Abstract
Major depressive disorder is a highly debilitating psychiatric disorder involving the dysfunction of different cell types in the brain. Microglia are the predominant resident immune cells in the brain and exhibit a critical role in depression. Recent studies have suggested that depression can be regarded as a microglial disease. Microglia regulate inflammation, synaptic plasticity, and the formation of neural networks, all of which affect depression. In this review, we highlighted the role of microglia in the pathology of depression. First, we described microglial activation in animal models and clinically depressed patients. Second, we emphasized the possible mechanisms by which microglia recognize depression-associated stress and regulate conditions. Third, we described how antidepressants (clinical medicines and natural products) affect microglial activation. Thus, this review aimed to objectively analyze the role of microglia in depression and focus on potential antidepressants. These data suggested that regulation of microglial actions might be a novel therapeutic strategy to counteract the adverse effects of devastating mental disorders.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-022-02492-0.
Keywords: Major depressive disorder, Microglial activation, Antidepressants, NLRP3 inflammasome, Neurogenesis, Kynurenine pathway
Background
Major depressive disorder (MDD) is a public health problem that affects approximately 322 million people worldwide [1]. During the coronavirus disease 2019 (COVID-19) pandemic, a meta‐analysis reported that 45% of COVID‐19 patients experienced depression [2]. Over 700,000 people die from suicide every year due to depressive disorder, which results in a heavy burden on individuals and society [3]. Clinical symptoms of MDD include persistent low mood, appetite loss, decreased interest in favorite activities, despair, sleepy disorders, and, in severe cases, suicidal behavior [4]. Unfortunately, due to its complexity and heterogeneity that are determined by genetic and environmental factors, the biological mechanisms underlying depression remain unclear. Currently, theories concerning depression primarily focus on the monoamine neurotransmitter depletion hypothesis, neuroplasticity hypothesis, and hypothalamus–pituitary–adrenal (HPA) axis hypothesis [5]. However, limitations are associated with these specific pathological mechanisms, such as an inability to explain the delayed effect of antidepressants and a lack of focus on cells other than neurons in the central nervous system (CNS) [6, 7].
Microglia are the first line in the innate immune system in the CNS and actively regulate microenvironmental changes in healthy and disordered brains. Microglial activation regulates inflammation, synaptic refinement, synaptic pruning, and neuronal connectivity [8]. Depression is considered a microglia-associated disorder (microgliopathy) [9]. Ample evidence suggests that microglia-mediated neuroinflammation interacts with all three theories correlated with MDD listed above [10, 11]. Neuroinflammation and the HPA axis are thought to function in a coordinated manner, and their dysregulation might mediate the onset of depression [10, 12–14]. Some clinical antidepressants affect the activation of microglia and neuroinflammation [15–17]. Nonsteroidal anti-inflammatory drugs appear to alleviate depressive symptoms by inhibiting microglial activation [18]. Minocycline is a semisynthetic tetracycline antibiotic that decidedly improves depressive-like behaviors by inhibiting microglial activation in the prefrontal cortex (PFC) and hippocampus (HIP) [19]. Microglial activation can be divided into the classic activation of M1 or alternate activation of M2 under optimal conditions [20]. Recently, pharmacological principles that modulate microglial polarization may provide beneficial treatments to alleviate the recurrence of psychiatric disorders [21–23].
Therefore, we conducted a systematic review using the Pubmed electronic database. Search themes included depression and microglia, neurogenesis, stress, and antidepressants. Articles from 1992 to 2021 were reviewed that focused on the connection between microglia and depression. In this review, we described the activation states of microglia in animal models and clinically depressed patients. We elucidated the mechanisms underlying depression and the therapeutic potential of targeting microglia. Finally, we highlighted the protective effects of antidepressants that act through modulating microglia in stress-induced animal models of depression.
Microglia: origin and function
Origin and development of microglia
Microglia were first described as a distinct cell type by Spanish neuroscientist Pio del Rio-Hortega in 1919 and account for approximately 5–10% of the total cell population in the brain [24]. The number of microglia in the adult mouse brain is estimated at 3.5 million, with variations in density across different regions. For example, more microglia are found in the HIP, substantia nigra (SN), olfactory telencephalon, and basal ganglia. Fewer microglia are observed in the fiber tracts, cerebellum, and the majority of brainstem [25]. Ontogenetically, recent fate-mapping studies have revealed that microglia originate from erythromyeloid progenitor cells in the developing embryonic yolk sac, and migrate into the embryonic CNS to differentiate into mature microglia. CNS microglia form a self-renewing cell population through proliferation and apoptosis throughout life of the individual [26, 27].
Characteristics and functions of microglia
As CNS immune effector cells, microglia are similar to peripheral macrophages with respect to their morphology and functions [28]. Under normal conditions, neurons serve a regulatory role in the CNS. Microglia provide protection and nutritional support to neurons, influence neuronal homeostasis, regulate synaptogenesis, and activate astrocytes (Fig. 1) [29, 30]. Resting microglia are characterized by a small soma with multiple symmetrically distributed protrusions that protrude at a rate of 0.4–3.8 μm/min to maintain either an extended or contracted state to sense changes in surrounding environment. Microglia are maintained in a relatively resting state in part by signals from neurons and astrocytes [31]. In response to challenges, such as tissue injury, pathogens, or other pathological processes, microglia quickly respond to the homeostatic imbalance and undergo considerable morphological transformation to provide defense mechanisms [32]. Specifically, the processes retract, the soma enlarges, and the ramification of the distal branches decreases. Activated microglia undergo additional morphological change and present a characteristic “amoeba-like” shape. The number of transformed microglia increases, and they migrate to the injured site at a rate of 1–2 μm/min for tissue repair [33, 34]. The activated microglia gradually shift from providing nutritional support and repairing neurons to neuronal dysfunction. These changes in function may be more persistent after continuous exposure to certain stimuli. This response further recruits peripheral innate immune cells (e.g., macrophages) and adaptive immune cells (e.g., B cells) to cross the blood–brain barrier and eventually lead to cognitive and emotional disorders [35–37].
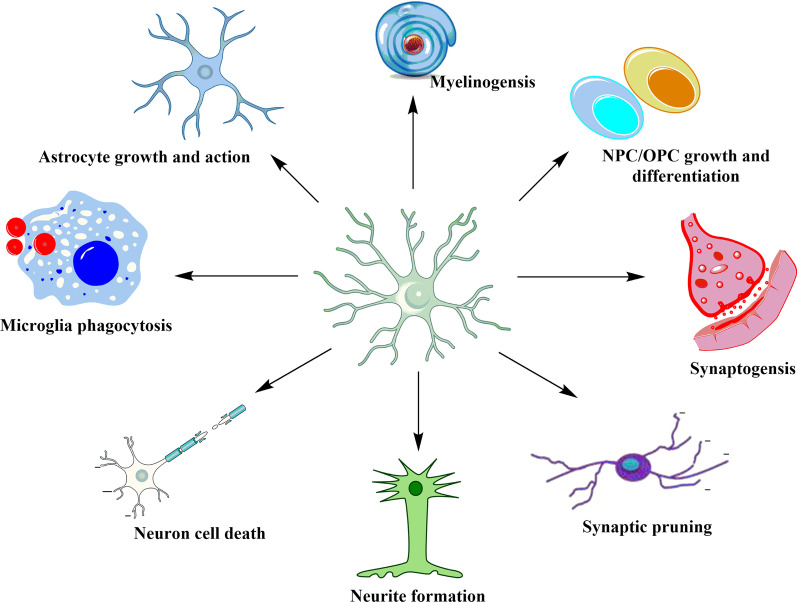
Fig. 1.
Multiple physiological roles of microglia in the CNS. Microglia are the center that maintain proper neuronal functioning of the CNS, including regulation of neuronal death, neurite formation, synaptic pruning, and synaptogenesis. In addition, microglia specify neural progenitor cell (NPC) and oligodendrocyte progenitor cell (OPC) growth and differentiation, facilitate myelination, and induce astrocyte activation. Lastly, microglia exhibit a phagocytic role concerning misfolded proteins and cellular debris [27]
The polarization of microglia
Microglia exist in a resting state under normal physiological conditions and carry out “immune surveillance” functions [38, 39]. When CNS damage or infection occurs, microglia can be broadly divided into the classic activation (M1) or alternative activation (M2) phenotype. M1 microglia result in pro-inflammatory cytokine release and increased expression of several differentiation marker 86 and 16/32 (CD86, CD16/32) and inducible nitric oxide synthase (iNOS). M2 microglia are inclined to express anti-inflammatory cytokines, arginase-1 (Arg1), transforming growth factor-β1 (TGF-β1), CD206, and chitinase-3-like-3 [22]. M1 microglia remove apoptotic cells, pathogens, and inhibit normal neuron growth, which adversely affects synaptic transmission. M2 microglia promote phagocytosis of cell fragments and misfolded proteins, tissue repair, and support neuron survival. Intriguingly, M2 microglia are driven by the coordinated regulation of multiple anti-inflammatory mediators and against M1-induced inflammation that ultimately achieve immune suppression and neuronal protection (Fig. 2) [40].
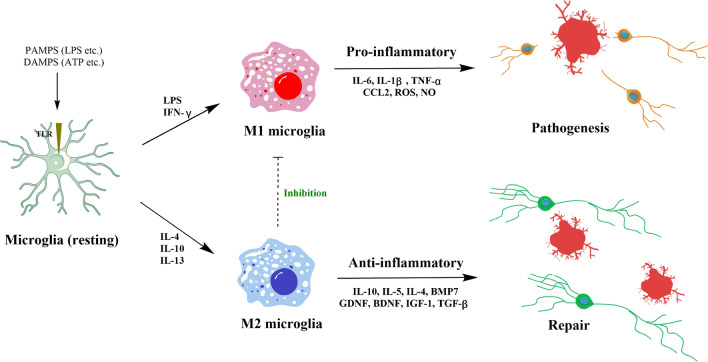
Fig. 2.
Microglial M1/M2 polarization and their regulatory functions in the CNS. Resting microglia are stimulated with pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) via toll-like receptors (TLRs). In the presence of lipopolysaccharide (LPS) and interferon-γ (IFN-γ), microglia polarize to the M1 phenotype and produce pro-inflammatory mediators including interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), CCL2, reactive oxygen species (ROS), and NO. In contrast, IL-4, IL-13, or IL-10 cause alternative activation into M2 microglia that inhibit M1 functions by releasing anti-inflammatory cytokines and neurotrophic factors [40]
However, the status of microglia may include a range of different but overlapping functional phenotypes that are in response to changes in their local environment. Novel single-cell technologies, such as single-cell RNA sequencing and cytometry by time-of-flight mass spectrometry, have emerged as superior methods to characterize immune cell types and states, the transition from normal to disease, and respond to therapy. For instance, microglia were isolated from LPS-injected mice that showed downregulation of homeostatic markers (e.g., Tmem119, Mef2c, P2ry13, P2ry12, and Siglech) and upregulation of inflammatory genes (e.g., Ccl2, Gpr84, and Nfkbia) by single-cell RNA sequencing and multicolor flow cytometry [41]. In the brains of a transgenic mouse model of Alzheimer’s disease (AD), a novel type of microglia (disease-associated microglia, DAM) was identified using single-cell analysis. Researchers further discovered that Trem2 is necessary for transformation from DAM stage-1 to DAM stage-2 [42]. With the development of unbiased and high-throughput analytical methods, it is possible to comprehensively characterize the spatial and temporal heterogeneity of microglia during CNS development and disease [43, 44]. Based on current research of microglial phenotypes, the classification using M1 and M2 subtypes is oversimplified and not universally accepted. We will use “pro-inflammatory” or “anti-inflammatory” microglia in subsequent descriptions to highlight the contribution of microglia in depression.
Microglia in depression
Clinical evidence
A growing body of research has shown that damage to the typical structure and function of microglia in the developing and adult brain is associated with the etiology of depressive disorders. Changes in microglia in different brain regions, including the PFC, HIP, anterior cingulate cortex (ACC), and amygdala, are involved in the development of depression. Previous studies have shown that considerable microglial activation occurs in the PFC and ACC during severe episodes of MDD. Microglial activation in ACC also is positively correlated with the severity of the depressive episode [45]. Positron emission tomography (PET) scans have shown that microglia are increased in ACC during episodes of MDD [46]. At the same time, the TSPO levels (a marker of microglial activation) were increased in MDD patients [47]. A cross-sectional study using 18F-FEPPA PET showed a strong relationship between the total distribution volume of TSPO and the duration of untreated MDD, total illness duration, and antidepressant exposure [48]. Correspondingly, in an autopsy study of patients with MDD, the concentrations of quinolinic acid (QUIN) produced by microglia were increased in the subgenual ACC and anterior midcingulate cortex of suicide victims [49]. In the dorsal ACC white matter from depressed suicide patients, microglial density was increased significantly, as identified by elevated gene expressions of ionized calcium-binding adapter molecule 1 (Iba-1), CD45, and monocyte chemoattractant protein-1 [50]. Another study reported similar results that were obtained in the prefrontal white matter [51]. Nevertheless, the role of microglia-mediated inflammation in the occurrence of depression is still controversial. Additional evidence demonstrated that microglia decrease in depression. In subjects with familial MDD, glia cells were clearly reduced while the number of neurons remained unchanged [52]. Brain imaging revealed cortical atrophy in the subgenual part of Brodmann’s area 24 [52]. A similar reduction in glial density was also observed in the orbital cortex, ACC, and dorsolateral PFC, based on a laminar analysis [53–55]. In addition, the numbers of glial cells were reduced, especially on the left side of the amygdala in MDD patients, when assessed using stereological methods [56]. However, no reduction in the numbers of glia was found in area 3b of the somatosensory cortex in patients with depression, suggesting that glial reduction in mood disorders is limited to specific brain structures [57].
In addition to depression disorder, microglial activation has been reported in other mental disorders such as anxiety [58], schizophrenia [59], and autism spectrum disorders [60, 61]. Anxiety disorders often emerge early in life and are associated with other diseases. Clinical reports on microglia and anxiety disorders remains extremely limited, except in animal models of depression [58]. A PET imaging study demonstrated increased microglial activity in patients with schizophrenia and persons at ultra-high risk of psychosis [62]. In autism, increases in microglia were observed in cortical areas (fronto-insular and visual cortex) of the brain [63]. Conversely, several studies reported no significant microgliosis or changes in the expression of glial cell markers in schizophrenia and autism [60]. Therefore, there are different opinions concerning microglial activation in the development of these mental diseases, which may be related to assessments during different stages of disease onset or individual heterogeneity. The treatment of depression should be personalized based on the status of major depression and microglial function in individual patients. It also is worth noting that suicide has a high prevalence in the long-term course of mental diseases. Postmortem analysis has revealed that an elevated microglial density in schizophrenia and depressive patients is associated with suicide [59], indicating altered microglial activity might be critical in psychiatric disorders.
Preclinical evidence
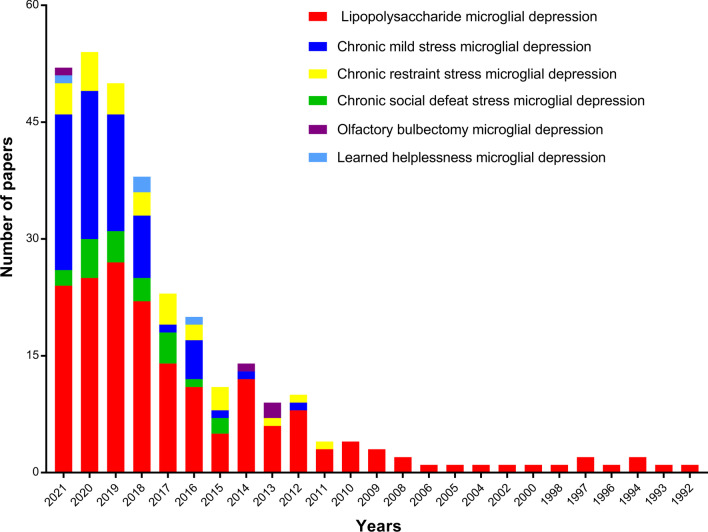
Considerable research has focused on the relationship between MDD and microglia in various animal models. Sustained microglial activation that exhibited high levels of pro-inflammatory cytokines has been observed in different brain regions [64–68], while inhibition of microglia alleviated depressive-type behaviors [69]. In this review, we illustrated the effects of microglial activation in animal models of depression, including acute/chronic stress and rodent pharmacological models. The search themes that were used included “LPS, chronic mild stress (CMS), chronic social defeat stress (CSDS), chronic restraint stress (CRS), olfactory bulbectomy (OBX), or learned helplessness (LH) combined with microglia and depression, respectively. The results indicated that microglia involved in nearly all models mentioned above. Among them, microglia were most evident in the models associated with LPS, CMS, and CRS, as well as CSDS-induced neuroinflammation, as shown in Fig. 3.
Fig. 3.
Annual changes in the number of published papers focused on animal models of depression and microglia
In a mouse model of acute depression, intraperitoneal injection of LPS activated the nod-like receptor pyrin containing 3 (NLRP3) expression and IL-1β cleavage in the HIP. Immunofluorescence staining showed that NLRP3 was primarily expressed in Iba-1 positive microglia when compared with control mice [70]. In BV2 microglial cells, LPS exposure induced an imbalance between the pro-inflammatory and anti-inflammatory microglial phenotype [71], activated TLR4/nuclear factor-kappa B (NF-κB) pathway, and downregulated TREM2 expression [72]. In the HIP and cortex of CMS-exposed mice, immunofluorescence staining revealed that the activated microglia (Iba-1 positive cells), as well as increased pro-inflammatory microglial markers (IL-1β, TNF-ɑ, IL-6, INF-γ, and iNOS) and decreased anti-inflammatory markers (Ym1, IL-4, IL-10, and Arg-1), suggesting a transformation of the microglial phenotype [73]. The CSDS paradigm also produces mood alterations and microglial activation, as well as ROS elevation. In addition, depletion of microglia using PLX5622 protects against behavioral abnormalities in the light–dark (LD) and social interaction (SI) tests [74]. These studies provide evidence that depressive-like behaviors and inflammation are present in chronic stress and pharmacological rodent models, which might be associated with persistent interference in microglia-related signaling. Other animal models that demonstrate an association between depression and microglia are shown in the Additional file 1: Table S1.
Since 1992, numerous studies have verified the concept that CNS microglia-mediated inflammation may contribute to depression and is closely related to regional selectivity and disease severity. Even though a strong correlation between microglia and depression has been observed in patients and animal models, determining whether microglial abnormalities actually play a significant role in depression remains challenging.
The role of microglia in the pathogenesis of depression
Microglia–neuron communication in depression
Microglia–neuron communication functions bi-directionally. Microglia impart considerable influence on numerous aspects of neuronal function. Similarly, neurons regulate microglial functions mainly through soluble factors such as chemokines, cytokines, and neurotransmitters. Among these factors, CX3CL1 and CD200 are primarily expressed by neurons, and their receptors, CX3CR1 and CD200R, are expressed on microglia [75]. Dysfunctional interactions between neurons and microglia are critical factors in severe neurological disorders, including depression, schizophrenia, and AD.
The communication between CX3CL1 and CX3CR1 contributes to the ability of microglia to maintain functional stability. Depending on the degree of brain damage, CX3CL1 leads to increased microglia pro-inflammation or maintenance of microglia in a quiescence state [76, 77]. In a LPS-induced depression model, the decreased expression of CX3CL1 and microglial activation were observed in the HIP [78]. The serum levels of CX3CL1 in patients with moderate–severe depression were higher than in the control group [79]. A similar observation was made for the plasma levels of CX3CL1 from MDD patients with co-morbid cocaine addiction [80]. Cx3cr1-deficient mice displayed transient microglial reductions during the early postnatal period and subsequent defects in synaptic pruning. Defective synaptic pruning has been associated with less effective synaptic transmission and decreased neural circuit formation, and social interaction, as well as increased repetitive behavior, which also have been observed in several neuropsychiatric disorders. These findings suggest that microglia-mediated disruption of synaptic pruning could be associated with neurodevelopmental and neuropsychiatric disorders [81]. More importantly, previous studies have suggested that hyper-ramified microglia (process branching and/or soma enlargement) is connected to depressive-like behavior in rodents. The CX3CR1-deficient mice showed definite resistance to repetitive swim stress-induced depressive-like behavior and microglia hyper-ramification changes compared to wild-type mice [82]. Also, CX3CR1-deficient mice also demonstrated the impairment in long-term potentiation (LTP). Treatment with an IL-1β receptor antagonist significantly reversed the cognitive function and synaptic plasticity impairments observed in CX3CR1-deficient mice [83]. The prenatal stress produced behavioral disturbances in anxiety and depressive behavior in adult offspring of rats. The underlying mechanism may be related to the upregulation of CXCL12 and its receptor, as well as decreased CX3CL1-CX3CR1 expression in the HIP and frontal cortices, whereas exogenous CX3CL1 application alleviated the observed changes [84, 85]. Furthermore, CX3CR1 deficiency has been shown to impair neuron–microglia responsiveness to chronic stress [86]. Treatment with antidepressants such as fluoxetine, venlafaxine, or tianeptine normalized these behavioral and biochemical alterations [84].
Recently, CD200-CD200R has been shown to be related to the pathogenesis of depression through animal models studies that focused on using different stress-inducing protocols. For instance, exposure to inescapable tail shock resulted in reduced CD200R level in the HIP, basolateral (BLA), and central nucleus of the amygdale [87]. Similar observations have been reported in male and female rats [88]. However, a paradoxical observation indicated that unavoidable foot shocks in rats reduced the transcription level of CD200R in the hypothalamus, but not the HIP [89]. These discrepancies between published reports concerning the HIP might be attributed to the use of different protocols by the two research groups. In an IFN-α-induced model of depression, vulnerable mice displayed increased levels of MHC-II, CD86, and CD200R. These mice displayed depressive-like behaviors characterized by increased immobility time in the forced swimming test (FST) and tail suspension test (TST), as well as decreased explorative behavior in the novel object exploration test [90].
Microglia and neurogenesis in depression
During brain development, microglia regulate synaptic transmission, prune neuronal synapses, and facilitate the formation of neural circuits. Once homeostasis is disturbed, microglia are converted into an active state and release pro-inflammatory cytokines, chemokines, and reactive oxidants. The pro-inflammatory microglia are involved with the upregulation of pro-inflammatory mediators, which is usually considered a harmful event. Whereas anti-inflammatory microglia display protective effects in neuronal survival and adult neurogenesis [91].
Activation of microglia is a critical mechanism in neurogenesis inhibition in the presence of inflammation and stress [9]. Hippocampal neurogenesis is a complex neurobiological process involving the generation and functional integration of newborn cells into brain neural circuits [92]. Several studies have demonstrated that stress strongly suppresses adult hippocampal neurogenesis. Both acute and chronic stress have been reported to reduce adult neurogenesis by decreasing neuroprogenitor proliferation and newborn cell survival [93–95], and also impair newborn neuron maturation [96, 97]. LPS infusion via a cannula for 4 weeks reduced the survival of new neurons in the subgranular zone/granule cell layer (SGZ/GCL) of the HIP, while 6 days of intracortical LPS infusion in rats did not affect the proliferation of new cells [98]. In addition, LPS-induced neuroinflammation suppressed proliferation and differentiation of neural stem cells in the dentate gyrus (DG) of the HIP, which was indicated by the decreased number of BrdU-, DCX- and NeuN-positive cells [99]. In an 8-week CMS-induced depression paradigm, C57BL/6 mice exhibited an increase in the number of Iba-1 positive cells and levels of IL-1β, IL-6, and TNF-α [100]. Another report on the CMS showed that an imbalance of peripheral inflammation markers and decreased CX3CL1/CX3CR1 immunoreactivity were associated with reduced numbers of BrdU/Ki-67/DCX+ (nascent, proliferating and DCX-associated) cells in the DG of the HIP [101]. Furthermore, in a graded study of the CMS for 5, 6, or 7 weeks, the investigators found that the pro- or anti-inflammatory microglial changes in the cortex and HIP, as well as BrdU/DCX+ cells, decreased in the DG [102]. Similar results related to the relationship between depressive-like behaviors and changes in microglia and neurogenesis have been reported in psychosocial stress models, including maternal separation [103], maternal sleep deprivation [104], chronic water-immersion restraint stress (CWIRS) [105], and CSDS [106, 107]. On the other hand, IL-4 and IL-10 induce alternative activation of anti-inflammatory factors that play a critical neuroprotective function in tissue remodeling and nerve regeneration. Treatment with IL-4 markedly inhibited IL-1β-caused depressive behavior by regulating glial activation and neurotransmitter levels in the HIP [108]. IL-4 stimulation increased the expression of insulin-like growth factor-1 in microglia, which has been reported to promote neurogenesis [109, 110]. Recent research demonstrated that IL-4-driven Arg1+ microglia modulate stress resilience through brain-derived neurotrophic factor (BDNF)-dependent neurogenesis in CMS mice [111]. IL-10-stimulated microglia enhanced the proliferation of NPCs but did not affect neuronal differentiation. IL-10-secreting microglia supported neuronal differentiation and the survival of newly formed cells in vitro [112]. CX3CR1CreERIL-10 knockout mice exhibited depression- and anxiety-like behaviors, along with decreased NR2B (N-methyl-D-aspartate receptor (NMDAR) subunit) and synaptophysin (SYP) levels in the mPFC, and increased NR2B and postsynaptic densitin-95 (PSD95) levels in the amygdala [113]. Furthermore, hippocampal neurogenesis was reduced in CX3CR1-deficient mice, and antagonizing CX3CR1 resulted in increased hippocampal IL-1β level and decreased neurogenesis in young rats [114]. Minocycline, a microglial inhibitor, reversed the pathogenic phagocytic potential of neurotoxic microglia, and reduced the negative phenotypes associated with reduced neurogenesis in depression models induced by chronic stress and LPS [115]. Therefore, these data suggest that microglia are correlated with reduced neurogenesis induced by exposure to stress and, in turn, may be responsible for the development of depressive-like behaviors.
Microglia-mediated activation of NLRP3 in depression
The NLRP3 inflammasome is a multi-molecule complex containing cytosolic NLRP3, adaptor protein ASC, and pro-caspase-1 precursor. NLRP3 plays a vital role in production of pro-inflammatory cytokines during the stress process [116]. The NLRP3 inflammasome is currently considered to be an essential molecular platform for regulating pro-inflammatory cytokines release. NF-κB nuclear translocation is an indispensable event in the initiation of NLRP3 activation, which further confirmed that NF-κB nuclear translocation might play a role in assembling the NLRP3 inflammasome. When NLRP3 is activated by repetitive stress, it modulates caspase-1 activation, which, in turn, promotes IL-1β and IL-18 maturation in microglia, where excessive secretion of cytokines contributes to the development and progression of MDD [117–119].
NLRP3 inflammasome activation has even been observed in depressive patients and numerous animal models of depression. Previous studies have revealed that the NLRP3 inflammasome was activated in blood cells from MDD patients, and the levels of IL-1β and IL-18 were increased in serum. The increase in pro-inflammatory factors was positively correlated with Beck Depression Inventory scores [120], suggesting that NLRP3 might have a critical role in mediating the development of depression [121]. In another experimental study, a sustained CMS procedure (12 weeks) enhanced the levels of IL-1, NLRP3, ASC, TLR2, NF-κB, p-IKKα, and IKKβ in rat PFC, while these alterations were reversed with fluoxetine [122]. Immunofluorescent analysis has confirmed that the NLRP3 inflammasome was primarily activated only in microglia in the PFC (Iba-1/NeuN+ cells) [122]. Using NLRP3 inflammasome inhibitors AC-YVAD-CMK and VX-765 significantly improved depressive-like behavior, as shown by improvements in sucrose intake in the sucrose preference test (SPT) and immobility time in the FST of CMS mice [94, 123]. Moreover, activation of P2X7R and NLRP3 inflammasome-associated proteins in hippocampal microglia could mediate depressive-like behaviors, which provide new therapeutic targets for depression [124]. In a LPS-induced acute depression mouse model, NLRP3, ASC, and caspase-1 mRNA expressions were remarkably increased compared to the control group [125]. In addition, IL-1β secretion was closely controlled by the NLRP3 inflammasome, which plays an important role in the pathogenesis of depression [108]. Minocycline has an acute antidepressant role in CRS, CUMS, and LH models by inhibiting microglia and NLRP3 activation [126–129]. Fluoxetine conferred an antidepressant effect in part by inhibiting NLRP3 inflammasome activation [130]. Clomipramine reversed LPS-induced increases in IL-1β, IL-6, TNF-α, and NLRP3 gene expressions in vivo, as well as in vitro in BV2 cells [131]. Electro-acupuncture treatment for 4 weeks markedly reversed CMS-induced increases in NLRP3 inflammasome-associated components (NLRP3, ASC, and caspase-1) and the expressions of mature IL-1β, as well as IL-18, TNF-α, IL-6, P2X7 receptor, and Iba-1 [132]. Iptakalim negatively regulates NLRP3 expression and, in turn, affects microglia-mediated neuroinflammation by inhibiting the activation of NLRP3/caspase-1 axis in the HIP of CMS mice [94]. These findings suggest that microglial NLRP3 activation is a central mediator involved in depressive-like behaviors in animal models and MDD patients.
Microglia-mediated kynurenine pathway in depression
Chronic stress stimulation or inflammation may contribute to tryptophan (TRP) metabolism associated with the kynurenine pathway (KP). The generation of neuroactive kynurenine metabolites leads to subsequent depressive-like symptoms [133]. KP metabolizes tryptophan into several bioactive metabolites in the brain, including QUIN (an NMDAR agonist) and kynurenic acid (KYNA, an α7-AChR and NMDAR antagonist). KYNA is primarily formed in astrocytes [134], which exhibit a neuroprotective function via their ability to eliminate glutamate spillover. QUIN is produced mainly by microglia [135] and has a strong excitotoxic role through enhancing NMDAR activation [136]. Microglia regulate KP balance by preferentially producing oxidative metabolites, including QUIN. Other metabolites of KP, including 3-hydroxykynurenine (3-HK) and anthralinic acid, do not directly affect neuronal activity but are involved in complicated pro-oxidation and anti-oxidation processes [137].
Recent studies have found that microglial QUIN expression was increased in the subgenual and supracallosal regions of the ACC in post-mortem brains of suicide patients with severe depression. In contrast, additional research revealed decreased QUIN in left CA1 or right CA2/3 areas of the HIP in uni- and bipolar depression patients [138]. Moreover, a meta-analysis revealed decreased KYNA and kynurenine (KYN) levels in patients with depression and increased QUIN level in antidepressant-free patients [139]. Similar results have been reported concerning the increase of QUIN in peripartum depression and adolescent MDD [140, 141]. In addition to clinical data, enhanced levels of 3-HK and QUIN have been observed in several models of depression [142, 143]. LPS and other infectious agents, including viruses, upregulate the release of inflammatory cytokines by binding to TLRs, which in turn directly and indirectly induce KP metabolism via pro-inflammatory cytokines [144]. Ketamine treatment could reduce the LPS-induced depressive-like alterations observed in the novelty suppressed feeding test (NSFT) and splash test (ST) [145]. In a genetic animal model, the level of KYNA was reduced in the PFC of Flinders sensitive line rats compared with Flinders resistant line control rats [146]. In addition, CMS contributed to QUIN production and its release from microglia in the HIP. QUIN resulted in the elevation of Glu level via NMDARs and mGluR1, as well as the increased expression of NR2B and mGluR1, which lead to depressive-like symptoms [147]. CRS exposure induced depressive-like behavior in C57BL/6 J mice, which was attributed partially to disruption of the neuroprotective/neurotoxic balance of the kynurenine metabolic pathway in the gut and brain [148]. Enzyme indoleamine 2,3-dioxygenase 1 (IDO1) is considered to be a rate-limiting enzyme for tryptophan metabolism in KP [149]. Treatment with the IDO inhibitor, 1-methyl-tryptophan, partially prevented CRS-induced depression- and anxiety-like changes [148]. The chronic forced swim test and tail suspension test in mice enhanced KYN/TRP and reduced the 5-HT/TRP ratio, which indicated activation of IDO1 [150]. In the poststroke depression (PSD) mouse model, 3-hydroxyanthranilate 3,4-dioxygenase (HAAO), QUIN, IDO1, Iba-1, and ROS were remarkably increased in the nucleus accumbens (NAc), HIP, and hypothalamus. At the same time, treatment with aripiprazole ameliorated the abnormal behaviors in PSD mice that were accompanied by decreased levels of IDO1, HAAO, QUIN, and ROS [151]. IDOInh is a specific inhibitor of IDO1 and has been shown to reverse fear learning and memory in CSDS-induced depressed mice by decreasing KYN and 3-HK levels in the blood and brain [152]. Consequently, it has been proposed that kynurenine metabolites directly interact with microglial activity, which provides a reliable target for investigating the mechanisms underlying antidepressant drugs.
Effects of antidepressants on microglia
Currently, numerous reports using different experimental models have indicated that antidepressants, including clinical and plant-based drugs, exert their antidepressant effects, in part, by regulating microglial phenotypes. Regulation of microglia has been proposed as a potentially effective therapeutic strategy in chronic inflammatory diseases [29, 30]. In this section, we summarized the current knowledge of antidepressants (clinical medicine and natural products) that act against microglial dysfunction in stress-induced depression models (Tables 1 and 2).
Table 1.
Traditional antidepressants regulate the microglia-mediated neuroinflammation in animal models of depression
| Treatment | Animal models | Behavioral test | Analyzed regions | Microglia | Pro-inflammation | Anti-inflammation |
|---|---|---|---|---|---|---|
| Fluoxetine [154] | CSDS | OFT, EPM, SIT | Serum | — | ELISA: TNF-α ↓, HMGB1 ↑ | — |
| Hip | Iba-1(–) |
mRNA:TNF-α, IL-1β, RAGE, TLR4 ↑ Proteins: T-HMGB1, T-p65, IκB ↑, TLR4 ↓ |
mRNA: Arg-1, CD206 ↑ | |||
| Fluoxetine [170] | CMS | Nest-building test, Dexamethasone suppression test | Cortical | CD11 b/P2X7R+↓ | — | — |
| Amygdala | CD11 b/P2X7R+↓ | — | — | |||
| HIP | CD11 b/P2X7R+↓ | — | — | |||
| Fluoxetine [105] | CWIRS | SPT, FST, TST | HIP | Proteins: CD68, Iba-1 ↓ | Proteins: IL-1β, TNF-α, iNOS ↓ | — |
| Fluoxetine [155] | LPS | SPT, FST | HIP | Iba-1/COX-2+↓ in DG |
ELISA: IL-1β, IL-6, TNF-α Proteins: TLR4, NLRP3 ↓ |
— |
| Citalopram [101] | CMS | SPT, FST | HIP | Iba-1+↑; CX3CL1+↑, CX3CR1+↑ in DG | — | — |
| Escitalopram [16] | CMS | OFT, SPT, TST, FST, NSFT | HIP | — | Proteins: IL-6, IL-1β ↓ | IL-10 (–) |
| Cerebral cortex | — | Protein: TNF-α ↓ | IL-10 (–) | |||
| Imipramine [107] | CSDS | SIT, SPT, TST, FST | Serum | — | ELISA: IL-1β, IL-6, TNF-α ↓ | — |
| HIP | Iba-1+↓ in DG |
mRNA: IL-1β, IL-6, TNF-α ↓ Proteins: p-p65/p65, cleved-Caspase-3, ac-NF-κB ↓ |
— | |||
| Sertraline [171] | CMS | SPT, TST, FST | PFC | Iba-1/HMGB1+↓ | Proteins: TNF-α, IL-1β ↓ | — |
| Serum | — | ELISA: TNF-α, IL-1β, NO ↓ | — | |||
| Sertraline [172] | CMS | TST, FST | Brain | Protein: Iba-1 ↓ | Proteins: TNF-α, iNOS, IL-1β, p-p65-NF-κB, p65-NF-κB, p-IκB-α ↓ | — |
| Serum | — | ELISA: TNF-α, IL-1β ↓ | — | |||
| Vortioxetine [17] | LPS | OFT, SPT, NORT | Dorsal HIP | mRNA: CD14, CD86 ↑ | mRNA: TNF-α, IL-6, IDO1 ↑ | mRNA: IL-4, TGF-β1 ↑ |
| Ventral hippocampus (vHIP) | mRNA: CD14, CD86, CD11b ↑ | mRNA: IL-1β, IL-6, IDO1 ↑ | mRNA: IL-4, IL-1Rα ↑ | |||
| Imipramine [173] | Repeated social defeat | OFT, social avoidance test | Plasma | — | ELISA: IL-6, CORT ↓ | — |
| Brain | Proteins: CD45, CD11b ↓ | — | — | |||
| Imipramine [156] | LH | — | Hilus | Iba-1+↓ | — | — |
| Clomipramine [131] | LPS | TST, FST | HIP | Iba-1+↓ | mRNA: IL-1β, TNF-α, IL-6 ↓ | — |
| Agomelatine [174] | LPS | — | vHIP | mRNA: CD11b, CX3CL1, CX3CR1 ↓, CD68 ↑ | — | — |
| Melation [70] | LPS | TST, FST | HIP | Iba-1/NLRP3+↓ | Proteins: pro-IL-1β, IL-1β, NLRP3, caspase-1 | — |
| SRT2104 [175] | CMS | OFT, SPT, TST, FST | HIP | Iba-1+, CD11b+ CD45low+ (–) |
mRNA: IL-6, IL-1β, iNOS ↓ CD11b+ MHCII+↓ |
mRNA: IL-10, TGF-β, Arg-1 ↑ CD11b+CD206+↑ |
| Pioglitazone [169] | CMS | OFT, SPT, TST, FST | HIP | Iba-1+↓ | mRNA: IL-1β, IL-6, TNF-α, iNOS, CCL2 ↓ | mRNA: Ym1, Arg1, IL-4, IL-10, TGF-β ↑ |
| Clemastine [176] | CMS | SPT, TST, FST | Serum | — | ELISA: IL-1β, TNF-α ↓ | — |
| HIP | Protein: Iba-1 ↓ | Proteins: IL-1β, TNF-α, iNOS ↓ | Protein: Arg-1 ↑ | |||
| Minocycline [163] | OBX + spinal nerve ligation | OFT | PFC | — | mRNA: IL-1β, IL-6 ↑ | mRNA: MRC2, IL-10 ↑ |
| Minocycline [14] | CMS | SPT, FST, EMP | Serum | — | CORT ↓ | — |
| HIP | mRNA: CD11b, IFN-γ/IL-4, IFN-γ/IL-10 ↓ |
mRNA: IFN-γ, TNF-α, IL-1β, IL-17 ↓ Protein: CD11b ↓ |
mRNA: TGF-β1, IL-4, IL-10, IL-13 ↑ | |||
| Memantine [177] | OBX | Emotional behavior, TST, FST | HIP | Iba-1+↓ | Proteins: p-IkBα, p-p65-NF-κB, TNF-α, IL-6 ↓ | — |
| Iptakalim [94] | CMS | SPT, TST, FST | Serum | — | ELISA: IL-1β ↓ | — |
| HIP | MAC-1+↓ in DG |
mRNA: TNF-α, IL-6 Proteins: p65, IL-1β, NLRP3, caspase-1 ↓ |
mRNA: IL-10 ↑ | |||
| Simvastatin [178] | LPS or CMS | SPT, FST, NSFT | HIP | Iba-1+↓ | Proteins: p65-NF-κB, IL-1β, TNF-α, IL-6 ↓ | — |
| ONO-2952 [179] | CSDS | OFT, EMP, TST, FST | NAc | — | Proteins: TNF-α, IL-6 ↓ | — |
| BLA | DHE/Iba1+↓ | Proteins: IL-1β, IL-6, IL-12 ↓ | — | |||
| PFC | — | Proteins: IL-1β, IL-12 ↓ | — | |||
| vHip | — | Protein: IL-12 ↓ | — | |||
| Iptakalim [180] | CRS | OFT, FST | Hypothalamus | TNF-α/CD11b+↓ | mRNA: TNF-α, TLR4, IL-1β ↓ | — |
| Caffeine [105] | CWIRS | SPT, FST, TST | HIP |
Protein: Iba-1 ↓ CD68+↓ |
Proteins: CD68, iNOS, TNF-α, IL-1β ↓ | — |
| Apelin-13 [181] | CWIRS | OFT, SPT, TST, FST | HIP | Iba-1+↑, Iba-1/iNOS+↑, Iba-1/Arg-1+↓ in CA1 | Proteins: IL-1β, IL-6, iNOS ↓ | Proteins: IL-1β, IL-6, Arg-1 ↑ |
| Melatonin [101] | CMS | SPT, FST | HIP | Iba-1+, CX3CL1+, CX3CR1+↑ in DG | — | — |
| ω-3 polyunsaturated fatty acids [182] | Ovariectomized | SPT, FST, NSFT, TST | HIP | Iba-1+↓ in DG |
mRNA: IL-1β, IL-6, TNF-α, CD68 ↓ Proteins: p-p65, IκB, iNOS ↓ |
mRNA: IL-4, IL-10, CD206, Arg-1 ↑ Protein: Arg-1 ↑ |
| Fingolimod [183] | CMS | OFT, SPT, FST, WMW | HIP | mRNA: Iba-1 ↑ |
ELISA: IL-1β, IL-6, TNF-α ↓ mRNA: NLRP3, ACS, caspase-1, iNOS, CD16 ↓ Proteins: iNOS, CD16, NLRP3, ACS, caspase-1 ↓ |
ELISA: IL-10 ↑ mRNA: Arg-1, CD206 ↑ Proteins: Arg-1, CD206 ↑ |
↑upregulated; ↓downregulated; (–) no significant difference;—no explicit data
Table 2.
Plant-derived natural compounds and formulations that regulate microglia-mediated neuroinflammation in animal models of depression
| Treatment | Animal models | Behavioral test | Analyzed regions | Microglia | Pro-inflammation | Anti-inflammation |
|---|---|---|---|---|---|---|
| Crocin [226] | LPS | OFT, FST, TST, FST | HIP | — |
ELISA: IL-1β, IL-18, TNF-α ↓ Proteins: CD16/32, iNOS, p-65-NF-κB ↓ |
Protein: CD206 ↑ |
| Baicalin [186] | CMS | OFT, FST, TST, FST | HIP | — | Proteins: IL-1β, IL-6, TNF-α, TLR4 ↓ | — |
| Catalpol [205] | CMS | OFT, EPM, FST | HIP | Iba-1+↓ |
mRNA: IL-1β, TNF-α, iNOS, IL-6, CD206 ↓ Proteins: NLRP3, cleaved caspase-1, IL-1β ↓ ROS ↓ |
mRNA: CD206 ↑ |
| Ganoderic acid A [227] | MCAO + CMS | OFT, SPT | HIP | — |
ELISA: TNF-α, IL-1β, IL-6 ↓ mRNA: TNF-α, IL-1β, IL-6, iNOS, CD68 ↓ Proteins: iNOS, CD68 ↓ |
ELISA: IL-10 ↑ mRNA: IL-10, CD206, Arg-1 ↑ Proteins: CD206, Arg-1 ↑ |
| Ginsenoside Rb1 [73] | CMS | OFT, SPT, TST, FST | HIP | Iba-1+↓ | mRNA: TNF-α, IL-1β ↓ | mRNA: TGF-β, Arg-1 ↑ |
| Cortex | Iba-1+↓ | mRNA: TNF-α, IL-1β ↓ | mRNA: TGF-β, Arg-1 ↑ | |||
| Salvianolic acid B [213] | CMS | SPT, TST, FST | HIP | Iba-1+(–) |
ELISA: TNF-α ↓ mRNA: IL-1β, TNF-α ↓ |
ELISA: IL-10 ↑ mRNA: IL-10, TGF-β ↑ |
| Cortex | Iba-1+↓ |
ELISA: TNF-α ↓ mRNA: IL-1β, TNF-α ↓ |
ELISA: IL-10 ↑ mRNA: IL-10, TGF-β ↑ |
|||
| Curcumin [215] | GWI + CRS | OLT, NORT, NSFT | HIP | Iba-1+↓ | — | — |
| Ginsenoside Rb1 [197] | CRS | OFT, TST, FST | HIP | Protein: Iba-1 ↓ | Proteins: TNF-α, IL-1β ↓ | — |
| Arctigenin [171] | CMS | SPT, TST, FST | Serum | — | ELISA: TNF-α, IL-1β, NO ↓ | — |
| PFC |
Iba-1/HMGB1+↓ Protein: Iba-1 ↓ |
Proteins: TNF-α, IL-1β, IDO ↓ | — | |||
| Astragalin [231] | CMS | SPT, TST, FST | HIP | Iba-1+↓ | Proteins: Nuclear p65-NF-κB, NLRP3, cleaved caspase-1, cleaved IL-1β, cleaved gasdermin D ↓ | — |
| Saikosaponin-d [190] | LPS | OFT, FST, TST, FST | HIP | Iba-1+↓ in CA1 | Proteins: CD68, IL-1β, IL-6, TNF-α, HMGB1, TLR4, p65-NF-κB, p-IκB-α ↓ | — |
| Asperosaponin VI [228] | LPS | OFT, FST | HIP | Iba-1+↓ |
mRNA: IL-1β, IL-6, TNF-α, iNOS ↓ Proteins: IL-1β, TNF-α, TLR4, p-NF-κB/NF-κB ↓ |
— |
| PFC | Iba-1+, iNOS/ Iba-1+↓ |
mRNA: IL-1β, IL-6, TNF-α, iNOS ↓ Proteins: IL-1β, TNF-α, TLR4, p-NF-κB/NF-κB ↓ |
||||
| 5-O-Methylvisammioside [233] | LPS | OFT, FST, FST, EPM | HIP | — | Proteins: NF-κB, IκB-α ↓ | — |
| Scutellarin [230] | LPS | OFT, SPT, FST | HIP | Iba-1+↓ |
Proteins: NLRP3, caspase-1, IL-1β ↓ ROS ↓ |
— |
| Ginsenoside Rg1 [200] | CMS | SPT, FST | vmPFC | Iba-1+↓ |
mRNA: IL-1β, IFN-γ, TNF-α ↓ ELISA: IL-1β, IFN-γ, TNF-α ↓ |
— |
| Ginsenoside Rg3 [201] | LPS | — | Cortex | Iba-1+↓ | — | — |
| Hypothalamus | Iba-1+↓ | — | — | |||
| Brain | iNOS+, COX-2+, Iba-1+↓ | mRNA: IL-1β, IL-6, TNF-α | — | |||
| Magnolol [219] | CMS | OFT, SPT, FST, TST | HIP |
Iba-1+↓ in DG; Iba-1/CD16/32+↓, Iba-1/CD206+↑ |
— | — |
| Brain | — | ELISA: TNF-α, IL-1β, IL-6, IL-12 ↓ |
ELISA: IL-4, IL-10 ↑ mRNA: Arg1, Ym1, Fizz1, Klf4 ↑ |
|||
| Geniposide [208] | LPS | SPT, TST, FST, OFT | HIP | — |
ELISA: TNF-α, IL-6 ↓ Proteins: CD86 ↓, CD206 ↑ |
— |
| Serum | — | ELISA: TNF-α, IL-6 ↓ | — | |||
| Arctiin [210] | CMS | TST, FST, OFT, SPT | PFC |
Iba-1+/HMGB1+↓ Protein: Iba-1 ↓ |
Proteins: TNF-α, IL-1β, iNOS, HMGB1 ↓ | — |
| Serum | — | ELISA: TNF-α, IL-1β, iNOS ↓ | ||||
| Ginkgolide B [234] | Depression in post myocardial infarction | OFT, SPT | Median raphe nucleus | — |
ELISA: IL-1β ↓ mRNA: IL-1β ↓ |
— |
| HIP | Iba-1+↓ |
ELISA: IL-1β ↓ mRNA: IL-1β ↓ |
— | |||
| Cortex | — |
ELISA: IL-1β ↓ mRNA: IL-1β ↓ |
— | |||
| Leonurine [235] | CMS | TST, FST, SPT | HIP | — | Proteins: IL-1β, IL-6, TNF-α, p-IKKβ/IKKβ, p-p65/p65 ↓ | — |
| Hesperidin [232] | CMS | FST, SPT, OFT | PFC | Iba-1+↓ |
mRNA: NLRP3, caspase-1, ASC ↓ Proteins: NLRP3, caspase-1, ASC ↓ ELISA: IL-1β, IL-6, TNF-α ↓ |
— |
| 20(S)-Protopanaxadiol [229] | CMS | SPT, TST, FST | HIP | Iba-1+↓ | Proteins: iNOS, COX-2, acetylated p65 (ac-p65) ↓ | — |
| Cortex | Iba-1+↓ | — | — | |||
| Ferulic acid [236] | CMS | SPT, FST | PFC | — |
mRNA: IL-6, IL-1β, TNF-α, NF-κB, CD11b ↓ Proteins: NLRP3, caspase-1 ↓ |
— |
| Resveratrol [224] | LPS | OFT, TST, FST | HIP | Iba-1+↓ in DG-SGZ | — | — |
| (+)-Sesamin [69] | CMS | OFT, FST, TST, EMP | HIP | Protein: Iba-1 ↓ | mRNA: COX-2, iNOS, IL-1β, TNF-α ↓ | — |
| Prelimbic cortex | Protein: Iba-1 ↓ | mRNA: COX-2, iNOS, IL-1β, TNF-α ↓ | — | |||
| Quercetin [187] | OBX | OFT, FST | HIP | — | ELISA: TNF-α, IL-6 ↓ | — |
| Cortex | — | ELISA: TNF-α, IL-6 ↓ | — | |||
| Theaflavins [246] | LPS | TST | HIP | MIP-1α/CD11b+, TNF-α/CD11b+↓ in microglia | ELISA: TNF-α, IL-1β ↓ | — |
| Epigallocatechin-3-gallate [99] | LPS | — | HIP | Iba-1+↓ in DG |
mRNA: IL-1β, IL-6, TNF-α ↓ Proteins: TLR4, Rel A, pRel A ↓ ELISA: IL-1β, IL-6, TNF-α ↓ |
— |
| Gypenosides [192] | CMS | SPT, TST | HIP | Iba-1+↓ in DG |
mRNA: IL-6 ↓ Proteins: IL-6, TNF-α ↓ |
— |
| Kososan [237] | CSDS | Social avoidance test | HIP | Iba-1+↓ | — | — |
| Aquilariae Lignum ethanol extracts [238] | CRS | — | HIP | Iba-1+↓ | Proteins: TNF-α, IL-1β, iNOS ↓ | — |
| Rosemary Extracts [239] | CRS | OFT, TST, FST | Serum | — | ELISA: IL-1β, TNF-α ↓ | — |
| HIP | Protein: Iba-1 ↓ | Proteins: IL-1β, TNF-α, p-p65-NF-κB ↓ | — | |||
| Radix Polygalae extract [240] | CRS | OFT, SPT, NSFT | PFC | Iba-1+↓ |
mRNA: NLRP3, IL-1β, IL-6, IL-18, TNF-α ↓ Proteins: NLRP3, ASC, cleaved caspase-1 |
— |
| Bangpungtongsung-san [241] | Reserpine | OFT, TST, FST | HIP | — | mRNA: IL-1β, IL-6, TNF-α ↓ | — |
| XingnaoJieyu decoction [242] | MCAO + CMS | OFT, FST | Cortex | Iba-1+↓ | ELISA: TNF-α, IL-6, IL-1β ↓ | — |
| HIP | Iba-1+↓ | ELISA: TNF-α, IL-6, IL-1β ↓ | — | |||
| Ganoderma lucidum polysaccharides [243] | CSDS | OFT, SPT, TST, FST | HIP | Iba-1+↓ | Proteins: IL-1β, TNF-α ↓ | Proteins: IL-10, BDNF ↑ |
| Water extract of Armillaria mellea (Vahl) P. Kumm [244] | CMS | OFT, FST | Cerebral | Protein: Iba-1 ↓ | Proteins: IL-1β, TNF-α ↓ | — |
| Xiaoyaosan [245] | CMS | SPT, OFT, FST, NSFT | HIP | Iba-1+↓ | mRNA: COX-2 ↓ | — |
| Myelophil [96] | CMS | OFT, TST, FST | HIP | Iba-1+↓ in CA1, DG, CA3 |
ELISA: IL-1β, TNF-α ↓ Proteins: NLRP3, ASC, pro-IL-1β, IL-1β ↓ |
— |
↑upregulated; ↓downregulated; (–) no significant difference;—no explicit data
Clinical antidepressive drugs
Clinical antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs), have exhibited effects on microglial activation and neuroinflammation [153]. Other compounds, including minocycline, melation, FCPR16, pioglitazone, iptakalim, and caffeine, also influence microglial polarization and depressive-like behavior [91], as shown in Table 1.
The SSRIs (fluoxetine, citalopram, and escitalopram) exhibit antidepressant roles in LPS, CMS, CSDS, and CWIRS models, as seen by alleviating nest-building deficits, reducing immobility time in the FST and TST, and increasing sucrose intake in the SPT (Table 1). These behavioral changes were accompanied by decreases in the microglial marker Iba-1 expression in different brain regions. In addition, pro-inflammatory factors levels (IL-1β, TNF-α, and NO) were downregulated, and anti-inflammatory factors levels (IL-4, IL-10, Arg-1, and TGF-β1) were upregulated [16, 101, 105, 154, 155]. Tricyclics, including clomipramine and imipramine, reversed depressive behaviors induced by LPS, LH, and CSDS by reducing the number of activated hippocampal microglia (Table 1). These traditional antidepressants promote the activation of anti-inflammatory microglia to release anti-inflammatory cytokines and neurotrophic factors, suggesting that reducing inflammation may be part of the function of clinical antidepressants [107, 131, 156]. Cytokines levels are strongly correlated with the efficacy of antidepressant treatment in patients. For example, higher levels of IL-6 and TNF-α are more commonly found in treatment-resistant patients than responders [157]. Nevertheless, some results revealed conflicting views that antidepressants might sometimes increase the inflammatory load in the brain. For instance, citalopram treatment elevated the levels of TNF-α and IFN-γ in the PFC, and these effects were inhibited by the anti-inflammatory agent ibuprofen [158]. Phenelzine, a monoamine oxidase inhibitor, has been shown to enhance the microglia-mediated immune responses by increasing the expressions of iNOS, TNF-α, and IL-6 in LPS-treated BV-2 cells. It has been verified that phenelzine also increased the levels of NO, TNF-α, and IL-6 via the NF-κB signaling pathway in LPS-activated primary microglia cells [159]. These differences may depend on regional brain specificity and heterogeneity among depression models and patients, but the conflicting causes and mechanisms need further study. Ketamine has exhibited potential antidepressant effects in vivo and in vitro by inhibiting microglia-mediated neuroinflammation [160]. Partial depletion of microglia with PLX3397 blocked the rapid and sustained antidepressant effects of (R)-ketamine (an isomer of ketamine), suggesting that antidepressant effects of ketamine may be partly attributed to microglial activation [161].
Minocycline is a potential agent for the treatment of depression [19]. In several studies, chronic minocycline treatment clearly alleviated depressive-like symptoms by inhibiting microglia and HPA axis hyperactivity. The powerful neuroprotective effects of minocycline were mainly mediated by modulating pro-inflammation and anti-inflammation balance in the PFC and HIP, as well as upregulating the BDNF-mediated synaptic plasticity in stress models [14, 162–164]. Meanwhile, CMS-induced changes in behavior, hippocampal LTP, CD11b expression, NLRP3 inflammasome, BDNF, and p-GluR1 levels were restored with chronic minocycline treatment [128, 165–168]. Peroxisome proliferator-activated receptor γ (PPARγ) is a nuclear receptor that regulates inflammation and microglial polarization and is a potential treatment target in MDD. Pioglitazone, a PPARγ agonist, significantly ameliorated depressive-like behaviors in CMS mice by regulating the expression of pro-inflammatory markers (IL-1β, IL-6, TNF-α, iNOS, and CCL2) together with anti-inflammatory markers (Ym1, Arg1, IL-4, IL-10, and TGF-β) in the HIP [169]. Other drugs that play a potential antidepressant role in modulating microglial phenotype are shown in Table 1.
Plant-derived natural compounds and formulations with antidepressant properties
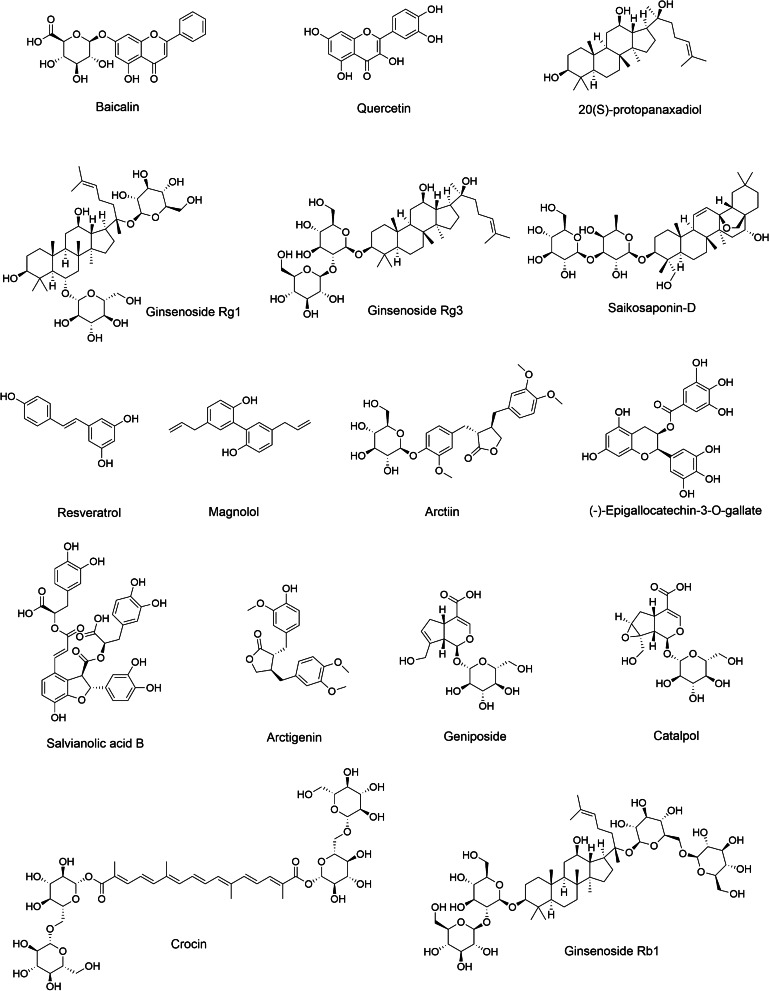
Recently, the regulatory properties of some plant-derived natural compounds and formulations have shown efficacy in treating depression. In this review, the antidepressive effect of natural products such as ginsenosides (Rg1, Rg3, and Rb1), resveratrol, salvianolic acid B, and magnolol on microglia-mediated neuroinflammation are summarized in Table 2. The chemical structures of several natural compounds that may act as potential inhibitors of pro-inflammatory microglial activation in MDD are illustrated in Fig. 5.
Fig. 5.
Chemical structures of several plant-derived natural compounds that act as potential inhibitors of M1-type microglia activation in animal models of depression
Flavonoids
Flavones are subgroups of flavonoids composed of a backbone of 2-phenylchromen-4-ketone, and their biological activities have been reported in vitro and in vivo [184]. Flavonoids achieve their antidepressive effects through modulating microglial activation and NLRP3 inflammasomes. Baicalin, a flavonoid found in the root of Scutellaria, could ameliorate depressive behaviors in CMS model [185, 186]. Oral administration of baicalin (20, 40 mg/kg/day) for 3 weeks significantly improved sucrose intake, locomotor activities, and behavioral despair in CMS rats. The mechanism of baicalin against CMS-induced depression was partially achieved by increasing levels of DCX, neuron-specific enolase (NSE), and BDNF, reducing oxidative stress, and modulating the GSK3/NF-κB/NLRP3 signal cascade in the HIP [185]. In addition, baicalin supplementation (30, 60 mg/kg/day, p.o.) for 3 weeks also inhibited TLR4 activation and pro-inflammatory cytokine secretion in CMS mice. Furthermore, it was confirmed that the inhibition might be realized through PI3K/AKT/FoxO1 pathway in LPS-stimulated BV2 cells [186]. Quercetin is a flavanol present in apples, onions, and berries [37]. Treatment with quercetin (40, 80 mg/kg/d, p.o.) for 2 weeks significantly reversed OBX-induced increase in immobility time in the FST, possibly by inhibiting TNF-α, IL-6, and caspase-3 in the HIP and cortex. Co-treatment with minocycline (25 mg/kg, p.o.) significantly potentiated its protective effects when compared to treatment with quercetin alone [187]. Treatment with quercetin (40 mg/kg/day, p.o.) for 2 weeks alleviated depressive-like behavior in the SPT and FST of LPS-challenged rats via regulation of BDNF-related imbalance of Copine 6 and TREM1/2 in the HIP and PFC [188].
Terpenoids
Terpenoids, especially triterpenoids, monoterpenes and sesquiterpenes, are the most abundant natural compounds found in several vegetables and fruits. Recently, some terpenoids have been reported to have preventive efficacy against neurological diseases by modulating the activation of microglia, especially on depression. Saponins, which are triterpene glycosides, are abundant in some plants, and their general biological activities have been summarized [189]. Saikosaponin-D is a triterpenoid saponin derived from Radix bupleuri and exhibits anti-inflammatory, anti-oxidative, and other pharmacological activities [190]. Previous studies have suggested that treatment with saikosaponin-D (0.75, 1.50 mg/kg/day, p.o.) for 3 weeks remarkably ameliorated the CMS-induced depressive-like behaviors in rats, mainly by improving HPA axis functions and hippocampal neurogenesis [191]. Moreover, additional research confirmed that saikosaponin-D administration ameliorated LPS-induced depressive-like behaviors, as shown by increased sucrose consumption in the SPT and decreased immobility time in the TST and FST. These performances appear to be mediated by inhibition of microglial activation and regulation of the high mobility group box 1 (HMGB1)/TLR4/NF-κB signaling pathway [190]. Gypenosides, the major ingredients of Gynostemma pentaphyllum, exert a neuroprotective function in the CNS. Treatment with gypenosides (50, 100 mg/kg/day, p.o.) for 4 weeks significantly relieved depressive-like behaviors of CMS mice in the TST and FST, which was mediated, in part, by inhibiting microglial activation and NF-κB signaling and increasing BDNF levels in the HIP [192, 193].
Ginsenosides are one of ginseng’s most biologically active ingredients and have a triterpenoid glycoside structure [194]. Direct and indirect evidence support that some ginsenosides (such as ginsenoside Rg3, Rb1, and Rg1) induce the anti-inflammatory actions of macrophages and microglia [195]. Ginsenoside Rb1 is a typical 20(S)-protopanaxadiol-type saponin and exerts significant antidepressive effects in chronic stress models [73, 196, 197]. It is known that Rb1 regulates microglial activation, protects neurons from inflammatory and oxidative damage, and promotes neurogenesis [198, 199]. Recently, treatment with Rb1 (20 mg/kg/d, p.o.) in CMS-treated mice for consecutive 4 weeks alleviated depressive-like behaviors in the SPT, TST, and FST mainly through PPARγ-mediated transitions in microglial phenotype (TNF-α, IL-1β, TGF-β, and Arg-1) in the HIP and cortex [73]. Rb1 attenuated a decrease in BDNF and the ratio of p-AKT/AKT expression, and increased IL-1β, TNF-α, and Iba-1 levels in the HIP in a CRS model. In line with in vivo reports, Rb1 lowered the protein expressions of IL-1β and TNF-α in BV-2 cells [197]. In addition to Rb1, ginsenoside Rg1 and Rg3 exhibited potential anti-inflammatory effects, which protected against microglial activation in stress-injured rodents [200, 201]. A previous study reported that chronic pretreatment with ginsenoside Rg1 (40 mg/kg/d, i.p.) for 5 weeks significantly suppressed inflammatory responses by alleviating microglial and astrocyte activation through decreased overexpression of IL-1β, IFN-γ, and TNF-α. These effects were accompanied by attenuation of dendritic spine and synaptic defects, and upregulation of synaptic-related proteins in the ventral medial prefrontal cortex (vmPFC). Ginsenoside Rg1 inhibited neuronal apoptosis by increasing Bcl-2 and decreasing cleaved caspase-3 and caspase-9 expressions after CMS exposure. Furthermore, ginsenoside Rg1 increased the nuclear factor erythroid 2-related factor (Nrf2) and inhibited p38 mitogen-activated protein kinase (p-p38 MAPK) and p65-NF-κB activation in the vmPFC [200]. Moreover, ginsenoside Rg3 at oral doses of 20 and 30 mg/kg attenuated upregulation of TNF-α, IL-1β, and IL-6 mRNA expression in brains of C57BL/6 mice after systemic LPS injection. Morphological activation of microglia, and Iba-1, cyclooxygenase-2 (COX-2), iNOS protein expression was reduced with Rg3 treatment [201]. Our previous studies also found that ginsenosides Rd and Re (10, 20, or 40 mg/kg, p.o.) have potentially neuroprotective and anti-inflammatory properties, as manifested by significantly reducing the expression of hippocampal pro-inflammatory factors and NLRP3 inflammasome related protein, as well as enhancing endogenous antioxidant factor Nrf2, and mediating PI3K-AKT and BDNF signaling pathways in mice exposed to CRS [129, 202]. Taken together, the anti-inflammatory effects of ginsenosides have been confirmed, and the negative regulation of pro-inflammatory cytokines and enzymes has been found to underlie the anti-inflammatory properties of ginsenosides in pro-inflammatory microglia in depression. However, additional studies are needed to investigate whether other ginsenosides or their metabolites can relieve depressive symptoms through modulating microglial phenotype and the molecular mechanisms involved in neuroinflammation.
Iridoid glycosides
Catalpol, an iridoid glucoside, is primarily isolated from Radix rehmannia and is commonly used as a traditional Chinese medicine [203]. Previous studies have been reported that catalpol exhibits a wide range of pharmacological effects, including exhibiting anti-diabetic, anti-tumor, anti-inflammatory, and antioxidant activities. Growing evidence indicates that catalpol has a robust antidepressant effect that acts through its anti-inflammatory and anti-oxidative properties in vitro and in vivo [203, 204]. Catalpol (5, 10, or 20 mg/kg/day, i.g.) administration for 5 weeks ameliorated CMS-induced depressive-like behavior in the SPT, and its underlying mechanisms might be at least partially ascribed to reducing HPA axis dysfunction, upregulating BDNF and its specific binding receptor tyrosine kinase B (TrkB), downregulating COX-2 expression, thus reducing prostaglandin E2 level in the brain [204]. It was also confirmed that catalpol at 20 mg/kg decreased the expression of NLRP3 inflammasome-associated proteins and inhibited pro-inflammatory microglial polarization in the HIP (IL-1β, TNF-α, and iNOS) [205]. Geniposide, a type of iridoid glycoside extracted from the ripe fruit of Gardenia jasminoides Ellis, exhibits numerous bioactivities, including anti-diabetic, anti-oxidative, and anti-inflammatory actions [206]. It has been demonstrated that administration of geniposide (10, 40 mg/kg/day, p.o.) for 1 or 3 weeks ameliorated LPS- and CMS-induced depressive-like behavior in the SPT, TST, and FST by regulating the Bruton’s tyrosine kinase (BTK)/TLR4/NF-κB, BDNF/TrkB, and BTK/JAK2/STAT1 signaling pathways [207, 208]. Also, the beneficial effect of geniposide on anxiety- and depressive-like behaviors in mice may possibly act through induction of microglial polarization towards anti-inflammatory phenotype and inhibition of IL-6 and TNF-α release [208].
Phenylpropanoids
Arctigenin and its glycoside, arctiin, are the major active ingredients of the dried ripe fruit of Arctiumlappa L. Arctiin can be metabolized into arctigenin by human intestinal microflora after oral consumption [209]. Arctiin has antidepressive effects that appear to act through inhibition of the NF-κB mediated HMGB1/TLR4 and TNF-α/TNF receptor 1 (TNFR1) pathways, which consequently attenuated microglial activation and neuroinflammation [210]. Similarly, arctigenin showed antidepressive effects by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB signaling pathways [171]. Salvianolic acid B (SalB) is one of the phenolic acid compounds derived from Salvia miltiorrhiza, which has various pharmacological effects, including anti-inflammatory, antioxidant, and anti-apoptotic [211, 212]. CMS mice treated with SalB (20 mg/kg/day, i.p.) for 3 weeks significantly reversed decreased sucrose preference index in the SPT and increased immobility time in the FST and TST. In addition, the decreased expression of IL-1β and TNF-α and increased expression of IL-10 and TGF-β were accompanied by increased apoptosis (cleaved-caspase 3+) and microglial activation (Iba-1+) in the HIP and cortex that were reversed after SalB treatment [213]. Curcumin is a representative natural phenolic compound of turmeric and a potential candidate for regulation of brain function due to its antioxidant and anti-inflammatory properties. In a CRS model, systemic administration of curcumin (10, 20, or 30 mg/kg, i.p.) daily for 3 weeks significantly attenuated oxidative stress and lipid peroxidation, prevented apoptosis, and increased antioxidant defense activity [214]. Curcumin (10 mg/kg, i.p.) also was beneficial for maintaining improved memory and mood functions after 30 days of daily therapy in a Gulf War Illness (GWI) with stress-treatment model. At the molecular level, enhanced numbers of DCX+ cells, decreased numbers of Iba-1/ED-1+ cells, and elevated antioxidant genes with normalized mitochondrial respiration might be the basis of the regulatory mechanism that is mediated by curcumin treatment [215]. Magnolol, a hydroxylated biphenyl compound extracted from Magnolia tree, has endothelial cell protective, antioxidant, and anti-inflammatory functions [216]. It is noteworthy that magnolol exhibits antidepressant effects, as evident by increased sucrose consumption and decreased the immobility time in the SPT, TST, and FST. In an OBX model, magnolol (50, 100 mg/kg/day, p.o.) treatment for 2 weeks produced antidepressant-like effects by enhancing neurogenesis (as indicated by BrdU positive cells) in the HIP [217]. In a CMS model, treatment with magnolol (20, 40 mg/kg/day, p.o.) for 4 weeks increased BDNF expression and normalized serotonergic system [218]. In agreement with previous studies, the protective effect of magnolol (50, 100 mg/kg/day, p.o.) given for 3 weeks to CMS-treated female mice also appeared to be associated with inhibition of pro-inflammatory microglia (TNF-α, IL-1β, and IL-6) and activation of anti-inflammatory microglia (IL-4, IL-10, Arg1, Ym1, Fizz1, and Klf4) via Nrf2/hemeoxygenase (HO-1)/NLRP3 signaling pathway [219].
Others
Resveratrol is one of the most well-known dietary stilbenoids. Resveratrol confers health benefits due to its antioxidant, anti-inflammatory, anti-aging, and immune-regulatory activities [220]. Resveratrol is found in the skin of red grapes, peanuts, and other medicinal plants. Recent studies have found that resveratrol ameliorated depressive-like behaviors in several animal models [221–223]. In a LPS model, mice were treated for 2 weeks with resveratrol (20 mg/kg/day, i.p.), which abrogated the increased immobility in the FST and TST. Immunohistochemical staining revealed that resveratrol reversed increased microglial activation (Iba-1+) and inhibition of neurogenesis (BrdU/DCX+) in the DG [224]. Moreover, 3 doses of resveratrol (20 mg/kg/day, i.p.) promoted the activation of sirtuin type 1 and blocked the decline of hippocampal neurogenesis triggered by ethanol exposure during early postnatal life [225]. The carotenoids crocin was able to regulate microglial activation associated with neurological disorders. Crocin decreased the expression of LPS-induced NO, TNF-α, and ROS production in BV-2 cells and improved locomotor activity, sucrose intake, and reduced immobility time in LPS-treated Kunming mice [226]. The health benefits associated with crocin might be due to its potent ability to regulate the NLRP3 inflammasome and NF-κB, as well as anti-inflammatory phenotypic conversion [226]. Moreover, emerging data have shown that plant-derived compounds can exert neuroprotective effects that prevent neurological disorders in depression. For instance, ganoderic acid A [227], asperosaponin VI [228], 20(S)-protopanaxadiol [229], scutellarin [230], astragalin [231], hesperidin [232], 5-O-methylvisammioside [233], ginkgolide B [234], leonurine [235], ferulic acid [236], and (+)-sesamin [69] were found to inactivate microglia in stress-induced depression models. Recent information concerning the antidepressant activity of phytochemicals through regulation of microglial polarization is illustrated in Table 2. In addition, several herbal extracts and traditional formulations reported to improve depression-like behavior by regulating microglia are shown in Table 2 [237–245].
Conclusions
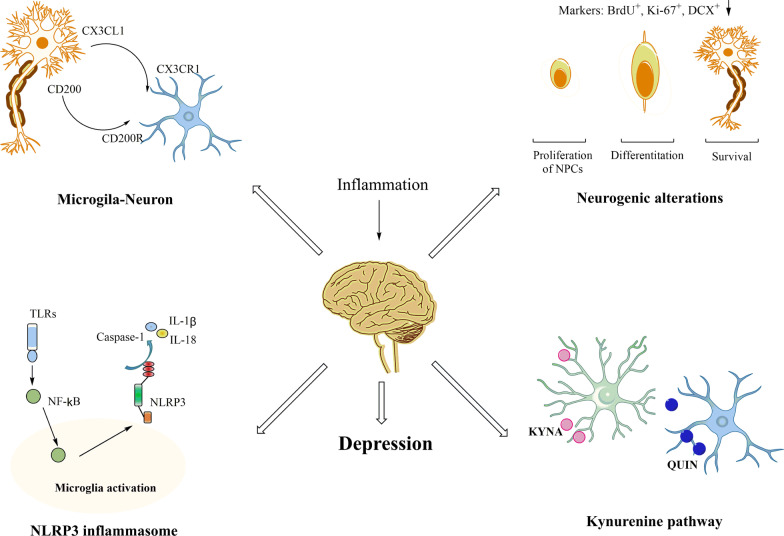
This review has summarized the current knowledge regarding microglial activation in depression as seen in clinical and preclinical studies. Microglia are the primary resident immune cells that are currently considered as a critical link between neurological and immunological activity in the CNS [247]. These cells modulate neuronal function not only during an inflammatory response, but also during developmental synaptic pruning and plasticity in healthy brains [27]. The heterogeneous states of activated microglia exist on a continuum ranging from neuroprotection to neurotoxic/pathogenic activity (Figs. 1 and 2) [248]. In this review, we summarized microglial activation in depressed patients and animal models (Additional file 1: Table S1), as well as the possible mechanisms associated with the pathogenesis of MDD (Fig. 4). Moreover, we described the therapeutic role of traditional antidepressants and phytochemicals in stress-induced depression models (Tables 1 and 2).
Fig. 4.
Schematic diagram of microglial activation involved in the pathogenesis of depression. Dysfunction of neuron–microglia interactions, particularly the CX3CL1-CX3CR1 and CD200-CD200R interactions, lead to behavioral and biochemical alterations. In addition, the most relevant markers of the NLRP3 inflammasome and neurogenesis used in the reviewed studies are indicated. We also included the generation of neuroactive kynurenine metabolites in depression disorders
Recently, natural compounds have been considered to be potential agents for the prevention or treatment of neuropsychiatric diseases due, in part, to their antioxidant and anti-inflammatory activities [249]. Numerous studies have shown that natural compounds and formulations are beneficial in the treatment of depression through their ability to regulate microglial functions, as listed in Table 2. The chemical structures of these bioactive compounds are highlighted in Fig. 5. Most compounds have been shown to modulate inflammatory response, oxidative stress, and ameliorate symptoms of depression through inhibiting microglial activation. However, additional research is needed to determine whether these compounds promote the transformation of microglia with a pro-inflammatory phenotype to an anti-inflammatory phenotype, or whether they depend on regulation of downstream signaling pathways that are activated by microglia. In a pathological state, changes in microglial phenotypes depend on the disease stage and severity. Therefore, developing protocols that control the stage-specific conversion of pro-inflammatory/anti-inflammatory actions in appropriate time frames might provide better therapeutic outcomes. Thus, changing the activity of microglia to a stable state through pharmacological or non-pharmacological methods will be the focus of future research, which also may provide new ideas for targeted treatment of depression. Moreover, it has been shown that some polyphenolic compounds are not completely absorbed via the gastrointestinal tract but still possess anti-inflammatory actions due to metabolites that are produced by intestinal microflora [250]. However, information concerning the role of these activated metabolites through microglia regulation is still minimal. Therefore, the discovery of potential inhibitors against microglial activation from metabolites may also be another promising strategy for the prevention of depression in the future.
In addition, data primarily obtained from studies that used specific microglial manipulation methods help improve our understanding of the mechanisms underlying neurogenesis, inflammation, and neurotransmitter metabolism dysfunction in depression. Genetic and pharmacological methods that directly or indirectly modify the activation status of microglia have been used and achieved some success, such as the Cx3cr1−/− or Cd200-deficient mouse models and the minocycline or PLX4497 treatment models [75, 251]. Moreover, it should be noted that the dichotomy of microglial activation states (M1/M2) is an oversimplified conceptual framework. This dichotomy is not generally accepted as it may not accurately reflect the heterogeneous microglial profiles that can be observed in complicated homeostatic or disease conditions [252–254]. The spatial and temporal distribution of microglial subsets can be better assessed using newly developed unbiased and high-throughput methods. Novel single-cell techniques enable scientists to overcome such limitations and reveal the surprising context-dependent heterogeneity of microglia [247, 255]. Specific subsets and key targets of microglia have been revealed in the development of AD and regulators such as TREM2 that control disease severity has been identified [42]. Currently, advanced technologies and tools are being used to comprehensively decipher the role of microglial heterogeneity in the pathology of depression, particularly in the modulation, inhibition, and stimulation of contextually relevant microglia functions. These technologies also are being used to target microglia to explore the treatment of neuropsychiatric diseases, which remains to be explored further.
Taken together, the data analyzed in this review suggest that microglial changes mainly affect the regulation of inflammatory response, neurogenesis, and tryptophan metabolism with respect to the development of depression. However, other factors should not be ignored. The discovery of natural compounds that exert antidepressant effects by inhibiting microglial activation may contribute to the effectiveness of preventing and treating depression. Additional information regarding the neuroprotective properties of these compounds that act through the regulation of microglial phenotypes remains to be explored in the future. High-performance omics technologies are expected to provide more effective molecular targets and identify additional specific signaling pathways in drug screening and disease diagnosis.
Supplementary Information
Additional file 1: Table S1. The animal models of depression involving microglial changes.
Acknowledgements
The authors would like to thank the Hotchkiss Brain Institute, the National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, and Natural Sciences and others for their financial contributions. The authors have no competing financial interests with respect to the work described here. All figures were generated with BioRender and ChemDraw.
Abbreviations
- MDD
Major depressive disorder
- COVID-19
Coronavirus disease 2019
- HPA
Hypothalamus–pituitary–adrenal
- CNS
Central nervous system
- PFC
Prefrontal cortex
- HIP
Hippocampus
- vHIP
Ventral hippocampus
- BLA
Basolateral
- SN
Substantia nigra
- NPC
Neural progenitor cell
- OPC
Oligodendrocyte progenitor cell
- CD86, CD16/32
Differentiation marker 86 and 16/32
- iNOS
Inducible nitric oxide synthase
- Arg1
Arginase-1
- TGF-β1
Transforming growth factor-β1
- BDNF
Brain-derived neurotrophic factor
- DAM
Disease-associated microglia
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
Danger-associated molecular patterns
- TLRs
Toll-like receptors
- LPS
Lipopolysaccharide
- IFN-γ
Interferon-γ
- IL-1β
Interleukin-1β
- TNF-α
Tumor necrosis factor-α
- ROS
Reactive oxygen species
- ACC
Anterior cingulate cortex
- QUIN
Quinolinic acid
- PET
Positron emission tomography
- Iba-1
Ionized calcium-binding adapter molecule 1
- CMS
Chronic mild stress
- CSDS
Chronic social defeat stress
- CRS
Chronic restraint stress
- OBX
Olfactory bulbectomy
- LH
Learned helplessness
- NLRP3
Nod-like receptor pyrin containing 3
- NF-κB
Nuclear factor-kappa B
- SPT
Sucrose preference test
- FST
Forced swimming test
- TST
Tail suspension test
- LD
Light–dark
- SIT
Social interaction test
- NORT
Novel object recognition test
- AD
Alzheimer’s disease
- SGZ/GCL
Subgranular zone/granule cell layer
- CWIRS
Chronic water-immersion restraint stress
- NMDAR
N-Methyl-d-aspartate receptor
- SYP
Synaptophysin
- PSD95
Postsynaptic densitin-95
- SSRIs
Selective serotonin reuptake inhibitors
- TCAs
Tricyclic antidepressants
- TRP
Tryptophan
- KP
Kynurenine pathway
- KYNA
Kynurenic acid
- 3-HK
3-Hydroxykynurenine
- KYN
Kynurenine
- NSFT
NOvelty suppressed feeding test
- ST
Splash test
- IDO1
Enzyme indoleamine 2, 3-dioxygenase 1
- PSD
Poststroke depression
- BDNF
Brain-derived neurotrophic factor
- HAAO
3-Hydroxyanthranilate 3, 4-dioxygenase
- NAc
Nucleus accumbens
- LTP
Long-term potentiation
- PPARγ
Peroxisome proliferator-activated receptor γ
- NSE
Neuron-specific enolase
- vmPFC
Ventral medial prefrontal cortex
- COX-2
Cyclooxygenase-2
- TrkB
Tyrosine kinase B
- BTK
Bruton’s tyrosine kinase
- HMGB1
High mobility group box 1
- TNFR1
TNF receptor 1
- SalB
Salvianolic acid B
- GWI
Gulf war illness
Author contributions
All authors participated in writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Beijing Young Top-Notch Talent Support project (2018000021223ZK36), Beijing Talents Project (2020A38), Beijing Hospitals Authority Youth Programme (QML20181901), National Natural Science Foundation of China (82171526 and 31970918), Beijing Biobank of Clinical Resources-Mental Disorders (BBCR-MD), and Beijing Municipal Administrationof Hospitals Incubating Program (PX2018065).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Haixia Wang and Yi He contributed equally in writing of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gang Wang, Email: gangwangdoc@ccmu.edu.cn.
Jian Yang, Email: yangjian@ccmu.edu.cn.
References
- 1.Smith K. Mental health: a world of depression. Nature. 2014;515:181. doi: 10.1038/515180a. [DOI] [PubMed] [Google Scholar]
- 2.Deng JW, Zhou FW, Hou WT, Silver Z, Wong CY, Chang O, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486:90–111. doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Depression. World Health Organization. Published [February 2017]. Updated [30 January 2020]. Accessed [25 June, 2019]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/depression.
- 4.Pollak DD, Rey CE, Monje FJ. Rodent models in depression research: classical strategies and new directions. Ann Med. 2010;42:252–264. doi: 10.3109/07853891003769957. [DOI] [PubMed] [Google Scholar]
- 5.Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model-are we there yet? Behav Brain Res. 2018;341:79–90. doi: 10.1016/j.bbr.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Frazer A, Benmansour S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 2002;7:23–28. doi: 10.1038/sj.mp.4001015. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Ge TT, Leng YS, Pan ZX, Fan J, Yang W, et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast. 2017;2017:6871089. doi: 10.1155/2017/6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng SL, Chen JG, Wang F. Microglia: a central player in depression. Curr Med Sci. 2020;40:391–400. doi: 10.1007/s11596-020-2193-1. [DOI] [PubMed] [Google Scholar]
- 9.Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. 2021;53:151–171. doi: 10.1111/ejn.14720. [DOI] [PubMed] [Google Scholar]
- 11.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapp ZM, Godbout JP, Kokiko-Cochran ON. A tilted axis: maladaptive inflammation and HPA axis dysfunction contribute to consequences of TBI. Front Neurol. 2019;10:345. doi: 10.3389/fneur.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker DJ, Zimmer C, Larriva M, Healy SD, Spencer KA. Early-life adversity programs long-term cytokine and microglia expression within the HPA axis in female Japanese quail. J Exp Biol. 2019;222:jeb187039. doi: 10.1242/jeb.187039. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Zhang YP, Li YY, Liu BP, Wang HY, Li KW, et al. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res. 2019;356:348–357. doi: 10.1016/j.bbr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Wang H, Wang JL, Wang YJ, Zhu Q, Wang CN, et al. Hippocampal salt-inducible kinase 2 plays a role in depression via the CREB-regulated transcription coactivator 1-cAMP response element binding-brain-derived neurotrophic factor pathway. Biol Psychiatry. 2019;85:650–666. doi: 10.1016/j.biopsych.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhong QP, Yu H, Huang C, Zhong JH, Wang HT, Xu JP, et al. FCPR16, a novel phosphodiesterase 4 inhibitor, produces an antidepressant-like effect in mice exposed to chronic unpredictable mild stress. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:62–75. doi: 10.1016/j.pnpbp.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Alboni S, Benatti C, Colliva C, Radighieri G, Blom JMC, Brunello N, et al. Vortioxetine prevents lipopolysaccharide-induced memory impairment without inhibiting the initial inflammatory cascade. Front Pharmacol. 2020;11:603979. doi: 10.3389/fphar.2020.603979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiat. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 19.Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed Pharmacother. 2008;62:308–311. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- 21.Pusic KM, Pusic AD, Kemme J, Kraig RP. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia. 2014;62:1176–1194. doi: 10.1002/glia.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Chiba K. Role of microglial M1/M2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel) 2014;7:1028–1048. doi: 10.3390/ph7121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang LJ, Zhang JQ, You ZL. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. 2018;12:306. doi: 10.3389/fncel.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-W. [DOI] [PubMed] [Google Scholar]
- 26.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med. 2019;25:967–979. doi: 10.1016/j.molmed.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci. 2017;18:2135. doi: 10.3390/ijms18102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198. doi: 10.3389/fncel.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dibaj P, Nadrigny F, Steffens H, Scheller A, Hirrlinger J, Schomburg ED, et al. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010;58:1133–1144. doi: 10.1002/glia.20993. [DOI] [PubMed] [Google Scholar]
- 34.Carbonell WS, Murase S, Horwitz AF, Mandell JW. Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci. 2005;25:7040–7047. doi: 10.1523/JNEUROSCI.5171-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Kim YK, Na KS. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:117–126. doi: 10.1016/j.pnpbp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Hung WL, Ho CT, Pan MH. Targeting the NLRP3 inflammasome in neuroinflammation: health promoting effects of dietary phytochemicals in neurological disorders. Mol Nutr Food Res. 2020;64:e1900550. doi: 10.1002/mnfr.201900550. [DOI] [PubMed] [Google Scholar]
- 38.Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity. 2016;44:505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Le WD. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 41.Sousa C, Golebiewska A, Poovathingal SK, Kaoma T, Pires-Afonso Y, Martina S, et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018;19:e46171. doi: 10.15252/embr.201846171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s Disease. Cell. 2017;169:1276–1290. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 44.Chen WT, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s Disease. Cell. 2020;182:976–991. doi: 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 45.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiat. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8:57. doi: 10.1186/s13550-018-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Sagar AP, Kéri S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:1–7. doi: 10.1016/j.pnpbp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry. 2018;5:339–347. doi: 10.1016/S2215-0366(18)30048-8. [DOI] [PubMed] [Google Scholar]
- 49.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Schnieder TP, Trencevska I, Rosoklija G, Stankov A, Mann JJ, Smiley J, et al. Microglia of prefrontal white matter in suicide. J Neuropathol Exp Neurol. 2014;73:880–890. doi: 10.1097/NEN.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/S0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 54.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/S0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 55.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 56.Bowley MP, Drevets WC, Ongür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/S0006-3223(02)01404-X. [DOI] [PubMed] [Google Scholar]
- 57.Ongür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. doi: 10.1002/(SICI)1096-9861(19981130)401:4<480::AID-CNE4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 58.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. doi: 10.1155/2013/608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiat. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- 62.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, et al. Microglia in the cerebral cortex in autism. J Autism Dev Disords. 2012;42:2569–2584. doi: 10.1007/s10803-012-1513-0. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Su WJ, Chen Y, Wu TY, Gong H, Shen XL, et al. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci Rep. 2016;6:23742. doi: 10.1038/srep23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu LL, Li JM, Su WJ, Wang B, Jiang CL. Sex differences in depressive-like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain Behav Immun. 2019;81:188–197. doi: 10.1016/j.bbi.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M. Differential microglial activation between acute stress and lipopolysaccharide treatment. J Neuroimmunol. 2009;207:24–31. doi: 10.1016/j.jneuroim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 68.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao YH, Wang QX, Jia MZ, Fu SC, Pan JR, Chu CQ, et al. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J Nutr Biochem. 2019;64:61–71. doi: 10.1016/j.jnutbio.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, et al. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y, Xu YZ, Wang YR, Wang YJ, He L, Jiang ZZ, et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain Behav Immun. 2015;50:298–313. doi: 10.1016/j.bbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Zhang JW, Zheng YL, Luo Y, Du Y, Zhang XJ, Fu JL. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Zhang LJ, Tang MM, Xie XF, Zhao QY, Hu N, He H, et al. Ginsenoside Rb1 induces a pro-neurogenic microglial phenotype via PPARγ activation in male mice exposed to chronic mild stress. J Neuroinflammation. 2021;18:171. doi: 10.1186/s12974-021-02185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lehmann ML, Weigel TK, Poffenberger CN, Herkenham M. The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. J Neurosci. 2019;39:5594–5605. doi: 10.1523/JNEUROSCI.0184-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chamera K, Trojan E, Szuster-Głuszczak M, Basta-Kaim A. The potential role of dysfunctions in neuron-microglia communication in the pathogenesis of brain disorders. Curr Neuropharmacol. 2020;18:408–430. doi: 10.2174/1570159X17666191113101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Wu XM, Luo QQ, Huang S, Yang Q-WQ, Wang FX, et al. CX3CL1/CX3CR1-mediated microglia activation plays a detrimental role in ischemic mice brain via p38MAPK/PKC pathway. J Cereb Blood Flow Metab. 2015;35:1623–1631. doi: 10.1038/jcbfm.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zanier ER, Marchesi F, Ortolano F, Perego C, Arabian M, Zoerle T, et al. Fractalkine receptor deficiency is associated with early protection but late worsening of outcome following brain trauma in mice. J Neurotrauma. 2016;33:1060–1072. doi: 10.1089/neu.2015.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang MM, Lin WJ, Pan YQ, Li YC. Fibroblast growth factor 2 modulates hippocampal microglia activation in a neuroinflammation induced model of depression. Front Cell Neurosci. 2018;12:255. doi: 10.3389/fncel.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merendino RA, Di Pasquale G, De Luca F, Di Pasquale L, Ferlazzo E, Martino G, et al. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate-severe depression. Mediators Inflamm. 2004;13:205–207. doi: 10.1080/09511920410001713484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.García-Marchena N, Barrera M, Mestre-Pintó JI, Araos P, Serrano A, Pérez-Mañá C, et al. Inflammatory mediators and dual depression: potential biomarkers in plasma of primary and substance-induced major depression in cocaine and alcohol use disorders. PLoS ONE. 2019;14:e0213791. doi: 10.1371/journal.pone.0213791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 82.Hellwig S, Brioschi S, Dieni S, Frings L, Masuch A, Blank T, et al. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav Immun. 2016;55:126–137. doi: 10.1016/j.bbi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, et al. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31(45):16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trojan E, Ślusarczyk J, Chamera K, Kotarska K, Głombik K, Kubera M, et al. The modulatory properties of chronic antidepressant drugs treatment on the brain chemokine-chemokine receptor network: a molecular study in an animal model of depression. Front Pharmacol. 2017;8:779. doi: 10.3389/fphar.2017.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ślusarczyk J, Trojan E, Wydra K, Głombik K, Chamera K, Kucharczyk M, et al. Beneficial impact of intracerebroventricular fractalkine administration on behavioral and biochemical changes induced by prenatal stress in adult rats: possible role of NLRP3 inflammasome pathway. Biochem Pharmacol. 2016;113:45–56. doi: 10.1016/j.bcp.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav Immun. 2016;55:114–125. doi: 10.1016/j.bbi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 87.Frank MG, Fonken LK, Annis JL, Watkins LR, Maier SF. Stress disinhibits microglia via down-regulation of CD200R: a mechanism of neuroinflammatory priming. Brain Behav Immun. 2018;69:62–73. doi: 10.1016/j.bbi.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fonken LK, Frank MG, Gaudet AD, D'Angelo HM, Daut RA, Hampson EC, et al. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun. 2018;70:257–267. doi: 10.1016/j.bbi.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blandino P, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 90.Wachholz S, Eßlinger M, Plümper J, Manitz MP, Juckel G, Friebe A. Microglia activation is associated with IFN-α induced depressive-like behavior. Brain Behav Immun. 2016;55:105–113. doi: 10.1016/j.bbi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 91.Nieto-Quero A, Chaves-Peña P, Santín LJ, Pérez-Martín M, Pedraza C. Do changes in microglial status underlie neurogenesis impairments and depressive-like behaviours induced by psychological stress? A systematic review in animal models. Neurobiol Stress. 2021;15:100356. doi: 10.1016/j.ynstr.2021.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559:98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ito N, Nagai T, Yabe T, Nunome S, Hanawa T, Yamada H. Antidepressant-like activity of a Kampo (Japanese herbal) medicine, Koso-san (Xiang-Su-San), and its mode of action via the hypothalamic-pituitary-adrenal axis. Phytomedicine. 2006;13:658–667. doi: 10.1016/j.phymed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Lu M, Yang JZ, Geng F, Ding JH, Hu G. Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. Int J Neuropsychopharmacol. 2014;17:1501–1510. doi: 10.1017/S1461145714000285. [DOI] [PubMed] [Google Scholar]
- 95.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee JS, Kim WY, Jeon YJ, Lee SB, Lee DS, Son CG. Antidepressant-like activity of Myelophil attenuation of microglial-mediated neuroinflammation in mice undergoing unpredictable chronic mild stress. Front Pharmacol. 2019;10:683. doi: 10.3389/fphar.2019.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Llorens-Martín M, Jurado-Arjona J, Bolós M, Pallas-Bazarra N, Ávila J. Forced swimming sabotages the morphological and synaptic maturation of newborn granule neurons and triggers a unique pro-inflammatory milieu in the hippocampus. Brain Behav Immun. 2016;53:242–254. doi: 10.1016/j.bbi.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seong KJ, Lee HG, Kook MS, Ko HM, Jung JY, Kim WJ. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in mice. Korean J Physiol Pharmacol. 2016;20:41–51. doi: 10.4196/kjpp.2016.20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng J, Chen M, Zhu JX, Li CF, Zhang QP, Geng D, et al. FGF-2 signaling activation in the hippocampus contributes to the behavioral and cellular responses to puerarin. Biochem Pharmacol. 2019;168:91–99. doi: 10.1016/j.bcp.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 101.Vega-Rivera NM, Ortiz-López L, Granados-Juárez A, Estrada-Camarena EM, Ramírez-Rodríguez GB. Melatonin reverses the depression-associated behaviour and regulates microglia, fractalkine expression and neurogenesis in adult mice exposed to chronic mild stress. Neuroscience. 2020;440:316–336. doi: 10.1016/j.neuroscience.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 102.Zhang JQ, Xie XF, Tang MM, Zhang J, Zhang BY, Zhao QY, et al. Salvianolic acid B promotes microglial M2-polarization and rescues neurogenesis in stress-exposed mice. Brain Behav Immun. 2017;66:111–124. doi: 10.1016/j.bbi.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 103.Han Y, Zhang LJ, Wang QZ, Zhang DD, Zhao QY, Zhang JQ, et al. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology. 2019;107:37–45. doi: 10.1016/j.psyneuen.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 104.Zhao QY, Peng C, Wu XH, Chen YB, Wang C, You ZL. Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiol Dis. 2014;68:57–65. doi: 10.1016/j.nbd.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 105.Mao ZF, Ouyang SH, Zhang QY, Wu YP, Wang GE, Tu LF, et al. New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: inhibition of CORT-induced microglia activation. FASEB J. 2020;34:10998–11014. doi: 10.1096/fj.202000146RR. [DOI] [PubMed] [Google Scholar]
- 106.Jiang N, Lv JW, Wang HX, Lu C, Wang Q, Xia TJ, et al. Dammarane sapogenins alleviates depression-like behaviours induced by chronic social defeat stress in mice through the promotion of the BDNF signalling pathway and neurogenesis in the hippocampus. Brain Res Bull. 2019;153:239–249. doi: 10.1016/j.brainresbull.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Jiang N, Lv J, Wang HX, Huang H, Wang Q, Lu C, et al. Ginsenoside Rg1 ameliorates chronic social defeat stress-induced depressive-like behaviors and hippocampal neuroinflammation. Life Sci. 2020;252:117669. doi: 10.1016/j.lfs.2020.117669. [DOI] [PubMed] [Google Scholar]
- 108.Park HJ, Shim HS, An K, Starkweather A, Kim KS, Shim I. IL-4 inhibits IL-1β-induced depressive-like behavior and central neurotransmitter alterations. Mediators Inflamm. 2015;2015:941413. doi: 10.1155/2015/941413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 110.Forster R, Sarginson A, Velichkova A, Hogg C, Dorning A, Horne AW, et al. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019;33:11210–11222. doi: 10.1096/fj.201900797R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang JQ, Rong PJ, Zhang LJ, He H, Zhou T, Fan YH, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. 2021;7:eabb9888. doi: 10.1126/sciadv.abb9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qi FF, Zuo ZJ, Yang JH, Hu SS, Yang Y, Yuan QF, et al. Combined effect of BCG vaccination and enriched environment promote neurogenesis and spatial cognition via a shift in meningeal macrophage M2 polarization. J Neuroinflammation. 2017;14:32. doi: 10.1186/s12974-017-0808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang L, Liu C, Li WY, Ma YQ, Huo SJ, Ozathaley A, et al. Depression-like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL-10 from microglia. Brain Behav Immun. 2021;97:68–78. doi: 10.1016/j.bbi.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 114.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, et al. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bassett B, Subramaniyam S, Fan Y, Varney S, Pan H, Carneiro AMD, et al. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav Immun. 2021;91:519–530. doi: 10.1016/j.bbi.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 116.Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, et al. Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol Psychiatry. 2018;23:556–568. doi: 10.1038/mp.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Impellizzeri D, Mazzon E, Paterniti I, Esposito E, Cuzzocrea S. Effect of fasudil, a selective inhibitor of Rho kinase activity, in the secondary injury associated with the experimental model of spinal cord trauma. J Pharmacol Exp Ther. 2012;343:21–33. doi: 10.1124/jpet.111.191239. [DOI] [PubMed] [Google Scholar]
- 119.Singhal G, Baune BT. Microglia: an interface between the loss of neuroplasticity and depression. Front Cell Neurosci. 2017;11:270. doi: 10.3389/fncel.2017.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 121.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 122.Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, et al. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int J Neuropsychopharmacol. 2015;18(8):pyv006. doi: 10.1093/ijnp/pyv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yue N, Huang HJ, Zhu XC, Han QQ, Wang YL, Li B, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14:102. doi: 10.1186/s12974-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther. 2014;20(2):119–124. doi: 10.1111/cns.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dai JJ, Ding ZF, Zhang J, Xu W, Guo QL, Zou WY, et al. Minocycline relieves depressive-like behaviors in rats with bone cancer pain by inhibiting microglia activation in hippocampus. Anesth Analg. 2019;129:1733–1741. doi: 10.1213/ANE.0000000000004063. [DOI] [PubMed] [Google Scholar]
- 127.Arakawa S, Shirayama Y, Fujita Y, Ishima T, Horio M, Muneoka K, et al. Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol Biochem Behav. 2012;100:601–606. doi: 10.1016/j.pbb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 128.Liu MC, Li J, Dai P, Zhao F, Zheng G, Jing JF, et al. Microglia activation regulates GluR1 phosphorylation in chronic unpredictable stress-induced cognitive dysfunction. Stress. 2015;18:96–106. doi: 10.3109/10253890.2014.995085. [DOI] [PubMed] [Google Scholar]
- 129.Wang HX, Lv JW, Jiang N, Huang H, Wang Q, Liu X. Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother Res. 2021;35:2523–2535. doi: 10.1002/ptr.6947. [DOI] [PubMed] [Google Scholar]
- 130.Du RH, Tan J, Sun XY, Lu M, Ding JH, Hu G. Fluoxetine inhibits NLRP3 inflammasome activation: implication in depression. Int J Neuropsychopharmacol. 2016;19(9):pyw037. doi: 10.1093/ijnp/pyw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gong W, Zhang S, Zong Y, Halim M, Ren Z, Wang Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15–25. doi: 10.1016/j.jad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Yue N, Li B, Yang L, Han QQ, Huang HJ, Wang YL, et al. Electro-acupuncture alleviates chronic unpredictable stress-induced depressive- and anxiety-like behavior and hippocampal neuroinflammation in rat model of depression. Front Mol Neurosci. 2018;11:149. doi: 10.3389/fnmol.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garrison AM, Parrott JM, Tuñon A, Delgado J, Redus L, O'Connor JC. Kynurenine pathway metabolic balance influences microglia activity: targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology. 2018;94:1–10. doi: 10.1016/j.psyneuen.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78(4):842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 135.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115:1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 136.Birner A, Platzer M, Bengesser SA, Dalkner N, Fellendorf FT, Queissner R, et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE. 2017;12(2):e0172699. doi: 10.1371/journal.pone.0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dantzer R. Role of the kynurenine metabolism pathway in inflammation-induced depression: preclinical approaches. Curr Top Behav Neurosci. 2017;31:117–138. doi: 10.1007/7854_2016_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Müller UJ, et al. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? Eur Arch Psychiatry Clin Neurosci. 2015;265(4):321–329. doi: 10.1007/s00406-014-0562-0. [DOI] [PubMed] [Google Scholar]
- 139.Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25. doi: 10.1016/j.neubiorev.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 140.Achtyes E, Keaton SA, Smart L, Burmeister AR, Heilman PL, Krzyzanowski S, et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun. 2020;83:239–247. doi: 10.1016/j.bbi.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Öztürk M, Yalın Sapmaz Ş, Kandemir H, Taneli F, Aydemir Ö. The role of the kynurenine pathway and quinolinic acid in adolescent major depressive disorder. Int J Clin Pract. 2021;75:e13739. doi: 10.1111/ijcp.13739. [DOI] [PubMed] [Google Scholar]
- 142.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609–1616. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mithaiwala MN, Santana-Coelho D, Porter GA, O'Connor JC. Neuroinflammation and the kynurenine pathway in CNS disease: molecular mechanisms and therapeutic implications. Cells. 2021;10:1548. doi: 10.3390/cells10061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verdonk F, Petit AC, Abdel-Ahad P, Vinckier F, Jouvion G, de Maricourt P, et al. Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav Immun. 2019;81:361–373. doi: 10.1016/j.bbi.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 146.Liu XC, Erhardt S, Goiny M, Engberg G, Mathé AA. Decreased levels of kynurenic acid in prefrontal cortex in a genetic animal model of depression. Acta Neuropsychiatr. 2017;29:54–58. doi: 10.1017/neu.2016.31. [DOI] [PubMed] [Google Scholar]
- 147.Chen HB, Li F, Wu S, An SC. Hippocampus quinolinic acid modulates glutamate and NMDAR/mGluR1 in chronic unpredictable mild stress-induced depression. Sheng Li Xue Bao. 2013;65:577–585. [PubMed] [Google Scholar]
- 148.Deng YY, Zhou MF, Wang JF, Yao JX, Yu J, Liu WW, et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes. 2021;13:1–16. doi: 10.1080/19490976.2020.1869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Imbeault S, Goiny M, Liu X, Erhardt S. Effects of IDO1 and TDO2 inhibition on cognitive deficits and anxiety following LPS-induced neuroinflammation. Acta Neuropsychiatr. 2020;32:43–53. doi: 10.1017/neu.2019.44. [DOI] [PubMed] [Google Scholar]
- 150.Thomas J, Khanam R, Vohora D. Augmentation of antidepressant effects of venlafaxine by agomelatine in mice are independent of kynurenine pathway. Neurochem Int. 2016;99:103–109. doi: 10.1016/j.neuint.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 151.Koo YS, Kim H, Park JH, Kim MJ, Shin YI, Choi BT, et al. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism contributes to poststroke depression induced in mice by ischemic stroke along with spatial restraint stress. Oxid Med Cell Longev. 2018;2018:2413841. doi: 10.1155/2018/2413841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fuertig R, Azzinnari D, Bergamini G, Cathomas F, Sigrist H, Seifritz E, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun. 2016;54:59–72. doi: 10.1016/j.bbi.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 153.Kopschina Feltes P, Doorduin J, Klein HC, Juárez-Orozco LE, Dierckx RA, Moriguchi-Jeckel CM, et al. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol. 2017;31:1149–1165. doi: 10.1177/0269881117711708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu LM, Zhao ZX, Lu LW, Liu JQ, Sun J, Dong JC. Icariin and icaritin ameliorated hippocampus neuroinflammation via mediating HMGB1 expression in social defeat model in mice. Int Immunopharmacol. 2019;75:105799. doi: 10.1016/j.intimp.2019.105799. [DOI] [PubMed] [Google Scholar]
- 155.Cheng J, Chen M, Wan HQ, Chen XQ, Li CF, Zhu JX, et al. Paeoniflorin exerts antidepressant-like effects through enhancing neuronal FGF-2 by microglial inactivation. J Ethnopharmacol. 2021;274:114046. doi: 10.1016/j.jep.2021.114046. [DOI] [PubMed] [Google Scholar]
- 156.Iwata M, Ishida H, Kaneko K, Shirayama Y. Learned helplessness activates hippocampal microglia in rats: a potential target for the antidepressant imipramine. Pharmacol Biochem Behav. 2016;150:138–146. doi: 10.1016/j.pbb.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 157.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 158.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108(22):9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chung HS, Kim H, Bae H. Phenelzine (monoamine oxidase inhibitor) increases production of nitric oxide and proinflammatory cytokines via the NF-κB pathway in lipopolysaccharide-activated microglia cells. Neurochem Res. 2012;37:2117–2124. doi: 10.1007/s11064-012-0833-y. [DOI] [PubMed] [Google Scholar]
- 160.Tan SJ, Wang Y, Chen K, Long ZF, Zou J. Ketamine alleviates depressive-like behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol Pharm Bull. 2017;40:1260–1267. doi: 10.1248/bpb.b17-00131. [DOI] [PubMed] [Google Scholar]
- 161.Zhang K, Yang C, Chang LX, Sakamoto A, Suzuki T, Fujita Y, et al. Essential role of microglial transforming growth factor-β1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-β1. Transl Psychiatry. 2020;10:32. doi: 10.1038/s41398-020-0733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu N, Tang XH, Pan W, Xie ZM, Zhang GF, Ji MH, et al. Spared nerve injury increases the expression of microglia M1 markers in the prefrontal cortex of rats and provokes depression-like behaviors. Front Neurosci. 2017;11:209. doi: 10.3389/fnhum.2017.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1–M2 microglial gene expression in a rat model of depression. Brain Behav Immun. 2014;42:147–156. doi: 10.1016/j.bbi.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 164.Majidi J, Kosari-Nasab M, Salari AA. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res Bull. 2016;120:1–13. doi: 10.1016/j.brainresbull.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 165.Peng ZL, Zhang C, Yan L, Zhang YP, Yang ZY, Wang JJ, et al. EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippocampal apoptosis signaling in a chronic stress-induced rat model of depression. Int J Mol Sci. 2020;21:1769. doi: 10.3390/ijms21051769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang YL, Han QQ, Gong WQ, Pan DH, Wang LZ, Hu W, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation. 2018;15:21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 168.Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex. 2013;23:1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- 169.Zhao QY, Wu XH, Yan S, Xie XF, Fan YH, Zhang JQ, et al. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. J Neuroinflammation. 2016;13:259. doi: 10.1186/s12974-016-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farooq RK, Tanti A, Ainouche S, Roger S, Belzung C, Camus V. A P2X7 receptor antagonist reverses behavioural alterations, microglial activation and neuroendocrine dysregulation in an unpredictable chronic mild stress (UCMS) model of depression in mice. Psychoneuroendocrinology. 2018;97:120–130. doi: 10.1016/j.psyneuen.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 171.Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol. 2020;177:5224–5245. doi: 10.1111/bph.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu Y, Xu X, Jiang T, Jin L, Zhao XD, Cheng JH, et al. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Int Immunopharmacol. 2019;67:119–128. doi: 10.1016/j.intimp.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 173.Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav Immun. 2016;57:293–303. doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Molteni R, Macchi F, Zecchillo C, Dell'agli M, Colombo E, Calabrese F, et al. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol. 2013;23:1645–1655. doi: 10.1016/j.euroneuro.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 175.Duan CM, Zhang JR, Wan TF, Wang Y, Chen HS, Liu L. SRT2104 attenuates chronic unpredictable mild stress-induced depressive-like behaviors and imbalance between microglial M1 and M2 phenotypes in the mice. Behav brain Res. 2020;378:112296. doi: 10.1016/j.bbr.2019.112296. [DOI] [PubMed] [Google Scholar]
- 176.Su WJ, Zhang T, Jiang CL, Wang W. Clemastine alleviates depressive-like behavior through reversing the imbalance of microglia-related pro-inflammatory state in mouse hippocampus. Front Cell Neurosci. 2018;12:412. doi: 10.3389/fncel.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takahashi K, Nakagawasai O, Nemoto W, Kadota S, Isono J, Odaira T, et al. Memantine ameliorates depressive-like behaviors by regulating hippocampal cell proliferation and neuroprotection in olfactory bulbectomized mice. Neuropharmacology. 2018;137:141–155. doi: 10.1016/j.neuropharm.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 178.Yu XB, Zhang HN, Dai Y, Zhou ZY, Xu RA, Hu L-F, et al. Simvastatin prevents and ameliorates depressive behaviors via neuroinflammatory regulation in mice. J Affect Disord. 2019;245:939–949. doi: 10.1016/j.jad.2018.11.086. [DOI] [PubMed] [Google Scholar]
- 179.Nozaki K, Ito H, Ohgidani M, Yamawaki Y, Sahin EH, Kitajima T, et al. Antidepressant effect of the translocator protein antagonist ONO-2952 on mouse behaviors under chronic social defeat stress. Neuropharmacology. 2020;162:107835. doi: 10.1016/j.neuropharm.2019.107835. [DOI] [PubMed] [Google Scholar]
- 180.Zhao XJ, Zhao Z, Yang DD, Cao LL, Zhang L, Ji J, et al. Activation of ATP-sensitive potassium channel by iptakalim normalizes stress-induced HPA axis disorder and depressive behaviour by alleviating inflammation and oxidative stress in mouse hypothalamus. Brain Res Bull. 2017;130:146–155. doi: 10.1016/j.brainresbull.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 181.Zhou SH, Chen SS, Xie WX, Guo XX, Zhao JF. Microglia polarization of hippocampus is involved in the mechanism of Apelin-13 ameliorating chronic water immersion restraint stress-induced depression-like behavior in rats. Neuropeptides. 2020;81:102006. doi: 10.1016/j.npep.2020.102006. [DOI] [PubMed] [Google Scholar]
- 182.Wu B, Song QG, Zhang YK, Wang CS, Yang MQ, Zhang J, et al. Antidepressant activity of ω-3 polyunsaturated fatty acids in ovariectomized rats: role of neuroinflammation and microglial polarization. Lipids Health Dis. 2020;19:4. doi: 10.1186/s12944-020-1185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guo YX, Gan XH, Zhou HF, Zhou HJ, Pu SY, Long X, et al. Fingolimod suppressed the chronic unpredictable mild stress-induced depressive-like behaviors via affecting microglial and NLRP3 inflammasome activation. Life Sci. 2020;263:118582. doi: 10.1016/j.lfs.2020.118582. [DOI] [PubMed] [Google Scholar]
- 184.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8:281–290. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 185.Zhang CYY, Zeng MJ, Zhou LP, Li YQ, Zhao F, Shang ZY, et al. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. Int Immunopharmacol. 2018;64:175–182. doi: 10.1016/j.intimp.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 186.Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16:95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rinwa P, Kumar A. Quercetin suppress microglial neuroinflammatory response and induce antidepressant-like effect in olfactory bulbectomized rats. Neuroscience. 2013;255:86–98. doi: 10.1016/j.neuroscience.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 188.Fang K, Li HR, Chen XX, Gao XR, Huang LL, Du AQ, et al. Quercetin alleviates LPS-induced depression-like behavior in rats regulating BDNF-related imbalance of copine 6 and TREM1/2 in the hippocampus and PFC. Front Pharmacol. 2019;10:1544. doi: 10.3389/fphar.2019.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 190.Su J, Pan YW, Wang SQ, Li XZ, Huang F, Ma SP. Saikosaponin-d attenuated lipopolysaccharide-induced depressive-like behaviors via inhibiting microglia activation and neuroinflammation. Int Immunopharmacol. 2020;80:106181. doi: 10.1016/j.intimp.2019.106181. [DOI] [PubMed] [Google Scholar]
- 191.Li HY, Zhao YH, Zeng MJ, Fang F, Li M, Qin TT, et al. Saikosaponin D relieves unpredictable chronic mild stress induced depressive-like behavior in rats: involvement of HPA axis and hippocampal neurogenesis. Psychopharmacology. 2017;234:3385–3394. doi: 10.1007/s00213-017-4720-8. [DOI] [PubMed] [Google Scholar]
- 192.Dong SQ, Zhang QP, Zhu JX, Chen M, Li CF, Liu Q, et al. Gypenosides reverses depressive behavior via inhibiting hippocampal neuroinflammation. Biomed Pharmacother. 2018;106:1153–1160. doi: 10.1016/j.biopha.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 193.Mu RH, Fang XY, Wang SS, Li CF, Chen SM, Chen XM, et al. Antidepressant-like effects of standardized gypenosides: involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology. 2016;233:3211–3221. doi: 10.1007/s00213-016-4357-z. [DOI] [PubMed] [Google Scholar]
- 194.Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Im DS. Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of. Biomolecules. 2020;10:444. doi: 10.3390/biom10030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang GL, He ZM, Zhu HY, Gao YG, Zhao Y, Yang H, et al. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng. J Ethnopharmacol. 2017;204:118–124. doi: 10.1016/j.jep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 197.Guo Y, Xie JP, Zhang LC, Yang LL, Ma JQ, Bai YF, et al. Ginsenoside Rb1 exerts antidepressant-like effects via suppression inflammation and activation of AKT pathway. Neurosci Lett. 2021;744:135561. doi: 10.1016/j.neulet.2020.135561. [DOI] [PubMed] [Google Scholar]
- 198.Li DW, Zhou FZ, Sun XC, Li SC, Yang JB, Sun HH, et al. Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen Res. 2019;14:1814–1822. doi: 10.4103/1673-5374.257536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang D, Zhao SX, Pan JW, Wang Z, Li Y, Xu XX, et al. Ginsenoside Rb1 attenuates microglia activation to improve spinal cord injury via microRNA-130b-5p/TLR4/NF-κB axis. J Cell Physiol. 2021;236:2144–2155. doi: 10.1002/jcp.30001. [DOI] [PubMed] [Google Scholar]
- 200.Fan CQ, Song QQ, Wang P, Li Y, Yang M, Yu SY. Neuroprotective effects of ginsenoside-Rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 201.Park SM, Choi MS, Sohn NW, Shin JW. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol Pharm Bull. 2012;35:1546–1552. doi: 10.1248/bpb.b12-00393. [DOI] [PubMed] [Google Scholar]
- 202.Wang HX, Jiang N, Lv JW, Huang H, Liu XM. Ginsenoside Rd reverses cognitive deficits by modulating BDNF-dependent CREB pathway in chronic restraint stress mice. Life Sci. 2020;258:118107. doi: 10.1016/j.lfs.2020.118107. [DOI] [PubMed] [Google Scholar]
- 203.Yang CJ, Shi ZY, You LT, Du YY, Ni J, Yan D. Neuroprotective effect of catalpol anti-oxidative, anti-inflammatory, and anti-apoptotic mechanisms. Front Pharmacol. 2020;11:690. doi: 10.3389/fphar.2020.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang JM, Yang LH, Zhang YY, Niu CL, Cui Y, Feng WS, et al. BDNF and COX-2 participate in anti-depressive mechanisms of catalpol in rats undergoing chronic unpredictable mild stress. Physiol Behav. 2015;151:360–368. doi: 10.1016/j.physbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 205.Wang YL, Wu HR, Zhang SS, Xiao HL, Yu J, Ma YY, et al. Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl Psychiatry. 2021;11:353. doi: 10.1038/s41398-021-01468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu SN, Zheng ML, Li YX, He L, Chen T. The protective effect of Geniposide on diabetic cognitive impairment through BTK/TLR4/NF-κB pathway. Psychopharmacology. 2020;237:465–477. doi: 10.1007/s00213-019-05379-w. [DOI] [PubMed] [Google Scholar]
- 207.Chen T, Liu SN, Zheng ML, Li YX, He L. The effect of geniposide on chronic unpredictable mild stress-induced depressive mice through BTK/TLR4/NF-κB and BDNF/TrkB signaling pathways. Phytother Res. 2021;35:932–945. doi: 10.1002/ptr.6846. [DOI] [PubMed] [Google Scholar]
- 208.Zheng ML, Li K, Chen T, Liu SN, He L. Geniposide protects depression through BTK/JAK2/STAT1 signaling pathway in lipopolysaccharide-induced depressive mice. Brain Res Bull. 2021;170:65–73. doi: 10.1016/j.brainresbull.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 209.Wang W, Pan Q, Han XY, Wang J, Tan RQ, He F, et al. Simultaneous determination of arctiin and its metabolites in rat urine and feces by HPLC. Fitoterapia. 2013;86:6. doi: 10.1016/j.fitote.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 210.Xu X, Zeng XY, Cui YX, Li YB, Cheng JH, Zhao XD, et al. Antidepressive effect of arctiin by attenuating neuroinflammation via HMGB1/TLR4- and TNF-α/TNFR1-mediated NF-κB activation. ACS Chem Neurosci. 2020;11:2214–2230. doi: 10.1021/acschemneuro.0c00120. [DOI] [PubMed] [Google Scholar]
- 211.Wang SX, Hu LM, Gao XM, Guo H, Fan GW. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res. 2010;35(7):1029–1037. doi: 10.1007/s11064-010-0151-1. [DOI] [PubMed] [Google Scholar]
- 212.Liu CS, Cheng Y, Hu JF, Zhang W, Chen NH, Zhang JT. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb 761) Acta Pharmacol Sin. 2006;27:1137–1145. doi: 10.1111/j.1745-7254.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 213.Zhang JQ, Wu XH, Feng Y, Xie XF, Fan YH, Yan S, et al. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: involvement of the neuroinflammatory pathway. Acta Pharmacol Sin. 2016;37:1141–1153. doi: 10.1038/aps.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother. 2017;87:223–229. doi: 10.1016/j.biopha.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 215.Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, Shetty AK. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav Immun. 2018;69:499–514. doi: 10.1016/j.bbi.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ranaware AM, Banik K, Deshpande V, Padmavathi G, Roy NK, Sethi G, et al. Magnolol: a neolignan from the magnolia family for the prevention and treatment of cancer. Int J Mol Sci. 2018;19:2362. doi: 10.3390/ijms19082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Matsui N, Akae H, Hirashima N, Kido Y, Tanabe S, Koseki M, et al. Magnolol enhances hippocampal neurogenesis and exerts antidepressant-like effects in olfactory bulbectomized mice. Phytother Res. 2016;30:1856–1861. doi: 10.1002/ptr.5695. [DOI] [PubMed] [Google Scholar]
- 218.Li LF, Lu J, Li XM, Xu CL, Deng JM, Qu R, et al. Antidepressant-like effect of magnolol on BDNF up-regulation and serotonergic system activity in unpredictable chronic mild stress treated rats. Phytother Res. 2012;26:1189–1194. doi: 10.1002/ptr.3706. [DOI] [PubMed] [Google Scholar]
- 219.Tao WW, Hu YW, Chen ZY, Dai YX, Hu Y, Qi MM. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine. 2021;91:153692. doi: 10.1016/j.phymed.2021.153692. [DOI] [PubMed] [Google Scholar]
- 220.Navarro G, Martínez-Pinilla E, Ortiz R, Noé V, Ciudad CJ, Franco R. Resveratrol and related stilbenoids, nutraceutical/dietary complements with health-promoting actions: industrial production, safety, and the search for mode of action. Compr Rev Food Sci Food Saf. 2018;17:808–826. doi: 10.1111/1541-4337.12359. [DOI] [PubMed] [Google Scholar]
- 221.Ali SH, Madhana RM, Athira KV, Kasala ER, Bodduluru LN, Pitta S, et al. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids. 2015;101:37–42. doi: 10.1016/j.steroids.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 222.Ge JF, Peng L, Cheng JQ, Pan CX, Tang J, Chen FH, et al. Antidepressant-like effect of resveratrol: involvement of antioxidant effect and peripheral regulation on HPA axis. Pharmacol Biochem Behav. 2013;114:64–69. doi: 10.1016/j.pbb.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 223.Ge L, Liu LW, Liu H, Liu S, Xue H, Wang XE, et al. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol. 2015;768:49–57. doi: 10.1016/j.ejphar.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 224.Liu L, Zhang Q, Cai YL, Sun DY, He X, Wang L, et al. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget. 2016;7:56045–56059. doi: 10.18632/oncotarget.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xu L, Yang Y, Gao LX, Zhao JH, Cai YL, Huang J, et al. Protective effects of resveratrol on the inhibition of hippocampal neurogenesis induced by ethanol during early postnatal life. Biochim Biophys Acta. 2015;1852:1298–1310. doi: 10.1016/j.bbadis.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 226.Zhang L, Previn R, Lu L, Liao RF, Jin Y, Wang RK. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-κB and NLRP3 signaling pathway. Brain Res Bull. 2018;142:352–359. doi: 10.1016/j.brainresbull.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 227.Zhang L, Zhang L, Sui RB. Ganoderic acid A-mediated modulation of microglial polarization is involved in depressive-like behaviors and neuroinflammation in a rat model of post-stroke depression. Neuropsychiatr Dis Treat. 2021;17:2671–2681. doi: 10.2147/NDT.S317207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang JQ, Yi SN, Li YH, Xiao CH, Liu C, Jiang WK, et al. The antidepressant effects of asperosaponin VI are mediated by the suppression of microglial activation and reduction of TLR4/NF-κB-induced IDO expression. Psychopharmacology. 2020;237:2531–2545. doi: 10.1007/s00213-020-05553-5. [DOI] [PubMed] [Google Scholar]
- 229.Jiang N, Lv JW, Wang HX, Huang H, Wang Q, Zeng GR, et al. Ginsenoside 20(S)-protopanaxadiol attenuates depressive-like behaviour and neuroinflammation in chronic unpredictable mild stress-induced depressive rats. Behav brain res. 2020;393:112710. doi: 10.1016/j.bbr.2020.112710. [DOI] [PubMed] [Google Scholar]
- 230.Bian HT, Wang GH, Huang JJ, Liang L, Xiao L, Wang HL. Scutellarin protects against lipopolysaccharide-induced behavioral deficits by inhibiting neuroinflammation and microglia activation in rats. Int Immunopharmacol. 2020;88:106943. doi: 10.1016/j.intimp.2020.106943. [DOI] [PubMed] [Google Scholar]
- 231.Tong Y, Fu HL, Xia CB, Song W, Li YJ, Zhao JJ, et al. Astragalin exerted antidepressant-like action through SIRT1 signaling modulated NLRP3 inflammasome deactivation. ACS Chem Neurosci. 2020;11:1495–1503. doi: 10.1021/acschemneuro.0c00156. [DOI] [PubMed] [Google Scholar]
- 232.Xie LL, Gu ZM, Liu HZ, Jia BT, Wang YY, Cao M, et al. The anti-depressive effects of hesperidin and the relative mechanisms based on the NLRP3 inflammatory signaling pathway. Front Pharmacol. 2020;11:1251. doi: 10.3389/fphar.2020.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sun XL, Zhang TW, Zhao Y, Cai EB, Zhu HY, Liu SL. The protective effect of 5-O-methylvisammioside on LPS-induced depression in mice by inhibiting the over activation of BV-2 microglia through Nf-κB/IκB-α pathway. Phytomedicine. 2020;79:153348. doi: 10.1016/j.phymed.2020.153348. [DOI] [PubMed] [Google Scholar]
- 234.Ge YB, Xu W, Zhang LJ, Liu MY. Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1β in central nervous system. Int Immunopharmacol. 2020;85:106652. doi: 10.1016/j.intimp.2020.106652. [DOI] [PubMed] [Google Scholar]
- 235.Jia MM, Li CX, Zheng Y, Ding XJ, Chen M, Ding JH, et al. Leonurine exerts antidepressant-like effects in the chronic mild stress-induced depression model in mice by inhibiting neuroinflammation. Int J Neuropsychopharmacol. 2017;20:886–895. doi: 10.1093/ijnp/pyx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu YM, Shen JD, Xu LP, Li HB, Li YC, Yi LT. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int Immunopharmacol. 2017;45:128–134. doi: 10.1016/j.intimp.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 237.Ito N, Hirose E, Ishida T, Hori A, Nagai T, Kobayashi Y, et al. Kososan, a Kampo medicine, prevents a social avoidance behavior and attenuates neuroinflammation in socially defeated mice. J Neuroinflammation. 2017;14:98. doi: 10.1186/s12974-017-0876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee HY, Lee JS, Kim HG, Kim WY, Lee SB, Choi YH, et al. The ethanol extract of Aquilariae Lignum ameliorates hippocampal oxidative stress in a repeated restraint stress mouse model. BMC Complement Altern Med. 2017;17:397. doi: 10.1186/s12906-017-1902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guo Y, Xie JP, Li X, Yuan Y, Zhang LC, Hu WY, et al. Antidepressant effects of Rosemary extracts associate with anti-inflammatory effect and rebalance of gut microbiota. Front Pharmacol. 2018;9:1126. doi: 10.3389/fphar.2018.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhou YF, Yan MZ, Pan R, Wang Z, Tao X, Li CC, et al. Radix Polygalae extract exerts antidepressant effects in behavioral despair mice and chronic restraint stress-induced rats probably by promoting autophagy and inhibiting neuroinflammation. J Ethnopharmacol. 2021;265:113317. doi: 10.1016/j.jep.2020.113317. [DOI] [PubMed] [Google Scholar]
- 241.Park BK, Kim NS, Kim YR, Yang C, Jung IC, Jang IS, et al. Antidepressant and anti-neuroinflammatory effects of Bangpungtongsung-San. Front Pharmacol. 2020;11:958. doi: 10.3389/fphar.2020.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yan YM, Li T, Wang D, Zhao BB, Zhou Q. Antidepressant effect of Xingnao Jieyu decoction mediated by alleviating neuroinflammation in a rat model of post-stroke depression. J Tradit Chin Med. 2019;39:658–666. [PubMed] [Google Scholar]
- 243.Li HR, Xiao YH, Han L, Jia Y, Luo SL, Zhang DD, et al. Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of dectin-1 and the innate immune system. Brain Res Bull. 2021;171:16–24. doi: 10.1016/j.brainresbull.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 244.Lin YE, Wang HL, Lu KH, Huang YJ, Panyod S, Liu WT, et al. Water extract of Armillaria mellea (Vahl) P. Kumm. alleviates the depression-like behaviors in acute- and chronic mild stress-induced rodent models via anti-inflammatory action. J Ethnopharmacol. 2021;265:113395. doi: 10.1016/j.jep.2020.113395. [DOI] [PubMed] [Google Scholar]
- 245.Jiao HY, Yang HJ, Yan ZY, Chen JB, Xu MB, Jiang YM, et al. Traditional Chinese formula Xiaoyaosan alleviates depressive-like behavior in CUMS mice by regulating PEBP1-GPX4-mediated ferroptosis in the hippocampus. Neuropsychiatr Dis Treat. 2021;17:1001–1019. doi: 10.2147/NDT.S302443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ano Y, Ohya R, Kita M, Taniguchi Y, Kondo K. Theaflavins improve memory impairment and depression-like behavior by regulating microglial activation. Molecules. 2019;24:467. doi: 10.3390/molecules24030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Masuda T, Sankowski R, Staszewski O, Prinz M. Microglia heterogeneity in the single-cell era. Cell Rep. 2020;30:1271–1281. doi: 10.1016/j.celrep.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 248.DeRidder L, Sharma A, Liaw K, Sharma R, John J, Kannan S, et al. Dendrimer-tesaglitazar conjugate induces a phenotype shift of microglia and enhances β-amyloid phagocytosis. Nanoscale. 2021;13:939–952. doi: 10.1039/D0NR05958G. [DOI] [PubMed] [Google Scholar]
- 249.Hussain G, Huang J, Rasul A, Anwar H, Imran A, Maqbool J, et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: an updated review. Molecules. 2019;24:2213. doi: 10.3390/molecules24122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Espín JC, González-Sarrías A, Tomás-Barberán FA. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 251.Cao P, Chen CM, Liu A, Shan QH, Zhu X, Jia CH, et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron. 2021;109:2573–2589. doi: 10.1016/j.neuron.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 252.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 253.Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, et al. Antagonizing peroxisome proliferator-activated receptor γ facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell. 2018;17:e12774. doi: 10.1111/acel.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jia XN, Gao ZH, Hu HL. Microglia in depression: current perspectives. Sci China Life Sci. 2021;64:911–925. doi: 10.1007/s11427-020-1815-6. [DOI] [PubMed] [Google Scholar]
- 255.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–271. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The animal models of depression involving microglial changes.
Data Availability Statement
Not applicable.