Abstract
Microautoradiography was used to investigate substrate uptake by natural communities of uncultured bacteria from the genus Achromatium. Studies of the uptake of 14C-labelled substrates demonstrated that Achromatium cells from freshwater sediments were able to assimilate 14C from bicarbonate, acetate, and protein hydrolysate; however, 14C-labelled glucose was not assimilated. The pattern of substrate uptake by Achromatium spp. was therefore similar to those of a number of other freshwater and marine sulfur-oxidizing bacteria. Different patterns of radiolabelled bicarbonate uptake were noted for Achromatium communities from different geographical locations and indicated that one community (Rydal Water) possessed autotrophic potential, while the other (Hell Kettles) did not. Furthermore, the patterns of organic substrate uptake within a single population suggested that physiological diversity existed in natural communities of Achromatium. These observations are consistent with and may relate to the phylogenetic diversity observed in Achromatium communities. Incubation of Achromatium-bearing sediment cores from Rydal Water with 35S-labelled sulfate in the presence and absence of sodium molybdate demonstrated that this bacterial population was capable of oxidizing sulfide to intracellular elemental sulfur. This finding supported the role of Achromatium in the oxidative component of a tightly coupled sulfur cycle in Rydal Water sediment. The oxidation of sulfide to sulfur and ultimately to sulfate by Achromatium cells from Rydal Water sediment is consistent with an ability to conserve energy from sulfide oxidation.
Achromatium oxaliferum is a large sediment-dwelling bacterium found principally in freshwater and brackish environments (1, 12, 17, 32). Notable for its large size, the bacterium also precipitates intracellular calcium carbonate, a property which makes it unique among bacteria. Although the bacterium was first described in 1893 (30), little about its role in the sediments that it inhabits has been elucidated. A. oxaliferum belongs to the γ-subdivision of the class Proteobacteria and is related to a number of photosynthetic and nonphotosynthetic sulfur-oxidizing bacteria (12). Recent studies have shown that natural communities of A. oxaliferum previously thought to be homogeneous in fact comprised a number of phylogenetically, morphologically, and ecologically distinct subpopulations (6, 7, 9, 13). The degree of homology observed for Achromatium-derived 16S rRNA sequences (<97.5%) was consistent with the occurrence of different Achromatium species both within a single sediment community and at geographically separated locations (6, 7, 9, 13). Thus, it is clear that the majority of Achromatium communities studied in detail comprise several Achromatium species. While this fact was not known at the outset of the current study and was not taken into consideration in the experimental design, the genetic heterogeneity of natural communities of Achromatium has considerable bearing on the interpretation of the data from this study.
In addition to the phylogenetic affiliation of Achromatium spp. with known sulfur-oxidizing chemolithoautotrophs, the presence of sulfur inclusions within Achromatium spp. (17) and the predominance of these organisms in the micro-oxic zone of sediments (2, 8, 12) have suggested that these organisms may also be sulfur-oxidizing chemolithoautotrophs. However, until recently, no direct evidence to support this notion had been obtained. Ecophysiological studies demonstrated that Achromatium cells were capable of oxidizing reduced sulfur to sulfate (8), suggesting that they conserve energy from sulfur oxidation (31), but to date no information on carbon metabolism in Achromatium spp. has been available.
Microautoradiography has the potential to address a number of specific questions relating to substrate uptake by natural populations of uncultured bacteria and has been applied to the study of bacterial activity in a number of distinct environments e.g., references (15), (16), and (21). Bacteria from the genus Achromatium have eluded all attempts at cultivation, and in order to study their metabolic potential, we have applied microautoradiography to the study of carbon and sulfur metabolism in natural communities of these bacteria.
MATERIALS AND METHODS
Study sites and sampling.
Sediment samples containing Achromatium cells were collected from two locations in the United Kingdom. The sampling site at the margins of Rydal Water (54°27′N, 3°00′W) has been described previously (8, 12). The second site, Hell Kettles (36), is located to the south of Darlington, County Durham, United Kingdom (54°29′N, 1°33′W). Hell Kettles comprise two pools formed by the dissolution and collapse of underlying limestone strata sometime in the year 1179 (36). Samples were taken from Croft Kettle, the more southerly of the two pools. The pH of overlying waters from Rydal Water and Hell Kettles sediments was 6.5.
Grab samples of sediment containing Achromatium cells were obtained from both sites and used to prepare reconstituted sediment cores (8). All incubations were carried out with 10-ml-capacity glass vials (SH Scientific, Blyth, Northumberland, United Kingdom). Freshly collected sediment (5 ml) was added to each vial and covered with 5 ml of lake water. The reconstituted cores were preincubated for 1 day at room temperature before radiochemicals were added. Measurement of oxygen profiles in reconstituted cores by membrane inlet mass spectroscopy (8) suggested that this time scale was appropriate for reestablishment of redox gradients within the sediment.
Radiochemicals.
All radiochemicals were purchased from Amersham International (Little Chalfont, Buckinghamshire, United Kingdom). The uptake of radiolabel from sodium [14C]bicarbonate (2.0 GBq/mmol), [1,2-14C]acetic acid (2.07 GBq/mmol), d-[U-14C]glucose (11.2 GBq/mmol), l-U-14C-protein hydrolysate (>1.85 GBq/milliatom of carbon), and sodium [35S]sulfate (4.162 GBq/mmol) by Achromatium cells was tested.
Uptake of 14C-labelled compounds.
In separate incubations, 0.4 MBq of radiochemical was added. This quantity equated to 0.2 μmol of bicarbonate, acetate, and protein hydrolysate and 0.035 μmol of glucose. The final amount of glucose added was adjusted to 0.2 μmol by the addition of unlabelled glucose to provide similar added specific activities for all substrates. We recognize that the indigenous concentrations of these substrates will affect their specific activities in sediment cores. This fact is principally a consideration if quantitative inferences regarding the level of radiolabelled substrate assimilation by individual cells are made. Consequently, we have not interpreted the level of substrate uptake by individual Achromatium cells quantitatively. Control incubations containing a 2% (wt/vol) final concentration of buffered formaldehyde (pH 6.5, 0.1 M phosphate buffer) were also prepared. Incubations were performed in the dark for 3 h to limit the formation of 14CO2 from the degradation of organic substrates by heterotrophic bacteria (15, 16). All incubations were carried out at 20°C. Acetate, glucose, and amino acid uptake experiments were conducted with sediments sampled on 10 June 1997 (Hell Kettles) and 4 July 1997 (Rydal Water). Bicarbonate uptake experiments were conducted with sediments sampled on 18 March 1998 (Hell Kettles) and 28 April 1998 (Rydal Water).
Following incubation, Achromatium cells were crudely purified from sediments (4) and washed three times in filter-sterilized water to remove residual radioactivity. For some incubations containing NaH14CO3, an aliquot of the purified cells was decalcified with 0.1 M HCl (1 ml) prior to washing. Cells from all incubations were placed on gelatin-coated microscope slides. The slides were dried at 50°C and dipped in LM-1 autoradiography emulsion (Amersham International, Little Chalfont, Buckinghamshire, United Kingdom). The microautoradiography slides were exposed in a light-proof box at 4°C for 2 weeks (15), developed with Kodak GBX developer, and fixed with fixative-replenisher (Sigma, Poole, Dorset, United Kingdom). Microscopy was carried out with an Olympus BH-2 microscope fitted with an Olympus OM-10 35-mm camera. One hundred cells chosen randomly from each slide were examined, and the number of cells assimilating labelled substrates was recorded. Triplicate sediment cores prepared from the same sediment sample and incubated with labelled substrates were used to determine reproducibility. For example, the percentage of cells assimilating H14CO3− in replicate sediment cores from Hell Kettles sampled on 18 March 1998 was 52.33 ± 5.78 (mean ± 95% confidence interval). Bright-field micrographs were obtained with Kodak Ektachrome Elite 400 film and automatic exposure.
Fate of 35S from radiolabelled sulfate in Achromatium communities from Rydal Water.
Na235SO4 (0.5 Mbq) was added to cores sampled on 13 August 1997, producing a final total sulfate concentration of approximately 18 μM. Negative controls were prepared by the addition of buffered formaldehyde (2% [wt/vol] final concentration) or sodium molybdate (2 mM final concentration). Incubation was carried out for 18 h at 20°C. After incubation, the cells were extracted from the cores, partially purified, washed, placed on slides, and dipped as described above. Alternatively, after incubation with Na235SO4, partially purified, washed preparations of Achromatium cells were treated with methanol (300 μl) and incubated overnight to remove elemental sulfur. The extraction of elemental sulfur was determined microscopically, and the extracted cells were washed, placed on slides, and dipped as described above. The percentage of cells assimilating radiosulfur was determined by analyzing 100 randomly selected cells. The presence of radiolabelled elemental sulfur in the methanol extract was determined by an adaptation of a previously published method (35). Briefly, a 20-ml-capacity crimp-top vial (SH Scientific) containing a smaller vial into which lead acetate paper was placed was sealed with a butyl rubber septum and flushed with nitrogen. The methanol extract was injected into the larger outer vial, and 1 M chromous chloride (1 ml) was added. The reduction of dissolved elemental sulfur by chromous chloride to produce hydrogen sulfide was allowed to proceed at room temperature for 6 h (35). The apparatus was dismantled, and Pb35S trapped by the lead acetate paper was detected with a Geiger counter (Morgan series 900 mini-monitor; Mini Instruments Ltd., Burnham-on-Crouch, Essex, United Kingdom).
RESULTS AND DISCUSSION
The ability of freshwater colorless sulfur bacteria to couple sulfur oxidation to energy generation or biosynthesis has been a subject of debate (7, 10, 11, 23), and colorless sulfur bacteria have been categorized by their specific metabolic use of reduced sulfur species, inorganic carbon, and organic carbon (29). For example, obligate chemolithoautotrophs obtain all their cellular carbon from inorganic sources, deriving energy and reducing power from the oxidation of reduced sulfur species. Facultative chemolithoautotrophs are able to do the same but are also able to grow mixotrophically or heterotrophically; mixotrophy in sulfur bacteria encompasses the simultaneous use of both organic carbon and inorganic carbon for biosynthesis and energy generation from organic carbon and reduced sulfur compounds (29). Chemolithoheterotrophs conserve energy from sulfur oxidation and use organic carbon for biosynthesis. Sulfur-oxidizing chemoorganoheterotrophs are defined as organisms deriving no energy or reducing power from the oxidation of reduced sulfur, relying instead on organic carbon for both. The oxidation of sulfur in these bacteria has been attributed to detoxification processes (19, 29, 31). Given this nutritional diversity in sulfur bacteria, knowledge of the use of reduced sulfur species and of inorganic and organic carbon is essential for an understanding of the physiology and hence the ecological role of Achromatium.
Assimilation of organic and inorganic carbon in Achromatium communities.
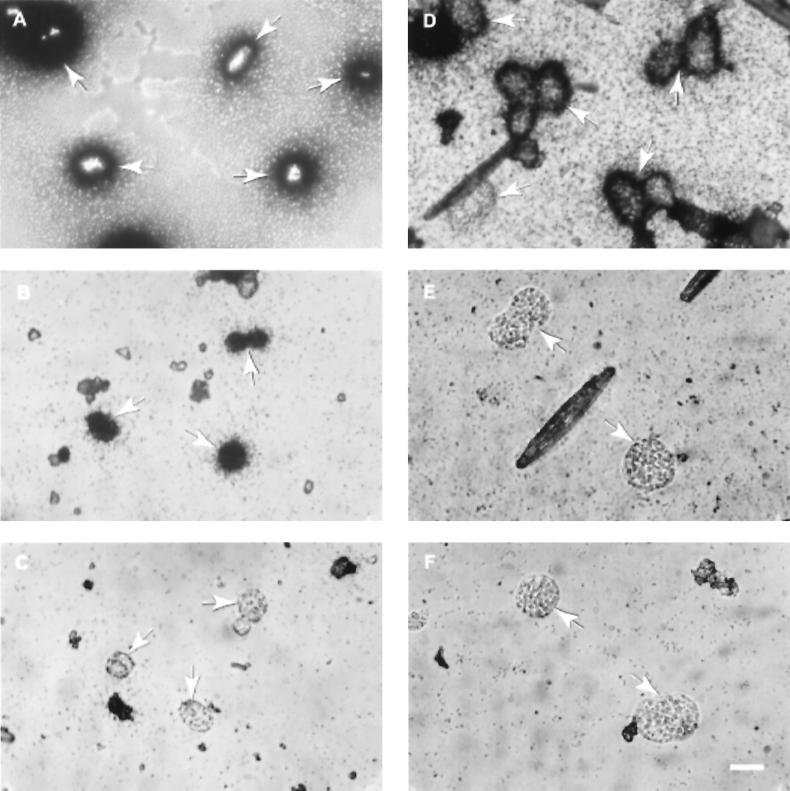
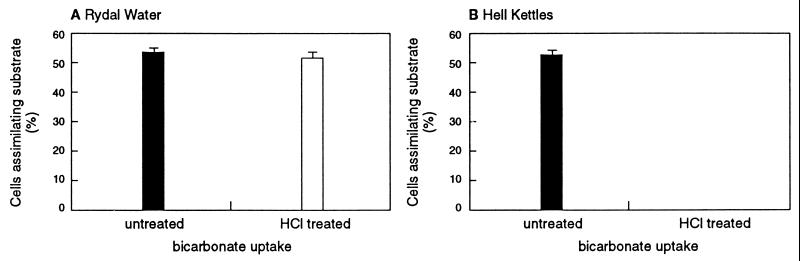
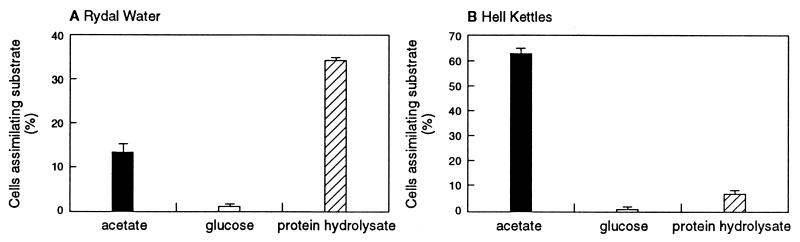
Achromatium cells from Rydal Water and Hell Kettles incorporated 14C from both organic and inorganic carbon sources (Fig. 1 to 3). A proportion of the cells present in both Rydal Water and Hell Kettles proved capable of assimilating carbon from acetate and protein hydrolysate (Fig. 2). The uptake of radiolabelled glucose was observed for only a very small number of cells (Fig. 2). In subsequent experiments, the uptake of radiolabelled bicarbonate was investigated. Although bicarbonate was assimilated by Achromatium cells from Rydal Water and Hell Kettles sediments (Fig. 1A and D and Fig. 3), following decalcification with dilute HCl, only cells from Rydal Water sediments retained the 14C label (Fig. 1B and E and Fig. 3). The incorporation of 14C from inorganic carbon into cellular organic material (and thus the potential to fix inorganic carbon for biosynthesis) apparently is a feature only of Achromatium cells present in Rydal Water sediments.
FIG. 1.
Microautoradiographs of Achromatium cells purified from sediment cores amended with 14C-labelled bicarbonate. (A to C) Cells from Rydal Water sediments. (D to F) Cells from Hell Kettles sediments. Cells from Rydal Water and Hell Kettles sediments both incorporated 14C from labelled bicarbonate (A and D). However, decalcification (see Materials and Methods) removed 14C from cells present in Hell Kettles sediments (E) but not from cells present in Rydal Water sediments (B). This result indicated that 14C was incorporated into cellular organic carbon in cells from Rydal Water but not in cells from Hell Kettles. Cells from formaldehyde-treated sediment cores did not incorporate any radiolabel (C and F). Note that in decalcified samples, cells incorporating the radiolabel are not visible within the halo of silver halide particles, presumably because removal of the calcite inclusions results in collapse of the cell and an even coating of photographic emulsion over the surface of the cell is obtained. In cells that retain intracellular calcite, a portion of the cell is raised above the surface of the emulsion. Achromatium cells or groups of cells are indicated by arrows. Cells from Hell Kettles are larger than those from Rydal Water (9). The scale bar represents 20 μm and applies to all micrographs.
FIG. 3.
Proportion of cells in Achromatium communities incorporating 14C-labelled inorganic carbon. Uptake of 14C from radiolabelled sodium bicarbonate by untreated and decalcified Achromatium cells from Rydal Water sediments and Hell Kettles sediments was measured. No decalcified cells from Hell Kettles that had incorporated 14C were detected. The data represent the mean ± standard error (n = 3). In sediment cores treated with formaldehyde (2% [wt/vol]), no Achromatium cells that had incorporated 14C were detected.
FIG. 2.
Proportion of cells in Achromatium communities incorporating 14C from radiolabelled organic substrates. Uptake of 14C from radiolabelled acetate, glucose, and protein hydrolysate by Achromatium cells from Rydal Water sediments and Hell Kettles sediments was measured. The data represent the mean ± standard error (n = 3). In sediment cores treated with formaldehyde (2% [wt/vol]), no Achromatium cells that had incorporated 14C were detected.
The incorporation of radiolabel was never noted in killed control samples; however, in all experiments, a proportion of cells did not assimilate the labelled substrates (e.g., in Rydal Water sediments, only 51.3% ± 2.2% [mean ± standard error] of the Achromatium cells assimilated carbon from bicarbonate). This result could indicate that the cells unable to assimilate the radiolabelled carbon sources were dead or moribund. However, whole-cell in situ hybridization with fluorescence-labelled oligonucleotide probes indicated that approximately 98% of the Achromatium cells present in the sediments studied were metabolically active (9). It has been shown with populations of marine bacterioplankton that there is almost a one-to-one correlation between the number of cells giving a positive signal with 16S rRNA-targeted fluorescence oligonucleotide probes and metabolically active cells, as determined by microautoradiography (16). Because we did not observe this one-to-one relationship between probe hybridization and assimilation of 14C-labelled substrates, it is possible that there are physiological differences between different members of the Achromatium community. This notion can be interpreted in three ways. First, a number of Achromatium cells possessed sufficient ribosomes to produce a positive probe signal but were not in a physiological state that allowed them to assimilate the substrates in the 3-h incubations used. Nevertheless, in experiments involving incubation for 24 h, no increase in the proportion of cells assimilating labelled substrates was noted. Second, in our experiments, the cells were present in a heterogeneous sediment environment. Thus, genetically identical cells may have been exposed to different physicochemical conditions even if they were in close proximity. Under different conditions, the same cell type may express different metabolic pathways. When cells were purified from the sediment, cells from different microenvironments were intermixed. Consequently, the discrepancy between these two measures of cellular activity could have been the result of the presence of cells that were metabolically active but not expressing the requisite metabolic pathway for assimilation of the labelled substrates used. A third explanation is that cells that bound the specific 16S rRNA-targeted oligonucleotides were metabolically active but lacked the genetic potential to assimilate particular substrates. If this were the case, then the differential substrate uptake by cells in an Achromatium community might correspond to the genetic diversity observed in natural Achromatium communities (9).
The presence of coexisting, ecologically distinct subpopulations in Achromatium communities may also explain the pattern of acetate and amino acid uptake by Achromatium cells in sediment cores prepared from the same bulk sediment sample (Fig. 2). For example, although radiocarbon from acetate and protein hydrolysate was assimilated by Achromatium cells from both Hell Kettles and Rydal Water sediments, 34.3% ± 1.3% of cells from Rydal Water sediment assimilated 14C from protein hydrolysate, while only 13.3% ± 1.9% assimilated 14C from acetate; in Hell Kettles sediment, 62.7% ± 1.8% of cells assimilated labelled carbon from acetate, but only 7.3% ± 1.2% assimilated labelled carbon from protein hydrolysate. The hypothesis that genetically distinct Achromatium subpopulations assimilate different substrates remains to be tested. By combining whole-cell in situ hybridization with microautoradiography (20, 25, 27), it may be possible to establish if this is the case.
As with the pattern of organic substrate utilization in a single Achromatium community, the difference in bicarbonate uptake between the Hell Kettles and Rydal Water communities may relate to the evolutionary divergence of the Achromatium spp. present in these sediments (9). Achromatium-derived 16S rRNA sequences from Hell Kettles occupied lineages distinct from those from Rydal Water (9), and the clear evolutionary divergence of the two populations is consistent with the observed differences in inorganic carbon assimilation.
The substrates assimilated by Achromatium communities were similar to those used by freshwater and marine sulfur-oxidizing bacteria, such as Thiothrix, Thioploca, and Beggiatoa spp. (10, 18, 19, 21, 22, 33). Some representatives of these bacterial genera are able to utilize simple organic compounds for either energy or biosynthesis, and others fix inorganic carbon. However, given the differential bicarbonate uptake patterns observed for the two Achromatium communities studied here (Fig. 3), it may be concluded that some Achromatium spp., namely, the cells present in Rydal Water sediments which assimilate bicarbonate or bicarbonate plus acetate, are potentially chemolithoautotrophs or mixotrophs. On the other hand, the cells present in Hell Kettles sediments which do not assimilate bicarbonate but which do assimilate acetate and deposit elemental sulfur may be chemoorganoheterotrophs or chemolithoheterotrophs. An alternative interpretation of these data is that cells from Hell Kettles and Rydal Water have the same physiological capabilities and express either chemolithoautotrophic/mixotrophic physiology or chemoorganoheterotrophic/chemolithoheterotrophic growth depending on the availability of substrates in the sediment. Facultative autotrophy of this nature has been demonstrated for marine Beggiatoa sp. strain MS-81-6 (10). This isolate not only grew chemolithoautotrophically but also used a range of simple organic carbon sources for energy generation and biosynthesis. However, when it was provided with organic carbon, ribulose-1,5-bisphosphate carboxylase/oxygenase activity was considerably lower than that in strictly chemolithoautotrophically growing cells (10).
In support of the existence of physiological differences between the Hell Kettles and Rydal Water communities, rather than the expression of different physiologies depending on environmental conditions, a homologue of the ribulose-1,5-bisphosphate carboxylase/oxygenase large-subunit gene (rbcL) was detected in DNA extracts from pure preparations of Achromatium cells from Rydal Water by PCR (13), whereas an rbcL homologue was never detected in DNA extracts from Hell Kettles Achromatium cells, despite exhaustive efforts and multiple rounds of PCR amplification (13). Nonetheless, because many heterotrophic bacteria are able to assimilate CO2 via alternative enzymatically catalyzed pathways for the synthesis of some metabolic intermediates (5, 34), autotrophy in the Achromatium community present in Rydal Water sediments remains to be unequivocally confirmed.
Fate of 35S-labelled sulfate in Achromatium-bearing sediments.
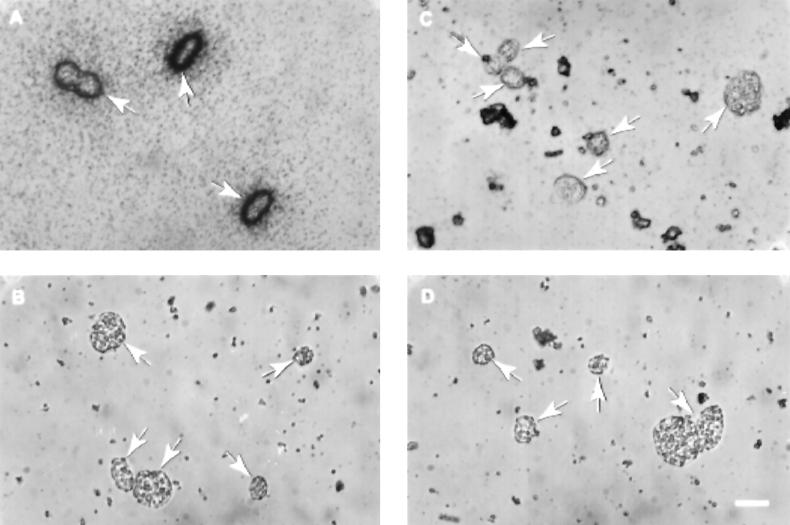
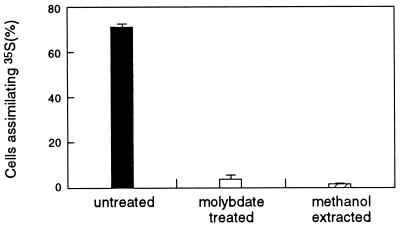
Microautoradiographic studies of Achromatium communities in Rydal Water sediment clearly demonstrated that sulfur from 35S-labelled sulfate was incorporated into Achromatium cells (Fig. 4A and 5). However, this process was inhibited in the presence of sodium molybdate (Fig. 4B and 5). Given that sodium molybdate probably inhibits the binding and transport of sulfate across the cell membrane (24) and blocks the formation of adenosine-5′-phosphosulfate (APS), an important intermediate in both assimilatory and dissimilatory sulfate reduction (26), there are two possible explanations for these results. First, following the reduction of sulfate to sulfide by dissimilatory sulfate-reducing bacteria, the sulfide was oxidized by Achromatium cells to elemental sulfur, which was deposited intracellularly. When dissimilatory sulfate reduction was inhibited by the addition of sodium molybdate, the production of sulfide and its subsequent conversion to elemental sulfur by Achromatium cells were prevented. Second, Achromatium cells accumulated 35S from radiolabelled sulfate via an assimilatory sulfate reduction pathway and incorporated it into cellular organic sulfur, a process inhibited in the presence of molybdate (28). The first alternative was confirmed by extraction of the radiolabelled cells with methanol to remove elemental sulfur. This procedure resulted in the removal of radioactivity (Fig. 4C and 5). To preclude the possibility that the methanol treatment removed intracellular 35S-labelled sulfate or organosulfur compounds, the methanol extract was treated with chromous chloride. Unlike elemental sulfur, sulfate and organosulfur compounds are not reduced to sulfide by this treatment (3). Consequently, upon treatment with chromous chloride, these compounds remain in the methanol phase, whereas elemental sulfur is converted to 35S-sulfide and can be trapped by lead acetate paper, with the formation of lead sulfide. The recovery of 35S as lead sulfide by this procedure confirmed that 35S-elemental sulfur was present within Achromatium cells.
FIG. 4.
Microautoradiographs of Achromatium cells purified from Rydal Water sediment cores amended with 35S-labelled sodium sulfate. (A) Cells from a sediment core treated with radiolabelled sodium sulfate. (B) Cells from a sediment core treated with radiolabelled sodium sulfate and 2 mM sodium molybdate. (C) Cells from a sediment core treated with radiolabelled sodium sulfate and extracted with methanol to remove intracellular elemental sulfur prior to exposure. (D) Cells from a sediment core treated with radiolabelled sodium sulfate and formaldehyde (2% [wt/vol]). Achromatium cells are indicated by arrows. The scale bar represents 20 μm and applies to all micrographs.
FIG. 5.
Proportion of cells incorporating 35S from radiolabelled sodium sulfate in the Achromatium community from Rydal Water sediments. The data represent the mean ± standard error (n = 3). In sediment cores treated with formaldehyde (2% [wt/vol]), no Achromatium cells that had incorporated 35S were detected.
This study and a previous study (8) have provided the first evidence that Achromatium can oxidize sulfide to intracellular elemental sulfur and ultimately to sulfate and support the proposal that Achromatium is involved in the oxidative side of a tightly coupled sulfur cycle in Rydal Water sediments (8). Studies on the mixotrophic sulfur-oxidizing bacterium Thiothrix nivea have indicated that the complete oxidation of sulfide to sulfate is consistent with a potential for energy generation from sulfur oxidation (31). This ability has been clearly demonstrated for at least a proportion of the cells from Rydal Water sediments. It is interesting to note that Achromatium cells present in Rydal Water sediments, in addition to oxidizing sulfide to sulfate, have autotrophic potential. This potential is evident from inorganic carbon fixation and the presence of genes homologous to rbcL. Moreover, sequences homologous to APS reductase genes (aprBA), which code for an enzyme involved in the oxidation of sulfite to sulfate (11, 14), were detected in DNA extracted from Achromatium cells purified from Rydal Water (13). The enzyme APS reductase catalyzes the AMP-dependent oxidation of sulfite to APS by use of an unknown electron acceptor. Substrate-level phosphorylation of APS catalyzed by either ATP or ADP sulfurylase then results in the formation of sulfate, with the release of ADP or ATP (11).
Microautoradiography experiments with 35S-labelled sulfate were not conducted with Hell Kettles sediments because the sulfate concentration in these samples was ca. 10 mM (13). Providing a specific activity of radiolabelled sulfate equivalent to that in experiments with Rydal Water sediments would have required the presence of 270 MBq of radioactivity in each sediment core; this was clearly impractical. Furthermore, experiments comparable to those conducted with Rydal Water sediments that indicated the oxidation of reduced sulfur to sulfate by Achromatium (8) were not possible with Hell Kettles sediments due to the difficulties of measuring a small increase in sulfate concentration against high background levels of sulfate. Although the sulfur-oxidizing activity of Achromatium communities in Hell Kettles sediments has not been investigated, this population did not exhibit autotrophic potential (Fig. 3B) and lacked detectable rbcL and aprBA homologues (13). Nevertheless, microscopic examination of cells from this population showed that they did contain intracellular elemental sulfur. Some studies have suggested that heterotrophic sulfur bacteria may oxidize sulfide and deposit intracellular elemental sulfur as a mechanism for the detoxification of metabolically produced hydrogen peroxide (19, 29). Alternatively, other pathways exist for energy generation from sulfur oxidation. These do not involve APS reductase but instead utilize sulfite:acceptor oxidoreductase to oxidize sulfite to sulfate, leaving the possibility open that Achromatium cells from Hell Kettles are chemolithoheterotrophs.
Conclusions.
Without access to pure cultures, determining the physiology and ecological role of Achromatium has proved difficult (13). This task has been further complicated by the fact that populations of Achromatium originally thought to contain a single species (A. oxaliferum) have now been shown to comprise several ecologically distinct species (9, 13). A simple interpretation of the data presented in this study without this understanding of Achromatium diversity would be that the organism is mixotrophic, utilizing simple organic compounds as well as conserving energy from the oxidation of reduced sulfur species to sulfate and, when conditions allow, fixing inorganic carbon for biosynthesis. However, this simple interpretation is not valid, and Achromatium spp. appear to be nutritionally heterogeneous.
For instance, the Achromatium spp. present in Rydal Water sediments are phylogenetically distinct from those in Hell Kettles sediments, and at least a proportion of the cells from Rydal Water can fix inorganic carbon. In contrast, no Achromatium cells from Hell Kettles showed autotrophic potential. Given that, of these two populations, only Rydal Water Achromatium harbored sequences homologous to rbcL and contained genes involved in the oxidation of sulfite to sulfate (aprBA), the data presented in this study suggest that genetically distinct Achromatium communities have different physiological properties. Thus, cells from Hell Kettles, which deposit intracellular elemental sulfur, assimilate acetate but not inorganic carbon, and do not contain detectable rbcL and aprBA genes are likely to be chemoorganoheterotrophs or chemolithoheterotrophs, as defined by Robertson and Kuenen (29). Without pure cultures, however, the effect of reduced inorganic sulfur on growth yield, for example, cannot be determined, and it is not currently possible to distinguish which of these physiologies is correct. Furthermore, because of the genetic heterogeneity of the Achromatium community, both physiological types may be present. In contrast, at least some of the cells in Rydal Water sediments have the capacity to assimilate organic and/or inorganic carbon, can oxidize reduced sulfur to sulfate, and contain rbcL and aprBA homologues. These observations are consistent with the presence of facultative chemolithoautotrophs or mixotrophs, as defined by Robertson and Kuenen (29), in this Achromatium community. Tentative evidence also suggests that physiological diversity exists within single Achromatium communities.
Consequently, future work in this area should focus on establishing the nutritional requirements of different Achromatium subpopulations by use of methods which combine phylogenetic identification of individual cells with the microautoradiographic techniques used here (20, 25, 27). Only then will it be possible to understand the ecological role of this well-documented but still poorly understood bacterial genus.
ACKNOWLEDGMENTS
Financial support from the Natural Environment Research Council (NERC) (grant GR3/9148) to I.M.H., R.W.P., and J.G.J. is gratefully acknowledged. R.H. was supported by NERC/CASE studentship GT4/95/235/F.
REFERENCES
- 1.Babenzien H-D. Achromatium oxaliferum and its ecological niche. Zentralbl Mikrobiol. 1991;146:41–49. [Google Scholar]
- 2.Babenzien H-D, Sass H. The sediment-water interface—habitat of the unusual bacterium Achromatium oxaliferum. Arch Hydrobiol. 1997;48:247–251. [Google Scholar]
- 3.Canfield D E, Raiswell R, Westrich J T, Reaves C M, Berner R A. The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chem Geol. 1986;54:149–155. [Google Scholar]
- 4.de Boer W E, La Rivière J W M, Schmidt K. Some properties of Achromatium oxaliferum. Antonie Leeuwenhoek. 1971;37:553–563. doi: 10.1007/BF02218525. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin M. Prokaryotic life cycles. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes: a handbook on the biology of bacteria; ecophysiology, isolation, identification, applications. 2nd ed. Vol. 1. New York, N.Y: Springer-Verlag; 1992. pp. 209–240. [Google Scholar]
- 6.Glöckner F R, Babenzien H-D, Wulf J, Amann R. Phylogeny and diversity of Achromatium oxaliferum. Syst Appl Microbiol. 1999;22:28–38. doi: 10.1016/S0723-2020(99)80025-3. [DOI] [PubMed] [Google Scholar]
- 7.Gray, N. D., and I. M. Head. New insights on old bacteria: diversity and function of morphologically conspicuous sulfur bacteria in aquatic systems. Hydrobiologia, in press.
- 8.Gray N D, Pickup R W, Jones J G, Head I M. Ecophysiological evidence that Achromatium oxaliferum is responsible for the oxidation of reduced sulfur species to sulfate in a freshwater sediment. Appl Environ Microbiol. 1997;63:1905–1910. doi: 10.1128/aem.63.5.1905-1910.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray N D, Howarth R, Rowan A, Pickup R W, Jones J G, Head I M. Natural communities of Achromatium oxaliferum comprise genetically, morphologically, and ecologically distinct subpopulations. Appl Environ Microbiol. 1999;65:5089–5099. doi: 10.1128/aem.65.11.5089-5099.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagen K D, Nelson D C. Organic carbon utilization by obligately and facultatively autotrophic Beggiatoa strains in homogeneous and gradient cultures. Appl Environ Microbiol. 1996;62:947–953. doi: 10.1128/aem.62.3.947-953.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagen K D, Nelson D C. Use of reduced sulfur species by Beggiatoa spp.: enzymology and physiology of marine and freshwater strains in homogeneous and gradient cultures. Appl Environ Microbiol. 1997;63:3957–3964. doi: 10.1128/aem.63.10.3957-3964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Head I M, Gray N D, Clarke K J, Pickup R W, Jones J G. The phylogenetic position and ultrastructure of the uncultured bacterium Achromatium oxaliferum. Microbiology. 1996;142:2341–2354. doi: 10.1099/00221287-142-9-2341. [DOI] [PubMed] [Google Scholar]
- 13.Head, I. M., N. D. Gray, R. Howarth, K. J. Clarke, R. W. Pickup, and J. G. Jones. Achromatium oxaliferum: understanding the unmistakable. Adv. Microb. Ecol., in press.
- 14.Hipp W M, Pott A S, Thum-Schmitz N, Faath I, Dahl C, Trüper H G. Towards the phylogeny of APS reductases and sirohaem sulfite reductases in sulfate-reducing and sulfur-oxidizing prokaryotes. Microbiology. 1997;143:2891–2902. doi: 10.1099/00221287-143-9-2891. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe H-G. Determination and properties of actively metabolizing heterotrophic bacteria in the sea, investigated by means of micro-autoradiography. Mar Biol. 1976;36:291–302. [Google Scholar]
- 16.Karner M, Fuhrman J A. Determination of active marine bacterioplankton: a comparison of universal 16S rRNA probes, autoradiography, and nucleoid staining. Appl Environ Microbiol. 1997;63:1208–1213. doi: 10.1128/aem.63.4.1208-1213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Rivière J W M, Schmidt K. Morphologically conspicuous sulfur-oxidizing eubacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes: a handbook on the biology of bacteria; ecophysiology, isolation, identification, applications. 2nd ed. Vol. 4. New York, N.Y: Springer-Verlag; 1992. pp. 3934–3947. [Google Scholar]
- 18.Larkin J M, Shinabarger D L. Characterization of Thiothrix nivea. Int J Syst Bacteriol. 1983;33:841–846. [Google Scholar]
- 19.Larkin J M, Strohl W R. Beggiatoa, Thiothrix and Thioploca. Annu Rev Microbiol. 1983;37:341–367. doi: 10.1146/annurev.mi.37.100183.002013. [DOI] [PubMed] [Google Scholar]
- 20.Lee N, Nielsen P H, Andreasen K H, Juretschko S, Nielsen J L, Schleifer K-H, Wagner M. Combination of in situ hybridization and microautoradiography—a new tool for structure-function analyses in microbial ecology. Appl Environ Microbiol. 1999;65:1289–1297. doi: 10.1128/aem.65.3.1289-1297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier S, Gallardo V A. Nutritional characteristics of two marine thioplocas determined by autoradiography. Arch Microbiol. 1984;139:218–220. [Google Scholar]
- 22.McGlannan M F, Makemson J C. HCO3− fixation by naturally occurring tufts and pure cultures of Thiothrix nivea. Appl Environ Microbiol. 1990;56:730–738. doi: 10.1128/aem.56.3.730-738.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson D C. Physiology and biochemistry of filamentous sulfur bacteria. In: Schlegel H G, Bowien B, editors. Autotrophic bacteria. Madison, Wis: Science Tech Publishers; 1989. pp. 219–238. [Google Scholar]
- 24.Newport P J, Nedwell D B. The mechanisms of inhibition of Desulfovibrio and Desulfotamaculum species by selenate and molybdate. J Appl Bacteriol. 1988;65:419–423. [Google Scholar]
- 25.Nielsen P H, Andreasen K, Lee N, Wagner M. Use of microautoradiography and fluorescent in situ hybridisation for characterization of microbial activity in activated sludge. Water Sci Technol. 1999;39:1–9. [Google Scholar]
- 26.Oremland R S, Capone D G. Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol. 1988;10:285–383. [Google Scholar]
- 27.Ouverney C C, Fuhrman J. Combined microautoradiography-16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl Environ Microbiol. 1999;65:1746–1752. doi: 10.1128/aem.65.4.1746-1752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardee A B, Prestidge L S, Whipple M B, Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966;241:3962–3969. [PubMed] [Google Scholar]
- 29.Robertson L A, Kuenen J G. The colorless sulfur bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes: a handbook on the biology of bacteria; ecophysiology, isolation, identification, applications. 2nd ed. Vol. 1. New York, N.Y: Springer-Verlag; 1992. pp. 385–413. [Google Scholar]
- 30.Schewiakoff W. Über einen neuen bakterienähnlichen Organismus des Süßwassers. Heidelberg, Germany: Habilitationsschrift; 1893. [Google Scholar]
- 31.Schmidt T M, Arielli B, Cohen Y, Padan E, Strohl W R. Sulfur metabolism in Beggiatoa alba. J Bacteriol. 1987;169:5466–5472. doi: 10.1128/jb.169.12.5466-5472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starr M P, Skerman V D B. Bacterial diversity: the natural history of selected morphologically unusual bacteria. Annu Rev Microbiol. 1965;19:407–454. doi: 10.1146/annurev.mi.19.100165.002203. [DOI] [PubMed] [Google Scholar]
- 33.Strohl W R, Schmidt T M. Mixotrophy of the colorless, sulfide-oxidizing, gliding bacteria Beggiatoa and Thiothrix. In: Strohl W R, Tuovinen O H, editors. Microbial chemoautotrophy. Columbus: Ohio State University Press; 1984. pp. 79–95. [Google Scholar]
- 34.Strohl W R, Cannon G, Shively J M, Güde H, Hook L A, Lane C A, Larkin J M. Heterotrophic carbon metabolism by Beggiatoa alba. J Bacteriol. 1981;148:572–583. doi: 10.1128/jb.148.2.572-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulrich G A, Krumholz L R, Suflita J M. A rapid and simple method for estimating sulfate reduction activity and quantifying inorganic sulfur species. Appl Environ Microbiol. 1997;63:1627–1630. doi: 10.1128/aem.63.4.1627-1630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler D B, Whitton B A. Ecology of Hell Kettles. 1. Terrestrial and sub-aquatic vegetation. Vasculum. 1971;56:25–37. [Google Scholar]