Abstract
Purpose
To report two cases of multifocal recurrent pleomorphic adenoma of the lacrimal gland, and to highlight the clinical and magnetic resonance imaging findings.
Observations
The authors present two patients with recurrent pleomorphic adenoma of the lacrimal gland. During their previous primary surgical resection at outside institutions, one patient reportedly had a macroscopically complete excision, while the second patient had violation of the pseudocapsule. Both patients had multiple recurrent nodular lesions detected on magnetic resonance imaging with extension beyond the surgical field of the primary resection. Both underwent subsequent lateral orbitotomy with resection of all identifiable nodules and histopathology was consistent with pleomorphic adenoma. In one patient, two nodules were found two months after the surgery, which grew slowly over the last four years. The second patient had no clinical or radiologic sign of recurrence at last follow up, three years after resection of multinodular recurrence.
Conclusions and importance
The two cases demonstrate the challenges in the management of multifocal recurrence of lacrimal gland pleomorphic adenoma. The multicentric nature of recurrent lesions in these two cases increase the risk of future recurrence, malignant transformation, and morbidity caused by surgery and radiation. Magnetic resonance imaging is the imaging study of choice, but it may still be inadequate in identifying all the nodules.
Keywords: Pleomorphic adenoma, Multifocal recurrence, Lacrimal gland
1. Introduction
Lacrimal gland pleomorphic adenoma (LGPA) accounts for approximately 12–25% of all lacrimal gland tumors.1 Incomplete excision, violation of the pseudocapsule and incisional biopsy are reported to be associated with increased risks of recurrence and malignant transformation.1 The multifocal nature of recurrent LGPA and its imaging findings have not been widely discussed in the literature. This report highlights the clinical and magnetic resonance imaging (MRI) findings in 2 patients with multiple benign recurrent nodules of LGPA. This study was conducted in compliance with the rules and regulations of the Health Insurance Portability and Accountability Act and the Declaration of Helsinki as amended in 2013.
1.1. Case 1
A 29-year-old female presented in 2017 for evaluation of a recurrent pleomorphic adenoma (PA) in the left orbit. She had a history of a left anterior orbitotomy in 2011 at another institution with en-bloc resection of lacrimal gland mass that was consistent with PA. Reportedly, the pseudocapsule was macroscopically intact on removal of the lesion but histopathology records were not available to confirm the completeness of the excision. On routine follow-up in 2017, MRI demonstrated several round T2 hyperintense, enhancing foci in the left superolateral orbit, in the region of the resected left lacrimal gland, with at least 4 measurable lesions, all of which measured 4–6 mm in size (Fig. 1A). Retrospectively, these tiny enhancing foci were present on two previous MRIs, dating back to 2014. On computed tomography (CT) imaging, there was no sign of bony erosion, excavation or remodeling in the area of these foci. The patient was subsequently referred to our institution for further management.
Fig. 1.
MRI of the recurrent pleomorphic adenoma of the lacrimal gland (patient #1). A. Axial T1 post contrast image on first presentation to our institution, demonstrating multiple tiny enhancing nodules representing the recurrent pleomorphic adenoma in the superolateral left orbit (arrow). B. Axial T1 post contrast image obtained 2 months after surgical intervention, showing 2 enhancing nodules (arrow). C. Axial T1 post contrast image demonstrating increase in size of the nodules on follow-up after 4 years (arrow).
On presentation to our institution, patient had 2mm of relative ptosis of the left upper eyelid, otherwise she had normal visual function, intraocular pressure, extraocular movements, and had absence of proptosis. Patient underwent a left lateral orbitotomy with a bone flap for resection of 5 nodular lesions in the area of the previously resected lacrimal gland and superonasal upper eyelid orbital fat pocket (Fig. 2). Histopathological examination confirmed PA without evidence of malignant transformation (Fig. 3). On follow-up imaging 2 months later, MRI demonstrated 2 foci in the superolateral left orbit (Fig. 1B), which may represent either residual or recurrent tumor. Given the benign nature of the resected lesions, a decision was made to do close serial observation of the remaining lesions. Additional surgical intervention could result in further periocular and ocular morbidity, including worsening of her ptosis and dry eye syndrome.
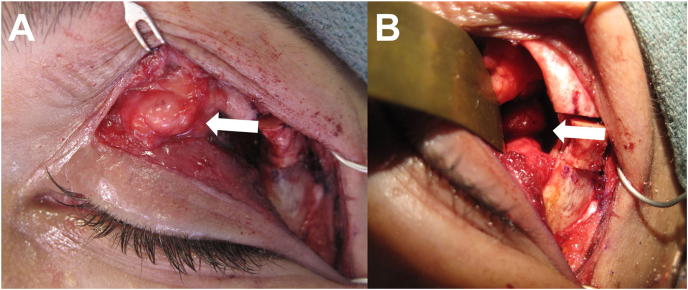
Fig. 2.
Intraoperative findings of patient #1, illustrating the multifocal nature of recurrent pleomorphic adenoma, with lesions in the superonasal upper eyelid orbital fat (A) and superolateral orbit (B).
Fig. 3.
Histological examination of resected orbital tissue of case 1 with hematoxylin and eosin stain. A. At low power, the specimen showed fibrosis with multinodular (recurrent) pleomorphic adenoma. Residual background lacrimal gland acini are not identified. B. At higher power, four distinct nodules with myxoid background are seen in this field all with pushing borders. C. At high-power (200x) the recurrent pleomorphic adenoma shows a predominance of monomorphic spindled myoepithelial cells in myxoid stroma.
During the most recent evaluation in 2021, 4 years after resection of multifocal recurrent lesions, the patient remained asymptomatic except for the presence of moderately severe dry eye syndrome for which she uses frequent lubrication and autologous serum drops to remain comfortable. MRI over the course of 4 years of follow up has shown slow growth of the two nodular lesions (Fig. 1C), hence continuation of close surveillance with clinical examination and imaging study seems appropriate.
1.2. Case 2
A 46-year-old female presented in 2018 with occasional pain and pressure sensation around the left orbit. She had a history of a left anterior orbitotomy with bone flap and resection of a LGPA in 2011 at another institution. Reportedly, the pseudocapsule was violated intraoperatively and histopathology found focal extension of PA to the inked margins. She was followed with annual MRI without evidence of recurrence until February of 2018. MRI showed multiple distinct nodular enhancing foci identified in the extraconal space extending from superior to lateral left orbit with a sessile enhancing lobulated lesion along the lateral orbital wall abutting the lateral rectus muscle. An incisional biopsy was done at an outside institution. Histopathology showed a neoplasm consistent with PA, present at inked margins. The patient was subsequently referred to our institution for further management.
At the time of presentation to our institution, she had 2 mm relative proptosis of the left eye, otherwise she had normal visual function, intraocular pressure, and extraocular movements. MRI showed multiple small T2 hyperintense, enhancing nodules in the superolateral left orbit (Fig. 4). A left lateral orbitotomy with bone flap was performed for gross resection of multiple nodular lesions throughout the orbit, including anterior upper eyelid fat, posterior superolateral intraconal space (27 mm from the rim), and adjacent to the lateral and superior recti muscles (Fig. 5). The intraconal nodules were not observed on the pre-operative MRI, likely due to susceptibility artifact from metals in the oral cavity and small size of nodules. Histopathology of the lesions was consistent with PA without signs of malignant transformation. Patient has been closely followed for 3 years without clinical or radiological evidence of recurrence. Surveillance will be continued with clinical examinations and imaging at least once a year.
Fig. 4.
MRI of the recurrent pleomorphic adenoma of the lacrimal gland (patient #2). A. Coronal T1 post contrast image demonstrating multiple tiny enhancing nodules in the superolateral left orbit (arrow). B. Axial T2 image shows the recurrent adenoma as multiple hyperintense nodules (arrow).
Fig. 5.
Intraoperative findings of patient #2 with pearl-like nodules of pleomorphic adenoma in the anterior upper eyelid fat pad (A) and superolateral orbit (B).
2. Discussion
Our two patients presented here demonstrate the challenging situation of multifocal, albeit benign, recurrence pattern of LGPA after previous resection. The number of recurrences and presumably “seeding” of the operative site during previous surgical procedures are the likely explanation for this multifocal pattern of recurrence. In case 1, some of the recurrent nodules were localized all the way in the nasal fat pads of the upper eyelid, while case 2 had lesions in the posterior intraconal space, 27mm from the orbital rim. This highlights the observation that recurrences can be seen not only in the lacrimal gland fossa but in a wider area that may have been “seeded” during the previous surgery.
There are a multitude of risk factors associated with recurrent PA in the head and neck region. It is widely accepted that LGPA need to be resected completely with a margin of normal tissue.1 Incomplete excision, violation of the pseudocapsule and incisional biopsy are believed to increase the risk of recurrence.1 In a case series of 136 cases of LGPA described by Font and Gamel, the 5-year recurrence rate was 32% in cases where biopsy was performed prior to excision, in contrast to a rate of 3% if the primary surgery removed the lesion with intact capsule.2 Most of the recurrences occur more than 5 years from the primary resection of the PA.3, 4, 5 The chance of curative treatment is lower after a first recurrence as risk of a second recurrence is reported to be higher, approximately 70%.2 In addition to benign recurrence, malignant transformation is also of concern in recurrent disease,6 about 10% recurrent PA undergo malignant transformation by 20 years and 20% by 30 years.2
The concept of tumor pseudopodia, first described by Patey in 1958,7 is a possible explanation for tumor recurrence after seemingly macroscopically intact removal of PA. Pseudopodia describes microscopically visible small lobulations that may extend outside the presumed capsule.7 This is a plausible explanation for the recurrence in case 1. Although the histopathology report from the primary resection was not available for review, photographs shared by the original surgeon suggested gross total resection of the original lacrimal gland mass. Case 2 in our series reportedly had violation of the pseudocapsule during the primary surgery and histopathology found tumor extension to the inked margin. Capsular breach can result in “tumor spillage”, where PA cells may be seeded in the operative field, permitting the growth of multiple new lesions.8 Some have argued that the term “residual disease” rather than “recurrence” should be used in these instances, since most patients were likely never disease free.9
There are very few reports in the literature of benign recurrence patterns for lacrimal gland PA and most of the attention has been on malignant transformation of LGPA.2, 3, 4, 5,10 We found only one report that mentioned a multifocal pattern of recurrence.4 McNab and Satchi described a case of benign recurrent LGPA that showed multiple abnormal soft tissue nodules, all localized to the lateral orbital region, adjacent to small areas of bone scalloping. Only computed tomography images were provided in this report, presumably due to the fact that the report was published more than 10 years ago.4 The patient was treated with local resection and no recurrence was detected at 9 months.4 Little has been published on the MRI findings in recurrent LGPA. MRI of our cases demonstrated multifocality of the tiny pearl-like enhancing nodules, that are few millimeters in diameter, with appearance of hyperintensity on T2 images. In addition to the lacrimal gland fossa, these recurrent lesions were found in different compartments of the orbit. Case 1 had lesions in the superonasal orbital fat while case 2 had lesions in the posterior intraconal space. These are not typical areas that are violated during removal of a lacrimal gland mass, raising the possibility that PA “tumor spillage” from primary resection could extend a wide region. Most nodules for recurrent PA of the parotid gland are reported to be less than 1mm in the head and neck literature, and Wittekindt et al reported the mean number of nodules was 26 with maximal number of 266.8,9 Although MRI is the imaging study of choice for recurrent PA, it can also be inadequate in identifying all the nodules as was the case in our second patient.8
The management of recurrent PA is challenging due to its multicentricity with numerous nodules, increasing possibility of new recurrence, risk of malignant transformation, and morbidity caused by surgery to remove all the nodules in a wide field in the orbit. In the two most recent case series on patients with benign recurrent LGPA,3,4 6 out of 10 patients had local resection without eye removal, 2 out of 10 underwent exenteration, 1 patient who was previously treated with exenteration, underwent craniofacial resection with excision of the entire orbit and adjacent orbital roof for the recurrence, and 1 patient declined treatment. None of the patient in these series had tumor recurrence after definitive surgery, with follow up period ranging from 9 months to 18 years.3,4 The use of adjuvant radiotherapy in recurrent parotid PA is controversial,11 and this has not been discussed in the literature for recurrent LGPA. There is also a lack of well-designed studies in the head and neck literature outlining the role of radiotherapy in management of recurrent PA.11 Both of our cases were treated with local resection, one has 2 remaining nodules that has shown slow growth over the last 4 years, and the other showed no sign of recurrence after 3 years of follow up. The risk of malignant transformation in cases with residual recurrent nodules is not well defined in the literature. Given the lack of evidence on the benefit of adjuvant radiation therapy for LGPA and the associated significant ocular morbidity, especially in the background of dry eye syndrome from multiple previous lacrimal gland surgery, the addition of radiation does not seem justified when the recurrent LGPA has been histologically proven to be a benign recurrence. An extensive discussion was also carried out with the patient on the benefits and risks of additional interventions versus close observation given the complexity of the clinical scenario. Our patient elected to proceed with continuation of surveillance with clinical examination and imaging.
3. Conclusions
In conclusion, we want to raise awareness on the multifocal nature of recurrent LGPA and the challenges in its management. To reduce ocular and orbital morbidity associated with additional surgical resection or adjuvant radiation, small remaining nodules can be observed so long as most of the nodules have been biopsied and confirmed to be benign. MRI is the imaging study of choice given the possibility of numerous nodules throughout a wide region in the orbit and in areas distant from the lacrimal gland fossa. However, it is important to note that MRI may still be inadequate in identifying all the nodules, especially the ones that are very small in size.
4. Patient consent
This report does not contain any personal information that could lead to the identification of the patient.
Acknowledgement and Disclosures
We thank Yimin Geng, MS, MSLIS, from the Research Medical Library of the University of Texas MD Anderson Cancer Center for her assistance in literature search. The authors (J.Z., A.M.P, J.M.D, B.E) have no financial interest related to the manuscript to disclose. There is no funding or grant support for this study. All authors attest that they meet the current ICMJE criteria for authorship.
References
- 1.Lai T., Prabhakaran V.C., Malhotra R., Selva D. Pleomorphic adenoma of the lacrimal gland: is there a role for biopsy? Eye (Lond) 2009;23(1):2–6. doi: 10.1038/eye.2008.16. [DOI] [PubMed] [Google Scholar]
- 2.Font R., Jw G. In: Ocular and Adnexal Tumors. Jakobiec F.A., editor. Aesculapius Publishers Inc; Birmingham, AL: 1978. Epithelial tumors of the lacrimal gland: an analysis of 265 cases. [Google Scholar]
- 3.Currie Z.I., Rose G.E. Long-term risk of recurrence after intact excision of pleomorphic adenomas of the lacrimal gland. Arch Ophthalmol. 2007;125(12):1643–1646. doi: 10.1001/archopht.125.12.1643. [DOI] [PubMed] [Google Scholar]
- 4.McNab A.A., Satchi K. Recurrent lacrimal gland pleomorphic adenoma: clinical and computed tomography features. Ophthalmology. 2011;118(10):2088–2092. doi: 10.1016/j.ophtha.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Ni C., Cheng S.C., Dryja T.P., Cheng T.Y. Lacrimal gland tumors: a clinicopathological analysis of 160 cases. Int Ophthalmol Clin. 1982;22(1):99–120. doi: 10.1097/00004397-198202210-00009. [DOI] [PubMed] [Google Scholar]
- 6.Tom A., Bell D., Ford J.R., et al. Malignant mixed tumor (Carcinoma Ex pleomorphic adenoma) of the lacrimal gland. Ophthalmic Plast Reconstr Surg. 2020;36(5):497–502. doi: 10.1097/IOP.0000000000001625. [DOI] [PubMed] [Google Scholar]
- 7.Patey D.H., Thackray A.C. The treatment of parotid tumours in the light of a pathological study of parotidectomy material. Br J Surg. 1958;45(193):477–487. doi: 10.1002/bjs.18004519314. [DOI] [PubMed] [Google Scholar]
- 8.Witt R.L., Eisele D.W., Morton R.P., et al. Etiology and management of recurrent parotid pleomorphic adenoma. Laryngoscope. 2015;125(4):888–893. doi: 10.1002/lary.24964. [DOI] [PubMed] [Google Scholar]
- 9.Wittekindt C., Streubel K., Arnold G., et al. Recurrent pleomorphic adenoma of the parotid gland: analysis of 108 consecutive patients. Head Neck. 2007;29(9):822–828. doi: 10.1002/hed.20613. [DOI] [PubMed] [Google Scholar]
- 10.Henderson J., Campbell R., Farrow G., Garrity J. third ed. Raven Press; New York, NY: 1994. Orbital Tumors. [Google Scholar]
- 11.Kanatas A., Ho M.W.S., Mücke T. Current thinking about the management of recurrent pleomorphic adenoma of the parotid: a structured review. Br J Oral Maxillofac Surg. 2018;56(4):243–248. doi: 10.1016/j.bjoms.2018.01.021. [DOI] [PubMed] [Google Scholar]