Abstract
Background/Aims
Autophagy is a process that allows the degradation of detrimental components through the lysosome to maintain cellular homeostasis under variable stimuli. SQSTM1 is a key molecule involved in functional autophagy and is linked to different signaling pathways, oxidative responses, and inflammation. Dysregulation of autophagy is reported in a broad spectrum of diseases. Accumulation of SQSTM1 reflects impaired autophagy, which is related to carcinogenesis and progression of various tumors, including hepatocellular carcinoma (HCC). This study investigated SQSTM1 protein expression in HCC and its relation to the clinicopathological features and the likelihood of tumor recurrence after radiofrequency ablation (RFA).
Methods
This study included 50 patients with cirrhotic HCC of Barcelona Clinic Liver Cancer stages 0/A-B eligible for RFA. Tumor and peritumor biopsies were obtained just prior to local ablation and assessed for tumor pathological grade and SQSTM1 expression by immunohistochemistry. Patients were followed for one year after achieving complete ablation to detect any tumor recurrence.
Results
Serum alpha-fetoprotein level (U = 149.50, P = 0.027∗) and pathological grade of the tumor (χ2 = 12.702, P = 0.002∗) associated significantly with the tumor response to RFA. SQSTM1 expression level was significantly increased in HCC compared to the adjacent peritumor cirrhotic liver tissues (Z = 5.927, P < 0.001∗). Significant direct relation was found between SQSTM1 expression level in HCC and the pathological grade of the tumor (H = 33.789, P < 0.001∗). On follow-up, tumor and peritumor SQSTM1 expression levels performed significantly as a potential predictor of the overall survival, but not the disease recurrence.
Conclusions
SQSTM1 expression could determine aggressive HCC, even with reasonable tumor size and number, and identify the subset of HCC patients with short overall survival and unfavorable prognosis. SQSTM1 expression could not predict post-RFA intrahepatic HCC recurrence. SQSTM1 may be a potential biomarker and target for the selection of HCC patients for future therapies.
Keywords: SQSTM1, hepatocellular carcinoma, radiofrequency ablation, tumor recurrence
Abbreviations: AFP, Alpha fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CTP, Child-Turcotte-Pugh; CT, Computed tomography; ELISA, Enzyme-linked immunosorbent assay; FNAC, Fine-needle aspiration cytology; HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus; Keap1, Kelch-like ECH-associated protein 1; mTORC1, mammalian target of rapamycin complex 1; mRECIST, modified Response Evaluation Criteria in Solid Tumors; MRI, Magnetic resonance imaging; Nrf2, Nuclear factor erythroid 2-related factor 2; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; RFA, Radiofrequency ablation; SQSTM/p62, Sequestosome 1/protein 62
Liver cancer is the fourth leading cause of cancer-related deaths worldwide, with most cases detected at late stages and an incidence-to-mortality ratio that approaches 1.1 Hepatocellular carcinoma (HCC) represents about 75–85% of primary liver cancers and constitutes a major public health burden.2 Radiofrequency ablation (RFA) is the best treatment option for HCC patients of early stages as per the Barcelona Clinic Liver Cancer (BCLC) staging algorithm who are not suitable for/refusing resection or liver transplantation.3 A complete response is expected in most of the tumors less than 2.5 cm, while the response rates decreases significantly with increasing the size of the tumor.4 Despite the recent progress in HCC diagnosis and intervention, only one-third of patients are candidates for curative or life-extending locoregional therapies, and the overall prognosis remains unsatisfactory due to the major obstacles of tumor recurrence and metastasis that may occur after any type of treatment.5 Elucidating the molecular mechanisms of tumor pathogenesis and growth would allow the development of effective molecule-targeted therapies, and ultimately help patients with HCC to achieve a favorable prognosis.6
Autophagy is a highly-regulated lysosome-dependent process that catabolizes intracellular components to maintain cellular homeostasis under a variety of stimuli like cytokine stimulation, diverse pathogens, nutrient starvation, or accumulation of misfolded proteins and damaged organelles.7 Dysregulation of autophagy is involved in a broad spectrum of diseases such as cancer, heart diseases and neurodegenerative diseases.8 Research focused on sequestosome 1 (SQSTM1/p62) accumulation as a substrate of impaired autophagy whereby failure of its regulation causes oxidative stress and contributes to HCC tumorigenesis.9
SQSTM1/p62 is a scaffolding protein member of the Src family that serves multiple functions in bone metabolism, inflammatory and oxidative stresses, inclusion body formation and tumorigenesis.9,10 Most of SQSTM1/p62 protein in the cell is distributed not only in the cytoplasm, but also in the nucleus and lysosomes, and its main function is to deliver damaged proteins and organelles for phagocytosis and lysosomal degradation.10 Among many regulatory stimuli, SQSTM1/p62 gene transcription is mostly induced by nuclear factor erythroid 2-related factor 2 (Nrf2) in response to oxidative stress, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in response to inflammatory stress, both of which are also activated by SQSTM1/p62, thus establishing two interlocked positive feedback loops.11,12 Additionally, post-translational regulation of SQSTM1/p62 depend upon autophagic machinery, whereby SQSTM1/p62 protein is rapidly and constantly degraded by autophagy.13 SQSTM1/p62 over-expression due to upregulation and/or inefficient degradation is used as an indicator of autophagic impairment and has been linked to different cancers, including HCC.14 It participates in the activation of mammalian target of rapamycin complex 1 (mTORC1) for nutrient sensing, NF-κB during inflammation and apoptosis, and Kelch-like ECH-associated protein 1 (Keap1)/Nrf2 for antioxidant response; whereby alterations in all these pathways have been associated with cancer development.15,16 However, there is still a lot of confusion about its role in most malignant tumors.
Therefore, the present study was designed to investigate SQSTM1 protein expression in HCC and its relation to the clinicopathological features and the likelihood of tumor recurrence after RFA.
Material/methods
This study included 50 patients with newly diagnosed definite HCC on top of hepatitis C virus (HCV)-related liver cirrhosis who were referred to the Hepatobiliary Unit at the Alexandria University Hospitals. Patients with preserved liver function and early stages HCC [BCLC stage 0: single lesion less than 2 cm, and BCLC stage A: single lesion less than 5 cm or up to three lesions not greater than 3 cm each] were included in the study. Patients with active HCV infection received direct-acting antivirals after HCC treatment. Patient collection started in May 2018 went on till June 2019 with longitudinal follow-up for a year. Exclusion criteria involved cirrhotic patients of CTP class C, patients with tumors nearby the gall bladder or vasculature or subcapsular tumors, patients with tumor-in-vein thrombus or metastasis, or those who received previous HCC treatment. Patients were assigned to RFA, and tissue samples were obtained from the tumors and peritumor cirrhotic liver just prior to ablation. The research was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the Ethics Committee of the Alexandria Faculty of Medicine (IRB No. 00007555). An informed consent was obtained from all subjects included in the study.
All patients included in the study were evaluated clinically as regard manifestations of liver cirrhosis and malignancy, with complete clinical examination and routine laboratory investigations, including complete blood picture and liver test assay. The severity of chronic liver disease was determined on the basis of CTP classification. Serum alpha-fetoprotein (AFP) levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit [Diagnostic Automation/Cortez Diagnostics. Catalog No. 5101Z].17 Radiological evaluation depended on a recent triphasic computed tomography (CT) abdomen and/or dynamic magnetic resonance imaging (MRI) performed within 4 weeks before intervention to confirm the diagnosis of HCC based on the characteristic enhancement pattern of contrast hyper-enhancement in the arterial phase “wash-in” and hypo-enhancement in the portal or delayed phase “wash-out,” and to determine the site, size, and number of tumors and to exclude vascular invasion or metastasis.18 The stage of HCC was determined according to the BCLC staging classification.19
Ablation Procedure
All ablations were performed by the same hepatologist (the author named A.A., who achieved about 20 years of experience in local ablation therapy of HCC). Mono-polar RFA apparatus [RITA Medical 1500X - ANGIODYNAMICS IntelliFlow - Boston Scientific Generator RF300. Power input: 100–240 V, 6 A, 50/60 Hz] was used as the radiofrequency energy source. A 15 cm long, 17 G, cool-tip electrode with a 2 cm long exposed metallic tip was used to deliver energy to the tissues. A standard grounding pad was placed on each of the patient’s thighs. Wattage and duration used for ablation were chosen based on the manufacturer’s guides. Percutaneous thermal ablation of the tumor was performed under ultrasound guidance and deep sedation in the presence of an anesthetist with continuous hemodynamic monitoring. Either sub-costal and inter-costal approaches were used according to the site of the target lesion(s) to avoid damaging large vessels, gall bladder wall, or hepatic capsule, and then the radiofrequency needle was directly inserted through the skin and positioned at the center of the tumor under ultrasound guidance. The radiofrequency energy was applied for 8–12 min in each treatment session according to the size of the tumor. Tumors larger than 3.5 cm needed multiple overlapping ablations. All ablations aimed at reaching at least 0.5 mm margin of nontumorous liver parenchyma. To prevent bleeding or tumor seeding, the intrahepatic needle track was treated with thermo-coagulation while the electrode was being removed.3 Ultrasound-guided fine-needle aspiration cytology (FNAC) smears from the tumors and peritumor cirrhotic liver tissues were obtained by separate needles just prior to RFA for histological examination.
Histopathology and Immunohistochemistry
All histologic evaluations were performed by the same pathologist who was blinded to the patients’ code (the author named N.B., who achieved almost 30 years of experience in histopathological examination). Tissue biopsies obtained from patients were fixed in 10% formalin solution, embedded in paraffin, sectioned (5 μm), dehydrated, cleared, and subsequently coverslipped using DPX as a mounting medium. HCC was classified into trabecular, pseudo-glandular, and compact morphology based on criteria laid down by the world health organization.20 HCC grade was assigned as regards the criteria of Edmondson and Steiner grading system.21 Concerning the patients who had multiple focal lesions; the biopsy was taken from the largest tumor nodule.
Smeared slides were permeabilized by shortly dipping in 0.1% solution of Triton X100 with gentle agitation [Thermo Scientific, Carpinteria, CA, USA]. Smears were incubated with 3% hydrogen peroxide for 10 min at room temperature to block endogenous peroxidases. Antigen retrieval was performed by boiling the slides in 2% citrate buffer for 10 min. Subsequently, sections were incubated overnight at 4◯ C with SQSTM1 monoclonal antibody [anti-SQSTM1/p62 antibody [2C11]-(ab56416), Abcam, USA] at an optimal dilution.22 Sections were then incubated with the one-step secondary antibody for 30 min at room temperature. Immune complexes were visualized using 3,3′-diaminobenzidine [Dako, Carpinteria, CA, USA]. Slides were counterstained with light hematoxylin, dehydrated, and coverslipped. All incubations were performed at room temperature in a humidity chamber unless otherwise stated. Positive controls were included with each run.
A point-counting technique was mounted to estimate SQSTM1 expression level using an Olympus magnifying microscope [CH 20 BIMF 200, Olympus, China]. It was calculated as a percentage, either as the number of positive cells to the number of positive and negative cells or counting the number of positive cells in 100 cells. Finally, the expression was graded on a scale from 0 to 3 as follows: 0 (Negative expression: No staining), 1 (Weak expression: Less than 30% of the smear showed positive staining), 2 (Moderate expression: Between 30 and 60% of the smear showed positive staining), 3 (Strong expression: Greater than 60% of the smear showed positive staining).23
Follow-up and Monitoring of Response to Treatment
All radiological evaluations were performed by the same radiologist who was blinded to the patients’ code, and the post-treatment response of tumors was assessed on the basis of the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.24 Tumor response to RFA was assessed by triphasic CT done 4 weeks post-intervention. All patients were followed using triphasic CT every 3 months after complete ablation for 1 year to detect any tumor recurrence. Patients with residual activity were retreated by another session of RFA, and an extra follow-up triphasic CT was performed after another 4 weeks. Complete response was defined as the disappearance of any intra-tumorous arterial phase enhancement in all target lesions. Partial response was defined as at least a 30% decrease in the sum of the diameters of viable target lesions with arterial phase enhancement, taking as reference the baseline sum of the diameters of target lesions. Local hepatic recurrence of the tumor was defined as reactivation at the ablated target tumor lesion recorded since treatment started. Distant hepatic recurrence of the tumor was defined as the appearance at other hepatic sites of new target tumor lesion unrecorded since treatment started.
Statistical Analysis
Data were fed to the computer and analyzed using the Statistical Package for Social Sciences software version 20.0. (SPSS. Armonk, NY: IBM Corporation). Quantitative data were described as range, mean ± standard deviation and median. Qualitative data were described as numbers and percentages. Kolmogorov–Smirnov test was used to verify the normality of data distribution. The statistical significance of the obtained results was judged at a P < 0.05 level. All calculated P values were two-tailed. Student’s t-test was used to compare between two groups for normally distributed numerical variables. Mann–Whitney test (U) was used to compare between two groups for non-normally distributed numerical variables. Chi-square test (χ2) was used for comparison between two groups with respect to categorical variables, and Fisher’s Exact test (FE) with Monte Carlo correction (MC) was appropriately applied when more than 20% of the cells have expected count less than 5. Wilcoxon signed ranks test (Z) was applied to compare between two groups for non-normally distributed categorical variables. Comparisons between more than two groups as regards normally distributed numerical variables were performed by the one-way analysis of variance (ANOVA) test with post hoc Tukey’s analysis. Comparisons between more than two groups as regards non-normally distributed numerical variables were performed by the Kruskal–Wallis test (H) with post hoc Dunn’s analysis. Logistic regression analysis was used to identify the variables predicting the outcome. Cox proportional hazards model was used to assess certain variables as potential predictors of overall survival.
Results
Baseline clinical, biochemical, radiological, and pathological data of patients included in the study were shown in Table 1.
Table 1.
Distribution of Clinical, Biochemical, Radiological, and Pathological Data of Patients Included in the Study.
| Baseline Characteristics | Distribution (n = 50) |
|---|---|
| Age | 55.64 ± 7.866 |
| Gender | |
|
42 (84.0%) |
|
8 (16.0%) |
| Child-Turcotte-Pugh class | |
|
24 (48.0%) |
|
26 (52.0%) |
| Hemoglobin concentration (g/dL) | 12.12 ± 1.554 |
| Platelet count (×103/cmm) | 118.36 ± 55.650 |
| Leucocyte count (×103/cmm) | 5.74 ± 1.858 |
| Serum aspartate aminotransferase (U/L) | 80.36 ± 54.588 |
| Serum alanine aminotransferase (U/L) | 65.88 ± 28.400 |
| Serum Albumin (g/dL) | 3.08 ± 0.374 |
| Serum Bilirubin (mg/dL) | 1.40 ± 0.475 |
| Prothrombin activity (%) | 70.38 ± 11.450 |
| Serum alpha-fetoprotein (ng/dL) | 152.78 ± 313.132 |
| Number of tumors | |
| Mean value | 1.46 ± 0.706 |
| Distribution: | |
| 1 nodule | 33 (66.0%) |
| 2 nodules | 11 (22.0%) |
| ≥3 nodules | 6 (12.0%) |
| Size of tumors | |
| Mean value | 3.25 ± 0.892 |
| Distribution: | |
| <2 cm | 3 (6.0%) |
| 2–3 cm | 20 (40.0%) |
| >3 cm–≤5 cm | 24 (54.0%) |
| Pathological grade of tumors | |
| Grade I (well-differentiated) | 9 (18.0%) |
| Grade II (moderately-differentiated) | 31 (62.0%) |
| Grade III (poorly-differentiated) | 10 (20.0%) |
Response to RFA Therapy in Relation to Different Clinico-chemical Parameters and Tumor Characteristics
For the treatment of initial tumors, 59 overall RFA sessions were performed. The mean number of RFA sessions needed per patient was 2.38 ± 1.02, and patients with incomplete responses underwent another RFA session(s) to get complete ablation of the target lesions. Within 12 months of follow-up, 36 (72%) patients responded efficiently to RFA, while 14 (28%) patients of the studied cohort did not respond satisfactorily to RFA and witnessed tumor recurrence.
No procedure-related deaths were observed. In our study, major complications of RFA were observed in 2 patients, one case had a hepatic abscess that was treated by ultrasound-guided percutaneous drainage, and one case had intra-peritoneal bleeding managed conservatively. Minor complications were observed in 13 patients and included periprocedural pain in 5 patients, a postablation syndrome in 4 patients, asymptomatic pleural effusion not requiring drainage in 2 patients, and skin burn in 2 patients.
There was no significant statistical difference between patients who were recurrence-free after RFA and those who showed recurrence as regard age and gender of the patients (P = 0.279 and P = 0.197 respectively), CTP class (P = 0.580), the number of tumor nodules (P = 0.064), and the size of tumor nodules (P = 0.933). However, the level of serum AFP (U = 149.50, P = 0.027∗) and the pathological grade of the tumor (χ2 = 12.702, P = 0.002∗) were found to be significantly associated with the tumor response to RFA, whereby poorly differentiated HCC and higher AFP levels were found among patients who experienced tumor recurrence (Table 2).
Table 2.
Response of the Tumor to Radiofrequency Ablation in Relation to the Clinical, Biochemical, Radiological, and Pathological Parameters.
| Parameter | Tumor Response to RFA |
P Value | Test of Significance | |
|---|---|---|---|---|
| Responder (n = 36) | Nonresponder (n = 14) | |||
| Age | ||||
| Range | 41–75 | 46–72 | ||
| Median | 54.50 | 51.50 | P = 0.279 | U = 202.00 |
| Mean ± SD | 56.22 ± 7.885 | 54.14 ± 7.702 | ||
| Gender | ||||
| Male | 32 (88.9%) | 10 (71.4%) | FEP = 0.197 | – |
| Female | 4 (11.1%) | 4 (28.6%) | ||
| Child-Turcotte-Pugh class | ||||
| A | 2 (22.2%) | 2 (40.0%) | FEP = 0.580 | – |
| B | 7 (77.8%) | 3 (60.0%) | ||
| Serum alpha-fetoprotein | ||||
| Range | 1–2000 | 5.42–815 | ||
| Median | 40.00 | 109.00 | P = 0.027∗ | U = 149.50 |
| Mean ± SD | 126.45 ± 333.051 | 220.48 ± 253.149 | ||
| Number of tumors | ||||
| Mean value | 1.36 ± 0.683 | 1.71 ± 0.726 | P = 0.056 | U = 178.00 |
| Distribution: | ||||
| 1 nodule | 27 (75.0%) | 6 (42.9%) | ||
| 2 nodules | 5 (13.9%) | 6 (42.9%) | P = 0.064 | χ2 = 5.507 |
| ≥3 nodules | 4 (11.1%) | 2 (14.3%) | ||
| Size of tumors | ||||
| Mean value | 3.31 ± 0.905 | 3.10 ± 0.875 | P = 0.579 | U = 220.00 |
| Distribution: | ||||
| <2 cm | 2 (5.6%) | 1 (7.1%) | ||
| 2–3 cm | 14 (38.9%) | 6 (42.9%) | P = 0.933 | χ2 = 0.140 |
| >3 cm–≤5 cm | 20 (55.6%) | 7 (50.0%) | ||
| Pathological grade of tumors | ||||
| Grade I | 9 (25.0%) | 0 (0.0%) | ||
| Grade II | 24 (66.7%) | 7 (50.0%) | P = 0.002∗ | χ2 = 12.702 |
| Grade III | 3 (8.3%) | 7 (50.0%) | ||
U: Mann–Whitney test.
χ2: Chi-square test.
FE: Fisher’s Exact test.
P: P value for association between different categories.
∗: Statistically significant at P ≤ 0.05.
SQSTM1 Expression in HCC and Peritumor Tissues
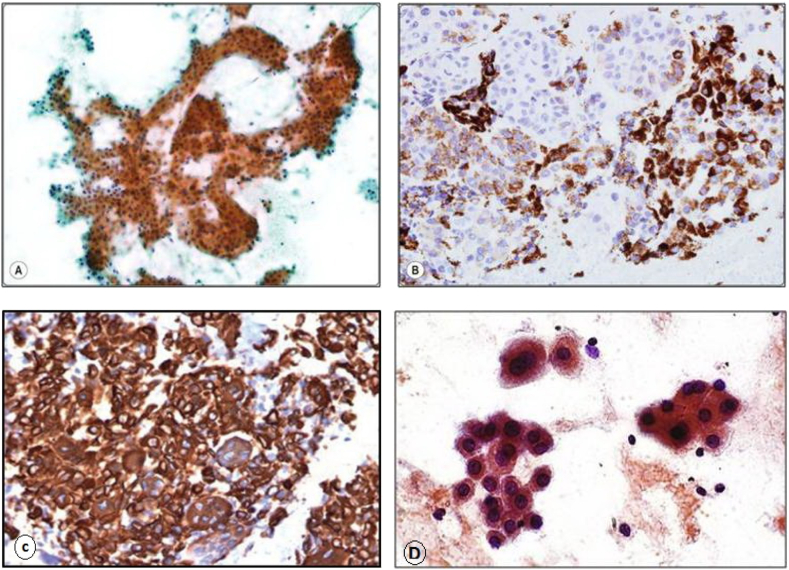
SQSTM1 expression was observed in the form of cytoplasmic staining in the cellular population of HCC and peritumor cirrhotic liver tissues (Figure 1). SQSTM1 expression in tumor was weak in 37 (74%) cases and moderate in 13 (26%) cases. However, SQSTM1 expression in peritumor cirrhotic liver tissues was weak in 39 (78%) cases and moderate in 11 (22%) cases. None of the patients showed neither negative nor strong SQSTM1 expression.
Figure 1.
SQSTM1/p62 immunostaining: (A) Diffuse cytoplasmic staining of neoplastic hepatocytes in grade I HCC (SQSTM1/p62 antibody, streptavidin-peroxidase technique, ×200 magnification). (B) Variable cytoplasmic staining of neoplastic hepatocytes in grade II HCC (SQSTM1/p62 antibody, streptavidin-peroxidase technique, ×200 magnification). (C) Intense cytoplasmic staining of neoplastic hepatocytes in grade III HCC (SQSTM1/p62 antibody, streptavidin-peroxidase technique, ×400 magnification). (D) Positive cytoplasmic staining was observed in a few cellular clusters of reactive hepatocytes of peritumor cirrhotic liver tissue (SQSTM1/p62 antibody, streptavidin-peroxidase technique, ×400 magnification).
Tumor SQSTM1 expression levels ranged between 15.0 and 42.0, with a mean of 27.70 ± 6.873. In peritumor cirrhotic liver tissues, SQSTM1 expression levels ranged between 11.0 and 40.0, with a mean of 25.62 ± 6.946. SQSTM1 expression level was significantly increased in HCC compared to the adjacent peritumor cirrhotic liver tissues (Z = 5.927, P < 0.001∗) (Table 3).
Table 3.
SQSTM1 Expression in Hepatocellular Carcinoma and Peritumor Cirrhotic Liver Tissues.
| SQSTM1 expression | Tumor tissues | Peritumor cirrhotic liver tissues | P Value | Test of Significance |
|---|---|---|---|---|
| Range | 15–42 | 11–40 | P < 0.001∗ | Z = 5.927 |
| Median | 28.00 | 26.00 | ||
| Mean ± SD | 27.70 ± 6.873 | 25.62 ± 6.946 |
Z: Wilcoxon signed ranks test.
P: P value for comparing between tumor and peritumor expressions.
∗ Statistically significant at P ≤ 0.05.
SQSTM1 Expression Level in HCC in Relation to Tumor Characteristics and Post-treatment Tumor Recurrence
On classifying the patients as regards the number of tumors (single nodule vs. multiple nodules), the analysis did not show a significant relation between SQSTM1 expression level in HCC and the number of tumors (P = 0.118) (Table 4). Also, nonsignificant relation was found between SQSTM1 expression level in HCC and the maximum size of the tumor on classifying the patients as those with size Max. ≤3 cm vs. others with size Max. >3 cm (P = 0.361) (Table 4).
Table 4.
Tumor SQSTM1 Expression Level in Relation to the Number and the Maximum Size of Tumors.
| Tumor SQSTM1 expression | Number of tumors |
P Value | Test of Significance | |
|---|---|---|---|---|
| Single (n = 33) | Multiple (n = 17) | |||
| Range | 15–41 | 18–42 | ||
| Median | 27.00 | 28.00 | P = 0.118 | U = 204.50 |
| Mean ± SD | 26.27 ± 5.719 | 30.47 ± 8.179 | ||
| Tumor SQSTM1 expression |
Size of tumors |
P Value | Test of Significance | |
| ≤3 cm (n = 22) | >3 cm (n = 28) | |||
| Range | 15–42 | 17–41 | ||
| Median | 28.00 | 27.50 | P = 0.361 | U = 261.50 |
| Mean ± SD | 28.64 ± 6.932 | 26.96 ± 6.861 | ||
U: Mann Whitney test.
P: P value for comparing between the two categories.
∗: Statistically significant at P ≤ 0.05.
Significant direct relation was found between SQSTM1 expression level in HCC and the pathological grade of the tumor (H = 33.789, P < 0.001∗), hence SQSTM1 positively expressed lesions tended to be of poorly differentiated nature (Table 5).
Table 5.
Tumor SQSTM1 Expression Level in Relation to the Pathological Grade of Tumors.
| Tumor SQSTM1 expression | Pathological grade of tumors |
||
|---|---|---|---|
| Grade I (n = 9) | Grade II (n = 31) | Grade III (n = 10) | |
| Range | 18–20 | 15–31 | 35–42 |
| Median | 20.00 | 28.00 | 39.00 |
| Mean ± SD | 19.33 ± 1.000 | 26.58 ± 3.566 | 38.70 ± 2.263 |
| P Value | P < 0.001∗ | ||
| Significance between groups | P1 = 0.005∗, P2 < 0.001∗, P3 < 0.001∗ | ||
| Test of Significance | H = 33.789 | ||
H: Kruskal–Wallis test, pairwise comparison between each two groups was done using post hoc test (Dunn’s for multiple comparisons test).
P: P value for comparing between the different studied groups.
P1: P value for comparing between patients with grade I and grade II.
P2: P value for comparing between patients with grade I and grade III.
P3: P value for comparing between patients with grade II and grade III.
∗: Statistically significant at P ≤ 0.05.
SQSTM1 expression level in HCC showed significantly direct relation to the frequency of post-RFA recurrence of the tumor (U = 75.50, P < 0.001∗), hence SQSTM1 intensely expressed lesions tended to be of recurrent presentation (Table 6). Among 14 patients who experienced recurrence after RFA, 9 cases showed medium SQSTM1 expression, and 5 cases showed weak SQSTM1 expression. However, nonsignificant relation was found between SQSTM1 expression level in HCC and the type of hepatic recurrence of the tumor on classifying the patients as those with local recurrence vs. those with distant recurrence (P = 0.606) (Table 6).
Table 6.
Tumor SQSTM1 Expression Level in Relation to the Frequency and the Type of Hepatic Recurrence of Tumors.
| Tumor SQSTM1 expression | Frequency of tumor recurrence |
P Value | Test of Significance | |
|---|---|---|---|---|
| No recurrence (n = 36) | Yes recurrence (n = 14) | |||
| Range | 15–40 | 26–42 | ||
| Median | 26.50 | 33.00 | P < 0.001∗ | U = 75.50 |
| Mean ± SD | 25.33 ± 5.777 | 33.79 ± 5.727 | ||
| Tumor SQSTM1 expression |
Type of tumor recurrence |
P Value | Test of Significance | |
| Local (n = 9) | Distant (n = 5) | |||
| Range | 26–42 | 28–41 | ||
| Median | 31.00 | 38.00 | P = 0.606 | U = 18.00 |
| Mean ± SD | 33.00 ± 5.874 | 35.20 ± 5.805 | ||
U: Mann Whitney test.
P: P value for comparing between the two categories.
∗: Statistically significant at P ≤ 0.05.
Predictors of Post-treatment Tumor Recurrence
Logistic regression analysis assessed potential explanatory variables for possible association with the tumor recurrence and revealed that none of the studied variables showed significant impact on post-RFA tumor recurrence, and hence, no parameter tended to be of good predictive value (Table 7).
Table 7.
Logistic Regression Analysis for Potential Predictors of Tumor Recurrence After Radiofrequency Ablation.
| Variables | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.97 (0.857–1.095) | 0.609 | ||
| Gender | 5.67 (0.452–70.740) | 0.179 | ||
| Child-Turcotte-Pugh class | 0.43 (0.060–3.044) | 0.396 | ||
| Size of tumors | 0.84 (0.286–2.467) | 0.751 | ||
| Number of tumors | 1.50 (0.290–7.746) | 0.630 | ||
| Pathological grade of tumors | 0.25 (0.002–39.083) | 0.594 | ||
| Serum alpha-fetoprotein | 1.00 (0.996–1.002) | 0.355 | ||
| Tumor SQSTM1 expression | 1.38 (0.638–2.983) | 0.413 | ||
| Peritumor SQSTM1 expression | 1.07 (0.523–2.197) | 0.850 | ||
OR: Odds ratio.
CI: Confidence interval.
#: All variables with P < 0.05 were included in the multivariate analysis.
∗: Statistically significant at P ≤ 0.05.
Predictors of Post-treatment Patient Survival
The total number of deaths in the study was 11 (22%) patients, and the main causes of death were hepatic failure and tumor progression. The total 1-year survival rate was 78%, and the mean overall survival was 11.318 ± 0.256. Potential explanatory variables were evaluated to explore possible relations to the post-treatment patient survival using Cox proportional hazards model. In the univariate analysis, multiple tumor lesions, high HCC grade, history of incomplete ablation, tumor reactivation, distant tumor recurrence, and SQSTM1 expression levels (in both tumor and peritumor tissues) performed significantly as potential predictors of patient survival. None of these variables had a significant impact over the other as an independent predictor of overall survival in the multivariate analysis (Table 8).
Table 8.
Cox Proportional Hazards Model for Potential Predictors of Overall Survival in the Study.
| Variables | Univariate |
Multivariate# |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 0.931 (0.846–1.024) | 0.143 | ||
| Size of tumors | 1.711 (0.501–5.849) | 0.392 | ||
| Number of tumors | 3.617∗(1.058–12.35) | 0.040∗ | 1.268 (0.278–5.778) | 0.759 |
| Pathological grade of tumors | 7.461∗ (2.175–25.58) | 0.001∗ | 0.459 (0.009–24.001) | 0.700 |
| Serum alpha-fetoprotein | 1.000 (0.999–1.002) | 0.586 | ||
| Incomplete ablation | 8.107∗ (2.350–27.97) | 0.001∗ | 4.286 (0.789–23.289) | 0.092 |
| Local recurrence | 8.388∗ (2.437–28.86) | 0.001∗ | 0.747 (0.082–6.774) | 0.795 |
| Distant recurrence | 8.053∗ (2.131–30.43) | 0.002∗ | 2.431 (0.334–17.719) | 0.381 |
| Tumor SQSTM1 expression | 1.191∗ (1.079–1.315) | 0.001∗ | 0.913 (0.502–1.663) | 0.767 |
| Peritumor SQSTM1 expression | 1.216∗ (1.097–1.348) | <0.001∗ | 1.314 (0.781–2.212) | 0.303 |
HR: Hazards ratio.
CI: Confidence interval.
#: All variables with P < 0.05 were included in the multivariate analysis.
∗: Statistically significant at P ≤ 0.05.
Discussion
This study describes our clinical experience with RFA in the treatment of HCC at a single institution. RFA, the modality of treatment in this study, is a widely accepted alternative to surgery in the treatment of HCC that has been considered potentially curative and safe with limited complications.25 In the present study, the rate of RFA effectiveness with complete response of the tumor after the first session was 72%. Our results were comparable to those of Cabibbo et al.26 and Sparchez et al.27 who stated that RFA effectiveness rates were 72.18% and 70.67%, respectively.
In the present study, no procedure-related deaths were reported, while the proportion of major complications (4.54%) was consistent with Koda et al.28 who reported close incidence of major complications (3.5%), whereas Giorgio et al.29 reported a much lower incidence of major complications (1.99%). Within 12 months follow-up period, our study revealed that tumor recurrence rate was 28%, which is close to the rates found in other studies,30,31 while a lower recurrence rate of 1.4% had been reported by Shiina et al.32 in 1170 HCC patients after RFA within the first year. Moreover, an Egyptian study reported that both tumor size larger than 2.8 cm and multinodular HCC were significant independent risk factors for tumor recurrence within one year after RFA.33 Meanwhile, the level of serum AFP and the pathological grade of the tumor were found to be significantly associated with the tumor response to RFA in our study, whereby poorly-differentiated HCC and higher AFP levels were found among patients who experienced tumor recurrence.
In the present study, SQSTM1 expression level was significantly increased in HCC compared to the adjacent peritumor cirrhotic liver tissues. Some studies indicated that the expression of SQSTM1/p62 protein was higher in different tumor tissues (including HCC) than in normal human tissues.34, 35, 36 In line with our results, Bao et al.35 reported that SQSTM1/p62 is increased in 100% of HCC tissues compared to the surrounding nontumorous liver tissues suggesting that human HCCs are autophagy defective and SQSTM1/p62 might represent a novel marker of HCC. In a recent study, Chava et al.36 found that 84% of HCC tissues were positive for SQSTM1/p62 expression and the number of SQSTM1/p62-positive cells was significantly higher in HCC compared to the adjacent cirrhotic liver tissue. In the same study, no statistically significant differences were observed in the expression of SQSTM1/p62 protein between different HCC etiologies, and tissue sections from eight normal livers that had neither cirrhosis nor HCC showed no evidence of SQSTM1/p62 expression. On the other hand, two studies found that SQSTM1/p62 protein level was lower in colorectal cancer and gastric cancer compared with corresponding normal tissues.37,38 Moreover, other studies reported no significant difference of SQSTM1/p62 protein level between tumor tissues and normal tissues in prostate cancer and glioblastoma.39,40
The potential valuable use of SQSTM1/p62 as a biomarker for distinguishing well-differentiated HCC from dysplastic nodules was demonstrated on immunohistochemistry and yielded a good sensitivity of 84.4% with a good specificity of 81.09%.41 Combining SQSTM1/p62 to both aminoacylase-1 and glypican-3 biomarkers significantly improved this discriminative capacity and increased sensitivity up to 93.8% and specificity to 95.2%. Interestingly, Inami et al.9 detected the presence of SQSTM1/p62 and Keap1 double-positive aggregates in tissues of patients with hepatitis, liver cirrhosis, and non-HCC tumors, although at lower incidence compared with those in HCC and HCC-adjacent tissues. Different from the high percentage of SQSTM1/p62 expression incidence in HCC tissues, Lu et al.42 demonstrated that about 33% of HCC cases exhibited readily detectable staining of SQSTM1/p62 protein in the cytoplasm of all malignant cells in cancer nodules, whereas it was undetectable in adjacent nonmalignant liver cells. This low incidence is not surprising because the immunofluorescence assay and positive criteria used in this study were different from those used in more recent ones. Additionally, the antibodies to SQSTM1/p62 antigen in human sera were detectable in 21.1% of HCC patients; however, in sera from patients with conditions that are known to be precursor diseases to HCC, including asymptomatic hepatitis B surface antigen carriers, acute and chronic hepatitis, no antibodies to SQSTM1/p62 were detected.43
The histological grade is an indicator of the biological aggressiveness and progression of any solid tumor.44 HCC of higher histologic grades has a greater chance of producing early infiltration of adjacent tissues and vascular invasion than do tumors of a lower histologic grade.26
In the present study, the level of SQSTM1/p62 expression showed a trend to increase with increasing histological grade of HCC. Significant differences were detected between the levels of SQSTM1/p62 expression in different HCC grades and in the corresponding peritumor cirrhotic liver tissues. Qian et al.45 found that the frequency of SQSTM1/p62 expression in HCC patients was 67.5%, and demonstrated that none of the grade I HCC cases was positive for SQSTM1/p62 immunoreactivity, whereas 70% of grade II HCC cases and 71% of grade III HCC cases were positive for SQSTM1/p62 expression. This study concluded that poorly-differentiated HCC exhibited a significantly higher expression level of SQSTM1/p62 than well-differentiated one suggesting that SQSTM1/p62 may be a prognostic indicator. In contrast, Lage and colleagues found that neoplastic transformation was associated with unambiguously reduced SQSTM1/p62 content, and the extent of decrease of SQSTM1/p62 expression corresponded to the histological grade of differentiation of HCC cells.46 However, these discrepancies might be explained by the possibility of the use of a different method for SQSTM1/p62 antigen detection, which was a monoclonal antibody that recognized a glypican-related p62.
In the current study, we found nonsignificant relation between SQSTM1/p62 expression level in tumor tissues and recurrence of the disease within a year of follow-up. Lin et al.47 investigated the role of different autophagy-related markers in HCC as prognostic factors of disease recurrence and found that SQSTM1/p62 expression in tumor and adjacent nontumorous tissues was not significantly associated with HCC recurrence. Excitingly, Xiang et al.48 reported that SQSTM1/p62 over-expression in patients who suffered HCC related to chronic hepatitis B and aflatoxin B1 exposure was associated with a lower rate of disease-free and overall patient survival after tumor ablation. Also, Aigelsreiter and colleagues found that the presence of tumor cell-associated p62-containing intracellular hyaline bodies (cytoplasmic inclusions found in a subset of HCC) was associated with significantly shortened overall survival and a trend toward shorter disease-free survival.49 Additionally, Xu et al.50 found that the markedly higher level of phosphorylated p62 in HCC samples compared to their adjacent nontumorous tissues correlated with shorter overall survival durations. The pooled results of two studies indicated that SQSTM1/p62 over-expression in cancer patients significantly predicted poor overall survival and disease-free survival, reflecting the general roles of SQSTM1/p62 in cancer prognosis.34,47
In the current study, multiple focal lesion, histological grade, history of incomplete ablations, reactivation, the appearance of distant recurrence, and SQSTM1/p62 expression level (both in tumor and peritumor cirrhotic tissues) all had a significant impact on overall survival within a year of follow-up, although not independently. Excitingly, Aigelsreiter et al.49 identified the presence of p62-containing intracellular hyaline bodies in HCC and the macroscopic tumor-in-vessel invasion as independent predictors of poor overall survival.
In conclusion, the present study postulated that SQSTM1 expression in cancer cells could bring up information about the tumor behavior, making the identification of aggressive tumors possible, even with reasonable tumor size and number, and could determine the subset of HCC patients with short overall survival and unfavorable prognosis. SQSTM1 expression could not predict intrahepatic HCC recurrence after RFA. SQSTM1 may be a potential biomarker and target for the selection of HCC patients for future therapies.
Since SQSTM1 immunoreactivity is implicated in the pathogenesis of HCC, it may be recommended that the validity of SQSTM1 expression to predict HCC behavior after local ablation should be extensively studied in prospective longitudinal studies of large-scale population and longer follow-up period with inclusion of patients with etiologies of chronic liver disease other than HCV infection and the use of different modalities of locoregional treatment of HCC. Furthermore, it is imperative to explore how SQSTM1 enhances HCC development and whether SQSTM1 suppression could be exploited for therapy to prevent or slow the progression of the disease and improve the patients’ survival.
Credit authorship contribution statement
Amr Abdel-Moety: concept and design of the work; performance of radiofrequency ablation; drafting and revising the work and final approval of the submitted version. Nahed Baddour: histopathological examination and immunohistochemical analysis; drafting and revising the work and final approval of the submitted version. Perihan Salem: analysis and interpretation of data; drafting and revising the work and final approval of the submitted version. Hesham El-Tobgy: acquisition of data for the work; drafting and revising the work and final approval of the submitted version. Assem El-Shendidi: analysis and interpretation of data for the work; drafting and revising the work and final approval of the submitted version.
Conflicts of interest
The authors have none to declare.
Acknowledgements
The authors would like to deeply thank Professor Sherif Shama (who has achieved almost 20 years of experience in diagnostic and interventional radiology) for his great help and support in the radiographic evaluation of HCC and assessment of response to therapy.
Funding
None.
References
- 1.Akinyemiju T., Abera S., Ahmed M., et al. Global Burden of Disease Liver Cancer Collaboration The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78(suppl 1):113–124. doi: 10.1159/000315239. [DOI] [PubMed] [Google Scholar]
- 4.Kalra N., Gupta P., Chawla Y., Khandelwal N. Locoregional treatment for hepatocellular carcinoma: the best is yet to come. World J Radiol. 2015;7:306–318. doi: 10.4329/wjr.v7.i10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet J.M., Zucman-Rossi J., Pikarsky E., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 6.Llovet J.M., Villanueva A., Lachenmayer A., Finn R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 7.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi A.M.K., Ryter S.W., Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 9.Inami Y., Waguri S., Sakamoto A., et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komatsu M., Kageyama S., Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res. 2012;66:457–462. doi: 10.1016/j.phrs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Jain A., Lamark T., Sjottem E., et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Z., Umemura A., Sanchez-Lopez E., et al. NF-kappa B restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan M.J., Thorburn A. Measuring autophagy in the context of cancer. Adv Exp Med Biol. 2016;899:121–143. doi: 10.1007/978-3-319-26666-4_8. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Martın P., Saito T., Komatsu M. p62/SQSTM1: ‘Jack of all trades’ in health and cancer. FEBS J. 2019;286:8–23. doi: 10.1111/febs.14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimmelman A.C., White E. Autophagy and tumor metabolism. Cell Metab. 2017;25:1037–1043. doi: 10.1016/j.cmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Z., Zender L. p62 in liver disease: good guy or bad guy? Cancer Cell. 2016;30:509–510. doi: 10.1016/j.ccell.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L., Liu J., Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gale P.R., Forner A., Llovet J.M., Mazzaferro V., Piscaglia F., Raoul J.-L., European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 20.Bosman F.T., Carneiro F., Hruban R.H., Theise N.D. In: 4th ed. Bosman F.T., Carneiro F., Hruban R.H., Theise N.D., editors. vol. 3. International Agency for Research on Cancer (IARC) Press; Lyon: 2010. WHO classification of tumours of the digestive system; pp. 217–225. (WHO Classification of Tumours). [Google Scholar]
- 21.Edmondson H.A., Steiner P.E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno Y., Shimada S., Akiyama Y., et al. DEPDC5 deficiency contributes to resistance to leucine starvation via p62 accumulation in hepatocellular carcinoma. Sci Rep. 2018;8:106. doi: 10.1038/s41598-017-18323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu Z.S., Niu X.J., Wang M. Management of hepatocellular carcinoma: predictive value of immunohistochemical markers for postoperative survival. World J Hepatol. 2015;7:7–27. doi: 10.4254/wjh.v7.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 25.Kettenbach J., Blum M., Kilanowicz E., Schwaighofer S.M., Lammer J. Percutaneous radiofrequency ablation of liver cell carcinoma: a current overview. Radiologe. 2004;44:330–338. doi: 10.1007/s00117-004-1031-y. [DOI] [PubMed] [Google Scholar]
- 26.Cabibbo G., Maida M., Genco C., et al. Survival of patients with hepatocellular carcinoma (HCC) treated by percutaneous radio-frequency ablation (RFA) is affected by complete radiological response. PLos One. 2013;8:e70016. doi: 10.1371/journal.pone.0070016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparchez Z., Mocan T., Radu P., et al. Prognostic factors after percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma. Impact of incomplete ablation on recurrence and overall survival rates. J Gastrointestin Liver Dis. 2018;27:399–407. doi: 10.15403/jgld.2014.1121.274.pro. [DOI] [PubMed] [Google Scholar]
- 28.Koda M., Murawaki Y., Hirooka Y., et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42:1058–1064. doi: 10.1111/j.1872-034X.2012.01025.x. [DOI] [PubMed] [Google Scholar]
- 29.Giorgio A., Merola M.G., Montesarchio L., et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma in cirrhosis: analysis of complications in a single centre over 20 years. Br J Radiol. 2017;90:20160804. doi: 10.1259/bjr.20160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S.H., Lim H.K., Choi D., et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: effect of histologic grade on therapeutic results. Am J Roentgenol. 2006;186(5 suppl l):S327–S333. doi: 10.2214/AJR.05.0350. [DOI] [PubMed] [Google Scholar]
- 31.Nouso K., Matsumoto E., Kobayashi Y., et al. Risk factors for local and distant recurrence of hepatocellular carcinoma after local ablation therapies. J Gastroenterol Hepatol. 2008;23:453–458. doi: 10.1111/j.1440-1746.2007.05120.x. [DOI] [PubMed] [Google Scholar]
- 32.Shiina S., Tateishi R., Arano T., et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montasser M.F., Shaker M.K., Albreedy A.M., Montasser I.F., El-Dorry A.K. Risk factors for early intrahepatic distant recurrence after radiofrequency ablation for hepatocellular carcinoma in Egyptian patients. J Dig Dis. 2014;15:676–683. doi: 10.1111/1751-2980.12190. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L., Wang Y., He J., Tang J., Lv W., Hu J. Cytoplasmic SQSTM1/p62 accumulation predicates a poor prognosis in patients with malignant tumor. J Cancer. 2018;9:4072–4086. doi: 10.7150/jca.26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao L., Chandra P.K., Moroz K., et al. Impaired autophagy response in human hepatocellular carcinoma. Exp Mol Pathol. 2014;96:149–154. doi: 10.1016/j.yexmp.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chava S., Lee C., Aydin Y., et al. Chaperone-mediated autophagy compensates for impaired macroautophagy in the cirrhotic liver to promote hepatocellular carcinoma. Oncotarget. 2017;8:40019–40036. doi: 10.18632/oncotarget.16685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang L.C., Fan C.W., Tseng W.K., et al. Immunohistochemical study of the Nrf2 pathway in colorectal cancer: Nrf2 expression is closely correlated to Keap1 in the tumor and Bach1 in the normal tissue. Appl Immunohistochem Mol Morphol. 2013;21:511–517. doi: 10.1097/PAI.0b013e318282ac20. [DOI] [PubMed] [Google Scholar]
- 38.Cao Q.H., Liu F., Yang Z.L., et al. Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res. 2016;8:3831–3847. [PMC free article] [PubMed] [Google Scholar]
- 39.Okada M., Oikawa M., Miki Y., et al. Immunohistochemical assessment of ATG7, LC3, and p62 in ameloblastomas. J Oral Pathol Med. 2014;43:606–612. doi: 10.1111/jop.12177. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X., Zhong W., Huang H., et al. Autophagy defects suggested by low levels of autophagy activator MAP1S and high levels of autophagy inhibitor LRPPRC predict poor prognosis of prostate cancer patients. Mol Carcinog. 2015;54:1194–1204. doi: 10.1002/mc.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin G.Z., Dong H., Yu W.L., et al. A novel panel of biomarkers in distinction of small well-differentiated HCC from dysplastic nodules and outcome values. BMC Cancer. 2013;13:161. doi: 10.1186/1471-2407-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu M., Nakamura R.M., Dent E.D., et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol. 2001;159:945–953. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J.Y., Chan E.K., Peng X.X., Tan E.M. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–1110. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nanashima A., Yano H., Yamaguchi H., et al. Immunohistochemical analysis of tumor biological factors in hepatocellular carcinoma: relationship to clinicopathological factors and prognosis after hepatic resection. J Gastroenterol. 2004;39:148–154. doi: 10.1007/s00535-003-1265-x. [DOI] [PubMed] [Google Scholar]
- 45.Qian H.L., Peng X.X., Chen S.H., Ye H.M., Qiu J.H. p62 expression in primary carcinomas of the digestive system. World J Gastroenterol. 2005;11:1788–1792. doi: 10.3748/wjg.v11.i12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lage H., Kellner U., Tannapfel A., Dietel M. Expression of a glypican-related 62-kDa antigen is decreased in hepatocellular carcinoma in correspondence to the grade of tumor differentiation. Virchows Arch. 2001;438:567–573. doi: 10.1007/s004280000377. [DOI] [PubMed] [Google Scholar]
- 47.Lin C.W., Chen Y.S., Lin C.C., et al. Autophagy-related gene LC3 expression in tumor and liver microenvironments significantly predicts recurrence of hepatocellular carcinoma after surgical resection. Clin Transl Gastroenterol. 2018;9:166. doi: 10.1038/s41424-018-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang X., Qin H.G., You X.M., et al. Expression of p62 in hepatocellular carcinoma involving hepatitis B virus infection and aflatoxin B1 exposure. Cancer Med. 2017;6:2357–2369. doi: 10.1002/cam4.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aigelsreiter A., Neumann J., Pichler M., et al. Hepatocellular carcinoma with intracellular hyaline bodies have a poor prognosis. Liver Int. 2017;37:600–610. doi: 10.1111/liv.13325. [DOI] [PubMed] [Google Scholar]
- 50.Xu D., Li X., Shao F., et al. The protein kinase activity of fructokinase A specifies the antioxidant responses of tumor cells by phosphorylating p62. Sci Adv. 2019;5:eaav4570. doi: 10.1126/sciadv.aav4570. [DOI] [PMC free article] [PubMed] [Google Scholar]