Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) contributes to a large proportion of liver disease burden in the world. Several groups have studied the prevalence of NAFLD in the Indian population.
Aim
A systematic review of the published literature and meta-analysis was carried out to estimate the prevalence of NAFLD in the Indian population.
Methods
English language literature published until April 2021 was searched from electronic databases. Original data published in any form which had reported NAFLD prevalence in the Indian population were included. The subgroup analysis of prevalence was done based on the age (adults or children) and risk category, i.e., average-risk group (community population, participants of control arm, unselected participants, hypothyroidic individuals, athletes, aviation crew, and army personnel) and high-risk group (obesity or overweight, diabetes mellitus, coronary artery disease, etc.). The prevalence estimates were pooled using the random-effects model. Heterogeneity was assessed with I2.
Results
Sixty-two datasets (children 8 and adults 54) from 50 studies were included. The pooled prevalence of NAFLD was estimated from 2903 children and 23,581 adult participants. Among adults, the estimated pooled prevalence was 38.6% (95% CI 32–45.5). The NAFLD prevalence in average-risk and high-risk subgroups was estimated to be 28.1% (95% CI 20.8–36) and 52.8% (95% CI 46.5–59.1), respectively. The estimated NAFLD prevalence was higher in hospital-based data (40.8% [95% CI 32.6–49.3%]) than community-based data (28.2% [95% CI 16.9–41%]). Among children, the estimated pooled prevalence was 35.4% (95% CI 18.2–54.7). The prevalence among non-obese and obese children was 12.4 (95% CI 4.4–23.5) and 63.4 (95% CI 59.4–67.3), respectively.
Conclusion
Available data suggest that approximately one in three adults or children have NAFLD in India.
Keywords: fatty liver, steatohepatitis, metabolic syndrome, obesity, diabetes mellitus
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BMI, Body mass index; CI, Confidence interval; CAD, Coronary artery disease; DM, Diabetes mellitus; GBD, Global burden of disease; GDM, Gestational diabetes mellitus; GDP, Gross domestic product; HC, Healthy control; IGT, Impaired glucose tolerance; NAFLD, Non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; NPCDCS, National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke; OSA, Obstructive sleep apnea; PCOS, Polycystic ovarian syndrome; UT, Union Territories
The entity of non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum from simple steatosis to non-alcoholic steatohepatitis (NASH), which can progress to liver fibrosis, cirrhosis, and hepatocellular carcinoma. The global prevalence of NAFLD is estimated to be 25%,1 with a higher prevalence in the Middle East and South America and the lowest in Africa. The prevalence of NASH is estimated to be 1.5%–6.5%.1 Global burden of disease (GBD) 2017 estimated the annual incidence of NASH cirrhosis to be 367,780 in 2017, which has almost doubled from that in 1990.2 In the future, NASH is expected to be the most common cause of chronic liver disease and indication for liver transplantation.2
The presence of certain characteristics has been identified for the development of NAFLD. The prevalence of NAFLD is found to be higher among those with diabetes (55.5%–59.7%),3, 4, 5 overweight or obesity (64.6%–95%),6, 7, 8 and metabolic syndrome (73%).9
The prevalence of adult NAFLD in India has been reported between 6.7% and 55.1%.10,11 Of all cases with an asymptomatic elevation of liver enzymes, NAFLD may be responsible for almost one-third.12 Furthermore, explant histology data from liver transplant centers suggest that two-third of the patients with ‘cryptogenic’ cirrhosis had NAFLD.13 The prevalence of pediatric NAFLD in India varies from 7.3% to 22.4% in the healthy population.14,15 The prevalence of NAFLD increases with age.16
The prevalence of prediabetes, diabetes, and metabolic syndrome among adults in India is 19–22%, 15–19%, and 30%, respectively, and is increasing in both urban and rural areas.17,18 With the increasing prevalence of diabetes, obesity, and metabolic syndrome, NAFLD prevalence is expected to increase and cause an increased burden on health resources. To plan strategies for the future and be ready to address this public health problem, it is important to know the burden of the disease and its health impact. The global meta-analysis did not include any study from India. Multiple studies on NAFLD prevalence are available from India. However, these suffer from certain limitations, including small sample sizes, predefined selection of patients (high-risk), and the absence of data regarding the prevalence of NASH, which represents the severe form of liver disease. In the absence of large sample size, pan-India studies, the exact burden of NAFLD in India is not known. Therefore, we did a systematic review and meta-analysis of all studies published from India.
Methods
Design
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for conducting the study.
Search Strategy
We searched electronic databases including Pubmed/Medline, Embase, Scopus, and Google scholar. The search strategy (Supplementary file 1) included the various terms used for fatty liver disease, the name of states, and major cities of the country. Cross-references from the published articles were manually searched to retrieve the additional literature.
Inclusion and Exclusion Criteria
We included English language literature published as full text before April 2021. The studies were included if they reported original data on the prevalence of fatty liver disease in the Indian population in any form, such as original articles, letters to the editor, brief communications, or short reports. We excluded abstracts, review articles, and non-English language literature. The studies that reported NAFLD prevalence in India based on ultrasound as the imaging modality were selected for data extraction and analysis. In studies reporting the prevalence of NAFLD based on multiple modalities, including ultrasound, we included the reported prevalence based on the latter modality.
Study Participants
We included patients across all age groups. We also included studies reporting NAFLD prevalence in high-risk populations such as those with obesity, diabetes, and metabolic syndrome.
Selection of Studies
The literature search was performed by AG. Two independent reviewers (S and AG) screened the title and abstract of all studies identified. Full-text articles were obtained for the relevant studies satisfying the inclusion criteria. The data were extracted independently by authors AE and BB. Extracted data were cross-checked by an independent author (TPS). The data extraction was supervised by S and AG, and any disagreement between the authors was resolved by consensus.
Data Extraction
The following data were extracted from the studies: author name, year of publication, study design, sample size, age group (<18 years and >18 years) of the participants, study setting, number of study centers, characteristics of the study population, risk category of the participants, residence, and diagnostic criteria used for the diagnosis of NAFLD. The study population was classified as average-risk (community population, participants of control arm, unselected participants, hypothyroidic individuals, athletes, aviation crew, and army personnel) or high-risk (obesity or overweight, prediabetes, diabetes mellitus, coronary artery disease, metabolic syndrome, obstructive sleep apnea, women with polycystic ovarian syndrome, and people with elevated liver enzymes).
Quality Assessment of the Studies
The quality of the included studies was assessed with the use of a modified checklist for studies reporting prevalence data.19 The checklist includes a set of ten questions on different methodological quality parameters of a prevalence study. The response to each of the questions was marked as either “Low Risk” or “High risk”. The overall quality of each of the studies was assessed as poor quality, average quality, and high quality.
Statistical Analysis
The NAFLD prevalence data from individual studies were summarized as proportions with 95% confidence intervals (CIs). The heterogeneity between studies was assessed with I2 statistics. The presence of substantial heterogeneity was adjudged using the I2 statistic (I2 ≥ 50%). The prevalence estimates from individual studies were pooled with a random-effects model because of marked heterogeneity among studies. Publication bias was assessed using Egger's test with funnel plots. The data were analyzed with STATA software, version 16 (StatCorp LLC, College Station, TX, USA). Subgroup analyses were performed for age group, gender, risk category, and urban/rural populations.
Results
Overall
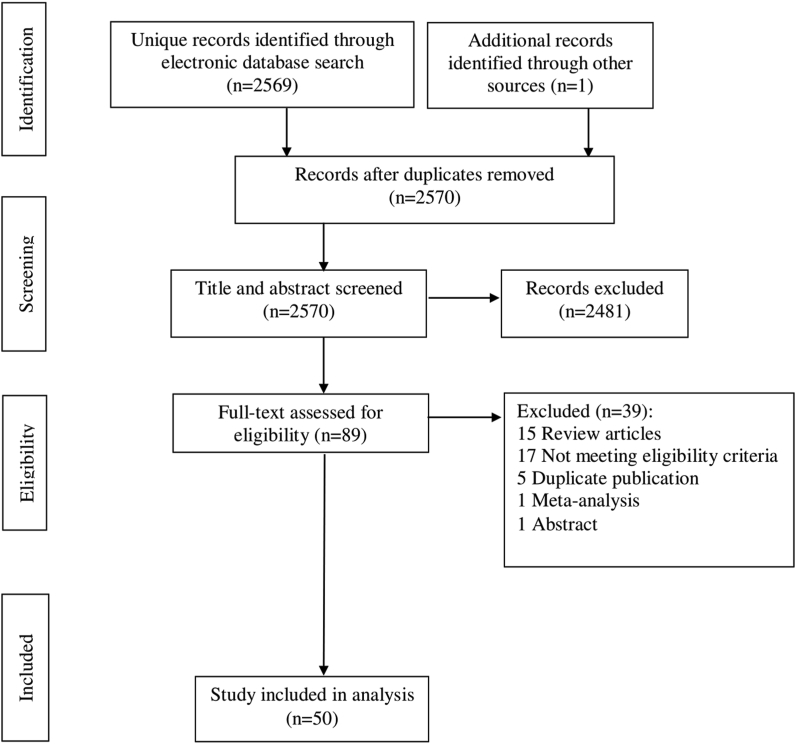
The literature search identified 50 studies5,9, 10, 11, 12,14,15,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 (Figure 1, PRISMA flow chart) which provided NAFLD prevalence among children (n = 8) and adults (n = 54). The characteristics of the included studies are summarized in Table 1. Included studies summarized the data from 26,484 participants grouped into 62 datasets.
Figure 1.
PRISMA flow diagram of the study selection.
Table 1.
Characteristics of the Included Studies.
| Author, year (ref.) | Study population, setting, design | Number of participants | Risk category | NAFLD prevalence (%) |
|---|---|---|---|---|
| Studies in children (age < 18 years) | ||||
| Chaturvedi, 201261 | OPD visitors, urban, cross-sectional | 100 | Average | 3% |
| Parray, 201215 | Students, urban, cross-sectional | 1115 | Average | 7.3% |
| Pawar, 201645 | Overweight and obese children, urban, cross-sectional | 100 | High | 62% |
| Das, 201714 | Students, urban, cross-sectional | 961 | Average | 22.4% |
| Jain, 201855 | Overweight children, urban, cross-sectional | 208 | High | 62.5% |
| Goyal, 201858 | Obese students, urban, cross-sectional | 160 | High | 66.2% |
| Bansal, 201860 | OPD visitors, urban, cross-sectional | 159 | Average | 21.4% |
| Gupta, 202062 | Obese children (BMI > 27 kg/m2), rural, cross-sectional | 100 | High | 62% |
| Studies in adults (age > 18 years) | ||||
| Madan, 200412 | People with elevated ALT, urban, cross-sectional | 67 | High | 35.8% |
| Singh, 200422 | Patient's relatives, urban, cross-sectional | 159 | Average | 24.5% |
| Gupte, 200456 | Patients with DM-2, urban, cross-sectional | 100 | High | 49% |
| Amarapurkar, 200751 | General population, urban, cross-sectional | 730 | Average | 18.9% |
| Banerjee, 200832 | Patients with DM-2, urban, cross-sectional | 47 | High | 65.9% |
| Chandran, 200830 | Patients with DM-2, urban, cross-sectional | 52 | High | 44.2% |
| Mohan, 200954 | General population, urban, cohort | 541 | Average | 32.0% |
| Prashanth, 200941 | Patients with DM-2, urban, cross-sectional | 204 | High | 62.2% |
| Sanyal, 200924 | Patients with DM-2/IGT and HC, urban, cross-sectional | 310 (DM-2/IGT) 160 (HC) |
High (DM-2/IGT) Average (HC) |
58.1% (DM-2/IGT) 20% (HC) |
| Chadha, 201031 | Aviation crew, urban, cross-sectional | 2589 | Average | 2.9% |
| Kalra, 20135 | Patients with DM-2, urban, cross-sectional | 924 | High | 56.5% |
| Thiruvagounder, 201023 | Patients with CAD, urban, cohort | 149 | High | 46.3% |
| Agarwal, 201144 | Patients with DM-2, urban, cross-sectional | 124 | High | 57.2% |
| Jayarama, 201235 | Patients with DM-2 and HC, urban, cross-sectional | 50 (DM-2) 50 (HC) |
High (DM-2) Average (HC) |
60% (DM-2) 20% (HC) |
| Anbalagan, 201220 | General population, urban, cohort | 409 | Average | 24.7% |
| Madanagobalane, 201253 | Psoriasis and HC, urban, cross-sectional | 333 (psoriasis) 330 (HC) |
Average | 17.4% (psoriasis) 8.6% (HC) |
| Karoli, 201327 | Women with PCOS and HC, urban, cross-sectional | 54 (PCOS) 55 (HC) |
High (PCOS) Average (HC) |
66.7% (PCOS) 25.4% (HC) |
| Mishra, 201321 | Non-diabetic adults, urban, cross-sectional | 645 | Average | 15.6% |
| Vendhan, 201425 | DM-1, urban, cross-sectional | 736 | High | 27.7% |
| Ajmal, 201438 | Patients with CAD, urban, cross-sectional | 104 | High | 69.2% |
| Srinivas, 201529 | Participants in health camp, urban, cross-sectional | 1075 | Average | 45.7% |
| Anurag, 201550 | Patient's relatives, rural, cross-sectional | 302 | Average | 28.1% |
| Majumdar, 201652 | General population, rural, cross-sectional | 176 | Average | 30.7% |
| Barik, 201628 | General population, rural, cohort | 4961 | Average | 11.8% |
| Sharma, 201734 | General population, urban, cross-sectional | 207 | Average | 28.5% |
| Choudhary, 201749 | Liver donors, urban, cross-sectional | 573 | Average | 11.3% |
| Gupta, 201859 | Patients with hypothyroidism, urban, cross-sectional | 50 | Average | 24% |
| Bhatt, 201837 | Patients with OSA and BMI >23 kg/m2, urban, cross-sectional | 240 | High | 70% |
| Vanjiappan, 201842 | Patients with DM-2, urban, cross-sectional | 300 | High | 61% |
| Jain, 201855 | Parents of overweight children, urban, cross-sectional | 380 | Average | 66.3% |
| Rajput, 201943 | Patients with prediabetes and HC, urban, cross-sectional | 100 (prediabetes) 100 (HC) |
High (prediabetes) Average (HC) |
59% (prediabetes) 26% (HC) |
| Chalmers, 201946 | General population, rural and urban, cross-sectional | 960 (rural) 1129 (urban) |
Average | 43.4% (rural) 55.2% (urban) |
| Harsha, 201948 | Women with PCOS, urban, cross-sectional | 60 | High | 38.3% |
| Duseja, 201911 | Male blood donors, urban, cross-sectional | 986 | Average | 53.5% |
| Arya, 202047 | Patients with DM-2, urban, cross-sectional | 100 | High | 50% |
| Karam, 201957 | Patients with metabolic syndrome, urban, cross-sectional | 100 | High | 34% |
| Goyal, 20209 | Patients with metabolic syndrome and HC, urban, cross-sectional | 100 (metabolic syndrome) 100 (HC) |
High (metabolic syndrome) Average (HC) |
73% (metabolic syndrome) 38% (HC) |
| Chakraborty, 202010 | Women with PCOS and HC, urban, cross-sectional | 70 (PCOS) 60 (HC) |
High (PCOS) Average (HC) |
38.6% (PCOS) 6.66% (HC) |
| Grewal, 202139 | Patients with hypothyroidism and HC, rural, cross-sectional | 100 (hypothyroid) 100 (HC) |
Average | 63% (hypothyroid) 28% (HC) |
| Kubihal, 202140 | Women with GDM and HC, urban, cross-sectional | 201 (GDM) 108 (HC) |
Average | 62.7% (GDM) 51.85% (HC) |
| Atri, 202036 | Women with BMI >32.5 kg/m2, urban, cross-sectional | 106 | High | 73.6% |
| Agarwal, 202133 | Celiac disease, urban, cohort | 304 | Average | 24.0% |
| Das, 201026 | General population, rural, cohort | 1911 | Average | 9.8% |
Abbreviations: OPD, outpatient department; BMI, body mass index; ALT, alanine aminotransferase; DM-1, type 1 diabetes mellitus; DM-2, type 2 diabetes mellitus; CAD, coronary artery disease; HC, healthy controls; PCOS, polycystic ovarian syndrome; IGT, impaired glucose tolerance; OSA, obstructive sleep apnea; GDM, gestational diabetes mellitus.
NAFLD in Children
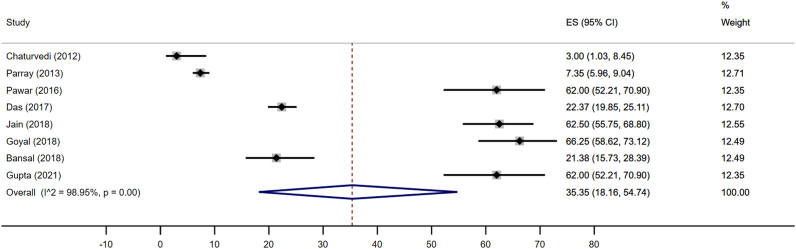
The pediatric data were collected either in school (n = 4) or hospital (n = 4) and included 2903 children. Pediatric studies represented only eight states and/or Union Territories (UTs). The pooled estimates of NAFLD prevalence were 35.4% (95% CI 18.2–54.7; I2 99%) (Figure 2). Four data points included 560 obese or overweight children (560/2903; 19.3%), which explains a high prevalence of NAFLD in children. On subgroup analysis, the pooled estimate among non-obese children and obese children was 12.4 (95% CI 4.4–23.5) and 63.4 (95% CI 59.4–67.3), respectively. Data collected from school and hospital-based sources showed NAFLD prevalence of 36.8% (95% CI 14.6–62.5%; I2 99.2%) and 33.8% (95% CI 8–66.5%; I2 98.4%), respectively.
Figure 2.
Pooled estimates of NAFLD prevalence by the random-effects model in children.
Data from four studies (n = 2336) were analyzed to estimate gender-specific NAFLD prevalence. The NAFLD prevalence among boys and girls was 36.8% (95% CI 13–64.6; I2 98.6%) and 37.1% (95% CI 15.3–62.1; I2 98.3%) respectively.
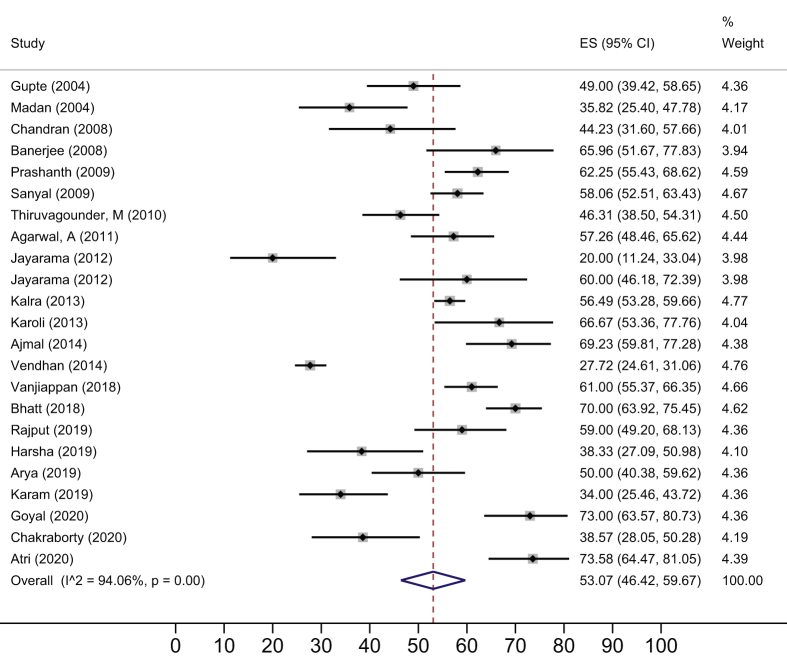
NAFLD in Adults
The prevalence data in the adult population were collected from either community (n = 9) or the hospital (n = 45). Most of the data for adults were collected from the urban (n = 47) population. The total number of study participants was 23,581. The single-center studies were conducted in 15 states/UTs (Delhi 12, Tamil Nadu 10, Haryana 6, West Bengal 5, Uttar Pradesh and Maharashtra 4 studies each, Karnataka 3, Kerala 2, and one each from Andhra Pradesh, Chandigarh, Odisha, Puducherry, Rajasthan, Uttarakhand, and Jammu & Kashmir). One study was multicentric.
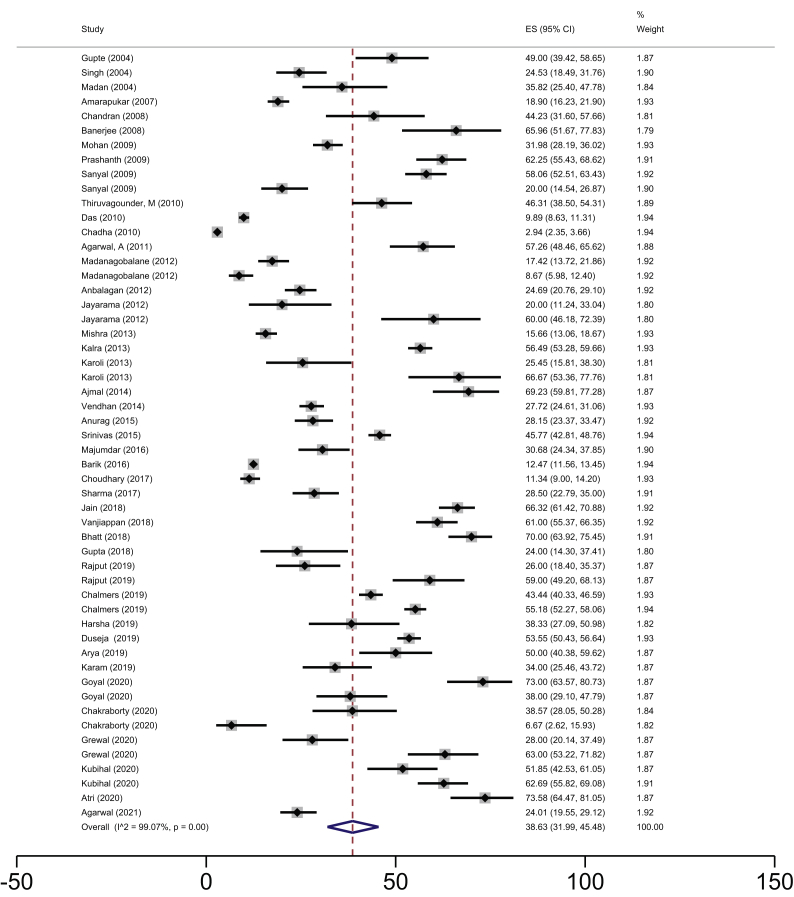
The overall pooled estimate of NAFLD prevalence among the adult population was 38.6% (95% CI 32–45.5; I2 99.1%) (Figure 3). The gender-specific NAFLD prevalence was estimated from 16 datasets (n = 10,282) which provided information on gender distribution. The gender-specific NAFLD prevalence was 39.4% (95% CI 27.7–51.7%; I2 98.5%) among males and 35.4% (95% CI 23.5–48.3%; I2 98.5%) among females.
Figure 3.
Pooled estimates of NAFLD prevalence by the random-effects model in adults.
The estimated NAFLD prevalence in community-based studies and hospital-based studies was found to be 28.2% (95% CI 16.9–41%; I2 99.4%) and 40.8% (95% CI 32.6–49.3%; I2 98.9%), respectively.
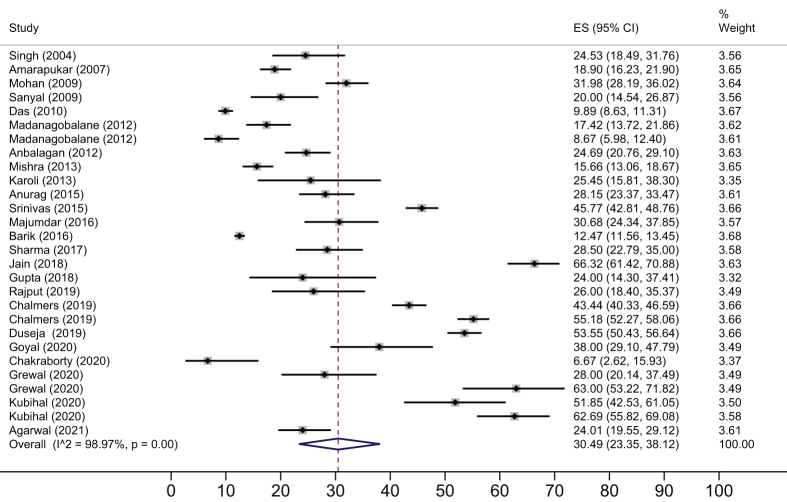
The NAFLD prevalence in average-risk (Figure 4) and high-risk (Figure 5) subgroups was 28.1% (95% CI 20.8–36.0; I2 99.2%) and 52.8% (95% CI 46.5–59.1; I2 93.8%) respectively. The NAFLD prevalence in the rural population was 29.2% (95% CI 17.8–42; I2 99.1%) whereas in the urban population, it was 40.0% (95% CI 32.4–48; I2 98.3%).
Figure 4.
Pooled estimates of NAFLD among average-risk adults.
Figure 5.
Pooled estimates of NAFLD among high-risk adults.
On subgroup analysis of data from urban populations, the NAFLD prevalence among average and high-risk populations was 27.8% (95% CI 18.3–38.3; I2 99.3%) and 52.8% (95% CI 46.5–59.1; I2 93.8%), respectively. State-wise prevalence of NAFLD among adults with average and high-risk population is shown in Table 2.
Table 2.
State-wise Prevalence of Non-alcoholic Fatty Liver (NAFLD) Among the Adult Population.
| State | Average-risk population |
High-risk population |
Overall∗ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Data sets included | Total number of study participants | NAFLD (%) | Data sets included | Total number of study participants | NAFLD (%) | Data sets included | Total number of study participants | NAFLD (%) | |
| Northern and Central India | |||||||||
| Delhi | 7 | 3742 | 16.7 | 5 | 601 | 60.4 | 12 | 4343 | 22.8 |
| Uttar Pradesh | 1 | 55 | 25.5 | 3 | 258 | 61.2 | 4 | 313 | 55.0 |
| Haryana | 5 | 1049 | 22.5 | 1 | 100 | 59 | 6 | 1149 | 25.7 |
| Uttarakhand | 1 | 207 | 28.5 | No data | – | – | 1 | 207 | 28.5 |
| Jammu and Kashmir | 1 | 50 | 24 | No data | – | – | 1 | 50 | 24 |
| Chandigarh | 1 | 986 | 53.5 | No data | – | – | 1 | 986 | 53.5 |
| Sub-total | 16 | 6089 | 24.2 | 9 | 959 | 60.5 | 25 | 7048 | 29.1 |
| Eastern India | |||||||||
| Odisha | 1 | 159 | 24.5 | No data | – | – | 1 | 159 | 24.5 |
| West Bengal | 3 | 6762 | 11.9 | 2 | 357 | 59.1 | 5 | 7119 | 14.3 |
| Sub-total | 4 | 6921 | 12.2 | 2 | 357 | 59.1 | 6 | 7278 | 14.5 |
| Western India | |||||||||
| Maharashtra | 2 | 1032 | 21.6 | 2 | 304 | 57.9 | 4 | 1336 | 29.9 |
| Rajasthan | 1 | 645 | 15.7 | No data | – | – | 1 | 645 | 15.7 |
| Sub-total | 3 | 1677 | 19.3 | 2 | 304 | 57.9 | 5 | 1981 | 25.2 |
| Southern India | |||||||||
| Tamil Nadu | 5 | 2446 | 30.7 | 5 | 1206 | 36.3 | 10 | 3652 | 32.6 |
| Karnataka | 1 | 50 | 20 | 2 | 199 | 49.7 | 3 | 249 | 43.8 |
| Kerala | 2 | 2089 | 49.8 | No data | – | – | 2 | 2089 | 49.8 |
| Andhra Pradesh | No data | – | – | 1 | 60 | 38.3 | 1 | 60 | 38.3 |
| Puducherry | No data | – | – | 1 | 300 | 61 | 1 | 300 | 61 |
| Sub-total | 8 | 4585 | 39.3 | 9 | 1765 | 42.1 | 17 | 6350 | 40.1 |
Abbreviation: NAFLD, non-alcoholic fatty liver disease.
∗Details provided for 53 out of the 54 data points. One study had participants from multiple states; state-wise distribution of participants was not available.
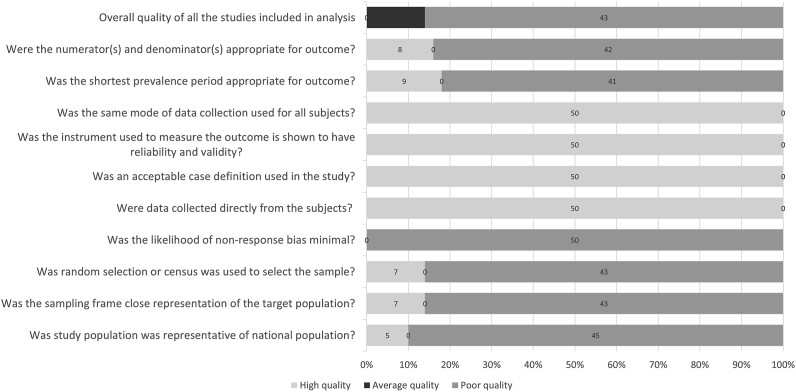
Quality of the Included Studies
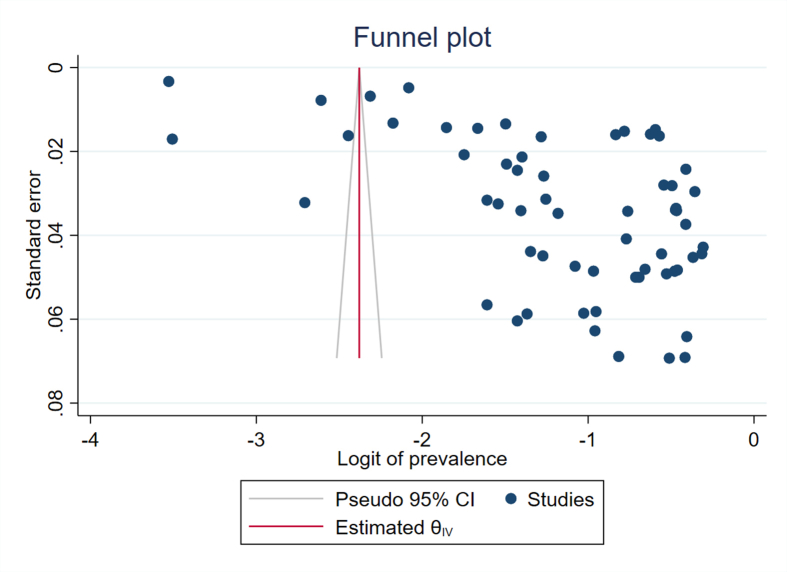
Most of the studies, selected for data extraction, were of poor quality (Figure 6). The overall quality of the included studies was either intermediate (n = 7) or poor (n = 43). Visual analysis of the funnel plot showed marked asymmetry (Supplementary Figure 1). A regression-based Egger test for small-study effects using the random-effects model showed a small-study effect (P = 0.0001). The funnel asymmetry may be due to more than 99% heterogeneity between the studies in our meta-analysis.
Figure 6.
Summary of the quality of the included studies.
Discussion
The overall pooled prevalence of NAFLD in India is 38.6% among adults and 35.4% among children. The prevalence is similar in males and females. Our analysis suggests that the prevalence of NAFLD in Indian urban and rural populations is higher than the average estimated global prevalence of 25%.
We calculated an overall pooled prevalence of NAFLD among children (<18 years of age) in India of 35.4% (95% CI: 18.2–54.7%), with a similar prevalence in boys and girls. The pooled prevalence of NAFLD among obese children was around 60%, five times greater than that in non-obese children. In contrast, a recent global meta-analysis estimated NAFLD prevalence among children from the general population to be 7.6% (95% CI: 5.5–10.3%) and among those under follow-up in obesity clinics to be 34.2% (95% CI: 27.8–41.2%), with a higher prevalence in Asian obese children.63 In our analysis, one-fifth of the included young individuals were obese/overweight, which could explain such high prevalence. Other possible reasons to explain this include significant heterogeneity among the studies included and sampling bias. We found no significant difference in NAFLD prevalence among studies conducted in school (36.8%, 95% CI 14.6–62.5%) or hospital (33.8%, 95% CI 8–66.5%) settings.
India is a young country; as per the 2011 census, 29.5% of the 1.2 billion population i.e., 354 million were <15 years, and another 121 million belonged to the age group of 15–19 years. If extrapolated to the overall population, our study results would suggest approximately 168 million children with NAFLD in India alone. The proportion of children and adolescents who are overweight/obese is around 20% and is increasing in both low-income and high socio-economic groups.64 The prospective obesity-related risks are alarming, as weight gain during school years carries a higher risk of NAFLD than weight gain in late adulthood.65 Since the prevalence of NAFLD increases with age, the overall proportion of adults with NAFLD in India may continue to rise over the next few decades.16 These predictions are extremely important from the public health perspective.
We found an estimated pooled prevalence of NAFLD among adults to be 38.6%. The NAFLD prevalence increased from average-risk to high-risk subgroups, which was expected as per the available literature.66 NAFLD has associations with other diseases like diabetes, obesity, metabolic syndrome, hypertension, dyslipidemia, coronary artery disease, obstructive sleep apnea, and hypothyroidism, which are progressively increasing in rural and urban populations in India.17,18,67 Physical activity is associated with a reduced risk of NAFLD.68
Our analysis did not reveal any significant difference between NAFLD prevalence among males (39.4%; 95% CI 27.7–51.7%) and females (35.4%; 95% CI 23.5–48.3%). In comparison, a global meta-analysis reported that women have a lesser incidence of NAFLD but with a higher risk of progression to advanced fibrosis.69 Interestingly, the prevalence of NAFLD in the rural population was 29.2% and in the urban population was 40.0%. Of the studies included in our meta-analysis, 44/50 (88.0%) were from the urban cohort, 40 were hospital-based and 4 were community-based. In contrast, 6/50 were from the rural cohort and included 4 community-based and 2 hospital-based. Very few studies have assessed the prevalence of NAFLD in both urban and rural cohorts simultaneously.46 A higher prevalence of NAFLD was observed among hospital-based studies as compared to community-based ones (40.8%, 95% CI 32.6–49.3% versus 27.2%, 95% CI 15.7–40.6%, respectively). This could have led to an overestimation of NAFLD prevalence in our analysis. Reasons for increased prevalence in the urban population include a more sedentary lifestyle, decreased physical activity, a high-calorie diet, and higher rates of obesity.65,70,71 Prevalence of diabetes is higher among those living in urban compared with those in rural areas.18 A recent meta-analysis reported a higher prevalence of metabolic syndrome among adults living in urban areas (32%) compared with those in tribal areas (28%) and rural areas (22%).17 Gut microbiome may play a role in the pathogenesis of NAFLD. A recent study reported differences in the gut microbiome among those living in rural and urban India.72 Future studies should explore the possible reasons for these differences in the rural and urban prevalence of NAFLD in India.
As per India's last two population censuses, the number of urban dwellers increased from 286 million in 2001 to 377 million in 2011. These numbers are expected to increase, which may add to the overall health burden of the country.
We could not assess the burden of the severe forms of NAFLD-non-alcoholic steatohepatitis and fibrosis, which actually determine the outcome in patients with NAFLD,73 as this information was not reported in most studies.
In patients with NAFLD, the most common cause of mortality in those without cirrhosis is coronary artery disease, whereas, in those with cirrhosis, liver-related deaths are common.66 Therefore, it is important to recognize and estimate the burden of the associated comorbidities in these patients.
Keeping the burden of NAFLD in mind, the Government of India has recently integrated NAFLD into the National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS) to target early diagnosis and management. To translate this goal into reality is a huge task.
Strengths
This is the first meta-analysis of all published studies on NAFLD from India.
Limitations
The majority of the data was from hospital-based studies. There were very few community-based population-based studies. The data from the rural population were under-represented. There were almost no data on NASH or lean NASH. Only 15/35 states/UTs were represented. No data were available on the prevalence of NAFLD in various weight categories, i.e, overweight, obesity I, obesity II, etc. We used ultrasound as the modality for the diagnosis of NAFLD. The limitations of ultrasound include subjective assessment, poor diagnostic accuracy for detecting mild steatosis, and a significant intra- and inter-observer variability. The included studies showed marked heterogeneity. This marked heterogeneity could be due to differences in the characteristics of the study designs, study setting, and study population. The included studies were of different designs such as case-control, cohort, and cross-sectional. These studies were conducted in varied clinical settings such as schools, clinics, hospitals, and the general population. The study population also differed in terms of major factors such as age, risk factors for NAFLD, comorbidities, etc. Due to a limited number of studies available for each subgroup, we were forced to merge the data from heterogeneous studies.
For a country with a population of 1.3 billion, the available data included only 23,581 individuals above 18 years of age and 2903 less than 18 years. Most of the available studies have included only a small number of patients, which may not be representative of the entire population. We could not assess the demographic details of NAFLD, including the number of males/females affected, age groups, BMI categories (lean versus obese), associated comorbidities (diabetes, hypertension, metabolic syndrome, or polycystic ovary disease), outcomes, and changing dietary patterns, as these data were available in few studies only. Also, it would be interesting to assess the prevalence of NAFLD over time, which was not possible in the present analysis due to the paucity of studies. Overall, the quality of studies included in the analysis was either high-risk (86%) or intermediate-risk (14%). Our estimate of NAFLD prevalence may not be accurate. Future prospective studies with large numbers and inclusion of all subgroups are required for the estimation of NAFLD prevalence in India.
Implication
We need to conduct larger and better-designed studies to generate reliable data on NAFLD in the Indian population.
In conclusion, the NAFLD burden in India is high among children and adults. Well-designed community-based studies are needed to assess the prevalence of NAFLD across India and plan resource allocation to mitigate the disease burden.
Credit authorship contribution statement
Shalimar: concept, data extraction, manuscript writing, and critical revision, Anshuman Elhence: data extraction and manuscript writing, Bhavik Bansal: data extraction and manuscript writing, Hardik Gupta: data extraction and manuscript writing, Abhinav Anand: data extraction and manuscript writing, Thakur Prashant Singh: data extraction and manuscript writing, and Amit Goel: concept, data extraction, manuscript writing, and critical revision.
Conflicts of interest
The authors have none to declare.
Acknowledgment
Ms. Sabreena is acknowledged for coordination and data maintenance.
Funding information
None.
Ethics clearance
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.11.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure.
Funnel plot showed marked asymmetry.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Zhai M., Liu Z., Long J., et al. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11:5195. doi: 10.1038/s41598-021-84577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Golabi P., de Avila L., et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Dai W., Ye L., Liu A., et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra S., Vithalani M., Gulati G., et al. Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (SPRINT) J Assoc Phys India. 2013;61:448–453. [PubMed] [Google Scholar]
- 6.Bellentani S., Saccoccio G., Masutti F., et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 7.de Barros F., Setúbal S., Martinho J.M., et al. The correlation between obesity-related diseases and non-alcoholic fatty liver disease in women in the pre-operative evaluation for bariatric surgery assessed by transient hepatic elastography. Obes Surg. 2016;26:2089–2097. doi: 10.1007/s11695-016-2054-y. [DOI] [PubMed] [Google Scholar]
- 8.Feijó S.G., Lima J.M., Oliveira M.A., et al. The spectrum of non-alcoholic fatty liver disease in morbidly obese patients: prevalence and associate risk factors. Acta Cir Bras. 2013;28:788–793. doi: 10.1590/s0102-86502013001100008. [DOI] [PubMed] [Google Scholar]
- 9.Goyal A., Arora H., Arora S. Prevalence of fatty liver in metabolic syndrome. J Fam Med Prim Care. 2020;9:3246–3250. doi: 10.4103/jfmpc.jfmpc_1108_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty S., Ganie M.A., Masoodi I., et al. Fibroscan as a non-invasive predictor of hepatic steatosis in women with polycystic ovary syndrome. Indian J Med Res. 2020;151:333–341. doi: 10.4103/ijmr.IJMR_610_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duseja A., Najmy S., Sachdev S., et al. High prevalence of non-alcoholic fatty liver disease among healthy male blood donors of urban India. JGH Open. 2019;3:133–139. doi: 10.1002/jgh3.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan K., Batra Y., Panda S.K., et al. Role of polymerase chain reaction and liver biopsy in the evaluation of patients with asymptomatic transaminitis: implications in diagnostic approach. J Gastroenterol Hepatol. 2004;19:1291–1299. doi: 10.1111/j.1440-1746.2004.03446.x. [DOI] [PubMed] [Google Scholar]
- 13.Nayak N.C., Jain D., Vasdev N., Gulwani H., Saigal S., Soin A. Etiologic types of end-stage chronic liver disease in adults: analysis of prevalence and their temporal changes from a study on native liver explants. Eur J Gastroenterol Hepatol. 2012;24:1199–1208. doi: 10.1097/MEG.0b013e32835643f1. [DOI] [PubMed] [Google Scholar]
- 14.Das M.K., Bhatia V., Sibal A., et al. Prevalence of nonalcoholic fatty liver disease in normal-weight and overweight preadolescent children in Haryana, India. Indian Pediatr. 2017;54:1012–1016. doi: 10.1007/s13312-017-1202-3. [DOI] [PubMed] [Google Scholar]
- 15.Parry I.A., Bhat R.A., Khan I. The prevalence of non-alcoholic fatty liver disease and its association with metabolic syndrome and obesity in pediatric population of north India. J Metab Syndrome. 2012;1:118. [Google Scholar]
- 16.Negro F. Natural history of NASH and HCC. Liver Int. 2020;40(suppl 1):72–76. doi: 10.1111/liv.14362. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamoorthy Y., Rajaa S., Murali S., Rehman T., Sahoo J., Kar S.S. Prevalence of metabolic syndrome among adult population in India: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranasinghe P., Jayawardena R., Gamage N., Sivanandam N., Misra A. Prevalence and trends of the diabetes epidemic in urban and rural India: a pooled systematic review and meta-analysis of 1.7 million adults. Ann Epidemiol. 2021;58:128–148. doi: 10.1016/j.annepidem.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Hoy D., Brooks P., Woolf A., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Anbalagan V.P., Venkataraman V., Vamsi M., Deepa M., Mohan V. A simple Indian diabetes risk score could help identify nondiabetic individuals at high risk of non-alcoholic fatty liver disease (CURES-117) J Diabetes Sci Technol. 2012;6:1429–1435. doi: 10.1177/193229681200600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra S., Yadav D., Gupta M., Mishra H., Sharma P. A study of carotid atherosclerosis in patients with non-alcoholic fatty liver disease. Indian J Clin Biochem. 2013;28:79–83. doi: 10.1007/s12291-012-0286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S.P., Nayak S., Swain M., et al. Prevalence of nonalcoholic fatty liver disease in coastal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 23.Thiruvagounder M., Khan S., Sheriff D.S. Non-alcoholic fatty liver disease (NAFLD) - is it an emerging risk factor for coronary artery disease?: preliminary study in a local Indian population. Sultan Qaboos Univ Med J. 2010;10:221–226. [PMC free article] [PubMed] [Google Scholar]
- 24.Sanyal D., Mukhopadhyay P., Pandit K., Mukhopadhyay S., Chowdhury S. Central obesity but not generalised obesity (body mass index) predicts high prevalence of fatty liver (NRFLD), in recently detected untreated, IGT and type 2 diabetes Indian subjects. J Indian Med Assoc. 2009;107:755–758. [PubMed] [Google Scholar]
- 25.Vendhan R., Amutha A., Anjana R.M., Unnikrishnan R., Deepa M., Mohan V. Comparison of characteristics between nonobese and overweight/obese subjects with nonalcoholic fatty liver disease in a South Indian population. Diabetes Technol Therapeut. 2014;16:48–55. doi: 10.1089/dia.2013.0165. [DOI] [PubMed] [Google Scholar]
- 26.Das K., Das K., Mukherjee P.S., et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 27.Karoli R., Fatima J., Chandra A., Gupta U., Islam F.-U., Singh G. Prevalence of hepatic steatosis in women with polycystic ovary syndrome. J Hum Reprod Sci. 2013;6:9–14. doi: 10.4103/0974-1208.112370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barik A., Shah R.V., Spahillari A., et al. Hepatic steatosis is associated with cardiometabolic risk in a rural Indian population: a prospective cohort study. Int J Cardiol. 2016;225:161–166. doi: 10.1016/j.ijcard.2016.09.120. [DOI] [PubMed] [Google Scholar]
- 29.Srinivas M., Srinivasan V., Mohan M.B., Varghese J., Venkataraman J. A study of gender-wise risk association between fatty liver and metabolic syndrome components (Asia-Pacific criteria) in a South Indian urban cohort. Indian J Gastroenterol. 2015;34:38–42. doi: 10.1007/s12664-014-0525-4. [DOI] [PubMed] [Google Scholar]
- 30.Chandran S.R., Vishnuram P. Hepatic profile of type 2 diabetes mellitus patients in a tertiary care hospital. J Assoc Phys India. 2008;56:645. [PubMed] [Google Scholar]
- 31.Chadha D.S., Sharma S., Sivasankar R., Kudva N., Sabhiki G., Behl A. Abdominal sonography in the medical evaluation of aviation aspirants. Aviat Space Environ Med. 2010;81:965–969. doi: 10.3357/asem.2749.2010. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee S., Ghosh U.S., Dutta S. Clinicopathological profile of hepatic involvement in type-2 diabetes mellitus and its significance. J Assoc Phys India. 2008;56:593–599. [PubMed] [Google Scholar]
- 33.Agarwal A., Singh A., Mehtab W., et al. Patients with celiac disease are at high risk of developing metabolic syndrome and fatty liver. Intest Res. 2021;19:106–114. doi: 10.5217/ir.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma V.K., Malhotra A., Chauhan T.K. Evaluation of liver stiffness in healthy individuals by sonoelastography. J Clin Diagn Res. 2017;11:TC13–TC16. [Google Scholar]
- 35.Jayarama N., Sudha R. A study of non-alcoholic fatty liver disease (NAFLD) in type 2 diabetes mellitus in a tertiary care centre, southern India. J Clin Diagn Res. 2012;6:243. [Google Scholar]
- 36.Atri A., Jiwanmall S.A., Nandyal M.B., et al. The prevalence and predictors of non-alcoholic fatty liver disease in morbidly obese women - a cross-sectional study from southern India. Eur Endocrinol. 2020;16:152–155. doi: 10.17925/EE.2020.16.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt S.P., Guleria R., Vikram N.K., Vivekanandhan S., Singh Y., Gupta A.K. Association of inflammatory genes in obstructive sleep apnea and non alcoholic fatty liver disease in Asian Indians residing in north India. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajmal M.R., Yaccha M., Malik M.A., et al. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients of cardiovascular diseases and its association with hs-CRP and TNF-α. Indian Heart J. 2014;66:574–579. doi: 10.1016/j.ihj.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grewal H., Joshi S., Sharma R., Mittal P., Goel A. Non-alcoholic fatty liver disease in patients with hypothyroidism presenting at a rural tertiary care centre in north India. Trop Doct. 2021;51:181–184. doi: 10.1177/0049475520945058. [DOI] [PubMed] [Google Scholar]
- 40.Kubihal S., Gupta Y., Shalimar, et al. Prevalence of non-alcoholic fatty liver disease and factors associated with it in Indian women with a history of gestational diabetes mellitus. J Diabetes Investig. 2021;12:877–885. doi: 10.1111/jdi.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prashanth M., Ganesh H.K., Vima M.V., et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Phys India. 2009;57:205–210. [PubMed] [Google Scholar]
- 42.Vanjiappan S., Hamide A., Ananthakrishnan R., Periyasamy S.G., Mehalingam V. Nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and its association with cardiovascular disease. Diabetes Metab Syndr. 2018;12:479–482. doi: 10.1016/j.dsx.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Rajput R., Ahlawat P. Prevalence and predictors of non-alcoholic fatty liver disease in prediabetes. Diabetes Metab Syndr. 2019;13:2957–2960. doi: 10.1016/j.dsx.2019.07.060. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal A.K., Jain V., Singla S., et al. Prevalence of non-alcoholic fatty liver disease and its correlation with coronary risk factors in patients with type 2 diabetes. J Assoc Phys India. 2011;59:351–354. [PubMed] [Google Scholar]
- 45.Pawar S.V., Zanwar V.G., Choksey A.S., et al. Most overweight and obese Indian children have nonalcoholic fatty liver disease. Ann Hepatol. 2016;15:853–861. doi: 10.5604/16652681.1222101. [DOI] [PubMed] [Google Scholar]
- 46.Chalmers J., Ban L., Leena K.B., et al. Cohort profile: the Trivandrum non-alcoholic fatty liver disease (NAFLD) cohort. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arya S., Haria J.M., Mishra A. To study the occurrence of non-alcoholic fatty liver disease (NAFLD) in type -II diabetes mellitus. J Assoc Phys India. 2020;68:51. [Google Scholar]
- 48.Harsha Varma S., Tirupati S., Pradeep T.V.S., Sarathi V., Kumar D. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065–1069. doi: 10.1016/j.dsx.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 49.Choudhary N.S., Saraf N., Saigal S., et al. Prediction of nonalcoholic fatty liver in prospective liver donors. Clin Transplant. 2017;31 doi: 10.1111/ctr.12890. [DOI] [PubMed] [Google Scholar]
- 50.Anurag L., Aniket S., Shalik J., Amarja L., Dhananjay R., Sachin J. Non-alcoholic fatty liver disease prevalence and associated risk factors--A study from rural sector of Maharashtra. Trop Gastroenterol. 2015;36:25–30. doi: 10.7869/tg.241. [DOI] [PubMed] [Google Scholar]
- 51.Amarapurkar D., Kamani P., Patel N., et al. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 52.Majumdar A., Misra P., Sharma S., Kant S., Krishnan A., Pandav C.S. Prevalence of nonalcoholic fatty liver disease in an adult population in a rural community of Haryana, India. Indian J Publ Health. 2016;60:26–33. doi: 10.4103/0019-557X.177295. [DOI] [PubMed] [Google Scholar]
- 53.Madanagobalane S., Anandan S. The increased prevalence of non-alcoholic fatty liver disease in psoriatic patients: a study from South India. Australas J Dermatol. 2012;53:190–197. doi: 10.1111/j.1440-0960.2012.00905.x. [DOI] [PubMed] [Google Scholar]
- 54.Mohan V., Farooq S., Deepa M., Ravikumar R., Pitchumoni C.S. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 55.Jain V., Jana M., Upadhyay B., et al. Prevalence, clinical & biochemical correlates of non-alcoholic fatty liver disease in overweight adolescents. Indian J Med Res. 2018;148:291. doi: 10.4103/ijmr.IJMR_1966_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupte P., Amarapurkar D., Agal S., et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 57.Karam P., Shanthi B., Selvi K. Study of prevalence of non-alcoholic fatty liver disease in already diagnosed metabolic syndrome patients in selected South Indian population. Indian J Public Health Res Develop. 2019:4166–4171. [Google Scholar]
- 58.Goyal P., Thapa B.R., Sharma N.R., Menon J., Bhatia A. Nutritional assessment in obese children with and without non-alcoholic fatty liver disease (NAFLD) in an urban area of Punjab, India. Indian J Public Health Res Develop. 2018;9 210-207. [Google Scholar]
- 59.Gupta A., Aggarwal R., Yousuf S., Sharma T., Gupta A.K. A study to find out the association between subclinical hypothyroidism/clinical hypothyroidism and NAFLD in subjects aged 18 to 65 years. JK Sci. 2018;20:161–168. [Google Scholar]
- 60.Bansal M, Vohra R, Sood A, Bhardwaj P. Correlation between metabolic, liver profile, dietary habits and ultrasound scan determined non-alcoholic fatty liver disease changes in children aged 6-18 years with body mass index. Accessed June 23, 2021. https://sljch.sljol.info/articles/abstract/10.4038/sljch.v47i2.8477/.
- 61.Chaturvedi K., Vohra P. Non-alcoholic fatty liver disease in children. Indian Pediatr. 2012;49:757–758. doi: 10.1007/s13312-012-0161-y. [DOI] [PubMed] [Google Scholar]
- 62.Gupta N., Jindal G., Nadda A., Bansal S., Gahukar S., Kumar A. Prevalence and risk factors for nonalcoholic fatty liver disease in obese children in rural Punjab, India. J Family Community Med. 2020;27:103–108. doi: 10.4103/jfcm.JFCM_287_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson E.L., Howe L.D., Jones H.E., Higgins J.P.T., Lawlor D.A., Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ranjani H., Mehreen T.S., Pradeepa R., et al. Epidemiology of childhood overweight & obesity in India: a systematic review. Indian J Med Res. 2016;143:160–174. doi: 10.4103/0971-5916.180203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 66.Miptah H.N., Ramli A.S., Mohamad M., Hashim H., Tharek Z. Non-alcoholic fatty liver disease (NAFLD) and the cardiovascular disease (CVD) risk categories in primary care: is there an association? BMC Fam Pract. 2020;21:238. doi: 10.1186/s12875-020-01306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jarvis H., Craig D., Barker R., et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu S., Cai X., Sun Z., et al. Association between physical activity and risk of nonalcoholic fatty liver disease: a meta-analysis. Therap Adv Gastroenterol. 2017;10:701–713. doi: 10.1177/1756283X17725977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balakrishnan M., Patel P., Dunn-Valadez S., et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61–71.e15. doi: 10.1016/j.cgh.2020.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elhence A., Shalimar Treatment of non-alcoholic fatty liver disease - current perspectives. Indian J Gastroenterol. 2020;39:22–31. doi: 10.1007/s12664-020-01021-2. [DOI] [PubMed] [Google Scholar]
- 71.Fan J.-G. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28(suppl 1):11–17. doi: 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 72.Das B., Ghosh T.S., Kedia S., et al. Analysis of the gut microbiome of rural and urban healthy Indians living in sea level and high altitude areas. Sci Rep. 2018;8:10104. doi: 10.1038/s41598-018-28550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor R.S., Taylor R.J., Bayliss S., et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–1625.e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.