Abstract
Duct-dependent congenital heart disease requires attentive therapeutic management since the only source of pulmonary blood flow in newborns is provided by the patent ductus arteriosus. The patency of the duct is the main objective in the first hours of life and it is guaranteed by prostaglandin E1 infusion, but it is not a long-term solution for this type of cardiac malformation. In order to augment pulmonary blood, there are two types of interventions that can be performed: a classical surgical shunt or stenting of the ductus arteriosus, a fairly new alternative to cardiac palliative surgery. Case selection for this type of procedure is essential regarding the patients’ outcome.
We present the management of a newborn diagnosed with (pseudo)atretic pulmonary valve, large ventricular septal defect and patent ductus arteriosus, who underwent an interventional procedure to secure pulmonary blood flow by placing a drug-eluting stent in the ductus arteriosus. The patient’s evolution was not uneventful, several complications appeared, but after three months of neonatal intensive care we were able to discharge him in good clinical condition.
Keywords:congenital heart disease, patent ductus arteriosus, stent, shunt, palliation.
INTRODUCTION
Congenital heart disease (CHD) with duct-dependent circulation can require an emergency therapeutic approach. The patency of ductus arteriosus (DA) can be mandatory to ensure systemic or pulmonary blood flow early in life. Neonates with pulmonary atresia will benefit from duct-dependent pulmonary blood flow. In patients with left heart obstructive lesions, patent ductus arteriosus will provide systemic blood flow.
Up to the moment of intervention, either surgical or transcatheter, the ductus arteriosus is maintained open by administration of continuous infusion with prostaglandin E1.
The surgical approach consists of an aortopulmonary palliative shunt named Blalock–Taussig (BT), after the names of the cardiac surgeon and cardiologist who imagined it for the first time. Considering the inability to maintain the ductus arteriosus patent outside the hospital only with medication, a surgical systemic-to-pulmonary shunt can be an option until the time of corrective repair of the malformation. Blalock–Taussig shunt consists of an anastomosis between the subclavian artery to the pulmonary artery, the subclavian artery needs to be ligated distally and routed downwards to the ipsilateral pulmonary artery. This procedure suffered several modifications. Nowadays, the modified BT shunt is done by placing a tube graft (Gore-Tex) to create a connection between the subclavian artery and the pulmonary artery. Blalock–Taussig shunt has significant postoperative risks such as thrombosis, chylothorax, and even recurrent laryngeal nerve injury.
The use of an intravascular stent designed to maintain ductal patency in an animal model was reported for the first time in 1991 (1, 2). Given the opportunity to provide pulmonary/systemic blood flow, duct stenting is an alternative to surgery in selected patients.
Indications for ductal stenting include high surgical risk or non-availability of cardiac surgery, interventional cardiologist preference, intention to maintain the ductal patency for more than two weeks, and ductal morphology on echocardiography (3). Ductal anatomy can also represent an exclusion criterion, because in some cases of tortuous ducts stent implantation may be technically impossible. Exclusion criteria also include the presence of a PDA-related branch pulmonary artery stenosis, insufficiently constricted PDA, weight less than 2.5 kg (4). Complications described in DA stenting vary from those met in cardiac catheterization (vessel injury or occlusion) to those specifically encountered in this kind of intervention. Even an unprepared medical team can lead to a major complication because the procedure itself can cause ductus injury, spasm, or occlusion during wire/catheter positioning, which can require emergency surgery, thus a hybrid operating room may be needed. Among ductal stenting, complications are also stent embolization, technical failures, and early ductal restenosis, more commonly seen if a part of the duct is left partially unstented (5). Over-shunting through the stented DA can represent a postprocedural complication due to the excessive pulmonary blood flow, but heart failure is uncommon (5, 6).
According to American Heart Association guidelines, there are two conditions when ductal stenting represents a class II recommendation (7):
- It is reasonable to stent an anatomically suitable ductus arteriosus in an infant with cyanotic CHD who has more than one source of pulmonary blood flow (eg, antegrade pulmonary blood flow or collateral blood flow) but who requires additional pulmonary blood flow from the stented ductus for a relatively short period of time (three to six months) – class IIa, level of evidence B;
- It might be reasonable to stent an anatomically suitable PDA in an infant with cyanotic CHD whose sole source of pulmonary blood flow is the ductus – class IIb, level of evidence C.
Furthermore, ductal stenting should not be performed in an infant with cyanotic CHD who has obvious proximal pulmonary artery stenosis in the proximity of the ductal insertion (7).
Ductal stenting can be performed using a wide variety of coronary artery stents as drug-eluting stents and bare-metal stents (BMS). The morphology of the PDA regarding the length, diameter, and tortuosity has high importance in choosing the most suitable stent.
CASE REPORT
We present the case of a one-month-old boy who was transferred to our service for the management of a congenital cardiac malformation. He was born at term (40 gestational weeks, birthweight 2850 g, APGAR score 8/9) after an uneventful pregnancy, but the prenatal echocardiography raised the suspicion of cardiac malformation. Except for II/VI-degree cardiac murmur and oxygen saturation between 80-85% in room air, no other signs and symptoms of the disease were noticed.
At 24 hours after birth, the patient was diagnosed by echocardiography with pulmonary atresia with large (8 mm) subaortic ventricular septal defect, dextroposition of the aorta (50%), atrial septal defect, patent ductus arteriosus, and major aortopulmonary collaterals. After the diagnosis was confirmed, continuous infusion with prostaglandin E1 was started (0.05 ug/kg/min) to prevent duct closure. Thoracic angioCT showed hypoplastic pulmonary artery trunk and branches with a large patent ductus arteriosus (4/3,5 mm).
At the moment of admission to the Neonatal Intensive Care Unit (NICU), the patient was not a candidate for cardiovascular surgery because the bacteriological screening revealed positive pharyngeal culture for Escherichia coli and positive nasal culture for Methicillin-susceptible Staphylococcus aureus, a major source for postoperative complications. In our case, the main indication of ductal stenting was to provide bridging palliation by the time of definitive surgery.
The interventional procedure was performed under general anesthesia. Prostaglandin E infusion was discontinued a few hours before stent implantation since it was known that it would make the ductus restrictive, providing a suitable site to anchor the stent (Eilers & Qureshi, 2020).
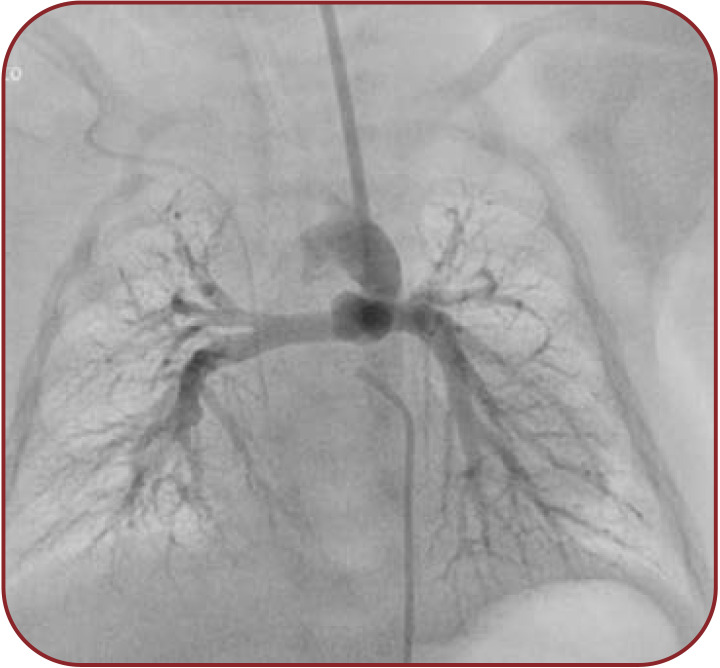
The vascular access was obtained by 4 Fr arterial sheath for the femoral artery and 5 Fr arterial sheath for the left carotid artery. Our approach through the left carotid artery was based on ductal morphology and we used a retrograde arterial approach (femoral artery) because, in the case of pulmonary valve (pseudo)atresia, we do not benefit from the patent right ventricle outflow tract. After obtaining vascular access, the patient received heparin 100 IU/kg. In the beginning, the technique consists of contrast angiography in anteroposterior and left anterior oblique projection (AP, LAO 60° Cranial 30°) in order to visualize the anatomy and measure the dimensions of the PDA. We identified a 5x12 mm patent ductus arteriosus tubular shaped, type C Kritchenko (Figure 1).
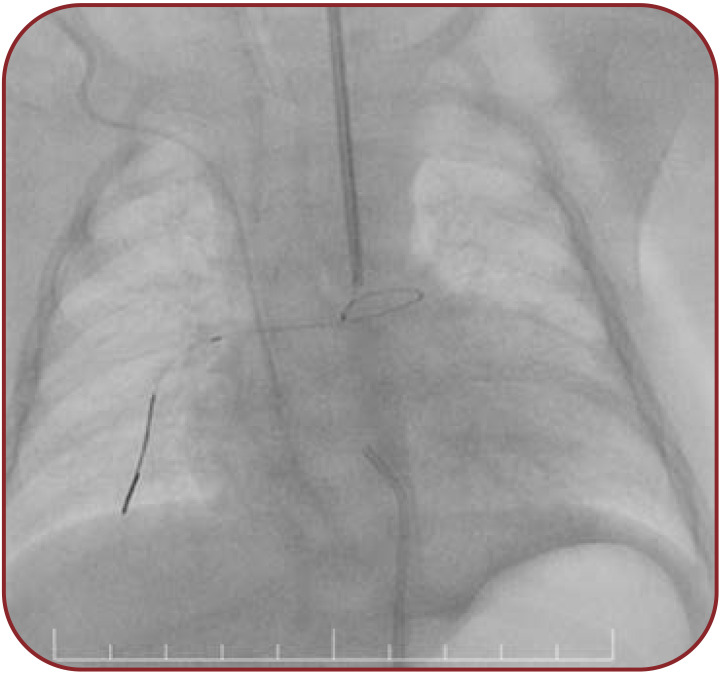
After angiography, a 5F Judkins Right (JR) catheter is positioned in the PDA ampulla. Once the 0.014” guidewire crossed the PDA, the tip was secured in a distal right pulmonary artery branch (Figure 2).
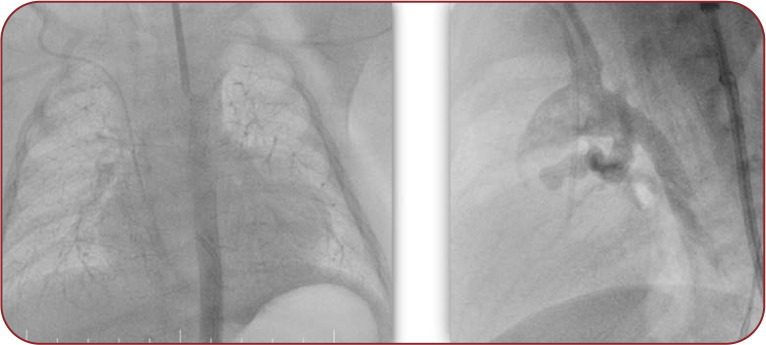
A 4.5 x 16 mm Sirolimus-eluting stent (Biomime) was advanced from the carotid access. We performed inflation of the stent up to 5 mm diameter without any difficulty and complication. Thereafter, we repeated the angiogram to assess the stent position (Figure 3, Figure 4 left). The procedure was a success, considering that the stent was positioned in the ductus arteriosus and the pulmonary blood flow was good.
The entire procedure lasted 90 minutes, with a radiation exposure time of 21 minutes.
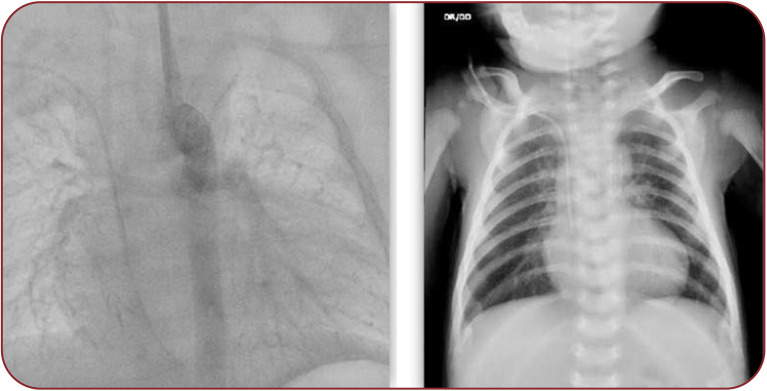
Following the procedure, heparin infusion was continued for several days (16), the efficacy being evaluated with regular ACT investigation in combination with antiplatelet therapy. After stent placement, oxygen saturation increased up to normal value. Chest X-ray showed vertical position of the stent into the thorax, near the spine, without any fracture of the stent (Figure 4, right).
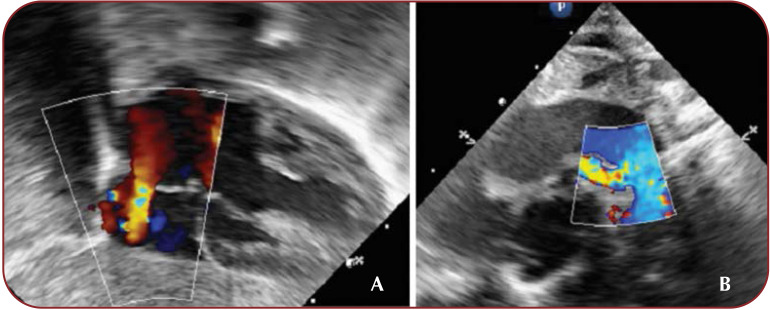
Postprocedural transthoracic echocardiography showed biventricular dysfunction (LVEF 30-35%, RVEF 20-25%) with hypokinetic and dilated right ventricle, biventricular hypertrophy. The stented PDA is shown with unrestrictive but slightly turbulent transductal flow, 3.5 millimeters in diameter (Figure 5). No obstruction of the aortic arch (abdominal aorta velocity measured 0.4 m/sec) and pericardial effusion were detected. Inotropic support with Adrenaline and Milrinone was started and then adjusted with Noradrenaline and Dopamine.
In evolution, the patient developed left concentric ventricular hypertrophy with severe biventricular hypertrophy. A moderate systolic dysfunction was noticed, with an ejection fraction measured at 45%. The blood flow through the stented PDA was unrestrictive, the maximal transductal velocity was measured at 2.2 m/s at the pulmonary end of the ductus. The imaging was conclusive for a large subaortic ventricular septal defect, overriding of the aorta, and ascending aorta ectasia. Abdominal aorta velocity was measured at 0.5 m/sec. Also, blood pressure (BP) measurements found 70/46 mm Hg on the lower limb, 69/46 mm Hg on the right hand and 68/44 mm Hg on the left hand, hence differential BP was negative.
During hospitalization, before percutaneous intervention, several convulsive episodes with clonic movements in the upper limbs and oral commissure occurred, which required anticonvulsant therapy (Phenytoin and Phenobarbital). We could not establish the exact etiology, given that imagistic investigations were unavailable at that moment, but all neurological manifestations were managed with medication. Also, multiple febrile episodes occurred, Pseudomonas aeruginosa being the infectious agent confirmed in tracheal aspirate, nasal secretion, and pharyngeal exudate cultures.
One month after the interventional procedure, throughout the entire NICU hospitalization, the patient presented a slightly improved status, and infection was controlled with antibiotics, allowing the moment of corrective surgery. Surgical management proposed closure of the VSD and ASD with a biological patch, enlargement of the pulmonary annulus, resection of both infundibular and trabecular portions, and exclusion of the ductal stent.
Extubation was attempted repeatedly, but central cyanosis, respiratory distress, and mixed acidosis occurred. Therefore, ventilatory support remained the best option for two more weeks after the intervention. From the cardiovascular perspective, the inotropic dosage was successfully decreased.
The patient was discharged from NICU after three months in good clinical status, without any form of respiratory distress, and saturation was 100% in room air. Treatment recommendations with a diuretic (Furosemide) and anticonvulsant (Phenobarbital) were given for one month, followed by dose adjustments after medical reassessment.
DISCUSSION
Although it is considered that modified BT shunt represents a palliative surgery that can be performed without cardiopulmonary bypass, in newborns this procedure carries a higher morbidity and mortality risk than in older infants. The procedural risk-benefit ratio still needs to be assessed for both procedures.
In two studies regarding the clinical outcomes after both procedures, researchers concluded that placing a stent in the DA is non-inferior to modified BT shunt (8, 9).
Our only available option was to maintain the ductus arteriosus patent by placing a stent into the DA until the baby could be taken up for cardiac surgery because the positive bacteriological status and low weight (3.7 kg) increased the perioperative risk. Among indications of this procedure, ductus anatomy was a key contributor to a successful PDA stenting.
A study conducted by Roggen et al over an 18-year period aimed to determine whether ductus morphology was associated with different procedural outcomes. The authors concluded that ductus arteriosus morphology influenced the technique, the most difficult to the stent approach being the “vertical” ductus arteriosus originating from the transverse aortic arch (typically seen in patients with extreme tetralogy of Fallot and pulmonary atresia) (as in our case), ductus arteriosus from innominate/subclavian artery to the main pulmonary artery, “intermediate” ductus arteriosus with a more angulated course (90–135°), typically found in severe pulmonary stenosis associated with single ventricle morphology. “Vertical” and “intermediate” types of ducti arteriosus frequently exhibit tortuosity (10).
Besides having full equipped catheterization laboratory and medical team, it is very important to have adequate coronary artery stent dimensions available, taking into account different measurements of the PDA made by echocardiography or angioCT. It has been shown that fluoropolymercoated everolimus-eluting stents were superior to BMS with less intimal proliferation, less need for unplanned reinterventions but with similar pulmonary artery growth to BMS (11). As a general rule, a 4.0-mm diameter stent is selected for a bodyweight of 3.0 to 3.5 kg, and a 4.5-mm diameter for infants weighing more than 3.5 kg (6).
After the procedure, our patient presented a seriously degraded general status with central cyanosis, respiratory insufficiency managed by mechanical ventilation, biventricular dysfunction, and pulmonary hypertension in need of inotropic support (Adrenaline, Noradrenaline, Milrinone, Dopamine) and pulmonary vasodilator medication (Sildenafil).
Guidelines show that palliation obtained from ductal stenting is of shorter duration than the one obtained from a surgical BT shunt, with the risk of reintervention being 89% at six months and 55% at 12 months after the procedure, most frequently because of restenosis (4, 5). In our case, the reintervention occurred 40 days after stent implantation, but we could achieve total repair of the malformation.
CONCLUSIONS
Stenting of the ductus arteriosus by the usage of premedicated coronary artery stents in selected cases of congenital heart diseases with ductus-dependent pulmonary blood flow is a procedure approved by the US Food and Drug Administration and also an interventional alternative to the surgical palliative approach, the Blalock-Taussig shunt. Although it is an innovative procedure that carries a lower risk of complications than open-heart surgery, the management of these patients represents a major challenge.
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
Contrast injection at the connection between the aortic arch and patent ductus arteriosus with visualization of the patent ductus arteriosus anatomy, the pulmonary artery and its branches.
FIGURE 2.
Guidewire positioned in the distal right pulmonary artery through the patent ductus arteriosus
FIGURE 3.
Different projections used in angiography to assess stent position: anteroposterior (left image) and left anterior oblique (right image)
FIGURE 4.
Imaging the stent implantation in the ductus arteriosus at control angiography (left image) and X-ray (right image)
FIGURE 5.
A. Biventricular hypertrophy, left-to-right ASD shunt. B. Color Doppler flow of the stent from the suprasternal view
Contributor Information
Hiyam MAHMOUD, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania; Royal Brompton Hospital, London, United Kingdom.
Tammam YOUSSEF, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Eliza CINTEZA, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Cristiana VOICU, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Adrian BALAN, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania; “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania.
Irina MARGARINT, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Cristina FILIP, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Georgiana NICOLAE, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Gabriela DUICA, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Alin NICOLESCU, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Ileana BARASCU, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania.
Catalin CIRSTOVEANU, “Marie Curie” Emergency Children’s Hospital, Bucharest, Romania; “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
References
- 1.Moore JW, KirbyWC, Lovett EJ, O’Neill JT. Use of an intravascular prothesis (stent) to establish and maintain short-term patency of the ductus arteriosus in new born lambs. Cardiovasc Intervent Radiol. 1991;14:299–301. doi: 10.1007/BF02578454. [DOI] [PubMed] [Google Scholar]
- 2.Coe JY, Olley PM. A novel method to maintain ductus arteriosus patency. J Am Coll Cardiol. 1991;18:837–841. doi: 10.1016/0735-1097(91)90810-v. [DOI] [PubMed] [Google Scholar]
- 3.Udink Ten Cate FEA, Sreeram N, Hamza H, et al. Stenting the arterial duct in neonates and infants with congenital heart disease and duct-dependent pulmonary blood flow: A multicenter experience of an evolving therapy over 18 years. Catheter Cardiovasc Interv. 2013;82:E233–E243. doi: 10.1002/ccd.24878. [DOI] [PubMed] [Google Scholar]
- 4.Sivakumar K, Pavithran S, Sonawane B, et al. Serum Sirolimus Levels After Implantation of Third Generation Drug Eluting Cobalt Chromium Coronary Stent in Ductus Arteriosus in Neonates with Duct-Dependent Pulmonary Circulation. Pediatric Cardiology. 2020;41:1354–1362. doi: 10.1007/s00246-020-02381-4. [DOI] [PubMed] [Google Scholar]
- 5.Eilers L, Qureshi AM. Advances in Pediatric Ductal Intervention: an Open or Shut Case? Current Cardiology Reports. 2020;22:14. doi: 10.1007/s11886-020-1266-x. [DOI] [PubMed] [Google Scholar]
- 6.Alwi M, Mood MC. Stenting of Lesions in Patent Ductus Arteriosus with Duct-Dependent Pulmonary Blood Flow. Focus on Case Selection, Techniques and Outcome. Interv Cardiol Clin. 2013;2:93–113. doi: 10.1016/j.iccl.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Feltes TF, Bacha E, Beekman RH 3, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–2652. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 8.Bentham JR, Zava NK, Harrison WJ, et al. Duct stent- ing versus modified Blalock-Taussig shunt in neonates with duct-dependent pulmonary blood flow: associations with clinical outcomes in a multicenter national study. Circulation. 2018;137:581–588. doi: 10.1161/CIRCULATIONAHA.117.028972. [DOI] [PubMed] [Google Scholar]
- 9.Glatz AC, Petit CJ, Goldstein BH, et al. Com- parison between patent ductus arteriosus stent and modified Blalock- Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: insights from the Congenital Catheterization Research Collaborative. Circulation. 2018;137:589–601. doi: 10.1161/CIRCULATIONAHA.117.029987. [DOI] [PubMed] [Google Scholar]
- 10.Roggen M, Cools B, Brown S, et al. Can ductus arteriosus morphology influence technique/outcome of stent treatment? Catheter Cardiovasc Interv. 2020;95:1149–1157. doi: 10.1002/ccd.28725. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal V, Dhillon GS, Penny DJ, et al. Dug-Eluting Stents Compared With Bare Metal Stents for Stenting the Ductus Arteriosus in Infants With Ductal-Dependent Pulmonary Blood Flow. Am J Cardiol. 2019;124:952–959. doi: 10.1016/j.amjcard.2019.06.014. [DOI] [PubMed] [Google Scholar]