Abstract
Alzheimer’s disease is the most common type of dementia which has both cognitive and non-cognitive disabilities. Recent research has proved that sleep deprivation and insomnia have been related to the pathophysiology of Alzheimer's disease and would influence the symptoms and progression of the disease. We look at the current research that supports the idea that the lack of sleep relates to cognitive decline and dementia, with an emphasis on Alzheimer's disease. We integrated the various possible mechanisms of sleep deprivation leading to Alzheimer’s disease and cognitive decline. The role of neuroinflammation, generation of reactive oxidative species and sleep disturbances play a central role in tau generation and Aβ deposition. An approach to manage sleep changes can widely prevent the cognitive decline of Alzheimer’s disease.
Keywords:sleep deprivation, dementia, Alzheimer’s disease, neuroinflammation, neurodegeneration.
INTRODUCTION
Alzheimer’s disease (AD) is the most common type of dementia which has both cognitive and non-cognitive disabilities.( 1) It is generally known that the majority of patients with AD have sleep problems, which increase as the illness progresses (2). Initially, sleep deprivation was thought to be the sequel of the neurodegenerative process. However, recent investigations suggest that sleep problems can show up years before cognitive impairment and thus become a plausible biomarker for early detection and intervention. Sleep difficulties were linked to a poorer course of events, including serious neuropsychiatric and cognitive symptoms and a decreased standard of living (3, 4). This review brings together the various possible mechanisms of how sleep deprivation can lead to cognitive decline and AD.
Sleep deprivation and amyloid beta (Aβ) protein
Amyloid peptides are 39–43 residue proteolytic products generated by amyloid precursor protein cleavage. Soluble Aβ 40 and insoluble Aβ 42 are the two main isoforms of Aβ, with Aβ 42 exhibiting a higher proportion in AD and vulnerable to aggregate. More than 90% of Aβ is produced in the form of Aβ 40 in a healthy state and less than 5% in the form of Aβ 42. Many researchers have looked at the links between subjective and objective sleep measurements, rising Aβ levels, and poor cognitive function in the elderly, indicating that inadequate sleep may raise the likelihood of poor cognitive outcomes. The building up of Aβ is a significant source of synaptic dysfunction and neurotransmission deterioration, both of them playing a critical part in the development of Alzheimer's disease (5, 6).
Several studies found that inadequate sleep causes the aggregation of soluble Aβ. One mechanism is that, during SWS (slow wave sleep), the brain may be able to remove metabolic waste more effectively. Another possible mechanism is based on the fact that neuronal firing is lower in SWS relative to alertness or rapid eye movement (REM) sleep, thus sleep loss could result in higher neuronal activity, which would augment the soluble Aβ (7).
The aggregation of Aβ encourages the formation of reactive oxidative species (ROS), to interact with many proteins and lipid fragments leading to potentially "toxic" oxidized proteins and peroxided lipids. Uninterrupted Aβ aggregation and/or sustained elevation of Aβ would trigger a chronic inbuilt immune reaction by microglial activation via immunological receptors such as Toll-like Receptors 2 (TLR2), TLR4, TLR6, and their co-receptors such as CD14, CD36, and CD47. These are capable of destroying the normally functioning neurons through direct phagocytosis (8). The accumulated amyloid-beta peptides ultimately lead to cholinergic neuron degeneration, changes in glutamine transmission, and, most significantly, synapse, dendritic spine loss, and cell death.
Many studies demonstrated that Aβ accumulation was closely linked to changes in the circadian rhythm. In a study, CSF A 42 levels were shown to be significantly higher in insomniacs and strongly related to Pittsburgh sleep quality index (PSQI) scores (9). In another study, the brains of 20 healthy study respondents aged between 22 and 72 years were scanned by positron emission tomography (PET) following 31 hours of sleep deprivation. Beta-amyloid increased about 5% in these individuals. All changes were observed in the thalamus and hippocampus, the areas that are liable to be injured in the early phase of AD (10). In another study, data indicating the function of the precuneus in promoting slow-wave sleep, nocturnal awakenings were shown to have a substantial relationship with Aβ deposition in the precuneus (11). Also, Aβ accumulation produces sleep pattern abnormalities in both mice and humans (12).
However, the relationship between sleep and amyloid accumulation over time remains a mystery. Amyloid accumulation on PET rises at various rates among amyloid-negative and amyloid-positive people (13). We do not know if certain individuals would have been more likely to have elevated amyloid than others, regardless of sleep, if they were not adjusted for their initial amyloid levels.
Sleep deprivation and tau protein
Tau is the most important microtubule-associated protein (MAP) of a neuron, which is essential for the stability of internal microtubules. It is a phosphoprotein, like MAP1 and MAP2, and the extent of phosphorylation determines its function. In the normal brain, tau has 2–3 moles of phosphate per mole of protein, which seems to be ideal for tubulin interaction and stimulation of microtubule formation (14, 15).
Recent research has looked into the link between tau disease and several elements of sleep. In a study, it has been observed that in cognitively normal persons, not just Aβ but also tau deposits have been linked to sleep problems (16). In a study by Nicholas et al it was found that CSF concentration of amyloid-β and tau were increased with sleep loss when measured by mass spectrometry. Unphosphorylated tau protein is increased by sleep deprivation, while changes in phosphorylation are site-specific, which implies that the physiological process involved in the modulation of site-specific phosphorylation of tau is altered in sleep deprivation, resulting in hyperphosphorylation of tau protein (17). Yet, in another study, in which lumbar catheters were implanted in six people (aged 30–60 years) for a whole night of regular sleep and a whole night of sleep deprivation, findings showed that, during sleep deprivation, CSF tau rose by more than 50%, whereas Aβ increased by 30% (18).
In AD, tau protein loses its ability to attach to microtubules and becomes inefficient in keeping the cytoskeleton properly structured in the axonal process. Conformational alterations and misfolding in the normal structure of tau encourage this abnormal activity, which leads to tau abnormal aggregation into fibrillary formations inside the neurons of patients with AD (19, 20). The pathological characteristics of tau protein have been linked to hyperphosphorylation, glycation, acetylation, nitration, ubiquitination, proteolytic cleavage (truncation), structural alterations, and other modifications (21-23). Tubulin is almost tenfold more abundant than tau in a mature neuron; therefore, virtually the bulk of tau protein in the cell is attached to microtubules. Abnormally phosphorylated cytosolic tau in AD-affected neurons does not bind to tubulin and does not stimulate the assembly of microtubules. Instead, this protein prevents the formation of microtubules and disturbs their structure. Furthermore, normal tau formed from microtubules in the cytosolic phase is displaced by hyperphosphorylated tau protein, which increases up to 40% in the brain cytosol of AD patients and is not polymerized into paired helical filaments (PHFs) or forms neuro fibrillary tangles. AD phosphorylated-tau (AD P-tau) also removes the other two main neuronal MAPs, including MAP1 and MAP2, from the microtubule lattice. This hazardous property of AD P-tau is exclusively attributable to its aberrant phosphorylation since dephosphorylation restores its normal function (22, 23). Also, recent studies in mice have shown that sleep deprivation enhanced brain proteome phosphorylation, such as microtubule affinity regulating kinase 2 (MARK2). Phosphorylation activates MARK2, which then phosphorylates tau. Sleep has also been found to affect protein phosphorylation at synapses. As a result, variations in kinase and phosphatase activation during SWA might promote tau hyperphosphorylation (26, 27). Also, in humans tau has a half-life of 23 days after translation, but the half-life is of few hours after the entry into the cerebrospinal fluid (CSF) of the brain, which indicates that sleep-induced changes in tau concentrations are due to alteration in the release process when compared to the production one (28).
However, more study is needed to establish that sleep deprivation raises tau levels in the brain, because blood levels are not always predictive of brain levels. Higher tau levels in the blood following sleep deprivation might indicate that the brain is cleaning away rather than accumulating the protein.
Sleep deprivation and glymphatic system
In the brain, the glymphatic–lymphatic system plays an essential role in the clearance of extracellular metabolites and waste materials. The water channel aquaporin-4 (AQP4), which is found in the vascular endfeet of astrocytes, helps to sustain this fluid transport system (27). The glymphatic system is more effective when sleeping, although it is unclear whether sleeping at the right time increases the function of glymphatic system. Slow-wave sleep is considered to be affected by age-related neuronal and cortical grey matter loss, notably in the prefrontal cortex (30). During alertness, glymphatic clearance is reduced by 90%, and protein clearance from the brain intima is twice as much during sleep than wakefulness. Norepinephrine levels drop during natural sleep, causing the extracellular space in the brain to expand, which is further resulting in less resistance to fluid flow. This is evidenced in enhanced interstitial solute clearance and better CSF infiltration along with the perivascular spaces. Delta oscillation of slow-wave sleep causes regular and synchronized depolarization of neuronal bundles for 20-30 seconds. This raises glymphatic activity and increases CSF influx into the interstitial cavities, improving interstitial solute clearance (29). Glymphatic clearance of accumulated beta-amyloid and tau proteins is crucial for protection from AD.
However, the glymphatic system deteriorates with age, which implies a link between sleep disruption and symptom development in neurodegenerative dementias. Removal of misfolded proteins and other cellular debris is typically effective in mice, although capacity declines over time and fails near the end of the reproductive lifetime. This was proven by a reduction of 80–90% in glymphatic clearance in old mice, which might explain at least part of the increasing accumulation of amyloid-beta in aged brains (30). The perivascular polarisation of AQP4 is greatest during the rest phase, and AQP4 deletion removes the difference in glymphatic clearance between day and night. The loss of polarisation of AQP4 water channels is seen in sleep deprivation; further, sleep deprivation causes alterations of AQP4 expression. Some AQP4 single nucleotide polymorphisms (SNPs) were linked to lower self-reported sleep quality and a higher amyloid load in people, which implies that AQP4 genetic variation can affect both amyloid buildup and sleep quality (31).
When compared to healthy controls, AD patients have more perivascular hypertrophy. Surprisingly, perivascular space expansion has been linked to decreased sleep quality. Population research discovered a connection between larger perivascular spaces and sleep disturbances, leading to the idea that sleep deprivation might cause structural alterations in the perivascular gaps. In another study, a link between the number of increased perivascular spaces and sleep deprivation has been demonstrated (32, 33). These findings suggest that disturbed sleep and circadian dysfunctions as a result of AD are owing to direct cell injury in the brain areas that govern sleep and wakefulness, at least in part, and that sleep deprivation might hasten disease development by storing metabolic waste. In both animal and clinical studies, slow (delta) waves that occur during the non-rapid eye movement (NREM) sleep are linked to increased glymphatic inflow and amyloid clearance, suggesting that deep sleep is especially essential for brain clearance. The sleeping position has been also identified as a potential risk factor for neurodegeneration. Tiny clinical research in humans found that individuals with neurodegenerative diseases sleep more in the supine posture than healthy controls, who prefer lateral positions (34). The rate of amyloid clearance is also affected by the head posture. As a result, even something as basic as the posture of the head might influence the positive effects of sleep, showing that fluid dynamics may play a role in brain clearing.
Sleep deprivation and respiratory disorders in Alzheimer’s disease
Obstructive sleep apnea (OSA) is a disorder characterized by intermittent hypoxia, hyper/hypocapnia, substantial sleep fragmentation, oxidative stress and a persistent low-grade systemic inflammatory response caused by brief episodes of recurrent upper airway obstruction during sleep. A recent analysis found that 30% of men and 12% of women are affected by OSA between 30 and 70 years of age (35). A meta-analysis of nine observational studies revealed that OSA was likely to increase the cognitive decline or disease severity in AD patients, whereas another meta-analysis of five studies found that OSA was more prevalent in AD patients (36, 37). Apart from this, a study looked at the extent of Alzheimer's-like indicators in autopsy specimen of 34 hippocampal regions and 24 brainstem regions of people with OSA; it was discovered that plaques and neurofibrillary tangles first appeared in a nearby cortical area in AD, followed by the hippocampus, before mounting to the rest of the cortex; the plaques had a greater relationship with severe sleep apnea, despite the presence of both plaques and neurofibrillary tangles in patients with OSA (40).
Patients with dementia were demonstrated to have a link between OSA symptoms and cognitive impairment. In a study, it was shown that the time to AD onset was shorter in patients with OSA. It also revealed that women with OSA had a 2.28-fold higher chance of acquiring the illness later in life when compared to controls, while men had a 1.42-fold higher risk. Short sleep duration of fewer than six hours as well as a mean total sleep time greater than nine hours were associated with a significantly higher risk of AD in persons with OSA than sleep length of six to nine hours (41). In another study, it was found that people with severe dementia had worse OSA than those with mild to moderate or no dementia, and vice versa (42).
Obstructive sleep apnea electroencephalography (EEG) abnormalities include reduced slow-wave and spindle activity during NREM sleep as well as slowing of the EEG during REM sleep. This is associated with an increased neural activity that is intimately linked to episodes of apnea and accompanying EEG arousals, leading to sleep deprivation and fragmentation. The pivotal function of sleep in the regulation of CNS amyloid load and tau protein levels has been advocated as the possible mechanism for the cognitive decline in OSA (43, 44).
OSA-induced brain damage is well-known in experimental animal models. Obstructive sleep apnea can cause neuroinflammatory and neurotrophic alterations in afflicted and vulnerable people. Microglia, the resident mononuclear phagocytes, may be chronically primed to various activators in the brains of OSA patients, including chronic vascular alterations such as cerebrovascular dysregulation, local ischemia, cerebral microinfarcts, chronic Aβ exposure (45). Microglia and astrocytes can generate cytokines, interleukins, nitric oxide (NO) and other implicit lethal substances on exposure, worsening the neuroinflammatory response, indicating that AD and OSA share the neuroinflammatory route in the genesis of dementia. Exogenous and endogenous variables have also been proposed as potential modifiers of the innate immune response generated by Aβ-exposed microglia. Obesity and systemic inflammation cause the impact by an increase in the persistent neuroinflammatory drive. In a recent pilot study, treatment of severe OSA with CPAP in patients with mild-to-moderate AD significantly slowed the cognitive decline over a period of three years (46). This suggests that OSA is one of the modifiable risk factors for AD, emphasizing the importance of OSA early diagnosis and treatment.
Neurotrophic factors in Alzheimer’s disease
Brain-derived neurotrophic factors (BDNF) play an important role in enhancing neurogenesis and neurotransmission by synaptic growth promotion, and synaptic plasticity modulation (47). The prerequisite for memory formation, which includes long term potentiation (LTP), is maintained by the action of BDNF at tropomyosin receptor kinase B (TrkB); TrkB receptors also support cholinergic function. The change in either the expression of nerve growth factor (NGF) or BDNF, or in the levels of TrkA or TrkB receptors may result in degeneration of neurons and inebriated memory formation (48). Studies suggested that increased peripheral BDNF levels were protective against AD in older adults. Alzheimer’s disease risk was lowered to 33% while increasing the BDNF levels by one standard deviation (49). Reports have confirmed that impaired hippocampal BDNF expression, impaired memory, and cognition were observed in a selective loss of REM sleep (48). Further SWA was higher in the BDNF injected hemisphere of rats. Experimentally in knockout mice it has been shown that intact BDNF was essential for the modulation and homeostatic regulation of REM sleep in both sexes (49).
Nerve growth factor is the founder member of the neurotrophin family. It plays an important role in regulating the differentiation, growth, survival, and plasticity of certain cell types, including the cholinergic neurons in the central and peripheral systems (50). Pro NGF is the precursor form of NGF and its mature form is the mNGF. Both proNGF and mNGF are biologically active (51). The NGF acts through the tropomyosin receptor kinase A (TrkA, high-affinity receptor) and p75 (also known as low-affinity NGF receptor, LNGFR, or p75 neurotrophin receptor, p75NTR), respectively (52). Mature NGF is metabolized by the matrix metalloproteinase 9 (MMP9), which is up-regulated in AD. Metabolism of NGF is altered in AD, leading to accumulation of proNGF levels and reduction of mNGF. On the whole, (i) altered NGF maturation, (ii) skewed TrkA/p75 receptor ratio, (iii) inefficient axonal transport and signaling, (iv) Aβ induced modulation of NGF receptors, (v) suboptimal ACh innervation induced inflammatory response, and (vi) Aβ cytotoxicity can all together affect memory and hence contribute to the development of AD. The expression of BDNF is altered in hippocampus and parahippocampal areas in both normal and pathological aging and also in psychiatric illness (53). These changes are related to plastic changes in the episodic memory in the frontal cortex and entorhinal cortex.
Neuroinflammation in Alzheimer’s disease
A large number of studies have attributed AD pathogenesis to neuroinflammation, where inflammatory markers such as interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-18 (IL-18), tumor necrosis factor-á (TNF-á), interferons (IFN), and interleukin-12 (IL-12) lead to neuronal death and eventually to deposition of Ab and tau protein (54-56).
Sleep deprivation results in an increased expression of IL-1, IL-6, IL-18, TNF-α and C reactive protein (CRP) (57, 58). Also, studies have proven that adequate sleep was required to clear Ab protein out of the brain, and inadequate sleep was leading to accumulation of these b amyloid proteins which are directly linked with AD pathogenesis (16, 59). Irwin et al observed that a partial sleep deprivation triggerred inflammatory factors such as nuclear factor κB (NF-κB), activator protein 1, and STAT; apart from this, there was an increase in mRNA expression of other proinflammatory cytokines (60).
Interleukin-1 seems to accumulate in the hippocampus of AD brain due to stress injury and it has a substantial impact on the hippocampal synaptic function (61). Interleukin-1 beta exerts multiple effects in the brain – especially, it has an essential role in hippocampal synaptic function, which is writhed in AD pathogenesis (62). Further, IL-1 plays the main role in the evolution of plaque, it promotes the synthesis and processing of APP adding to AD pathogenesis (63, 64). Sleep deprivation can disrupt the blood-brain barrier (BBB) due to inflammation and further worsen AD neurodegenerative changes (65). It has been observed that BBB was breaking in the hippocampal region prior to the onset of hippocampal atrophy seen in early AD (66).
Inflammation and sleep deprivation (observed in sleep apnoea) lead to ROS generation, again adding on to apoptotic neuronal cells and neurodegeneration (67, 68). In 1994, Reimund proposed the theory that the waking state caused ROS production and sleep helped to clear this (69). Later it was demonstrated that sleep caused resistance to oxidative stress. Moreover, it was seen that the neural ROS pool drived the sleep needs. Hence, it can be stated that neuronal ROS levels regulate sleep (70). Yet, another study observed that sleep deprivation in mice resulted in learning impairment, decreased brain mitochondrial ATP levels and synaptic plasticity regulatory proteins. Also, ROS and inflammatory cytokine levels were increased in the hippocampus (71). Sleep deprivation resulted in significant impairment of long term potentiation (LTP) in the hippocampus as the NMDA/AMPA ratio of CA1 pyramidal cells in response to Schaffer collateral stimulation was depressed (72). The sleep/wake cycle is important in the proliferation, migration and differentiation of oligodendrocytes; also, sleep deprivation lowers myelin thickness, and "oxidative stress "is the primary pathognomic cause of the early onset of AD. In fact, sleep deprivation causes AD and AD produces sleep disruption (73).
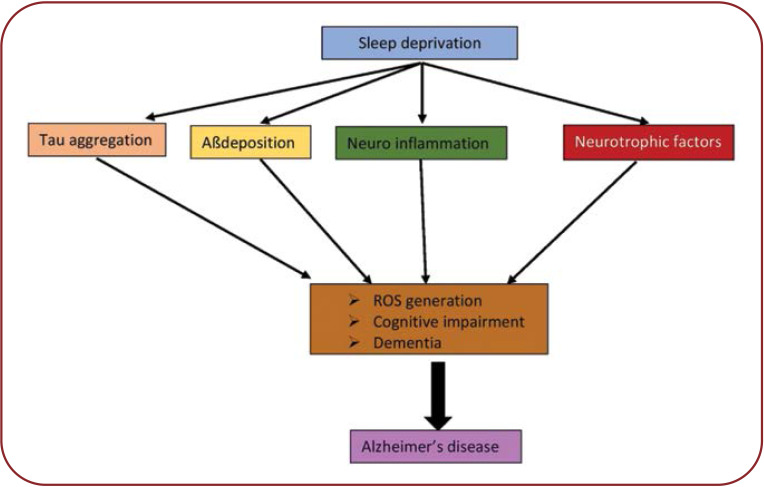
Sleep deprivation can cause apoptotic neuronal cell death and neurodegeneration by producing ROS and neuroinflammation, disrupting the BBB and further leading to atrophic changes in the hippocampus typical of AD (Figure 1).
Therapeutics for sleep disturbances in Alzheimer’s disease
Existing evidence implicates that sleep disruptions have been linked to both the pathophysiology of AD and the risk of developing the disease in the future. Subsequently, accelerated AD pathogenesis causes deterioration in cognitive performance. Current AD therapy focuses on slowing the progression of the illness rather than modifying its pathophysiology. Though studies are still required to establish that sleep therapies modify disease progression in AD patients, focusing on the sleep-wake cycle, right now may be an appropriate, non-invasive strategy to alleviate the symptoms of Alzheimer’s dementia (74).
In people with AD, the non-pharmacological approach is often regarded as the first line of treatment. During the day, persons with AD should be advised to exercise and stroll outside. Stimulants like coffee and tea should be consumed in moderation. Bright light therapy (BLT) is a chronotherapeutic strategy used to address circadian abnormalities in AD patients, which has been shown to enhance nighttime sleep, reduce the nocturnal awakening, prolong the daytime awakening, lower agitational behaviour in the evening, and enhance cognition (75). Nighttime exposure to noise and light should be reduced. Sleep hygiene practices and relaxation techniques such as meditation and yoga can help with insomnia (76). New innovative treatments with direct current stimulation (tDCS) in the <-Hz range, which have experimentally proved to be effective in memory consolidation in patients with schizophrenia, attention- deficit hyperactivity disorders and those with temporal lobe epilepsy, can also be tried in AD (77). Melatonin, trazodone, and ramelteon are the most widely utilized medications for the pharmacological management of sleep problems in AD (78).
CONCLUSIONS
We can now state that increased wakefulness and disturbed sleep acutely lead to increased Aβ production and decreased Aβ clearance as well as tau pathology, which are in particular seen in AD. Adding on to AD pathogenesis is neuroinflammation and ROS generation as a consequence of sleep deprivation. Patients with AD have sleep disturbances, which in turn can cause AD. Obstructive sleep apnoea seen in sleep disturbances further worsens by generating ROS and neuroinflammation. Hence, sleep is critical for the proper function of many organ systems, particularly the brain. There is a vicious cycle: patients with AD exhibit disturbances in the timing and duration of the sleep cycle, primarily manifested as increased wakefulness at night and excessive daytime sleep, while sleep disturbance can cause AD. Efficient and effective sleep is more than just a luxury. It can be one of the preventable causes of neurodegeneration and cognitive decline. Relaxation techniques and sleep hygiene practices can be encouraged for cognitive improvement.
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
Possible mechanisms of Alzheimer’s disease. Sleep deprivation causes Aβ and tau deposition and can cause neuroinflammation along with disruption in levels of neurotrophic factors. These mechanisms produce reactive oxygen species and neurodegeneration, further causing cognitive impairment and dementia, all together leading to the development of Alzheimer’s disease
Contributor Information
Archana GAUR, Department of Physiology, All India Institute of Medical Science, Bibinagar, Hyderabad, Telangana, India.
Ariyanachi KALIAPPAN, Department of Anatomy, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana, India.
Yuvaraj BALAN, Department of Biochemistry, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana, India.
Varatharajan SAKTHIVADIVEL, Department of General Medicine, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana, India.
Kalpana MEDALA, Department of Physiology, All India Institute of Medical Science, Bibinagar, Hyderabad, Telangana, India.
Madhusudhan UMESH, Department of Physiology, All India Institute of Medical Science, Bibinagar, Hyderabad, Telangana, India.
References
- 1. . 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 2.Prinz PN, Vitaliano PP, Vitiello MV, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon J-F, Petit D, Latreille V, Montplaisir J. Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharm Des. 2008;14:3430–3445. doi: 10.2174/138161208786549353. [DOI] [PubMed] [Google Scholar]
- 4.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58:480–486. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 5.Mander BA, Winer JR, Walker MP. Sleep and Human Aging. Neuron. 2017;94:19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Liu Y, Hao W, et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 9.Chen D-W, Wang J, Zhang L-L, et al. Cerebrospinal Fluid Amyloid-β Levels are Increased in Patients with Insomnia. J Alzheimers Dis. 2018;61:645–651. doi: 10.3233/JAD-170032. [DOI] [PubMed] [Google Scholar]
- 10.Shokri-Kojori E, Wang G-J, Wiers CE, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA. 2018;115:4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45:104–120. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köpke E, Tung YC, Shaikh S, et al. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 15.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucey BP, McCullough A, Landsness EC, et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med 2019. [DOI] [PMC free article] [PubMed]
- 17.Barthélemy NR, Liu H, Lu W, et al. Sleep Deprivation Affects Tau Phosphorylation in Human Cerebrospinal Fluid. Ann Neurol. 2020;87:700–709. doi: 10.1002/ana.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holth JK, Fritschi SK, Wang C, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Sierra F, Ghoshal N, Quinn B, et al. Conformational changes and truncation of tau protein during tangle evolution in Alzheimer’s disease. J Alzheimers Dis. 2003;5:65–77. doi: 10.3233/jad-2003-5201. [DOI] [PubMed] [Google Scholar]
- 20.Ghoshal N, García-Sierra F, Wuu J, et al. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 21.Carrell RW, Gooptu B. Conformational changes and disease--serpins, prions and Alzheimer’s. Curr Opin Struct Biol. 1998;8:799–809. doi: 10.1016/s0959-440x(98)80101-2. [DOI] [PubMed] [Google Scholar]
- 22.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Min S-W, Cho S-H, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JZ, Gong CX, Zaidi T, et al. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 26.Brüning F, Noya SB, Bange T, et al. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019. [DOI] [PubMed]
- 27.Wang Z, Ma J, Miyoshi C, et al. Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature. 2018;558:435–439. doi: 10.1038/s41586-018-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato C, Barthélemy NR, Mawuenyega KG, et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018;97:1284–1298. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessen NA, Munk ASF, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner’s Guide. Neurochem Res. 2015;40:2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Léger D, Debellemaniere E, Rabat A, et al. Slow-wave sleep: From the cell to the clinic. Sleep Med Rev. 2018;41:113–132. doi: 10.1016/j.smrv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Reddy OC, van der Werf YD. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020;10:868. doi: 10.3390/brainsci10110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rainey-Smith SR, Mazzucchelli GN, Villemagne VL, et al. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Transl Psychiatry. 2018;8:47. doi: 10.1038/s41398-018-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berezuk C, Ramirez J, Gao F, et al. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015;38:853–858. doi: 10.5665/sleep.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Brutto OH, Mera RM, Del Brutto VJ, Castillo PR. Enlarged basal ganglia perivascular spaces and sleep parameters. A population-based study. Clin Neurol Neurosurg. 2019;182:53–57. doi: 10.1016/j.clineuro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Levendowski DJ, Gamaldo C, St. Louis EK, et al. Head Position During Sleep: Potential Implications for Patients with Neurodegenerative Disease. J Alzheimers Dis. 2019;67:631–638. doi: 10.3233/JAD-180697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bubu OM, Brannick M, Mortimer J, et al. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep. 2017;40:4–16. doi: 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- 39.Emamian F, Khazaie H, Tahmasian M, et al. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front Aging Neurosci. 2016;8:78. doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen JE, Benediktsdottir B, Cook E, et al. Alzheimer’s disease neuropathology in the hippocampus and brainstem of people with obstructive sleep apnea. Sleep 2019. [DOI] [PubMed]
- 41.Siachpazidou DI, Stavrou VT, Astara K, et al. Alzheimer’s Disease in Patients with Obstructive Sleep Apnea Syndrome. Tanaffos. 2020;19:176–185. [PMC free article] [PubMed] [Google Scholar]
- 42.Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39:258–263. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 43.Cedernaes J, Osorio RS, Varga AW, et al. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Troussière A-C, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405–408. doi: 10.1136/jnnp-2013-307544. [DOI] [PubMed] [Google Scholar]
- 47.Lu B, Nagappan G, Guan X, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 48.Allen SJ, Watson JJ, Dawbarn D. The neurotrophins and their role in Alzheimer’s disease. Curr Neuropharmacol. 2011;9:559–573. doi: 10.2174/157015911798376190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein G, Beiser AS, Choi SH, et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahmani M, Rahmani F, Rezaei N. The Brain-Derived Neurotrophic Factor: Missing Link Between Sleep Deprivation, Insomnia, and Depression. Neurochem Res. 2020;45:221–231. doi: 10.1007/s11064-019-02914-1. [DOI] [PubMed] [Google Scholar]
- 51.Levi-Montalcini R. The nerve growth factor and the neuroscience chess board. Prog Brain Res. 2004;146:525–57. [PubMed] [Google Scholar]
- 52.Iulita MF, Cuello AC. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol Sci. 2014;35:338–348. doi: 10.1016/j.tips.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav Brain Res. 2011;221:515–526. doi: 10.1016/j.bbr.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front Cell Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.J P, A O, D T, L D, M B, E M, et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain Behav Immun. [Internet]. 2017 May [cited 2021 Oct 22];62. Available from: https://pubmed.ncbi.nlm.nih.gov/28161476/ [DOI] [PubMed]
- 56.Jh C, Kf K, Jh L, et al. Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1-42-induced Alzheimer’s disease model rats. PloS One [Internet] 2015 [cited 2021 Oct 22];10(2). Available from: https://pubmed.ncbi.nlm.nih.gov/25658940/ [DOI] [PMC free article] [PubMed]
- 57.Minter MR, Taylor JM, Crack PJ. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem. 2016;136:457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 58.S Y, B S, Rl C, D K-S. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res [Internet] 2009 [cited 2021 Oct 22];29(7). Available from: https://pubmed.ncbi.nlm.nih.gov/19450150/ [DOI] [PubMed]
- 59.Wt S, Jm R, Jm M, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol [Internet] 2001 [cited 2021 Oct 22];107(1). Available from: https://pubmed.ncbi.nlm.nih.gov/11150007/ [DOI] [PubMed]
- 60.A DM, Yb J, D P. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging [Internet] 2014 [cited 2021 Oct 22];35(8). Available from: https://pubmed.ncbi.nlm.nih.gov/24629673/ [DOI] [PubMed]
- 61.Huang C-W, Hsu S-W, Tsai S-J, et al. Genetic effect of interleukin-1 beta (C-511T) polymorphism on the structural covariance network and white matter integrity in Alzheimer’s disease. J Neuroinflammation. 2017;14:12. doi: 10.1186/s12974-017-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch MA. Interleukin-1 beta exerts a myriad of effects in the brain and in particular in the hippocampus: analysis of some of these actions. Vitam Horm. 2002;64:185–219. doi: 10.1016/s0083-6729(02)64006-3. [DOI] [PubMed] [Google Scholar]
- 63.Dursun E, Gezen-Ak D, Hanağası H, et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J Neuroimmunol. 2015;283:50–57. doi: 10.1016/j.jneuroim.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S, Wu MD, Shaftel SS, et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J Neurosci. 2013;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer’s disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitwell JL, Dickson DW, Murray ME, et al. Neuroimaging correlates of pathologically-defined atypical Alzheimer’s disease. Lancet Neurol. 2012;11:868. doi: 10.1016/S1474-4422(12)70200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mittal M, Siddiqui MR, Tran K, et al. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Zhang SXL, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–316. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43:231–233. doi: 10.1016/0306-9877(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 70.Hill VM, O’Connor RM, Sissoko GB, et al. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLOS Biol. 2018;16:e2005206. doi: 10.1371/journal.pbio.2005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J, Dou Y, Ladiges WC. Adverse Neurological Effects of Short-Term Sleep Deprivation in Aging Mice Are Prevented by SS31 Peptide. Clocks Sleep. 2020;2:325–333. doi: 10.3390/clockssleep2030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lloret A, Esteve D, Lloret MA, et al. Is Oxidative Stress the Link Between Cerebral Small Vessel Disease, Sleep Disruption, and Oligodendrocyte Dysfunction in the Onset of Alzheimer’s Disease? Front Physiol. 2021;12:1330. doi: 10.3389/fphys.2021.708061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuang H, Zhu Y-G, Zhou Z-F, et al. Sleep disorders in Alzheimer’s disease: the predictive roles and potential mechanisms. Neural Regen Res. 2021;16:1965–1972. doi: 10.4103/1673-5374.308071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCurry SM, Pike KC, Vitiello MV, et al. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59:1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prehn-Kristensen A, Munz M, Göder R, et al. Transcranial Oscillatory Direct Current Stimulation During Sleep Improves Declarative Memory Consolidation in Children With Attention-deficit/hyperactivity Disorder to a Level Comparable to Healthy Controls. Brain Stimulat. 2014;7:793–799. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 78.Urrestarazu E, Iriarte J. Clinical management of sleep disturbances in Alzheimer’s disease: current and emerging strategies. Nat Sci Sleep. 2016;8:21–33. doi: 10.2147/NSS.S76706. [DOI] [PMC free article] [PubMed] [Google Scholar]