Abstract
The long-term physical and mental sequelae of COVID-19 are a growing public health concern, yet there is considerable uncertainty about their prevalence, persistence and predictors. We conducted a comprehensive, up-to-date meta-analysis of survivors’ health consequences and sequelae for COVID-19. PubMed, Embase and the Cochrane Library were searched through Sep 30th, 2021. Observational studies that reported the prevalence of sequelae of COVID-19 were included. Two reviewers independently undertook the data extraction and quality assessment. Of the 36,625 records identified, a total of 151 studies were included involving 1,285,407 participants from thirty-two countries. At least one sequelae symptom occurred in 50.1% (95% CI 45.4-54.8) of COVID-19 survivors for up to 12 months after infection. The most common investigation findings included abnormalities on lung CT (56.9%, 95% CI 46.2–67.3) and abnormal pulmonary function tests (45.6%, 95% CI 36.3–55.0), followed by generalized symptoms, such as fatigue (28.7%, 95% CI 21.0–37.0), psychiatric symptoms (19.7%, 95% CI 16.1–23.6) mainly depression (18.3%, 95% CI 13.3–23.8) and PTSD (17.9%, 95% CI 11.6–25.3), and neurological symptoms (18.7%, 95% CI 16.2–21.4), such as cognitive deficits (19.7%, 95% CI 8.8–33.4) and memory impairment (17.5%, 95% CI 8.1–29.6). Subgroup analysis showed that participants with a higher risk of long-term sequelae were older, mostly male, living in a high-income country, with more severe status at acute infection. Individuals with severe infection suffered more from PTSD, sleep disturbance, cognitive deficits, concentration impairment, and gustatory dysfunction. Survivors with mild infection had high burden of anxiety and memory impairment after recovery. Our findings suggest that after recovery from acute COVID-19, half of survivors still have a high burden of either physical or mental sequelae up to at least 12 months. It is important to provide urgent and appropriate prevention and intervention management to preclude persistent or emerging long-term sequelae and to promote the physical and psychiatric wellbeing of COVID-19 survivors.
Subject terms: Depression, Prognostic markers
Introduction
Severe acute respiratory syndrome-2 (SARS-COV-2) is the novel coronavirus causing the coronavirus disease 2019 (COVID-19) pandemic [1]. By April 2022, COVID-19 had caused 488 million confirmed cases and 6.1 million deaths globally. The clinical features, pathogenesis, transmission, diagnosis, and complications of patients with COVID-19 during the acute phase have been comprehensively described [2, 3], however, survivors of acute COVID-19 still risk long-term sequelae affecting multiple body organs systems, including the brain and nervous system. Understanding the long-term health burden of COVID-19 is essential to allow timely identification and treatment of affected patients and appropriate allocation of healthcare resources.
Investigations of longitudinal epidemiology and follow-up have uncovered a large variety of long-term sequelae in survivors of the COVID-19 pandemic [4–8]. Studies have reported that as many as 80% of patients discharged after hospitalization had at least one symptom persisting 2–6 months after disease onset, including fatigue, muscle weakness, and sleep difficulties, affecting body systems ranging from cardiopulmonary to psychiatric and neurological [4, 6, 9, 10]. Additionally, more than half of the patients had abnormal lung computed tomography (CT) images and impaired pulmonary function [4, 11, 12]. In the absence of effective treatment, the persistence of these sequelae may cause chronic or even permanent suffering in COVID-19 survivors, negatively affecting their quality of life and delaying returning to work for those who were at working age [13, 14].
Due to the novel nature of the COVID-19 pandemic, evidence of long-term sequelae is still emerging. Considering that SARS-COV-2 is in the same beta-coronavirus clade as SARS-CoV-1 and MERS-CoV, it is possible that some of the chronic problems encountered by survivors of SARS and MERS may also apply to COVID-19 survivors. Previous studies show that SARS survivors were identified to have markedly lower health status and exercise capacity at 1 year after acute illness, and 24% still experienced impaired lung diffusing capacity by that time [15]. A 15-year follow-up study of SARS survivors indicated a 2-year recovery time after rehabilitation for pulmonary interstitial damage and functional decline to resolve [16]. Chronic physical illness has been shown to increase the risk of developing psychiatric disorders, therefore long-term sequelae in any body system may further impact on survivors’ mental health. One study showed high levels of psychiatric distress among MERS survivors even 12 months after acute infection, with 42% of patients experiencing signs of post-traumatic stress disorder (PTSD) and 27% experiencing depression [17]. Based on these findings, we predict that the sequelae of the COVID-19 pandemic are likely to remain a significant global challenge in the months and years ahead.
Most studies of the sequelae of COVID-19 to date are heterogeneous. Accordingly, there is uncertainty about the relative prevalence of different symptoms and radiographic findings, their longevity and the factors that predict them. Although a recent meta-analysis estimated the prevalence of long-term effects of COVID-19, it included only 15 studies with a follow-up duration less than 4 months from infection and did not identify influential factors correlated with health sequelae [18]. A better understanding of the convalescent phase among COVID-19 survivors would help direct clinical care and tailor public health strategies in the post-epidemic era. In this meta-analysis, we aimed to (1) quantitatively estimate the pooled prevalence of long-term sequelae overall, as well as those of specific organ systems, in particular, neuropsychiatric symptoms; (2) to evaluate whether patient characteristics and clinical factors have different impacts on COVID-19 long-term sequelae; and (3) to explore the biomarkers and mechanism of such sequelae. We systematically review the current evidence of the long-term health consequences of COVID-19 infection which, to our knowledge, is the largest and most wide-ranging analysis of its kind to date. We identify important emerging trends that can inform further research and healthcare provision targeted at this urgent, expanding health needs.
Methods
We conducted this systematic review and meta-analysis in accordance with the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Supplementary Table S1) [19]. We registered the protocol of this study on the PROSPERO platform (Registration Number: CRD42021228197).
Data sources and searches
We searched and updated the PubMed, Embase, and Cochrane Library databases for studies published in English up to Sep 30th, 2021. The search strategy was developed by the senior authors (Supplementary Appendix 1). Search terms included coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, COVID-19, SARS-COV-2, 2019-nCoV, and novel coronavirus in conjunction with recovery, discharge, survival, and sequelae. The reference lists of original articles and reviews in the field were also manually examined to identify additional studies. References were managed with EndNote software version X9.3. Given that this field is developing rapidly, we also searched the preprint server medRxiv for studies published before Sep 30, 2021, with the terms “COVID-19” and “sequelae” in the title or abstract.
Study selection
Articles were included if they met the following criteria: (1) original research; (2) follow-up studies including patients recovering from COVID-19; (3) reported at least one symptom, relevant laboratory or examination result (such as lung imaging, lung function tests or blood tests) of post-acute COVID-19 or (4) provided raw data that allowed the calculation of the estimates. We used 4 weeks after initial infection as a cut-off for post/long-COVID syndrome [20]. In our study, 10 studies [21–30] with follow up duration of 2–4 weeks from discharge from hospitals were included, because of their average duration of more than 4 weeks after onset of infection, considering an average period in hospital of 22 days from illness onset [31]. The exclusion criteria were as follows: (1) the study was a review article or an abstract; (2) the study did not report the persistent symptoms of COVID-19 during the recovery stage; (3) the study was a case report based on a single patient.
The full texts of the identified articles were reviewed by two authors (NZ and YMZ). Disagreements on the inclusion of articles were resolved by consensus or involvement of a senior expert (YPB).
Data extraction and quality assessment
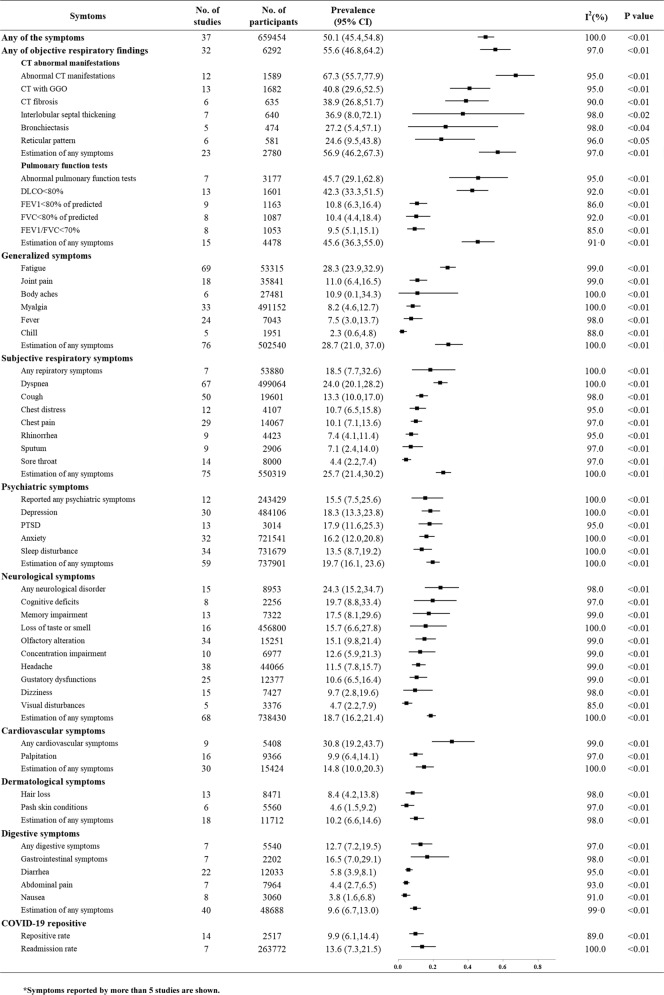
Data were extracted using Excel software with respect to study information (author, geographical location, publication year, sample size, severity of participants during acute infection and follow-up duration), population characteristics (mean age, male proportion), and reported health sequelae of COVID-19. Objective respiratory findings included CT abnormalities and abnormal pulmonary function tests. Subjective respiratory symptoms included dyspnea, cough, chest distress, chest pain, sputum, sore throat, and pharyngeal symptoms. Generalized symptoms included fatigue, sweat, joint pain, fever, myalgia, functional decline, moving difficulty, body aches, weight loss, and chills. Psychiatric sequelae included anxiety, mood disorder, depression, post-traumatic stress disorder (PTSD), sleep disturbance, stress, obsessive thinking, irritability, suicidality, behavior changes, hallucinations and neurological sequelae including cognitive deficit, headache, olfactory dysfunction, gustatory dysfunction, dementia, memory impairment, concentration impairment, visual disturbances, parasthesia, confusion, hearing loss, tremor, verbal problems, and dizziness. Objective Cardiovascular findings as well as subjective cardiovascular symptoms of palpitation, sinus bradycardia, hypertension, arrythmia or palpitations and angina pectoris. Digestive symptoms included gastrointestinal symptoms, nausea, abdominal pain, swallowing problems and diarrhea. Hair loss, pruritus and pash skin conditions were also reported. Nucleic re-positive and hospital readmission rates were included. Symptoms reported by more than 5 studies are shown in Fig. 2, while those reported by fewer than five studies are listed in the supplementary file. Four investigators (NZ, YMZ, CL, and QDL) extracted data independently.
Fig. 2. The pooled prevalence of sequelae by organ system and specific symptoms of COVID-19.
(Symptoms reported by more than five studies are shown in Fig. 2). In total, 50.1% of patients were estimated to have at least one symptom at follow-up, and the objectively examined respiratory system was most commonly affected, followed by generalized symptoms, subjective respiratory symptoms, psychiatric symptoms, and the neurological system. The prevalence of patients re-testing positive by SARS-COV-2SARS-COV-2 nucleic acid PCR and readmission to the hospital were estimated to be 9.9% and 13.6%, respectively.
The risk of bias of the included studies was assessed by the criteria of the Newcastle–Ottawa quality assessment scale. Based on the NOS criteria, we assigned a maximum of four stars for selection bias, two stars for comparability evaluation, and three stars for exposure and outcome assessment. Studies with fewer than five stars were considered low quality; five to seven stars, moderate quality; and more than seven stars, high quality. Four investigators (NZ, YMZ, CL, and QDL) independently appraised each item of the scale. Disagreements were settled by joint review with an experienced methodologist (YPB).
Data synthesis and analysis
Meta-analysis was performed to estimate the pooled prevalence of each sequelae among COVID-19 survivors after discharge from the hospital. The main affected systems included the respiratory, generalized, psychiatric, neurological, cardiovascular, dermatological, and digestive systems. The prevalence of any symptom within a specific organ system was estimated by pooling the most common symptoms related to that system, reported in one study. The I2 index was calculated to assess the between-study heterogeneity, and the Cochrane Q-test was used to determine statistical significance. An I2 value >50% or a chi-square p value <0.05 was considered substantial heterogeneity. Pooled rates with 95% confidence intervals (95% CIs) were calculated using the random-effect model if heterogeneity existed; otherwise, the fixed-effect model was used.
Subgroup analyses and meta-regression on the estimated prevalence of any symptom in each system were performed by stratification with mean age of study participants, the male proportion of the studies, the follow up duration, the severity of acute infection of COVID-19 (community patients, mild or moderate hospitalized patients and hospitalized patients who were admitted to intensive care) and whether study was conducted in high-income country or middle- or low-income country by World Bank income groups [32]. Funnel plots and Egger’s test were used to assess the presence of any publication biases. All analyses were performed with R Software (version 4.0.3).
Results
Characteristics of included studies
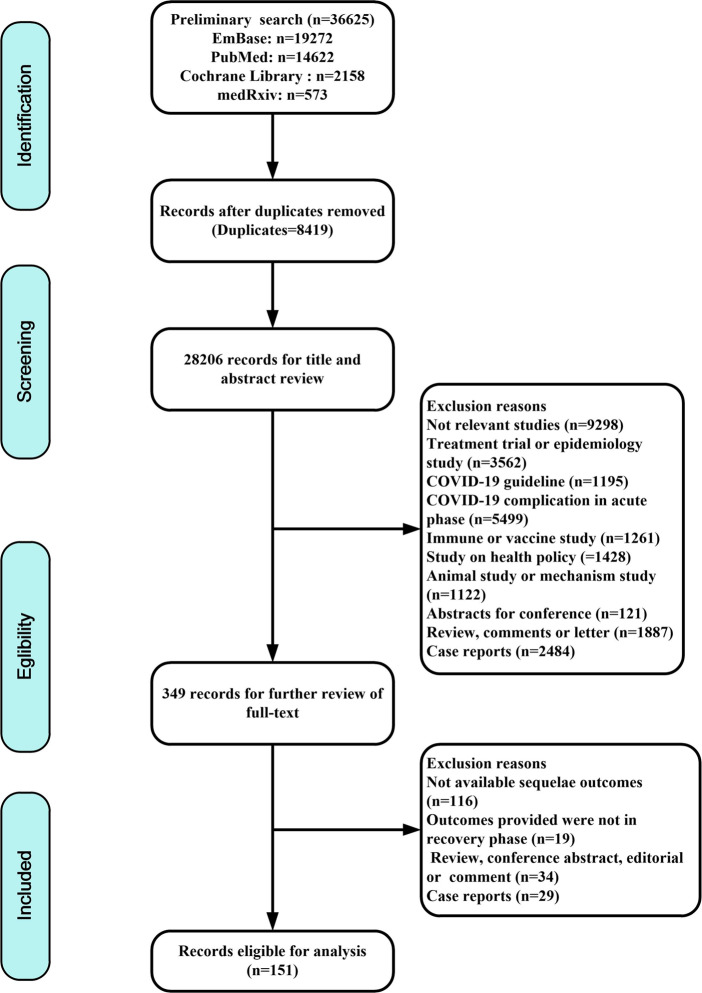
The search strategy yielded a total of 36,625 articles, with 19,272 from EMBASE, 14,622 from PubMed, 2158 from Cochrane Library and 573 from medRxiv. After the removal of duplicates, the titles and abstracts of 28,206 records were screened. A total of 349 were assessed for eligibility by full-text review, and 198 did not meet the inclusion criteria and were therefore excluded. Finally, 151 eligible studies involving 1,285,407 participants were included (Fig. 1).
Fig. 1.
Flow chart of study selection.
The characteristics of the included studies and their corresponding quality scores are reported in Supplementary Table S2. These studies covered 32 countries (35 in China, 23 in the USA, 21 in Italy, 12 in Spain, 9 in the UK, 4 in France, 4 in Germany, 4 in India, 3 in Norway, 3 in Belgium, 3 in the Netherlands, 3 in Switzerland, 3 in Sweden, 2 in Austria, 2 in Bangladesh, 2 in Iran, 2 in Mexico, one each for Australia, Brazil, Canada, Croatia, Egypt, Finland, Ireland, Japan, Nigeria, South Korea, Thailand, The Republic of North Macedonia, Russia, Turkey, United Arab Emirates and a multinational study). Most studies were prospective (n = 110), 21 were cross-sectional and 20 were retrospective. Overall, across the 151 studies, the long-term effects of COVID-19 on seven organ systems and 102 specific symptoms were included in this meta-analysis. Findings related to the respiratory system (90, 59.6%) were most commonly reported, including lung CT abnormalities (n = 23), pulmonary function tests (n = 15) or subjective respiratory symptoms (n = 75). This was followed by studies reporting generalized symptoms (n = 76, 50.3%), neurological effects (n = 68, 45.0%), psychiatric symptoms (n = 59, 39.1%), digestive symptoms (n = 40, 26.5%), cardiovascular symptoms (n = 30, 19.9%) and dermatological symptoms (n = 18, 11.9%). Fourteen (9.3%) studies reported the prevalence of cases who retested positive for COVID-19. Seventeen studies identified patients with varying severity of infection, involving 1026 community patients, 3114 mild or moderate hospitalized cases and 605 severe hospitalized cases. The follow-up duration was up to 12 months after discharge from the hospital.
Long-term sequelae by organ systems and specific symptoms among recovered survivors
The pooled prevalence of system sequelae
Figure 2 summarizes the pooled estimates of overall and specific symptoms in multiple organ systems in COVID-19 survivors after recovery. A total of 50.1% (95% CI 45.4–54.8, from thirty-seven studies, 659,454 participants) of participants were estimated to have at least one self-reported symptom up to the 12-month follow-up.
Of all organ systems, the respiratory system was most commonly affected with an estimated 55.6% (95% CI 46.8–64.2) of patients experiencing abnormalities in any objective examination results based on 32 studies (6292 participants), including either abnormalities on lung CT (56.9%, 95% CI 46.2–67.3) or abnormal pulmonary function testing (45.6%, 95% CI 36.3–55.0). Self-reported respiratory symptoms from 75 studies (550,319 participants) were estimated to be present in 25.7% of patients (95% CI 21.4–30.2). Generalized symptoms were also prevalent (28.7%, 95% CI 21.0–37.0) in 76 studies (502,540 participants), including 28.3% (95% CI 23.9–32.9) of survivors reporting fatigue. Psychiatric symptoms were frequently reported, with a pooled prevalence estimated to be 19.7% (95% CI 16.1–23.6) by pooling 59 studies (737,901 participants). Neurological symptoms still affected a considerable proportion of patients (18.7%, 95% CI 16.2–21.4) based on 68 studies (738,430 participants). Cardiovascular symptoms, digestive symptoms and dermatological symptoms were estimated to affect 14.8% (95% CI 10.0–20.3), 9.6% (95% CI 6.7–13.0) and 10.2% (95% CI 6.6–14.6) of survivors up to 12 months post-discharge from the hospital, respectively. The prevalence of patients re-testing positive by SARS-COV-2 nucleic acid PCR after a period of negative testing was estimated to be 9.9% (95% CI 6.1–14.4), although whether this represents true reinfection is unclear. The prevalence of readmission to the hospital was estimated to be 13.6% (95% CI 7.3–21.5). Other specific sequelae in COVID-19 survivors are shown in Fig. 2 and Supplementary Figs S1–S13.
Effect of clinical characteristics and demographic factors on system symptoms
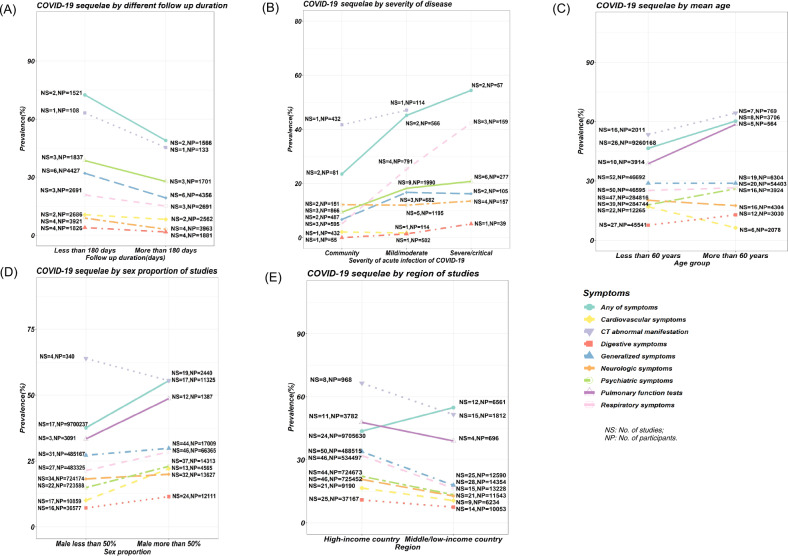
We conducted subgroup analyses for follow-up duration in longitudinal studies, the severity of acute infection, mean age, proportion of male participants, and income level (Fig. 3, and Supplementary Figs S14–S20). We found that longer follow-up duration was associated with lower reported prevalence of sequelae (Fig. 3A). The temporal trend in symptom sequelae showed that the proportion of patients with at least one sequelae symptom decreased from 56.0% (at 1–3 months) to 48.7% (at 3–6 months) and 37.8% (at 6–12 months). The prevalence of sequelae in each specific organ system increased according to the severity of the acute infection, from the lowest prevalence in community cases to the highest prevalence among those who were hospitalized as severely or critically ill patients (Fig. 3B). Subgroup analyses showed that elderly and male participants were at increased risk of reporting any symptoms (Fig. 3C, D). For the specific organ systems, elderly participants were at significantly increased risk of abnormal pulmonary function tests, whereas younger and male participants reported a significant higher prevalence of cardiovascular symptoms. In addition, COVID-19 survivors who lived in a high-income country had an increased risk of respiratory symptoms, generalized symptoms, and neurological symptoms (Fig. 3E).
Fig. 3. Subgroup analysis of sequelae of COVID-19 by mean age, sex proportion, region of studies, severity of disease and follow-up duration.
NS No. of studies, NP No. of participants. COVID-19 survivors of senior age, male sex, living in a high-income country, had a more severe health statuses at acute infection and within 6 months since recovery appeared to have higher prevalence of long-term sequelae than their contrast groups; A Subgroup analysis among different follow-up duration; B Subgroup analysis among asymptomatic participants in the community, mild/moderate patients and severe patients in hospital; C Subgroup analysis by mean age of participants; D Subgroup analysis by sex proportion of studies; E Subgroup analysis between high-income country and middle or low-income country.
Long-term neuropsychiatric symptoms among recovered survivors
The pooled prevalence of neuropsychiatric symptoms
Psychiatric problems and neurological symptoms were most reported after respiratory and generalized symptoms. We specifically estimated the pooled prevalence of each sequela of neuropsychiatric symptoms (Fig. 2). Psychiatric symptoms among recovered survivors were reported as, 18.3% (95% CI 13.3–23.8) for depression, 17.9% (95% CI 11.6–25.3) for PTSD, 16.2% (95% CI 12.0–20.8) for anxiety and 13.5% (95% CI 8.7–19.2) for sleep disturbance. Reports of mild/moderate anxiety (41.2%, 95% CI 7.5–80.6) were significantly more common than severe/very severe anxiety (6.0%, 95% CI 1.7–12.6%) (P = 0.04). For neurological symptoms, cognitive deficits (19.7%, 95% CI 8.8–33.4), memory impairment (17.5%, 95% CI 8.6–29.6) and loss of taste or smell (15.7%, 95% CI 6.6–27.8) were most commonly reported.
Effect of clinical characteristics and demographic factors on neuropsychiatric symptoms
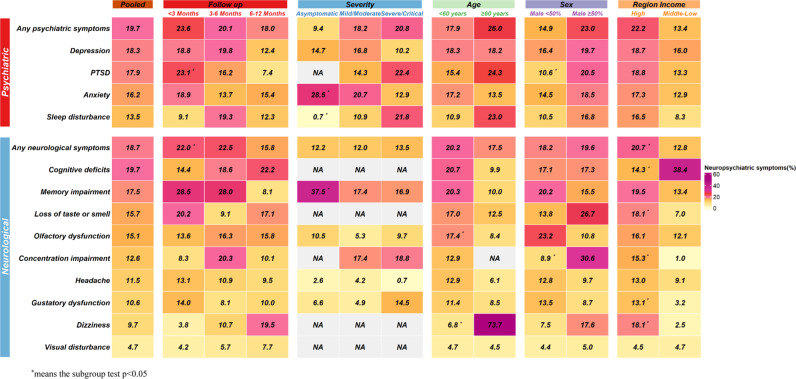
We did subgroup analysis to examine the longitudinal resolution of neuropsychiatric symptoms (Fig. 4, Supplementary Fig. S16), neurological symptom decreased from 22.0% (95% CI 17.1–27.3, at <3 months), and 22.5% (95% CI 13.4–33.0, at 3–6 months) to 15.8% (95% CI 13.4–18.4, at 6–12 months) (P = 0.04). Survivors reported declining psychiatric symptoms of 23.6% (95% CI 16.1–32.0, at <3 months), 20.1% (95% CI 11.2–30.8, at 3–6 months) and 18.0% (95% CI 12.5–24.3, at 6–12 months), although there was no significant difference across these time-points. Symptom of PTSD, however, was found to significantly resolve over the time, from 23.1% (95% CI 10.9–37.9) at <3 months, to 16.2% (95% CI 9.7–24.0) at 3–6 months and 7.4% (95% CI 4.3–11.2) at 6–12 months (p < 0.01). Specifically, the most prevalent psychiatric outcomes at 6–12 months after initial infection were anxiety (15.4%, 95% CI 8.0–24.6), depression (12.4%, 95% CI 5.8–21.1) and sleep disturbance (12.3%, 95% CI 4.8–22.6). For neurological symptoms, cognitive deficits (22.2%, 95% CI 19.6–25.0), dizziness (19.5%, 95% CI 0.5–55.1) and loss of taste or smell (17.1%, 95% CI 4.9–34.6%) were most prevalent at the 6–12 month time-point.
Fig. 4. The pooled prevalence of neuropsychiatric sequelae and subgroup analysis.
83 studies investigated neuropsychiatric symptoms, prevalence of the specific symptoms were as follows: 19.7% for any psychiatric illness, 18.3% for depression, 17.9% for PTSD, 16.2% for anxiety and 13.5% for sleep disturbance in psychiatric symptoms and 18.7% for any neurological symptoms, 19.7% for cognitive deficits, 17.5% for memory impairment, and 15.7% for loss of taste or smell in neurological symptoms. Patients were found to exhibit neuropsychiatric symptoms even 6 months after infection and mild patients who were not hospitalized were also presented with neuropsychiatric syndromes.
Subgroup analysis for the severity of the acute infection were conducted (Fig. 4). The prevalence of sequelae in specific neuropsychiatric symptom including any psychiatric symptoms and sleep disturbance increased according to the severity of the acute infection, from the lowest prevalence in asymptomatic community subjects to the highest prevalence among those who were hospitalized as severely or critically ill patients. Sleep disturbance was more commonly found in severely or critically ill subjects (21.8%, 95% CI 6.6–42.2) compared to those with hospitalized with mild or moderate acute illness (10.9%, 95% CI 1.6–26.4), and asymptomatic or community-based patients (0.7%, 95% CI 0.09–1.8) (p < 0.01). In contrast, anxiety was more frequent in asymptomatic or community-based patients (28.5%, 95% CI 23.9–33.3), compared to those mildly or moderately unwell hospitalized patients (20.7%, 95% CI 9.4–34.8) and severely or critically ill patients (12.9%, 95% CI 7.3–19.6) (p < 0.01). Notably, mild cases who were not hospitalized experienced a wide variety of neuropsychiatric symptoms months after recovery. Among the mild cases, the most common outcomes were memory impairment (37.5%, 95% CI 28.1–47.5), anxiety (28.5%, 95% CI 23.9–33.3) and depression (14.7%, 95% CI 2.2–35.0). (Fig. 4, Supplementary Fig. S20).
Subgroup analyses were further performed regarding the mean age, proportion of male participants and income level regarding neuropsychiatric symptoms, (Fig. 4, Supplementary Fig. S16–S20). Older people suffered more from dizziness (age ≥60 years: 73.7%, 95% CI 64.3–82.1 vs. age <60: 6.8%, 95% CI 2.2–13.5, p < 0.01), whereas younger adults are at higher risk of olfactory dysfunction (age <60: 17.4%, 95% CI 10.7–25.3 vs. age ≥60: 8.4%, 95% CI 4.5–13.3, p = 0.04). A higher proportion of male participants had a significant effect on the prevalence of PTSD (male proportion ≥ 50%: 20.5, 95% CI 12.4–30.0 vs. male proportion < 50%: 10.6, 95% CI 6.4–15.7, p = 0.04) and concentration impairment (male proportion ≥ 50%: 30.6, 95% CI 27.0–34.4 vs. male proportion < 50%: 8.9, 95% CI 2.9–17.6, p < 0.01). In addition, COVID-19 survivors who lived in a high-income country had an increased risk of, concentration impairment (high-income country: 15.3, 95% CI 7.3–25.5 vs. middle- or low-income country: 1.0, 95% CI 0.7–1.4, p < 0.01), dizziness (high-income country: 18.1, 95% CI 4.0–39.3 vs. middle- or low-income country: 2.5, 95% CI 1.1–4.2, p = 0.03) and gustatory dysfunction (high-income country: 13.1, 95% CI 8.1–19.1 vs. middle- or low-income country: 3.2, 95% CI 1.3–5.7, p < 0.01), whereas cognitive deficits were more found in middle- or low-income country (high-income country: 14.3, 95% CI 4.2–28.8 vs. middle- or low-income country: 38.4, 95% CI 31.8–45.1, p < 0.01).
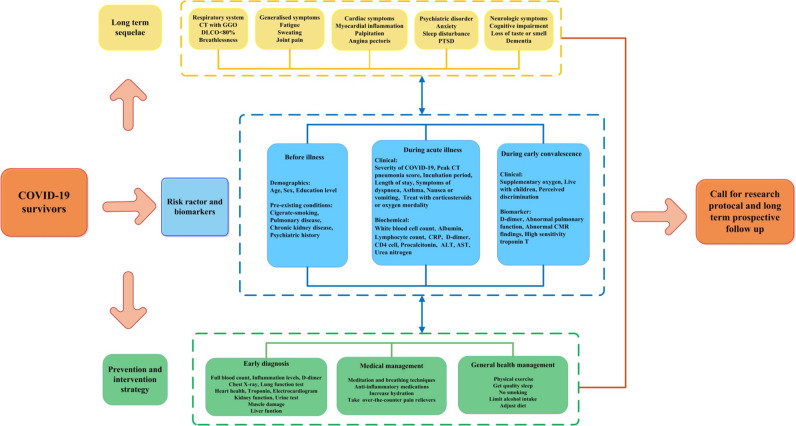
Predictive risk factors and biomarkers
To help clinicians efficiently identify survivors most at risk of long-term sequelae, we summarized the evidence of potential predictive risk factors and biomarkers in Fig. 5 and Supplementary Table S3. Potential risk factors that were identified were categorized into three groups: pre-illness, during acute illness and during the convalescent stage. In terms of pre-illness factors, individuals who were older [4, 33], male [5, 34] and had pre-existing conditions such as pulmonary disease (particularly COPD) [5, 34], those with a history of psychiatric illness [35], and cigarette smokers [4, 5] had a significantly higher risk of medium- or long-term COVID-19 sequelae. During the acute illness stage, those who suffered from severe COVID-19 illness (admitted to the ICU or requiring mechanical ventilation) [4, 5, 36, 37], required longer hospital stays [38–41] and those with abnormal objective test results (higher peak CT pneumonia score [33], higher level of CRP [5, 33, 34], elevated white blood cell count [37, 42], lower albumin [33], higher level of D-dimer [33], higher level of glucose [33] or higher level of ALT [37, 42]) were at greater risk of long-term sequelae. SARS-COV-2-associated cytokines, including interleukin (IL)-6, IL-1b, tumor necrosis factor (TNF), and IL-17, which are known to disrupt the blood-brain barrier and could facilitate the entry of the virus and consequent brain damage, were associated with persistent neurological symptoms [43]. After discharge from the hospital, survivors who had no access to oxygen supplementation or those with abnormal objective test results (abnormal pulmonary function, especially DLCO [38, 44], decreasing D-dimer [12], or abnormal cardiac magnetic resonance (CMR) findings [45]) were also at higher risk of experiencing long-term effects of COVID-19. Furthermore, patients who report living with children [36] and perceived stigma [36] involving verbal abuse and rejection from family, relatives, neighbors, and community were also at increased risk.
Fig. 5. Framework of long-term sequelae, risk factors, and prevention and intervention strategies for long COVID-19.
Potential risk facotrs were categorized into three groups: the pre-illness group included age, sex and pre-existind conditions; the acute illness stage group included severity of illness, hospital length and abnormal objective tests during hospitalization; the convalescent stage included access to oxygen supplementation, abnormal objective tests during follow up and perception of stigma from community.
Quality control and publication bias
According to the quality assessment criteria, twelve studies (7.9%) were graded as high quality, 124 (82.2%) as medium quality, and 15 (9.9%) as low quality (Supplementary Table S2). Meta-regression found no effect of study quality on the estimation of sequelae prevalence.
We observed an effect of small study size when estimating the prevalence of abnormal pulmonary function tests, generalized symptoms, and respiratory and neurological sequelae across studies (Egger’s test: p < 0.001, Supplementary Table S4) but not that of other organ systems (Egger’s test: p > 0.05) and funnel plot (Supplementary Fig. 21). Applying the leave-one-out sensitivity analysis did not significantly alter the pooled estimates of the prevalence of sequelae, indicating that no individual study significantly influenced the results (Supplementary Fig. S22).
Discussion
This study is, to our knowledge, the largest and most wide-ranging systematic review and meta-analysis to date, comprehensively summarizing current evidence on the long-term physical and mental sequelae of COVID-19 in the convalescent phase. Our results found that approximately half of survivors had multiple organ sequelae after discharge from the hospital and within up to 12 months of follow-up. Unsurprisingly, the most common sequelae involved the respiratory system, followed by generalized symptoms, psychiatric symptoms, and neurological symptoms, which were also of similarly high prevalence. Additionally, our study is also the first to perform subgroup analyses and found that the prevalence and burden of long-term physical and mental sequelae among COVID-19 survivors increased with older age, higher male proportion, living in a high-income country, severity in acute infection, and decreased with the extension of follow-up time. Prospective cohort studies with large sample sizes that aim to further characterize the long-term sequelae of COVID-19 are needed, especially in lower-income countries. Future studies with large sample sizes, long-term follow-up and exploring biomarkers and underlying pathological mechanisms will be addressed to better understand, prevent and heal physical conditions and mental well-being.
Respiratory abnormalities remain a key issue in survivors of COVID-19. Although only a quarter of survivors reported respiratory symptoms, including dyspnea, cough and chest distress, more than 50% of survivors displayed objective persistent impairment on lung CT scans and pulmonary function tests. Furthermore, previous studies have reported that lower lymphocytes and asthma during hospitalization were associated with the presence of long-term subjective respiratory symptoms [40, 42]. This finding indicates that it is necessary to carry out CT scans and pulmonary function tests during follow-up of COVID-19 in the convalescent phase. Given that the potential risk of pulmonary injury may last months to years [46], further research is required to confirm the risk factors and mechanism for respiratory sequelae and to identify treatments that prevent pulmonary fibrosis in survivors of COVID-19 [47]. Our study also reported significant cardiovascular sequelae associated with SARS-COV-2 infection, with 14.8% of survivors reporting cardiovascular symptoms and palpitation being the most common symptoms. Unlike previous studies that indicate an association between cardiovascular injury and fatal cases among SARS and MERS patients [31, 48, 49], some studies found that cardiac conditions are mostly mild in COVID-19 survivors [40, 50]. Our study demonstrated that 28.3% of patients experienced lasting fatigue after recovering from their acute illness, which is similar to previous findings in SARS patients, approximately one-third of whom reported persistent fatigue within the follow-up period of 18 to 40 months post-infection [17, 51]. A lengthy post-disease fatigue will not only impair the quality of life of individuals but also have adverse impacts on employers and governments; thus, multidisciplinary input to manage fatigue should be of particular concern.
COVID-19 is also anticipated to take a toll on the mental and neuropsychiatric health of survivors in the long term [43]. Approximately one-fifth of COVID-19 survivors in this review demonstrate psychiatric symptoms during the 12 months after recovery. These estimates are similar to those in survivors of SARS and MERS, in whom psychiatric illness may linger beyond 6 months [17, 51, 52]. Neuroinflammation induced by SARS-COV-2 infection and reduction of neurotransmitters related to interferon- or IL-based immunotherapy may correlate with a prolonged psychiatric syndrome. In addition, studies demonstrated that increased public restrictions, stressful medical care in hospital, concerns about infecting others as well as stigma may have considerable impacts on the mental health of survivors [53–55]. Depression following pandemics is a most significant public health concern. Our study reported a long-term prevalence of 18.3%, and another study concerning COVID-19 suggested an overall depression prevalence of 27.9% [53]. It is also important to note that even mildly affected patients who didn’t require to hospital can exhibit a high prevalence of long term psychiatric symptoms, including anxiety and depression.
In our study, neurological consequences were also identified, with an estimated prevalence of 11.4%. Memory impairment, cognitive deficits and loss of taste or smell were the predominant symptoms in this system. Among them, 15.1% and 10.6% of patients experience persistent olfactory and gustatory dysfunction, respectively, after recovery, which are highly specific to COVID-19 infection. Such neurological deficits may be due to brain damage caused by neuroinflammation and hypoxic injury in relation to viral invasion [56, 57]. A study investigated brain image changes in the UK Biobank participants and found significant longitudinal reduction in gray matter thickness among COVID-19 survivors. Studies indicated that alterations of olfactory and memory function may predict long term consequence such as Alzheimer’s disease or other forms of dementia [58, 59]. More efforts aimed to elucidate the mechanisms of and develop early screening and interventions for the psychiatric and neurological sequelae of COVID-19 need to be addressed. One such study is the long-term database and biobank launched in the United States to collect information about neurological problems specifically associated with COVID-19 [60].
Since the medical community has begun to recognize those affected by long-term COVID-19 sequelae [7], we summarize the relevant factors in the included articles to identify the potential predictive biomarkers for different sequelae. First, people who are male [34], with pre-existing pulmonary disease [34], and severe acute disease during hospitalization with COVID-19 [4, 34], who do not receive oxygen treatment during follow-up [12] and have decreasing D-dimer levels [12] are at higher risk of having any sequelae after discharge from the hospital. Patients who are older, have a longer incubation period, higher peak CT pneumonia score, abnormal biomarkers (lower albumin level [33], higher urea nitrogen level [33], higher serum sodium concentration [33], higher glucose [33], higher CRP [33], higher D-dimer [33]), longer hospital stay length [38, 39], treated with oxygen during hospitalization [38], and with abnormal pulmonary function detected during follow-up tend to have long-term abnormal CT manifestations. Female patients [4, 38] with chronic kidney disease [5], smoking history or COPD history [5, 36], who have been admitted to the ICU or have severe COVID-19 [4, 5] in the hospital, would be at higher risk of long-term abnormal pulmonary function. In addition, female patients with older age [4] who have abnormal biomarkers during hospitalization, including higher AST [42], ALT [42] and serum troponin-I [42], or detected with abnormal pulmonary function tests [38, 44] during follow-up, are more likely to have persistent generalized symptoms such as fatigue. Meta-regression analyses based on age, male proportion, and follow-up duration as continuous variables were also performed, and the results supported these findings of the subgroup analyses (Supplementary Table S5). Patients with a history of pulse ≥90 beats per min during hospitalization [40], had symptoms of dyspnea [40] and had lower lymphocytes [42] during hospitalization, and those who had high-sensitivity troponin T markers [45] and abnormal CMR findings [45] tended to be vulnerable to cardiovascular-related sequelae.
Some factors increased the risk of long-term psychiatric and neurological problems among COVID-19 survivors, including patients who suffer from a history of psychiatric illness or pulmonary disease [5, 35, 61], who have severe acute COVID-19 [4, 62], who are not treated with corticosteroids in the hospital [36], who live with children [36], and who perceive discrimination after discharge [36]. In addition, sleep disturbance, anxiety and depression were most commonly found in female patients, whereas PTSD was more common in male patients [4, 5, 63]. Previous studies suggested that quarantine and delays in returning to work were also associated with worse mental health outcomes among infected individuals during the COVID-19 outbreak [14, 53]. For digestive sequelae, the most influential factors included elevation of certain biomarkers (CD4, CRP, ALT, white blood cells, lymphocytes and procalcitonin) and treatments with corticosteroids and PPIs [37]. Although predictive factors and biomarkers have been proposed in some follow-up studies, further prospective studies that systematically correlate risk factors with clinical outcomes should be established. To address these key questions, updated results from ongoing, prospective, longitudinal studies involving interdisciplinary and multinational researchers are eagerly awaited [47, 64].
The findings from this study should be interpreted with caution in the context of its limitations. A weakness of the currently available data, and therefore of this review, was the high heterogeneity between studies, with most I-squared estimates >50%. The heterogeneity may be due to different definitions of cases, a variety of diagnostic criteria, and different follow-up durations across studies. Subgroup analysis has shown that age, sex proportion, region of studies, severity of acute infection and follow-up duration all have impacts on the overall estimations of the prevalence and are likely to explain some of these variations. Endeavors on the internationally agreed definition and management of long-term sequelae among COVID-19 survivors will help decrease heterogeneity of prevalence across studies. Second, the estimated prevalence of any symptoms in a specific organ system is based on self-reported, and neuropsychiatric symptoms are mainly self-reported. Although we pooled the subjective symptoms and objective examination tests for the respiratory and cardiovascular system, the objectively detected evidence is limited, such as only one study reported cardiac inflammation. More prospective cohort studies with larger sample sizes, longer follow up periods and objective examinations, such as brain imaging are needed. Third, these results are need interpreted cautiously, because we could not verify that the self-report long-term sequelae was definitely caused by COVID-19 infection. Detailed medical evaluation of these patients is clearly needed to prevent or at least minimize the possibility that symptoms due to another disease are erroneously attributed to “long COVID” [65]. Fourth, our analysis found and reviewed only work done with the Alpha variant, and no study reported data on those who contracted the Delta or Omicron variants. We did not involve the effect of vaccination because of the limited number of studies reporting vaccination status. With the spread of new variants and with many people vaccinated, future studies would quite usefully observe whether similar symptom changes are found with these two new viral variants and whether the vaccines offer a layer of protection from these newer variants. Finally, risk stratification studies with biomarker-guided interventions and longer follow-up durations in larger populations could contribute to a better understanding of sequelae.
Conclusion
This meta-analysis provides a comprehensive overview of the current state of knowledge of the long-term physical and mental sequelae of COVID-19 and the risk factors associated with it. Our findings suggest that about half patients who have recovered from COVID-19 have a burden of any long-term sequelae in the 12 months after hospital discharge. It is important to follow up with these patients and appropriately manage any persistent or emerging long-term sequelae in both physical and psychiatric domains. The interaction between physical and mental sequelae and the effect for the health and social well-being in the long term should be addressed in the future.
Supplementary information
Author contributions
LL, YPB, and JS were responsible for the study design. NZ, YMZ, CL, LL, QDL, SYN, and HM were responsible for the literature search and study selection. NZ, YMZ, CL, QDL, and YPB were responsible for the data extraction and quality assessment. NZ, YMZ, WY, HQC, and LL were responsible for the statistical analysis and manuscript drafting. ALK, AL, WY, CL, MVV, TK, TTF, JLY, HQS, XDT, YML, MVV, JS, YPB, and LL were responsible for critical revision of the manuscript, and NZ, ALK, AL, TK, MVV, JS, YPB, and LL contributed to review and approval of the final version of the manuscript.
Funding
This study was supported by grants from the National Key Research and Development Program of China (No. 2021YFC0863700, 2019YFA0706200) and the National Natural Science Foundation of China (No. 82171514, 81761128036, 81821092, and 31900805).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Na Zeng, Yi-Miao Zhao, Wei Yan.
Contributor Information
Jie Shi, Email: shijie@bjmu.edu.cn.
Yan-Ping Bao, Email: baoyp@bjmu.edu.cn.
Lin Lu, Email: linlu@bjmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01614-7.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;324:782–93. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of sars-cov-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4:e2036142. doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondratiuk AL, Pillay TD, Kon OM, Lalvani A. A conceptual framework to accelerate the clinical impact of evolving research into long COVID. Lancet Infect Dis. 2021;21:756–7. [DOI] [PMC free article] [PubMed]
- 8.Nehme M, Braillard O, Chappuis F, Courvoisier DS, Guessous I. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med. 2021;174:1252–60. [DOI] [PMC free article] [PubMed]
- 9.Brito J, Silva B, da Silva P, Cortez-Dias N, Silva D, Agostinho J, et al. Cardiovascular complications of COVID-19. Heart Mind. 2020;4:67–74. doi: 10.4103/hm.hm_28_20. [DOI] [Google Scholar]
- 10.Allam H, Kinsara A, A Alrajawi A, Tuiama T. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Heart Mind. 2020;4:123–5. doi: 10.4103/hm.hm_34_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020;76:396–8. [DOI] [PMC free article] [PubMed]
- 13.Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325:2015–6. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Shi L, Que J, Lu Q, Liu L, Lu Z, et al. The impact of quarantine on mental health status among general population in China during the COVID-19 pandemic. Mol Psychiatry. 2021;26:4813–22. [DOI] [PMC free article] [PubMed]
- 15.Hui DS, Wong KT, Antonio GE, Tong M, Chan DP, Sung JJ. Long-term sequelae of SARS: Physical, neuropsychiatric, and quality-of-life assessment. Hong Kong Med J. 2009;15:21–3. [PubMed] [Google Scholar]
- 16.Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: A 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Shin HS, Park HY, Kim JL, Lee JJ, Lee H, et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in middle east respiratory syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-COV-2 infection: Illness beyond acute infection and public health implications. JAMA. 2020;324:2251–2. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- 21.Deng W, Guang TW, Yang M, Li JR, Jiang DP, Li CY, et al. Positive results for patients with COVID-19 discharged form hospital in Chongqing, China. BMC Infect Dis. 2020;20:429. doi: 10.1186/s12879-020-05151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BM, Yang QQ, Zhao LY, Xie W, Si XY. Epidemiological characteristics of COVID-19 patients in convalescence period. Epidemiol Infect. 2020;148:e108. doi: 10.1017/S0950268820001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. [DOI] [PMC free article] [PubMed]
- 24.Osikomaiya B, Erinoso O, Wright KO, Odusola AO, Thomas B, Adeyemi O, et al. ‘Long COVID’: Persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infectious Diseases. 2021;21:304. [DOI] [PMC free article] [PubMed]
- 25.Peng J, Liu ZY, Yu XJ, Chen XY, Zhang K, Liu Y, et al. Antibody response in COVID-19 patients with and without re-positive RT-PCR results during the convalescent phase. Arch Virol. 2021;166:2299–303. doi: 10.1007/s00705-021-05132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Liu E, Xie J, Smyth RL, Zhou Q, Zhao R, et al. A follow-up study of children infected with SARS-COV-2 from western China. Ann Transl Med. 2020;8:623. doi: 10.21037/atm-20-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan N, Wang W, Gao Y, Zhou J, Ye J, Xu Z, et al. Medium term follow-up of 337 patients with coronavirus disease 2019 (COVID-19) in a Fangcang shelter hospital in Wuhan, China. Front Med (Lausanne) 2020;7:373. doi: 10.3389/fmed.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Liu L, Yang B, Li R, Luo J, Huang J, et al. Clinical characteristics of 134 convalescent patients with COVID-19 in Guizhou, China. Respir Res. 2020;21:314. doi: 10.1186/s12931-020-01580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J, Zhou R, Chen F, Tang G, Wu K, Li F, et al. Incidence, clinical course and risk factor for recurrent PCR positivity in discharged COVID-19 patients in Guangzhou, China: A prospective cohort study. PLoS Negl Trop Dis. 2020;14:e0008648. doi: 10.1371/journal.pntd.0008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M, Wong CK, Lau YM, Lee JCY, Tam FCC, Lau YM, et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS ONE. 2021;16:e0246732. [DOI] [PMC free article] [PubMed]
- 31.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The world bank.World bank country and lending groups. January 30, 2021; Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 33.Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur Respir J. 2021;57:2003481. [DOI] [PMC free article] [PubMed]
- 35.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D, Baumeister RF, Veilleux JC, Chen C, Liu W, Yue Y, et al. Risk factors associated with mental illness in hospital discharged patients infected with COVID-19 in Wuhan, China. Psychiatry Res. 2020;292:113297. doi: 10.1016/j.psychres.2020.113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng J, Li Y, Li J, Shen L, Zhu L, Liang Y, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol. 2021;6:344–6. doi: 10.1016/S2468-1253(21)00076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir Med. 2021;9:747–54. [DOI] [PMC free article] [PubMed]
- 39.Wei J, Yang H, Lei P, Fan B, Qiu Y, Zeng B, et al. Analysis of thin-section ct in patients with coronavirus disease (COVID-19) after hospital discharge. J Xray Sci Technol. 2020;28:383–9. doi: 10.3233/XST-200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–27. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang L, Yang B, Jiang N, Fu W, He X, Zhou Y, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020;35:e418. doi: 10.3346/jkms.2020.35.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Sar-van der Brugge S, Talman S, Boonman-de Winter L, de Mol M, Hoefman E, van Etten RW, et al. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir Med. 2021;176:106272. doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–73. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–50. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song WJ, Hui CKM, Hull JH, Birring SS, McGarvey L, Mazzone SB, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: Role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–44. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badawi A, Ryoo SG. Prevalence of comorbidities in the middle east respiratory syndrome coronavirus (MERS-COV): A systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam MH, Wing YK, Yu MW, Leung CM, Ma RC, Kong AP, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch Intern Med. 2009;169:2142–7. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 52.Lee AM, Wong JG, McAlonan GM, Cheung V, Cheung C, Sham PC, et al. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry. 2007;52:233–40. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- 53.Shi L, Lu ZA, Que JY, Huang XL, Liu L, Ran MS, et al. Prevalence of and risk factors associated with mental health symptoms among the general population in China during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2014053. doi: 10.1001/jamanetworkopen.2020.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Shi L, Que J, Lu Q, Liu L, Lu Z, et al. The impact of quarantine on mental health status among general population in China during the COVID-19 pandemic. Mol Psychiatry. 2021;26:4813–22. doi: 10.1038/s41380-021-01019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan K, Gong YM, Liu L, Sun YK, Tian SS, Wang YJ, et al. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: A meta-analysis and systematic review. Mol Psychiatry. 2021;26:4982–98. doi: 10.1038/s41380-021-01036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiatry. 2021;78:682–3. [DOI] [PMC free article] [PubMed]
- 57.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–83. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S. The chronic neuropsychiatric sequelae of COVID-19: The need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021;17:1056–65. doi: 10.1002/alz.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-COV-2 is associated with changes in brain structure in UK biobank. Nature. 2022;604:697–707. [DOI] [PMC free article] [PubMed]
- 60.Rubin R. Collecting data about COVID-19-related brain symptoms. JAMA. 2021;325:712. doi: 10.1001/jama.2021.0791. [DOI] [PubMed] [Google Scholar]
- 61.Vassalini P, Serra R, Tarsitani L, Koukopoulos A E, Borrazzo C, Alessi F, et al. Depressive symptoms among individuals hospitalized with COVID-19: Three-month follow-up. Brain Sciences. 2021; 11. [DOI] [PMC free article] [PubMed]
- 62.Liu L, Ni SY, Yan W, Lu QD, Zhao YM, Xu YY, et al. Mental and neurological disorders and risk of COVID-19 susceptibility, illness severity and mortality: A systematic review, meta-analysis and call for action. EClinicalMedicine. 2021;40:101–11. doi: 10.1016/j.eclinm.2021.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747–58. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laine C, Cotton D. COVID-19: Evaluation and care of patients with persistent symptoms following acute SARS-COV-2 infection. Ann Intern Med. 2021;174:1159–60. doi: 10.7326/M21-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matta J, Wiernik E, Robineau O, Carrat F, Touvier M, Severi G, et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.