Abstract
Objective:Comprehensive characterization of potential frailty determinants, including sociodemographic, clinical, dietary, psychological, cognitive and systemic inflammation parameters.
Methods:A rural cohort of 186 subjects aged 60-89 years recruited from a community-based study in Crete, Greece (the Cretan Aging Cohort). Frailty was assessed with the Simple “Frail” Questionnaire Screening Tool.
Results:Univariate analyses revealed significant (a) positive associations (p<0.01) between frailty and age, widowhood, Geriatric Depression Scale (GDS) score, waist circumference, polypharmacy, IL-6 and (b) negative associations between frailty and frequency of contact with friends, Mini Mental State Examination (MMSE), and adherence to the Mediterranean diet. Multivariate analyses revealed a significant independent contribution of the following variables to frailty: age (B=0.035, p<0.001), GDS score (B=0.041, p=0.034), polypharmacy (B=0.568, p<0.001), waist circumference (B=0.015, p=0,006), plasma IL-6 levels (B=0.189, p=0.004), and adherence to the Mediterranean diet (B=-0.036, p=0.015).
Conclusion:Older age, depression symptoms, polypharmacy, waist circumference, poor adherence to Mediterranean diet and IL-6 plasma levels are associated with increased frailty.
Keywords:frailty, polypharmacy, Mediterranean diet, depression, systemic inflammation, elderly.
INTRODUCTION
Frailty is characterized by physical decline and increased vulnerability to various stressors, and it has been linked to adverse health outcomes such as hospitalizations and mortality (1, 2). Frailty determinants and underlying pathophysiological mechanisms are still obscure, whilst their identification would contribute to appropriate clinical interventions (3, 4). This is particularly important in the COVID-19 era since elderly people with frailty and COVID-19 illness are more prone to death (5).
Certain parameters have been proposed as potential frailty determinants. Chronic inflammation is one such factor, with several inflammatory biomarkers (including IL-6, and TNF-α) being linked to frailty risk (6, 7). Furthermore, depression and cognitive decline have been associated with frailty (8, 9). Unhealthy dieting habits may also contribute to frailty, as evidenced by studies linking obesity with frailty (10, 11). Conversely, Mediterranean diet (characterized by high amounts of olive oil, fruits, vegetables, cereals, legumes, and nuts, moderate amounts of fish and dairy products, low quantities of meat and meat products, and moderate consumption of wine) has been associated with vitality and robustness (11, 12). However, to date the relative contributions and interactions of these frailty determinants have not been examined.
This study aimed to assess physical, emotional, cognitive, and inflammatory correlates of frailty among elderly participants drawn from the Cretan Aging Cohort.
METHODS
Study design
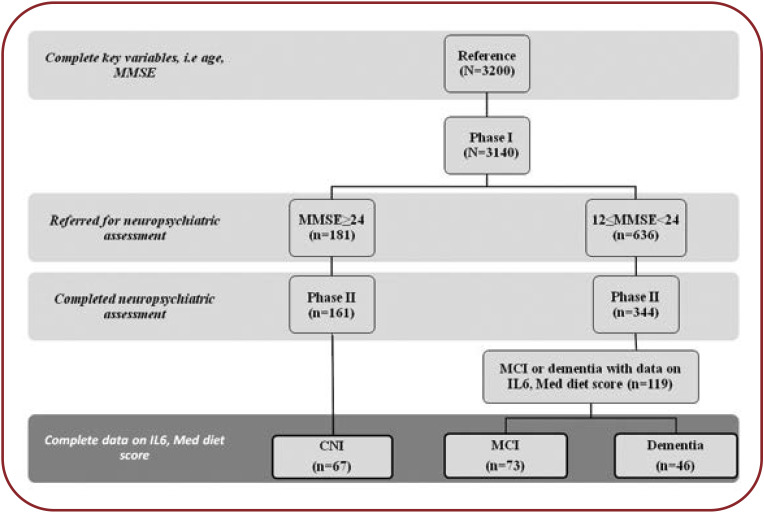
The Cretan Aging Cohort consists of 3140 community dwelling adults aged 60–100 years, who had visited a representative set of 11 Primary Health Care (PHC) facilities in the district of Heraklion in Crete, Greece, for routine care. The study was completed between March 2013 and June 2015. The primary aim of this study was to investigate the prevalence of cognitive decline and risk factors associated with its occurrence. Most participants resided in rural areas of the district (86.2%) and had achieved no more than six years of formal education (82.7%). The study was conducted in two phases, as previously described (9, 13, 14).
In phase I, all participants were interviewed by appropriately trained nursing staff using a semi-structured questionnaire recording demographics (age, sex, marital status, education, place of residence), lifestyle (smoking, frequency of alcohol consumption), medication and comorbidities. Weight and height were also measured to calculate the body mass index (BMI) along with waist circumference (WC) as an index of central obesity. Global cognitive status was evaluated with the Mini Mental State Examination (MMSE) (15) using a universal cutoff of 23/24 points to indicate possible cognitive impairment.
Participants with MMSE<24 (n=636) were referred for neuropsychiatric, neuropsychological and geriatric evaluation by a multidisciplinary team of physicians to establish a final diagnosis (phase II). Of those invited to participate in phase II, 344 persons were tested and received a provisional diagnosis of mild cognitive impairment (MCI) or dementia, as previously described (9). The phase II sample pool was supplemented by 181 persons with MMSE ≥24 points in phase I, from which 161 completed the comprehensive neuropsychiatric assessment, and these persons comprised the cognitively non-impaired participants (CNI) (Figure 1).
One hundred eighty six individuals from the phase II sample pool were randomly chosen to provide blood samples for measuring pro-inflammatory cytokines (Figure 1). Current analyses were performed in this sub-sample of 186 individuals who had complete demographic, anthropometric and dietary data, data on cognitive and frailty status and measurements of inflammatory markers (TNF-α and IL-6 plasma levels).
Frailty assessment
Frailty score was estimated by the use of the Simple "FRAIL" Questionnaire Screening Tool, proposed by the International Association of Nutrition and Aging (16). This score examines five components, including Fatigue, Resistance, Ambulation, Illness, and Loss of weight, and it can be administered in 30 seconds. Fatigue was measured by asking respondents how much time they felt tired during the past four weeks, with responses of "gall of the time" or "most of the time" scoring 1 point. Resistance was assessed by asking participants if they had difficulty walking up 10 steps alone without resting and without aids, and Ambulation was evaluated by asking if they had difficulty walking 100 meters alone without aids; "yes" responses were each scored as 1 point. Presence of five or more illnesses was scored with 1 (with 0 for 4 or fewer illnesses). The illnesses included hypertension, diabetes, cancer (except minor skin cancer), chronic lung disease, heart attack, congestive heart failure, angina, asthma, arthritis, stroke, and kidney disease. Loss of weight was scored 1 for reported weight decline of 5% or greater within the past year. Polypharmacy was defined as receiving more than four medications. Frailty scores range from 0.5 (i.e., 0=best to 5=worst) and represent frail (3.5), pre-frail (1.2), and robust (0) status. Participant functionality was evaluated using the Katz score, computed during the clinical interview and used to characterize patients as fully independent (Katz Score ≥5) versus partially dependent on others (Katz Score ≤4) (17).
Dietary assessment
Dietary patterns were assessed using the Greek validated semiqualitative Food Frequency Questionnaire (FFQ) (18). A score was calculated assessing the degree of adherence to the Mediterranean diet for each participant (19). Calculation of the Med Diet Score involves rating the frequency of weekly consumption of 11 food groups (non-refined cereals, fruits, vegetables, legumes, potatoes, fish, meat and meat products, poultry, full fat dairy, olive oil use and alcohol) on a scale from 0 to 5. Individuals reporting no consumption of food groups characterizing the Mediterranean dietary pattern were assigned a score of 0, rare consumption was rated as 1, 1-3 times monthly was rated as 2, 1-2 times a week was rated as 3, 3-6 times a week was rated as 4 and daily consumption was rated as 5. Reverse scoring was used for items least characteristic of the Mediterranean diet (i.e., 0 for daily consumption, 4 for rare consumption and 5 for no consumption). The total score ranges from 0 to 55, with higher scores indicating greater adherence to the Mediterranean diet. Routine alcohol consumption was graded as follows: 0=alcohol consumption once in a year or no use,1=alcohol consumption less than once in the month, 2=alcohol consumption 1-5 times in the month, 3=alcohol consumption 3-4 times in the week, 4=alcohol consumption 5-7 times in the week, 5=alcohol consumption two or more times in the day). Social networking was indexed by the number of participants’ contacts with friends and relatives during the past month. Presence of depressive symptomatology was assessed using the Greek validated version of Geriatric Depression Scale (20) with scores above 7/15 indicating depression.
Circulating biomarker (IL-6, TNF-α) measurements IL-6 and TNF-α were quantified as previously described (21). Briefly, fasting (morning) blood samples were collected, transferred to EDTA-containing tubes and refrigerated until centrifugation for plasma isolation. Plasma was aliquoted and kept in deep freeze (-80oC) until assayed. Plasma TFN-α and IL-6 levels were measured by commercially available kits (Human TNF-alpha Quantikine HS ELISA and Human IL-6 Quantikine HS ELISA kits respectively, R&D Systems Europe, Abington, UK).
Statistical analyses
Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Normally distributed variables were analyzed with parametric tests, whilst non-normally distributed variables were either log-transformed prior to parametric analysis or analyzed with non-parametric tests.
Frailty subgroup differences were assessed through two-way ANOVAs for continuous variables (followed by pairwise comparisons) or χ2 tests for categorical variables. All pairwise comparisons were evaluated at p=0.017.
Correlates of overall frailty score (as a semicontinuous variable) were firstly assessed through Pearson or Spearman correlations in univariate analysis for demographic (age, male gender, education level in years), social/lifestyle factors (widowhood, frequency of contact with α and relatives [as an index of the frequency of social interactions], smoking, frequency of alcohol consumption, Med Diet Score), emotional status (GDS score), BMI, polypharmacy, cognitive status (MMSE score), and plasma levels of pro-inflammatory cytokines (IL-6, TNF-α).
Hierarchical linear regression models were applied to evaluate the independent contribution of individual covariates to overall frailty score. At first, traditional factors showing a trend for overall frailty score (p<0.1) were entered in a multivariate analysis model, followed by adding each pro-inflammatory cytokine and Med Diet Score together in the second step, to evaluate the additive contribution of proinflammatory biomarkers and adherence degree to the Mediterranean diet. Additional hierarchical models were applied to assess the independent role of systemic inflammation versus Mediterranean diet in a step-wise manner.
RESULTS
Descriptive participant characteristics
Our sample of 186 participants (63 men; 33.9%) had an average 75.38 (SD=7.30) years of age and 4.94 (SD=3.0) years of education. Among participants, 42 (22.6%) were widowed, 132 (71%) were fully functionally independent and 54 (29%) partially dependent. Mean BMI was 29.94 (SD=4.75), WC was 103.2 cm (SD=11.4), and mean Med diet score was 34.11 (SD=4.39). In terms of cognitive status, 46 individuals had dementia, 73 had mild cognitive impairment (MCI), and 67 were cognitively non-impaired (CNI). In terms of lifestyle, 17 (9.1%) were current smokers, and 36 persons (19.4%) reported consuming alcohol more than three times/week. Mean number of contacts with friends/month was 3.96 (SD=4.98). Participants scored an average of 23.25 (SD=5.27) points on the MMSE, 3.79 (SD=3.59) points on the GDS, and frailty score of 0.77 (SD=1.03) points. Mean values of TNF-α and IL-6 were 1.16 pgr/mL (SD=0.58) and 1.39 pgm/mL (SD=0.96), respectively.
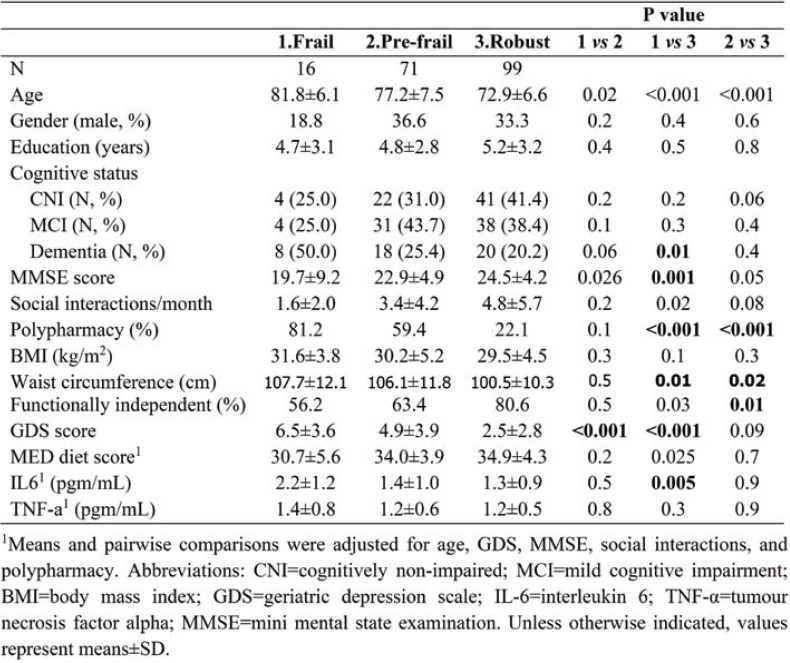
Frailty subgroup comparisons (Table 1) indicated that they were comparable on gender (p>0.2), education (p>0.4), TNF-a levels (p>0.1), and rates of persons without dementia (CNI or MCI; p>0.06). However, there was a higher rate of persons with dementia in the frail as compared to the robust subgroup (p=0.01), whilst persons in the frail subgroup displayed higher average levels of IL-6 (p<0.001), and lower average MMSE score (p=0.001). Moreover, higher rates of polypharmacy were found in both the frail and prefrail subgroups as compared to the robust subgroup (p<0.001), whereas higher GDS scores were found among both frail and prefrail participants as compared to the robust subgroup (p<0.001).
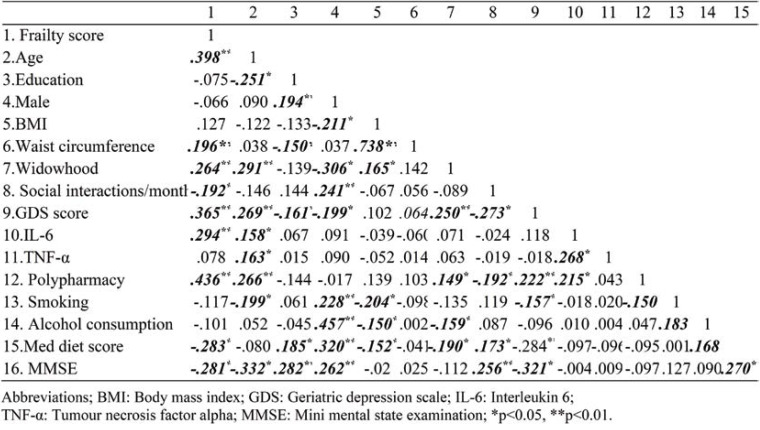
Univariate analysis: effects of age, widowhood, depression, inflammation and polypharmacy There were significant positive associations (p <0.01) between frailty score and age, widowhood, GDS score, polypharmacy, waist circumference, and IL-6 (Table 2). Also, there were significant negative associations (p<0.05) between frailty score and frequency of contact with friends, the Med diet score, and MMSE. The correlation between frailty score and TNF-α was negligible (r=0.078) and therefore the latter was not included in subsequent analyses.
Multivariate analyses
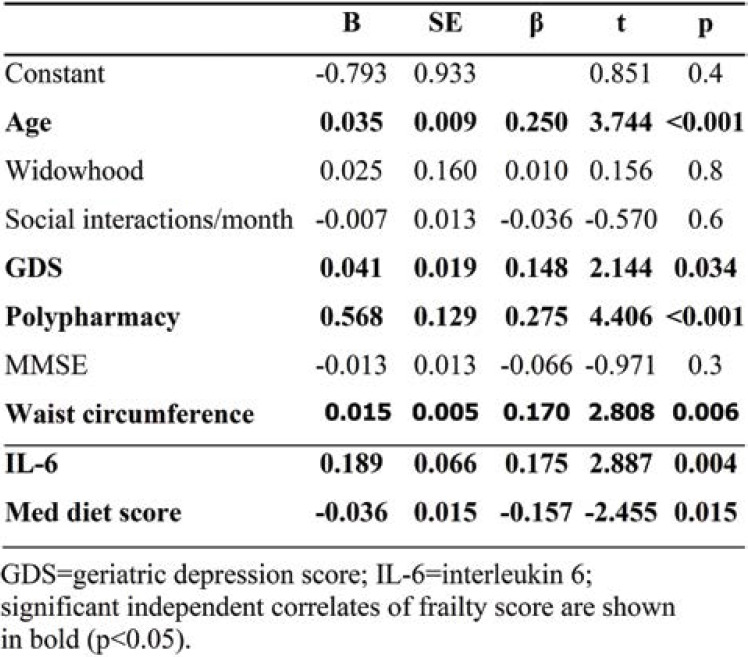
In hierarchical linear regression models, frailty score served as the dependent variable, whilst age, widowhood, contact with friends, GDS, polypharmacy, waist circumference, and MMSE were used as independent covariates. Step-wise inclusion of IL-6 and Med Diet Score into the model resulted in significant increase in R² (R² change=0.054, F[2,177] = 7.623, p=.001, total adjusted R²=0.39), suggesting that IL-6 and the level of adherence to the Mediterranean diet collectively accounted for a significant amount of variance in overall frailty score after controlling for the rest of the aforementioned factors (Table 3).
In further hierarchical multiple regression analyses, we assessed if IL-6 alone significantly contributed to overall frailty after controlling for Med Diet Score, and vice versa. In these models, age, GDS score, polypharmacy, widowhood, social interactions, MMSE score, and IL-6 or Med Diet Score were entered together in the first step whilst Med Diet Score or IL-6, respectively, were introduced in the second step. Med Diet Score alone made a significant additional contribution to overall frailty score (total sample: R² change=0.021, F[1,177] = 6.03, p=0.015). Likewise, a significant contribution of IL-6 alone to the overall frailty score was also observed (total sample: R² change=0.029, F[1,177] = 8.33, p=0.004).
DISCUSSION
The novelty of this study is the exploration of interactions of sociodemographic, clinical, cognitive and emotional variables as well as of systemic inflammation and Mediterranean diet in moderating overall frailty status in the elderly in a comprehensive way. Using a sub-sample of 186 individuals aged 60-89 years from the Cretan Aging Cohort, we found that the frailty score was positively associated with age, depression symptoms, plasma IL-6 levels, polypharmacy (a surrogate marker of medical comorbidities), and waist circumference (a marker of central obesity) whilst negatively associated with adherence to the Mediterranean diet (an indicator of healthy diet). Our results highlight the significant additive effect of emotional status (depression symptoms), lifestyle factors (Med Diet Score), central obesity, and systemic inflammation (IL-6) in accounting for physical frailty among the elderly, suggesting the complex content of frailty and providing a novel framework for more efficient frailty assessment.
Observational studies have linked chronic inflammation with the presence of frailty in the elderly (6, 7). These have focused on IL-6, TNF-α and CRP, well-established cytokines produced by a wide range of immune cells such as monocytes and macrophages (6, 7, 22) as well as other cell types. Of those, IL-6 and CRP have been consistently linked to frailty in a recent metanalysis (7). The underlying mechanisms might involve induction of sarcopenia, progression of co-existing chronic diseases and cytokine-mediated regulation of depression (6). Accordingly, our work reveals a significant association of serum IL-6 with frailty much more so than TNF-α. Crucially, this was additive on top of other established risk factors, highlighting a promising independent predictor of frailty.
Multiple studies have linked nutrition to frailty (11, 23). Underlying mechanisms are poorly understood, since nutrition affects multiple biological processes, from protein synthesis and oxidative stress to energy storage and consumption as well as cell signaling modification (24). Nutrition is also known to be related with cognitive decline and Alzheimer’s disease either via circulation of direct stimulators (pro-inflammatory cytokines) in central nervous system with blood or indirect by modulating gut microbiome and metabolites (25, 26). However, specific nutritional interventions focusing on the Mediterranean diet have produced controversial results as to their effect on frailty (12). Here, we highlight the independent role of nutrition in determining frailty status, integrating its effect with more traditional risk factors, such as age, as well as with biological processes, such as systemic inflammation.
Depression has been linked to frailty risk in observational studies (8). The underlying mechanism may be multilayered, involving nutrition, adherence to medical treatments, and underlying neurohormonal disturbances such as inflammation (6). Interestingly, this relationship appears to be bidirectional, with frailty increasing prospective risk for depression (27, 28). Our work identifies depression symptoms as a significant determinant of frailty, independently to other studied correlates. Depression may thus be mechanistically linked to frailty via core neuropsychiatric mechanisms independently of physical pathophysiological processes.
Body mass index and WC are indicators of general and central obesity and they are variously correlated with frailty in the literature. In our study, we found that WC was significantly associated with frailty, whilst BMI was not. These findings are in accordance with those from the literature which report that WC is a better predictor of frailty than BMI in a cohort of Chinese elderly individuals, and that BMI reveals a U-shaped relationship with frailty with the lowest prevalence of frailty observed between 25 and 29.9 kg/m² values of BMI (29, 30). In our study, the interaction of IL-6 with WC did not come out significant (π=0.5) in the multiple regression model, so we could claim that IL-6 and central obesity appeared to exert independent effects on frailty (i.e., the effect of IL-6 does not vary with the degree of central obesity).
The main strength of our study is the inclusion of multiple variables as potential determinants of frailty such as sociodemographic, dietary, pharmacological, psychological, cognitive and inflammatory variables. Furthermore, it was conducted in a well characterized sample. All participants have attended comprehensive neuropsychological and geriatric assessments by specialized physicians and neuropsychologists. Limitations of our study are represented by the rather modest sample size of participants and overrepresentation of elderly individuals who were born and raised in rural areas. Another limitation of the study was that the frailty status assessment was done by a scale validated in a group of patients of African American descent, and not Caucasians, but this scale included subjective measurements of frailty and was easier for screening use. The comprehensive statistical approach applied as well as the detailed phenotyping and naturalistic character of our cohort provided an overall strong message warranting further investigation.
CONCLUSION
Our study advances the field by integrating traditional risk factors with nutritional, psychological and biological features to provide a comprehensive evaluation of frailty in the elderly. Our data suggest that age, central obesity, polypharmacy, depression and IL-6 independently increase the probability of frailty in the elderly. Adherence to Mediterranean diet reduces this risk. Furthering our understanding of the relationships among the aforementioned variables may have important implications for the development of public health and clinical care interventions against frailty.
Conflict of interests: none declared
Financial support: none declared.
Ethical approval: The study was approved by the Bioethics Committee of the University Hospital of Heraklion, Crete, Greece (protocol number: 13541, 20/11/2010).
Informed consent: A written informed consent for study participation was provided by all participants.
FIGURE 1.
Study flowchart. MMSE=mini mental state examination; CNI=cognitively non-impaired;
TABLE 1.
Summary of sociodemographic, somatometric, cognitive, and clinical characteristics of the study sample
TABLE 2.
Correlations between frailty score, physical, lifestyle, depression symptoms, inflammatory markers, and overall cognitive status (N=186)
TABLE 3.
Results of hierarchical multiple regression predicting frailty score in the total sample (N=186)
Contributor Information
Symeon H. PANAGIOTAKIS, Internal Medicine Department, Heraklion University Hospital, Heraklion, Crete, Greece
Panagiotis SIMOS, Psychiatry Department, Medical School, University of Crete, Heraklion, Crete, Greece.
Maria BASTA, Psychiatry Department, Medical School, University of Crete, Heraklion, Crete, Greece.
Ioannis ZAGANAS, Neurology Department, Medical School, University of Crete, Heraklion, Crete, Greece.
Garyfalia S. PERYSINAKI, Nephrology Department, Rethymnon General Hospital, Rethymnon, Crete, Greece
Ioannis AKOUMIANAKIS, Internal Medicine Department, Heraklion University Hospital, Heraklion, Crete, Greece.
Chariklia TZIRAKI, MELABEV, Research Institute, Community Elders Clubs and Institute for Gerontological Data Bases, Hebrew University, Jerusalem, Israel.
Christos LIONIS, Clinic of Social and Family Medicine, Medical School, University of Crete, Heraklion, Crete, Greece.
Alexandros VGONTZAS, Psychiatry Department, Medical School, University of Crete, Heraklion, Crete, Greece.
Dimitrios BOUMPAS, Internal Medicine Department, Medical School, University of Athens, Athens, Greece.
References
- 1.Ke, LS. Frailty in the elderly: A concept analysis. J Nurs. 2013;173:489–495. doi: 10.6224/JN.60.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston, J, et al. Frailty in Older Adults: Evidence for a Phenotype. Journals Gerontol Ser A Biol Sci Med Sci. 2003;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Ntanasi E, Yannakoulia M, Mourtzi N, et al. Prevalence and Risk Factors of Frailty in a Community-Dwelling Population: The HELIAD Study. Journal of Aging and Health. 2020;32:14–24. doi: 10.1177/0898264318801735. [DOI] [PubMed] [Google Scholar]
- 4.Alexandre TDS, Corona LP, Brito TRP, et al. Gender Differences in the Incidence and Determinants of Components of the Frailty Phenotype Among Older Adults: Findings From the SABE Study. J Aging Health. 2018;30:190–212. doi: 10.1177/0898264316671228. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soysal P, Stubbs, B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. A. geing Res Rev. 2017;36:78–87. doi: 10.1016/j.arr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Panagiotakis SH, Simos P, Zaganas I, et al. Self-reported fatigue as a risk index for dementia diagnosis. Eur Geriatr Med. 2018;9:211–217. doi: 10.1007/s41999-017-0020-4. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29:43–48. doi: 10.1007/s40520-016-0709-0. [DOI] [PubMed] [Google Scholar]
- 11.Bonnefoy M, Berrut, G, Lesourd B, et al. Frailty and nutrition: Searching for evidence. J Nutr Heal Aging. 2015;19:250–257. doi: 10.1007/s12603-014-0568-3. [DOI] [PubMed] [Google Scholar]
- 12.Veronese N, Stubbs B, Noale M, et al. Adherence to a Mediterranean diet is associated with lower incidence of frailty: A longitudinal cohort study. Clin Nutr. 2017;37:1492–1497. doi: 10.1016/j.clnu.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaganas IV, Simos P, Basta M, et al. The Cretan Aging Cohort: Cohort Description and Burden of Dementia and Mild Cognitive Impairment. Am J Alzheimers Dis Other Demen. 2019;34:23–33. doi: 10.1177/1533317518802414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basta M, Simos P, Vgontzas, A., et al. Associations between sleep duration and cognitive impairment in mild cognitive impairment. J Sleep Res. 2019;28:e12864. doi: 10.1111/jsr.12864. [DOI] [PubMed] [Google Scholar]
- 15.Fountoulakis KN, Tsolaki M, Chantzi H, Kazis A. Mini Mental State Examination (MMSE): A validation study in Greece. Am J Alzheimers Dis Other Demen. 2000;15:342–345. [Google Scholar]
- 16.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged african americans. J Nutr Heal Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 18.Katsouyanni K. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int J Epidemiol. 1997;26:S118–S127. doi: 10.1093/ije/26.suppl_1.s118. [DOI] [PubMed] [Google Scholar]
- 19.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16:559–568. doi: 10.1016/j.numecd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Fountoulakis KN, Tsolaki M, Iacovides A, et al. The validation of the short form of the geriatric depression scale (GDS) in Greece. Aging Clin Exp Res. 1999;11:367–372. doi: 10.1007/BF03339814. [DOI] [PubMed] [Google Scholar]
- 21.Basta M, Koutentaki E, Vgontzas A, et al. Objective Daytime Napping is Associated with Disease Severity and Inflammation in Patients with Mild to Moderate Dementia. J Alzheimers Dis. 2020. [DOI] [PubMed]
- 22.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Hao Q, Su L, et al. Adherence to the Mediterranean Diet and the Risk of Frailty in Old People: A Systematic Review and Meta-Analysis. J Nutr Health Aging. 2018;22:613–618. doi: 10.1007/s12603-018-1020-x. [DOI] [PubMed] [Google Scholar]
- 24.Mathers JC. Nutritional modulation of ageing: Genomic and epigenetic approaches. Mech Ageing Dev. 2006;127:584–589. doi: 10.1016/j.mad.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Nagpal R, Neth BJ, Wang S, et al. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–542. doi: 10.1016/j.ebiom.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrattan AM, McGuinness B, McKinley MC, et al. Diet and Inflammation in Cognitive Ageing and Alzheimer's Disease. Curr Nutr Rep. 2019;8:53–65. doi: 10.1007/s13668-019-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaughan L, Corbin AL, Goveas JS. Depression and frailty in later life: A systematic review. Clin Interv Aging. 2015;10:1947–1958. doi: 10.2147/CIA.S69632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buigues C, Padilla-Sánchez C, Fernández Garrido, J, et al. The relationship between depression and frailty syndrome: A systematic review. Aging Ment Heal. 2015;19:762–772. doi: 10.1080/13607863.2014.967174. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, Body Mass Index, and Abdominal Obesity in Older People. Journals Gerontol Ser A Biol Sci Med Sci. 2010;65A:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 30.Liao Q, Zheng Z, Xiu S, Chan P. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res. 2018;30:1319. doi: 10.1007/s40520-018-0933-x. [DOI] [PubMed] [Google Scholar]