Abstract
The past decade has seen the convergence of a series of new insights that arose from genetic and systems analyses of Alzheimer’s disease (AD) with a wealth of epidemiological data from a variety of fields; this resulted in renewed interest in immune responses as important, potentially causal components of AD. Here, we focus primarily on a review of human data which has recently yielded a set of robust, reproducible results that exist in a much larger universe of conflicting reports stemming from small studies with important limitations in their study design. Thus, we are at an important crossroads in efforts to first understand at which step of the long, multiphasic course of AD a given immune response may play a causal role and then modulate this response to slow or block the pathophysiology of AD. We have a wealth of new experimental tools, analysis methods, and capacity to sample human participants at large scale longitudinally; these resources, when coupled to a foundation of reproducible results and novel study designs, will enable us to monitor human immune function in the CNS at the level of complexity that is required while simultaneously capturing the state of the peripheral immune system. This integration of peripheral and central perturbations in immune responses results in pathologic responses in the central nervous system parenchyma where specialized cellular microenvironments composed of multiple cell subtypes respond to these immune perturbations as well as to environmental exposures, comorbidities and the impact of the advancing life course. Here, we offer an overview that seeks to illustrate the large number of interconnecting factors that ultimately yield the neuroimmune component of AD.
Subject terms: Neuroscience, Psychiatric disorders
Introduction
Through the vagaries of history and the impact of major narratives in each field, neuroimmunology and neurodegeneration in general as well as Alzheimer’s disease (AD) in particular were modestly interconnected in the late 20th and early 21st centuries. The rapid development of tools with which to examine the peripheral immune system led to a focus on acute inflammation on the one hand while neuronal dysfunction and loss dominated on the other. A recent renaissance in the study of glial cells coupled with the results of human genetic studies in AD has led to the re-discovery of the role of the immune system in AD. The immune system has a multiplicity of roles—causal and reactive—in AD, consistent with the broad array of interconnected cell types and molecular programs that, together, are captured under this rubric. Further, because of its nature that includes monitoring the organism and responding to perturbations, the immune system is deeply integrated into every organ system and with inter-organ communications such as those provided by the nervous and endocrine systems to maintain homeostasis of the organism in the face of environmental exposures. As a result, many different cell types outside of the hematopoietic system interact with and modulate immune cells or contribute to immune responses, as these responses are both modulated by their microenvironment and their molecular backbones have been repurposed in other cellular contexts over the course of vertebrate evolution. While model systems—particularly mammalian ones—can be useful to assess targeted questions, the rapid evolution of the immune system given the selective pressures of pathogens and other factors has led to numerous differences in the molecular composition of mammalian immune systems, limiting the direct translatability of insights. We are thus faced with the task of characterizing a complex, distributed and highly plastic system. With different immune responses working at different tempo, the lack of an acute inflammatory component that directly causes AD long hampered attention to this area of investigation. Certain systematic evaluations of immune responses in relation to the course of AD have now begun; yet, many of the ongoing efforts remain focused on a subset of immune phenomena.
One of the critical challenges that we face in this thematic area relates to the plethora of partially correlated outcome measures that are used in human studies: intuitively, it is likely that different cells and immune responses are involved in different aspects and at different times along the long trajectory of this disease. It is even possible that the same molecular pathway may actually have opposing effects over the course of disease, and thus it is essential to be very precise in understanding whether an analysis is assessing associations with a pathologic diagnosis of AD (or that of its component proteinopathies of amyloid and tau) or a clinical outcome such as AD dementia. All of these traits are correlated and co-exist in individuals, particularly with advancing age, so great care needs to be taken in interpreting individual results and integrating them into an overview. Further, there is a host of aging-related comorbidities such as (1) neurovascular disease, obesity, diabetes, among others [1, 2], (2) metabolic changes such as altered mitochondrial function [3], and (3) other neuropathologic processes (TDP-43 and α-synuclein proteinopathies) [4–6] that coexist and interact with AD-related processes [7, 8] in the older brain. Without accounting for such confounders, one must be cautious in attributing molecular events to AD. Here, we attempt to sketch a broad overview of the component parts of the human immune system in AD, to highlight key new insights and point to areas where further development is urgently needed.
Early hints of neuroimmunological contributions to AD
Alzheimer himself observed glial changes and clustering of glial cells around plaques in post-mortem tissue, thereby providing the first evidence for microglia playing a role in a neurodegenerative disease in 1910 [9, 10]. After developing a novel method to stain the brain in the early 1900s, Cajal described protoplasmic and fibrous astrocytes while calling the parenchymal cells the “third element” [11]. Only when Río-Hortega joined Cajal’s laboratory and further developed existing staining methods, were ramified microglia stained for the first time and further characterized as phagocytic cells in the brain [12]. Río-Hortega further stated that the “nomadic” nature of microglia is best observed during neurodegenerative processes in which they adapt migratory and phagocytic activity from their resting state, suggesting a role for these cells in neurodegeneration [13]. After that time, the field of microglia research evolved slowly for many decades and only started to gain more attention in the 1960s and 1970s [13].
Later in the 1980s, Canadian scientists reported HLA-DR+ and reactive microglia in the gray matter of post-mortem AD patient tissue throughout the cortex with higher concentrations around senile plaques [13, 14]. These HLA-DR-positive microglia were further described to phagocytose dying neurons in the AD cortex [14]. At the time, the authors questioned whether the activation of the identified reactive microglia was induced by a pathological process within the central nervous system (CNS) or derived from infiltrating peripheral monocytes in response to the emergence of pathology [14]. This manuscript illustrates the conception that the role of immune cells in AD was primarily reactive, following the appearance of a pathologic insult intrinsic to the CNS parenchyma.
This observation was elaborated in 1988 with a description of the presence of HLA-DR+ T cells in AD brain tissue as well as instances of apposition between putative microglia and T cells, representing the first evidence for potential interaction of microglia and T cells in AD [15]. These investigators further report AD-specific staining of astrocytes with the Natural Killer cell marker Leu-11, for the first time suggesting a role for astrocytes in AD pathology [15]. Around the same time, another report described increased Interleukin-1 (IL-1) and S100-positive astrocytes in AD brain tissue, further confirming a role for astrocytes in AD and suggesting that the observed astrogliosis in AD may be promoted by increased IL-1 expression [16]. These and other early studies began to highlight reliable markers for the staining of glial cells and paved the way for future investigations of the contributions of the (neuro-) immunological responses to AD.
Recent studies renewing interest in the immunological contribution to AD
Once genome wide association studies began to identify robust associations between susceptibility to AD and genetic variation (primarily single nucleotide polymorphisms), the loci implicated by the top-scoring variants clearly harbored genes that were relatively specific to myeloid cells, such as CD33 and TREM2 [17–19]. In addition, statistical analyses soon returned an enrichment for genes involved in immune responses [20], and a polarization of the functional consequences of AD susceptibility variants towards altered gene expression in myeloid cells [21]. Myeloid cells are a key component of the innate immune system that includes dendritic cells, monocytes, macrophages, and microglia. This narrative has been consistently reinforced as additional AD loci have been discovered, and currently, more than 1/3 of the described susceptibility loci harbor genes that may be expressed in myeloid cells [18, 22–24]. In addition to studies of common variants, whole exome and genome sequencing studies have identified multiple different rare variants that influence AD risk in TREM2 but also in PLCG2, ABCA7, ABI3, SORL1, ECE2, PLD3 among others that influence AD risk [19, 25–29]. Another important locus implicated in AD susceptibility is the Major Histocompatibility Complex (MHC), a gene dense, genetically complex region. While there appears to be a robust association with the MHC, the magnitude of the effect is modest, similar to the other common AD susceptibility variants and much smaller than nearby associations with inflammatory diseases such as multiple sclerosis (MS) [30]. Given the extensive long-range linkage disequilibrium in the MHC, it is difficult, at this time, to definitively know which gene may be involved. The MHC association is centered on a group of MHC class II genes, but one should be cautious about jumping to the conclusion that these Class II genes, involved in antigen presentation, are the target genes, as the true causal gene could be hundreds of kilobases away, and the MHC is gene-dense. Interestingly, while certain MHC Class II alleles associated with rheumatoid arthritis (RA) may have an effect on AD-related traits, polygenic scores for RA and MS are not related to AD susceptibility or other AD-related traits [31]. This suggests that a propensity for inflammatory diseases centered on autoimmune dysregulation does not appear to affect AD: the molecular immune pathways that are vulnerable to dysregulation and contribute to AD may be distinct from those involved in classical inflammatory diseases.
A minority of myeloid cell-related variants have been shown to influence AD-related intermediate traits such as the accumulation of amyloid for the CD33 risk allele [32], increased burden of neuritic plaques and neurofibrillary tangles for a TREM1 allele [33], and activation of phospholipase C-gamma (PLCγ2) downstream of TREM2 signaling for the PLCG2 protective variant [34]. However, for the TREM2 variant—where most of the data have been accumulated from mouse models—the functional consequences in humans remain unclear, limiting the community’s ability to pursue therapeutic options. From genetic studies, it appears that sTREM2 may be most relevant in terms of AD as surface expression of TREM2 is not related to AD susceptibility while sTREM2 levels clearly are [28, 35].
While the functional consequences of individual AD susceptibility variants are gradually emerging, their interaction is also beginning to be understood, with a connection between CD33 and TREM2 [36] as well as an effect of PU.1 on the expression of multiple AD genes [37]. Further, a shared evolutionary history of a subset of these myeloid variants suggests that they may be working together in the same pathway [38]. Thus, we need to better understand whether certain molecular pathways are preferentially involved in AD susceptibility. An important limitation of these in silico analyses is that the various types of myeloid cells share many transcriptional programs, making it difficult to ascertain whether AD susceptibility is due to the involvement of CNS resident microglia, meningeal macrophages, infiltrating monocytes, or even perhaps dendritic cells working in the periphery.
Cellular players
Peripheral monocytes
Infiltration of peripheral immune cells including peripheral monocytes is associated with neuroinflammation and blood brain barrier (BBB) relaxation in the context of aging or neurodegenerative disease, including AD [39, 40]. While brain-resident microglia have received most of the attention in AD recently, infiltrating monocytes are largely indistinguishable from microglia after activation, and investigation of a role for blood-derived peripheral monocytes in AD has found renewed interest. Both cell types are included in Fig. 1. However, studies on the role of peripheral monocytes in AD remain rare. In one study, pro-inflammatory cytokine expression by peripheral monocytes was noted throughout the course of AD, except for its earliest prodromal stages [41]. Another study assessing human peripheral blood mononuclear cells (PBMC) derived from donors with different ages and including AD patients reported an age-dependent decline in Aβ uptake which was more pronounced in AD patients, suggesting that impaired Aβ uptake by monocytes might be involved in AD pathogenesis [42]. The authors further suggested the promotion of monocytic phagocytosis as a therapeutic strategy [42]. While some single cell (sc) or single nucleus (snuc) RNA sequencing studies using human AD tissue were not able to identify peripheral monocytes in tissue specimens [43, 44], our group recently identified a discrete set of myeloid cells as monocytes in a scRNAseq study using human cerebral cortex autopsy and surgical samples, characterized by FCN1, VCAN, and LYZ expression [45]. Their exact source remains unclear, although these monocytes are much more frequent in these samples than T cells, suggesting that most of them do not represent monocytes from blood that happened to be in a vessel within the sample being processed, as T cells are more frequent than myeloid cells in peripheral blood.
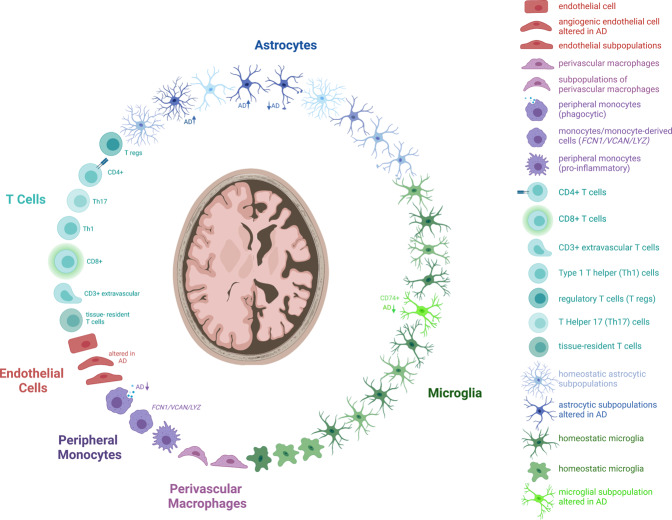
Fig. 1. Cellular players in AD.
(Neuro-) immune cells and their described subtypes relevant in AD pathology including astrocytes (blue), microglia (green), perivascular macrophages (purple), peripheral monocytes (dark violet), endothelial cells (red) and T cells (turquoise).
Data from mammalian in vivo models must be considered carefully given the many differences between the human and murine immune systems. There is some evidence that murine monocytes may contribute to amyloid proteinopathy models, although there are conflicting reports about the direction of the effect [46–51]. There are also conflicting reports on whether monocytes or microglia have a higher phagocytic capacity for Aβ engulfment [52–54]. Thus, even with the additional tools available to murine in vivo experiments, the jury remains out as monocytes display quite a lot of plasticity in downregulating proteins that are used as lineage markers [55]. Once in the brain, monocytes can actively promote neuroinflammation through the activation of microglia as shown in APP/PS1 mice [56, 57]. These results highlight the challenges we face as we explore a multi-phasic process in which immune responses have different roles at different times. Further, another important limitation of in vivo models—beyond the differences in molecular architecture due to millions of years of separate evolution—relates to the non-physiologic nature of accelerated proteinopathy models that are frequently used to model aspects of AD. The kinetics of immune responses are finely tuned; the compressed murine life span and accelerated time course of proteinopathies doubtlessly distort the magnitude and nature of immune responses. Overall, both neuroprotective and detrimental functions have been suggested for infiltrating monocytes, but much more characterization is needed to refine their role in the different phases of AD.
T Cells
As early as the 1980s, T cells of different types have been reported to be present in samples of AD cortex, with HLA-DR-positive T cells being localized in close proximity to plaques and tangles [15]. Around the same time another study reported increased numbers of T cells in the hippocampus and cortex of AD subjects compared to controls [58]. Another such report further suggested that these T cells most likely represent activated memory cells rather than fully differentiated effector cells [59]. A study assessing peripheral blood mononuclear cells discovered an AD signature consisting of increased CD8+CD45RA memory T cells which were negatively associated with cognition; they were further found to be clonally expanded in the cerebrospinal fluid (CSF) of individuals with AD [60]. Another group, however, reported no changes in CD8+ effector memory T cells while they showed an upregulation of late stage effector T cells in the peripheral blood of AD patients [61]. A third, independent study, however, could not reproduce the latter results [62].
Other studies elaborate this collection of observations, reporting higher numbers of extravascular CD3+ T cells in human AD post-mortem brain correlating with Tau but not amyloid plaque pathology; the authors further suggest that T cell extravasation might be driven by tau-related neurodegenerative changes and speculate that T cells might play a crucial role in the amyloid-independent phase of AD pathology [63]. A recent study further confirmed increased CD8+ T cell numbers in human AD post-mortem hippocampus which was also shown in a murine APP/PS1 amyloid proteinopathy model [64]. Depletion of CD8+ T cells in APP/PS1 mice changed neuronal- and synapse-related gene expression in the hippocampus, suggesting that CD8+ T cells infiltrate the AD brain and might have direct effects on synaptic plasticity, thereby contributing to neuronal dysfunction [64]. However, these results should be interpreted carefully as the ablation of CD8+ T cells might act peripherally and therefore indirectly affect gene expression in the hippocampus. Additional studies reporting a slight increase in circulating CD4+ T cells and a decrease of CD8+ T cells at the same time exist without significant changes of the CD4+/CD8+ ratio [65]. A decrease in naïve CD4+ T cells along with higher numbers of late-differentiated cells as well as activated CD4+CD25+ T cells was also reported by others [66]. An additional study examining the effect of different oligomers of the N-terminal domain of the HypF protein from Escherichia coli as a model system to elicit differential immune responses since this protein fragment yields different well-characterized oligomers (Type A and Type B). Several different effects were noted in human PBMCs exposed to the different oligomers, with, for example, a reduction in CD4+ T cells when exposed to HypF-N type B oligomers but not when exposed to type A HypF-N oligomers, and CD8+ T cells were unaffected [67]. This illustrates the heterogeneity in responses that can be elicited among the varied immune cell types found in peripheral blood with a well-characterized perturbation.
A study assessing CD4+ T cells in peripheral blood found a decrease in CD4+ effector T cells, but an increase in CD4+ memory cells in individuals with AD [61]. As just pointed out for CD4+ and CD8+ T cell subtypes, the occurrence of AD-related changes in regulatory T cells (Treg) is currently under debate [66, 68, 69]. One report recently assessed the levels of defined subtypes of regulatory T cells (CD4+/CD25high/CD127low-neg) including Resting (analyzed CD45RA+/CD25dim), Activated (CD45RA-/CD25bright), and Secreting (CD45RA-/CD25dim) cells, reporting a decrease in the total Treg population as well as the Resting subtype of Tregs in peripheral blood samples from AD subjects compared to healthy individuals [68]. Additionally, in the study introduced above, exposure of human PBMCs also elicited differential responses with type A HypF-N oligomers having a more pronounced effect of increasing the proportion of CD4+/CD25high/FoxP3+ Treg cells after stimulation, although the functional capacity of these cells was not significantly different [67]. The direction of the association between Tregs and AD remains unclear: are Tregs playing a protective role? One has to be careful in the interpretation of such data when causality has not been established using longitudinal data or perturbation studies. One study using the APP/PS1 mouse model noted accelerated cognitive impairment upon depletion of Tregs; this observation was correlated with reduced numbers of plaque-associated microglia. Further, amplification of regulatory T cells reversed this effect, suggesting a role for Tregs in modulating the response of microglia to amyloid β accumulation, although human data are lacking to confirm this observation [70]. A more recent study investigating Treg function in AD subjects found the suppressive function of Tregs on effector T cells to be compromised in AD subjects when compared to individuals with mild cognitive impairment (MCI) or controls; the authors further showed that Treg function could be enhanced upon ex vivo expansion of Tregs, suggesting Tregs as potential targets to modulate the inflammatory status in AD [71]. However, similar to the earlier study, causality was not established, and other studies have appeared which report no significant changes in the regulatory T cell compartment (CD4+ CD25+ FoxP3+ CD127low) between AD subjects and age-matched controls [66]. It joins other studies assessing T cell frequency and responses which did not observe any significant differences between individuals with AD and healthy controls nor any correlation between antigen-specific T cell responses and clinical parameters including age, gender and cognitive score [62]. In another case, T cells derived from elderly patients showed increased Aβ-reactivity when compared to young individuals; however, no changes were seen between older healthy and AD individuals [72, 73]. The HypF-N oligomers discussed above appear to also influence effector T cell differentiation including that of Th1 and Th17 cells as well as cytokine secretion [67]. This suggests that Aβ could have effects that are not antigen-specific on the profile of the older peripheral immune system. An additional recent study reported increased levels of circulating Th17 cells in AD individuals compared to age- and sex-matched controls, which is consistent with this hypothesis [74]. Finally, the T cell receptor gamma (TRG) repertoire was further shown to be reduced in AD individuals, and specific AD-associated clonotype features of TRGs derived from blood and brain cell populations were described but the relevance of such T cell subpopulations with responses to specific antigens remains to be elucidated [75].
Thus, the role of T cells in AD is rather unclear at this juncture. Many intriguing changes in T cell populations and function have been reported, but they are difficult to assemble into a coherent picture because—beyond technical differences among the studies—each of these studies is small and most do not account for the host of possible confounders that could also influence their results. Age-related effects on T cell populations are strong and have probably not been well evaluated or accounted for. Overall, the discordant results on T cells in the literature preclude clear conclusions at this time, but they do highlight the need for large, well-designed evaluations of the role of this lymphocyte population. As discussed recently, further studies are needed to replicate observed changes in a longitudinal rather than cross-sectional fashion [76]. An overview of the described subpopulations is included in Fig. 1.
The glymphatic system
The meningeal lymphatic system plays an important role in the maintenance of brain homeostasis by being functionally linked to the exchange of soluble content between the cerebrospinal fluid and the interstitial fluid [77]. The efferent paravascular glial lymphatic (glymphatic) system comprises a perivascular network for the transport of lymphatic fluid and is connected to the peripheral lymphatic system [78], ultimately draining into the thoracic duct. Immune cells exiting the parenchyma can travel through these vessels, although their characteristics in humans remain poorly understood. The glymphatic system exits the CNS through the meninges, the cranial nerves and the larger vessels [78]. A more detailed perspective on the glymphatic system as a new important player in neurophysiology is available elsewhere [77]. Since the discovery of the glymphatic system as an alternative route to the BBB for Aβ clearance from the interstitial fluid about a decade ago [79, 80], its possible role in AD pathology was elaborated by further studies showing that ablation of the glymphatic system in young mice increases the severity of amyloid proteinopathy, leading to Aβ deposition in the meninges as well as recruitment of local macrophages [77, 81]. Since Aβ deposition in human dura from AD patients has been observed and a decline in meningeal lymphatic function with age has been described [81], one might speculate that the glymphatic system may aggravate or even contribute to AD pathogenesis by favoring Aβ deposition if its function is impaired. Based on these findings a recent study proposed a role for the APOE gene as favoring premature shrinkage of meningeal lymphatic vessels, thereby impairing meningeal lymphatic functions and eventually causing reduced clearance of Aβ, inflammatory mediators or immune cell egress, favoring AD progression [82]. Modulation of glymphatic vessel diameter by increased expression of vascular endothelial growth factor C in young and old mice improved spatial learning and memory exclusively in old mice, suggesting the meningeal lymphatic system as an interesting novel approach to modulating AD pathobiology [81]. However, the molecular nature and distribution of glymphatics in the human brain remain much less well understood and are the focus of ongoing investigations.
Perivascular macrophages
Perivascular macrophages (PVMs) constitute a highly specialized population of myeloid cells residing in the perivascular spaces that are limited by the glial basement and the vascular basement membranes and serve important functions in the clearance of interstitial fluid and metabolic waste by functioning as conduits for the uptake of cerebrospinal fluid [83, 84]. Perivascular macrophages are involved in BBB integrity, lymphatic drainage as well as immune functions including phagocytosis and antigen presentation. They have been implicated in Aβ clearance in mice [85–88]. Currently, no data on PVM function in human AD pathology are available, which might be partially explained by the lack of distinct markers for human PVMs as well as the availability of appropriate specimens to study these cells. Future studies are required to define the existence of spatial or temporal subpopulations of human PMVs as well as their function in AD.
Endothelial cells
Highly specialized endothelial cells connected by tight junctions and adherens junctions, lining cerebral microvessels constitute the BBB, the interface between the central nervous system and the systemic circulation [89]. The BBB plays an important role in neuroinflammation as the conduit of bone-marrow derived cells into the CNS parenchyma, and its dysfunction is associated with AD-related changes [89]. A recent study performing a single-nucleus transcriptome analysis of nuclei isolated from AD or control prefrontal cortex samples reported the presence of a subpopulation of angiogenic endothelial cells in individuals with AD [90]. These angiogenic endothelial cells showed increased expression of angiogenic growth factors and their receptors—such as ERG, FLT1, and VWF—as well as of genes involved in antigen-presentation via MHC class I (MHC I), suggesting the activation of an acute inflammatory response program [90]. Such cells could contribute to the activation and proliferation of the T cell populations described above [60, 91]. Similarly, changes in endothelial transporter activity also likely contribute to altered BBB permeability, as region-specific reduced glucose utilization in individuals with AD is a consistent finding [92]. Additionally, Aβ is known to be transported actively to and from the CNS via BBB endothelial cells through transcytosis. While low density lipoprotein receptor related protein 1 (LRP1) controls Aβ transport from the brain to the periphery, RAGE promotes Aβ influx in the CNS [93–95]. Expression changes of Aβ receptors might contribute to Aβ levels in the CNS and indeed multiple clinical studies demonstrated a correlation between AD pathology and low expression of LRP1 as well as high expression of RAGE on endothelial cells, which may promote Aβ accumulation in the parenchyma [89, 96]. The development of novel models to study endothelial cells, such as iPSC-derived brain endothelial cells [97] as well as single cell genomics will promote further studies deciphering phenotypes and functions of endothelial cells in AD. Novel unpublished data from our group, derived from a single-nucleus RNA sequencing study suggest the existence of several endothelial subpopulations (Fig. 1) whose identity and function in AD remain to be further determined [98].
Astrocytes
Astrocytes have been recognized as important players within the neuroimmune axis as they have the ability to modulate both innate and adaptive immunity, as shown for MS and other CNS diseases [99, 100]. Many astrocyte functions have been linked to AD, including reactive astrogliosis characterized by functional and morphological remodeling of astrocytes [101]. Reactive astrocytes have been observed around amyloid plaques in post-mortem tissue from individuals with AD and have been proposed to be involved in sustaining the inflammatory process in AD through the secretion of pro-inflammatory cytokines such as TNF-α, IL-1β and COX2 expression through NFkB activation [102, 103]. Astrogliosis is mainly associated with early to moderate stages of AD, while later stages characterized by severe dementia have been noted to have astroglial atrophy in humans, and a similar response is seen in advanced stages of certain mouse models. These changes may relate to the loss of synaptic connectivity seen in AD given the central role of astrocytes in maintaining synaptic transmission [104, 105].
In comparison to other cellular players in the neuroimmunology of AD, single nucleus transcriptomic studies have already started to characterize astrocytes in AD in greater depth and have identified several subpopulations of astrocytes in the healthy human brain as well as in the human AD brain (Fig. 1) [44, 90, 98]. Two of these studies report shifts in the frequency of astrocyte subtypes, generally away from the homeostatic state, in the context of AD [90, 98]. However, results are difficult to integrate at this time given the small sample sizes of individual studies and variation in cluster definitions among these studies; a clearer picture should emerge soon and prioritize astrocytic subtypes and specific transcriptional programs as studies become larger and a more stable cluster structure emerges. A major challenge may be that, like microglia, these cells are plastic and that, for many astrocytic subtypes, there may not be a clear boundary between subtypes: astrocytes are probably best seen as being distributed across gradients oriented towards different poles of extreme differentiation. Once a clearer structure of the population of astrocyte subtypes emerges, the extent to which they contribute to immune responses vs. other more specialized astrocytic functions in AD will become clearer.
Microglia
As the CNS-resident phagocyte and a key element of the parenchyma with a multiplicity of roles, microglia have been studied extensively in AD. While the involvement of microglia in AD-associated neuroinflammatory processes was noted very early, genome-wide association studies provided an inflection point in the study of this cell type as they clearly implicated myeloid cells as playing a causal role in the onset of AD [17–20, 22–24, 38]. Around the same time, association studies using transcriptomic and proteomic data have elaborated this role, although the direction of these associations remains ambiguous, and further work is needed to validate the proposed sequence of events implicating genes such as TYROBP which encodes a protein that interacts directly with TREM2, a genetically identified AD susceptibility gene. Beyond individual genes, network approaches showed that genes found in AD risk loci converged in glial-related modules of co-expressed genes and proteins [106], that these results are found in multiple different datasets [107], and that microglia are likely to be involved, in different ways, at different stages of AD, contributing to both amyloid and tau proteinopathy [108]. Many of the relevant pathways remain to be dissected mechanistically. The most studied gene to date is probably TREM2 [106], which is primarily expressed in myeloid cells in the CNS. Extensive work in murine models has offered a number of proposed mechanisms, but these have yet to be validated and translated to human subjects [109, 110]. In humans, the function of TREM2 in AD remains unclear, with genetic studies pointing to soluble TREM2 as the form that may be most relevant [111]. A recent study using iPSC-derived microglia also suggests a role for TREM2 in microglial metabolism as microglia derived from individuals with TREM2 AD R47H risk variant exhibited metabolic deficits ranging from reduced mitochondrial respiratory capacity to incapacity of performing immunometabolic switching to glycolysis [112]. Interestingly, APOE, one of the risk genes with a large effect on the risk of developing late-onset AD, has been proposed as a ligand for TREM2, contributing to increased phagocytosis of apoptotic neurons in primary mouse microglia [113]. Understanding the underlying mechanisms of TREM2 and APOE interaction, its impact on synaptic function and cognitive impairment in AD pathogenesis will be important as it will connect two well-validated genetic risk factors [114]. Microglial APOE expression strongly increases during aging and in AD as shown by multiple human and mouse studies [115–117]. Currently, data on the effects of APOE on microglial function exist only from mouse studies; human datasets are, as yet, too small to interrogate this question effectively [117]. Additional AD-specific risk genes with enriched expression in myeloid cells include the transmembrane receptor CD33, the glycoprotein clusterin and the complement receptor CR1 among others [118, 119]. PLCG2 is interesting, being expressed in microglia and other myeloid cells (where it has been mostly studied in relation to AD [120]), but it is also expressed in T cells.
Microglia can bind Aβ oligomers and Aβ fibrils via several cell surface receptors including Toll-like receptors (TLR2, TLR4, TLR6, TLR9), CD14, CD36, CD47, and α6β1 integrin among others, resulting in microglial activation and production of pro-inflammatory cytokines and activation of the inflammasome [105, 121–123]. Aβ-induced microglial pro-inflammatory cytokines include IL-1β, IL-6, TNF, and IFN-γ which can, in turn, induce the expression of β-secretase, the enzyme cleaving APP to generate pathogenic Aβ, thereby potentially contributing to amyloid plaque formation [105]. Constant activation of microglia through extracellular Aβ, neuronal debris and chronic vascular changes of older age might prime microglia, rendering them more susceptible towards acute inflammatory stimuli and might lead to chronic neuroinflammation in the context of an ongoing inflammatory process such as extracellular Aβ deposition [114]. Microglial priming might also result from microglial senescence through accelerated aging as well as systemic immune challenges from the peripheral immune system throughout life [124]. Murine microglia are able to adapt their phenotype depending on their history of exposure to inflammatory stimuli to yield either a weaker (immune tolerance) or stronger (immune training) reaction to subsequent inflammatory stimuli, a concept termed “innate immune memory” [125]. Microglial priming and innate immune memory were both shown to affect the progression of proteinopathy in murine models [125, 126].
Compromised microglial phagocytic capacity in AD is well described and has been associated with several processes including downregulation of Aβ phagocytosis as a result of chronic exposure to Aβ [127] and, in humans, the functional consequences of certain AD susceptibility variants, such as the CD33 allele [32]. These and many other observations point towards an impairment of microglial phagocytosis in the accumulation of amyloid proteinopathy; however, there is mounting evidence that other aspects of microglial function relate to tau proteinopathy and perhaps to other processes such as cognitive decline following the accumulation of tau. One study proposed that TREM2 and TYROBP may be implicated more in microglial senescence, a distinct process not directly related to pathology, and it identified distinct transcriptional programs of microglia as associated with either amyloid or tau [108]. Further, the role of microglia in tau proteinopathy seems to relate, at least in part, to microglial “activation”, defined morphologically [128]. Activated microglia also seem to engage in the accumulation of tau aggregates [108]; whether phagocytosed or extracellular tau leads to the activation of microglia, however, remains unclear [129]. Further evidence suggests that microglia might secrete seed-competent tau into the extracellular space and may therefore play a critical role in the spreading of tau protein [129]. An additional analysis of mouse and human microglia examining tauopathy reports dynamic changes of microglia in the course of pathology, starting from proliferating microglia characterized by increased type-1 interferon response at early stages, to a transient phagocytic phenotype followed by a late stage characterized by partially impaired function potentially caused by tau-induced DNA damage and by increased type 2 interferon signaling [130]. Given that amyloid and tau pathology (and other neuropathologies) coexist in the aging brain, different subsets of microglia may coexist in the same piece of tissue that could account for part of this perceived heterogeneity in response [108, 131].
Single cell transcriptomics has started to yield a higher-resolution map of microglial heterogeneity, which is illustrated in Fig. 1 [45, 90, 98]. Several of these microglial subsets have been linked to AD [45, 90]. One study using single nucleus transcriptomics identified 13 microglial subpopulations and highlighted one of them as contributing to AD; this microglial subset was proposed to be associated with synaptic pruning and cytokine response and to be reduced in AD, suggesting its contribution to the disparity in complement signaling and synaptic pruning in AD [90]. Another study deploying single cell RNA sequencing of live microglia isolated from human cortical samples identified nine microglial subclusters and reports one microglial cluster to be specifically reduced in frequency in AD tissue [45]. This cluster was defined by high expression of CD74 and enriched in AD-related genes [45]. Whether the cluster plays a neuroprotective role in the aged brain remains to be determined. As with astrocytes, these single cell approaches and emerging spatial transcriptomic technologies should provide critical new insights, but results are difficult to integrate together and are not robust at this time given small sample sizes.
Cellular communities in AD
While recent studies deploying single nucleus or single cell transcriptomic profiling (snuc or sc RNAseq) have begun to uncover the different subsets of neuroimmune cell types in the human brain [44, 45, 98], the interplay of these subpopulations in the non-AD and AD brain has not yet been studied in detail. Small scale analyses of snucRNAseq data from human prefrontal cortex samples proposed that specific subpopulations of excitatory neurons, astrocytes and oligodendrocytes may be associated with features of AD pathology [44]. Another study of this type went further, developing a model of cellular communities using snucRNAseq data and mapping the reciprocal relationships among communities composed of specific endothelial, astrocytic, microglial subtypes, and their relation to neuronal subtypes. Further, it validates the proposed role of these communities in AD by leveraging large bulk cortex RNAseq data that have proper statistical power [98]. This structure to single nucleus data is not surprising given that there are clear micro-environments within the AD cortex, such as the collection of morphologically altered cells surrounding a neuritic plaque: microglia and dystrophic astrocytes have long been noticed around plaques [14, 132]. Emerging technologies such as spatial transcriptomics will be critical to (1) further delineating this concept, assessing whether these communities of different cellular subtypes (Fig. 2) are topologically defined microenvironments or represent different cell types responding to the same molecular signal and (2) relating these communities to neuropathologic indices that are found in the aging and AD brain. Almost certainly, measuring and ultimately perturbing the function of these communities will be essential, conceptually, in therapeutic development as the summary output of the community is likely to have a greater impact on outcomes than individual cellular programs.
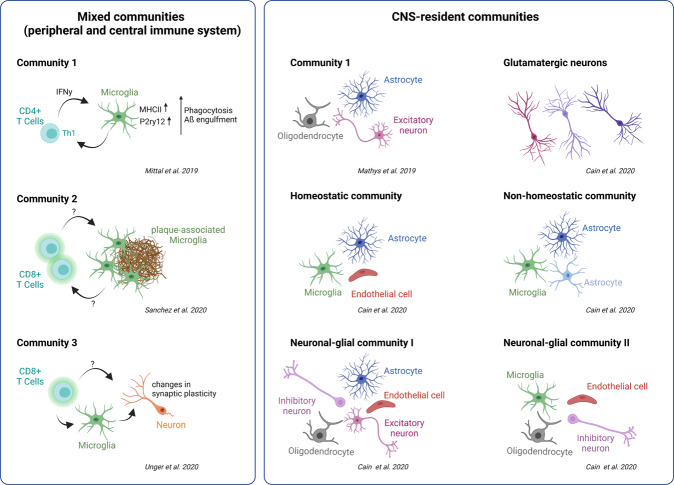
Fig. 2. Cellular communities in AD.
Illustration of cellular communities comprised of different CNS and peripheral immune cells as well as neurons in recent publications ([44, 64, 98, 275, 276]). They include both CNS-resident and mixed peripheral/CNS communities.
Crosstalk among the different neuro-immunological players in AD
These emerging communities highlight the fact that immune responses involve the coordinated activities of multiple different cell types that are communicating through physical interaction, localized paracrine signaling and systemic signals that engage the neuro-endo-immunological axis. A plethora of cytokines and other signaling molecules have been implicated in neuroinflammatory processes and progression in AD [105]. Whether those cytokines are causally involved in AD pathogenesis or whether they are the result of reactions to the deposition of amyloid plaques, tau proteinopathy, and neurodegeneration within the course of the disease has not been determined in humans [133]. Here, we briefly highlight current data on two important mediators, IL-6 and CRP which have been explored as serum biomarkers for AD, to illustrate the state of understanding of systemic signals of inflammation.
IL-6, a classical pro-inflammatory cytokine, is elevated in individuals with AD [134], including within and sourrounding amyloid plaques, and it has been studied as an AD biomarker in human serum and CSF samples [135]. However, results are inconsistent, reporting either decreased, unchanged, or increased IL-6 levels in individuals with AD [135–138]. In fact, a more recent meta-analysis did not confirm any signifcant changes in IL-6 levels in the serum or CSF of elderly with AD when analyzing data derived from 1645 elderly with Alzheimer’s disease and 14,363 controls [139]. Thus, current data do not support its applicability as a systemic biomarker for AD, although more refined study designs leveraging endophenotypes that capture the different elements of AD and other analytes relevant to IL-6 function (such as the level of soluble IL6R) may be needed to determine the role of this important mediator of systemic inflammation.
C-reactive protein (CRP) is an acute phase protein whose synthesis is induced through increased IL-6 secretion by macrophages or T cells. Like IL-6, CRP has been discussed as a systemic biomarker for AD; however, results are also inconsistent, suggesting either an increase [140–142] or decrease [143] in plasma CRP levels in AD. An additional study reports no differences in circulating CRP between AD patients and healthy controls; however, it suggests a link between CRP and APOE as APOEε4 carriers had lower circulating CRP levels than APOEε4 non-carriers [138]. A meta-analysis further revealed no significant differences in peripheral CRP levels between AD and non-AD individuals [139]. Thus, a general, relatively non-specific activation of systemic inflammation is not apparent in AD. There are certainly changes in these two and many other biomarkers, but they will need to be deployed in a much more targeted manner to contribute to our understanding and monitoring of the long course of AD. These two and other markers may be involved transiently in the presymptomatic, minimally symptomatic, or dementia phase of the disease.
Contribution of previous (neuro-) inflammatory events to AD
These association studies with systemic mediators of inflammatory responses have spurred interest into evaluating the role of conditions that can alter such mediators. Here, we provide an overview of the environmental exposures and life experiences (Fig. 3) that can modulate immune responses in the brain and have the most evidence to date in relation to AD.
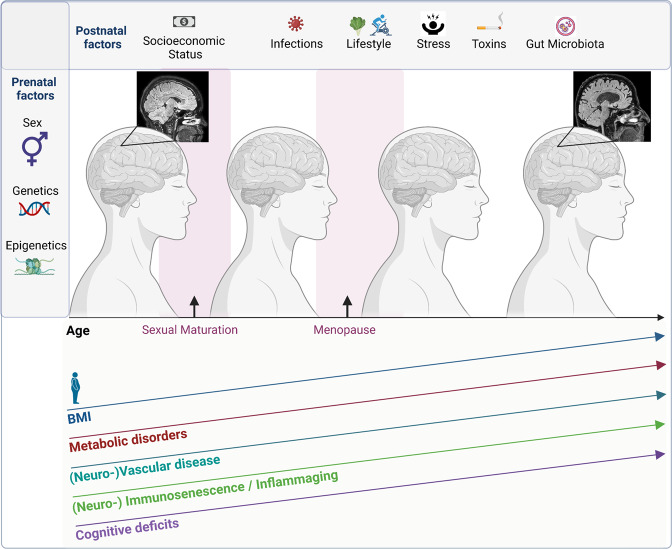
Fig. 3. Pre- and postnatal factors influencing AD.
Overview of Factors influencing AD including prenatal factors to postnatal factors ranging from socioeconomic status, lifestyle, exposure to infections, toxins and stress as well as composition of the gut microbiota and aging increase in BMI as well as comorbidities such as metabolic or vascular disease also increase the risk for AD development.
Infections
A role for microbes in the pathogenesis of AD has been postulated since the 1950s [144] with a growing number of reports presenting evidence for bacterial and viral pathogens contributing to AD [145–147]. The infectious hypothesis proposes a causal role for viral, bacterial or fungal infections in AD [148]. It can be summarized as follows: pathogen-induced inflammation leads to tissue damage which contributes to Aβ aggregation and deposition of tangles, which may promote further inflammation [149]. Alternatively, Aβ and tau may have a role in anti-inflammatory responses and only accumulate once their aggregates cannot be cleared sufficiently by microglia due to reduced phagocytic capacity as a consequence of natural aging [150].
This putative dual protective and damaging role for Aβ has been suggested by findings showing that oligomerized Aβ can function as an antimicrobial peptide that protects against fungal and bacterial infections by binding microbial cell walls and entrapping microbes through the formation and propagation of β-amyloid fibrils. Further, bacterial as well as Herpesviridae infections of the brain were associated with accelerated Aβ deposition in human and mouse studies, suggesting that Aβ might play a role in innate immunity and that inflammatory stimuli might drive Aβ proteinopathy [150–152]. A range of pathogens has been suggested as potential drivers of AD with herpesviruses being the most studied viral family in this context [153].
A recent analysis of three independent multi-omic datasets including genomic, transcriptomic, proteomic and histopathological data derived from non-sterile brain autopsy samples sought to identify pathogen-related sequences and reported increased levels of multiple viral species, including human herpesvirus 6A and human herpesvirus 7 across several regions in post-mortem brain samples of individuals with AD. These viral sequences were associated with altered expression of genes involved in APP metabolism and associated with AD risk, suggesting a link between certain viral species and AD [154]. However, a re-analysis of some of these data supplemented by targeted evaluation of HHV-6 could not confirm these associations with AD [155]. Moreover, interferon-based responses that are seen in brain autopsy data could be engaged by other, sterile processes and are not, in themselves, evidence of a viral etiology. Nonetheless, the low frequency of viral sequences and their lack of association with AD-related traits does not mean that these viruses did not have a role much earlier in the disease in initiating a cascade of events that contribute to AD. Thus, more work remains to be performed to explore the role of prevalent viruses such as herpesviruses [156] (including the varicella zoster and Epstein–Barr viruses) in the etiology of AD, ideally with dedicated data generation instead of repurposing data generated for other reasons.
Recently, periodontitis and chlamydia have also been associated with AD suggesting a link to chronic low-grade inflammation and AD [157–159]. However, longitudinal data are lacking so far to address possible causal relationships between these microbial species and AD; they could simply be coexisting or be the manifestation of an indirect impact of low-grade systemic inflammation on the kinetics of immune responses within the CNS parenchyma. Specifically, chronic inflammation may contribute to Aβ overproduction in AD, and increased Aβ deposition might be caused by continuous stimuli in a chronic inflammatory context and/or by impaired microglial phagocytic capacity owed to natural aging or another process [150].
COVID-19
Based on our current knowledge of viral infections as well as neuroinflammation representing potential risk for AD onset and progression, the current pandemic caused by SARS-CoV-2 poses multiple questions with regards to its influence on AD onset and progression in individuals with COVID-19. Besides advanced age, cardiovascular disease and metabolic disorders constitute the biggest known risk factors for SARS-CoV-2 infection [160]. At the same time, Type 2 Diabetes (T2D) and neurovascular comorbidities lead to greater predisposition for AD development [161].
One of the characteristics of severe COVID-19 infection is the so called “cytokine storm” associated with higher systemic levels of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6 [162], all of which are also increased in elderly individuals. This systemic signature has been described by the term “inflamm-aging” (for more details on “inflamm-aging” see the dedicated section below) [163, 164]. A recent report suggests that elevated blood glucose levels resulting from T2D might aggravate AD and COVID-19 pathology through increased activity of Interferon regulatory factor 5 (IRF5), a regulator of the cytokine storm [165]. Interferons play a central role in the response to viral infection, causing the release of pro-inflammatory cytokines that enter the central nervous system and could contribute to the emergence of the microglial response observed from a case series of brain autopsies from patients with COVID-19 [166]; current evidence from PCR and immunohistochemistry studies suggests that SARS-CoV-2 can enter the brain but is predominantly detected in vascular and immune cells rather than neurons [166, 167]. In the brain, SARS-CoV-2 as well as IRF5 and pro-inflammatory cytokines might activate microglia leading to increased pro-inflammatory cytokine secretion [165]. However, the severity of neuroimmune activation did not seem to be associated with the presence of SARS-CoV-2 [166]. It is still too early to tell whether COVID-19 will have an impact on AD risk [165], but two recent independent retrospective cohort studies investigating neurological and psychological outcomes each in a comprehensive cohort of COVID-19 survivors report substantial neurological and psychiatric morbidity 6 months after COVID-19 infection [168, 169]. Further, a “long covid” neuropsychiatric syndrome is apparent in many individuals and is undergoing further characterization [170].
Overall, the implications of SARS-CoV-2 in regards to predisposition to neurodegeneration and AD development will become clearer over time. It is probable that SARS-CoV-2 exposure might contribute to AD development in vulnerable subpopulations, particularly in those which are at higher risk of COVID-19 and AD due to age, genetic predisposition or comorbidities.
Metabolic disorders
Metabolic disorders including obesity and T2D are described to pose an increased risk for AD development [171]. For T2D for instance, a dual role in increasing AD risk as well as in exacerbating neurological symptoms of AD has been proposed [172]. Insulin resistance as well as T2D have been shown to increase the risk for mild cognitive impairment and its progression to AD [173, 174]. Interestingly, a second substrate of insulin-degrading enzyme, that is genetically implicated in T2D and AD, is Aβ, and reduced degradation of Aβ has been reported in the context of excessive insulin circulation, providing an additional link between insulin resistance and AD [175, 176]. Moreover, hyperinsulinemia is a known risk factor for T2D which in turn is associated with a higher AD risk [177].
Adipose tissue of obese individuals, high fat diet as well as hypertension and T2D have all been linked to increased levels of pro-inflammatory cytokines including IL-6 and TNF-α [178–180] that suggest an impact on peripheral, chronic inflammation. Increased systemic levels of pro-inflammatory cytokines might in turn cause blood brain barrier leakage, entry of pro-inflammatory mediators, and microbial metabolites into the CNS, leading to chronic neuroinflammation via microglial activation. This, in turn, may favor neurodegeneration including AD potentially via Aβ accumulation [176, 181] or through acceleration of Tau proteinopathy [108]. Existing data illustrate the complex relationship between genetic risk factors, life style, comorbidities and AD development; the direct mechanisms by which metabolic disorders may cause changes in the immune system that favor the development of AD remain to be elucidated [176].
The role of the gut-brain axis in neuroimmune contributions to AD
In the context of metabolic diseases and environmental exposures, the gut-brain axis represents another important link whose role in health and disease has been increasingly studied in recent years. The gut-brain axis describes the complex communication between the enteric and the central nervous system using neural, endocrine and immune signals [182]. In fact, the gut microbiota, constitutes a major part of the gut-brain axis as it is a dynamic ecosystem that can be remodeled through environmental or lifestyle changes, through the aging process and many AD risk factors including T2D, obesity, chronic stress as well as through gut dysbiosis which has been associated with AD (Fig. 3) [176, 183]. These factors are interconnected; for example, reduced gut microbial diversity in obese people was reported to be associated with higher pro-inflammatory cytokine levels in peripheral blood cells [184]. Further, reduced gut biodiversity observed in elderly people, specifically of Bifidobacterium and Lactobacillus—species that are actively involved in the synthesis of aminobutyric acid (γ-Aminobutyric acid, GABA)—has been proposed to contribute to brain dysfunction associated with cognitive decline [185, 186]. Changes in the microbial composition of elderly people have been described by several studies, reporting a shift towards proteolytic as well as pro-inflammatory bacterial species (summarized in [185]). One of the first published studies showed an association between brain amyloidosis, peripheral inflammation, and increased pro-inflammatory as well as reduced anti-inflammatory gut microbiota in cognitively impaired elderly [187]. An increase in pro-inflammatory microbial species was further described in the 5xFAD mouse model and has been linked to increased APP levels in the brain as well as different districts of the gut, suggesting that the gut microbiota has the potential to modulate innate immunity, thereby affecting amyloidosis and AD progression [188]. One study characterizing the gut microbiome from AD patients reported reduced microbial diversity as well as changes in its composition including a reduction in Firmicutes and Bifidobacterium as well as an increase in Bacteroidetes when compared to age- and sex-matched controls. The authors further report a correlation between AD-specific CSF markers and the relative abundance of bacterial genera [189]. Mechanistically, information regarding the mechanisms by which the gut microbiome can influence the human brain remains sparse; however, it is known that the gut microbiota can synthesize and/or mimic a range of neuroimmune active substances including acetylcholine, melatonin, histamine, catecholamines, GABA, or 5-hydroxytryptamine (5-HT serotonin) [185, 190]. The murine literature offers a number of hypotheses such as effects on amyloid proteinopathy [183, 191, 192]. Moreover, intestinal bacteria can also secrete Lipopolysaccharide (LPS) and LPS levels in blood plasma of individuals with AD have been shown to be increased in comparison to age-matched controls [193], potentially causing inflammatory reactions in peripheral- as well as neuroimmune cells. In addition, gut bacteria were previously shown to regulate microglial development, homeostasis and function via short-chain fatty acids (SCFA), and microbiota-derived bacterial fermentation products, suggesting that a complex microbiota is required to maintain microglial function throughout life [194]. Interestingly, age-related changes in the human microbiome were also characterized by a loss of genes related to SCFA metabolism [195], which might directly impact microglia function. Similarly, microbial metabolites derived from dietary tryptophan have been shown to modulate the inflammatory state of astrocytes [196]. At the same time, age-related gut dysbiosis has been proposed to impact cognitive and behavioral changes perhaps through changes in gut permeability and increased peripheral inflammation, leading to neuroinflammation [197]. Given the results on reduced microbial diversity in human AD patients as well as their role in the synthesis of immunologically and neuroimmunologically active substances, one current hypothesis on how the gut microbiota might influence AD pathophysiology is through either directly affecting microglial function or via indirect effects modulated by the peripheral immune system. Along these lines, there is also evidence showing that host innate immunity can be modulated by remodeling the gut microbiota, leading to changes in amyloid deposition and neuronal plasticity, suggesting an interesting role for probiotics in AD treatment [176].
Sexual dimorphism in neuroinflammation and AD development
Women face a 1.6–3 fold higher risk of AD when compared to men [198–201]. While men diagnosed with AD seem to progress to death quicker, women with increased tau pathology can exhibit greater cognitive resilience [202, 203]. The underlying causes for sex differences in prevalence and vulnerability to AD are currently subject to a lively debate in the field [204], with an increasing amount of studies trying to delineate sex-specific clinical associations [205]. Among women, changes in hormonal exposure—such as early sudden, surgical menopause—are associated with more rapid cognitive decline and a greater burden of neuritic amyloid plaque on autopsy [206]. Higher risk for MCI or AD has also been associated with female carriers of the APOEε4 haplotype [207–209], connecting sex with a gene whose importance in microglial function has been increasingly investigated (as discussed above), and it connects microglia to hormonal and metabolic alterations associated with menopause [210, 211]. The implications of sexual dimorphism of neuroimmune cells on the development of AD as well as other neurodegenerative diseases, however, are only beginning to being explored. Recently, differences in microglial morphology in post-mortem parietal cortical tissue from AD patients have been described in relation to sex with microglia from male AD brains showing process retraction and ameboid morphology while female microglia were more complex, heterogenous with a morphology dominated by rod-shaped microglia [212]. Additionally, higher CD68 immunoreactivity was detected in the parietal cortex of male AD individuals when compared to female AD brain tissue, suggesting higher phagocytic activity [212]. Data focusing on sexual dimorphism of human astrocytes in the context of AD are currently not available. The function of both microglia and astrocytes appears to be influenced by sex, although most of the available data comes from the murine literature, and its relevance is not yet clear.
Differences in microglial transcriptional patterns related to sex have been described throughout the lifespan [213]. Microglia express steroid hormone receptors and are responsive to estrogen, testosterone, and other sex hormones; yet, the interplay of age, sex, and context make for a complex picture of sex-related altered function in mice that remains to be systematically evaluated, particularly in the context of proteinopathy models [214, 215]. Male or female microglia can be more “pro-inflammatory” depending on the condition being assessed [198, 213–220].
Neuro-immunosenescence and inflamm-aging in AD
With aging being the central risk factor for AD and many other forms of neurodegeneration, understanding cellular senescence might advance our understanding of the underlying causes for the emergence and progression of neurodegenerative diseases including AD. Immunosenescence—best illustrated by the decline in the proportion of individuals who mount a humoral response to vaccination with advancing age—is the gradual alteration of immune function and changes in the proportion of certain leukocytes, such as memory T cells, in older age [221]. It begins to become manifest in the fifth and sixth decades of life, when the earliest stages of AD are also appearing, and it is also influenced by comorbidities that influence AD, such as obesity and T2D [222–224]. However, the relation of immunosenescence to AD remains poorly characterized in humans. Further, the interaction of the aging peripheral leukocytes and aging resident CNS cells such as microglia and astrocytes remains unclear in humans.
Human immunologists have begun to pursue larger studies that will provide a robust description of those immune responses and cell subtypes that are changing with aging [225, 226]. This will then enable investigators to assess how these features of peripheral immunosenescence relate to AD. Understanding the resident CNS cells that contribute to immune responses is far more challenging since they are difficult to access; typically, they are only available from deceased individuals at autopsy or from excess neurosurgical samples. So, we do not have access to young “healthy” microglia and astrocytes, and we are left to disentangle the effects of age on these cells from those of the multiplicity of disease conditions and comorbidities that are found in older people or in individuals undergoing surgical resections for brain tumor or epilepsy. Nonetheless, it is clear that microglia and astrocytes change with age in humans. In one example, statistical modeling proposed different microglial transcriptional programs associated with aging, amyloid proteinopathy, and tau proteinopathy [108]. Datasets from purified microglia and microglial nuclei will also help to address this question, but the sample sizes are, as yet, too small and heterogeneous to clearly delineate the effect of aging in the absence of other factors [45, 227, 228]. Aged human microglia possess an activated morphology with some cells showing a dystrophic phenotype, referring to several abnormal changes in their cytoplasmic structure [229, 230]. Further, reduced process length, reduced arborization and less branching of microglia from human neocortex autopsy samples has been reported with aging which was enhanced in AD microglia when compared to age-matched control microglia [231]. In particular phagocytosis may be affected, as it is by certain AD susceptibility alleles, such as the one in CD33 [32, 232–234]. Interestingly, microglia may age in a region-dependent manner, suggesting region-specific microglial sensitivity to dysregulation and involvement in neurodegeneration might exist [235]. This highlights the need for large-scale, systematic evaluations of microglial senescence in humans.
Using p16INK4a as a marker for cellular senescence, a study identified a significant population of p16INK4a positive astrocytes in the frontal cortex of AD patients when compared to age-matched non-AD control subjects, suggesting a link between increased age of astrocytes and risk for sporadic AD [236]. More broadly, reactive astrocytes have been described in specific brain regions in aging brains that are the primary targets for synaptic loss or age-related cognitive decline, such as the hippocampus and frontal cortex. These astrocytes are characterized by increased expression of genes related to immune responses and synapse elimination [237, 238]. These and other studies suggest a phenomenon of astrosenescence as being relevant to AD, but it will require much more extensive investigation to characterize it in humans.
Modeling of neuroimmune contributions to AD
With the advancement of in vitro models including iPSC-derived CNS cell types as well as organoids, options for more complex model systems than monocultures of human cell lines are growing rapidly. Relevant in vitro models are aiming to recapitulate Aβ accumulation, p-tau aggregation as well as neuroinflammation [239]. We are still at the stage of identifying the individual elements of immune responses involved in AD, but the rudimentary human model systems provide an important early foundation with which to iteratively improve our understanding by testing hypotheses emerging from human association studies.
Three dimensional human neural cell culture model systems for AD with characteristics of Aβ deposition and p-tau have started to emerge [240]; however, they do not allow one to fully model the interactions of different cell types in disease [241]. One of the first models incorporating the neuroinflammatory component of AD consisted of a human AD triculture system comprised of neurons and astrocytes differentiated from human neural precursor cells (hNPCs) and an SV40- immortalized human microglial cell line using a microfluidic platform [239]. Through the overexpression of human Aβ in hNPCs, pathological AD signatures including Aβ deposition, p-tau as well as high levels of IFN-y were recapitulated, and microglial migration, pro-inflammatory cytokine secretion as well as microglia-induced neuronal loss through IFN-y and TLR4-dependent mechanisms were observed [239]. As suggested by the authors, instead of using an immortalized microglial cell line for future 3D culture models, iPSC-derived microglia including from lines bearing AD-risk associated genotypes such as TREM2 or CD33 could further improve current 3D AD culture models.
Within the last few years, several different protocols for the generation of primary human microglia to study neurodegeneration in vitro were published including monocyte-derived microglia [242–244] as well as more recently iPSC-derived microglia [245–247]. Targeted gene-editing of iPSC-derived microglia using CRISPR/Cas9 technology to study the effects of AD-risk genes will further provide novel insights on the role of microglia in AD. Moreover, human iPSC-derived microglia were also successfully integrated into mouse brains, offering novel routes for studying the role of human microglia in neurodegeneration in vivo [248, 249].
Studying neuroimmunological contributions to AD in vivo
The more than 200 murine “AD models”—more appropriately referred to as accelerated proteinopathy models or mice carrying an AD risk allele discovered in humans—will play an important role in helping investigators dissect molecularly specific questions in vivo, but they also have several important limitations. Since this is not a focus of this review, we direct readers to an overview of the current animal modeling landscape for AD, the difficulties the field faces with the lack of standardized approaches for reliable comparison of results obtained from different model systems as well the question of translatability [250]. Another review complements the first one, delving into detailed information on the technical details of existing model systems as well as on the cognitive tests used to assess cognitive impairment in existing models [251]. Overall, caution is recommended in interpreting results from murine studies, particularly those that go beyond testing very focused, mechanistic questions given the many differences between mouse and human neuroimmune biology.
The existing possibilities to study the immune responses involved in human AD in vivo, while still limited, are growing rapidly and need to be harnessed at the proper scale to address key questions such as the sequence of events along the causal chain leading from AD risk factors to a clinical syndrome. We need to precisely map in which step of this sequence a given immune response plays a role. Current tools consist of either nuclear medicine-based imaging techniques or the assessment of CSF- or blood-derived biomarkers for diagnosis or monitoring of AD progression.
Positron emission tomography (PET) scans using a selective ligand for Translocator Protein, 18 kDa, a microglial marker, or Monoamine Oxidase B, an astrocytic marker, are being performed, and the reagents are evolving quickly, with third-generation ligands now being used [252]. However, their properties need better definition in large-scale studies. They will be very important in helping to validate possible causal relationships that emerge from autopsy studies; in one example, the relation of amyloid proteinopathy, tau proteinopathy, microglial activation, and other factors such as olfactory loss were assessed together to map the interconnections of these factors in cognitive decline [253, 254]. Similarly, as reduced glucose transport constitutes a feature of AD that might even precede neurodegeneration and brain atrophy in AD, F-2-fluoro-2-deoxy-d-glucose-PET for functional imaging could be another important tool to deploy [89]. However, new ligands are definitely needed, and the rapidly enriching set of single cell/nucleus data should help to highlight new target proteins for ligand development.
CSF characterization is already an important component of clinical evaluations in AD, with validated biomarkers such as Aβ1–42, T-tau, and P-tau181, and an emerging set of immune-related biomarkers such as sTREM2, GFAP, YKL-40 and others are now being tested at reasonable scale so that we can begin to delineate exactly where, in the AD sequence of events, an individual marker is most relevant [255]. This study also illustrates the ongoing translation of markers from CSF to blood, which will allow studies to be pursued at much larger scale, although understanding in which context to deploy serum vs. CSF biomarkers remains a critical question [256]. Other reviews summarize this rapidly evolving field in more detail [257]; overall, no single immune response marker will be sufficient given the multiplicity of ways in which the immune system may be involved in AD. Further, the fact that multiple processes are ongoing in parallel in the aging brain means that the development of an optimal immune response panel will involve a multiplicity of targets and most likely both, blood and CSF sampling.
Therapeutic strategies
To date, clinical trials have failed to show prevention of the onset or a reduction in the progression of AD. Recently, Aducanumab, a human monoclonal antibody directed against a conformational epitope exclusively found on toxic Aβ oligomers has been granted accelerated approval by the United States Food and Drug Administration without a successful Phase III trial. Aducanumab was shown to reduce plaque size and plaque burden and increased plaque clearance along with reduced cognitive decline in the AD mouse model Tg2576 as well as in Phase I clinical trials [258], suggesting that the observed effects of Aducanumab might be at least partially mediated by microglia. This is of particular interest, as it suggests that even an anti-amyloid antibody might indirectly modulate the neuroimmune response in AD. Other data, however, suggest that Aducanumab might act mainly through restoring intracellular calcium homeostasis, thereby reestablishing neuronal network function [259]. However, the failure to meet primary endpoints in the pivotal trials highlight the fact that our current trial designs need further refinement, that we do not fully understand the sequence of events leading to AD, and that we need a broader range of targets to pursue [260]. The latter message has been heeded in the past decade, as evidenced by the Accelerating Medicines Partnership for AD (an effort to diversify the portfolio of AD targets funded by the National Institute of Aging and pharma partners) and its Agora site presenting assembled data supporting a range of different targets, with an important component of immune-related targets https://agora.ampadportal.org/genes/genes-router:genes-list [261]. Further, there has also been an effort in Pharma to ground therapeutic development efforts in targets emerging from human genetic studies given that some studies suggest greater success in development efforts for such targets [262, 263]. In AD, this has led to intense interest on the immune-related AD susceptibility genes, but even in the case of TREM2, where the target gene was evident from the beginning, or CD33, where the pathogenic mechanism was described early, such “validated” targets remain far from the targets typically pursued by industry. This has led to other efforts such as the PHAGO consortium [264] funded by Pharma and the European Union to develop the foundation of functional characterization and robust tools needed for target validation and, ultimately, the range of clinical tools needed to assess target engagement, in vivo mechanism, and clinical efficacy. A more detailed overview on selected promising immune targets for manipulating AD pathology including TREM2, CD33, CR1, CD36, CX3CR1 among others is provided elsewhere [265].
Genetic studies highlighting immune responses as targets in AD are further supported by epidemiological evidence reporting associations between the chronic use of non-steroidal anti-inflammatory drugs and a lower incidence of AD or the link with inflammatory diseases such as rheumatoid arthritis and AD or an intriguing reduction of AD among individuals with leprosy treated with Dapsone [266–270]. Thus, it is likely that a diversity of target genes and approaches will be useful and that combination of treatments is almost certainly what will ultimately be needed to manage AD like T2D is managed today. Some drugs may directly interact with genetically defined targets, but others may well emerge from the efforts described above to modulate the gut microbiota or modulate pathways involved in the estrogen and related hormonal observations in AD [271–274]. For biologists, it is important to realize that drug development is an iterative process that goes hand-in-hand with biomarker development and an understanding of the underlying heterogeneity of the target population: the path forward will go through a detailed characterization of the immune responses involved in the trajectory of the disease and likely modulation not outright suppression or activation of immune responses over time to slow down or ideally stop the progression of disease.
A moment of opportunity
The renaissance of interest in the immune component of AD has converged with the deployment of high-dimensional data generation and analysis at proper scale over the past decade to establish a framework of robust, reproducible observations. This framework based on human samples remains sparse today, but it provides a critical foundation for the design of the next generation of studies which will require large-scale, multi-center studies to yield definitive results. As noted above, study designs also have to become more precise, measuring critical endophenotypes to enable us to understand the temporal sequence of events and to deconstruct the multiplicity of immune responses involved in different aspects of AD that are occurring concurrently in the aging brain. Molecular profiles based on samples of bulk tissue have their uses, but the accelerating pace of single cell work—particularly in ways that capture spatial patterns—will provide the next inflection point in our understanding of cellular communities in the CNS parenchyma. Classical histological studies have clearly established the existence of certain microenvironments, but our understanding of their molecular composition remains limited without highly multiplexed data capture from individual cells. Understanding the composition of and inter-cellular communication within these communities is one of our key tasks in the short term to identify those molecular signals that could be modulated as part of a pharmacological toolkit to slow or ultimately halt the progression of AD.
A wealth of observations has accumulated over the last three decades in the field of AD that are related to or potentially influence immune responses involved in the disease. In this review, we sought to assemble a broad perspective of our current understanding to highlight the fact that (1) immune responses in AD involve many cell types beyond the microglia that have garnered most of the attention, (2) that these responses are highly plastic, evolving based on communications from nearby cells and the broader state of the immune system of an individual, (3) that we lack many of the tools that are needed to map and monitor immune responses in living subjects, especially in earlier stages of disease, (4) that many existing studies are under-powered, yielding a confusing mass of contradictory reports, (5) that comorbidities and life experiences such as infections are likely to play a role, (6) that different immune responses appear to be involved at different stages along the path to AD and (7) that aging has a fundamental and multifactorial impact on the cells and the kinetics of immune responses. We therefore want to emphasize that there will not be a one-size-fits-all solution: AD will not be amenable to a single solution where a deleterious immune cell population is eliminated. Rather, the model for future intervention will resemble therapeutic approaches to inflammatory diseases in which a large toolkit of immunomodulatory agents tweak an immune response by the correct amount in a selected subset of patients who are at the appropriate stage of the disease for a given agent. Simply put, one can think of this task as using an “immune rheostat” that can be dialed up and down to address a specific task, such as limiting amyloid proteinopathy, with a series of additional rheostats to modulate other processes such as tau proteinopathy or synaptic loss; each rheostat can be engaged alone or in combination depending on the state of the patient. Further, this conceptual model highlights the need to achieve a delicate balance with highly targeted immunomodulation, as excessive correction will create adverse events and a broadly acting agent will likely engage different off-target effects. Finally, it also highlights the urgent need for a large toolkit of biomarkers with which to precisely measure the state of immune responses within and without the CNS.
Our understanding of murine neuroimmunology has expanded dramatically and much more extensively than its human counterpart given the lifespan of mice and the many genetic tools available for murine studies. These insights are very valuable, but, as seen with classical inflammatory diseases, there are many differences between the mouse and human immune systems and probably even more differences with how these immune systems engage with their respective CNS given the complexity of the human brain. Here, we elected to focus primarily on a review of human observations, bringing in selected insights from murine studies. Integrating the two sets of observations is a vastly important task, but we did not pursue it here since we sought to establish a framework for human studies. While murine studies will continue to play an important role, they need to be much more grounded in human biology to be relevant: the best studies today dissect mechanistic questions that we cannot tackle in human participants and are very constrained in their interpretation.
Overall, we are at an exciting conjecture in the study of immune responses in AD: despite the many challenges that we face, we have a large kit of new tools that will enable rapid progress in coming years. And, we have the aggregate experience of modulating the immune system for the treatment of inflammatory diseases, management of transplantation, and, increasingly, cancer therapy. While early evidence suggests that many of these existing agents may not be relevant to AD, developing new therapeutic agents will proceed quickly given this experience, and, similarly, biomarker development for more accurate outcome measures should proceed rapidly. Some early agents are already in clinical trials, and this is essential to grow our understanding of immunomodulation in AD, even if they are unsuccessful. Early translation to human studies is essential and promises a breakthrough in coming years, with new therapies that engage specific CNS immune responses likely to emerge in the next five years.
Acknowledgements
We thank Dr. Korhan Buyukturkoglu for providing us with the MR images used for completion of Fig. 3.
Author contributions
VH and PLD conceptualized the review, performed a critical review of the literature, wrote, edited, and revised the manuscript. VH created the figures which were edited by PLD.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the national institute on aging task force on comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62:275–80. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44:1055–68. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein H-U, Trumpff C, Yang H-S, Lee AJ, Picard M, Bennett DA, et al. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain. Molecular. Neurodegeneration. 2021;16:75. doi: 10.1186/s13024-021-00495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chornenkyy Y, Fardo DW, Nelson PT. Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Investig. 2019;99:993–1007. doi: 10.1038/s41374-019-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visanji NP, Lang AE, Kovacs GG. Beyond the synucleinopathies: alpha synuclein as a driving force in neurodegenerative comorbidities. Transl Neurodegener. 2019;8:28. doi: 10.1186/s40035-019-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moussaud S, Jones DR, Moussaud-Lamodière EL, Delenclos M, Ross OA, McLean PJ. Alpha-synuclein and tau: teammates in neurodegeneration? Mol Neurodegener. 2014;9:43. doi: 10.1186/1750-1326-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HS, White CC, Klein HU, Yu L, Gaiteri C, Ma Y, et al. Genetics of gene expression in the aging human brain reveal TDP-43 proteinopathy pathophysiology. Neuron. 2020;107:496–508.e496. doi: 10.1016/j.neuron.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabin JS, Yang HS, Schultz AP, Hanseeuw BJ, Hedden T, Viswanathan A, et al. Vascular risk and β-amyloid are synergistically associated with cortical tau. Ann Neurol. 2019;85:272–9. doi: 10.1002/ana.25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 10.Möller HJ, Graeber MB. The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci. 1998;248:111–22. doi: 10.1007/s004060050027. [DOI] [PubMed] [Google Scholar]
- 11.yCajal SR. Contribucion al conocimiento de la neuroglia del cerebro humano, 1913.
- 12.Del Rıo Hortega P. El ‘tercer elemento’de los centros nerviosos. I. La microglıa en estado normal. Bol Soc Esp Biol. 1920;8:68–92. [Google Scholar]
- 13.Sierra A, Paolicelli RC, Kettenmann H. Cien Anos de microbial: milestones in a century of microbial research. Trends Neurosci. 2019;42:778–92. doi: 10.1016/j.tins.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 14.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 15.Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9:339–49. doi: 10.1016/S0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 16.Sue W, Griffin T, Stanley LC, Ling C, White L, MacLeod V, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–5. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert JC, Grenier-Boley B, Chouraki V, Heath S, Zelenika D, Fievet N, et al. Implication of the immune system in Alzheimer’s disease: evidence from genome-wide pathway analysis. J Alzheimer’s Dis. 2010;20:1107–18. doi: 10.3233/JAD-2010-100018. [DOI] [PubMed] [Google Scholar]
- 21.Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344:519–23. doi: 10.1126/science.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–20. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5:e13950. doi: 10.1371/journal.pone.0013950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wes PD, Sayed FA, Bard F, Gan L. Targeting microglia for the treatment of Alzheimer’s disease. Glia. 2016;64:1710–32. doi: 10.1002/glia.22988. [DOI] [PubMed] [Google Scholar]
- 25.Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49:1373–84. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettens K, Brouwers N, Engelborghs S, De Deyn PP, Van Broeckhoven C, Sleegers K. SORL1 is genetically associated with increased risk for late-onset Alzheimer disease in the Belgian population. Hum Mutat. 2008;29:769–70. doi: 10.1002/humu.20725. [DOI] [PubMed] [Google Scholar]
- 27.Pottier C, Hannequin D, Coutant S, Rovelet-Lecrux A, Wallon D, Rousseau S, et al. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry. 2012;17:875–9. doi: 10.1038/mp.2012.15. [DOI] [PubMed] [Google Scholar]
- 28.Cruchaga C, Karch CM, Jin SC, Benitez BA, Cai Y, Guerreiro R, et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature. 2014;505:550–4. doi: 10.1038/nature12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao X, Cai F, Sun Z, Zhang Y, Wang J, Jiao B, et al. Identification of Alzheimer’s disease-associated rare coding variants in the ECE2 gene. JCI Insight. 2020;5:e135119. doi: 10.1172/jci.insight.135119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Multiple Sclerosis Genetics Consortium Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365:eaav7188. doi: 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felsky D, Patrick E, Schneider JA, Mostafavi S, Gaiteri C, Patsopoulos N, et al. Polygenic analysis of inflammatory disease variants and effects on microglia in the aging brain. Mol Neurodegener. 2018;13:38. doi: 10.1186/s13024-018-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–50. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Replogle JM, Chan G, White CC, Raj T, Winn PA, Evans DA, et al. A TREM1 variant alters the accumulation of Alzheimer-related amyloid pathology. Ann Neurol. 2015;77:469–77. doi: 10.1002/ana.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleineidam L, Chouraki V, Próchnicki T, van der Lee SJ, Madrid-Márquez L, Wagner-Thelen H, et al. PLCG2 protective variant p.P522R modulates tau pathology and disease progression in patients with mild cognitive impairment. Acta Neuropathol. 2020;139:1025–44. doi: 10.1007/s00401-020-02138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Yu J. Genome-wide association studies for cerebrospinal fluid soluble TREM2 in Alzheimer’s disease. Front Aging Neurosci. 2019;11:297. doi: 10.3389/fnagi.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan G, White CC, Winn PA, Cimpean M, Replogle JM, Glick LR, et al. CD33 modulates TREM2: convergence of Alzheimer loci. Nat Neurosci. 2015;18:1556–8. doi: 10.1038/nn.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang K-l, Marcora E, Pimenova AA, Di Narzo AF, Kapoor M, Jin SC, et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat Neurosci. 2017;20:1052–61. doi: 10.1038/nn.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raj T, Shulman JM, Keenan BT, Chibnik LB, Evans DA, Bennett DA, et al. Alzheimer disease susceptibility loci: evidence for a protein network under natural selection. Am J Hum Genet. 2012;90:720–6. doi: 10.1016/j.ajhg.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ní Chasaide C, Lynch MA. The role of the immune system in driving neuroinflammation. Brain Neurosci Adv. 2020;4:2398212819901082. doi: 10.1177/2398212819901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–.e386. doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Thome AD, Faridar A, Beers DR, Thonhoff JR, Zhao W, Wen S, et al. Functional alterations of myeloid cells during the course of Alzheimer’s disease. Mol Neurodegener. 2018;13:61. doi: 10.1186/s13024-018-0293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S-H, Tian D-Y, Shen Y-Y, Cheng Y, Fan D-Y, Sun H-L, et al. Amyloid-beta uptake by blood monocytes is reduced with ageing and Alzheimer’s disease. Transl Psychiatry. 2020;10:423. doi: 10.1038/s41398-020-01113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galatro TF, Holtman IR, Lerario AM, Vainchtein ID, Brouwer N, Sola PR, et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat Neurosci. 2017;20:1162–71. doi: 10.1038/nn.4597. [DOI] [PubMed] [Google Scholar]
- 44.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–7. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olah M, Menon V, Habib N, Taga MF, Ma Y, Yung CJ, et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat Commun. 2020;11:6129. doi: 10.1038/s41467-020-19737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6208–20. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–8. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Horiba M, Buescher JL, Huang D, Gendelman HE, Ransohoff RM, et al. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–85. doi: 10.1016/S0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unger MS, Schernthaner P, Marschallinger J, Mrowetz H, Aigner L. Microglia prevent peripheral immune cell invasion and promote an anti-inflammatory environment in the brain of APP-PS1 transgenic mice. J Neuroinflammation. 2018;15:274. doi: 10.1186/s12974-018-1304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jay TR, Hirsch AM, Broihier ML, Miller CM, Neilson LE, Ransohoff RM, et al. Disease progression-dependent effects of TREM2 deficiency in a mouse model of Alzheimer’s disease. J Neurosci. 2017;37:637–47. doi: 10.1523/JNEUROSCI.2110-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shukla AK, McIntyre LL, Marsh SE, Schneider CA, Hoover EM, Walsh CM, et al. CD11a expression distinguishes infiltrating myeloid cells from plaque-associated microglia in Alzheimer’s disease. Glia. 2019;67:844–56. doi: 10.1002/glia.23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. Faseb j. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 53.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–32. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, et al. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017;77:2266–78. doi: 10.1158/0008-5472.CAN-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly RJ, Minogue AM, Lyons A, Jones RS, Browne TC, Costello DA, et al. Glial activation in AβPP/PS1 mice is associated with infiltration of IFNγ-producing cells. J Alzheimer’s Dis. 2013;37:63–75. doi: 10.3233/JAD-130539. [DOI] [PubMed] [Google Scholar]
- 57.Minogue AM, Jones RS, Kelly RJ, McDonald CL, Connor TJ, Lynch MA. Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol Aging. 2014;35:1442–52. doi: 10.1016/j.neurobiolaging.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 58.Itagaki S, McGeer PL, Akiyama H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci Lett. 1988;91:259–64. doi: 10.1016/0304-3940(88)90690-8. [DOI] [PubMed] [Google Scholar]
- 59.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol. 2002;124:83–92. doi: 10.1016/S0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 60.Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577:399–404. doi: 10.1038/s41586-019-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Busse M, Michler E, von Hoff F, Dobrowolny H, Hartig R, Frodl T, et al. Alterations in the peripheral immune system in dementia. J Alzheimer’s Dis. 2017;58:1303–13. doi: 10.3233/JAD-161304. [DOI] [PubMed] [Google Scholar]
- 62.Dhanwani R, Pham J, Premlal ALR, Frazier A, Kumar A, Pero ME, et al. T cell responses to neural autoantigens are similar in Alzheimer’s disease patients and age-matched healthy controls. Front Neurosci. 2020;14:874. doi: 10.3389/fnins.2020.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merlini M, Kirabali T, Kulic L, Nitsch RM, Ferretti MT. Extravascular CD3+ T cells in brains of alzheimer disease patients correlate with tau but not with amyloid pathology: an immunohistochemical study. Neurodegener Dis. 2018;18:49–56. doi: 10.1159/000486200. [DOI] [PubMed] [Google Scholar]
- 64.Unger MS, Li E, Scharnagl L, Poupardin R, Altendorfer B, Mrowetz H, et al. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain, Behav, Immun. 2020;89:67–86. doi: 10.1016/j.bbi.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 65.Richartz-Salzburger E, Batra A, Stransky E, Laske C, Köhler N, Bartels M, et al. Altered lymphocyte distribution in Alzheimer’s disease. J Psychiatr Res. 2007;41:174–8. doi: 10.1016/j.jpsychires.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Pellicanò M, Larbi A, Goldeck D, Colonna-Romano G, Buffa S, Bulati M, et al. Immune profiling of Alzheimer patients. J Neuroimmunol. 2012;242:52–59. doi: 10.1016/j.jneuroim.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Leal-Lasarte M, Mannini B, Chiti F, Vendruscolo M, Dobson CM, Roodveldt C, et al. Distinct responses of human peripheral blood cells to different misfolded protein oligomers. Immunology. 2021;164:358–71. doi: 10.1111/imm.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciccocioppo F, Lanuti P, Pierdomenico L, Simeone P, Bologna G, Ercolino E, et al. The characterization of regulatory T-cell profiles in Alzheimer’s disease and multiple sclerosis. Sci Rep. 2019;9:8788. doi: 10.1038/s41598-019-45433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larbi A, Pawelec G, Witkowski JM, Schipper HM, Derhovanessian E, Goldeck D, et al. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J Alzheimer’s Dis. 2009;17:91–103. doi: 10.3233/JAD-2009-1015. [DOI] [PubMed] [Google Scholar]
- 70.Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–51. doi: 10.1093/brain/awv408. [DOI] [PubMed] [Google Scholar]
- 71.Faridar A, Thome AD, Zhao W, Thonhoff JR, Beers DR, Pascual B, et al. Restoring regulatory T-cell dysfunction in Alzheimer’s disease through ex vivo expansion. Brain Commun. 2020;2:fcaa112. doi: 10.1093/braincomms/fcaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giubilei F, Antonini G, Montesperelli C, Sepe-Monti M, Cannoni S, Pichi A, et al. T cell response to amyloid-beta and to mitochondrial antigens in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16:35–38. doi: 10.1159/000069991. [DOI] [PubMed] [Google Scholar]
- 73.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Investig. 2003;112:415–22. doi: 10.1172/JCI200318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oberstein TJ, Taha L, Spitzer P, Hellstern J, Herrmann M, Kornhuber J, et al. Imbalance of circulating Th17 and regulatory T cells in Alzheimer’s disease: a case control study. Front Immunol. 2018;9:1213. doi: 10.3389/fimmu.2018.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aliseychik M, Patrikeev A, Gusev F, Grigorenko A, Andreeva T, Biragyn A, et al. Dissection of the human T-cell receptor γ gene repertoire in the brain and peripheral blood identifies age- and Alzheimer’s disease-associated clonotype profiles. Front Immunol. 2020;11:12. doi: 10.3389/fimmu.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lutshumba J, Nikolajczyk BS, Bachstetter AD. Dysregulation of systemic immunity in aging and dementia. Front Cell Neurosci. 2021;15:652111. doi: 10.3389/fncel.2021.652111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100:375–88. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology. 2019;65:106–19. doi: 10.1159/000490349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111–147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–9. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mentis A-FA, Dardiotis E, Chrousos GP. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: a conceptual framework. Mol Psychiatry. 2021;26:1075–97. doi: 10.1038/s41380-020-0731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 84.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16:137–53. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 85.Yang T, Guo R, Zhang F. Brain perivascular macrophages: Recent advances and implications in health and diseases. CNS Neurosci Ther. 2019;25:1318–28. doi: 10.1111/cns.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA. 2009;106:1261–6. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thanopoulou K, Fragkouli A, Stylianopoulou F, Georgopoulos S. Scavenger receptor class B type I (SR-BI) regulates perivascular macrophages and modifies amyloid pathology in an Alzheimer mouse model. Proc Natl Acad Sci USA. 2010;107:20816–21. doi: 10.1073/pnas.1005888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park L, Uekawa K, Garcia-Bonilla L, Koizumi K, Murphy M, Pistik R, et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ Res. 2017;121:258–69. doi: 10.1161/CIRCRESAHA.117.311054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lau S-F, Cao H, Fu AKY, Ip NY. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc Natl Acad Sci. 2020;117:25800–9. doi: 10.1073/pnas.2008762117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature. 2019;571:205–10. doi: 10.1038/s41586-019-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferreira IL, Resende R, Ferreiro E, Rego AC, Pereira CF. Multiple defects in energy metabolism in Alzheimer’s disease. Curr Drug Targets. 2010;11:1193–206. doi: 10.2174/1389450111007011193. [DOI] [PubMed] [Google Scholar]
- 93.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Investig. 2012;122:1377–92. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pflanzner T, Kuhlmann CR, Pietrzik CU. Blood-brain-barrier models for the investigation of transporter- and receptor-mediated amyloid-β clearance in Alzheimer’s disease. Curr Alzheimer Res. 2010;7:578–90. doi: 10.2174/156720510793499066. [DOI] [PubMed] [Google Scholar]
- 95.Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a011452. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD, Miller MC, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–15. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 97.Oikari LE, Pandit R, Stewart R, Cuní-López C, Quek H, Sutharsan R, et al. Altered brain endothelial cell phenotype from a familial alzheimer mutation and its potential implications for amyloid clearance and drug delivery. Stem Cell Rep. 2020;14:924–39. doi: 10.1016/j.stemcr.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cain A, Taga M, McCabe C, Hekselman I, White CC, Green G, et al. Multi-cellular communities are perturbed in the aging human brain and with Alzheimer’s disease. bioRxiv 2020: 2020.2012.2022.424084.
- 99.Ponath G, Park C, Pitt D. The role of astrocytes in multiple sclerosis. Front Immunol. 2018;9:217. doi: 10.3389/fimmu.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Priego N, Valiente M. The potential of astrocytes as immune modulators in brain tumors. Front Immunol. 2019;10:1314. doi: 10.3389/fimmu.2019.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medeiros R, LaFerla FM. Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol. 2013;239:133–8. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58:831–8. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 104.Verkhratsky A, Rodrigues JJ, Pivoriunas A, Zorec R, Semyanov A. Astroglial atrophy in Alzheimer’s disease. Pflug Arch. 2019;471:1247–61. doi: 10.1007/s00424-019-02310-2. [DOI] [PubMed] [Google Scholar]
- 105.Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seyfried NT, Dammer EB, Swarup V, Nandakumar D, Duong DM, Yin L, et al. A multi-network approach identifies protein-specific co-expression in asymptomatic and symptomatic Alzheimer’s disease. Cell Syst. 2017;4:60–72.e64. doi: 10.1016/j.cels.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan YW, Al-Ouran R, Mangleburg CG, Perumal TM, Lee TV, Allison K, et al. Meta-analysis of the Alzheimer’s disease human brain transcriptome and functional dissection in mouse models. Cell Rep. 2020;32:107908. doi: 10.1016/j.celrep.2020.107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patrick E, Olah M, Taga M, Klein HU, Xu J, White CC, et al. A cortical immune network map identifies distinct microglial transcriptional programs associated with beta-amyloid and Tau pathologies. Transl Psychiatry. 2021;11:50. doi: 10.1038/s41398-020-01175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci. 2016;17:201–7. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 110.Kleinberger G, Brendel M, Mracsko E, Wefers B, Groeneweg L, Xiang X, et al. The FTD-like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. Embo J. 2017;36:1837–53. doi: 10.15252/embj.201796516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deming Y, Filipello F, Cignarella F, Cantoni C, Hsu S, Mikesell R, et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci Transl Med. 2019;11:eaau2291. doi: 10.1126/scitranslmed.aau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piers TM, Cosker K, Mallach A, Johnson GT, Guerreiro R, Hardy J, et al. A locked immunometabolic switch underlies TREM2 R47H loss of function in human iPSC-derived microglia. FASEB j. 2020;34:2436–50. doi: 10.1096/fj.201902447R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, et al. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2) J Biol Chem. 2015;290:26043–50. doi: 10.1074/jbc.M115.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 115.Rangaraju S, Dammer EB, Raza SA, Gao T, Xiao H, Betarbet R, et al. Quantitative proteomics of acutely-isolated mouse microglia identifies novel immune Alzheimer’s disease-related proteins. Mol Neurodegener. 2018;13:34. doi: 10.1186/s13024-018-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olah M, Patrick E, Villani A-C, Xu J, White CC, Ryan KJ, et al. A transcriptomic atlas of aged human microglia. Nat Commun. 2018;9:539. doi: 10.1038/s41467-018-02926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernandez CG, Hamby ME, McReynolds ML, Ray WJ. The role of APOE4 in disrupting the homeostatic functions of astrocytes and microglia in aging and Alzheimer’s disease. Front Aging Neurosci. 2019;11:14. doi: 10.3389/fnagi.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–43. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch Neurol. 2010;67:1473–84. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Andreone BJ, Przybyla L, Llapashtica C, Rana A, Davis SS, van Lengerich B, et al. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat Neurosci. 2020;23:927–38. doi: 10.1038/s41593-020-0650-6. [DOI] [PubMed] [Google Scholar]
- 121.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–66. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoeijmakers L, Heinen Y, van Dam A-M, Lucassen PJ, Korosi A. Microglial priming and Alzheimer’s disease: a possible role for (early) immune challenges and epigenetics? Front Hum Neurosci. 2016;10:398–398. doi: 10.3389/fnhum.2016.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neher JJ, Cunningham C. Priming microglia for innate immune memory in the brain. Trends Immunol. 2019;40:358–74. doi: 10.1016/j.it.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 126.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–7. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 127.Galloway DA, Phillips AEM, Owen DRJ, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790. doi: 10.3389/fimmu.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Felsky D, Roostaei T, Nho K, Risacher SL, Bradshaw EM, Petyuk V, et al. Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun. 2019;10:409. doi: 10.1038/s41467-018-08279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vogels T, Murgoci A-N, Hromádka T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Commun. 2019;7:109. doi: 10.1186/s40478-019-0754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rexach JE, Polioudakis D, Yin A, Swarup V, Chang TS, Nguyen T, et al. Tau pathology drives dementia risk-associated gene networks toward chronic inflammatory states and immunosuppression. Cell Rep. 2020;33:108398. doi: 10.1016/j.celrep.2020.108398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–.e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 132.Rozemuller JM, Eikelenboom P, Pals ST, Stam FC. Microglial cells around amyloid plaques in Alzheimer’s disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci Lett. 1989;101:288–92. doi: 10.1016/0304-3940(89)90547-8. [DOI] [PubMed] [Google Scholar]
- 133.Dionisio-Santos DA, Olschowka JA, O’Banion MK. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation. 2019;16:74. doi: 10.1186/s12974-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, et al. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1991;285:111–4. doi: 10.1016/0014-5793(91)80737-N. [DOI] [PubMed] [Google Scholar]
- 135.Park JC, Han SH, Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer’s disease: a brief review. BMB Rep. 2020;53:10–19. doi: 10.5483/BMBRep.2020.53.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anoop A, Singh PK, Jacob RS, Maji SK. CSF biomarkers for alzheimer’s disease diagnosis. Int J Alzheimer’s Dis. 2010;2010:606802. doi: 10.4061/2010/606802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu Y-Y, Hsu J-L, Wang H-C, Wu S-J, Hong C-J, Cheng IH-J. Alterations of the neuroinflammatory markers IL-6 and TRAIL in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2015;5:424–34. doi: 10.1159/000439214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eriksson UK, Pedersen NL, Reynolds CA, Hong MG, Prince JA, Gatz M, et al. Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer’s disease and dementia. J Alzheimer’s Dis. 2011;23:361–9. doi: 10.3233/JAD-2010-101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 2018;8:12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Locascio JJ, Fukumoto H, Yap L, Bottiglieri T, Growdon JH, Hyman BT, et al. Plasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer disease. Arch Neurol. 2008;65:776–85. doi: 10.1001/archneur.65.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Watanabe Y, Kitamura K, Nakamura K, Sanpei K, Wakasugi M, Yokoseki A, et al. Elevated C-reactive protein is associated with cognitive decline in outpatients of a general hospital: the Project in Sado for Total Health (PROST) Dement Geriatr Cogn Dis Extra. 2016;6:10–19. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vintimilla R, Hall J, Johnson L, O’Bryant S. The relationship of CRP and cognition in cognitively normal older Mexican Americans: a cross-sectional study of the HABLE cohort. Medicines. 2019;98:e15605. doi: 10.1097/MD.0000000000015605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yarchoan M, Louneva N, Xie SX, Swenson FJ, Hu W, Soares H, et al. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J Neurol Sci. 2013;333:9–12. doi: 10.1016/j.jns.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sjogren T, Sjogren H, Lindgren AG. Morbus Alzheimer and morbus Pick; a genetic, clinical and patho-anatomical study. Acta Psychiatr Neurol Scand Suppl. 1952;82:1–152. [PubMed] [Google Scholar]
- 145.Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, et al. Microbes and Alzheimer’s disease. J Alzheimer’s Dis. 2016;51:979–84. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mastroeni D, Nolz J, Sekar S, Delvaux E, Serrano G, Cuyugan L, et al. Laser-captured microglia in the Alzheimer’s and Parkinson’s brain reveal unique regional expression profiles and suggest a potential role for hepatitis B in the Alzheimer’s brain. Neurobiol Aging. 2018;63:12–21. doi: 10.1016/j.neurobiolaging.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, et al. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci. 2009;119:765–77. doi: 10.1080/00207450902782083. [DOI] [PubMed] [Google Scholar]
- 148.Sochocka M, Zwolińska K, Leszek J. The infectious etiology of Alzheimer’s disease. Curr Neuropharmacol. 2017;15:996–1009. doi: 10.2174/1570159X15666170313122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra372. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, et al. Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99:56–63.e53. doi: 10.1016/j.neuron.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Itzhaki RF. Corroboration of a major role for Herpes simplex virus type 1 in Alzheimer’s disease. Front Aging Neurosci. 2018;10:324. doi: 10.3389/fnagi.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64–82.e67. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Allnutt MA, Johnson K, Bennett DA, Connor SM, Troncoso JC, Pletnikova O, et al. Human herpesvirus 6 detection in Alzheimer’s disease cases and controls across multiple cohorts. Neuron. 2020;105:1027–.e1022. doi: 10.1016/j.neuron.2019.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seaks CE, Wilcock DM. Infectious hypothesis of Alzheimer disease. PLoS Pathog. 2020;16:e1008596–e1008596. doi: 10.1371/journal.ppat.1008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Balin BJ, Hammond CJ, Little CS, Hingley ST, Al-Atrache Z, Appelt DM, et al. Chlamydia pneumoniae: an etiologic agent for late-onset dementia. Front Aging Neurosci. 2018;10:302. doi: 10.3389/fnagi.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol. 2019;11:1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abbayya K, Puthanakar NY, Naduwinmani S, Chidambar YS. Association between Periodontitis and Alzheimer’s disease. North Am J Med Sci. 2015;7:241–6. doi: 10.4103/1947-2714.159325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lauc G, Sinclair D. Biomarkers of biological age as predictors of COVID-19 disease severity. Aging. 2020;12:6490–1. doi: 10.18632/aging.103052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21–e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–6. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Naughton SX, Raval U, Pasinetti GM. Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J Alzheimer’s Dis. 2020;76:21–25. doi: 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–29. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–75. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 168.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–27. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 172.Walker JM, Harrison FE. Shared neuropathological characteristics of obesity, type 2 diabetes and Alzheimer’s disease: impacts on cognitive decline. Nutrients. 2015;7:7332–57. doi: 10.3390/nu7095341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li JQ, Tan L, Wang HF, Tan MS, Tan L, Xu W, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry. 2016;87:476–84. doi: 10.1136/jnnp-2014-310095. [DOI] [PubMed] [Google Scholar]
- 174.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 175.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation. 2018;15:276. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimer’s Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, Milicic T, et al. Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: potential targets for an efficient preventive intervention. Int J Environ Res Public Health. 2014;11:3586–98. doi: 10.3390/ijerph110403586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112:1796–808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379–86. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 181.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. 2017;11:216. doi: 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–9. [PMC free article] [PubMed] [Google Scholar]
- 183.Friedland RP, Chapman MR. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017;13:e1006654. doi: 10.1371/journal.ppat.1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 185.Askarova S, Umbayev B, Masoud A-R, Kaiyrlykyzy A, Safarova Y, Tsoy A, et al. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front Cell Infection Microbiol. 2020;10:104. doi: 10.3389/fcimb.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–33. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 188.Brandscheid C, Schuck F, Reinhardt S, Schäfer KH, Pietrzik CU, Grimm M, et al. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J Alzheimer’s Dis. 2017;56:775–88. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- 189.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 191.Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–5. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, et al. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2009;206:121–4. doi: 10.1016/j.jneuroim.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, et al. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging. 2013;5:902–12. doi: 10.18632/aging.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scott KA, Ida M, Peterson VL, Prenderville JA, Moloney GM, Izumo T, et al. Revisiting Metchnikoff: age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav Immun. 2017;65:20–32. doi: 10.1016/j.bbi.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 198.Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione, et al. Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol. 2018;14:457–69. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- 199.Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15:451–2. doi: 10.1016/S1474-4422(16)00067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham study. Neurology. 1997;49:1498–504. doi: 10.1212/WNL.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 201.Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70:418–26. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dubal DB. Sex difference in Alzheimer’s disease: an updated, balanced and emerging perspective on differing vulnerabilities. Handb Clin Neurol. 2020;175:261–73. doi: 10.1016/B978-0-444-64123-6.00018-7. [DOI] [PubMed] [Google Scholar]
- 203.Ossenkoppele R, Lyoo CH, Jester-Broms J, Sudre CH, Cho H, Ryu YH, et al. Assessment of demographic, genetic, and imaging variables associated with brain resilience and cognitive resilience to pathological tau in patients With Alzheimer disease. JAMA Neurol. 2020;77:632–42. doi: 10.1001/jamaneurol.2019.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimer’s Dement. 2018;14:1171–83. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tang AS, Oskotsky T, Havaldar S, Mantyh WG, Bicak M, Solsberg CW, et al. Deep phenotyping of Alzheimer's disease leveraging electronic medical records identifies sex-specific clinical associations. Nat Commun. 2022;13:675. doi: 10.1038/s41467-022-28273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–9. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 208.Saunders AM, Strittmatter WJ, Schmechel D, St. George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele ϵ4 with late‐onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1467. doi: 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 209.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci. 1993;90:1977–81. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Osorio RS, et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS One. 2017;12:e0185926. doi: 10.1371/journal.pone.0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74:1178–89. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guillot-Sestier M-V, Araiz AR, Mela V, Gaban AS, O’Neill E, Joshi L, et al. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun Biol. 2021;4:711. doi: 10.1038/s42003-021-02259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yanguas-Casás N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol Neuroinflammation. 2020;7:13–22.. [Google Scholar]
- 214.Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, et al. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23:3501–11. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–74. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 216.Sárvári M, Hrabovszky E, Kalló I, Solymosi N, Likó I, Berchtold N, et al. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: rat and human studies identify strikingly similar changes. J Neuroinflammation. 2012;9:264. doi: 10.1186/1742-2094-9-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–20. doi: 10.1002/glia.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37:372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–63. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018;24:2773–.e2776. doi: 10.1016/j.celrep.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 221.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–36. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Parisi MM, Grun LK, Lavandoski P, Alves LB, Bristot IJ, Mattiello R, et al. Immunosenescence induced by plasma from individuals with obesity caused cell signaling dysfunction and inflammation. Obesity. 2017;25:1523–31. doi: 10.1002/oby.21888. [DOI] [PubMed] [Google Scholar]
- 223.Chiu Y-L, Tsai W-C, Hung R-W, Chen IY, Shu K-H, Pan S-Y, et al. Emergence of T cell immunosenescence in diabetic chronic kidney disease. Immun Ageing. 2020;17:31. doi: 10.1186/s12979-020-00200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thomas S, Rouilly V, Patin E, Alanio C, Dubois A, Delval C, et al. The Milieu Intérieur study—an integrative approach for study of human immunological variance. Clin Immunol. 2015;157:277–93. doi: 10.1016/j.clim.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 226.Márquez EJ, Chung C-H, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med. 2020;26:131–42. doi: 10.1038/s41591-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sankowski R, Bottcher C, Masuda T, Geirsdottir L, Sagar, Sindram E, et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci. 2019;22:2098–110. doi: 10.1038/s41593-019-0532-y. [DOI] [PubMed] [Google Scholar]
- 229.Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–53. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- 230.Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–12. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- 231.Davies DS, Ma J, Jegathees T, Goldsbury C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 2017;27:795–808. doi: 10.1111/bpa.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wong WT. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci. 2013;7:22–22. doi: 10.3389/fncel.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195.e191–112. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–60. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, et al. Microglial brain region−dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19:504–16. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016;323:170–82. doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 239.Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21:941–51. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–8. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Arber C, Lovejoy C, Wray S. Stem cell models of Alzheimer’s disease: progress and challenges. Alzheimer’s Res Ther. 2017;9:42. doi: 10.1186/s13195-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ryan KJ, White CC, Patel K, Xu J, Olah M, Replogle JM, et al. A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci Transl Med. 2017;9:eaai7635. doi: 10.1126/scitranslmed.aai7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sellgren CM, Sheridan SD, Gracias J, Xuan D, Fu T, Perlis RH. Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors. Mol Psychiatry. 2017;22:170–7. doi: 10.1038/mp.2016.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Banerjee A, Lu Y, Do K, Mize T, Wu X, Chen X, et al. Validation of induced microglia-like cells (iMG Cells) for future studies of brain diseases. Front Cell Neurosci. 2021;15:629279. doi: 10.3389/fncel.2021.629279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, et al. iPSC-Derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278–.e279. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016;22:1358–67. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat Neurosci. 2017;20:753–9. doi: 10.1038/nn.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xu R, Li X, Boreland AJ, Posyton A, Kwan K, Hart RP, et al. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat Commun. 2020;11:1577. doi: 10.1038/s41467-020-15411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Svoboda DS, Barrasa MI, Shu J, Rietjens R, Zhang S, Mitalipova M, et al. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc Natl Acad Sci. 2019;116:25293–303. doi: 10.1073/pnas.1913541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Götz J, Bodea L-G, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19:583–98. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- 251.Myers A, McGonigle P. Overview of transgenic mouse models for Alzheimer’s disease. Curr Protoc Neurosci. 2019;89:e81. doi: 10.1002/cpns.81. [DOI] [PubMed] [Google Scholar]
- 252.Cavaliere C, Tramontano L, Fiorenza D, Alfano V, Aiello M, Salvatore M. Gliosis and neurodegenerative diseases: the role of PET and MR imaging. Front Cell Neurosci. 2020;14:75. doi: 10.3389/fncel.2020.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zou J, Tao S, Johnson A, Tomljanovic Z, Polly K, Klein J, et al. Microglial activation, but not tau pathology, is independently associated with amyloid positivity and memory impairment. Neurobiol Aging. 2020;85:11–21. doi: 10.1016/j.neurobiolaging.2019.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Klein J, Yan X, Johnson A, Tomljanovic Z, Zou J, Polly K, et al. Olfactory impairment is related to tau pathology and neuroinflammation in Alzheimer’s disease. J Alzheimer’s Dis. 2021;80:1051–65. doi: 10.3233/JAD-201149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pereira JB, Janelidze S, Smith R, Mattsson-Carlgren N, Palmqvist S, Teunissen CE, et al. Plasma GFAP is an early marker of amyloid-beta but not tau pathology in Alzheimer’s disease. Brain. 2021;144:3505–16. doi: 10.1093/brain/awab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Prins S, Zhuparris A, Hart EP, Doll RJ, Groeneveld GJ. A cross-sectional study in healthy elderly subjects aimed at development of an algorithm to increase identification of Alzheimer pathology for the purpose of clinical trial participation. Alzheimer’s Res Ther. 2021;13:132. doi: 10.1186/s13195-021-00874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lee JC, Kim SJ, Hong S, Kim Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp Mol Med. 2019;51:1–10. doi: 10.1038/s12276-019-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 259.Kastanenka KV, Bussiere T, Shakerdge N, Qian F, Weinreb PH, Rhodes K, et al. Immunotherapy with aducanumab restores calcium homeostasis in Tg2576 mice. J Neurosci. 2016;36:12549–58. doi: 10.1523/JNEUROSCI.2080-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sabbagh MN, Cummings J. Open peer commentary to “Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen December 2019”. Alzheimer’s Dement. 2021;17:702–3. doi: 10.1002/alz.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.https://agora.ampadportal.org/genes/(genes-router:genes-list)
- 262.Xu Y, Kong J, Hu P. Computational drug repurposing for Alzheimer’s disease using risk genes from GWAS and single-cell RNA sequencing studies. Front Pharmacol. 2021;12:617537. doi: 10.3389/fphar.2021.617537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.King EA, Davis JW, Degner JF. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLOS Genet. 2019;15:e1008489. doi: 10.1371/journal.pgen.1008489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.https://www.phago.eu/news/
- 265.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–72. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 266.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–7. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–6. doi: 10.1212/WNL.59.6.880. [DOI] [PubMed] [Google Scholar]
- 268.Appleby BS, Nacopoulos D, Milano N, Zhong K, Cummings JL. A review: treatment of Alzheimer’s disease discovered in repurposed agents. Dement Geriatr Cogn Disord. 2013;35:1–22. doi: 10.1159/000345791. [DOI] [PubMed] [Google Scholar]
- 269.McGeer PL, Harada N, Kimura H, McGeer EG, Schulzer M. Prevalence of dementia amongst Elderly Japanese with Leprosy: apparent effect of chronic drug therapy. Dement Geriatr Cogn Disord. 1992;3:146–9. doi: 10.1159/000107010. [DOI] [Google Scholar]
- 270.Policicchio S, Ahmad AN, Powell JF, Proitsi P. Rheumatoid arthritis and risk for Alzheimer’s disease: a systematic review and meta-analysis and a Mendelian Randomization study. Sci Rep. 2017;7:12861. doi: 10.1038/s41598-017-13168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Vedin I, Cederholm T, Freund Levi Y, Basun H, Garlind A, Faxén Irving G, et al. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008;87:1616–22. doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- 272.Chen H, Liu S, Ji L, Wu T, Ji Y, Zhou Y, et al. Folic acid supplementation mitigates Alzheimer’s disease by reducing inflammation: a randomized controlled trial. Mediators Inflamm. 2016;2016:5912146–5912146. doi: 10.1155/2016/5912146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cerovic M, Forloni G, Balducci C. Neuroinflammation and the gut microbiota: possible alternative therapeutic targets to counteract Alzheimer’s disease? Front Aging Neurosci. 2019;11:284. doi: 10.3389/fnagi.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Simpkins JW, Perez E, Wang X, Yang S, Wen Y, Singh M. The potential for estrogens in preventing Alzheimer’s disease and vascular dementia. Ther Adv Neurol Disord. 2009;2:31–49. doi: 10.1177/1756285608100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sanchez JR, Marsh S, McIntyre L, Davtyan H, Walsh C, Blurton-Jones M. Cytotoxic T cells infiltrate the brain and interact with microglia to reduce Alzheimer’s disease pathogenesis. J Immunol. 2020;204:64.64–64.64. doi: 10.4049/jimmunol.204.Supp.64.4. [DOI] [Google Scholar]
- 276.Mittal K, Eremenko E, Berner O, Elyahu Y, Strominger I, Apelblat D, et al. CD4 T cells induce a subset of MHCII-expressing microglia that attenuates Alzheimer’s pathology. iScience. 2019;16:298–311. doi: 10.1016/j.isci.2019.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]