Significance
Multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), are autoimmune diseases characterized by accumulation of myeloid cells in the central nervous system (CNS). Both harmful and beneficial myeloid cells are present in EAE/MS, and a goal of MS therapy is to preferentially remove harmful myeloid cells. The receptor for CSF-1 (CSF-1R) is found on myeloid cells and is important for their survival. CSF-1R can bind two ligands, CSF-1 and IL-34, but it is not known whether their functions in EAE/MS differ. We found that blocking CSF-1 depleted only harmful myeloid cells in the CNS and suppressed EAE, whereas blocking IL-34 had no effect. Thus, we propose that blocking CSF-1 could be a therapy for MS.
Keywords: CSF-1R, EAE, multiple sclerosis, CSF-1, IL-34
Abstract
The receptor for colony stimulating factor 1 (CSF-1R) is important for the survival and function of myeloid cells that mediate pathology during experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). CSF-1 and IL-34, the ligands of CSF-1R, have similar bioactivities but distinct tissue and context-dependent expression patterns, suggesting that they have different roles. This could be the case in EAE, given that CSF-1 expression is up-regulated in the CNS, while IL-34 remains constitutively expressed. We found that targeting CSF-1 with neutralizing antibody halted ongoing EAE, with efficacy superior to CSF-1R inhibitor BLZ945, whereas IL-34 neutralization had no effect, suggesting that pathogenic myeloid cells were maintained by CSF-1. Both anti–CSF-1 and BLZ945 treatment greatly reduced the number of monocyte-derived cells and microglia in the CNS. However, anti–CSF-1 selectively depleted inflammatory microglia and monocytes in inflamed CNS areas, whereas BLZ945 depleted virtually all myeloid cells, including quiescent microglia, throughout the CNS. Anti–CSF-1 treatment reduced the size of demyelinated lesions and microglial activation in the gray matter. Lastly, we found that bone marrow–derived immune cells were the major mediators of CSF-1R–dependent pathology, while microglia played a lesser role. Our findings suggest that targeting CSF-1 could be effective in ameliorating MS pathology, while preserving the homeostatic functions of myeloid cells, thereby minimizing risks associated with ablation of CSF-1R–dependent cells.
Multiple sclerosis (MS) is an autoimmune disease characterized by accumulation of immune cells in inflamed areas of the central nervous system (CNS), which eventually become demyelinated lesions (1). Myeloid cells account for up to 78% of immune cells in active MS lesions (2), suggesting that they are the major mediators of pathology. In support of this notion, studies in experimental autoimmune encephalomyelitis (EAE), an animal model of MS, have demonstrated the essential role of myeloid cells in EAE pathology, as interventions that affect them, in particular, monocytes and conventional dendritic cells (cDCs), ameliorate or abrogate EAE (3, 4). However, despite evidence on the importance of myeloid cells in CNS autoimmunity, they have not been specifically targeted for MS therapy. This provides an opportunity for devising therapeutic approaches that target myeloid cells relevant to MS pathology.
Receptor for colony stimulating factor 1 (CSF-1R) is a cell-surface receptor tyrosine kinase that binds two ligands, CSF-1 (also known as M-CSF) and IL-34 (5). CSF-1R signaling facilitates survival and proliferation of myeloid cells, with either CSF-1 or IL-34 predominantly controlling the size of myeloid cell populations in various organs and tissues (5–7). CSF-1R is expressed by microglia, monocytes, and monocyte-derived cells, which comprise the bulk of myeloid cells in the CNS during MS and EAE (6). CSF-1R function is not intrinsically proinflammatory, as CSF-1R signaling in steady state induces a regulatory/homeostatic phenotype in macrophages, and a resting/quiescent phenotype in microglia (8, 9). However, in inflammation, CSF-1R function could indirectly be proinflammatory by perpetuating the survival and expansion of inflammatory myeloid cells. A recent study found an increase in both CSF-1R and CSF-1 in and around demyelinating lesions in cortical white matter of patients with progressive MS, and elevated CSF-1 in patients’ cerebrospinal fluid, whereas IL-34 was not increased, suggesting that CSF-1 drives the deleterious role of CSF-1R in MS (10). In the progressive EAE model, CSF-1R has been identified as a key regulator of the inflammatory response in the CNS, with CSF-1R and CSF-1 expression levels correlating with disease progression. Inhibition of CSF-1R with small molecule inhibitor in mice with EAE reduced the expression of proinflammatory genes, and the remaining microglia had homeostatic gene expression profile (10).
It has been shown that direct inhibition of CSF-1R kinase activity with small molecule inhibitors suppresses EAE pathology (11), but the effects of CSF-1R inhibition on particular cell subsets remain poorly understood. Different methods for reducing CSF-1R signaling, such as by antibodies (Ab) against the receptor or its individual ligands, have not been compared with small molecule inhibitors. The principal difference between these blocking methods is that small molecule inhibitors readily penetrate the entire CNS (12), whereas Abs have limited access, mainly to inflamed areas where the blood–brain barrier (BBB) has been compromised (6, 13). Furthermore, inhibitors of CSF-1R kinase activity could also affect other kinases (14), causing unwanted effects, especially over their prolonged use for therapy. In addition, direct inhibition of CSF-1R indiscriminately affects its systemic functions in their entirety, whereas individual neutralization of CSF-1 and IL-34 would have more nuanced effects, by preserving functions of the ligand that has not been targeted. These differences could lead to distinct outcomes in therapy of autoimmune neuroinflammation given that the types and numbers of myeloid cells affected by various means of CSF-1R inhibition can substantially differ. In addition, any indirect effects of CSF-1R inhibition on cells that do not express CSF-1R also remain largely uncharacterized.
Our and other studies show that small molecule inhibitors of CSF-1R cause a profound depletion of microglia (6, 12), which may be an important drawback in their use for therapy, as microglia play important roles in CNS homeostasis (9). It has also been shown that neurons can express CSF-1R during excitotoxic injury, contributing to their survival (9). Notably, it remains unknown whether neurons express CSF-1R in EAE and MS and if its expression would be of significance, but if so, restricting inhibition of CSF-1R signaling to inflamed areas of the CNS could be beneficial for neuronal survival. Thus, the ideal MS therapy would preferentially block CSF-1R signaling only in inflamed regions, affecting only inflammatory myeloid cells in them while sparing cells in the rest of the CNS, such as quiescent microglia. This may be accomplished by targeting CSF-1R ligands, CSF-1 and IL-34, which show spatial- and context-dependent differences in expression (15). Although some differences have been described (16), CSF-1 and IL-34 signaling via CSF-1R have similar effects at the cellular level (17), suggesting that the differences found in animals lacking CSF-1 or IL-34 are primarily due to differential expression patterns (18). IL-34 also binds to two additional receptors, PTP-ζ (19), which has functions in oligodendrocyte development and homeostasis (20), and CD138 (Syndecan-1), which modulates IL-34–dependent CSF-1R activity (21). CSF-1 is systemically the dominant CSF-1R ligand, with its serum concentrations approximately 10 times greater than that of IL-34 (22, 23). Importantly, CSF-1 is not highly expressed in the CNS, but its expression can be up-regulated by inflammation or injury (24), facilitating expansion of myeloid cells at the site of inflammation. In contrast to more widespread CSF-1 expression, IL-34 is primarily and constitutively expressed in the CNS and skin (25, 26). In steady state, IL-34 maintains survival of tissue-resident myeloid cells in the skin and CNS, as the primary effect of IL-34 knockout is lack of Langerhan’s cells and microglia, respectively (15). IL-34 is the predominant CSF-1R ligand in the CNS, accounting for 70% of total CSF-1R signaling in healthy brain (9).
In the present study, we sought to understand how CSF-1R inhibition affects immune cells in the CNS of mice with EAE and to determine how the effects of blocking CSF-1 and IL-34 may differ from blocking CSF-1R. We found that treatment with CSF-1R inhibitor BLZ945 suppresses EAE when given both prophylactically and therapeutically. Treatment efficacy correlated with a profound reduction in the numbers of monocytes, monocyte-derived dendritic cells (moDCs), and microglia, suggesting that loss of one or more of these cell types is responsible for EAE suppression. We also found that blocking CSF-1 but not IL-34 with Abs suppressed EAE. Importantly, anti–CSF-1 treatment preferentially depleted inflammatory myeloid cells, whereas quiescent microglia were preserved. These findings suggest that blocking CSF-1 may be a better therapeutic strategy for alleviating MS pathology than inhibiting CSF-1R itself.
Results
Blocking CSF-1R or CSF-1 but not IL-34 Suppresses EAE.
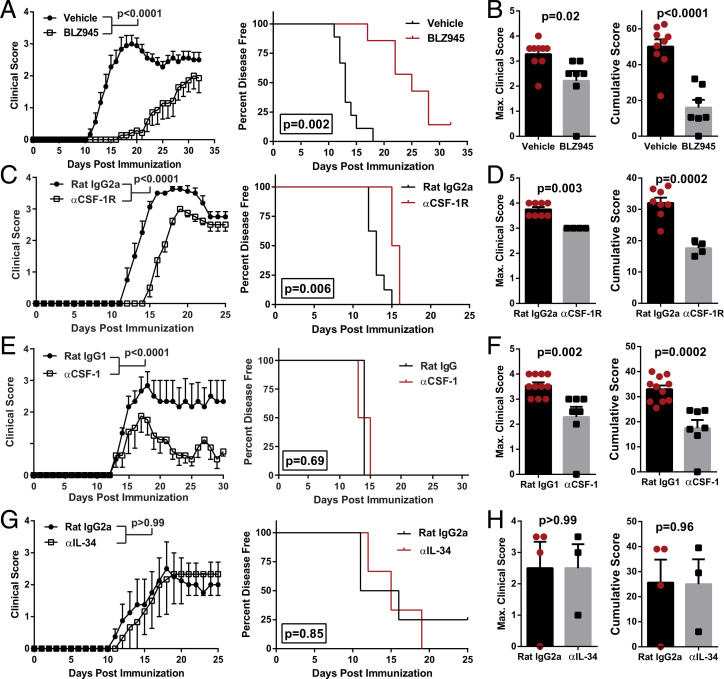
To determine the roles of CSF-1R, CSF-1, and IL-34 in EAE, we blocked them with either a small-molecule inhibitor or monoclonal Abs (MAbs). We blocked CSF-1R kinase function with BLZ945, a CNS-penetrant small molecule that, among other effects, efficiently depletes microglia within several days of treatment (12). Prophylactic treatment with BLZ945 delayed the onset of EAE (Fig. 1 A and B) and initially suppressed disease severity but did not preclude subsequent disease progression despite continuous treatment. We also blocked CSF-1R activity with MAb, which was less effective than BLZ945 in delaying EAE onset and reducing disease severity (Fig. 1 C and D). Surprisingly, anti–CSF-1R MAb (clone AFS98) failed to bind to microglia, as determined by flow cytometry, whereas it bound to monocytes (SI Appendix, Fig. S1A). It is unclear why this MAb did not bind to microglia and how that may have impacted its effect in EAE.
Fig. 1.
Blocking CSF-1R activity suppresses EAE. (A–D) C57BL/6J mice were immunized with MOG35-55 for EAE induction. Clinical course, maximum and cumulative clinical scores, and Kaplan–Meier plots depicting percent of disease-free animals over time are shown. Significance for clinical course data were calculated by two-way repeated measures ANOVA. Significance for maximum and cumulative clinical scores was calculated by Student’s t test. Error bars are SEM. Significance for Kaplan–Meier plots was calculated by comparing disease-free curves with the log-rank (Mantel–Cox) test. (A and B) EAE animals treated orally with BLZ945 (n = 9; 4 mg/day) or vehicle (n = 7; 20% Captisol) daily, starting from day of immunization. Data were compiled from two independent experiments. (C and D) Treatment with αCSF-1R MAb (n = 4) or control rat IgG2a (n = 8). MAbs were i.p. injected every other day (400 µg per dose). (E) Treatment with αCSF-1 MAb (n = 4) or control rat IgG1 (n = 3). One of three representative experiments with similar results are shown. (F) Maximum and cumulative clinical scores compiled from two independent experiments where mice were treated with either αCSF-1 MAb (n = 7) or control rat IgG1 (n = 11) and observed up to day 23 p.i. MAbs were i.p. injected every other day (200 µg per dose). (G and H) Treatment with αΙL-34 MAb (n = 3) or control rat IgG2a MAb (n = 4). MAbs were i.p. injected every other day (100 µg per dose).
We then tested how neutralization of either CSF-1 or IL-34 with MAbs would affect EAE. In contrast to blocking CSF-1R, neutralization of CSF-1 did not delay onset of disease (Fig. 1 E and F) but did continuously suppress its severity. Anti–IL-34 treatment did not suppress EAE (Fig. 1 G and H), although these data are limited by the small number of mice (n = 3). We tested whether our treatments with the MAbs, which were rat IgGs, induced an anti-rat IgG Abs in mice with EAE. Anti–CSF-1 and control isotype MAb induced similar low titers of anti-rat IgG, whereas anti–CSF-1R and anti–IL-34 MAbs induced notably higher anti-rat IgG titers (SI Appendix, Fig. S1B), indicating that anti-rat IgG responses may have reduced the effects of anti–CSF-1R and anti–IL-34 MAbs. To minimize the development of anti-rat IgG responses, we started anti–IL-34 treatment after the onset of clinical disease, but similar to prophylactic treatment, this had no impact on disease (SI Appendix, Fig. S1C). We also found that intraperitoneal (i.p.) administration of recombinant CSF-1 did not worsen disease (SI Appendix, Fig. S1D). Overall, these data show that blocking CSF-1R signaling attenuates EAE and that CSF-1 is the relevant CSF-1R ligand for EAE pathology.
Inhibition of CSF-1R Signaling Depletes Myeloid Antigen-Presenting Cells (APCs) in the CNS during EAE.
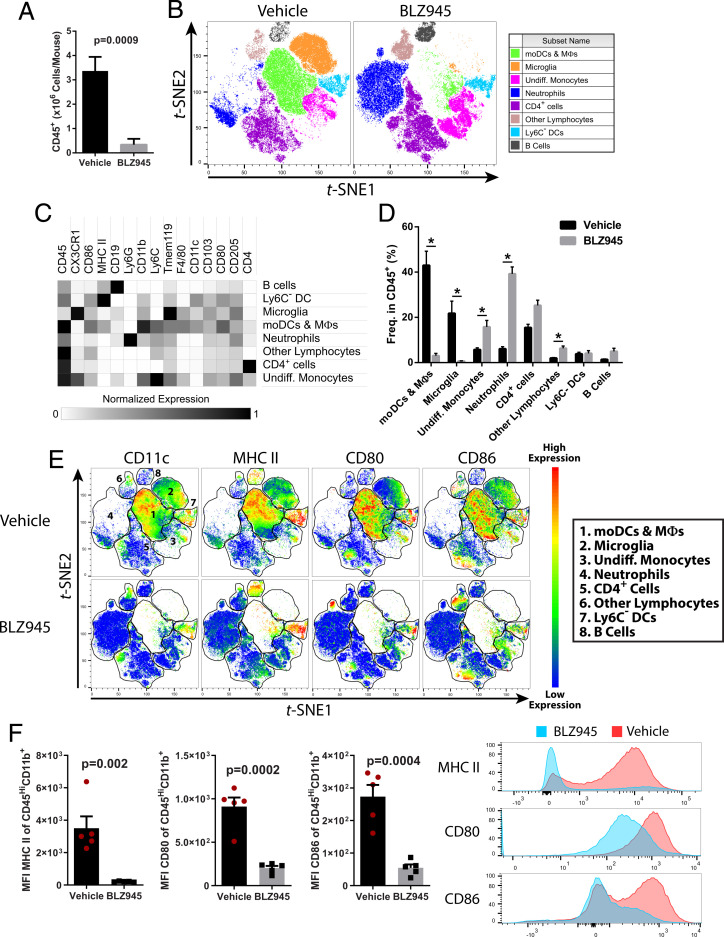
We characterized how prophylactic treatment with BLZ945 influenced CNS inflammation at the peak of EAE. BLZ945-treated animals had ∼90% reduced numbers of CD45+ cells in the CNS compared to vehicle-treated animals (Fig. 2A). All major types of immune cells were reduced in number, but CD11b+ cells were most impacted, including profound depletion of CD45LowCD11b+Tmem119+CX3CR1Hi microglia and CD45HiCD11b+CD11c+ myeloid DCs, including CD45HiCD11b+CD11c+Ly6GLow/-Ly6CHiMHCIIHi moDCs (SI Appendix, Fig. S2). We clustered flow cytometry data by t-stochastic neighbor embedding (t-SNE), and consistent with manual gating, the frequency of microglia and moDCs/macrophages was dramatically reduced (Fig. 2 B–E). In contrast, the frequency of neutrophils was increased, likely reflecting that they do not express CSF-1R (27) and are therefore not impacted by CSF-1R inhibition. Interestingly, the frequency of undifferentiated monocytes (CD45HiCD11b+Ly6CHiCD11c−MHCII−) increased, suggesting that primarily monocyte-derived cells are CSF-1R dependent. Overall, BLZ945 treatment markedly reduced the frequency of CD11c+ myeloid antigen-presenting cells (APCs) expressing MHCII, CD80, and CD86 (Fig. 2 E and F), suggesting that CSF-1R signaling maintains sufficient numbers of APCs in the CNS to drive inflammation during EAE. Lastly, we found differences in cytokine production by CD4+ T cells from BLZ945-treated mice, including lower frequency of GM-CSF+ cells (SI Appendix, Fig. S2G).
Fig. 2.
CSF-1R inhibition depletes myeloid APCs in the CNS of mice with EAE. C57BL/6J mice were immunized for EAE induction and treated orally with BLZ945 (4 mg/day) or vehicle control (20% captisol) daily, starting on the day of immunization. Mice were euthanized on day 15 p.i., and brains and spinal cords were pooled for cell isolation. (A) Numbers of CNS CD45+ cells (n = 7/group, combined from two independent experiments). (B) t-stochastic neighbor embedding plot depicting clustering of CD45+ cells (n = 5 mice per group). moDCs and macrophages were defined as CD45Hi CD11b+CD11c+Ly6C+MHCII+CD80+CD86+. Microglia were defined as CD45+CD11b+Tmem119+CX3CR1Hi cells. Undifferentiated monocytes were defined as CD45HiLy6CHiCD11c− cells that were overall MHCIILo/Neg. Neutrophils were defined as CD45+CD11b+Ly6GHi. CD4+ cells were defined as CD45+CD4+. Other lymphocytes were defined as CD45HiSSCLo. Ly6C− DCs were defined as CD45HiCD11b+CD11c+MHCIIHiLy6C− cells. B cells were defined as CD45+CD19+. (C) Heatmap showing normalized expression of markers used to identify clusters. (D) Quantification of clusters between vehicle- and BLZ945-treated mice with EAE. (E) Heatmap of CD11c, MHCII, CD80, and CD86 expression among CD45+ cells. (F) MFI of MHCII, CD80, and CD86 among CD45HiCD11b+ myeloid cells. Representative histograms showing fluorescence intensity of MHCII, CD80, and CD86 between vehicle- and BLZ945-treated animals is also shown. y-axis is frequency of cells as normalized to mode. Significance was calculated with Student’s t test. For (D),* indicates a P < 0.02. Error bars are SEM.
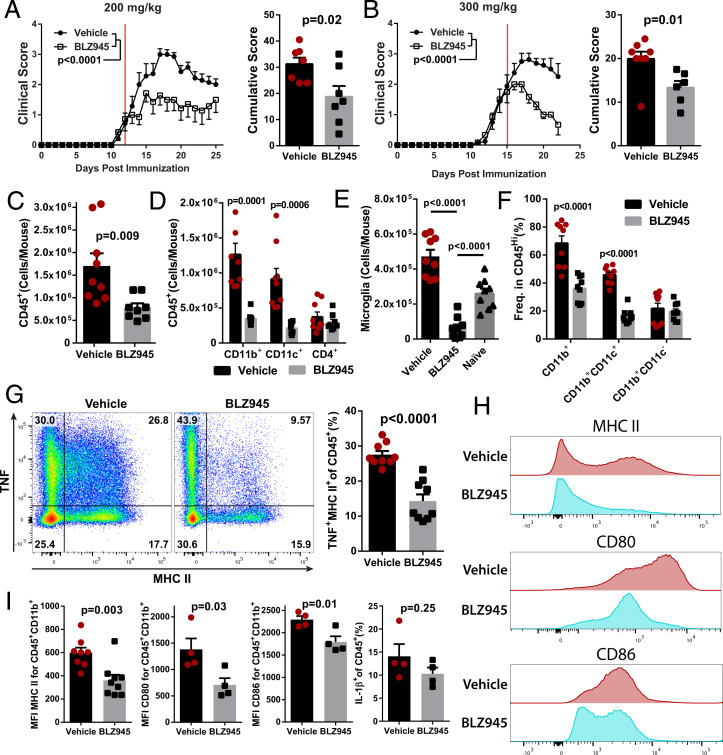
We next tested BLZ945 treatment during ongoing EAE, as that scenario is the most relevant to MS therapy. Therapeutic treatment with 200 mg/kg/day BLZ945 suppressed clinical EAE, but better efficacy was observed with 300 mg/kg/day (Fig. 3 A and B). To determine the acute effects of CSF-1R inhibition on immune cells in the CNS, we focused our analysis on mice treated with BLZ945 for 6 d, starting at a clinical score of 2.0. BLZ945-treated mice had reduced numbers of CD45+ cells in the CNS, primarily due to fewer CD11b+ and CD11c+ cells, including microglia and CD45HiCD11b+CD11c+ myeloid DCs (Fig. 3 C–F). Most immune cells depleted by BLZ945 treatment coexpressed CD11c, TNF, MHCII, CD80, and CD86, suggesting that inflammatory APCs are preferentially affected by CSF-1R inhibition (Fig. 3 G–I). IL-1β production was not affected in BLZ945-treated mice (Fig. 3I). Taken together, these data further indicate that inhibition of CSF-1R signaling suppresses EAE by reducing numbers of APCs in the CNS.
Fig. 3.
BLZ945 suppresses ongoing clinical EAE and reduces the number of myeloid APCs in the CNS. C57BL/6J mice were immunized and allowed to develop clinical signs of EAE before treatment with BLZ945 (n = 7) or vehicle (n = 8). (A) Clinical course and cumulative score for mice treated with 200 mg/kg BLZ945 starting at a clinical score of ∼1. The red line indicates start of treatment. (B) Mice treated with 300 mg/kg BLZ945 (n = 6) or vehicle (n = 8), starting at a clinical score of ∼2. A and B were compiled from two independent experiments. Significance for clinical course determined by two-way repeated measures ANOVA and by unpaired Student’s t test for cumulative scores. (C–I) Analysis of the CNS (pooled brain and spinal cords) by flow cytometry. (C) Number of CD45+ cells. (D) Number of CD45+ cells that also expressed CD11b, CD11c, or CD4. (E) Number of CD45LoCD11b+CX3CR1Hi microglia. Naïve mice were not immunized or otherwise manipulated. (F) Frequency of CD45Hi cells that also express CD11b and/or CD11c. (G) TNF and MHCII expression in CD45+ cells. (H and I) Expression of MHCII, CD80, and CD86 in CD45+CD11b+ cells. Significance for (C–I) was calculated by unpaired Student’s t test. P value corrections for multiple comparisons was performed by false discovery rate approach with Q = 0.01 as a cutoff. Error bars are SEM.
Blocking CSF-1 Depletes Inflammatory Myeloid APCs in the CNS without Affecting Quiescent Microglia.
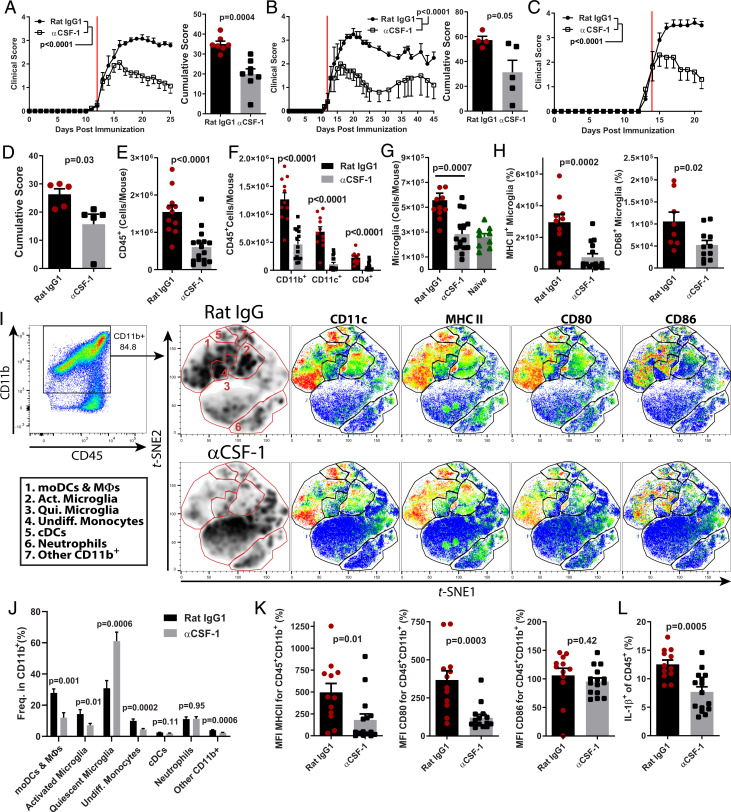
We next tested the therapeutic efficacy of anti–CSF-1 MAb treatment. Treatment initiated after onset of disease suppressed clinical EAE (Fig. 4A), and the suppression was maintained up to 45 d after EAE induction, which was the longest period tested (Fig. 4B). Similar to BLZ945, anti–CSF-1 MAb suppressed disease even when treatment was initiated during its more advanced stage (Fig. 4 C and D). Mice treated with anti–CSF-1 MAb had fewer CD45+ immune cells in the CNS, including CD11b+, CD11c+, and CD4+ cells (Fig. 4 E and F). The treatment decreased frequency of CD11b+CD11c+ myeloid DCs in the CNS (SI Appendix, Fig. S3A), and among CD11b+ myeloid cells, we observed reduced frequencies of CD11c+ microglia, moDCs, cDCs, and other CD11c+ cells, indicating that DC populations were preferentially affected (SI Appendix, Fig. S3B). Anti–CSF-1 treatment reduced the numbers of microglia to those in naïve mice (Fig. 4G) without depleting the entirety of microglia, as was the case with BLZ945. Importantly, the reductions in microglia numbers in anti–CSF-1-treated mice were primarily due to loss of activated inflammatory microglia, which expressed MHCII and/or CD68 (Fig. 4H).
Fig. 4.
CSF-1 controls the population size of inflammatory myeloid cells in the CNS during EAE. (A) Clinical course and cumulative score of mice with EAE treated with αCSF-1 MAb starting after disease onset (n = 7 per group; compiled from two independent experiments). The red line denotes day that treatment was started. Mice were treated with 200 μg MAb per day. (B) Clinical course and cumulative scores of mice treated long term with αCSF-1 MAb. Mice were treated daily until day 25 p.i. and then every other day for the duration of the experiment. (C and D) Clinical course and cumulative score for mice treated with αCSF-1 MAb, starting at clinical score of 2.0 (n = 4 to 5 per group). (E–L) Characterization of immune cells from the CNS of control MAb– and αCSF-1 MAb–treated mice. (E) Number of CD45+ cells. (F) Numbers of CD11b+, CD11c+, and CD4+ cells. (G) Numbers of CD45+CD11b+CX3CR1HiTmem119+ microglia in control-treated, αCSF-1 MAb–treated, and naïve mice. (H) Numbers of MHCII+ and CD68+ microglia in control MAb- and αCSF-1 MAb–treated mice. (I) t-stochastic neighbor embedding analysis of CD45+CD11b+ cells. moDCs and macrophages were defined as CD45HiCD11b+CD11c+Ly6C+MHCII+CD80+CD86+. Activated microglia were defined as CD45+CD11b+Tmem119+CX3CR1HiMHCII+CD68+/− cells. Quiescent microglia were defined as CD45+CD11b+Tmem119+CX3CR1HiMHCII−CD68−. Undifferentiated monocytes were defined as CD45HiLy6CHiCD11c− cells that were overall MHCIILo/Neg. Neutrophils were defined as CD45+CD11b+Ly6GHi. cDCs were defined as CD45HiCD11b+CD11c+MHCIIHiLy6C−CD26+ cells. Other CD11b+ cells were defined as expressing CD11c and CX3CR1 but did not express markers for antigen presentation. (J) Quantification of clusters from (I). (K) Median fluorescence intensity of MHCII, CD80, and CD86 in CD45+CD11b+ cells. (L) Frequency of IL-1β+ cells among CD45+ cells. Significance was calculated with unpaired Student’s t test. Error bars are SEM. Significance for clinical course was calculated by two-way ANOVA.
We then quantified how anti–CSF-1 treatment affected the composition of myeloid cells in the inflamed CNS. Anti–CSF-1 treatment reduced the frequencies of moDCs, macrophages, activated microglia and undifferentiated monocytes but did not affect the frequency of neutrophils (Fig. 4 I and J). This resulted in a large increase in the frequency of quiescent microglia among CD11b+ cells. Similar to BLZ945-treated mice, anti–CSF-1 treatment resulted in a decrease in median fluorescence intensity (MFI) for MHCII and CD80 but not CD86 (Fig. 4K), suggesting that inflammatory APCs were preferentially impacted by anti–CSF-1 treatment. Consistent with this, anti–CSF-1-treated mice had a lower frequency of TNF+MHCII+ cells in their CNS, including moDCs (SI Appendix, Fig. S3 C and D). An important pathogenic function of moDCs in EAE is production of IL-1β (28). A reduced frequency of IL-1β–producing cells was also observed in the CNS of anti–CSF-1-treated mice (Fig. 4L) but not in BLZ945-treated mice (Fig. 3I). Together, these data suggest that neutralization of CSF-1 preferentially depletes infiltrating and resident inflammatory myeloid cells, without affecting quiescent microglia.
Anti–CSF-1 Treatment Depletes Myeloid Cells in White Matter Lesions, but not in the Gray Matter.
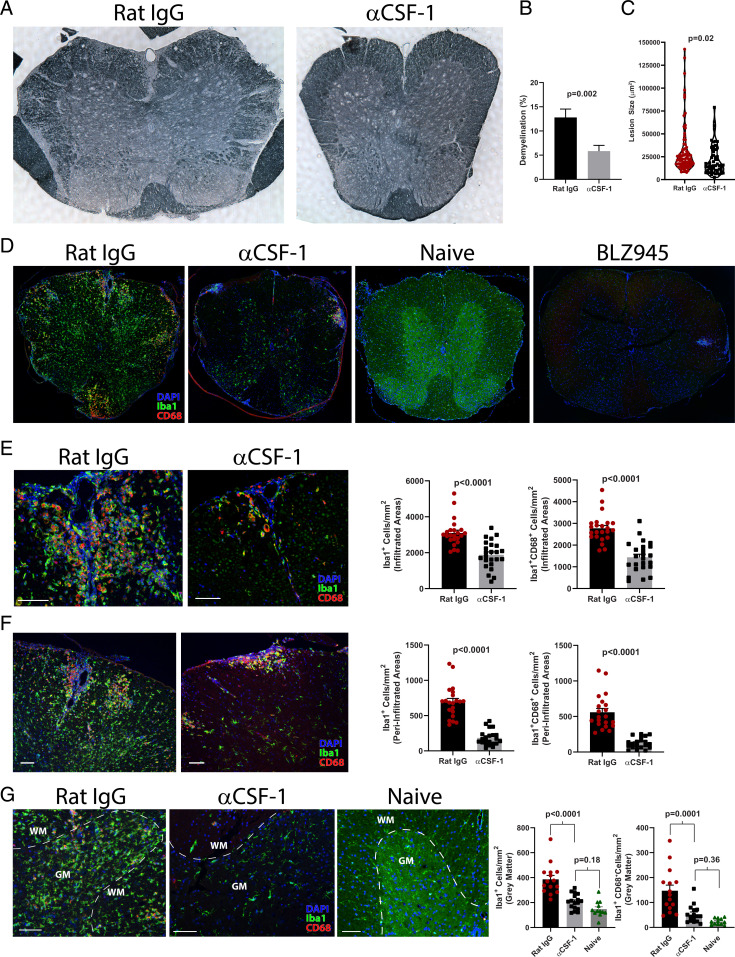
We further tested whether treatment with anti–CSF-1 MAb preferentially depletes inflammatory myeloid cells by examining their distribution in the CNS by microscopy. Anti–CSF-1-treated mice with EAE had reduced white matter demyelination and smaller spinal cord lesions when compared to control mice (Fig. 5 A–C). Immunostaining and analysis by confocal microscopy showed that mice treated with control isotype MAb had large numbers of Iba+ cells throughout the spinal cord (Fig. 5 D–G). In contrast, mice treated with anti–CSF-1 MAb had notably fewer Iba+ cells and were similar to healthy naïve controls. BLZ945 treatment depleted virtually all Iba+ cells in both white and gray matter. Anti–CSF-1-treated mice also had reduced density of Iba1+ and Iba+CD68+ inflammatory myeloid cells both within regions infiltrated with immune cells and in immediately surrounding areas but not further into the gray matter (Fig. 5 E and F). Treatment with anti–CSF-1 MAb reduced numbers of gray matter microglia to levels found in naïve mice (Fig. 5G). Together, these data indicate that anti–CSF-1 treatment primarily targets inflammatory myeloid cells in CNS lesions without depleting quiescent microglia in the gray matter. Furthermore, the depletion of inflammatory myeloid cells in inflamed areas of the white matter precluded alterations to microglia in normal-appearing white and gray matter at sites distant from inflamed areas of the white matter, indicating an overall reduction in CNS inflammation.
Fig. 5.
Anti–CSF-1 treatment reduces lesion burden without depleting quiescent microglia. (A) Sudan black stained spinal cord sections from rat IgG- and anti–CSF-1-treated mice with EAE euthanized on day 17 to 19 p.i. (n = 5 mice per group). Spinal cords were split into four pieces of equal length, and one section from each piece is included in the analysis. (B) Degree of demyelination expressed as a percentage of demyelinated white matter. (C) Violin plot showing demyelinating lesion size in rat IgG– and αCSF-1–treated mice. (D) Confocal microscopy of spinal cord sections stained with DAPI and Abs against Iba1 and CD68 from rat IgG–, anti-CSF-1–, and BLZ945-treated EAE mice, and naïve mice. (E) Representative images and quantification of cell density of Iba1+ and Iba1+CD68+ cells within lesions infiltrated with immune cells from rat IgG– and αCSF-1–treated mice. (F) Representative images and quantification of cell density of Iba1+ and Iba1+CD68+ cells within a 100-µm–wide area surrounding lesions infiltrated with immune cells. (G) Representative images and quantification of cell density of Iba1+ and Iba1+CD68+ cells in gray matter of rat IgG– or αCSF-1–treated and naïve mice (all Scale bars, 100 µm). For (E and F), individual lesions from five mice per group are analyzed. Significance was calculated with unpaired Student’s t test. Error bars are SEM. Significance for comparisons between more than two groups was calculated by one-way ANOVA.
CSF-1R Inhibition Depletes Myeloid DCs and Monocytes in Peripheral Lymphoid Compartments.
Treatment with BLZ945 delayed onset of disease, whereas anti–CSF-1 treatment did not. This difference could be due to diminished priming of encephalitogenic T cell responses in peripheral lymphoid organs of BLZ945-treated mice, resulting in failure to initiate disease in the CNS. To test this possibility, we treated immunized mice with either BLZ945 or anti–CSF-1 MAb and euthanized them during the priming phase of EAE, on day 8 p.i. We quantified the immune cells in blood, draining lymph nodes (dLN), and spleen and found no difference in overall numbers of CD45+ cells in any tissues examined from BLZ945- or anti–CSF-1-treated mice compared to control animals (SI Appendix, Fig. S4). We did, however, observe a decrease in the numbers of CD11b+ cells in all tissues examined from BLZ945-treated mice but not from anti–CSF-1-treated mice (SI Appendix, Fig. S4 A–C). We noted a decrease in CD11b+CD11c+ cells in some peripheral lymphoid organs in both BLZ945- and anti–CSF-1-treated mice, including a decrease in moDCs. Notably, numbers of monocytes were reduced in all examined tissues from BLZ945-treated mice but not from anti–CSF-1-treated mice (SI Appendix, Fig. S5 A–F).
We tested whether reductions in myeloid DCs in the spleen and dLNs would diminish MOG35-55–specific T cells responses but did not find reduced proliferation of cells from either BLZ945- or anti–CSF-1-treated animals when compared to control animals (SI Appendix, Fig. S5 G and H). We also measured antigen-specific proliferation at day 16 p.i. and found a reduction in proliferation of splenocytes from BLZ945-treated mice but not of cells from dLNs. The reduction was likely due to fewer APCs, rather than to intrinsic differences in APC function, as coculture of equal numbers of CD11c+ cells purified from spleens of vehicle- or BLZ945-treated mice with CD4+ T cells from 2D2 mice elicited similar levels of proliferation (SI Appendix, Fig. S5 I and J). These data show that myeloid DCs are impacted by CSF-1R inhibition, but this only modestly affects the development of myelin antigen-specific responses. Thus, delayed onset of disease in BLZ945-treated animals is likely due to factors other than impaired development of MOG35-55–specific T cells responses.
CSF-1R Signaling Promotes Survival/Proliferation of Bone Marrow-Derived moDCs, but not Their APC Function.
We tested how CSF-1R signaling influences the numbers of DCs by generating bone marrow-derived dendritic cells (BMDCs) with GM-CSF and IL-4 (29) and neutralizing CSF-1 and CSF-1R with MAbs. Cultures with either anti–CSF-1 or anti–CSF-1R MAbs contained fewer CD11c+MHCIIHi DCs (SI Appendix, Fig. S6A). This was correlated with a decreased ratio of live/dead cells after lipopolysaccharide (LPS) treatment (SI Appendix, Fig. S6B), suggesting that survival of BMDCs was negatively impacted by the absence of CSF-1R signaling. CSF-1R signaling was not important for the development of APC function of BMDCs, as there was only a small reduction in the frequency of CD11c+MHCII+ among live CD11b+ cells (SI Appendix, Fig. S6 C and D), and coculture with 2D2 CD4+ T cells revealed no differences in eliciting the proliferation of 2D2 T cells (SI Appendix, Fig. S6E). To confirm that these findings are applicable to monocyte-derived BMDCs, we purified CD11b+Ly6G−Ly6CHi monocytes from the BM of CD45.1+ mice, mixed them with total BM cells from CD45.2+ mice, and blocked CSF-1R signaling during their development into moDCs. Consistent with total BM cultures, blockade of CSF-1R signaling did not affect the frequency of CD11c+MHCIIHiCD45.1+ moDCs (SI Appendix, Fig. S6 F and G) but caused a ∼75% reduction in their numbers (SI Appendix, Fig. S6H). Together, these data indicate that CSF-1R signaling promotes the survival of moDCs, rather than their differentiation and APC function, which is consistent with the role of CSF-1R signaling in maintaining myeloid cell populations (5–7).
Blocking CSF-1R Signaling or CSF-1 Reduces Numbers of CCL2- and CCR2-Expressing Myeloid Cells in the CNS during EAE.
The numbers of monocytes/moDCs in the CNS of mice with EAE were greatly reduced during CSF-1R inhibition. Given that monocyte recruitment into the CNS via CCL2/CCR2 signaling is essential to EAE pathology (30) and that several reports have shown that CSF-1 induces CCL2 production by monocytes (31, 32), we examined CCL2 production in the CNS of BLZ945- and anti–CSF-1-treated mice with EAE. The vast majority of CCL2+ cells were CD45+ (SI Appendix, Fig. S8A). Among CD45+ cells, there was a reduction in numbers of CCL2+ cells in both BLZ945- and anti–CSF-1-treated animals (SI Appendix, Fig. S7 A and D). The majority of CCL2+ cells were CD11b+Ly6C+ (SI Appendix, Fig. S7 B, C, E, and F), indicating that monocyte-derived cells are a relevant source of CCL2 in the CNS during EAE. Most CCL2+ cells were TNF+MHCII+ inflammatory myeloid cells (SI Appendix, Fig. S8 B, C, F, and G). MFI for CCL2 among CCL2+ cells from anti–CSF-1-treated mice was also reduced (SI Appendix, Fig. S8E). We also examined CCR2+ cells from the CNS of BLZ945- and anti–CSF-1-treated mice. As with CCL2-producing cells, there was a reduction in numbers of CCR2+ cells (SI Appendix, Fig. S7 G and J). Most CCR2+ cells were CD45HiCD11b+Ly6C+ cells (SI Appendix, Fig. S7 H, I, K, and L), indicating that these were the same cells that produce CCL2. Indeed, nearly all CCL2+ cells were CCR2+CD11b+ cells in anti–CSF-1-treated mice (SI Appendix, Fig. S8K). Combined with our in vitro findings, these data suggest that antagonism of CSF-1R signaling inhibits the survival and proliferation of monocytes/moDCs, resulting in fewer CCL2-producing cells, which then reduces the recruitment of CCR2+ cells in the CNS during EAE.
Monocytes Remaining in the CNS of Anti–CSF-1-Treated Mice Have a Transcriptional Profile Consistent with a Prosurvival Phenotype.
Anti–CSF-1 treatments depleted most (>80%) but not all monocytes and monocyte-derived cells in the CNS of mice with EAE (SI Appendix, Fig. S10 A and B). To identify transcriptional changes that could have enabled some monocytes to persist despite diminished CSF-1 signaling, we sequenced their transcriptome after 6 d of treatment, a timepoint that correlated well with maximal disease suppression (gating strategy shown in SI Appendix, Fig. S9A). There were 412 genes differentially expressed between monocytes from anti–CSF-1- and control MAb-treated mice (SI Appendix, Fig. S10 C and D). We utilized the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics database (33) to identify gene ontology (GO) and KEGG pathway terms that were significantly enriched among the differentially expressed genes. Among GO terms identified as significantly enriched, the largest percentage of genes were involved in cell division (SI Appendix, Figs. S10E and S9B). Among enriched KEGG pathways in these monocytes, the module with the greatest number of genes was the PI3K-Akt signaling pathway (SI Appendix, Figs. S10F and S9C), which controls proliferation (34). Among genes in this pathway, a number of growth factor receptors and transcription factors were up-regulated, including Flt1 (VEGFR), Myb, Kit, Pdgfrb, and Fgfr1 (SI Appendix, Fig. S10G). These data suggest that monocytes in the CNS of anti–CSF-1-treated mice survive by up-regulation of alternative growth factor receptors, which compensate for diminished CSF-1R signaling.
BM-Derived Immune Cells Are Major Contributors to CSF-1R–Dependent Pathology in EAE.
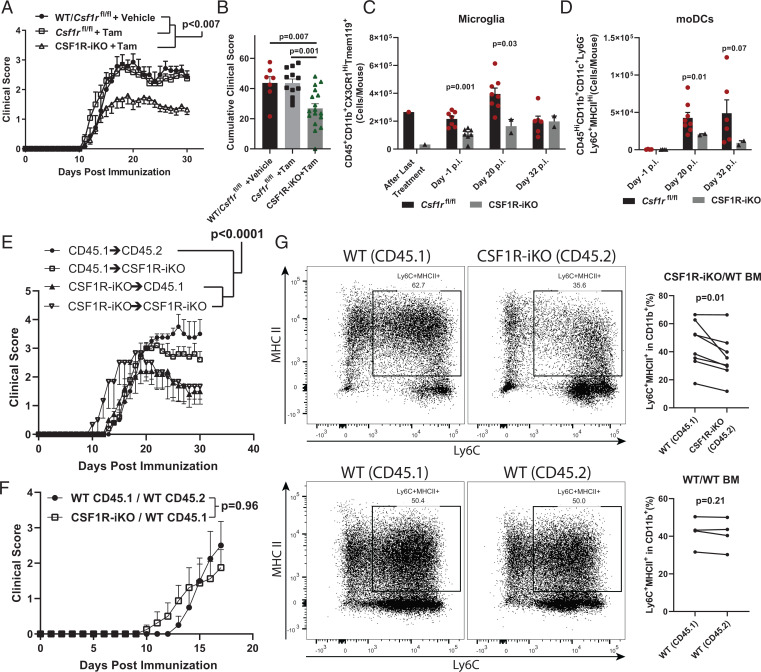
Inhibition of CSF-1R signaling during EAE, either by direct blocking of CSF-1R kinase activity or by neutralizing CSF-1, diminished numbers of moDCs and microglia, suggesting that disease suppression was due to reduced numbers of one or both of these cell types. To further test the role of CSF-1R signaling in monocytes and microglia in EAE, we developed a genetic model for inducible Csf1r deletion that circumvents the perinatal lethality of conventional Csf1r knockout mice (35). We crossed UBC-CreERT2 mice (36) and Csf1rfl/fl mice (37) to generate CSF1R-iKO mice with tamoxifen-inducible Cre-mediated deletion of Csf1r. Tamoxifen-treated adult CSF1R-iKO mice had 70 to 100% reduced CSF-1R protein in Western blots of cell lysates from the spleen, dLN, and CNS (SI Appendix, Fig. S11A). Next, we treated CSF1R-iKO mice with tamoxifen for 5 d and rested them for an additional 7 to 14 d before immunization to induce EAE. CSF1R-iKO mice developed milder EAE than control mice, without a delay in disease onset (Fig. 6 A and B and SI Appendix, Fig. S11B). The tamoxifen pretreatment led to sustained reduction of CNS moDCs for the duration of observation (∼30 d p.i.) (Fig. 6 C and D). In contrast, microglia were initially efficiently depleted, but by ∼30 d p.i. microglia numbers had recovered to near normal. Nevertheless, microglia were substantially reduced at the time of immunization and until disease peak (∼ 20 d p.i.). Thus, CSF1R-iKO mice enable efficient depletion of CSF-1R in adult mice without the developmental defects present in conventional CSF-1R knockout mice; the depletion of moDCs is longlasting, whereas the depletion of microglia is transient.
Fig. 6.
BM-derived immune cells are the major contributors to CSF-1R–dependent pathology. (A) Comparison between vehicle- (n = 7) and tamoxifen-treated (n = 17) Csf1rfl/fl mice and tamoxifen-treated CSF1R-iKO mice (n = 12). (B) Quantification of cumulative score from (C). Significance for clinical course determined by two-way repeated measures ANOVA and by unpaired Student’s t test for cumulative scores. (C) Time course showing numbers of microglia and (D) moDCs in tamoxifen-treated Csf1rfl/fl and CSF1R-iKO mice. Significance was calculated by unpaired Student’s t test. (E) WT and CSF1R-iKO mice were treated with treosulfan and received 107 WT or CSF1R-iKO BM cells. After 8-wk reconstitution, mice were treated with tamoxifen and immunized for EAE. The Right panel shows clinical course of EAE. Numbers of mice per group were 4, 5, 5, and 3 for group listed in legend from Top to Bottom. (F) Mixed BM chimera experiments. BM was ablated with teosulfan, then equal numbers of WT and CSF1R-iKO BM cells were cotransferred to recipients (total cell number transferred = 107 cells/mouse) via i.v. injection. After reconstitution period, mice were treated with tamoxifen, rested, and then immunized for EAE induction. Significance for BM chimera experiments was calculated by two-way repeated measures ANOVA. (G) Quantification of frequency of monocyte-derived APCs among CD11b+ cells from mixed BM chimeras in (F). Significance was determined by two-tailed paired Student’s t test.
To determine the relative contributions of CSF-1R signaling in monocytes and microglia to EAE, we generated BM chimeras by reconstituting treosulfan-conditioned wild-type (WT) and CSF1R-iKO recipient mice with either WT or CSF1R-iKO BM. We used the BM-conditioning agent treosulfan because it does not cross or affect the BBB, does not induce a cytokine storm, nor does it enable engraftment of peripheral myeloid cells into the CNS, as has been observed in irradiation-induced BM chimeras (38). Preconditioning with treosulfan was highly efficient for establishing chimerism, with >90% of peripheral immune cells in chimera mice originating from donor BM (SI Appendix, Fig. S11C). Following 8 wk of BM reconstitution, mice were treated with tamoxifen to knockout Csf1r and then immunized to induce EAE. Both WT and CSF1R-iKO mice reconstituted with WT BM developed typical EAE, whereas WT mice reconstituted with CSF1R-iKO BM developed attenuated EAE, similar to CSF1R-iKO mice reconstituted with CSF1R-iKO BM (Fig. 6E). These data show that BM-derived cells are a major contributor to CSF-1R–dependent pathology during EAE, whereas microglia play a less important role.
Lastly, we sought to understand the role of CSF-1R in survival of monocytes during EAE. We utilized mixed-BM chimeras generated by reconstitution of WT mice with WT and CSF1R-iKO BMs (1:1). Reconstitution with 50% WT BM is sufficient to drive the development of typical EAE (3). Indeed, mice reconstituted with a mixture of WT and CSF1R-iKO BMs and pretreated with tamoxifen developed EAE equivalent to mice reconstituted with WT BM (Fig. 6F). In the CNS, we found a lower frequency of CSF1R-iKO monocyte-derived cells when compared to WT cells (Fig. 6G and SI Appendix, Fig. S11D). These data suggest that CSF-1R signaling maintains monocytic cells in the CNS during EAE.
DISCUSSION
We show that blocking CSF-1R, CSF-1, and IL-34 has differential effects on EAE. Overall, blocking either CSF-1R or CSF-1 resulted in suppression of clinical disease and diminished CNS inflammation and demyelination. Notably, blocking CSF-1R and CSF-1 produced distinct effects on the composition of immune cells in the CNS during EAE. Numbers of microglia and monocyte-derived cells were the most reduced by CSF-1R inhibition. Treatment with BLZ945 depleted virtually all microglia, whereas anti–CSF-1 treatment preferentially depleted inflammatory microglia, reducing total numbers of microglia similar to those in naïve mice. Moreover, depletion of myeloid cells in anti–CSF-1-treated mice was limited to CNS lesions and adjacent areas, while gray matter microglia had a similar appearance as naïve mice. The notably lower microglia depletion by anti–CSF-1 MAb than with BLZ945 did not result in less effective disease suppression but rather improved long-term therapeutic efficacy when compared to BLZ945 treatment, suggesting that full therapeutic benefit can be achieved without applying a maximally ablative approach. Limited depletion of microglia by anti–CSF-1 MAb may therefore be advantageous in MS therapy, as it carries fewer potential risks than widespread microglia depletion likely would. Favoring a milder approach for depletion of myeloid cells is supported by a finding that PLX5622, a commonly used inhibitor of CSF-1R kinase activity, in addition to depleting microglia, also causes long-term and widespread systemic changes to myeloid and lymphoid compartments (39). It is likely that other small molecule inhibitors of CSF-1R, including BLZ945, induce similar changes, whereas blocking CSF-1 would induce fewer systemic changes because of compensatory IL-34 signaling and more restricted tissue distribution of the MAb compared to small molecule inhibitors, as exemplified by our findings in the CNS. For example, other organs with blood barriers, such as testes, eye, and thymus, all of which express CSF-1R (40), would likely be less affected by anti–CSF-1 MAb than a small molecule inhibitor.
The limited microglia depletion by anti–CSF-1 MAb is likely due to the presence of IL-34 in noninflamed CNS areas, where it maintains homeostatic microglia survival. Indeed, it has been shown that systemic injections of anti–CSF-1 MAb do not deplete microglia (41). This is consistent with studies showing that IL-34 accounts for ∼70% of CSF-1R signaling in healthy brain (9) and that anti–CSF-1 MAb is unlikely to penetrate into the normal CNS parenchyma, as only a miniscule fraction of Abs crosses the intact BBB (6, 13). Thus, it is expected that neutralization of CSF-1 in the CNS occurs primarily in active lesions, where the BBB is leaky, resulting in localized depletion of inflammatory myeloid cells. This is analogous to the role of CSF-1R ligands in the skin, where IL-34 maintains Langerhans cells in steady state. However, during skin inflammation, IL-34 becomes dispensable, as infiltrated immune cells produce CSF-1 and maintain/expand numbers of Langerhans cells (25). It should be noted, however, that CSF-1R signaling is not in itself inherently proinflammatory by eliciting inflammatory phenotype in myeloid cells but can have such a net effect by simply maintaining their survival during inflammation. In fact, in the absence of inflammation, CSF-1R signaling induces an immunosuppressive/homeostatic M2 phenotype in macrophages and a resting/quiescent phenotype in microglia (8, 9). Hence, our observations on blockade of CSF-1R signaling in EAE are the net effect of abrogating both pro- and antiinflammatory functions of CSF-1R signaling, with the proinflammatory ones predominating. Together, our findings suggest that CSF-1 promotes inflammation in EAE by expansion of microglia and monocyte-derived myeloid cells, whereas IL-34 maintains microglia in noninflamed CNS areas, similar to the healthy CNS.
Our data suggests that IL-34 does not play a significant role in EAE pathology. This can be explained by notably more widespread and abundant expression of CSF-1 compared with IL-34 (42). It is likely that in most cases, CSF-1 can therefore compensate for lack of IL-34. This interpretation is supported by a study demonstrating that CSF-1 in inflamed sites becomes the dominant CSF-1R ligand, even in tissue (skin) where IL-34 but not CSF-1 is expressed in steady state (25). Thus, although our data suggest that blockade of IL-34 with MAb may have been incomplete due to the development of anti-rat IgG responses, it is probable that abundantly produced CSF-1 in CNS lesions mediates most CSF-1R signaling and that blockade of IL-34 therefore does not have an effect on EAE.
Anti–CSF-1 and BLZ945 treatments differed in that BLZ945 delayed the onset of clinical disease, whereas anti–CSF-1 MAb did not. This may be due to the greater capacity of BLZ945 to inhibit CSF-1R signaling because BLZ945 simultaneously blocks the actions of both CSF-1 and IL-34. In line with this concept, priming of encephalitogenic T cell responses in the periphery was more suppressed by BLZ945 than by anti–CSF-1 MAb, suggesting that IL-34 can compensate for the lack of CSF-1. Indeed, IL-34 is expressed in peripheral lymphoid organs (43) and therefore could, at least in part, substitute for the lack of CSF-1 signaling in anti–CSF-1-treated mice. This is substantiated by a greater degree of depletion of CD11b+ cells, including monocytes, in the periphery during BLZ945 treatment when compared to anti–CSF-1 treatment. However, IL-34 has immunoregulatory functions (21), and it is not clear that its net effect on the magnitude of encephalitogenic immune response during anti–CSF-1 treatment is substantially proinflammatory. Given that BLZ945 readily enters the uninflamed CNS, it is able to deplete CSF-1R–dependent CNS-resident myeloid cells needed for initiation and amplification of CNS inflammation, thus precluding/delaying onset of clinical disease. We hypothesize that this is the more important reason for delayed disease onset rather than diminished myelin-specific T cell responses. In support of this hypothesis, mice with EAE treated with anti–CSF-1R MAb had only a modest delay in disease onset, despite presumably similar effects in the periphery as BLZ945. Anti–CSF-1R MAbs would also be unable to cross the BBB and predeplete APCs in the CNS (44). This supports the view that depletion of CNS APCs by BLZ945 is the main reason for prolonged delay in disease onset. Similar to anti–CSF-1R, anti–CSF-1 does not cross the intact BBB and does not predeplete CNS APCs (41), which makes them available to initiate CNS inflammation. Hence, in contrast to BLZ945, anti–CSF-1 MAb can suppress already ongoing disease but not delay its development.
We also found that prophylactic treatment with BLZ945 resulted in worse overall outcomes when compared to therapeutic treatment. Despite a delay in disease onset, prophylactically treated mice eventually developed disease nearly as severe as control mice. By contrast, mice with EAE treated therapeutically with BLZ945 had sustained disease suppression. This may be explained by differences in myeloid cell composition caused by predepletion of CNS myeloid cells before initiation of CNS inflammation. Indeed, some myeloid cells, specifically microglia, have been found to persist, or even expand, during CSF-1R inhibition and to cause demyelination (45). The mechanism whereby microglia (and possibly other myeloid cells as well) become independent of CSF-1R signaling is unknown, but it has been proposed that CSF-2 and/or TREM-2 signaling can substitute for the CSF-1R signaling during inflammation (46, 47). However, it is unclear why during CSF-1R inhibition started after disease onset there is no exacerbation of disease and why, similarly, during anti–CSF-1 treatment there is no disease exacerbation. It appears that the overall effect of CSF-1/CSF-1R inhibition results from a complex interplay between multiple cell types (monocytes, macrophages, microglia, neurons, DCs, and T cells) that express CSF-1R, which is further complicated by IL-34 signaling. Findings by our group and others show that the role of CSF-1/IL-34/CSF-1R axis in inflammation is complex and has yet to be fully elucidated.
Our in vitro studies with BMDCs show that the primary effect of CSF-1R inhibition is limiting the number of myeloid DCs in these cultures rather than affecting their APC functions. These data are consistent with a body of literature showing that CSF-1R signaling in myeloid cells chiefly provides proliferative and antiapoptotic signals for maintenance of the population size (17). This concept is exemplified by differences between animals lacking CSF-1 and IL-34, in which CSF-1 knockout mice have reduced numbers of osteoclasts and monocytes but only a modest reduction in microglia (15). In contrast, IL-34 knockout mice have greatly reduced numbers of microglia and Langerhans cells but largely normal numbers of other tissue-resident macrophages (15). Thus, CSF-1R inhibition is likely to suppress inflammation in EAE by reducing the population size of inflammatory myeloid cells in the CNS.
Transcriptional profiling of monocytes that remained in the CNS after anti–CSF-1 treatment suggests that they avoid death via up-regulation of growth factor receptors known to promote myeloid cell survival, including Kit (48). Notably, up-regulation of these genes has been reported in myeloid cell cancers (49–53). However, a number of questions regarding these surviving monocytes remain, such as the following: are they a normally present subpopulation among CNS monocytes, or were they induced by neutralization of CSF-1; do they eventually succumb to early death compared to monocytes that have been receiving CSF-1R signaling; do all monocytes lacking CSF-1R signaling acquire this phenotype before death; and, what is the capacity of the surviving monocytes to perpetuate inflammation? It is possible that the altered phenotype of surviving monocytes is less proinflammatory because of diminished effector functions, such as cytokine and chemokine production.
Our in vivo studies indicate an additional mechanism of EAE suppression by inhibition of CSF-1R signaling, namely, reduced recruitment of immune cells to the CNS. We found reduction in numbers of both CCL2- and CCR2-expressing cells when mice were treated with BLZ945 or anti–CSF-1 MAb. Most cells that expressed CCL2 and CCR2 were monocytes/moDCs, which suggests a model whereby monocytes that infiltrate the CNS produce CCL2, thus amplifying inflammation by further recruitment of CCR2-expressing cells. Given that CSF-1R signaling in monocytes/macrophages induces CCL2 expression (31, 32), this suggests that its blockade reduces CNS inflammation by two mechanisms: 1) by reducing numbers of cells that produce CCL2 and 2) by reducing CCL2 production from surviving cells, which together amounts to greatly diminished CCL2 levels in the CNS during EAE. This is consistent with an essential role of CCL2 and CCR2 in EAE, since interventions that affect them attenuate disease (54, 55). It is also possible that in addition to monocytes, reduction in CCL2 directly affects recruitment of pathogenic CCR2+ Th cells to the CNS, given a report that CCR2 drives their recruitment to the CNS (54). Taken together with our result showing reduced GM-CSF and IL-1β production in the CNS, it is likely that CSF-1R inhibition suppresses EAE by depleting CCL2- and IL-1β–expressing APCs. IL-1β has an essential role in EAE (3, 28) by acting on CD4+ T cells to promote their proliferation and GM-CSF production. GM-CSF is also essential to EAE development by acting on monocytes to induce their proinflammatory phenotype and IL-1β production, thus completing a positive feedback loop that sustains inflammation (56). Inhibition of CSF-1R signaling likely interrupts this proinflammatory feedback loop, resulting in EAE suppression.
To determine the relative contributions of CSF-1R signaling in monocytes and microglia to EAE, we developed CSF1R-iKO mice. Our BM chimera experiments indicate that a lack of CSF-1R signaling in BM-derived cells, rather than in CNS-resident cells (e.g., microglia), is responsible for the EAE suppression. However, the transient nature of microglia depletion in CSF-1RiKO mice prevents us from concluding that microglia are entirely dispensable for EAE development. Our experiments with mixed BM chimeras support the view that CSF-1R functions as a growth factor receptor for monocytes and their progeny (5–7). However, it is worth noting that treating WT mice with BLZ945 induced more profound reduction in CNS moDCs than in mixed BM chimeras. This may be explained by the presence of WT monocytes in mixed BM-chimeras, which are able to produce CCL2 and therefore maintain recruitment of monocytes into the CNS. This is consistent with a model in which CSF-1R signaling maintains numbers of monocytes and cells that differentiate from them by promoting their survival/proliferation but also by potentiating their recruitment into the CNS via CCL2 production, which are both reported functions of CSF-1R (6, 7, 31, 32).
Therapies using anti–CSF-1 MAb and small molecule inhibitors of CSF-1R have been tested in multiple clinical trials for autoimmune and oncological diseases (57, 58). These trials have demonstrated that blockade of CSF-1/CSF-1R is well tolerated by patients (59). Targeting CSF-1/CSF-1R in MS has not been tested, but agents used in trials for other diseases would likely be suitable for testing in MS. Moreover, because CSF-1R signaling is not required by myeloid progenitors residing in the BM or CNS (for microglia) (17, 60), the effects of these treatments would be largely reversible. Indeed, there is complete repopulation of microglia within 1 wk after cessation of treatment with CSF-1R inhibitors (60). A treatment modality can be envisioned whereby blocking CSF-1R signaling for therapy of MS would follow an intermittent regimen, given for a period of time, instead of continuously. Thus, as a potential therapy for MS, targeting CSF-1/CSF-1R offers several advantages, including the potential of being readily translatable to clinical testing with already existing therapeutic agents.
Given that increased risk of progressive multifocal leukoencephalopathy (PML) via John Cunningham (JC) virus activation has been an issue for some MS therapies, the safety of CSF-1/CSF-1R targeting therapies for MS treatment will need to be thoroughly explored. Neutralization of CSF-1 during Listeria monocytogenes infection in mice has been shown to increase susceptibility to a certain extent but only during high colony forming unit (c.f.u.) i.v. infection, which mimics severe infection (61). In contrast, moderate c.f.u. infection did not result in increased susceptibility. Moreover, CSF-1R inhibition also increased susceptibility to West Nile Virus infection in mice (62), and CSF-1 production by γδ T cells has been shown to prevent recurrence of malaria infection in mice (63). These findings in mice suggest that targeting CSF-1/CSF-1R may increase risk for infections, but only long-term studies in MS patients would determine if that is the case. However, the safety of CSF-1/CSF-1R targeting agents has been thoroughly explored in the oncological setting in humans (59). Most adverse events were relatively mild and associated with a (typically) transient increase in liver enzymes caused by partial depletion of Kupffer cells (59). CSF-1/CSF-1R targeting agents are Food and Drug Administration-approved therapies for tenosynovial giant cell tumors (64). In these cancers, the most serious adverse events (although rare) were also associated with liver toxicity. Thus, although further exploration in autoimmunity is needed, it is likely that these drugs will have a similar safety profile in MS.
In conclusion, blocking CSF-1R signaling ameliorates EAE by depleting inflammatory APCs in the CNS. MS therapy with anti–CSF-1 MAb could be a preferred approach because, unlike small molecule inhibitors of CSF-1R, it preserves quiescent microglia and their homeostatic functions as well as other IL-34 functions, such as maintenance of Langerhans cells. Limited depletion of microglia by anti–CSF-1 treatment, however, does not diminish its therapeutic effect compared to BLZ945 treatment. Reducing CSF-1R signaling via neutralization of CSF-1 could therefore be a strategy for therapy of MS.
Materials and Methods
Detailed descriptions for all procedures are available in SI Appendix.
Mice and EAE Induction.
Mice used in this study were on C57BL/6J genetic background. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. CSF1R-iKO mice were generated by breeding UBC-CreERT2 (The Jackson Laboratories, Stock No. 007001) with Csf1rflox mice (The Jackson Laboratories, Stock No. 021212). CD45.1 mice (B6.SJL-Ptprca Pepcb/BoyJ, Stock No. 002014) were obtained from The Jackson Laboratories. EAE was induced by immunization with 200 µg MOG35-55 peptide (Genscript) in complete Freund's adjuvant and scored as previously described (65).
In Vivo Treatment.
BLZ945 (Selleck Chemicals and MedChemExpress) was prepared in 20% captisol and mice were treated with 4 to 6 mg/d by oral gavage. Recombinant CSF-1 (4 µg/dose; R&D Systems) was given to EAE mice by i.p. injection. All MAb treatments were also given by i.p. injection. Prophylactic treatments with anti–CSF-1 (200 µg/dose; clone: 5A1; Bio X Cell) started on day 0 p.i. and were given every other day until disease onset, when dosing was changed to every day. In therapeutic treatments, MAb was given every day, starting on days indicated in figures, for the duration of acute phase of the disease (typically days 11 to 25 p.i.), then switched to every other day. Control IgG1 (Clone: HPRN; Bio X Cell) were used to treat control mice. Mice were treated prophylactically with anti–IL-34 MAb (100 µg/dose; Clone: 780310; Novus Biologicals) every other day. For mice treated therapeutically with anti–IL-34 MAb, mice were treated after onset of disease with 55 µg/d i.p. Anti–CSF-1R MAb (400 µg/dose; Clone: AFS98; Bio X Cell) was given every other day. For anti–IL-34 and anti–CSF-1R MAbs, experiments, control IgG2A (Clone: 2A3; Bio X Cell) were given to control mice. For tamoxifen treatment via i.p. injection, 2 mg tamoxifen was injected per day for a total of five injections. Mice were then rested for 14 to 21 d before further manipulation. For tamoxifen treatment via oral gavage, mice were treated five times with 5 mg tamoxifen per day, then rested for 7 d.
Supplementary Material
Acknowledgments
We thank Katherine Regan for editing the manuscript. We thank Medac Germany for the kind gift of treosulfan for our BM chimera studies. Funding: This work was supported by a grant from the National Multiple Sclerosis Society (Grant No. RG-1803-30491) to B.C. This work was also partially supported by the NIH T32 training grant (Grant No. T32AI134646, National Institute of Allergy and Infectious Diseases).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2111804119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Lassmann H., Bradl M., Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 133, 223–244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramaglia V., et al. , Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. eLife 8, e48051 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croxford A. L., et al. , The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity 43, 502–514 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Giles D. A., Duncker P. C., Wilkinson N. M., Washnock-Schmid J. M., Segal B. M., CNS-resident classical DCs play a critical role in CNS autoimmune disease. J. Clin. Invest. 128, 5322–5334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Percin G. I., et al. , CSF1R regulates the dendritic cell pool size in adult mice via embryo-derived tissue-resident macrophages. Nat. Commun. 9, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hume D. A., MacDonald K. P., Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820 (2012). [DOI] [PubMed] [Google Scholar]
- 7.MacDonald K. P., et al. , The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J. Immunol. 175, 1399–1405 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Hamilton J. A., Achuthan A., Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 34, 81–89 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Chitu V., Gokhan Ş., Nandi S., Mehler M. F., Stanley E. R., Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends Neurosci. 39, 378–393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagan N., et al. , CSF1R signaling is a regulator of pathogenesis in progressive MS. Cell Death Dis. 11, 904 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nissen J. C., Thompson K. K., West B. L., Tsirka S. E., Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Exp. Neurol. 307, 24–36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyonteck S. M., et al. , CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freskgård P. O., Urich E., Antibody therapies in CNS diseases. Neuropharmacology 120, 38–55 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Denny W. A., Flanagan J. U., Small-molecule CSF1R kinase inhibitors; review of patents 2015-present. Expert Opin. Ther. Pat. 31, 107–117 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Colonna M., Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur. J. Immunol. 44, 1575–1581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulakirba S., et al. , IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci. Rep. 8, 256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley E. R., Chitu V., CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 6, a021857 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamichi Y., Udagawa N., Takahashi N., IL-34 and CSF-1: Similarities and differences. J. Bone Miner. Metab. 31, 486–495 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Nandi S., et al. , Receptor-type protein-tyrosine phosphatase ζ is a functional receptor for interleukin-34. J. Biol. Chem. 288, 21972–21986 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuboyama K., Fujikawa A., Suzuki R., Noda M., Inactivation of protein tyrosine phosphatase receptor type Z by pleiotrophin promotes remyelination through activation of differentiation of oligodendrocyte precursor cells. J. Neurosci. 35, 12162–12171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baghdadi M., et al. , Interleukin-34, a comprehensive review. J. Leukoc. Biol. 104, 931–951 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Sarahrudi K., et al. , Elevated levels of macrophage colony-stimulating factor in human fracture healing. J. Orthop. Res. 28, 671–676 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Wang H., Cao J., Lai X., Serum interleukin-34 levels are elevated in patients with systemic lupus erythematosus. Molecules 22, E35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo J., et al. , Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J. Exp. Med. 210, 157–172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., et al. , Nonredundant roles of keratinocyte-derived IL-34 and neutrophil-derived CSF1 in Langerhans cell renewal in the steady state and during inflammation. Eur. J. Immunol. 46, 552–559 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okubo M., et al. , Macrophage- colony stimulating factor derived from injured primary afferent induces proliferation of spinal microglia and neuropathic pain in rats. PLoS One 11, e0153375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawley C. A., et al. , Csf1r-mApple transgene expression and ligand binding in vivo reveal dynamics of CSF1R expression within the mononuclear phagocyte system. J. Immunol. 200, 2209–2223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C. C., Edelson B. T., New insights into the role of IL-1β in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Immunol. 198, 4553–4560 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz M. B., Strobl H., Schuler G., Romani N., GM-CSF monocyte-derived cells and langerhans cells as part of the dendritic cell family. Front. Immunol. 8, 1388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mildner A., et al. , CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132, 2487–2500 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Sierra-Filardi E., et al. , CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 192, 3858–3867 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Stutchfield B. M., et al. , CSF1 restores innate immunity after liver injury in mice and serum levels indicate outcomes of patients with acute liver failure. Gastroenterology 149, 1896–1909 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W., Sherman B. T., Lempicki R. A., Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Hemmings B. A., Restuccia D. F., PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 4, a011189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chitu V., Stanley E. R., Regulation of embryonic and postnatal development by the CSF-1 receptor. Curr. Top. Dev. Biol. 123, 229–275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruzankina Y., et al. , Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., Chen K., Zhu L., Pollard J. W., Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis 44, 328–335 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Capotondo A., et al. , Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc. Natl. Acad. Sci. U.S.A. 109, 15018–15023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei F., et al. , CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc. Natl. Acad. Sci. U.S.A. 117, 23336–23338 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasmono R. T., et al. , A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101, 1155–1163 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Rietkötter E., et al. , Anti-CSF-1 treatment is effective to prevent carcinoma invasion induced by monocyte-derived cells but scarcely by microglia. Oncotarget 6, 15482–15493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei S., et al. , Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J. Leukoc. Biol. 88, 495–505(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bézie S., et al. , IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J. Clin. Invest. 125, 3952–3964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mrdjen D., et al. , High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Marzan D. E., et al. , Activated microglia drive demyelination via CSF1R signaling. Glia 69, 1583–1604 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chitu V., et al. , Microglial homeostasis requires balanced CSF-1/CSF-2 receptor signaling. Cell Rep. 30, 3004–3019.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulland T. K., Wang Y., Colonna M., Regulation of microglial survival and proliferation in health and diseases. Semin. Immunol. 27, 410–415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brizzi M. F., Pavan M., Zini M. G., Avanzi G. C., Pegoraro L., Regulation of c-kit expression in human myeloid cells. Stem Cells 11 (suppl. 2), 42–48 (1993). [DOI] [PubMed] [Google Scholar]
- 49.Heo S. K., et al. , Targeting c-KIT (CD117) by dasatinib and radotinib promotes acute myeloid leukemia cell death. Sci. Rep. 7, 15278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin H., Wu Q., Cowell J. K., Ren M., FGFR1OP2-FGFR1 induced myeloid leukemia and T-cell lymphoma in a mouse model. Haematologica 101, e91–e94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao L., Ye P., Gonda T. J., The MYB proto-oncogene suppresses monocytic differentiation of acute myeloid leukemia cells via transcriptional activation of its target gene GFI1. Oncogene 33, 4442–4449 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Demoulin J. B., Montano-Almendras C. P., Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am. J. Blood Res. 2, 44–56 (2012). [PMC free article] [PubMed] [Google Scholar]
- 53.Song G., Li Y., Jiang G., Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets (Review). (Review). Oncol. Rep. 28, 1935–1944 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Kara E. E., et al. , CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat. Commun. 6, 8644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang D. R., Wang J., Kivisakk P., Rollins B. J., Ransohoff R. M., Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 193, 713–726 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paré A., et al. , IL-1β enables CNS access to CCR2hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 115, E1194–E1203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cassier P. A., et al. , CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 16, 949–956 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Genovese M. C., et al. , Results from a Phase IIA parallel group study of JNJ-40346527, an oral CSF-1R inhibitor, in patients with active rheumatoid arthritis despite disease-modifying antirheumatic drug therapy. J. Rheumatol. 42, 1752–1760 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Cannarile M. A., et al. , Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 5, 53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elmore M. R., et al. , Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin W., et al. , Function of CSF1 and IL34 in macrophage homeostasis, inflammation, and cancer. Front. Immunol. 10, 2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Funk K. E., Klein R. S., CSF1R antagonism limits local restimulation of antiviral CD8+ T cells during viral encephalitis. J. Neuroinflammation 16, 22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mamedov M. R., et al. , A Macrophage colony-stimulating-factor-producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity 48, 350–363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benner B., et al. , Pexidartinib, a novel small molecule CSF-1R inhibitor in use for tenosynovial giant cell tumor: A systematic review of pre-clinical and clinical development. Drug Des. Devel. Ther. 14, 1693–1704 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshimura S., et al. , IL-9 controls central nervous system autoimmunity by suppressing GM-CSF production. J. Immunol. 204, 531–539 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.