Keywords:atrial fibrillation, diabetes mellitus, anticoagulation.
INTRODUCTION
Atrial fibrillation (AF) and diabetes mellitus (DM) are frequent pathological conditions in cardiology. Their association is also frequent: approximately 15% of people with diabetes mellitus have AF and approximately 30% of AF cases occur in diabetic patients (1).
Diabetes mellitus is an independent risk factor for AF, and coexisting AF is a co-morbidity – a risk factor for the evolution of the diabetic patient.
The association of AF with DM is a situation that enables a holistic approach to AF with patient- centered management and corresponds to the recommendations of the 2020 ESC Guideline for the diagnosis and management of AF (1).
In this context, the management guidelines summarized in the ABC formula were formulated as follows: A = anticoagulation (avoid stroke); B = better symptom control and C = cardiovascular risk factors and comorbid conditions management) (2).
The implications of the association between AF and DM from a demographic, pathophysiological and therapeutic perspective are analysed in this paper.
General data. Epidemiology
The Framingham study (extended) was the first showing the significant increase in the risk of AF in patients with DM (4). Many subsequent studies have reported the reciprocal relationship between DM and AF. The prevalence of AF in patients with DM is estimated to be 15%, but approximately 30% of patients with AF have DM (5). The prevalence of AF is at least twice as high in DM compared to a non-diabetic person, however higher in cases of microangiopathy. Other comorbidities such as high blood pressure (HBP) should also be considered: the association of DM with HBP increases the risk of AF threefold compared to the absence of this association.
The prevalence and incidence of AF in DM and their determining factors are also reported in large populational studies. In a cohort study (Swedish) carried out during 2001-2013, the relationship between DM and the presence or development of AF in 421,855 diabetic subjects versus controls (2,131,223 non-diabetics) was followed. The risk of DM for incident AF was 28%, higher than in the control group (HR 1.41; p = 0.0001) (6). In a Danish study based on data from the Danish National Patient Register [5,081,087 people, of which 5% (253,374) with DM], DM was associated with a rate of development of AF of 19%. In an analysis by age groups (18-39; 40-64; 65-74; > 75 years), the rate of AF per 1000 people/years ranged from 0.2 to 1000 people/years in the first group to 20/1000 people/years in the elderly group (> 75 years) (7).
The developmental elements of AF are dependent, besides age, on both the duration of DM and level of glycaemic control. Thus, the glycaemic level is associated with an increased risk of AF; for example, a 1% increase in HbA1C is accompanied by an OR of 1.14. Likewise, the duration of DM and glycaemic control sum up their effects of increasing the risk of AF (8).
Overall, the analysis of studies and data from meta-analyses estimates that patients with type 2 DM have a 38.6% risk for incidental AF (9).
The evolution of patients with DM and AF is globally unfavorable, considering the relationships with mutual negative influence. Diabetes mellitus associated with AF increases the cardiovascular mortality of stroke, chronic kidney disease (CKD) and heart failure (HF). The risk of death may be 25-66% higher in patients with DM and AF than in non-diabetic ones and patients without AF. At the same time, AF is a high risk marker of negative evolution in DM, at least for stroke and HF.
In the ADVANCE study, patients with type 2 diabetes and AF had an increased risk of major cardiovascular events (stroke, HF, cardiovascular mortality) (10).
Not only type 2 diabetes has been associated with an increased rate of AF, but also type 1 diabetes. A Swedish study compared 36,253 patients with type 1 diabetes with 179,980 control subjects for a period of 9.7 years. The mean age of subjects was 30 years. A 50% increase in the risk of AF (HR 1.5) but a moderate increase in the risk of AF in men (HR 1.13) was reported in the data analysis.
The increased risk of AF in type 1 diabetes is influenced by inadecquate glycaemic control and renal impairment (11, 11 bis).
The role of pre-diabetes, of the metabolic syndrome, as predictors/risk factors for AF, is another chapter of interrelationships.
Pathophysiology
The pathophysiology of AF onset and development and its modulating factors in diabetic patients contains multiple mostly known pathogenic processes.
A scheme of pathogenic processes which lead from DM – the primary element – to atrial remodeling, arrhythmogenesis and AF was formulated by Wang (12). Broadly speaking, DM, a complex metabolic disorder related to insulin resistance, induces and generates (intermediate) pathogenic processes (unstable hyperglycemia, oxidative stress and inflammation) which, in isolation and in inter-correlations, lead to the final process of LA remodeling, arrhythmogenesis and atrial fibrillation.
Especially type 2 diabetes is an independent risk factor for AF and the starting point of the pathogenic chain. Diabetes can act in isolation as a metabolic factor at the cellular level or associated with risk factors/comorbidities (HBP, heart failure, coronary disease) which are also involved in atrial remodeling and arrhythmogenesis.
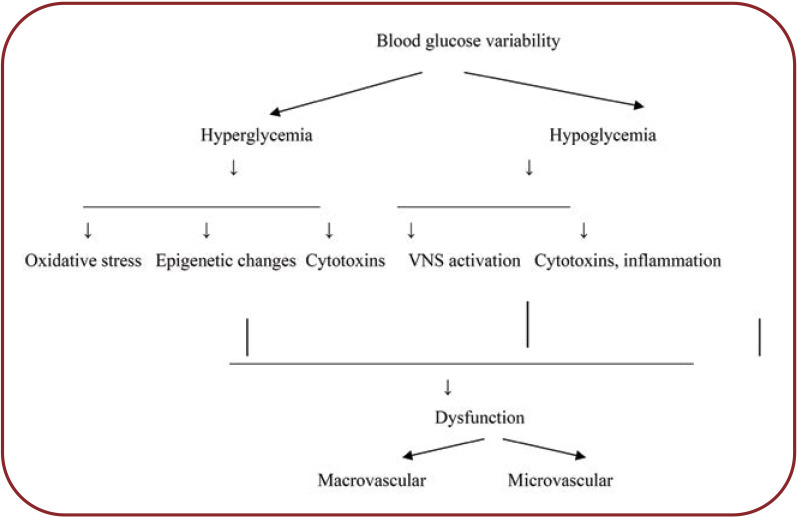
Chronic hyperglycemia is a key modulator of atrial remodeling and AF initiation (13). In addition to high blood glucose levels per se, blood glucose fluctuations and long-term blood glucose variability are associated with atrial fibrosis, oxidative stress, and susceptibility to AF (14). Long term DM and increased HbA1C are associated with a higher risk of AF (15). Hyperglycemia (DM) stimulates RAAS and the expression of growth factors (e.g., TGF beta) and subsequently, collagen synthesis. AG II is one of the most important modulators of fibrosis in DM (15).
Hyperglycemia also stimulates the production of advanced glycation end-product (AGE) and the AGE-RAGE system is considered an important mediator for the development of fibrosis in LA (16).
To summarize, hyperglycemia and its fluctuations, biological molecules (AG II, AGE, ROS) lead to increased deposits of collagen and atrial fibrosis, the main conditions in AF arrhythmogenesis.
Oxidative stress and inflammation, together with blood glucose level and blood glucose variations, are intermediate pathogenic modeling processes between DM and atrial remodeling. Increase of oxidative stress (ROS) at the atrial level activates biological pathways (nuclear factor Kappa B) that promote inflammation and fibrosis via increased TGF and TNFα (17). Inflammatory biomarkers are increased in serum and at the atrial level in AF. Atrial biopsies reported increased CRP levels. CRP, TNFá, and interleukin 6 (IL6) biomarkers were significantly increased in patients with DM, dilated LA, and incident AF (15, 17).
It is suggested that ROS is linked not only to the inflammatory process, but also to the promotion of AF and maintenance of arrhythmia. Oxidative stress is also linked to blood glucose fluctuations (18, 19).
Diabetes mellitus may be accompanied by autonomous remodeling. Autonomous neuropathy expressed at the cardiac, atrial and systemic level is the result of imbalance between parasympathetic activity (parasympathetic denervation) and stimulation of the sympathetic activity. Paroxysmal atrial fibrillation in young people with a normally structured heart is typically of vagal type.
Clinically, autonomous cardiac dysfunction is manifested by heart rhythm variability (20) and by numerous episodes of silent AF, identified in prolonged Holter monitoring, which can be a predictor of cerebral events (21).
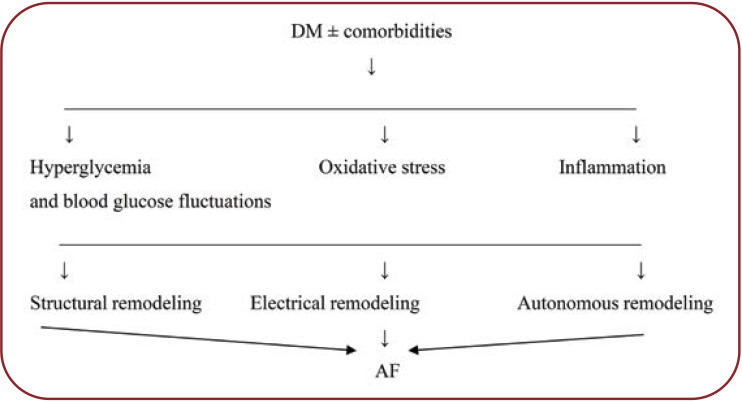
Atrial remodeling (LA) is the final pathogenic process and the substrate for the development and perpetuation of atrial fibrillation. As far as the pathogenic aspect is concerned, there are three interdependent types (mechanical, electrical, and neurogenic autonomous) of atrial remodeling in DM, but also in other conditions favoring or determining AF, all three types being also factors potentiated by arrhythmogenesis and AF (Fig. 1).
Structural remodeling (macro-, micro- and ultrastructural) is expressed morphologically and in imaging through inflammation and fibrosis, atrial dilation and alterations of the mechanical and electrical function (22). Atrial fibrosis is the result of the actions of a number of factors: oxidative stress, inflammation, increased AGE production and AGE-RAGE interaction, increased expression of growth factors, especially activated AG II (12). Under DM conditions, extensive atrial fibrosis and atrial dilation may also be the result of restrictive diabetic cardiomyopathy and increased ventricular filling pressure that stimulates the development of AF.
Other comorbidities of type 2 diabetes may be involved in the structural remodeling of AS, especially HBP, coronary disease, heart failure.
High blood pressure can be found in about 2/3 of diabetic patients. It is accompanied in evolution by ventricular remodeling (LVH) and possibly by atrial remodeling.
In practice there is a reciprocal relationship between HBP and AF. In diabetic persons, HBP is a risk factor for AF, and AF can be considered a manifestation of hypertensive heart disease (23). In the Framingham study, HBP doubled the risk of AF.
Atrial electrical remodeling develops in interrelation with structural (mechanical) remodeling. Electrophysiologically in DM there is a shortening of the action potential, a shortening of the refractory period and the process of dispersing refractoriness, the fragmentation of the excitation propagation front – conditions that predispose to the development and maintenance of AF (15).
The possible mechanism of electrical remodeling and the shortening of the refractory period is represented by the remodeling of ion channels, in which Ca2++ occupies an important place (15).
Features of atrial fibrillation in diabetes mellitus
There are no significant differences in the history of AF in a diabetic versus a non-diabetic patient. It usually begins as a new AF, which evolves in relation to structural and electrophysiological factors at the atrial level, to repeated recurrent AF, persistent AF, and finally permanent AF. The reported differences are the immediate success rate of pharmacological cardioversion (shorter) and the shorter duration of maintaining the sinus rhythm. Insufficient blood glucose control is an independent predictor of cardioversion failure.
Repeated episodes of silent AF are reported with an increasing prevalence depending on the monitoring methods (Holter, loop recorder) in both older, seemingly healthy people, or those with a higher CHA2DS2-VASC score, in people with recurrent AF or early post-stroke. The episodes lasting > 5-6 minutes are significant and have a higher 24-hour arrhythmic load. A higher prevalence of silent AF episodes has also been reported in diabetics. In a follow-up study of people aged <60 years for 37 months, the prevalence of subclinical AF was significantly higher in diabetic patients versus controls (11% vs 1.6%; p = 0.001) (21). In the reported study, a higher number of strokes (17.3% vs 5.9%; p <0.001) was noticed in the follow-up period, but also a higher prevalence of silent cerebral infarctions (61% vs 20%; p <0.01) (21). Future research using improved monitoring methods will show whether repeated episodes of silent AF in patients with DM are an independent predictor of AF.
The major feature of DM associated with AF is the significantly increased risk of cerebral and systemic embolic events. DM-associated AF has a higher risk of ischemic but not hemorrhagic stroke, lacunar stroke (compatible with microangiopathy), and recurrent stroke. The thromboembolic risk is correlated with blood glucose level, duration of DM, insulin treatment, and CHA2DS2-VASC score. A recent study investigated the effects of blood glucose status and AF duration using data from several Danish registries (5,363 patients with DM and AF). The studied lot was divided into three groups based on the HbA1C level (<6.5%; 6.6%-7.5%; and > 75%) as well as the DM duration <10 years and >10 years. The study follow-up duration was five years. The incidence of thromboembolic events per 1000 people/years was 1.92, 2.66 and 2.74, respectively, progressively increasing with the HbA1C level. The study showed that the associations were similar when DM was present <10 years (HR 2.39) for patients with HbA1C 6.5%-7.5% and HbA1C > 7.5% (7).
Several studies have also reported the interaction between AF and DM and the thromboembolic risk. For example, ATRIA study showed that the duration of DM > three years compared to that of less than three years was associated with an increased risk of ischemic stroke (adjusted HR 1.74) (24). The PREFER-AF study evaluated the role of insulin versus non-insulin treatment on thromboembolic risk in patients with DM and AF [5,717 (22.4%) patients with DM]. At one-year follow-up, insulin-requiring patients with DM and AF versus non-diabetic subject had HR 2.19, p = 0.001, and insulin-requiring versus non-insulin-requiring diabetic patients had HR 2.62, p <0.01 (25).
Overall, the annual incidence of stroke in AF with DM is between 3.6% and 8.6%. In patients with DM there is a state of hypercoagulability in relation to platelet activation, high inflammatory status, oxidative stress and insulin treatment.
Atrial fibrillation associated with DM is an important condition in the development and progression of HF in diabetic patients. The typical example is of the persistent AF, but also other atrial tachyarrhythmias, causes of arrhythmic cardiomyopathy. The progression of asymptomatic LV dysfunction to clinically manifested HF is directly related to the onset of AF, rate control deficit, and level of hyperglycemia, both in HF with reduced EF and with preserved EF. The pathophysiological and structural changes determined by DM in the myocardium and microcirculation, especially in long term DM or insufficient blood glucose control, are items that determine the development, progression and worsening of HF in diabetic patients (26).
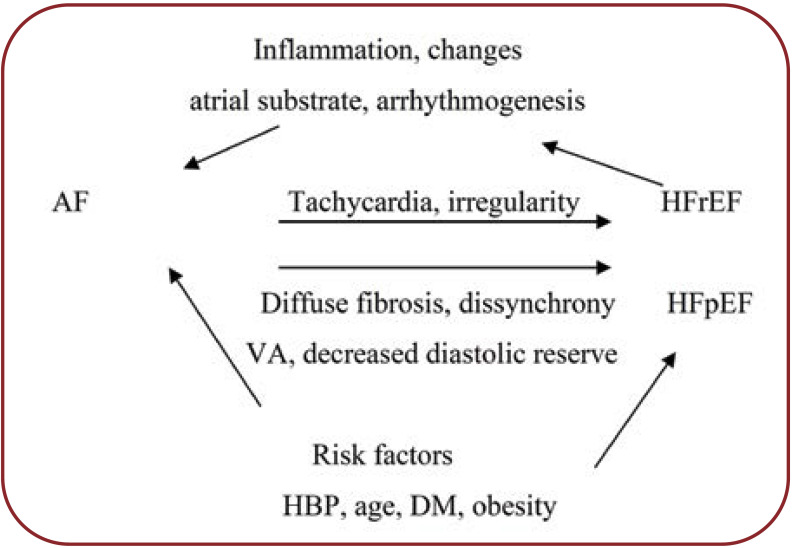
A synthesis of the pathophysiological factors that link AF to HF, in diabetics, is shown in Figure 3.
Management of atrial fibrillation associated with diabetes mellitus
Management of AF in patients with type 2 diabetes is not generally different from that recommended in people without diabetes. There are some features of the treatment that are worth emphasizing.
Anticoagulant treatment is the top priority in the management of AF associated with DM, in order to prevent stroke and systemic embolism (SE). The prevalence of AF is at least two times higher in diabetic patients compared to non-diabetics and non-valvular AF (NVAF) is the cause of 20-30% of ischemic stroke. AF incidence increases with DM duration and with insulin treatment.
There is an experience of over 60 years in the use of vitamin K-antagonist (VKA)-type oral anticoagulants (OACs) in the prevention of stroke in AF; warfarin reduces the risk of stroke by 60% and mortality by 20% versus placebo. The results of using VKA are good in terms of achieving a stable INR (INR 2-3.0), TTR> 70%, but with a hemorrhagic risk of 3-4% per year (27). The limitations of VKA are related to the unpredictable anticoagulant effect, narrow therapeutic window (INR 2.0-3.0), drug and food interactions, and the need for repeated INR control. The limitations of VKA-type medication versus benefits in stroke/SE prevention are arguments for using VKA as a second (alternative) option in the absence of novel oral anticoagulants (NOAC, DOAC). VKA-type oral anticoagulants have special indications especially in stage IV CKD.
The group of direct (or novel) oral anticoagulants (DOAC) and the positive experience of use in large clinical trials of AF imposed it as the main option (indication IA) in stroke prevention in NVAF (1).
The experience of using dabigatran (antithrombotic) and rivaroxaban, apixaban, edoxaban (anti-FXa) has been analysed in large clinical trials and meta-analyses versus VKA. The analyses referred to the RE-LY (dabigatran), ROCKET-AT (rivaroxaban), ARISTOTLE (apixaban), ENGAGETIKI 48 (edoxaban) studies. The mentioned studies did not specifically investigate the efficacy of DOAC versus warfarin in NVAF in diabetic patients, but type 2 diabetes accounted for 30-35% of the total analysed AF figures. No differences were reported in the sub-analyses for AF in DM compared to all cases in the studies (28).
A first meta-analysis from 2014, comprising the four core studies and including 71,683 participants (42,411 with DOAC and 29,272 warfarin), yielded conclusive results. Direct oral anticoagulants reduced stroke and systemic embolism in NVAF by 19% compared with warfarin (HR 0.81; p <0.0001), overall mortality (HR 0.9; p <0.0003) and intracerebral haemorrhage (HR 0.48; p <0.0001) (29).
Similar results are reported in the ARISTOPHANES study (2018) (30), which included 28,592 patients with AF and monitored the risk of stroke/SE and major bleeding in six cohorts – combinations of DOAC versus warfarin. The final results showed: apixaban HR 0.61; dabigatran HR 0.80; rivaroxaban HR 0.75; all of these anticoagulants were significantly associated with a lower stroke/SE rate compared to warfarin. Apixaban and dabigatran had a lower rate of major bleeding, HR 0.58 and HR 0.73, respectively, compared to warfarin, excepting rivaroxaban HR 1.01 (30).
In addition to the increased risk for AF, type 2 diabetes has also an increased risk/comorbidity for CKD, the presence of which may alter OAC treatment, especially for DOAC. Approximately 30-40% of people with type 2 diabetes have also CKD manifested by albuminuria, accompanied or not by the varied decrease of GFR. Renal elimination rate and the degree of renal dysfunction are taken into account in the treatment with DOAC in type 2 diabetes associated with AF. Up to GFR values of 30 mL/min, DOAC doses are those commonly recommended; for GFR values between 15-30 mL/min, lower doses of DOAC may be used, except dabigatran.
VKA-type oral anticoagulants, with strict monitoring of INR, are generally used in patients with DM, AF and stage III CKD; advanced CKD has an increased risk of bleeding and thrombosis. Intraglomerular haemorrhages and nephropathy, leading to acute kidney injury (AKI) have been reported in prolonged VKA administration (31, 32).
In the issue of stroke/SE prevention in AF associated with DM, three more aspects need to be mentioned.
In the ESC Treatment Guide of AF (2020), the risk of stroke/SE is assessed by the CHA2DS2-VASC score and the anticoagulant treatment with DOAC has an indication of grade I A for CHA2DS2-VASC ≥2 in men and ≥3 in women. In patients with DM and AF, anticoagulant therapy may also be considered for CHA2DS2-VASC ≥1 in men and ≥2 in women, in relation to particular aspects of each patient (e.g., large LA, high AHRE load on Holter exploration, long duration of DM, insulin treatment (1). Oral anticoagulant treatment may also be considered in diabetic patients with atrial high rate rhythm episode (AHRE) lasting more than 5-6 minutes and significant 24-hour arrhythmic load (Holter or other new recording techniques) (21).
Other intervention or surgical methods for preventing stroke in diabetic patients with AF (exclusion or occlusion of LA) have no special indications compared to recommendations in non-diabetic patients.
The hypoglycemic medication in the treatment of type 2 diabetes associated with AF has as main objective the stabilization of the blood glucose at a currently accepted level (100-130 mg/dL and HbA1C about 7.0) as well as the avoidance of blood glucose fluctuations, an important pathophysiological element in the development of atrial remodeling and AF.
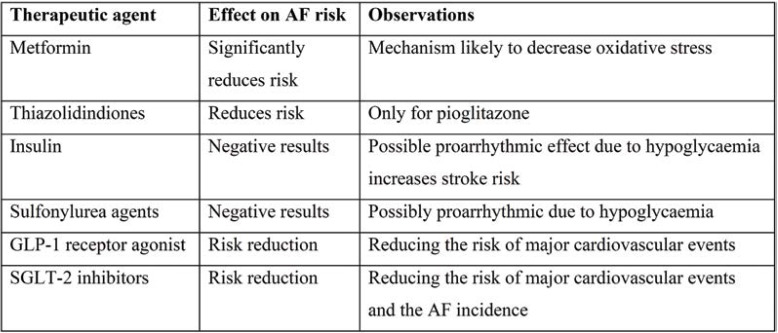
The seven major groups of hypoglycemic agents for type 2 diabetes – Metformin, Sulfonylurea (SU), Thiazolidindiones (TZD), Insulin, Dipeptidyl peptidase 4 inhibitors (DPP-4i), Glucagon- like peptide-1 receptor agonist (GLP-1) and Sodium-glucose co-transporter-2 inhibitors (SGLT-2) – act through different mechanisms and broadly stabilize the blood glucose level. However, their effects on atrial remodeling and decreasing AF incidence are different (33) (Table 1).
Overall, from the hypoglycaemic medication used in the treatment of type 2 diabetes associated with AF, only metformin and pioglitazone decreased the incidence of AF. Insulin has a negative effect on the incidence of AF, and GLP-1 agonists and SGLT 2 inhibitors have results that need to be confirmed. In the DACARE-Timi 48 study, dapagliflozin reduced the risk of AF-atrial flutter by 19% (p = 0.001). Reduction of arrhythmic events was found regardless of the presence or absence of AF or cardiovascular atherosclerotic disease.
In the treatment of DM associated or not with AF, it is important to stabilize the blood glucose level (as close as possible to the optimum) and to avoid blood glucose fluctuations, including hypoglycaemia (33).
Class I and III antiarrhythmics are widely used, with many indications in the treatment of AF in non-diabetic or diabetic patients. Pharmacological conversion of AF to SR, prevention of recurrence after pharmacological or electrical conversion, prevention of recurrence after AF ablation (temporary) are the main current recommendations for the use of antiarrhythmics and provided in the 2020 ESC Guidelines (1). Recommendations for rate control in AF with DM are similar in diabetic and non-diabetic people. Their limitations are related to the proarrhythmic effect, the negative effects on cardiac function in diabetic heart disease, but also on electrophysiological mechanisms and QT prolongation.
The antiarrhythmics flecainide, propafenone, dofetilide, amiodarone and sotalol each have indications for either pharmacological conversion or recurrence prevention, in relation to the presence of structural heart disease, HBP, and heart failure.
In addition to the special groups of medicines which are mentioned and used in the management of AF (with or without DM), other groups of medicines in special pathological conditions (rate control), HBP, heart failure, and renal dysfunction are also used as appropriate. Beta-blockers and diltiazem or less frequently digoxin for rate control in permanent AF; renin-angiotensin inhibitors possibly associated with non-dhydropyridine calcium blockers in the treatment of HBP. Renin-angiotensin inhibitors (ACE inhibitors or Ag II receptor blockers) used in the treatment of HBP reduce the risk of developing DM and are likely to act at the atrial level to reduce remodeling (37). Thiazolic diuretics used in the treatment of congestive HF increase the risk of developing DM, and antialdosterone diuretics could have a preventive effect on AF (38).
Heart failure and AF are interrelated. Heart failure of various etiologies or phenotypes favors the development of atrial remodeling, arrhythmogenesis and AF. Atrial fibrillation is a tachyarrhythmia that favors the occurrence and development of HF via various pathophysiological mechanisms in diabetic and non-diabetic patients.
A wide range of medicines (ACE-I, neprilysin/valsartan blockers), beta blockers, mineralocorticoid inhibitors and SGLT-2 inhibitors are used in the current treatment of HF. The use of SGLT-2 inhibitors (dapagliflozin, empagliflozin) is a key element in the treatment of HF in diabetic and non-diabetic patients (46).
Current data (AHA Session 2021) suggest that SGLT-2 inhibitors could also significantly reduce AF incidence and mortality in patients with electronic cardiac devices. One possible explanation would be the pressure reduction in LA due to the effects of SGLT-2.
Catheter ablation of atrial fibrillation via isolating the pulmonary veins by radiofrequency or cryoablation has become a common method of treatment for sinus rhythm (SR) restoration, with an efficiency of approximately 70-75% of patients. Atrial fibrillation ablation achieves (within certain limits) the restoration of SR, recurrence prevention, symptom improvement and finally, a better quality of life. Sinus rhythm restoration for an extended period of time or permanently reduces the risk of cerebral and systemic embolism, significantly improving EF of LV in structural heart diseases (1, 12). In addition, it avoids indefinite anticoagulant treatment (except for 2-3 months post-ablation) and its risks, but also antiarrhythmic treatment to prevent recurrences and pro-arrhythmic risk.
The experience of the first years after the introduction of AF ablation as well as the studies of recent years have demonstrated the benefits of ablation compared to antiarrhythmic treatment (40). The recent CABANA study, which included 2,024 patients with AF, followed the effects of catheter ablation versus medical therapy on cardiovascular events and mortality. After 48 months of follow-up, a reduction in stroke, mortality, severe bleeding was found, without reaching the threshold of statistical significance, except for the group with HF, for which the risk reduction was insignificant (41, 41 bis).
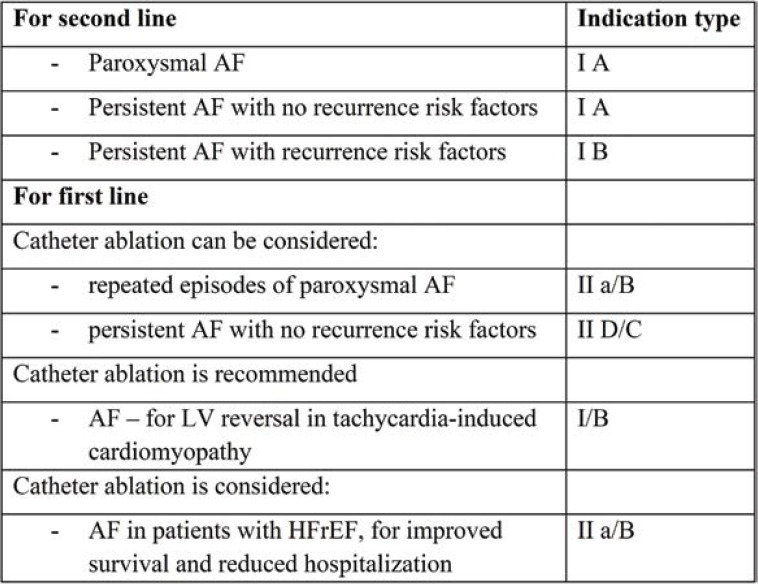
The recommendations for AF catheter ablation are similar in non-diabetic and diabetic patients. The groups of patients who have a recommendation for AF ablation are relatively large: paroxysmal or recurrent AF, persistent fibrillation, and paroxysmal or recurrent AF in patients with HF. The ESC Guidelines for the treatment of AF state that ablation is the second line of therapy after failure of treatment with antiarrhythmics or intolerance to class I and III antiarrhythmics. It also specifies the conditions of ablation as the first line of treatment in AF (prior to antiarrhythmic treatment) (Table 2).
The efficacy and safety of catheter ablation of paroxysmal AF, as the first line of treatment, is confirmed by a recently published meta-analysis. The results are analysed for two groups of patients: 609 with ablation versus 603 with antiarrhythmic treatment. The analysis of the results showed a reduction in the risk of AF recurrence of 36.6% (HR 0.64%) in ablation vs antiarrhythmic treatment. A 79% reduction in crossover rate was also reported during follow-up in patients randomized for catheter ablation versusantiarrhythmic treatment (HR 0.21; p <0.01) (42).
There are multiple risk factors for post-ablation recurrences, which must be considered in the ablation decision. The most important ones include LA size (indexed), AF duration, patient age, HBP, DM, renal dysfunction and substrate visualization on magnetic resonance imaging (MRI) (12).
Ablation limitations are related to post-ablation AF recurrence and possible interventional complications, which are relatively different in diabetic and non-diabetic patients. Post-ablation recurrence may be early (in the first 2-3 months) or late, either symptomatic or asymptomatic (silent AF). Ablation recurrences are more common in persistent AF than paroxysmal AF, in which the success rate can reach approximately 90%.
In relation to the methods and duration (Holter, loop recorder) of post-ablation monitoring, silent AF is identified, with one or more episodes of at least 30 seconds in 50% of patients. "Post-ablation arrhythmic load" may indicate a possible recurrence of AF and a different therapeutic orientation (22).
Relatively limited studies of catheter ablation of AF in diabetic patients indicate partially different results from those seen in non-diabetic people.
A meta-analysis of 1,464 patients with DM revealed an incidence of post-ablation recurrence in those with diabetes (40). Following the same line, a European Cooperative Clinical Study analysed the results obtained through AF ablation in 2,504 patients (of which 9% with DM) (44). In multivariate analysis, type 2 diabetes was an independent predictor of AF recurrence at one year (HR 1.39; p = 0.011). In addition, the study highlights several elements:
- Cryoablation and radiofrequency ablation had similar efficacy in diabetics and non-diabetics
- DM was associated with more frequent recurrence after ablation, but the evolution in paroxysmal AF was similar in patients with or without DM.
- Post-procedural complications (e.g., pericardial tamponade, stroke, other bleedings, phrenic paralysis, etc.) were similar in non-diabetic patients (44).
Overall, the recommendations for AF ablation are similar in people with or without DM, but type 2 diabetes is a predictor of post-ablation recurrence – including episodes of silent AF and arrhythmic load.
Problems with catheter ablation in AF or other interventional or relatively complex techniques are not different in patients with HF, diabetics or non-diabetics. Of note, in HC clinical trials, patients with type 2 diabetes are approximately 30% of all tested patients (45).
Commenting on the results of catheter ablation in HF with AF are beyond the scope of this paper
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
Simplified scheme of atrial fibrillation pathophysiology in diabetes mellitus [modified after (12)]
FIGURE 2.
Pathogenic mechanisms caused by blood glucose variability
FIGURE 3.
Pathophysiological interactions between AF and HF [modified after (26)]
TABLE 1.
Synthesis of hypoglycemic medication and the risk of AF (33-36)
TABLE 2.
Catheter ablation indications in AF [after (1)]
References
- 1.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrilation, developed in collaboration with EACTS, ESC, EHRA of the ESC. Europ Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–628. doi: 10.1038/nrcardio.2017.153. [DOI] [PubMed] [Google Scholar]
- 3.Dublin S, Glazer N, Smith NL, et al. Diabetes mellitus, glicemic control and risk of atrial fibrillation. J Gen. Intern Med. 2010;25:853–858. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 5.Staerk L, Sherer J.A, Ko D, et al. Atrial fibrillation, pathophysiology and clinical outcomes. Circ Res. 2017;120:1501–1517. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadi SS, Svensson AM, Pivodic A, et al. Risk of atrial fibrillation in person with type 2 diabetes and the excess risk in relation to glycemic control and renal function: a Swedish cohort study. Cardiovasc Diabetol. 2020;19:9. doi: 10.1186/s12933-019-0983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fangel MV, Nielsen PB, Kristensen JK. Glycemic status and thromboembolic risk in patients with atrial fibrillation and type 2 diabetes: a Danish cohort study. Circ Arrhythm Electrophysiol. 2019;12:e007030. doi: 10.1161/CIRCEP.118.007030. [DOI] [PubMed] [Google Scholar]
- 8.Palisgaard JL, Schjerning AM, et al. Risk of atrial fibrillation in diabetes mellitus: a national cohort study. Eur J Preventive Cardiology. 2016;23:621–627. doi: 10.1177/2047487315599892. [DOI] [PubMed] [Google Scholar]
- 9.Ugowe FE, Jackson LR, Thomas KL. Atrial fibrillation and diabetes mellitus. Circulation: Arrhythmia and Electrophysiology. 2019;12:e007351. doi: 10.1161/CIRCEP.119.007351. [DOI] [PubMed] [Google Scholar]
- 10.Heller SR, DM, FRCP and on behalf of the ADVANCE Collaborative Group. A Summary of the ADVANCE Trial. Diabetes Care. 2009;32(Suppl 2):S357–S361. doi: 10.2337/dc09-S339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlqvist S, Rosengren A, Gudbjörnsdottir S, et al. Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population: a prospective case-control study. Lancet Diabetes Endocrinol. 2017;5:799–807. doi: 10.1016/S2213-8587(17)30262-0. [DOI] [PubMed] [Google Scholar]
- 12.Wang ABS, Green JB, Halparin JL. Atrial fibrillation and diabetes mellitus. JACC review of the week. J Am Coll Cardiol. 2019;74:1107–1815. doi: 10.1016/j.jacc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 13.De Sensi F, De Potter T, Cresti A, et al. Atrial fibrillation in patients with diabetes: molecular mechanisms and therapeutic perspectives. Cardiovascular Diagnosis & Therapy. 2015;5:364–373. doi: 10.3978/j.issn.2223-3652.2015.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito S, Teshima Y, Fukui A, et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc Res. 2014;104:5–14. doi: 10.1093/cvr/cvu176. [DOI] [PubMed] [Google Scholar]
- 15.Bohne JL, Johnson D, Rose RA, et al. The association between diabetes mellitus and atrial fibrillation: Clinical and mechanistic insights. Front Physiol. 2019;10:135. doi: 10.3389/fphys.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato T, Yamashita T, Sekiguchi A, et al. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat”. Cardiovasc Electrophysiology. 2008;19:415–420. doi: 10.1111/j.1540-8167.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 17.Faria A, Persaud SJ. Cardiac oxidative stress in diabetes: mechanism and therapeutic potential. Pharmacol Ther. 2017;172:50–62. doi: 10.1016/j.pharmthera.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. J Am Med Assoc. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 19.Ziolo MT, Mohler PJ. Defining the role of oxidative stress in atrial fibrillation and diabetes. J Cardiovasc Electrophysiol. 2015;26:223–225. doi: 10.1111/jce.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benichou T, Pereira B, Mermillod M, et al. Heart rate variability in type 2 diabetes mellitus a systematic review and meta-analysis. PloS One. 2018;13:e0195166. doi: 10.1371/journal.pone.0195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marfella R, Sasso FC, Siniscalchi M. Brief episodes of silent atrial fibrillation predict clinical vascular brain disease in type 2 diabetes mellitus. JACC. 2013;62:525–530. doi: 10.1016/j.jacc.2013.02.091. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group – 2018 ESC/ESU Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner JM, Go AS, Chang Y, et al. Effect of diabetes and glycemic control of ischemic stoke risc in atrial fibrillation. ATRIA Study. J Am Coll Cardiol. 2016;67:239–247. doi: 10.1016/j.jacc.2015.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patti G, Lucerna M, Cavallari I, et al. Insulin-requiring versus noninsulin-requiring diabetes and thromboembolic risk. J Am Coll Cardiol. 2017;69:409–419. doi: 10.1016/j.jacc.2016.10.069. [DOI] [PubMed] [Google Scholar]
- 26.Sugumar H, Nanayakkara S, Prabhu S, et al. Pathophysiology of atrial fibrillation and heart failure: Dangerous interactions. Cardiol Clin. 2019;37:131–138. doi: 10.1016/j.ccl.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Kreutz R, Camm A.J, Rossing P. Concomitant diabetes with atrial fibrillation and anticoagulation management considerations. E. ur Heart J Suppliments. 2020;22:076–086. doi: 10.1093/eurheartj/suaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itzhaki Ben Zadok O, Eisen A. Use of non-vitamin K oral anticoagulants in people with atrial fibrillation and diabetes mellitus. Diabet Med. 2018;35:548–556. doi: 10.1111/dme.13600. [DOI] [PubMed] [Google Scholar]
- 29.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 30.Lip GYH, Keshisshian A, Xiaoyan Li, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. The ARISTOPHANES Study. Stroke. 2018;49:2933–2944. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost. 2016;14:461–467. doi: 10.1111/jth.13229. [DOI] [PubMed] [Google Scholar]
- 32.Brodsky S, Eikelboom J, Herbert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29:2787–2793. doi: 10.1681/ASN.2018070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen HY, Yang FY, Jong GP, et al. Antihyperglicemic drugs use and new-onset atrial fibrillation in eldery patients. Eur J Clin Invest. 2017;47:388–393. doi: 10.1111/eci.12754. [DOI] [PubMed] [Google Scholar]
- 34.Chang SH, Wu LS, Chiou MY, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus. Cardiovas Diabetol. 2014;13:123. doi: 10.1186/s12933-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu O, Azoulay L, Chen HY, et al. Sulfonylureas as initial treatment for type 2 diabetes and the risk of severe hypoglicemia. Am J Med. 2018;131:137. doi: 10.1016/j.amjmed.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 36.Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insight from the DECLARE – Timi 59 Trial. Circulation. 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- 37.Schneider MP, Hua TA, Böhm M, et al. Prevention of atrial fibrillation by renin-angiotensin system inhibition: a meta-analysis. J Am Coll Cardiol. 2010;15:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Neefs J, van den Berg NWE, Limpens J, et al. Aldosterone pathway blockade to prevent atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2017;231:155–161. doi: 10.1016/j.ijcard.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with EASD. Euro Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 40.Anselmino M, Matta M, D'ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace. 2015;17:1518–1525. doi: 10.1093/europace/euv214. [DOI] [PubMed] [Google Scholar]
- 41.Packer DL, Piccini JP, Monahan KH. Ablation versus drug therapy for atrial fibrillation in heart failure: rezults from CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imberti JF, Ding WY, Kotalczyk A, et al. Catheter ablation as a first-line treatment for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Heart. 2021;107:1630–1636. doi: 10.1136/heartjnl-2021-319496. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen JC, Johannessen A, Raatikainen P, et al. Radiofrequency ablation a initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 44.Creta A. Impact of type 2 diabetes mellitus on the outcomes of catheter ablation of atrial fibrillation. European multicenter study. Am J Cardiol. 2020;125:901–905. doi: 10.1016/j.amjcard.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 45.Terricabras M, Piccini JP, Verma A. Randomized clinical trials of catheter ablation of atrial fibrillation in congestive heart failure: knowuns and unmet needs. Cardiol Clin. 2019;37:167–176. doi: 10.1016/j.ccl.2019.01.003. [DOI] [PubMed] [Google Scholar]