, macrophages and concentration of VEGF of the second study [33] are presented in Table 2, Table 3, Table 4. The tables provide useful insights into the mechanism by which CRLX101 improves therapeutic outcome inducing normalization effects. The CRLX101 induced normalization of the vessels results in vessel decompression and increased vessel density, better tissue oxygenation and delivery of the drugs, an increase in the number of effector immune cells and M1-like TAMs and decrease in the population of all types of cancer cells, M2-like TAMs and VEGF levels. Therefore, nanomedicine efficacy can be improved when it induces normalization of the tumor microenvironment in a manner similar to metronomic therapy.
Table 2.
Model predictions specified to the treatment protocol of Conley et al. [33] presenting the normalized blood vessel diameter, vascular density, tumor oxygenation and drug concentration. The drug concentration is normalized by dividing with the concentration in the blood vessels. The parameters were calculated midway between the tumor center and periphery.
| Treatment | Normalized vessel diameter | Functional vascular density (cm−1) | Oxygen (mol/mm3) | Drug delivery |
|---|---|---|---|---|
| Control | 0.087 | 2.61 | 0.0927 | – |
| CRLX101 (with normalization) | 0.093 | 11.19 | 0.159 | 0.0389 |
| CRLX101 (without normalization) | 0.090 | 2.79 | 0.121 | 0.0316 |
Table 3.
Model predictions specified to the treatment protocol of Conley et al. [33] presenting the total population of each type of cancer cells. The cell population is normalized by division with the initial number of non-stem cancer cells. The parameters were calculated midway between the tumor center and periphery.
| Treatment | Cancer cells | Stem cell-like cancer cells | Induced cancer cells |
|---|---|---|---|
| Control | 11.05 | 3.73 | 2.90 |
| CRLX101 (with normalization) | 7.65 | 0.90 | 4.01 |
| CRLX101(without normalization) | 9.51 | 1.11 | 4.53 |
Table 4.
Model predictions specified to the treatment protocol of Conley et al. [33] presenting the total population of immune cells (NK cells, CD8+ T-cells, conventional CD4+ T-cells and Tregs), macrophages and VEGF concentration (dimensionless). The cell population is normalized by division with the initial number of non-stem cancer cells. The parameters were calculated midway between the tumor center and periphery.
| Treatment | Immune cells | M1-like TAMs | M2-like TAMs | VEGF |
|---|---|---|---|---|
| Control | 6.83 | 0.073 | 0.383 | 0.34 |
| CRLX101 (with normalization) | 17.65 | 0.234 | 0.081 | 0.12 |
| CRLX101(without normalization) | 10.18 | 0.141 | 0.154 | 0.21 |
3.2. Low and more frequent doses of Doxil normalize the mechanical tumor microenvironment to improve immunotherapy
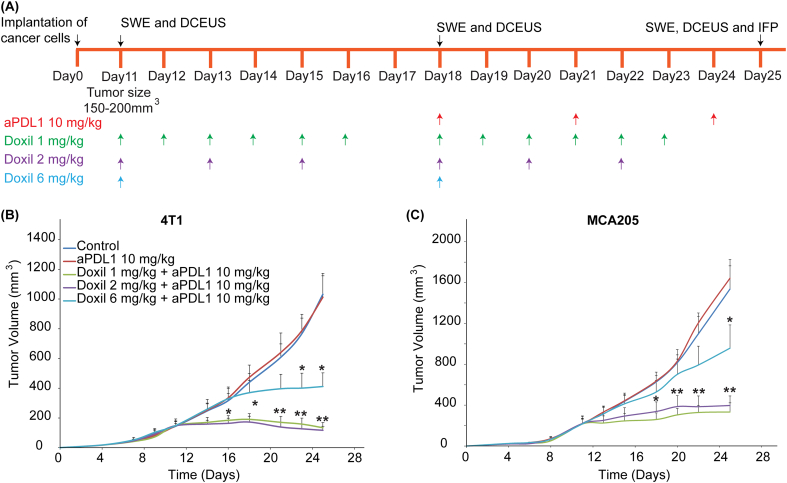
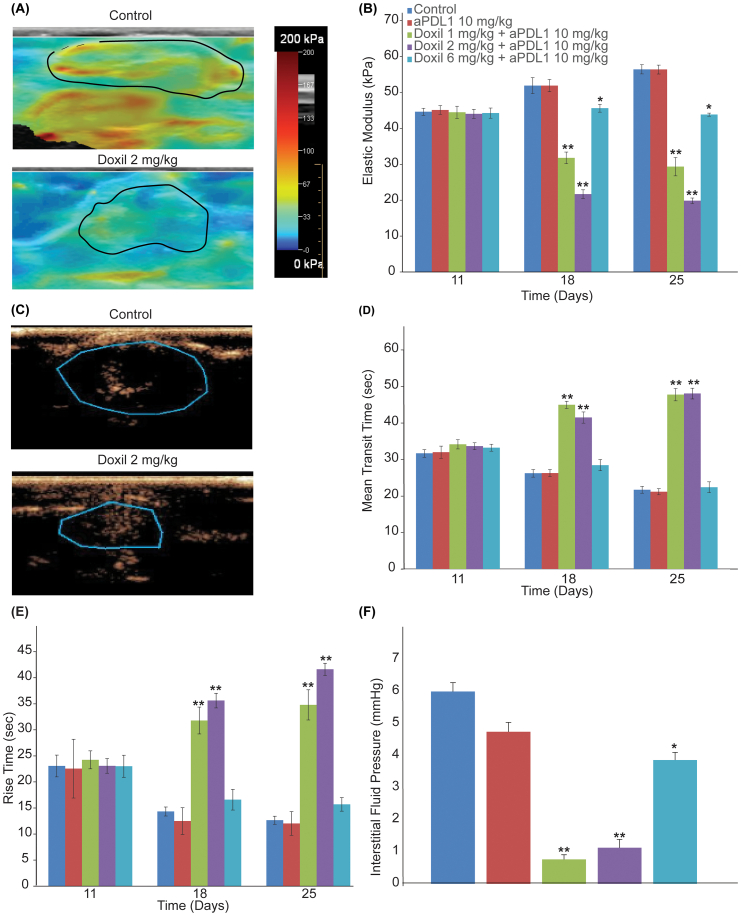
Guided by model predictions, we next set out to test their validity in two syngeneic models: murine 4T1 breast cancer and MCA205 fibrosarcoma tumors. Specifically, we investigated the normalization effects of the clinically approved for breast cancers nanomedicine, Doxil. Doxil is a liposomal formulation of doxorubicin, which can induce immunogenic cell death, normalize the tumor vasculature by increasing pericyte coverage and remodel the tumor extracellular matrix by reducing abnormal levels of collagen I and hyaluronan [37,38]. The treatment protocol for both cancer cell lines is summarized in Fig. 3A and included three different dosage schedules where the same amount of Doxil – on a weekly basis – was administered at different doses, namely 1 mg/kg (daily for six days), 2 mg/kg (every other day) and 6 mg/kg (once a week). Given that the elimination half-life of Doxil is 20–30 h, it was expected that the first two dose schedules would maintain continuous drug circulation in the blood. To monitor the effects of Doxil on normalizing the mechanical tumor microenvironment, SWE and DCEUS were performed in the beginning of the treatment (Day 11) and before the initiation of the second cycle (Day 18). For quantification of tumor elastic modulus, the region of interest was identified by ultrasound B-mode imaging and the average elastic modulus within this region was calculated by the ultrasound software. For quantification of tumor perfusion four measures were obtained by the produced time intensity curves, the mean transit time, rise time, peak intensity and area under the curve (Supplementary Fig. S1) [64,67]. Doxil treatment was able to reduce tumor stiffness (i.e., elastic modulus) and improve perfusion in a dose dependent manner. The normalization effects of the two lower and more frequent doses (1 mg/kg daily and 2 mg/kg every other day) were similar in both cancer cell lines, whereas the higher, less frequent dose reduced stiffness and improved perfusion in a less efficient way (Fig. 4A–E, Supplementary Figs. S2 and S3A–E). Reduction in tumor stiffness is due to the ability of Doxil to reduce extracellular matrix levels [37,38], while improvement in perfusion is associated with alleviation of intratumoral mechanical forces that decompresses tumor vessels but also due to improved pericyte coverage that induces vascular normalization by decreasing vessel leakinees (i.e., permeability) [37,38,51,63,[68], [69], [70], [71]]. Indeed, Doxil treatment was able to significantly reduce growth-induced residual stresses, as indicated by the reduction in the tumor opening (Supplementary Fig. S4) and reduce IFP (Fig. 4F and Supplementary Fig. S3F). Interestingly, during the first cycle of Doxil treatment (Days 11–17) only the low, frequent doses of Doxil were able to decrease tumor growth rate and thus, exhibit anti-tumor efficacy (Fig. 3B and C).
Fig. 3.
Improved efficacy of metronomic administration of Doxil combined with immunotherapy. (A) Experimental protocol of the in vivo studies in 4T1 and MCA205 tumors. When tumors reached an average volume of 150 mm3 for 4T1 and 200 mm3 for MCA205, shear wave elastography (SWE) and dynamic contrast-enhanced ultrasound (DCEUS) were performed prior to initiation of Doxil treatment. Three dose schedules were employed for Doxil: i) 1 mg/kg daily for six days, ii) 2 mg/kg every other day and iii) 6 mg/kg once a week. After completion of the first cycle of treatment (Day 18), SWE and DCEUS were repeated. During the second cycle of treatment an immune checkpoint inhibitor (ICI), aPDL1 (10 mg/kg), was added to the treatment protocol and was administered on days 18, 21 and 24. SWE and DCEUS were repeated prior tumor excision (Day 25). Tumor volume data for (B) 4T1 and (C) MCA205 tumors. Low and more frequent doses of Doxil (1 mg/kg and 2 mg/kg) were able to decrease tumor growth rate for the first treatment cycle and improve the efficacy of nano-immunotherapy in the second treatment cycle. Statistical analyses were performed by comparing the Doxil 6 mg/kg group with the control and the aPDL1 groups * and the Doxil 1 mg/kg and Doxil 2 mg/kg with all other treatment groups **, p ≤ 0.05, determined by t-test. Data are presented as mean ± SEM (n = 6 mice per group).
Fig. 4.
Low and more frequent doses of the nanomedicine Doxil normalize the tumor mechanical microenvironment in 4T1 tumors. (A) Representative SWE images of control and Doxil 2 mg/kg treated tumors on Day 18 (endpoint of 1st treatment cycle). Blue indicates compliant tissue and red indicates stiff tissue. The black line denotes the tumor border (region of interest). (B) Quantification of the average elastic modulus of the tumors on Days 11, 18 and 25. (C) Representative images of the distribution of microbubbles (contrast agents) during a bolus injection imaged with DCEUS in control and Doxil 2 mg/kg treated tumors on Day 18 (endpoint of 1st treatment cycle). The black line denotes the tumor border. (D) Mean transit time and (E) rise time of contrast agents for the different treatment groups. (F) Interstitial fluid pressure (IFP) levels at the end of the treatment protocol. Statistical analyses were performed by comparing the Doxil 6 mg/kg group with the control and aPDL1 groups * and the Doxil 1 mg/kg and Doxil 2 mg/kg with all other treatment groups **, p ≤ 0.05, determined by t-test. Data are presented as mean ± SEM (n = 6 mice per group). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
For the second cycle of Doxil treatment (Days 18–24), an anti-PDL1 immune checkpoint inhibitor was added to the treatment protocol to investigate whether pretreatment with a metronomic/normalizing dose of nanomedicine can improve the efficacy of nano-immunotherapy. Immunotherapy alone had no effect on tumor elastic properties or the various measures of perfusion (Fig. 4 and Supplementary Figs. S2–S4) and on tumor growth compared to control group (Fig. 3B and C). On the other hand, combinatorial treatment of Doxil with – aPDL1, improved the efficacy of the treatment even for the highest, less frequent dose of the nanomedicine (Fig. 3B and C) but the optimal efficacy of nano-immunotherapy was observed when nanomedicine was used in a metronomic and normalizing dose.
3.3. Optimizing the efficacy of nanoparticle formulations
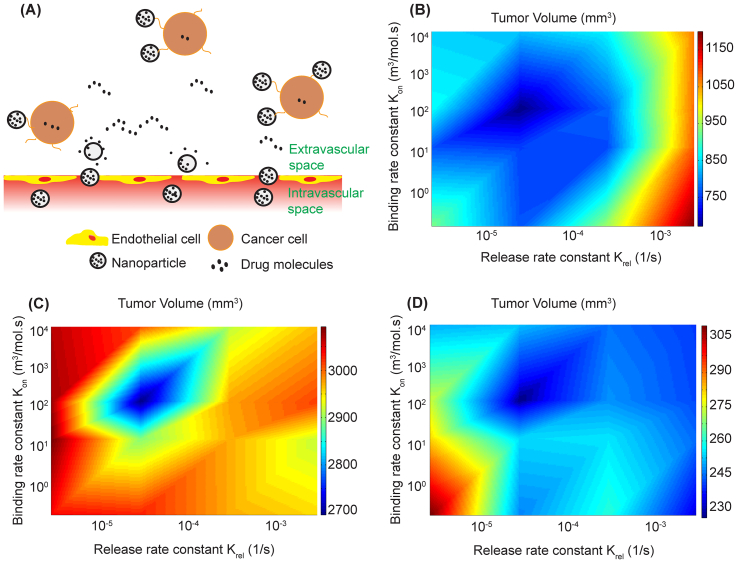
Nanoparticle size, drug release rate and – when applicable – binding affinity to cancer cells can determine the success of nanoparticle formulations on reducing tumor volume. To investigate whether an optimal combination of parameters exists, we incorporated into the mathematical model nanoparticle binding to cancer cells (Fig. 5A) and repeated the simulations for the protocol described in the study by Conley et al. [33] varying the drug release rate constant, krel, as well as the binding rate constant, kon, using a range of values from the literature. Simulations were also carried out for larger nanoparticles, 100 nm in diameter, that can carry a higher amount of the cytotoxic agent but as we have shown previously their delivery through normalized vessels is not improved owing to their large size [53]. For treatment with 20 nm particles we found the combination of the two rate constants that minimized tumor volume (Fig. 5B). Particles of this size can diffuse fast enough in the tumor interstitial space to distribute uniformly into the tumor provided that binding affinities are not so high as to trap the nanoparticles near the hyper-permeable tumor vessels nor the drug release rates so high that the nanoparticles lack sufficient time to diffuse. Either extreme can significantly reduce the efficacy of the treatment from its optimum. Model predictions for 100 nm particles using data for the load capacity of Doxil and the cytotoxic potential of doxorubicin [72] are shown in Fig. 5C. Our model is in agreement with experimental findings that transport of large nanoparticles across the pores of normalized tumor vessels remains limited [53]. As a result, efficacy of treatment is compromised but an optimal combination of parameters still exists which occurs at nearly the same combination of parameter values. It should be noticed, however, that the potency of doxorubicin is one-sixth that of camptothecin. To further explore the effect of drug potency on tumor volume, we carried out simulations assuming nanoparticles with the size and load capacity of Doxil and the potency of camptothecin. Interestingly, our model predicts the volume of the tumor was significantly reduced (Fig. 5D). Therefore, even though delivery of large particles is limited, its high load capacity and potency of the carrying drug may result in improved therapy.
Fig. 5.
(A) Schematic of nanoparticle transport accounting for particle binding to cancer cells. Effect of drug release constant, krel, and binding rate constant, kon, on tumor volume for (B) 20 nm particles specified to CRLX101, (C) 100 nm particles specified to Doxil and (D) 100 nm particles with increased potency of the carrying chemotherapy.
4. Conclusions
Nanomedicine and metronomic therapy have been regarded as two different approaches to treat cancer. Metronomic therapy aims not only to increase cancer cell killing compared to standard chemotherapy protocols but also to normalize the tumor microenvironment, improving blood vessel function and immune activation. Nanoparticles were developed for improved pharmacokinetic properties and preferential accumulation to tumor tissue owing to the EPR effect. Our analysis of preclinical experimental data suggests that these two approaches can be viewed using the same unified framework as strategies that normalize the tumor microenvironment by delivering lower, but more sustained levels of drug to the tumor than achieved with MTD. Additionally, we showed experimentally that low and more frequent doses of Doxil – compared to high and less frequent doses – decrease tumor stiffness, improve perfusion and exhibit enhanced anti-tumor effects either alone or in combination with immunotherapy. Use of appropriately-sized nanoparticles to normalize the tumor microenvironment could be advantageous over MTD chemotherapy because preferential targeting of tumor tissue could ameliorate adverse effects of MTD chemotherapy, whereas improved vascular function could increase the effectiveness of the immune system and importantly the intratumoral delivery of nanoparticles, which is the main barrier for the effective use of nanomedicines today. Importantly, to account for heterogeneity among different tumors types, we employed two tumor models, the 4T1 breast cancer and the MCA205 fibrosarcoma. Both tumor types are considered to be desmoplastic, having an abundant extracellular matrix, but they are expected to have differences in vascular permeability. The 4T1 tumors have hyper-permeable vessels and have been employed in several studies involving the transport of nanoparticles in tumors [37,38,53,73], whereas fibrosarcomas are considered less permeable [74], but there are not pertinent data for MCA205 tumors. Therefore, our results for the improved normalization effects of nanomedicine and metronomic therapy are valid despite heterogeneities in the EPR effect.
Our mathematical approach is subject to certain limitations. In our mathematical model, we assumed that enhancement in treatment efficacy of cancer nanomedicine is observed when vascular normalization (i.e., decrease of vessel wall pore size) was accounted for as it is indicated by the reduction in the IFP observed experimentally (Fig. 4). Also, we accounted only for changes in vessel wall diameter and pore size, and not the vessel morphology and effects of therapy on functional vascular density. Furthermore, we did not account for changes in the amount of collagen or cancer-associated fibroblasts owing to a desmoplastic response often observed in tumors, which would affect tumor growth. Incorporation of these parameters is not expected to change our results qualitatively.
In conclusion, the predictions of our mathematical model along with the experimental findings using two orthotopic and syngeneic tumor models provide evidence that therapeutic effects of nanomedicines can be improved when a metronomic dose schedule is employed. In addition, our results suggest that metronomic use of nanomedicine can further improve the efficacy of nano-immunotherpy. We should also notice that immune checkpoint inhibitors can also normalize tumor blood vessels and for that reason in some clinical studies for melanoma, lung and head and neck cancers, immunotherapy preceded chemotherapy in order to improve delivery of the chemotherapeutic drug [[75], [76], [77]]. In our study, a different strategy was employed having as a normalization agent the nanoparticle formulation.
Declaration of Competing Interest
Rakesh K. Jain received consultant fees from Elpis, Innocoll, SPARC, SynDevRx; owns equity in Accurius, Enlight, Ophthotech, SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund; and received a grant from Boehringer Ingelheim. Neither any reagent nor any funding from these organizations was used in this study.
Acknowledgments
This project was supported by Grants from the European Research Council (ERC-2013-StG-336839, ERC-2019-CoG-863955) and Grants co-financed by the European Regional Development Fund and the Republic of Cyprus through the Research and Innovation Foundation (INFRASTRUCTURE/1216/0052, POST-DOC/0718/0084) to T.S., Marie Skłodowska Curie Actions Individual Fellowship Global (Horizon 2020) (MSCA-IF-GF-2020-101028945) to C.V., Grants from the U.S. National Cancer Institute R01-CA208205, R01-CA259253, R01NS118929, U01-CA224348, U01CA261842, Outstanding Investigator Award R35-CA197743 and Grants from the National Foundation for Cancer Research, Jane's Trust Foundation, Nile Albright Medical Research Foundation, and Harvard Ludwig Cancer Center to R.K.J. and Grant R01 HL128168 to J.W.B.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2022.03.008.
Contributor Information
Rakesh K. Jain, Email: jain@steele.mgh.harvard.edu.
Triantafyllos Stylianopoulos, Email: tstylian@ucy.ac.cy.
Appendix A. Supplementary data
Supplementary material
References
- 1.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors, nature reviews. Clin. Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stylianopoulos T., Jain R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015;11:1893–1907. doi: 10.1016/j.nano.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chauhan V.P., Stylianopoulos T., Boucher Y., Jain R.K. Delivery of molecular and nanomedicine to tumors: transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1 [Google Scholar]
- 5.Stylianopoulos T., Munn L.L., Jain R.K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer. 2018;4:292–319. doi: 10.1016/j.trecan.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojha T., Pathak V., Shi Y., Hennink W.E., Moonen C.T.W., Storm G., Kiessling F., Lammers T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017;119:44–60. doi: 10.1016/j.addr.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges, nature reviews. Clin. Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 10.Kerbel R.S., Kamen B.A. The anti-angiogenic basis of metronomic chemotherapy. Nat. Rev. Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 11.Pasquier E., Kavallaris M., Andre N. Metronomic chemotherapy: new rationale for new directions, nature reviews. Clin. Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 12.Andre N., Carre M., Pasquier E. Metronomics: towards personalized chemotherapy?, nature reviews. Clin. Oncol. 2014;11:413–431. doi: 10.1038/nrclinonc.2014.89. [DOI] [PubMed] [Google Scholar]
- 13.Kerbel R.S. Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast Cancer. J. Mammary Gland Biol. Neoplasia. 2012;17:229–239. doi: 10.1007/s10911-012-9266-0. [DOI] [PubMed] [Google Scholar]
- 14.Kerbel R.S., Grothey A. Gastrointensinal cancer rationale for metronomic chemotherapy in phase III trials. Nat. Rev. Clin. Oncol. 2015;12:313–314. doi: 10.1038/nrclinonc.2015.89. [DOI] [PubMed] [Google Scholar]
- 15.Jedeszko C., Paez-Ribes M., Di Desidero T., Man S., Lee C.R., Xu P., Bjarnason G.A., Bocci G., Kerbel R.S. Postsurgical adjuvant or metastatic renal cell carcinoma therapy models reveal potent antitumor activity of metronomic oral topotecan with pazopanib. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.3010722. [DOI] [PubMed] [Google Scholar]
- 16.Kerbel R.S., Shaked Y. The potential clinical promise of ‘multimodality’ metronomic chemotherapy revealed by preclinical studies of metastatic disease. Cancer Lett. 2017;400:293–304. doi: 10.1016/j.canlet.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Cazzaniga M.E., Biganzoli L., Cortesi L., De Placido S., Donadio M., Fabi A., Ferro A., Generali D., Lorusso V., Milani A., Montagna E., Munzone E., Orlando L., Pizzuti L., Simoncini E., Zamagni C., Pappagallo G.L., G. Metronomic chemotherapy in advanced breast cancer study, treating advanced breast cancer with metronomic chemotherapy: what is known, what is new and what is the future? OncoTargets Ther. 2019;12:2989–2997. doi: 10.2147/OTT.S189163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simsek C., Esin E., Yalcin S. Metronomic chemotherapy: a systematic review of the literature and clinical experience. J. Oncol. 2019;2019:5483791. doi: 10.1155/2019/5483791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fares J.E., El Tomb P., Khalil L.E., Atwani R.W., Moukadem H.A., Awada A., El Saghir N.S. Metronomic chemotherapy for patients with metastatic breast cancer: review of effectiveness and potential use during pandemics. Cancer Treat. Rev. 2020;89:102066. doi: 10.1016/j.ctrv.2020.102066. [DOI] [PubMed] [Google Scholar]
- 20.Jain R.K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 21.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 22.Doloff J.C., Khan N., Ma J., Demidenko E., Swartz H.M., Jounaidi Y. Increased tumor oxygenation and drug uptake during anti-angiogenic weekly low dose cyclophosphamide enhances the anti-tumor effect of weekly tirapazamine. Curr. Cancer Drug Targets. 2009;9:777–788. doi: 10.2174/156800909789271503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cham K.K., Baker J.H., Takhar K.S., Flexman J.A., Wong M.Q., Owen D.A., Yung A., Kozlowski P., Reinsberg S.A., Chu E.M., Chang C.W., Buczkowski A.K., Chung S.W., Scudamore C.H., Minchinton A.I., Yapp D.T., Ng S.S. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br. J. Cancer. 2010;103:52–60. doi: 10.1038/sj.bjc.6605727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain R.K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain R.K. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkins C., Man S., Xu P., Shaked Y., Hicklin D.J., Kerbel R.S. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 27.Vives M., Ginesta M.M., Gracova K., Graupera M., Casanovas O., Capella G., Serrano T., Laquente B., Vinals F. Metronomic chemotherapy following the maximum tolerated dose is an effective anti-tumour therapy affecting angiogenesis, tumour dissemination and cancer stem cells. Int. J. Cancer. 2013;133:2464–2472. doi: 10.1002/ijc.28259. [DOI] [PubMed] [Google Scholar]
- 28.Pucci F., Garris C., Lai C.P., Newton A., Pfirschke C., Engblom C., Alvarez D., Sprachman M., Evavold C., Magnuson A., von Andrian U.H., Glatz K., Breakefield X.O., Mempel T.R., Weissleder R., Pittet M.J. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352:242–246. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu Lila A.S., Ishida T. Metronomic chemotherapy and nanocarrier platforms. Cancer Lett. 2017;400:232–242. doi: 10.1016/j.canlet.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y.L., Chang M.C., Cheng W.F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017;400:282–292. doi: 10.1016/j.canlet.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q., Xia R., Zheng W., Zhang L., Li P., Sun X., Shi J. Metronomic paclitaxel improves the efficacy of PD-1 monoclonal antibodies in breast cancer by transforming the tumor immune microenvironment. Am. J. Transl. Res. 2020;12:519–530. [PMC free article] [PubMed] [Google Scholar]
- 32.Parra K., Valenzuela P., Lerma N., Gallegos A., Reza L.C., Rodriguez G., Emmenegger U., Di Desidero T., Bocci G., Felder M.S., Manciu M., Kirken R.A., Francia G. Impact of CTLA-4 blockade in conjunction with metronomic chemotherapy on preclinical breast cancer growth. Br. J. Cancer. 2017;116:324–334. doi: 10.1038/bjc.2016.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conley S.J., Baker T.L., Burnett J.P., Theisen R.L., Lazarus D., Peters C.G., Clouthier S.G., Eliasof S., Wicha M.S. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res. Treat. 2015;150:559–567. doi: 10.1007/s10549-015-3349-8. [DOI] [PubMed] [Google Scholar]
- 34.Pham E., Yin M., Peters C.G., Lee C.R., Brown D., Xu P., Man S., Jayaraman L., Rohde E., Chow A., Lazarus D., Eliasof S., Foster F.S., Kerbel R.S. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 2016;76:4493–4503. doi: 10.1158/0008-5472.CAN-15-3435. [DOI] [PubMed] [Google Scholar]
- 35.Tian X., Nguyen M., Foote H.P., Caster J.M., Roche K.C., Peters C.G., Wu P., Jayaraman L., Garmey E.G., Tepper J.E., Eliasof S., Wang A.Z. CRLX101, a nanoparticle-drug conjugate containing camptothecin, improves rectal cancer chemoradiotherapy by inhibiting DNA repair and HIF1alpha. Cancer Res. 2017;77:112–122. doi: 10.1158/0008-5472.CAN-15-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt K.T., Chau C.H., Strope J.D., Huitema A.D.R., Sissung T.M., Price D.K., Figg W.D. Antitumor activity of NLG207 (formerly CRLX101) in combination with enzalutamide in preclinical prostate cancer models. Mol. Cancer Ther. 2021;20:915–924. doi: 10.1158/1535-7163.MCT-20-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panagi M., Voutouri C., Mpekris F., Papageorgis P., Martin M.R., Martin J.D., Demetriou P., Pierides C., Polydorou C., Stylianou A., Louca M., Koumas L., Costeas P., Kataoka K., Cabral H., Stylianopoulos T. TGF-beta inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics. 2020;10:1910–1922. doi: 10.7150/thno.36936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mpekris F., Panagi M., Voutouri C., Martin J.D., Samuel R., Takahashi S., Gotohda N., Suzuki T., Papageorgis P., Demetriou P., Pierides C., Koumas L., Costeas P., Kojima M., Ishii G., Constantinidou A., Kataoka K., Cabral H., Stylianopoulos T. Normalizing the microenvironment overcomes vessel compression and resistance to Nano-immunotherapy in breast Cancer lung metastasis. Adv. Sci. 2021;8:2001917. doi: 10.1002/advs.202001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J.U., Maharjan R., Pangeni R., Jha S.K., Lee N.K., Kweon S., Lee H.K., Chang K.Y., Choi Y.K., Park J.W., Byun Y. Modulating tumor immunity by metronomic dosing of oxaliplatin incorporated in multiple oral nanoemulsion. J. Control. Release Off. J. Control. Release Soc. 2020;322:13–30. doi: 10.1016/j.jconrel.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Pangeni R., Subedi L., Jha S.K., Kweon S., Kang S.H., Chang K.Y., Choi J.U., Byun Y., Park J.W. Improvements in the oral absorption and anticancer efficacy of an oxaliplatin-loaded solid formulation: pharmacokinetic properties in rats and nonhuman primates and the effects of oral metronomic dosing on colorectal cancer. Int. J. Nanomedicine. 2020;15:7719–7743. doi: 10.2147/IJN.S267424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mpekris F., Baish J.W., Stylianopoulos T., Jain R.K. Role of vascular normalization in benefit from metronomic chemotherapy. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1994–1999. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mpekris F., Voutouri C., Baish J.W., Duda D.G., Munn L.L., Stylianopoulos T., Jain R.K. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 2020;117:3728–3737. doi: 10.1073/pnas.1919764117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faivre C., Barbolosi D., Pasquier E., Andre N. A mathematical model for the administration of temozolomide: comparative analysis of conventional and metronomic chemotherapy regimens. Cancer Chemother. Pharmacol. 2013;71:1013–1019. doi: 10.1007/s00280-013-2095-z. [DOI] [PubMed] [Google Scholar]
- 44.Schattler H., Ledzewicz U., Amini B. Dynamical properties of a minimally parameterized mathematical model for metronomic chemotherapy. J. Math. Biol. 2016;72:1255–1280. doi: 10.1007/s00285-015-0907-y. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues D.S., Mancera P.F.A., Pinho S.T.R. Understanding the antiangiogenic effect of metronomic chemotherapy through a simple mathematical model. Phys. A. 2016;464:251–266. [Google Scholar]
- 46.Ciccolini J., Barbolosi D., Meille C., Lombard A., Serdjebi C., Giacometti S., Padovani L., Pasquier E., Andre N. Pharmacokinetics and pharmacodynamics-based mathematical modeling identifies an optimal protocol for metronomic chemotherapy. Cancer Res. 2017;77:4723–4733. doi: 10.1158/0008-5472.CAN-16-3130. [DOI] [PubMed] [Google Scholar]
- 47.Ledzewicz U., Wang S., Schattler H., Andre N., Heng M.A., Pasquier E. On drug resistance and metronomic chemotherapy: a mathematical modeling and optimal control approach. Math. Biosci. Eng. MBE. 2017;14:217–235. doi: 10.3934/mbe.2017014. [DOI] [PubMed] [Google Scholar]
- 48.Bodzioch M., Bajger P., Forys U. Angiogenesis and chemotherapy resistance: optimizing chemotherapy scheduling using mathematical modeling. J. Cancer Res. Clin. Oncol. 2021;147:2281–2299. doi: 10.1007/s00432-021-03657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozlowska E., Suwinski R., Giglok M., Swierniak A., Kimmel M. Mathematical model predicts response to chemotherapy in advanced non-resectable non-small cell lung cancer patients treated with platinum-based doublet. PLoS Comput. Biol. 2020;16 doi: 10.1371/journal.pcbi.1008234. e1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabizon A., Shmeeda H., Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 51.Voutouri C., Panagi M., Mpekris F., Stylianou A., Michael C., Averkiou M.A., Martin J.D., Stylianopoulos T. Endothelin inhibition potentiates Cancer immunotherapy revealing mechanical biomarkers predictive of response. Adv. Ther. 2021:2000289. [Google Scholar]
- 52.Goldman A., Majumder B., Dhawan A., Ravi S., Goldman D., Kohandel M., Majumder P.K., Sengupta S. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat. Commun. 2015;6:6139. doi: 10.1038/ncomms7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chauhan V.P., Stylianopoulos T., Martin J.D., Popovic Z., Chen O., Kamoun W.S., Bawendi M.G., Fukumura D., Jain R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang W., Huang Y., An Y., Kim B.Y. Remodeling tumor vasculature to enhance delivery of intermediate-sized nanoparticles. ACS Nano. 2015;9:8689–8696. doi: 10.1021/acsnano.5b02028. [DOI] [PubMed] [Google Scholar]
- 55.Theek B., Baues M., Gremse F., Pola R., Pechar M., Negwer I., Koynov K., Weber B., Barz M., Jahnen-Dechent W., Storm G., Kiessling F., Lammers T. Histidine-rich glycoprotein-induced vascular normalization improves EPR-mediated drug targeting to and into tumors. J. Control. Release Off. J. Control. Release Soc. 2018;282:25–34. doi: 10.1016/j.jconrel.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffon-Etienne G., Boucher Y., Brekken C., Suit H.D., Jain R.K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 57.Padera T.P., Stoll B.R., Tooredman J.B., Capen D., di Tomaso E., Jain R.K. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 58.Stylianopoulos T., Martin J.D., Snuderl M., Mpekris F., Jain S.R., Jain R.K. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 2013;73:3833–3841. doi: 10.1158/0008-5472.CAN-12-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mpekris F., Angeli S., Pirentis A.P., Stylianopoulos T. Stress-mediated progression of solid tumors: effect of mechanical stress on tissue oxygenation, cancer cell proliferation, and drug delivery. Biomech. Model. Mechanobiol. 2015;14:1391–1402. doi: 10.1007/s10237-015-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Dieras V., Hegg R., Im S.A., Shaw Wright G., Henschel V., Molinero L., Chui S.Y., Funke R., Husain A., Winer E.P., Loi S., Emens L.A., Investigators I.M.T. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 61.Adams S., Diamond J.R., Hamilton E., Pohlmann P.R., Tolaney S.M., Chang C.W., Zhang W., Iizuka K., Foster P.G., Molinero L., Funke R., Powderly J. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 2019;5:334–342. doi: 10.1001/jamaoncol.2018.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fadnes H.O., Reed R.K., Aukland K. Interstitial fluid pressure in rats measured with a modified wick technique. Microvasc. Res. 1977;14:27–36. doi: 10.1016/0026-2862(77)90138-8. [DOI] [PubMed] [Google Scholar]
- 63.Stylianopoulos T., Martin J.D., Chauhan V.P., Jain S.R., Diop-Frimpong B., Bardeesy N., Smith B.L., Ferrone C.R., Hornicek F.J., Boucher Y., Munn L.L., Jain R.K. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dietrich C.F., Averkiou M.A., Correas J.M., Lassau N., Leen E., Piscaglia F. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344–351. doi: 10.1055/s-0032-1313026. [DOI] [PubMed] [Google Scholar]
- 65.Strouthos C., Lampaskis M., Sboros V., McNeilly A., Averkiou M. Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010;57:1296–1310. doi: 10.1109/TUFFC.2010.1550. [DOI] [PubMed] [Google Scholar]
- 66.Averkiou M., Keravnou C.P., Izamis M.L., Leen E. Evaluation of perfusion quantification methods with ultrasound contrast agents in a machine-perfused pig liver. Ultraschall Med. 2018;39:69–79. doi: 10.1055/s-0042-104645. [DOI] [PubMed] [Google Scholar]
- 67.Xin L., Yan Z., Zhang X., Zang Y., Ding Z., Xue H., Zhao C. Parameters for contrast-enhanced ultrasound (CEUS) of enlarged superficial lymph nodes for the evaluation of therapeutic response in lymphoma: a preliminary study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:5430–5438. doi: 10.12659/MSM.907293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V., Stylianopoulos T., Mousa A., Han X., Adstamongkonkul P., Popovic Z., Bawendi M.G., Boucher Y., Jain R.K. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumor blood vessels. Nat. Commun. 2013;4 doi: 10.1038/ncomms.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papageorgis P., Polydorou C., Mpekris F., Voutouri C., Agathokleous E., Kapnissi-Christodoulou C.P., Stylianopoulos T. Tranilast-induced stress alleviation in solid tumors improves the efficacy of chemo- and nanotherapeutics in a size-independent manner. Sci. Rep. 2017;7:46140. doi: 10.1038/srep46140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y., Cao J., Melamed A., Worley M., Gockley A., Jones D., Nia H.T., Zhang Y., Stylianopoulos T., Kumar A.S., Mpekris F., Datta M., Sun Y., Wu L., Gao X., Yeku O., Del Carmen M.G., Spriggs D.R., Jain R.K., Xu L. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl. Acad. Sci. U. S. A. 2019;116:2210–2219. doi: 10.1073/pnas.1818357116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voutouri C., Mpekris F., Papageorgis P., Odysseos A.D., Stylianopoulos T. Role of constitutive behavior and tumor-host mechanical interactions in the state of stress and growth of solid tumors. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104717. e104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eikenberry S. A tumor cord model for doxorubicin delivery and dose optimization in solid tumors. Theor. Biol. Med. Model. 2009;6:16. doi: 10.1186/1742-4682-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Igarashi K., Cabral H., Hong T., Anraku Y., Mpekris F., Stylianopoulos T., Khan T., Matsumoto A., Kataoka K., Matsumoto Y., Yamasoba T. Vascular bursts act as a versatile tumor vessel permeation route for blood-borne particles and cells. Small. 2021;17 doi: 10.1002/smll.202103751. e2103751. [DOI] [PubMed] [Google Scholar]
- 74.Hobbs S.K., Monsky W.L., Yuan F., Roberts W.G., Griffith L., Torchilin V.P., Jain R.K. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saint-Jean M., Fronteau C., Peuvrel L., Khammari A., Varey E., Quereux G., Dreno B. Chemotherapy efficacy after first-line immunotherapy in 18 advanced melanoma patients. Medicine. 2020;99 doi: 10.1097/MD.0000000000021329. e21329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schvartsman G., Peng S.A., Bis G., Lee J.J., Benveniste M.F.K., Zhang J., Roarty E.B., Lacerda L., Swisher S., Heymach J.V., Fossella F.V., William W.N. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–95. doi: 10.1016/j.lungcan.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 77.Saleh K., Daste A., Martin N., Pons-Tostivint E., Auperin A., Herrera-Gomez R.G., Baste-Rotllan N., Bidault F., Guigay J., Le Tourneau C., Saada-Bouzid E., Even C. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur. J. Cancer. 2019;121:123–129. doi: 10.1016/j.ejca.2019.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material