Abstract
Objective: Stroke represents a major cause of upper limb motor impairment among stroke survivors, resulting in functional disability and affecting negatively their quality of life. Thus, it is imperative that stroke rehabilitation be efficient. Up to the present, several intervention methods have been proposed in an attempt to improve recovery potential poststroke, transcranial direct current stimulation (tDCS) and mirror therapy (MT) being among them. The aim of this review is to investigate the utility of tDCS administration in conjunction with MT on chronic stroke population.
Methods: A literature research of two databases (MEDLINE and Scopus) was conducted in order to identify all relevant studies published between January 1st 2010 and September 30th 2021 that focused on the efficacy of the combined application of tDCS and MT on upper limb rehabilitation among chronic stroke patients.
Results: Three studies fulfilled the selection criteria and were included in the present review. Transcranial direct current stimulation application along with MT exhibited statistically significant increases in Box and block test, grip strength, Action research arm test score and Nottingham extended activities of daily living score within the experimental group compared to controls. The timing-dependent interaction effects seem to be of key importance, as sequentially delivered tDCS prior to MT is considered to be more advantageous and time-efficient compared to the concurrent application of tDCS and MT.
Conclusions: Application of tDCS in parallel with MT represents a promising neurorehabilitation tool for post-stroke patients regarding upper limb motor performance, movement efficiency and daily function. Future studies are needed in order to clarify whether sequential or concurrent tDCS and MT application is more beneficial.
Keywords:transcranial direct current stimulation, mirror therapy, stroke, upper extremity, rehabilitation.
INTRODUCTION
Stroke represents not only the second leading cause of death, but also the major source of acquired adult disability, as mean stroke incidence has flourished between 76 and 112 per 100,000 population per year worldwide (1). More than half of stroke patients are over 65 years of age (2), highlighting the fact that stroke constitutes an age-related disease with a constantly increasing burden, when taking into account both the world population’s growth and extended lifespan (3).
Stroke is usually associated with a great odd of reporting disability, as far as many individual domains are concerned (4-6). For example, during the chronic phase, up to 80% of stroke patients present with upper and lower limb motor impairment, while only 20% exhibit intact hand function (7). Upper limb impairment following stroke commonly refers to a combination of difficulty moving or coordinating the arm, hand or fingers, painful upper limb or hypoesthesia (8, 9). The persistence and disabling character of the aforementioned impairments explain why stroke survivors often exhibit difficulties executing and participating in several activities of daily living (ADL) and subsequent poor quality of life (10).
According to current stroke rehabilitation guidelines, brain remodeling as a result of neuroplasticity, could be facilitated through the implementation of various therapeutic interventions aiming at upper extremity retraining in order to enhance motor and functional recovery (11, 12). The most frequently applied rehabilitation strategies include intensive and task-oriented training movements, repetitive motor training, biofeedback, robotic training, as well as transcranial direct current stimulation (tDCS) and mirror therapy (MT) (13-16).
Non-invasive brain stimulation has been utilized not only as a prognostic tool poststroke (17), but also serving specific therapeutic purposes. Stimulating the human motor cortex, through the delivery of low intensity current to the scalp, tDCS is commonly used in stroke rehabilitation. It has been reported to induce structural neuronal changes, thus modulating the function of neural networks and enhancing the effectiveness of motor learning process. Interestingly, tDCS seems to be of key importance in promoting neuroplasticity. When applied alone, tDCS is proven to be beneficial not only for improving upper limb motor function in chronic stroke patients (18-20), but also for increasing ADL capacity (21, 22). Even though tDCS can be administered alone, it is usually applied in parallel with other neurorehabilitation techniques, in an attempt to positively affect restoration of motor impairment post stroke.
Mirror therapy is a cognitive intervention method which is widely used in clinical and research practice among stroke patients. This motor training technique takes advantage of the visual illusion effect, which provides the impression of a moving paretic upper extremity, thereby enhancing motor performance of the affected side through activation of the mirror neuron system. Mirror neurons are mainly localized in premotor cortex, supplementary motor area, primary somatosensory cortex and inferior parietal cortex. Mirror therapy application, as a stroke rehabilitation approach, has been demonstrated to promote upper limb motor function (23-26) and positively affect restoration of ADL abilities (25, 26). Apart from that, recently published evidence supports the implementation of MT mainly during the subacute phase of stroke, in order to maximize the benefits of the intervention (27). As far as acute and chronic stroke patients are concerned, MT alone is considered to be more effective in facilitating upper limb recovery compared to conventional rehabilitation approach or combined MT with conventional therapy (28).
Mirror therapy has been used in combination with electric stimulation, strength training or transcranial magnetic simulation showing a synergetic effect on hand dexterity improvement (29-31). Mirror therapy implementation along with tDCS after stroke constitutes a scientific field of investigation that attracts the attention of the multidisciplinary rehabilitation team. Thus, the present review aims to explore the efficacy of the combined application of MT and tDCS on upper extremity functional recovery in chronic stroke patients.
MATERIAL AND METHODS
The preferred reporting items for systematic reviews and meta-analyses list (PRISMA check list) was used to guide the present review (32). Our study methods were a priori designed.
Search strategy
Two databases (MEDLINE and Scopus) were selected to carry out the present literature search, which was conducted by one investigator (PV). In order to trace all relevant studies published between January 1st 2010 and September 30th 2021, the following keywords were used: “mirror therapy” AND [“transcranial direct current stimulation” OR “tDCS”] AND “stroke” AND [“upper extremity” OR “upper limb” OR “hand”]. All retrieved articles were also hand searched for any further potential eligible articles. Any disagreement regarding screening, or selection process, was solved by a second investigator (KV) until a consensus was reached.
Selection criteria
Only full-text original articles dealing with adults having suffered a stroke at least six months in advance and published in English language were included. Secondary analyses, reviews, guidelines, meeting summaries, comments, unpublished abstracts or studies conducted in animals were excluded. There was no restriction on study design or sample characteristics.
Data extraction
Data extraction was performed using a predefined data spreadsheet created in Excel. We recorded author, year of publication, number of participants, timing of intervention, study design, intervention duration, intervention frequency, tDCS characteristics (equipment, type of stimulation, procedure, stimulus intensity, sponge electrode area), MT protocol, outcome assessment tools, follow-up period and main results.
Data analysis
No statistical analysis or meta-analysis was performed due to the high heterogeneity among studies. Thus, the data were only descriptively analyzed.
RESULTS
Database searches
Overall, 287 records were retrieved from the database searching. Duplicates and irrelevant studies were excluded; hence, a total of five articles were selected. After screening the full text of the articles, three studies were eligible for inclusion (Figure 1).
Study characteristics
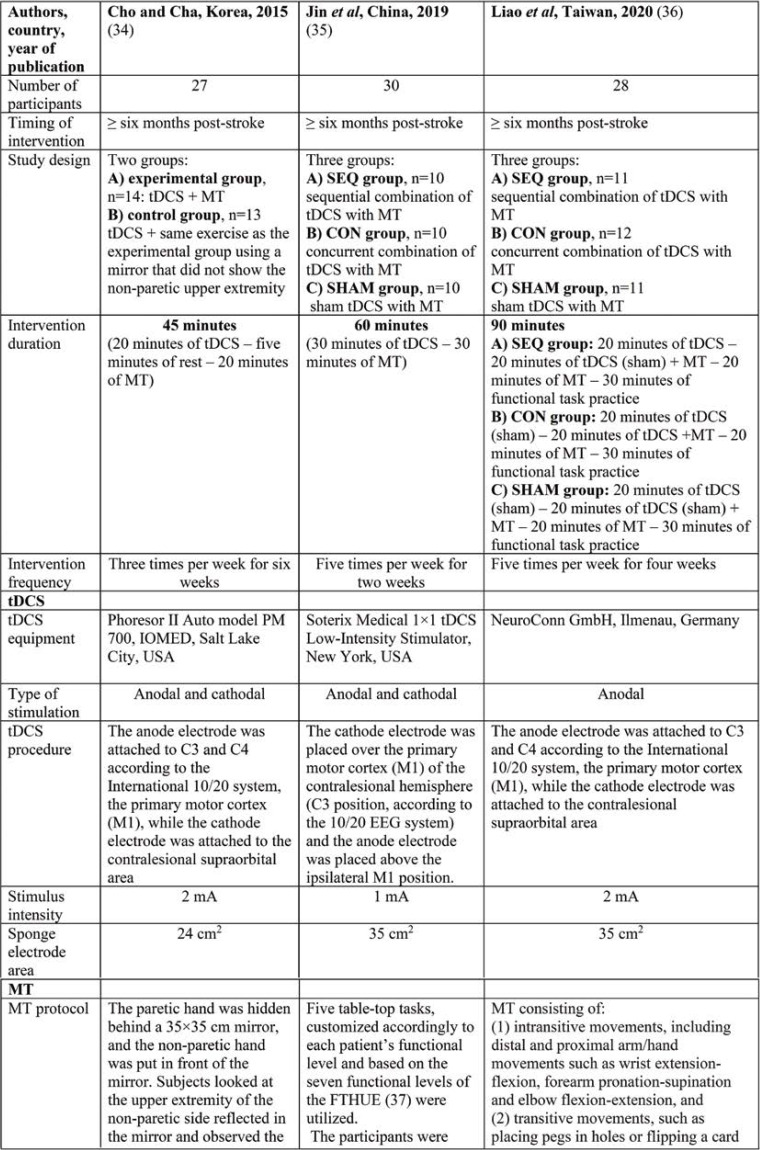
Three publications fulfilled our inclusion criteria. The reported number of participants who completed all training sessions ranged between 27 and 30 within each study, with an average of around 28.3 participants. Characteristics of included studies are presented in Table 1.
DISCUSSION
Disability resulting from a stroke insult still constitutes a challenging issue concerning stroke survivors, as the identification of optimal neurorehabilitation strategy, aiming to facilitate functional motor recovery poststroke, remains difficult and largely unexplored. Several studies have investigated the effect of tDCS and MT, solely or in combination, on upper limb functional recovery within six months of stroke onset, that is in subacute stroke patients who exhibit the greatest recovery potential (2, 33). The studies included in the present review aimed to explore the impact of combined tDCS and MT implementation on the rehabilitation of a chronic stroke population, that is after the time period that neurological function recovers naturally.
A synergistic approach with post-stroke application of both tDCS and MT seems a quite promising strategy, in order motor recovery of stroke patients to be further enhanced. Cho and Cha (34) investigated the influence of sequentially combined tDCS with MT on chronic stroke patients compared to tDCS administration in conjunction with motor training using a mirror that did not reflect the non-paretic upper extremity. The researchers reported statistically significant increases in the Box and block test (BBT) and grip strength within the experimental group more that those found in the control group, thus eliciting a link between the sequential use of tDCS and MT and the enhancement of manual dexterity and hand grip strength poststroke. The positive impact of the combined approach on functional motor recovery of chronic stroke patients was attributed to the role played by MT in motivating participants as well as the effect of tDCS on the function of the neuronal circuit in the motor cortex.
The effects of applying tDCS in parallel with MT in stroke survivors may not be just additive, implying that the interventions’ order of implementation could affect overall the rehabilitation process. With regards to the timing-dependent interaction effect, Jin et al (35) conducted a study and examined 30 chronic stroke patients who had been allocated to three different groups, depending on the intervention flow. Researchers delivered tDCS either prior or concurrently with movement practice of MT or not at all (sham-tDCS), in order to investigate how the timing would influence treatment effects poststroke. Their results revealed a greater improvement in the Action research arm test (ARAT) score when tDCS was administered simultaneously with MT compared to when tDCS was applied prior to MT or the sham condition, thus identifying a motor priming effect of concurrent-tDCS in conjunction with MT, as far as upper limb recovery is concerned. Although participants of the concurrent-tDCS group scored higher in the Fugl-Meyer assessment-upper extremity (FMA-UE) subscore and BBT post-intervention than the other two intervention groups, these differences did not achieve a significance level. The delivery of tDCS coupled with MT at the same time was found to be beneficial for restoration of motor performance after stroke, but the aforementioned approach did not yield to similar benefits regarding all reported outcomes.
The impact of timing-dependent effect of tDCS and MT in terms of motor performance, daily function, as well as upper extremity motor control was also investigated by Liao et al (36). Adult individuals with chronic stroke were involved in this study and assigned to three intervention groups, which were differentiated by the timing of tDCS application in relation to MT. As far as the clinical and kinematic outcomes were concerned, the researchers concluded that sequentially applied tDCS with MT was followed by a statistically significant increase in the Nottingham extended activities of daily living (NEADL) scale score, more than in the concurrent- or sham-tDCS group, thus indicating that the order of motor priming and training may influence restoration of ADL capacity. Similar results were not found with regard to FMA-UE scale, as motor impairment was recovered to a same degree between different intervention groups, thereby showing that the implementation of tDCS either simultaneously or prior to MT was not coupled with additional benefits on motor function. With respect to arm kinematics, it was demonstrated that the timing of tDCS could play a role in improving spatial and temporal upper limb motor control, as participants in the concurrent-tDCS groups were able to move their hand and arm more efficiently compared to the other groups.
Overall, the results of the present review should be considered as conflicting. Cho and Cha (34) found a significant enhancement in manual dexterity and grip strength when applying tDCS prior to MT than motor practice without mirror reflection, thus highlighting the fact that a sequential combination of tDCS with MT was able to promote motor recovery of upper limb during the chronic phase of stroke. By contrast, Liao et al (36) could not elicit a link between the use of tDCS in parallel with MT and motor function, as far as all intervention groups were concerned. With respect to ADL abilities and upper limb motor control, the researchers concluded that timing proved of key importance, as the sequential-tDCS group exhibited greater functional independence and movement efficiency than the concurrent-tDCS group or the sham condition. The outcomes of the study conducted by Jin et al (35) were inconsistent with those of the aforementioned studies, as the delivery of tDCS during the movement practice of MT was followed by benefits on motor recovery, only though regarding the ARAT score, but not also the other investigated clinical outcomes.
The heterogeneity of the included studies in terms of intervention protocols and outcome measures could be responsible for the differential results among studies. Cho and Cha (34) administered tDCS of 2 mA for 20 minutes, three times per week, for six weeks, while Jin et al (35) delivered low intensity tDCS of 1 mA for 30 minutes, in five sessions per weeks, across two weeks. Apart from that in the study of Liao et al (36), participants’ motor cortex was stimulated with anodal only tDCS of 2 mA that lasted 20 minutes, five days per week, for four consecutive weeks. Moreover, the applied MT protocol had variations between the three studies regarding the movement practice and performed upper limb tasks. As far as the outcome measures were concerned, both Cho and Cha (34) and Jin et al (35) evaluated the effects of combined application of tDCS with MT on motor function using only clinical measurements, while Liao et al (36) assessed the impact of neurorehabilitation interventions on functional independence and upper limb motor control, utilizing outcomes measures related to daily activities and movement kinematics. Several limitations of the included studies are also mentioned in Table 1. Future studies should address them, in order to optimize the outcomes and identify the most beneficial combination neurorehabilitation strategy for improving poststroke recovery.
CONCLUSIONS
Our findings indicate that the application of tDCS in conjunction with MT constitutes a promising approach, which is expected to enhance upper limb rehabilitation efficiency even in chronic stroke population. Although the results regarding the timing-dependent interaction effect of tDCS with MT on stroke patients’ recovery seem rather controversial, sequentially delivered tDCS prior to MT is considered to be more advantageous and time-efficient compared to concurrent application of tDCS and MT. Additional studies on the impact of the aforementioned neurorehabilitation approach are recommended, in order to identify the optimal combination strategy and provide further insight on the relationship between application of tDCS in parallel with MT and motor recovery in stroke patients.
Conflict of interests: none declared.
Financial support: This work was supported by the project “Study of the interrelationships between neuroimaging, neurophysiological and biomechanical biomarkers in stroke rehabilitation (NEURO-BIO-MECH in stroke rehab)” (MIS 5047286), which was implemented under the Action “Support for Regional Excellence”, funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund).
FIGURE 1.
Study flow diagram (PRISMA flowchart)
TABLE 1.
Characteristics of the included studies
TABLE 1.
Characteristics of the included studies
Contributor Information
Penelope VLOTINOU, Neurology Department, Democritus University of Thrace, Alexandroupolis, Greece.
Dimitrios TSIPTSIOS, Neurology Department, Democritus University of Thrace, Alexandroupolis, Greece.
Stella KARATZETZOU, Neurology Department, Democritus University of Thrace, Alexandroupolis, Greece.
Georgios KALOGIROU, Neurology Department, Democritus University of Thrace, Alexandroupolis, Greece.
Eleftherios STEFAS, Neurology Department, Democritus University of Thrace, Alexandroupolis, Greece.
Nikolaos AGGELOUSIS, Department of Physical Education and Sport Science, Democritus University of Thrace, Komotini, Greece.
Konstantinos VADIKOLIAS, Neurology Department, Democritus University of Thrace, Alexandroupolis, Greece.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. 2020;2:17. doi: 10.1186/s42466-020-00060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 4.Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13:171–177. doi: 10.1016/j.jstrokecerebrovasdis.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Katzan I, Thompson N, Uchino K, Lapin B. The most affected health domains after ischemic stroke. Neurology. 2018;90:e1364–e1371. doi: 10.1212/WNL.0000000000005327. [DOI] [PubMed] [Google Scholar]
- 6.Thrift A, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke. 2017;12:13–32. doi: 10.1177/1747493016676285. [DOI] [PubMed] [Google Scholar]
- 7.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 8.Pollock A, Farmer S, Brady M, et al. Interventions for improving upper limb function after stroke. Cohrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD010820.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jönsson AC, Lindgren I, Hallström B, et al. Prevalence and intensity of pain after stroke: a population-based study focusing on patients' perspectives. J Neurol Neurosurg Psychiatry. 2006;77:590–595. doi: 10.1136/jnnp.2005.079145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschini M, La Porta F, Agosti M, Massucci M, ICR2 group. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010;46:389–399. [PubMed] [Google Scholar]
- 11.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Chum M. Combination Transcranial Direct Current Stimulation and Virtual Reality for upper Extremity training in patients with Subacute stroke. Arch Phys Med Rehabil. 2014;95:431–438. doi: 10.1016/j.apmr.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 13.da Silva ESM, Santos GL, Catai AM, et al. Effect of aerobic exercise prior to modified constraint-induced movement therapy outcomes in individuals with chronic hemiparesis: a study protocol for a randomized clinical trial. BMC Neurol. 2019;19:196. doi: 10.1186/s12883-019-1421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wattchow K, McDonnell M, Hillier S. Rehabilitation Interventions for Upper Limb Function in the First Four Weeks Following Stroke: A Systematic Review and Meta-Analysis of the Evidence. Arch Phys Med Rehabil. 2018;99:367–382. doi: 10.1016/j.apmr.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Bondoc S, Booth J, Budde G, et al. Mirror Therapy and Task-Oriented Training for People With a Paretic Upper Extremity. Am J Occup Ther. 2018;72:7202205080. doi: 10.5014/ajot.2018.025064. [DOI] [PubMed] [Google Scholar]
- 16.Wolf TJ, Polatajko H, Baum C, et al. Combined Cognitive-Strategy and Task-Specific Training Affects Cognition and Upper-Extremity Function in Subacute Stroke: An Exploratory Randomized Controlled Trial. Am J Occup Ther. 2016;70:7002290010. doi: 10.5014/ajot.2016.017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karatzetzou S, Tsiptsios D, Terzoudi A, et al. Transcranial magnetic stimulation implementation on stroke prognosis. Neurol Sci. 2022;43:873–888. doi: 10.1007/s10072-021-05791-1. [DOI] [PubMed] [Google Scholar]
- 18.Marquez J, van Vliet P, McElduff P, et al. Transcranial Direct Current Stimulation (tDCS): Does it Have Merit in Stroke Rehabilitation? A Systematic Review. Int J Stroke. 2013;10:306–316. doi: 10.1111/ijs.12169. [DOI] [PubMed] [Google Scholar]
- 19.Bai X, Guo Z, He L, et al. Different Therapeutic Effects of Transcranial Direct Current Stimulation on Upper and Lower Limb Recovery of Stroke Patients with Motor Dysfunction: A Meta-Analysis. Neural Plast. 2019. pp. 1–13. [DOI] [PMC free article] [PubMed]
- 20.Butler AJ, Shuster M, O’Hara E, et al. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J Hand Ther. 2013;26:162–171. doi: 10.1016/j.jht.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J NeuroEng Rehabil. 2017;13:95. doi: 10.1186/s12984-017-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2016;21 doi: 10.1002/14651858.CD009645.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothgangel AS, Braun SM, Beurskens AJ, et al. The clinical aspects of mirror therapy in rehabilitation. Int J Rehabil Res. 2011;34:113. doi: 10.1097/MRR.0b013e3283441e98. [DOI] [PubMed] [Google Scholar]
- 24.Zeng W, Guo Y, Wu G, et al. Mirror therapy for motor function of the upper extremity in patients with stroke: A meta-analysis. J Rehabil Med. 2018;50:8–15. doi: 10.2340/16501977-2287. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Zhao Q, Zhang Y, Wu Q, et al. Effect of Mirror Therapy on Recovery of Stroke Survivors: A Systematic Review and Network Meta-analysis. Neuroscience. 2018;15:318–336. doi: 10.1016/j.neuroscience.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Thieme H, Morkisch N, Mehrholz J, et al. Mirror therapy for improving motor function after stroke. Cochrane Database Syst Rev 2018. [DOI] [PMC free article] [PubMed]
- 27.Toh SFM, Fong KNK. Systematic Review on the Effectiveness of Mirror Therapy in Training Upper Limb Hemiparesis after Stroke. Hong Kong Journal of Occupational Therapy. 2012;22:84–95. [Google Scholar]
- 28.Pérez-Cruzado D, Merchán-Baeza JA, González-Sánchez M, Cuesta-Vargas AI. Systematic review of mirror therapy compared with conventional rehabilitation in upper extremity function in stroke survivors. Aust Occup Ther Jl. 2016;64:91–112. doi: 10.1111/1440-1630.12342. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Yim J. Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation Combined with Task-Oriented Mirror Therapy Training on Hand Rehabilitation of Acute Stroke Patients. Med Sci Monit. 2018;24:743–750. doi: 10.12659/MSM.905636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrensberger M, Simpson D, Broderick P, et al. Unilateral Strength Training and Mirror Therapy in Patients With Chronic Stroke: A Pilot Randomized Trial. Am J Phys Med Rehabil. 2019;98:657–665. doi: 10.1097/PHM.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 31.Saavedra-García A, Moral-Munoz J, Lucena-Anton D. First Mirror therapy simultaneously combined with electrical stimulation for upper limb motor function recovery after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2020;35:39–50. doi: 10.1177/0269215520951935. [DOI] [PubMed] [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Kreisel SH, Hennerici MG, Bäzner H. Pathophysiology of stroke rehabilitation: the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis. 2007;23:243–255. doi: 10.1159/000098323. [DOI] [PubMed] [Google Scholar]
- 34.Cho HS, Cha HG. Effect of mirror therapy with tDCS on functional recovery of the upper extremity of stroke patients. J Phys Ther Sci. 2015;27:1045–1047. doi: 10.1589/jpts.27.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin M, Zhang Z, Bai Z, Fong KNK. Timing-dependent interaction effects of tDCS with mirror therapy on upper extremity motor recovery in patients with chronic stroke: A randomized controlled pilot study. J Neurol Sci. 2019;15:405. doi: 10.1016/j.jns.2019.116436. [DOI] [PubMed] [Google Scholar]
- 36.Liao WW, Chiang WC, Lin KC, et al. Timing-dependent effects of transcranial direct current stimulation with mirror therapy on daily function and motor control in chronic stroke: a randomized controlled pilot study. J Neuroeng Rehabil. 2020;17:101. doi: 10.1186/s12984-020-00722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong K, Ng B, Chan C, et al. Development of the Hong Kong version of the functional test for the hemiplegic upper extremity (FTHUE-HK). Hong Kong J Occup Ther. 2004;14:21–29. [Google Scholar]
- 39.Jebsen RH, Taylor N, Trieschmann RB, et al. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 40.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 41.Platz T, Pinkowski C, van Wijck F, et al. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer test, action research arm test and box and block test: a multicentre study. Clin Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 42.Chen HF, Lin KC, Wu CY, Chen CL. Rasch validation and predictive validity of the action research arm test in patients receiving stroke rehabilitation. Arch Phys Med Rehabil. 2012;93:1039–1045. doi: 10.1016/j.apmr.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Wu CY, Chuang LI, Lin KC, Hong WH. Responsiveness, minimal detectable change, and minimal clinically important difference of the Nottingham extended activities of daily living scale in patients with improved performance after stroke rehabilitation. Arch Phys Med Rehabil. 2011;92:1281–1287. doi: 10.1016/j.apmr.2011.03.008. [DOI] [PubMed] [Google Scholar]