Abstract
Background and aims
The role of Alfa-fetoprotein (AFP) in the management of hepatocellular carcinoma (HCC) is still debated, with differences in recommendations between international guidelines. We analyzed the relationship of the clinicopathological profile, prognostic features, and survival outcomes with baseline serum AFP levels in patients with HCC.
Methods
Retrospective analysis of a prospectively accrued dataset of consecutive HCC patients was done.
Results
508 treatment naive patients were included in the analysis. AFP at presentation was normal (<10 ng/ml) in 18% patients. Patients with very high AFP (>400 ng/ml) had poor hepatic reserves (higher mean serum bilirubin, AST, ALT, INR, and lower mean albumin) and advanced disease at presentation (higher incidence of extrahepatic metastasis, and less proportion of patients with well-differentiated tumors). AFP >400 ng/ml was an independent predictor for presence of portal vein tumor thrombosis (PVTT) (OR, 4.08; 95% CI, 2.34–7.12; P < 0.001), higher tumor size (OR, 2.19; 95% CI, 1.36–3.54, P = 0.001) and advanced BCLC stage (OR, 4.19; 95% CI, 2.51–7.03; P < 0.001). Two-third of patients with small HCC (MTD <3 cm) and more than half with early-stage HCC (BCLC stage 0/A) had elevated AFP levels. No significant relationship was seen between overall survival (OS) and baseline AFP in patients who underwent surgery, but median OS in patients subjected to nonsurgical therapies was 19.4,10.5 and 5.7 months in patients having AFP <10 ng/ml, 10–400 ng/ml and >400 ng/ml respectively (P = 0.003). AFP >400 ng/ml was an independent predictor of survival in patients receiving any form of therapy (HR = 2.23; 95% CI = 1.19–4.18, P = 0.012).
Conclusion
AFP as a biomarker still has a significant role to play in the management of HCC patients and is here to stay till the search for an ideal biomarker in HCC is over.
Keywords: alfa-fetoprotein, hepatocellular carcinoma, biomarker, portal vein tumor thrombosis, Barcelona clinic liver cancer staging
Abbreviations: AFP, Alfa-fetoprotein; BCLC, Barcelona clinic liver cancer; BSC, Best supportive care; EHM, Extrahepatic metastasis; HBV, Hepatitis B virus; HBHC, HBV or HCV related; HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus; MTD, Maximum tumor diameter; MDT, Multidisciplinary team; NAFLD, Nonalcoholic fatty liver disease; NBNC, Non B Non C related; OS, Overall survival; PVTT, Portal vein tumor thrombosis; RFA, Radiofrequency ablation; SBRT, Stereotactic body radiation therapy; TACE, Transarterial chemo-embolization; TARE, Transarterial radio-embolization
Hepatocellular carcinoma (HCC) is the commonest primary liver cancer, the sixth most common cancer overall, and the fourth most common cause of cancer-related mortality worldwide.1 Alpha-fetoprotein (AFP) is an alpha1 - globulin normally present in high concentration in fetal serum; serum AFP levels are extremely low in adults.2 AFP is the most widely used and studied tumor biomarker in HCC patients. AFP measurements are routinely used for the diagnosis, surveillance, and prognostication in HCC patients.
Existing western and Asian guidelines do not recommend the use of AFP for diagnosis of HCC due to its low sensitivity and specificity3, 4, 5, 6 and its optimal diagnostic threshold for HCC is still controversial.7 In addition, AFP levels can be raised due to other malignancies (gastric, gonadal) and benign causes like pregnancy and hepatitis B virus (HBV) and hepatitis C virus (HCV) related chronic liver disease.8, 9, 10, 11, 12 While its use as a surveillance tool is not mandatory as per western guidelines, most Asian societies and recent evidence suggest its use in addition to abdominal ultrasound.3, 4, 5, 6,13, 14, 15, 16
Studies on the use of AFP as a prognostic marker following treatment for HCC have shown variable results, with some studies showing baseline elevated AFP levels to be a robust predictor of poor overall survival (OS) and recurrence-free survival,17, 18, 19, 20, 21, 22, 23, 24, 25 while others did not find it to be a good prognostic indicator.26, 27, 28 AFP is not included in most current staging systems29 and decision algorithms of HCC treatment except in patients eligible for liver transplantation where AFP more than 1000 ng/mL is associated with a high rate of recurrence and poor prognosis.30, 31, 32
Available data on the utility of AFP have used the different cut off levels to evaluate mostly retrospective data and have largely focused on patients after hepatectomy for survival outcomes.33 There is a scarcity of data focusing on clinical and prognostic implications of AFP from India where HBV related chronic liver disease is the commonest cause of HCC; but nonviral etiologies specifically nonalcoholic fatty liver disease (NAFLD) are on a rising trend similar to Asia, which can impact baseline AFP levels.34 We planned a study to analyze the relationship of the clinicopathological profile, prognostic factors, and survival outcomes with serum AFP levels at the time of diagnosis in treatment naïve patients with HCC.
Methods
We performed a retrospective analysis of a prospectively collected dataset of 553 consecutive HCC patients registered in the hepatobiliary unit at the Tata Memorial Hospital, a referral cancer center in India, between June 2017 and September 2019. These patients were recruited on another ongoing HCC study approved by the institutional ethics committee (project number 1875). Patients who had received any form of treatment prior to presenting at our center were excluded from analysis (n = 45).
The patient’s demographic and clinical details were noted, along with investigation results. HCC was diagnosed as per EASL and AASLD guidelines.3,4 All patients with a histological confirmation of diagnosis (n = 138) [biopsy, n = 94 or post-resection histopathological examination (HPE), n = 44], had a grading as well/moderate/poor/undifferentiated tumors where possible by an expert gastrointestinal onco-pathologist at our center.
Serum AFP levels were measured at baseline for all patients by the Chemiluminescent Microparticle Immunoassay method. Patients were divided into three groups as per AFP levels - normal (<10 ng/ml), elevated (10–400 ng/ml), and very high (>400 ng/ml). Although many studies used a 20 ng/ml cutoff for AFP, we used a lower cutoff of <10 ng/ml. AFP levels decline to <10 ng/ml within 300 days of birth,35 and thus, 10 ng/ml may be the best cutoff for the normal range in adults; 400 ng/ml was taken as the upper cutoff value, as values beyond this are considered indicative of HCC in most studies. Size of largest tumor nodule was depicted as maximum tumor diameter (MTD) and divided into <3 cm, 3–5 cm, and >5 cm.
As per etiology, patients were divided into two broad groups- HBV or HCV related (HBHC) or Non-B Non-C related (NBNC) HCC. Patients with occult Hepatitis B (IgG Anti HBc + with detectable HBV DNA) without any other risk factor were included in HBHC related HCC group. Diagnosis of alcohol-related HCC was made based on the history of significant alcohol intake (≥40–60 gm per day for >10 years). Patients without a history of alcohol intake, negative for viral markers and other etiologies, with the presence or history of two of the metabolic risk factors were diagnosed as having a NAFLD associated HCC. Patients were diagnosed as having cirrhosis related to Budd-Chiari syndrome in an appropriate clinical setting and presence of radiological findings and exclusion of other causes. Etiology was labeled as cryptogenic when no cause could be identified after adequate evaluation.
Barcelona clinic liver cancer (BCLC)36 stage was noted. The treatment plan was made by a multidisciplinary team (MDT). Nonsurgical therapies included ablative therapies (radiofrequency ablation- RFA), loco-regional therapies (transarterial chemo-embolization- TACE, transarterial radio-embolization- TARE, stereotactic body radiation therapy - SBRT), and systemic therapies (Sorafenib/Lenvatinib as first-line therapy followed by Regorafenib or immunotherapy). Patients who did not receive any therapy or were planned for best supportive care (BSC) were excluded from survival analysis (n = 295). Patients were followed until mid-June 2020, or until the time of death.
Statistical Analysis
Data were collected and analyzed using the statistical package for the social sciences, version 23 (IBM Corp., Armonk, New York, USA). Mean and SD for continuous variables, and relative frequency for categorical variables, were used as indices of centrality and dispersion of the distribution. For categorical variables, the Chi-square and z test for proportions were used. Mann–Whitney U test was used to test the difference between two continuous categories, and the Kruskal–Wallis rank test to test the difference among the three groups. Dunn posthoc analysis was done to evaluate pairwise comparison among three groups.
The logistic regression analysis model was used to estimate the univariate and multivariate effects of the AFP levels regarding various prognostic variables (tumor size, PVTT, pathological grade, extrahepatic metastasis [EHM], and BCLC stage). Cut off of 40 years for young age, size above 5 cm, and AFP levels >400 ng/ml were used in regression analysis. Overall survival was estimated by the Kaplan–Meier method and compared by the log-rank test. The Cox proportional hazards model was used to estimate the univariate and multivariate effects of the different factors associated with overall survival in patients who received any form of cancer-directed treatment. P-value was considered significant if less than 0.05.
Results
Baseline Demography, Tumor Characteristics, and AFP Levels
A total of 508 patients were analyzed. AFP levels at presentation were normal (<10 ng/ml) in 92 patients (18%), between 10 and 400 ng/ml in 146 patients (29%) and >400 ng/ml in 270 (53%). HBV-related HCC was the commonest etiological subgroup overall (240 patients, 47%). Supplementary Table 1 depicts etiological breakup across AFP groups, as well as the total percentage of each etiology in the whole cohort. There was no significant difference in the proportion of patients with HBV and HCV infection across AFP groups. Comparison between three AFP groups, as well as pairwise comparison of demography, laboratory parameters, and tumor characteristics, are depicted in Table 1.
Table 1.
Comparison of Baseline Demographic, Laboratory, and Clinicopathological Parameters between AFP Groups.
| Parameter | AFP<10 n = 92 | AFP 10–400 n = 146 | AFP>400 n = 270 | P value | Pairwise comparisons P value |
||
|---|---|---|---|---|---|---|---|
| (a) vs (b) | (a) vs (c) | (b) vs (c) | |||||
| Age | 57.9 ± 13.5 | 58.3 ± 11.9 | 53.7 ± 12.6 | <0.0011 | 0.5712 | 0.0252 | <0.0012 |
| Gender | 0.7023 | ||||||
| Male | 82 (89.1%) | 125 (85.6%) | 237 (87.8%) | >0.054 | >0.054 | >0.054 | |
| Female | 10 (10.9%) | 21 (14.4%) | 33 (12.2%) | >0.054 | >0.054 | >0.054 | |
| Etiology | 0.1293 | ||||||
| HBHC | 49 (53.3%) | 85 (58.2%) | 174 (64.4%) | >0.054 | >0.054 | >0.054 | |
| NBNC | 43 (46.7%) | 61 (41.8%) | 96 (35.6%) | >0.054 | >0.054 | >0.054 | |
| Bilirubin | 1.7 ± 2.2 | 1.8 ± 1.4 | 2.3 ± 3.0 | <0.0011 | 0.0062 | <0.0012 | 0.4102 |
| Albumin | 3.6 ± 0.6 | 3.4 ± 0.6 | 3.4 ± 0.6 | 0.0241 | 0.0232 | 0.0752 | 0.4092 |
| AST | 82.7 ± 139.6 | 105.2 ± 78.7 | 155.7 ± 145.5 | <0.0011 | <0.0012 | <0.0012 | 0.0022 |
| ALT | 53.1 ± 54.2 | 67.7 ± 50.3 | 70.8 ± 53.8 | <0.0011 | <0.0012 | <0.0012 | 0.3942 |
| INR | 1.13 ± 0.22 | 1.17 ± 0.22 | 1.22 ± 0.31 | 0.0181 | 0.4592 | 0.0162 | 0.4752 |
| Extrahepatic metastasis (yes) | 13 (14.1%) | 27 (18.5%) | 69 (25.6%) | 0.0413 | >0.054 | >0.054 | >0.054 |
| PVTT (yes) | 21 (22.8%) | 56 (38.4%) | 157 (58.1%) | <0.0013 | <0.054 | <0.054 | <0.054 |
| MTD (cm) | 7.6 ± 3.5 | 8.3 ± 3.6 | 9.1 ± 3.4 | <0.0011 | 0.3392 | 0.0012 | 0.052 |
| BCLC stage | <0.0013 | ||||||
| 0 | 0 | 2 (1.4%) | 1 (0.4%) | >0.054 | >0.054 | >0.054 | |
| A | 16 (17.4%) | 11 (7.5%) | 5 (1.9%) | >0.054 | <0.054 | <0.054 | |
| B | 41 (44.6%) | 58 (39.7%) | 63 (23.3%) | >0.054 | <0.054 | <0.054 | |
| C | 25 (27.2%) | 58 (39.7%) | 149 (55.2%) | >0.054 | <0.054 | <0.054 | |
| D | 10 (10.9%) | 17 (11.6%) | 52 (19.3%) | >0.054 | >0.054 | >0.054 | |
| Child Pugh stage | 0.0383 | ||||||
| A | 41 (44.6%) | 60 (41.1%) | 98 (36.3%) | >0.054 | >0.054 | >0.054 | |
| B | 17 (18.5%) | 50 (34.2%) | 75 (27.8%) | <0.054 | >0.054 | >0.054 | |
| C | 8 (8.7%) | 12 (8.2%) | 37 (13.7%) | >0.054 | >0.054 | >0.054 | |
| Noncirrhotic | 26 (28.3%) | 24 (16.4%) | 60 (22.2%) | >0.054 | >0.054 | >0.054 | |
| Bilobar disease | 38 (41.3%) | 60 (41.1%) | 136 (50.4%) | 0.1163 | >0.054 | >0.054 | >0.054 |
| Pathological grade (n=138) | <0.0413 | ||||||
| Well | 17 (44.7%) | 9 (22%) | 8 (13.6%) | <0.054 | <0.054 | >0.054 | |
| Moderate | 14 (36.8%) | 20 (48.8%) | 30 (50.8%) | >0.054 | >0.054 | >0.05 | |
| Poor | 6 (15.8%) | 10 (24.4%) | 19 (32.2%) | >0.054 | >0.054 | >0.054 | |
| Undifferentiated | 1 (2.6%) | 2 (4.9%) | 2 (3.4%) | >0.054 | >0.054 | >0.054 | |
| MTD (cm) | <0.0053 | ||||||
| <3 | 10 (10.9%) | 10 (6.8%) | 10 (3.1%) | >0.054 | <0.054 | >0.054 | |
| 3–5 | 16 (17.4%) | 20 (13.7%) | 22 (8.1%) | >0.054 | <0.054 | >0.054 | |
| >5 | 66 (71.7%) | 116 (79.5%) | 238 (88.1%) | >0.054 | <0.054 | >0.054 | |
All values: Means ± Standard Deviation as continuous; Frequencies and percentage (%) as categorical. AFP (ng/ml), Albumin (G/dl), AST/ALT (iu/L), Bilirubin (mg/dl), MTD (cm).
1. Kruskal–Wallis test 2. Dunn posthoc method 3. Chi-square 4. z test to compare column proportions with adjusted P values (Bonferroni method).
On the evaluation of prognostic variables, there was an increase in the percentage of patients with PVTT (P=<0.001), EHM (P = 0.041), and larger MTD (<0.001) from lower to higher AFP groups. There was a statistically significant difference between three AFP groups in terms of BCLC stage at presentation (P=<0.001) with a trend toward an increase in BCLC C and BCLC D stage patients with AFP >400 ng/ml. 66.7% of patients with MTD <3 cm had above normal AFP levels.
Association Between AFP Levels and PVTT
46% of patients had macroscopic PVTT at presentation, diagnosed on imaging. On multivariate logistic regression, AFP levels >400 ng/ml (OR, 4.08; 95% CI, 2.34–7.12; P < 0.001) was an independent predictor of PVTT in HCC patients (Table 2). The OR for PVTT increased significantly with increasing AFP levels above normal.
Table 2.
Univariate and Multivariable Logistic Regression Analysis of the Association of AFP Levels With Portal Vein Tumor Thrombosis (PVTT).
| Variable | PVTT |
P value | OR (95% CI) (univariate) | OR (95% CI, P value) (multivariate) | |
|---|---|---|---|---|---|
| Absent n = 274 | Present n = 234 | ||||
| Gender | 0.0471 | ||||
| Female | 42 (15.3%) | 22 (9.4%) | Ref | ||
| Male | 232 (84.7%) | 212 (90.6%) | 1.74 (1.01–3.01) | 1.69 (0.95–3.01,P = 0.07) | |
| Age | 0.0341 | ||||
| ≤40 | 32 (11.7%) | 43 (18.4%) | 1.70 (1.03–2.79) | 1.30 (0.76–2.21,P = 0.335) | |
| >40 | 242 (88.3%) | 191 (81.6%) | Ref | ||
| Etiology | 0.0431 | ||||
| NBNC | 119 (43.4%) | 81 (34.6%) | Ref | ||
| HBHC | 155 (56.6%) | 153 (65.4%) | 1.45 (1.01–2.08) | 1.31 (0.89–1.92,P = 0.174) | |
| AFP(ng/ml) | |||||
| (mean ± SD) | 36314.2±128780.1 | 106346.9±300565.7 | <0.0012 | ||
| AFP(ng/ml) | |||||
| <10 | 71 (25.9%) | 21 (9%) | Ref | ||
| 10–400 | 90 (32.8%) | 56 (23.9%) | 2.10 (1.16–3.79, P = 0.014) | 2.00 (1.09–3.66,P = 0.024) | |
| >400 | 113 (41.2%) | 157 (67.1%) | 4.69 (2.73–8.09, P < 0.001) | 4.08 (2.34–7.12,p=<0.001) | |
| AFP(ng/ml) | <0.0011 | ||||
| ≤400 | 161 (58.8%) | 77 (32.9%) | Ref | ||
| >400 | 113 (41.2%) | 157 (67.1%) | 2.91 (2.02–4.18) | 4.08 (2.34–7.12,p=<0.001) | |
| MTD (cm) | <0.0011 | ||||
| ≤5 | 68 (24.8%) | 20 (8.5%) | Ref | ||
| >5 | 206 (75.2%) | 204 (91.5%) | 3.53 (2.07–6.02) | 2.89 (1.66–4.88,P value=<0.001) | |
All values: Means ± Standard Deviation as continuous; Frequencies and percentage (%) as categorical.
1.Chi-square test 2.Mann–Whitney U test.
MTD (Maximum tumor diameter); AFP (Alfa-fetoprotein); HBHC (Hepatitis B and hepatitis C); NBNC (Nonhepatitis B and non-hepatitis C).
Association Between AFP Levels and MTD
On multivariate logistic regression analysis, age ≤40 and AFP >400 ng/ml were independent predictors of MTD in patients of HCC (Table 3).
Table 3.
Univariate and Multivariable Logistic Regression Analysis of the Association of AFP Levels With Maximum Tumor Diameter in cm.
| Variable | Size |
P value | OR (95% CI) (univariate) | OR (95% CI, P value) (multivariate) | |
|---|---|---|---|---|---|
| <5 cm n = 88 | >5 cm n = 420 | ||||
| Gender | 0.8321 | NA | |||
| Male | 72 (81.8%) | 372 (88.6%) | Ref | ||
| Female | 16 (18.2%) | 48 (11.4%) | 1.72 (0.93–3.20) | ||
| Age | 0.0061 | ||||
| ≤40 | 4 (4.5%) | 71 (16.9%) | 4.27 (1.52–12.03) | 3.99 (1.41–11.29,P = 0.009) | |
| >40 | 84 (95.5%) | 349 (83.1%) | Ref | ||
| Etiology | 0.9321 | NA | |||
| HBHC | 53 (60.2%) | 255 (60.7%) | Ref | ||
| NBNC | 35 (39.8%) | 165 (39.3%) | 1.02 (0.64–1.63) | ||
| AFP (ng/ml) Mean ± SD | 58660.60 ± 240108.55 | 70650.01 ± 224775.15 | <0.0012 | NA | |
| AFP(ng/ml) | O.0011 | ||||
| <10 | 26 (29.5%) | 66 (15.7%) | Ref | ||
| 10–400 | 30 (34.1%) | 116 (27.6%) | 1.84 (0.59–5.64) | 1.51 (0.82–2.79,P = 0.185) | |
| >400 | 32 (36.4%) | 238 (56.7%) | 2.06 (0.73–5.76) | 2.79 (1.55–5.05,P = 0.001) | |
| AFP(ng/ml) | 0.0011 | ||||
| ≤400 | 56 (63.6%) | 182 (43.3%) | Ref | ||
| >400 | 32 (36.4%) | 238 (56.7%) | 2.29 (1.42–3.68) | 2.19 (1.36–3.54,P = 0.001) | |
All values: Means ± Standard Deviation as continuous; Frequencies and percentage (%) as categorical.
1.Chi-square test 2.Mann–Whitney U test.
MTD (Maximum tumor diameter); AFP (Alfa-fetoprotein); HBHC (Hepatitis B and hepatitis C); NBNC (Nonhepatitis B and nonhepatitis C).
Association Between AFP Levels and Pathological Grades
There was no statistically significant difference between well/moderately differentiated tumors compared with poorly/undifferentiated tumors in terms of age, gender, etiology of HCC, AFP levels, and size of the tumor (Table 4).
Table 4.
Univariate and Multivariable Logistic Regression Analysis of the Association of AFP Levels With Pathological Grade of Tumor.
| Variable | Pathological grade |
P value | OR (95% CI) [univariate] | OR (95% CI, P value) [multivariate] | |
|---|---|---|---|---|---|
| Well/moderate n = 98 | Poor/undifferentiated n = 40 | ||||
| Gender | 0.9671 | NA | |||
| Female | 10 (12.8%) | 5 (14.3%) | Ref | ||
| Male | 68 (87.2%) | 30 (85.7%) | 0.98 (0.32–2.98) | ||
| Age | 0.801 | NA | |||
| ≤40 | 16 (20.5%) | 6 (17.1%) | Ref | ||
| >40 | 62 (79.5%) | 29 (82.9%) | 0.88 (0.34–2.30) | ||
| Etiology | 0.111 | NA | |||
| NBNC | 49 (50%) | 14 (35%) | Ref | ||
| HBHC | 49 (50%) | 26 (65%) | 1.86 (0.87–3.98) | ||
| AFP (ng/ml) Mean ± SD | 50088.98 ± 213031.23 | 68513.40 ± 169304.82 | 0.0722 | NA | |
| AFP(ng/ml) | O.2891 | ||||
| <10 | 31 (31.6%) | 7 (17.5%) | Ref | ||
| 10–400 | 29 (29.6%) | 12 (30%) | 1.84 (0.63–5.29) | NA | |
| >400 | 38 (38.8%) | 21 (52.5%) | 2.44 (0.92–6.51) | NA | |
| AFP(ng/ml) | 0.1411 | NA | |||
| ≤400 | 60 (61.2%) | 19 (47.5%) | Ref | ||
| >400 | 38 (38.8%) | 21 (52.5%) | 1.75 (0.83–3.66) | ||
| MTD (cm) | 0.5711 | NA | |||
| ≤5 | 16 (16.3%) | 5 (12.5%) | Ref | ||
| >5 | 82 (83.7%) | 35 (87.5%) | 1.37 (0.46–4.02) | ||
All values: Mean ± Standard Deviation as continuous; Frequencies and percentage (%) as categorical.
1.Chi-square test 2.Mann–Whitney U test.
MTD (Maximum tumor diameter); AFP (Alfa-fetoprotein); HBHC (Hepatitis B and hepatitis C); NBNC (Nonhepatitis B and nonhepatitis C).
Association Between AFP Levels and BCLC Stage
Significantly higher proportion of patients with BCLC C/D stage at presentation had very high AFP (64.6%, P≤0.001) and MTD >5 cm (P = 0.001) as compared to BCLC 0/A/B stage. On multivariate logistic regression analysis, AFP levels >400 ng/ml (OR, 4.19; 95% CI, 2.51–7.03; P < 0.001) were an independent predictor of BCLC stage at presentation (Table 5).
Table 5.
Univariate and Multivariable Logistic Regression Analysis of the Association of AFP Levels With Barcelona Clinic Liver Cancer (BCLC) Stage.
| Variable | BCLC stage |
P value | OR (95% CI) (univariate) | OR (95% CI, P value) (multivariate) | |
|---|---|---|---|---|---|
| 0/A/B n = 197 | C/D n = 311 | ||||
| Gender | 0.381 | ||||
| Male | 169 (85.8%) | 275 (88.4%) | 1.27 (0.74–2.15) | ||
| Female | 28 (14.2%) | 36 (11.6%) | Ref | ||
| Age | 0.191 | ||||
| ≤40 | 24 (12.2%) | 51 (16.4%) | 1.41 (0.84–2.38) | ||
| >40 | 173 (87.8%) | 260 (83.6%) | Ref | ||
| Etiology | 0.1661 | ||||
| HBHC | 112 (56.8%) | 196 (63%) | 1.29 (0.89–1.86) | ||
| NBNC | 85 (43.2%) | 115 (37%) | Ref | ||
|
AFP (ng/ml) (mean ± SD) |
19253.82 ± 75818.01 | 99813.94 ± 279898.01 | <0.0012 | ||
| AFP(ng/ml) | <0.0011 | ||||
| <10 | 57 (28.9%) | 35 (11.2%) | Ref | ||
| 10–400 | 71 (36%) | 75 (24.1%) | 1.72 (1.01–2.93,P = 0.045) | 1.62 (0.94–2.80,P value = 0.083) | |
| >400 | 69 (35%) | 201 (64.6%) | 4.74 (2.87–7.84,p=<0.001) | 4.19 (2.51–7.03,P value=<0.001) | |
| AFP(ng/ml) | <0.0011 | ||||
| <400 | 128 (65%) | 110 (35.4%) | Ref | ||
| >400 | 69 (35%) | 201 (64.6%) | 3.39 (2.33–4.93) | 4.19 (2.51–7.03,P value=<0.001) | |
| MTD (cm) | <0.0011 | ||||
| ≤5 | 58 (29.4%) | 30 (9.6%) | Ref | ||
| >5 | 139 (70.6%) | 281 (90.4%) | 3.91 (2.40–6.35) | 3.35 (2.02–5.55,P value=<0.001) | |
All values: Mean ± Standard Deviation as continuous; Frequencies and percentage (%) as categorical.
1. Chi-square test 2. Mann–Whitney U test.
MTD (Maximum tumor diameter); AFP (Alfa-fetoprotein); HBHC (Hepatitis B and hepatitis C); NBNC (Nonhepatitis B and nonhepatitis C).
Association Between AFP Levels and EHM
21.5% of patients had evidence of EHM at presentation. Mean serum AFP levels were significantly higher in patients with EHM, but on multivariate logistic regression analysis, only age ≤40 years and MTD >5 cm were independent predictors of EHM (Table 6).
Table 6.
Univariate and Multivariable Logistic Regression Analysis of the Association of AFP Levels With Presence and Absence of ExtraHepatic Disease at Presentation.
| Variable | Extrahepatic metastasis |
P value | OR (95% CI) (Univariate) | OR (95% CI, P value) (multivariate) | ||
|---|---|---|---|---|---|---|
| Absent n = 399 | Present n = 109 | |||||
| Gender | 0.5731 | |||||
| Male | 347 (87%) | 97 (89%) | 1.21 (0.62–2.36) | NA | ||
| Female | 52 (13%) | 12 (11%) | Ref | |||
| Age | 0.0031 | |||||
| ≤40 | 49 (12.3%) | 26 (23.8%) | 2.24 (1.31–3.81) | 1.87 (1.09–3.22,P value = 0.023) | ||
| >40 | 350 (87.7%) | 83 (76.2%) | Ref | |||
| Etiology | 0.2781 | NA | ||||
| HBHC | 237 (59.4%) | 71 (65.1%) | 1.28 (0.82–1.99) | |||
| NBNC | 162 (40.6%) | 38 (34.9%) | Ref | |||
|
AFP (ng/ml) (mean ± SD) |
55420.64 ± 203280.01 |
116718.39 + 295452.47 |
0.0012 | |||
| AFP (ng/ml) | 0.0411 | |||||
| <10 | 79 (19.8%) | 13 (11.9%) | Ref | |||
| 10–400 | 119 (29.8%) | 27 (24.8%) | 1.38 (0.67–2.83, P = 0.382) | 1.26 (0.60–2.64,P = 0.536) | ||
| >400 | 201 (50.4%) | 69 (63.3%) | 2.09 (1.09–3.98, P = 0.026) | 1.69 (0.87–3.29,P = 0.119) | ||
| AFP (ng/ml) | 0.171 | |||||
| ≤400 | 198 (49.6%) | 40 (36.7%) | Ref | |||
| >400 | 201 (50.4%) | 69 (63.3%) | 1.69 (1.09–2.63) | |||
| MTD (cm) | <0.0011 | |||||
| ≤5 | 84 (21.1%) | 4 (3.7%) | Ref | |||
| >5 | 315 (78.9%) | 105 (96.3%) | 7.00 (2.51–19.55) | 5.95 (2.11–16.74,P value = 0.001) | ||
All values: Mean ± Standard Deviation as continuous; Frequencies and percentage (%) as categorical.
1. Chi-square test 2. Mann–Whitney U test.
MTD (Maximum tumor diameter); AFP (Alfa-fetoprotein); HBHC (Hepatitis B and hepatitis C); NBNC (Nonhepatitis B and nonhepatitis C).
AFP and Survival Depending Upon Treatment Received
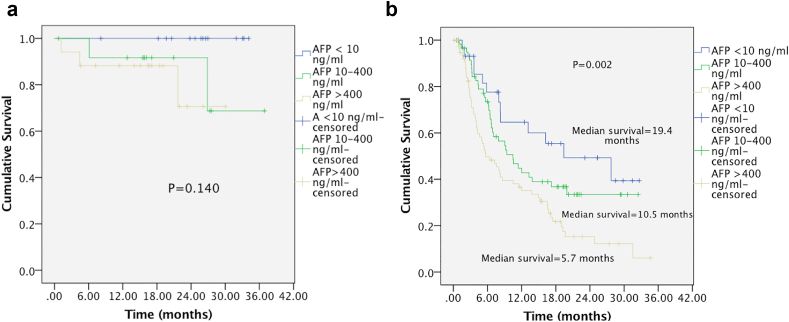
Analysis of the association between AFP levels and overall survival in patients who underwent surgery (n = 44) did not show any statistically significant relationship between postoperative OS across AFP groups (P = 0.140). Analysis of association between AFP levels and OS in patients who underwent nonsurgical therapies (ablative therapies, locoregional and systemic chemotherapy) [n = 169] showed a significant difference in median OS across AFP groups (P = 0.003). Median survival in months was 19.4, 10.5, and 5.7 in patients having AFP<10 ng/ml, AFP 10–400 ng/ml, and AFP >400 ng/ml, respectively (Figure 1). Supplementary Table 2 depicts the BCLC stage, AFP levels, and etiological breakup in patients who underwent surgery.
Figure 1.
Survival curves based on Kaplan–Meier analysis according to AFP level. (a) Patients who had undergone surgery (b) Patients who underwent nonsurgical therapies (Ablative, locoregional, or systemic chemotherapy).
Analysis of predictors of OS in patients who received either surgical or nonsurgical therapies using cox proportional hazard model on multivariate regression analysis showed AFP levels >400 ng/ml [HR = 2.23 (95% CI = 1.19–4.18, P = 0.012)], presence of macroscopic PVTT [HR = 2.65 (95% CI = 1.23–5.68, P = 0.012)] and EHM [HR = 2.42 (95% CI = 1.35–4.35, P = 0.003)] to be independent predictors of survival in these patients (Table 7).
Table 7.
Univariate and Multivariate Analysis of Predictors of Overall Survival in HCC Patients Who Were Planned for Any Form of Treatment.
| Variable | Comparison | HR (95% CI) (univariate) | HR (95% CI) (multivariate) |
|---|---|---|---|
| Gender | Female vs Male | 2.09 (1.02–4.29, P = 0.045) | 1.95 (0.94–4.08, P = 0.075 |
| Age (years) | ≤40 vs > 40 | 1.04 (0.63–1.71, P = 0.863) | NA |
| MTD (cm) | ≤5 vs > 5 | 2.89 (1.55–5.40, P = 0.001) | 1.65 (0.86–3.19, P = 0.134) |
| Etiology | NBNC vs HBHC | 1.12 (0.83–1.79, P = 0.315) | NA |
| Extrahepatic metastasis | Absent vs Present | 3.26 (2.08–5.09, P = 0.001) | 2.42 (1.35–4.35, P = 0.003) |
| BCLC stage | BCLC O/A/B vs BCLC C/D | 4.43 (2.98–6.59, P < 0.001) | 1.16 (0.48–2.79, P = 0.725) |
| AFP levels (ng/ml) | <10 | – | – |
| 10–400 | 2.18 (1.15–4.12, P = 0.016) | 1.85 (0.96–3.57, P = 0.068) | |
| >400 | 3.50 (1.91–6.38, P < 0.001) | 2.23 (1.19–4.18, P = 0.012)) | |
| PVTT | Absent vs present | 3.89 (2.65–5.70, P = <0.001) | 2.65 (1.23–5.68, P = 0.012) |
HR (Hazard ratio); CI (Confidence interval); NA (Not applicable in view of P > 0.05 on the univariate analysis); HBHC (Hepatitis B Hepatitis C related); NBNC (Nonhepatitis B hepatitis C related); AFP (Alfa-fetoprotein); PVTT (Portal vein tumor thrombosis): MTD (Maximum tumor diameter); BCLC (Barcelona clinic liver cancer).
Discussion
Almost 60 years after its discovery, the role of AFP in the management of HCC is still debated with differing recommendations by various international guidelines. Although used widely, the utility of AFP in clinical practice is limited by the reported low sensitivity at cut-off values maintaining sufficiently high specificity; the cut-off value of 200 ng/mL drops the sensitivity to 22% and levels >400 ng/mL in a high-risk patient are diagnostic of HCC with a specificity of >95 percent.37 Still none of the new tumor markers outperform the AFP so as to be widely adopted in clinical practice.38
Diagnostic Role of AFP
82% of HCC patients in our study had values of AFP above normal. The higher sensitivity reported in our analysis is greater than several previous reports and systematic reviews.39, 40, 41, 42, 43 Even patients with nonviral etiology (n = 200/508) for HCC had an almost similar proportion of AFP normal tumors (22%) in our analysis despite previous contrasting reports of the effect of viral etiology on AFP levels.17,44 Similar results have also been reported from China45 and Europe.39 An AFP cut off of >400 ng/ml, which is considered diagnostic as per previous reports,37 would include more than half of our patients. This is a larger proportion than several of previously published reports,39,43,46 but one study17 reported similar findings. The higher proportion of patients with elevated AFP above normal in our analysis can be attributed to variability in cut off threshold for normal AFP in different studies,46 and advanced disease at presentation due to predominance of HBV related HCC (49% had HBV related HCC and 60% were in BCLC C/D stage at presentation). Patients with HBV-related HCC are younger47 and present at an advanced stage because they are usually asymptomatic initially with preserved liver functions.18 Although the false-negative rate of AFP is significantly lesser in our analysis, it is still not sufficient to recommend it for a diagnostic role in view of previously reported poor specificity at this level.43 The optimal threshold of AFP is yet unclear.
Patients with AFP >400 ng/ml had poor hepatic reserves (higher mean serum bilirubin, AST, ALT, INR, and lower mean albumin) and advanced disease at presentation (higher incidence of PVTT, EHM, advanced BCLC stage, and significantly less proportion of patients with well-differentiated tumors) in our analysis supporting above observations. It has been suggested that AFP elevation might not only be just an epiphenomenon of malignant transformation but may also actively participate in tumor proliferation.45
Role of AFP in Surveillance
Early detection of HCC is the goal of surveillance therapies to improve survival in HCC patients.19,43 Major western guidelines do not recommend AFP for HCC surveillance3,4 based on the fact that almost 80% of small HCCs do not show increased levels of AFP, and the sensitivity decreases to 25% in tumors smaller than 3 cm.37 Supporting the role of AFP in screening for HCC is beyond the scope of our analysis, but we still had some noteworthy findings. Our subgroup analysis showed that two-thirds of patients with small HCC (MTD <3 cm) and more than half with early-stage HCC (BCLC stage 0/A) had AFP levels above the normal range. This is consistent with a multicenter case-control study, which showed a sensitivity of 66% at an AFP cutoff of 10.9 ng/mL in diagnosis of BCLC stage 0/A patients of HCC.48 A meta-analysis of 32 studies, including 13,367 patients, reported that ultrasound alone has a low sensitivity to detect early-stage HCC in patients with cirrhosis, and addition of AFP to ultrasound significantly increases sensitivity of early HCC detection in clinical practice.15 These findings support the recommendations of Asian guidelines for inclusion of AFP in surveillance protocols along with ultrasound abdomen.5,6,13,14 Use of combination biomarkers like des-gamma-carboxy prothrombin (DCP), an abnormal prothrombin molecule derived from an acquired defect in the post-translational carboxylation of the prothrombin precursor in malignant cells, and AFP-L3, an isoform of AFP characterized by the presence of a 1 – 6–linked residue on the AFP carbohydrate side chain along with AFP had shown superior detection of HCC in Asian cohorts but their use in routine clinical practice is limited to research settings and requires further validation. GALAD score encompassing patients’ gender (G), age (A), AFP-L3 (L), AFP (A), and DCP (D) has shown to improve detection of early-stage HCC in a German cohort.49
AFP and Age
Patients with AFP levels >400 ng/ml were significantly younger than patients with AFP levels of 10–400 ng/ml. Younger patients with HCC generally present at a more advanced stage and more often have chronic HBV infection.17,18 This was also supported by our analysis as young age (≤40 years) was an independent predictor of tumor size and EHM.
AFP and Prognosis
The impact of PVTT, tumor size, BCLC stage, EHM, and pathological grade on prognosis and overall survival is well known in HCC patients.20 We found that AFP >400 ng/ml is an independent predictor for the presence of PVTT, higher MTD, and more advanced BCLC stage at presentation. The odds ratio for PVTT increased significantly across each group of AFP levels, signifying the importance of even mildly raised AFP levels in pointing to its possibility. Our analysis included patients with only macroscopic PVTT, however high AFP levels could also predict the possibility of microscopic PVTT before surgery as reported in a retrospective analysis of postoperative pathological data on 170 HCC patients.50
These findings are consistent with previous reports of association of raised AFP to tumor size,18,39 BCLC stage,45 and the presence of PVTT;18 however, there are limited data evaluating these prognostic variables independent of multiple confounding parameters as we have done. Higher AFP levels at presentation were not independent predictors for EHM and degree of tumor differentiation in our analysis. Previous studies evaluating the association of degree of tumor differentiation with AFP levels have shown conflicting results, with some supporting25 and other reporting no association.17 Thus, our analysis supports the role of AFP at baseline as a significant prognostic factor in HCC patients.
AFP and Survival
Baseline AFP had no impact on postoperative OS in patients who underwent surgery in our analysis. The prognostic role of preoperative AFP is still a matter of debate with conflicting reports.19,22, 23, 24, 25, 26, 27, 28,39 Small number of patients who underwent surgery in our study prevents us from drawing any conclusions on this issue. However, there was a significant difference in median OS of patients across AFP groups who received nonsurgical treatment. These findings were also seen in another retrospective analysis;25 however, the cut off of AFP negative and positive tumors was not clear in this study. Also, whether patients who were planned for only BSC were included in the nonsurgical group was also unclear. We excluded patients planned for BSC from our survival analysis because we wanted to study the impact of baseline AFP levels in patients who received some HCC specific treatment because of the ongoing debate on the need to include AFP in treatment algorithms.33
Our analysis showed that AFP >400 ng/ml was an independent predictor of survival in patients who received either surgical or nonsurgical therapies for HCC. This effect of AFP was consistent even after adjusting for other clinical and prognostic variables related to HCC (Table 7). This is consistent with a large systematic review of 72 studies evaluating prognostic indicators in HCC;20 however, the AFP cut-off values varied in different studies.
Our study has a number of strengths. We analyzed the prospectively collected data reducing the chances of information bias. Our study depicts the most updated trend of the changing etiological profile of HCC in India (supplementary Table 1) in a large sample size.34 We have shown the relationship of AFP levels with multiple prognostic variables in a comprehensive way using multivariable logistic regression analysis. This is also the largest study, and to our knowledge, the only detailed analysis from the Indian subcontinent focusing on the clinical utility of AFP as a marker for diagnosis, screening, and prognosis in HCC patients.
Our study does have some limitations. Firstly, as many patients in the nonsurgical group received a combination of treatment modalities, we could not evaluate the impact of AFP levels on prognosis for individual treatment components. However, our analysis replicates the real-life scenario, where, usually, HCC patients who are not surgical candidates receive a combination of treatments depending upon the response to the initial treatment strategy. Secondly, the sample size of patients who underwent hepatectomy was too small (n = 44) for us to draw any meaningful conclusions in our analysis; however, multiple previous reports have shown similar results as mentioned before. Also, post-treatment AFP levels and their role in recurrence was not studied. Finally, being a tertiary care cancer center, referral bias could not be ruled out.
In our study, 82% of HCC patients had elevated AFP, with 53% having levels >400 ng/ml. Two-thirds of patients with small HCC (MTD <3 cm) and more than half with early-stage HCC (BCLC stage 0/A) had elevated AFP levels. Raised AFP levels were associated with worse liver function, more advanced disease at presentation, and aggressive tumor characteristics irrespective of etiology. Higher AFP levels were associated with poor overall survival in patients receiving nonsurgical treatment modalities and were an independent predictors of OS in patients planned for any tumor directed therapy. Thus, AFP as a biomarker still has a role to play in HCC patients with no other replacement in sight. Our study proves its utility in both diagnosis and prognostication, which can add value in planning treatment and follow up. Addition of AFP to treatment algorithms should therefore be considered. The role of adjuvant therapies in patients with high AFP levels requires further studies.
Credit authorship contribution statement
Vaneet Jearth and Dr Prachi S Patil contributed to study design, concept, and drafting of manuscript. Acquistion of data was done by Dr Vaneet Jearth, Dr Prachi S Patil Dr Shaesta Mehta, Dr Vidya Rao, Dr Sridhar Sundaram and Dr Vishal Seth. Statistical analysis was done by Dr Vaneet Jearth. Critical revision for important intellectual content was done by Dr Vaneet Jearth, Dr Prachi S Patil, Dr Mahesh Goel, Dr Munita Bal, and Dr Shraddha Patkar.
Conflicts of interest
The authors have none to declare.
Acknowledgements
We thank all consultants and residents of the Gastrointestinal Disease Management Group at Tata Memorial Hospital who helped in management of the patients. We also thank all the patients and their families for participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.11.006.
Source of funding
None.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ball D., Rose E., Alpert E. Alpha-fetoprotein levels in normal adults. Am J Med Sci. 1992 Mar;303:157–159. doi: 10.1097/00000441-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018 Jan;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019 Apr;70(4):817] J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Omata M., Cheng A.-L., Kokudo N., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A., Acharya S.K., Singh S.P., et al. 2019 update of Indian National Association for Study of the Liver consensus on prevention, diagnosis, and management of hepatocellular carcinoma in India: the Puri II recommendations. J Clin Exp Hepatol. 2020;10:43–80. doi: 10.1016/j.jceh.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singal A.G., Conjeevaram H.S., Volk M.L., et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012 May;21:793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Cheng Y., Sheng W., et al. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 9.El-Bahrawy M. α-Fetoprotein-Producing non-germ cell tumors of the urological system. Rev Urol. 2011;13:14–19. [PMC free article] [PubMed] [Google Scholar]
- 10.Sterling R.K., Wright E.C., Morgan T.R., et al. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107:64–74. doi: 10.1038/ajg.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasani P., Sangiovanni A., De Fazio C., et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology. 1999;29:1704–1707. doi: 10.1002/hep.510290604. [DOI] [PubMed] [Google Scholar]
- 12.Yang S., Kim G., Chung J., et al. Prediction of risk for hepatocellular carcinoma by response of serum α-fetoprotein to entecavir therapy. J Gastroenterol Hepatol. 2015;30:1175–1182. doi: 10.1111/jgh.12921. [DOI] [PubMed] [Google Scholar]
- 13.Poon D., Anderson B.O., Chen L.T., et al. Asian oncology summit. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian oncology summit 2009. Lancet Oncol. 2009 Nov;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 14.El-Serag H.B., Kanwal F. α-Fetoprotein in hepatocellular carcinoma surveillance: mend it but do not end it. Clin Gastroenterol Hepatol. 2013 Apr;11:441–443. doi: 10.1016/j.cgh.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 15.Tzartzeva K., Obi J., Rich N.E., et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018 May;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzartzeva K., Singal A.G. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev Gastroenterol Hepatol. 2018 Oct;12:947–949. doi: 10.1080/17474124.2018.1512855. [DOI] [PubMed] [Google Scholar]
- 17.Tangkijvanich P., Anukulkarnkusol N., Suwangool P., et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Peng S.Y., Chen W.J., Lai P.L., Jeng Y.M., Sheu J.C., Hsu H.C. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K., Imamura H., Matsuyama Y., et al. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:2795–2804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 20.Tandon P., Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanashima A., Taura N., Abo T., et al. Tumor marker levels before and after curative treatment of hepatocellular carcinoma as predictors of patient survival. Dig Dis Sci. 2011;56:3086–3100. doi: 10.1007/s10620-011-1796-6. [DOI] [PubMed] [Google Scholar]
- 22.Blank S., Wang Q., Fiel M.I., et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21:986–994. doi: 10.1245/s10434-013-3357-z. [DOI] [PubMed] [Google Scholar]
- 23.Ma W.J., Wang H., Teng L. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. doi: 10.1186/1477-7819-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santambrogio R., Opocher E., Costa M., et al. Hepatic resection for “BCLC stage A” hepatocellular carcinoma. The prognostic role of alpha-fetoprotein. Ann Surg Oncol. 2012;19:426–434. doi: 10.1245/s10434-011-1845-6. [DOI] [PubMed] [Google Scholar]
- 25.Bai D.S., Zhang C., Chen P., Jin S.J., Jiang G.Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017 Oct 9;7:12870. doi: 10.1038/s41598-017-12834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim J.H., Yoon D.L., Han S., et al. Is serum alpha-fetoprotein useful for predicting recurrence and mortality specific to hepatocellular carcinoma after hepatectomy? A test based on propensity scores and competing risks analysis. Ann Surg Oncol. 2012;19:3687–3696. doi: 10.1245/s10434-012-2416-1. [DOI] [PubMed] [Google Scholar]
- 27.Giannini E.G., Marenco S., Borgonovo G., et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56:1371–1379. doi: 10.1002/hep.25814. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda H., Kumada T., Kaneoka Y., et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol. 2008;49:223–232. doi: 10.1016/j.jhep.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Subramaniam S., Kelley R.K., Venook A.P. A review of hepatocellular carcinoma (HCC) staging systems. Chin Clin Oncol. 2013;2:33. doi: 10.3978/j.issn.2304-3865.2013.07.05. [DOI] [PubMed] [Google Scholar]
- 30.Lai Q., Iesari S., Melandro F., et al. The growing impact of alpha-fetoprotein in the field of liver transplantation for hepatocellular cancer: time for a revolution. Transl Gastroenterol Hepatol. 2017;2:72. doi: 10.21037/tgh.2017.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manas D., Burnapp L., Andrews P.A. Summary of the British transplantation society UK guidelines for living donor liver transplantation. Transplantation. 2016;100:1184–1190. doi: 10.1097/TP.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 32.Hakeem A.R., Young R.S., Marangoni G., Lodge J.P., Prasad K.R. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012 May;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 33.Muscari F., Maulat C. Preoperative alpha-fetoprotein (AFP) in hepatocellular carcinoma (HCC): is this 50-year biomarker still up-to-date? Translational Gastroenterology and Hepatology. 2020;5 doi: 10.21037/tgh.2019.12.09. 46-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tohra S., Duseja A., Taneja S., et al. Experience with changing etiology and nontransplant curative treatment modalities for hepatocellular carcinoma in a real-life setting—a retrospective descriptive analysis. J Clin Exp Hepatol. 2021;11:682–690. doi: 10.1016/j.jceh.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashyap R., Join A., Nalesnik M., et al. Clinical significance of elevated alpha-fetoprotein in adults and children. Dig Dis Sci. 2001;46:1709–1713. doi: 10.1023/a:1010605621406. [DOI] [PubMed] [Google Scholar]
- 36.Bruix J., Reig M., Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Schütte K., Schulz C., Link A., Malfertheiner P. Current biomarkers for hepatocellular carcinoma: surveillance, diagnosis and prediction of prognosis. World J Hepatol. 2015;7:139–149. doi: 10.4254/wjh.v7.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zacharakis G., Aleid A., Aldossari K. New and old biomarkers of hepatocellular carcinoma. Hepatoma Research. 2018;4:65. [Google Scholar]
- 39.Farinati F., Marino D., De Giorgio M., et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 40.Song P.P., Xia J.F., Inagaki Y., et al. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2016 Jan 7;22:262–274. doi: 10.3748/wjg.v22.i1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr B.I., Guerra V., Giannini E.G., et al. Low alpha-fetoprotein HCC and the role of GGTP. Int J Biol Markers. 2014;29:e395–e402. doi: 10.5301/jbm.5000092. [DOI] [PubMed] [Google Scholar]
- 42.Carr B.I., Akkiz H., Üsküdar O., et al. HCC with low- and normal-serum alpha-fetoprotein levels. Clin Pract (Lond). 2018;15:453–464. doi: 10.4172/clinical-practice.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuchiya N., Sawada Y., Endo I., Saito K., Uemura Y., Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015 Oct 7;21:10573–10583. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaminda S.R., Suchintha T., Anuk N.M., et al. Pre-treatment alpha feto protein in hepatocellular carcinoma with non-viral etiology - a prospective study. BMC Gastroenterol. 2017 Dec 6;17:142. doi: 10.1186/s12876-017-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An S.L., Xiao T., Wang L.M., et al. Prognostic significance of preoperative serum alpha- fetoprotein in hepatocellular carcinoma and correlation with clinicopathological factors: a single-center experience from China. Asian Pac J Cancer Prev. 2015;16:4421–4427. doi: 10.7314/apjcp.2015.16.10.4421. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Chen G., Zhang P., et al. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaoka Y. Survey and follow-up study of primary liver cancer in Japan-Report 13. Kanzo. 1999;40:288–300. [Google Scholar]
- 48.Marrero J.A., Feng Z., Wang Y., et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Best J., Bilgi H., Heider D., et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54:1296–1305. doi: 10.1055/s-0042-119529. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Yang W., Ren J. Risk factors of microvascular invasion in patients with hepatocellular carcinoma. Biomedical Research. 2018;29 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.