Abstract
Numerous studies have reported that patients with coronavirus disease 2019 (COVID-19) experience alterations in the sense of smell. Therefore, there is an increased need for a psychophysical evaluation tool for olfactory dysfunction in patients with COVID-19 without increasing the risk of virus spread. We applied a single-use sniffing-bead system, which consisted of small beads and a disposable plastic handpiece, to patients with COVID-19 presenting with olfactory dysfunction. The bead sets contained eight concentrations of 2-phenylethyl alcohol (PEA), which has been used in olfactory function tests for many years, and the lowest concentration at which the participant detected the PEA odor was recorded as the PEA threshold. The test was easily administered at the clinic during the consultation with the doctor without increasing the risk of virus spread. Based on the test results, anosmia was objectively confirmed in a patient with subjective anosmia, and another patient with subjective hyposmia was diagnosed with normosmia. Both patients started olfactory training after diagnosis. In conclusion, we present a system to psychophysically assess olfactory dysfunction in patients with COVID-19 using a universal odorant without the risk of virus spread, and suggest that this system might enable early diagnosis and management of patients with COVID-19.
Keywords: Diagnosis, tool, olfactory dysfunction, COVID-19
Introduction
Chemosensory dysfunction occurs in the early phase of coronavirus disease 2019 (COVID-19) with olfactory dysfunction preceding the diagnosis in 73% of patients, while 27% experience anosmia as an isolated symptom.1 Several recent studies regarding olfactory dysfunction in patients with COVID-19 were questionnaire-based. However, self-reported subjective symptoms do not consistently match measurement outcomes.2,3
In South Korea, psychophysical testing of olfactory function is based on the Sniffin’ Sticks test; however, they cannot be used in patients with COVID-19 due to the risk of virus transmission.
We have developed a sniffing-bead system that measures the olfactory detection threshold. It was originally developed as a screening tool for olfactory function evaluation in geriatric patients.4 We made minimal modifications to this sniffing-bead system such that it could be applied to patients with COVID-19 without the risk of virus spread. We introduced the system in seropositive patients with COVID-19. We used this sniffing-bead system to detect olfactory dysfunction and to evaluate symptom progress without the risk of virus spread.
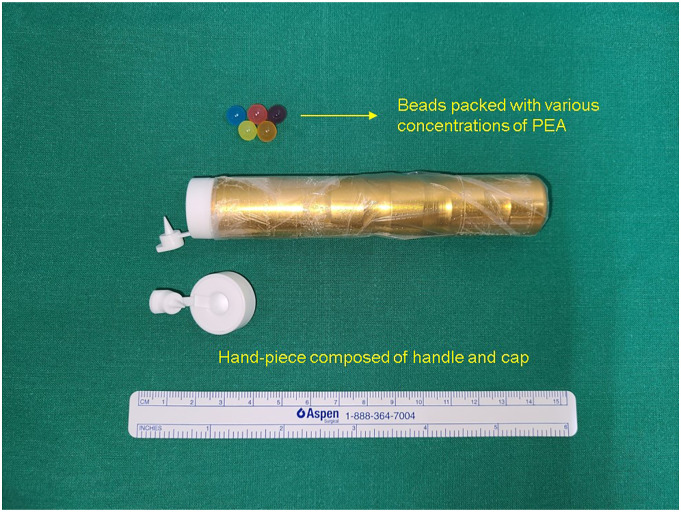
The process is demonstrated in Supplemental Video 1. Briefly, the sniffing-bead system consists of several small beads (7 mm in diameter) and a handpiece with a disposable plastic capsule that releases the odor after the bead is inserted into it and pierced (Figure 1). The beads are intended for single use, and the handpiece is wrapped in plastic vinyl. We used eight beads, each containing a different concentration of 2-phenylethyl alcohol (PEA). PEA has been used in olfactory function tests for many years worldwide, and its validity has been proved in our country also.4 PEA was diluted in distilled water in a ratio of 1:2 (from 0.078% to 10%). The lowest concentration at which the participant detected the PEA odor was recorded as the PEA threshold. Distilled water served as a negative control. The final diagnosis of the patient’s olfactory dysfunction was made as described in a previous report.4
Figure 1.
Sniffing-bead system used for the evaluation of olfactory dysfunction in patients with coronavirus disease 2019.
Case report
Case 1
A 25-year-old man was diagnosed with COVID-19 soon after arriving from a foreign country.
Subjectively, he scored the sense of olfaction as zero on a visual analog scale (range from 0 to 10, 0 = anosmia, 10 = normal olfactory function). In the sniffing-bead test, the patient could not detect the scent of PEA even at the highest concentration (10%). Based on previously established data,5 he was diagnosed with anosmia, which corresponded with his subjective assessment, and was started on olfactory training from that day. After 9 weeks, he noticed an improvement in symptoms and could identify the smell of PEA at a concentration of 0.625%.
Case 2
A 26-year-old man, diagnosed with COVID-19 with olfactory dysfunction, had a subjective score of 2 on the visual analog scale for hyposmia. In the sniffing-bead test, he detected the scent of PEA even at a low concentration (0.078%), which corresponded to “normosmia.”5 Therefore, although his subjective symptoms were scored as “hyposmia,” he was diagnosed with “normosmia” based on the sniffing-bead test. We encouraged him to perform olfactory training without medication and conducted a conventional olfactory function test after 2 weeks, the result of which also corresponded to “normosmia.”
Discussion
Early detection and evaluation of olfactory dysfunction may be valuable in the course of the disease and as a screening tool for COVID-19. However, the subjective recognition of olfactory dysfunction is not always consistent with measurement outcomes.
A previous study found that only 30% of patients who reported subjective olfactory dysfunction were confirmed to have olfactory dysfunction using an olfactory function test.2 Another study found that 38% of patients with COVID-19 recruited on the basis of self-reported olfactory dysfunction had a normal olfactory performance.3 Therefore, a psychophysical evaluation tool for olfactory dysfunction is needed in the evaluation of patients with COVID-19. Our sniffing-bead system is designed for single use only and can be easily used by a doctor using protective equipment, without the patient needing to visit any other facility for testing. It enables the psychophysical measurement of olfactory dysfunction in patients with COVID-19 at an early stage without the risk of potential virus transmission.
As olfactory identification exhibits culture-specificity, the odor must be modified for the test to be useful across different cultures; accordingly, culture-specific olfactory function tests have been developed worldwide. PEA, which was used in this study, has been used for olfactory function tests for many years in many countries, and its validity is established.6,7 Therefore, we propose that this system may be universally applicable in other countries, and in patients with other potential transmittable diseases in the future.
In some patients, the loss of olfactory function persists. Persistent abnormal olfactory scores have been reported in 37–52% of patients with COVID-19.1,8 Early diagnosis and intervention through olfactory training are important contributory factors for a better prognosis of post viral olfactory dysfunction. We suggest that the sniffing-bead system is beneficial for the early detection and intervention of olfactory dysfunction in patients with COVID-19 and helpful in reducing permanent disability. Furthermore, as observed in our case, this system may be applied in the evaluation of the progress of olfactory dysfunction symptoms after initiating the olfactory training in olfactory dysfunction patients.
Herein, we presented a novel system to assess olfactory dysfunction in patients diagnosed with COVID-19. This system can provide information about the olfactory detection threshold even in early-phase COVID-19 without the risk of transmission.
Supplementary Material
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the appropriate Institutional Review Board (2110-035-19390) of Chung-Ang university hospital.
Statement of Informed Consent: This study was approved by the appropriate Institutional Review Board (2110-035-19390), and getting informed consent was waived from the Institutional Review Board of Chung-Anug university hospital.
Supplemental Material: Supplemental material for this article is available online.
ORCID
Hyun Jin Min https://orcid.org/0000-0003-3075-1350
References
- 1.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC, III. COVID-19 anosmia reporting tool: Initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132-134. [DOI] [PubMed] [Google Scholar]
- 2.Vaira LA, Hopkins C, Salzano G, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42(7):1560-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42(7):1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min HJ, Kim SM, Han DH, Kim KS. The sniffing bead system, an olfactory dysfunction screening tool for geriatric subjects: a cross-sectional study. BMC Geriatr. 2021;21(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha JG, Kim J, Nam JS, et al. Development of a Korean culture-friendly olfactory function test and optimization of a diagnostic cutoff value. Clin Exp Otorhinolaryngol. 2020;13(3):274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okumura T, Kumazaki H, Singh AK, Touhara K, Okamoto M. Individuals with autism spectrum disorder show altered event-related potentials in the late stages of olfactory processing. Chem Senses. 2020;45(1):37-44. [DOI] [PubMed] [Google Scholar]
- 7.Welge-Lussen A, Looser GL, Westermann B, Hummel T. Olfactory source localization in the open field using one or both nostrils. Rhinology. 2014;52(1):41-47. [DOI] [PubMed] [Google Scholar]
- 8.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.