Abstract
Background
Effective means for early diagnosis are imperative to reduce death rate of non-small cell lung cancer (NSCLC) patients. We aimed to find out high-performance serologic markers to distinguish early-stage NSCLC patients from benign pulmonary nodule patients and healthy controls (HC). Cystatin-SN (CST1) is an active cysteine protease inhibitor of the CST superfamily, involving in the processes of inflammation and tumorigenesis. This is the first exploration of the diagnostic and prognostic values of serum CST1 in NSCLC.
Methods
We analyzed the transcriptome data from The Cancer Genome Atlas and the Gene Expression Omnibus database, screened biomarkers for NSCLC, and verified the candidate markers via the ONCOMINE database. Then, we performed ELISA, western blotting, and immunohistochemistry analysis to detect the expression levels of CST1 in NSCLC cell lines, tumor tissues, and serum samples of clinical cohorts.
Results
We identified 3 up-regulated secreted protein-encoding genes, validated the expression levels of CST1 in NSCLC tumor tissues and cell lines, and found that serum CST1 levels of NSCLC (4289 ± 2405 pg/mL) were significantly higher than those of PBN patients (1558 ± 441 pg/mL, P < .0001) and healthy controls (1529 ± 416 pg/mL, P < .0001). The AUC of the combination of CST1, Cytokeratin 19 fragment (Cyfra21-1), and Carcinoembryonic antigen (CEA) for distinguishing early-stage NSCLC from PBN/HC was as high as .914/0.925. Furthermore, our results suggested that the NSCLC patient with low serum CST1 level had a better survival rate.
Conclusions
Serum CST1 may serve as a novel diagnostic marker for differentiating early-stage NSCLC from PBN and HC, and could be used as a prognosis predictor in NSCLC patients.
Keywords: biomarker, cystatin-SN, diagnosis, non-small cell lung cancer, prognosis
Introduction
Non-small cell lung cancer (NSCLC) is the most common subtype of lung cancer, accounting for nearly 85% of lung cancer cases.1,2 Most NSCLC patients were initially diagnosed at advanced stage, of which the 5-year survival rate remains extremely poor; while the 5-year survival rate for the patients diagnosed at an early stage was as high as 71%-88%.3,4 Therefore, effective means for early diagnosis are imperative to reduce death rate of NSCLC.
As the gold standard for the diagnosis of NSCLC, the combination of bronchoscopy and pathological examination is an invasive and traumatic examination, leading to the poor compliance. Hematology testing is a convenient and safe diagnosis method, which plays an important role in the diagnosis and prognosis prediction of various tumors. Currently, serological markers including carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA21-1) are being employed extensively to diagnose NSCLC, as recommended by the National Academy of Clinical Biochemistry. 5 Although these serum markers exhibit high specificity (90%), their sensitivity is still quite low (50-60%), 6 which cannot meet the actual clinical needs. It is urgently necessary to develop innovative and high-performance diagnostic tumor markers.
Cystatin-SN (CST1), a secreted protein encoded by the CST1 gene, plays an important role in regulating the proteolytic activity of cysteine proteases and involving inflammation and tumorigenesis. 7 Numerous studies confirmed that high expression of CST1 was an independent indicator of poor prognosis in various cancers by multivariate analysis.8-12 In NSCLC, a study revealed that high level of CST1 in tumor tissue is associated with poor survival of patients through immunohistochemistry (IHC) analysis. 13 It has also been reported that the secreted protein CST1 can be detected in serum. 14 However, whether serum CST1 can serve as an independent predictor in NSCLC and aid the clinical diagnosis remains to be addressed.
In the present study, we identified CST1 as a candidate biomarker from the transcriptome data of TCGA and GEO database, and validated its expression in cell lines, tumor tissues and serum samples from NSCLC patients. We further evaluated the feasibility of detecting serum CST1 for differential diagnosis of clinical patients with NSCLC or benign nodules, and assessed its diagnostic efficacy and prognostic value.
Material and Methods
Patients and Specimens
In this retrospective clinical study, the NSCLC group included 201 NSCLC patients pathologically diagnosed at SYSUCC between December 2015 and February 2017. All patients met the following inclusion criteria: A clear pathological diagnosis of primary NSCLC was confirmed by two pathologists, and no surgery, chemotherapy or any other treatment was received before serum collection. A total of 94 patients with pulmonary benign nodules (PBN) were enrolled from SYSUCC between March 2015 and February 2016, which met the following criteria: Pulmonary benign nodules were identified by computed tomography (CT) scans, and at least 2-year follow-up showed no progression in the pulmonary nodules. Control samples were collected from 119 healthy volunteers without any malignant tumor or benign nodules confirmed by chest CT between March 2015 and February 2016. Each subject was asymptomatic without known disease, and underwent routine blood tests, serum biochemistry tests, liver function and kidney function tests in the PBN group and the healthy control (HC) group. A total of 19 NSCLC tumor tissues and adjacent non-tumor tissues were collected for IHC analysis. Besides, serum samples were retrospectively collected from 137 NSCLC patients who were treated at SYSUCC from May 2002 until April 2004 for survival analysis. The pathological stage was based on the American Joint Committee on Cancer (AJCC) 7th edition staging criteria. 15 Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article. All the procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Validation of Candidate Genes via the ONCOMINE Database
To validate the expression of candidate genes, we consulted the ONCOMINE database (http://www.ONCOMINE.org/), selected “cancer type” as “Non-small cell lung cancer”, chose all the transcriptome data of NSCLC, and we entered the gene symbols to be verified to obtain the expression levels in each study (the results of 32 studies were listed in supplement files).
Development of Enzyme-Linked Immunosorbent Assay (ELISA) for CST1
After screening multiple antibody pairs, a double antibody sandwich ELISA for CST1 antigen was developed using rabbit polyclonal antibody (1:2000, 16025-1-AP, Proteintech) as capture antibody and mouse monoclonal antibody (1: 6250, MAB1285-SP, R&D Systems) labeled with biotin as detection antibody. CST1 fusion protein (Ag9068, Proteintech) was used as standard/positive control. Other materials included 96-well plates (Corning), BSA (MPBio), PBS (Zhongshan Jinqiao), and TMB color reagent & stop solution (Kangwei Century). Low or high CST1 expression was defined by the median CST1 level (5463 pg/mL) determined in serum from NSCLC patients.
IHC Staining
The protocols are listed in the supplementary appendix.
Cell Culture
Human epithelial cells (16HBE and Beas2-B), and different NSCLC cell lines (A549, 95D, H1975, HCC827, PC-9, H358 and H460) were cultured in RPMI 1640 medium (Invitrogen) supplemented with 5-10% fetal bovine serum (Thermo Fisher Scientific) in a thermostatic incubator at 37°C with 5% CO2.
Western Blotting
Cells were lysed with sample buffer with proteinase/phosphatase inhibitors. The protein samples were separated by 6-15% SDS-PAGE, transferred to polyvinylidene difluoride membranes and then blocked with 5% skim milk for 45 min. The membranes were incubated with antibodies against CST1 (1: 400, 16025-1-AP, Proteintech) or β-actin (1:3000, A5441, Sigma-Aldrich) overnight at 4°C, followed by a horseradish peroxidase (HRP)-conjugated secondary antibody.
Statistical Analysis
Data was analyzed using Statistic Package for the Social Science 20.0 (SPSS, IBM). Nonparametric Mann-Whitney U-test or Kruskal-Wallis test were used to compare the differences in CST1 expression between two or more than two groups. The receiver operating characteristic curve (ROC) was established using GraphPad Prism 5 (San Diego, USA); the area under the curve (AUC), sensitivity, and specificity were utilized to evaluate the diagnostic efficacy. ELISA Calc was used to plot ELISA standard curve. Survival analysis was performed using the Kaplan-Meier method, and survival differences between the groups were determined by log-rank test. Multivariate analysis was performed using a Cox regression model. All statistical tests were two-sided and P < .05 was considered statistically significant.
Results
Identification of DEGs in NSCLC from the TCGA and GEO Database
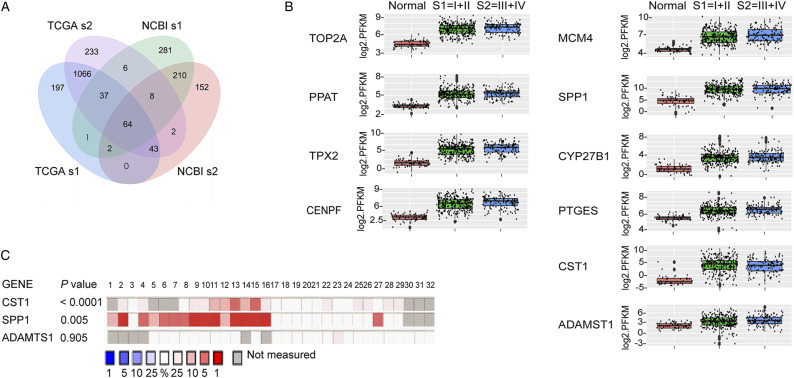
To obtain potential diagnostic indicators for lung cancer, we firstly screened and analyzed The Cancer Genome Atlas (TCGA) database and the Gene Expression Omnibus (GEO) database according to certain filter conditions by bioinformatics methods (Supplematal Table S1). A total of 64 overlapped differentially expressed genes (DEGs) were identified from early-stage (stage I and stage II) lung adenocarcinoma patients in both TCGA and GEO datasets (Figure 1A), of which 10 genes were up-regulated (Figure 1B) and 54 genes were down-regulated; all the DEGs are shown in Supplemenatl Figure S1. Considering that secreted proteins are much more likely to be detected in serum than nuclear proteins and membrane proteins especially under tumor progression, we picked out three up-regulated secreted protein-encoding genes as the candidate genes including Cystatin-SN (CST1), secreted phosphoprotein 1-osteopontin (SPP1) and a disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1) from 64 DEGs.
Figure 1.
Selection and verification of potential serum diagnostic indicators for NSCLC from the TCGA, NCBI, and Oncomine databases. A, in the TCGA and NCBI databases, 64 common genes were differentially expressed in early-stage lung adenocarcinoma patients, of which 10 genes were up-regulated and 54 genes were down-regulated; B, expression levels of 10 up-regulated genes in early-stage and advanced NSCLC compared with healthy controls; C, validation of the 3 candidate genes in 32 NSCLC studies from the ONCOMINE database, with ranking by the median expression levels of the genes in each study. s1, stage I; s2, stage II.
Verification of the Candidate Genes via the ONCOMINE Database
We validated the expression levels of three candidate genes in NSCLC-related studies using the ONCOMINE database. The results showed that CST1 (P < .0001) and SPP1 (P = .005) were significantly highly expressed in the NSCLC group compared with the HC group, while no significant difference was observed in ADAMTS1 (P = .905) (Figure 1C). Therefore, we speculated that CST1 and SPP1 may be suitable for the diagnosis of NSCLC. However, in view of numerous studies16,17 that had reported peripheral blood SPP1 was associated with the diagnosis, metastasis and recurrence of NSCLC, next we mainly focused on the diagnostic and prognostic values of CST1 for NSCLC.
Validation of CST1 Expression in NSCLC Cell Lines and Tumor Tissues
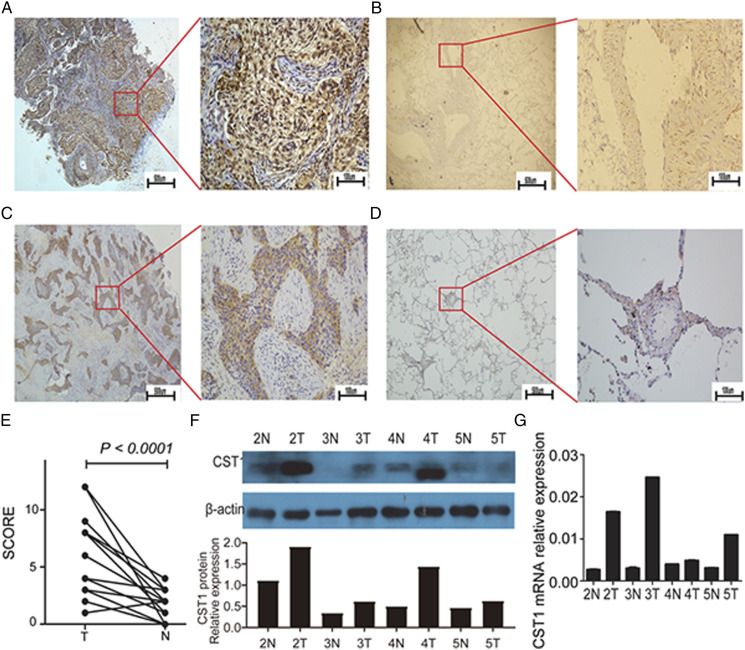
We further verified the expression of CST1 at the transcriptome level and the proteomic level in NSCLC samples. The expression of CST1 mRNA and protein in NSCLC tumor tissues were both significantly higher than that in adjacent tissues (Figure 2F-2G). Besides, IHC staining was performed to detect the expression levels of CST1 in 19 pairs of NSCLC tumor tissues and matched adjacent tissues. The results showed that the expression of CST1 protein was higher in 94.74% (18/19) of NSCLC tissues including lung adenocarcinoma and lung squamous cell carcinoma, compared with adjacent tissues (Figure 2A-2D), and the scores of CST1 levels were significantly higher in NSCLC tissues (P < .0001). Furthermore, compared with normal lung epithelial cells (16HBE and Beas2-B), there were significant differences observed in mRNA, cellular protein and supernatant protein levels of CST1 in 7 NSCLC cell lines (A549, 95D, H1975, HCC827, PC-9, H358 and H460) (Supplemenatl Figure S2A-2C).
Figure 2.
The expression of CST1 in NSCLC tumor tissues and adjacent tissues. Immunohistochemical (IHC) staining of CST1 in tumor tissues (A, left: 40 × and right: 200 ×) and adjacent normal tissues (B, left: 40 × and right: 200 ×) from lung adenocarcinoma patients; IHC staining of CST1 in tumor tissues (C, left: 40 × and right: 200 ×) and adjacent tissues (D, left: 40 × and right: 200 ×) from lung squamous cell carcinoma patients; Brown or tan particles are positively stained; E, CST1 protein expression levels were scored in 19 pairs of NSCLC tumor tissues and adjacent tissues; F, Western blotting of CST1 protein expression levels in 4 pairs of NSCLC tumor tissues and adjacent tissues and relative quantification analysis; G, the mRNA relative expression of CST1 in 4 pairs of NSCLC tumor tissues and adjacent tissues. T, the NSCLC tumor tissue; N, the adjacent normal tissue.
Serum CST1 Levels in NSCLC Patients
We recruited a total of 414 participants (201 NSCLC patients, 94 PBN patients and 119 healthy controls), of whom the main characteristics were presented in Supplemenatl Table S2.
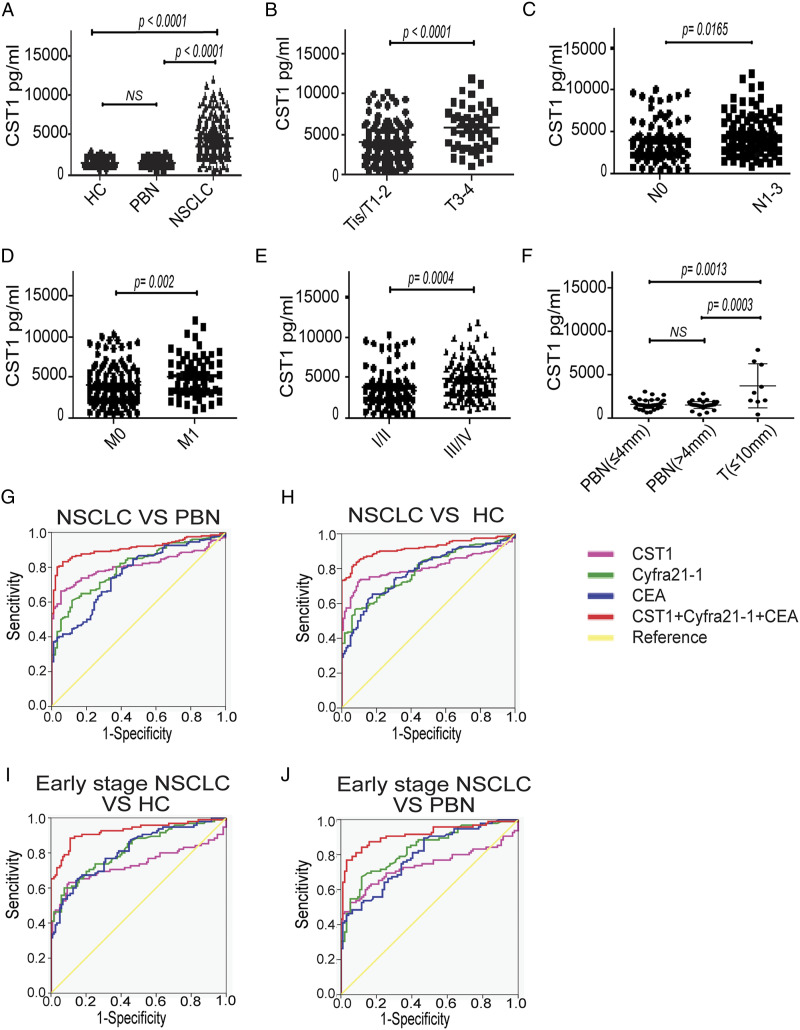
The level of serum CST1 in NSCLC patients (4289 ± 2405 pg/mL) was significantly higher than in PBN patients (1558 ± 441 pg/mL, P < .0001) and healthy controls (1529 ± 416 pg/mL, P < .0001). No significant difference was observed in serum CST1 level between the PBN group and the HC group (Figure 3A).
Figure 3.
Diagnostic efficiency of serum CST1 level for NSCLC. A, serum levels of CST1 in HC (n = 119), PBN patients (n = 94), and NSCLC patients (n = 201); B-E, serum CST1 levels in patients with stage T0–2 (n = 150) versus T3–4 (n = 45), N0 (n = 88) versus N1–3 (n = 102), M0 (n = 143) versus M1 (n = 58), or stage I–II (n = 94) versus III–IV (n = 107); F, serum CST1 levels in the PBN patients with the longest diameter of the nodule (Dmax) less than or equal to 4 mm (n = 49), PBN patients with Dmax more than 4 mm (n = 45), and NSCLC patients with Dmax less than or equal to 10 mm (n = 9); The ROC analysis of serum CST1, Cyfra21-1, CEA, and three combined indicators in distinguishing between NSCLC and PBN (G), NSCLC and HC (H), early-stage NSCLC and HC (I), early-stage NSCLC and PBN (J).
In addition, we analyzed the correlation between serum CST1 levels and the clinical characteristics of patients with NSCLC. We found that patients with stage T3-T4, N1-3 or M1 exhibited significantly higher median serum CST1 levels compared with patients with stage TIS-T2, N0 or M0, respectively (Table 1 and Figure 3B). We also observed significantly higher median serum CST1 levels in patients with stage III-IV compared with patients with stage I-II (Table 1 and Figure 3C-3E).
Table 1.
Clinical characteristics and serum CST1 levels of 201 NSCLC patients.
| Characteristics | No. | Percentage, % | CST1 (pg/mL) | P value |
|---|---|---|---|---|
| Age (years) | .130 | |||
| <60 | 111 | 55.22 | 3472 (492.4-11940) | |
| ≥60 | 90 | 54.78 | 4033 (378.3-9984) | |
| Gender | .072 | |||
| Male | 124 | 61.69 | 4134 (378.3-11940) | |
| Female | 77 | 38.31 | 3255 (416.3-11290) | |
| Type | .134 | |||
| AD | 159 | 79.10 | 7098 (416.3-11940) | |
| SCC | 28 | 13.93 | 5299 (378.3-10260) | |
| Others | 14 | 6.97 | 5233 (1151-9580) | |
| Grade | .455 | |||
| Well differentiated | 3 | 1.49 | 3990 (1535-7847) | |
| Middle differentiated | 66 | 32.84 | 3607 (530.4-9984) | |
| Poorly differentiated | 98 | 48.76 | 3800 (378.3-10420) | |
| missing | 34 | 16.92 | 4112 (416.3-11940) | |
| pT stage | .003 | |||
| pTis | 2 | 1.00 | 3312 (2024-4599) | |
| pT1 | 64 | 31.84 | 3295 (416.3-11940) | |
| pT2 | 84 | 41.79 | 3594 (378.3-10260) | |
| pT3 | 28 | 13.93 | 5553 (1438-11940) | |
| pT4 | 17 | 8.46 | 5763 (1045-11290) | |
| missing | 6 | 2.99 | 4004 (1724-8215) | |
| pN stage | .023 | |||
| pN0 | 88 | 43.78 | 3245 (378.3-9984) | |
| pN1 | 26 | 12.94 | 4274 (760-10260) | |
| pN2 | 60 | 29.85 | 3960 (1282-11290) | |
| pN3 | 16 | 7.96 | 6544 (3169-11940) | |
| missing | 11 | 5.47 | 3885 (1045-9922) | |
| pM stage | .002 | |||
| pM0 | 143 | 71.14 | 3586 (378.3-10260) | |
| pM1 | 58 | 28.86 | 4762 (1045-11940) | |
| TNM stage | .001 | |||
| I | 67 | 33.33 | 2974 (416.3-9547) | |
| II | 27 | 13.43 | 3472 (378.3-10260) | |
| III | 49 | 24.38 | 4168 (1438-9984) | |
| IV | 58 | 28.86 | 4762 (1045-11940) |
The CST1 level was expressed as the median with the range. Mann-Whitney U-test or Kruskal-Wallis test were used to compare the differences in CST1 levels between two or more than two groups. AD, adenocarcinoma; SCC, squamous carcinoma.
Besides, we further analyzed the correlation between serum CST1 levels and the PBN size. The 94 cases of PBN patients were divided into two groups by the size of PBN: the longest diameter of the nodule ≤4 mm (n = 49) and the longest diameter of the nodule >4 mm (n = 45). The basic characteristics were shown in Supplemenatl Table S3. There was no significant difference in serum CST1 level between the two group, while the serum CST1 levels of the NSCLC patients with the longest diameter of the tumor <10 mm were significantly higher than those in above two PBN groups (P < .01) (Figure 3F).
We also detected CST1 level in the serum of patients with pulmonary chronic inflammation, including chronic pneumonia, chronic obstructive pulmonary disease, pulmonary chronic granulomatous inflammation and so on. 40 patients with chronic inflammation, age and sex-matched 32 NSCLC patients and 24 healthy controls were included. We found that CST1 level in chronic inflammation patient group was higher than in healthy control group (P < .05) (Supplemenatl Figure S3), which suggested that inflammation may lead to the slight upregulation of CST1. However, the level of serum CST1 in NSCLC patients was significantly higher than that in patients with chronic inflammation (P < .001), which indicates that serum CST1 could still be a potential diagnostic biomarker for distinguishing NSCLC from healthy controls, PBN or chronic inflammation.
Diagnostic Efficiency of Serum CST1 for NSCLC Patients
The AUC of CST1 for differential diagnosis of NSCLC and PBN/HC was .809/0.808, the sensitivity was 90.80%/94.70%, and the specificity was 72.30%/66.30%, while the AUC of Cyfra21-1 and CEA in diagnosing NSCLC and PBN/HC was .799/0.788 and .758/0.783, respectively. The AUC of CST1 + Cyfra21-1 + CEA was as high as .910/0.919, the sensitivity was 83.20%/82.20%, and the specificity was 93.60%/93.30% (Supplemenatl Table S4, Figure 3G-3H).
CST1 identified early-stage NSCLC and PBN/HC patients with an AUC of .734/0.733, a sensitivity of 52.60%/63.20%, and a specificity of 94.70%/89.90%. Cyfra21-1 and CEA identified early NSCLC and PBN/HC patients with an AUC of .830/0.819 and .794/0.814, respectively. The AUC of CST1 + Cyfra21-1 + CEA for distinguishing early-stage NSCLC from PBN/HC was as high as .914/0.925, the sensitivity was 81.10%/88.40%, and the specificity was 90.40%/89.10% (Supplemenatl Table S5, Figure 3I-3J).
Serum CST1 is Associated with the Prognosis of NSCLC
In order to evaluate the predictive power for the prognosis of NSCLC, we examined the CST1 level in serum samples from 137 NSCLC participants. The characteristics of these patients were summarized in Table 2. We used the Cox risk regression model to investigate whether serum CST1 level can be used as a prognostic predictor for NSCLC patients. Univariate analysis suggested that T stage, N stage, M stage, and serum CST1 levels were significantly associated with NSCLC prognosis (P < .05) (Table 3). The above four factors were included in the multivariate analysis. The results showed that serum CST1 (Hazard Ratio (HR) = 1.605; 95% confidence interval (CI) = 1.016 - 2.537; P = .043) and TNM stage (HR = 1.596; 95% CI = 1.293 - 1.969; P < .0001) are independent prognostic predictors for overall survival (OS) of NSCLC patients (Table 3).
Table 2.
Clinical characteristics and serum CST1 levels of 137 NSCLC patients for survival analysis.
| Characteristics | No. | CST1 (pg/mL) | P value 1 | No. of low CST1 expression | No. of high CST1 expression | P value 2 |
|---|---|---|---|---|---|---|
| Age | .016 | .020 | ||||
| ≤60 | 82 | 3560 (1123-17706) | 63 | 19 | ||
| >60 | 55 | 4638 (1510-20000) | 32 | 23 | ||
| Gender | .510 | .617 | ||||
| Male | 99 | 4119 (1123-20000) | 72 | 27 | ||
| Female | 38 | 3469 (1227-20000) | 26 | 12 | ||
| Type | .546 | .826 | ||||
| AD | 83 | 3536 (1123-20000) | 56 | 27 | ||
| SCC | 46 | 4338 (1201-12374) | 33 | 13 | ||
| Others | 8 | 4107 (2949-8065) | 6 | 2 | ||
| pT stage | .067 | .704 | ||||
| pT1 | 18 | 3074 (1227-12374) | 13 | 5 | ||
| pT2 | 70 | 4261 (1253-20000) | 47 | 23 | ||
| pT3 | 27 | 3916 (1201-12730) | 19 | 6 | ||
| pT4 | 19 | 3386 (1123-20000) | 15 | 4 | ||
| missing | 3 | 6854 (5331-10434) | 1 | 2 | ||
| pN stage | .147 | .028 | ||||
| pN0 | 53 | 3401 (1227-12370) | 41 | 11 | ||
| pN1 | 14 | 3724 (1489-10375) | 11 | 3 | ||
| pN2 | 53 | 4119 (1201-20000) | 35 | 18 | ||
| pN3 | 15 | 6381 (1123-20000) | 6 | 9 | ||
| missing | 2 | 3091 (1704-6854) | 1 | 1 | ||
| pM stage | .085 | .004 | ||||
| pM0 | 111 | 3818 (1123-12730) | 83 | 28 | ||
| pM1 | 26 | 5745 (1201-20000) | 12 | 14 | ||
| TNM stage | .038 | .038 | ||||
| I | 32 | 3371 (1227-11271) | 25 | 7 | ||
| II | 27 | 3782 (1253-12730) | 22 | 5 | ||
| III | 53 | 4119 (1123-11135) | 36 | 17 | ||
| IV | 25 | 5510 (1201-20000) | 12 | 13 |
The CST1 level was expressed as the median with the range.
1Mann-Whitney U-test or Kruskal-Wallis test were used to compare the differences in CST1 levels between two or more than two groups. Low or high CST1 expression was defined by the median CST1 level (5463 pg/mL).
2Statistical significance was analyzed by chi-square test. AD, adenocarcinoma; SCC, squamous carcinoma.
Table 3.
Univariate and multivariate analysis for OS prediction of NSCLC patients.
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value 1 | HR | 95% CI | P value 1 | |
| Age | 1.032 | .687-1.548 | .881 | — | — | — |
| Gender | 1.067 | .695-1.639 | .766 | — | — | — |
| T stage | 1.564 | 1.211-2.018 | .001 | 1.154 | .867-1.536 | .325 |
| N stage | 1.420 | 1.153-1.749 | .001 | 1.484 | .577-3.814 | .842 |
| M stage | 3.516 | 1.885-6.559 | <.0001 | 1.040 | 1.293-1.969 | .413 |
| TNM stage | 1.622 | 1.316-2.000 | <.0001 | 1.596 | 1.293-1.969 | <.0001 |
| Serum CST1 | 1.747 | 1.112-2.742 | .015 | 1.605 | 1.016-2.537 | .043 |
1Statistical significance was analyzed by Cox regression model; - means the data was not given; HR, hazard ratio; CI, confidence interval.
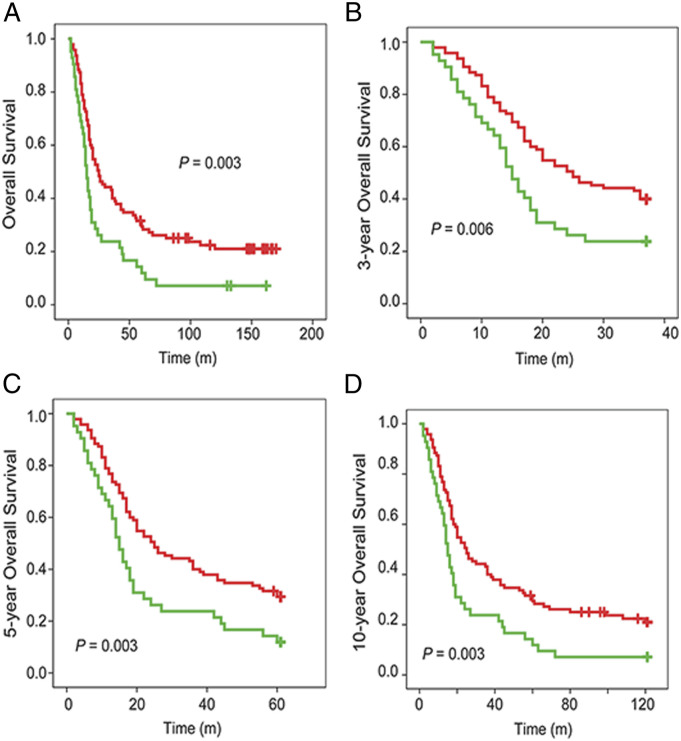
In this study, the Kaplan-Meier method was used to analyze the relationship between serum CST1 levels and the prognosis in 137 preoperative patients with NSCLC, and the log-rank test was used to compare the difference of survival curves of patients with high CST1 levels and low CST1 levels. All participants were followed up to 2017, of which the median survival time and mean survival time were 19 months and 45 months, respectively. The 1-, 3-, 5-, and 10-year OS rates were 72.99%, 35.04%, 23.36%, and 12.41%, respectively.
Survival analysis showed that 1-, 3-, 5-, and 10-year OS rates of NSCLC patients with high serum CST1 levels were 59.52%, 21.43%, 9.52%, and 7.14%, which were significantly lower than those with low serum CST1 levels (75.79%, 38.95%, 28.42%, and 14.74%, respectively) (Figure 4A-4D). These results suggested that serum level of CST1 was associated with poor prognosis of patients with NSCLC.
Figure 4.
Survival analysis of NSCLC patients stratified by median serum CST1 protein expression level. Kaplan-Meier curves show that patients with low CST1 expression had higher survival rates, including the total (A), 3-year (B), 5-year (C), and 10-year (D) OS rates. Red line, NSCLC patients with low CST1 expression; green line, NSCLC patients with high CST1 expression.
Discussion
Although the strategy for early diagnosis of lung cancer has been clinically recognized, the differential diagnosis of PBN and NSCLC is still an important issue to be resolved. 18 In the present study, we selected and verified a candidate gene CST1 from bioinformatics databases. We then validated the distinctions of serum CST1 levels in NSCLC patients (4289 ± 2405 pg/mL) compared with PBN patients (1558 ± 441 pg/mL) and healthy controls (1529 ± 416 pg/mL). Further analysis showed that serum CST1 levels of NSCLC patients in the early stage (I + II) were significantly lower than those in the terminal stage (III + IV), and meanwhile closely associated with the TNM stage.
CST1 is an active cysteine protease inhibitor of the CST superfamily, involving in the processes of inflammation and tumorigenesis. Numerous studies have reported that CST1 was a novel biomarker for several types of human cancer. Yoneda et al. found that CST1 were elevated in tumor tissues, serum samples and urine samples of patients with colorectal cancer compared with healthy controls. 14 Choi et al. have shown that CST1 was highly up-regulated, and involved in tumorigenesis of gastric cancer through T cell factor-mediated proliferative signaling; 9 clinical pathological analyses also revealed that high CST1 expression was closely related to the pTNM stage, which was consistent with our study. Furthermore, Cao et al. indicated that high expression of CST1 in tumor tissues was a significant prognostic indicator of recurrence, metastatic risk, and poor survival in patients with surgically resected NSCLCs. 13
This is the first exploration of the relationship between serum CST1 and NSCLC, and whether serum CST1 can serve as an independent diagnostic indicator for distinguishing between early-stage NSCLC and PBN. We performed the ROC analysis to clarify the role of serum CST1 in the diagnosis of NSCLC. The serum CST1 levels could be used to distinguish early-stage NSCLC patients from healthy subjects and PBN patients with the AUC of .733 and .734, respectively. Considering that the single indicator CST1 may not meet the needs of clinical diagnosis, we combined Cyfra21-1, CEA and CST1 to improve the diagnostic efficacy for early-stage NSCLC, with the AUC of combined indicators for differential diagnosis between early-stage NSCLC and PBN/HC patients reaching .914 and .925, respectively. Therefore, CST1-based combined indicators provided a great potential to recognize early-stage NSCLC and PBN cases.
We also explored the mechanisms involved in the relationship between CST1 and the prognosis of NSCLC. It has been previously reported that Cathepsin B (CTSB) concentration and activity were positively correlated with poor prognosis of tumors.19,20 CST1 can counteract the inhibitory effect of Cystatin C (CST3) on CTSB activity and indirectly lead to enhanced CTSB activity. 21 Moreover, CST1 and CTSB could delay cell senescence by inhibiting abnormal accumulation of glycogen. Overall, CST1 may be involved in regulating of CST3 and CTSB activity, thus indirectly affecting the prognosis.
In this study, we found that high serum CST1 levels were positively correlated with the poor prognosis of NSCLC patients, and the correlation was likely to persist throughout the course of disease progression. Our results also showed that 1-, 3-, 5-, and 10-year OS rates for NSCLC patients with high serum CST1 levels were 59.52%, 21.43%, 9.524%, and 7.143%, which were significantly lower than those with low serum CST1 levels (75.79%, 38.95%, 28.42%, and 14.74%). Moreover, we provided evidence that serum CST1 (HR = 1.605; P = .043) was an independent risk factor affecting OS of patients with NSCLC. These findings suggested that CST1 expression may regulate the malignant properties of tumor cells, leading to a poor prognosis in NSCLC patients.
We also acknowledged the limitations of the present study. All participants in this study were enrolled from the single center, which may cover a narrower range. Multicenter cohort should be conducted in future to further strengthen these results. The expression levels of upstream and downstream effectors of CST1 should be examined, which could provide further evidence of the functions of CST1. Additional investigations of the detailed mechanisms of CST1 expression acting on NSCLC should be performed.
Conclusions
Serum CST1 was a prognostic predictor for NSCLC, and could be combined with Cyfra21-1 and CEA for distinguishing early-stage NSCLC patients from PBN cases and healthy controls. However, whether serum CST1 will become a novel clinical biomarker for NSCLC still needs to be validated in a larger sample size of cohorts in future.
Supplemental Material
Supplemental Material for Identification and Validation of Serum CST1 as a Diagnostic Marker for Differentiating Early-Stage Non-Small Cell Lung Cancer from Pulmonary Benign Nodules by Yanzhen Lai, Yu Wang, Yaxian Wu, Meng Wu, Shan Xing, Ying Xie, Shulin Chen, Xiaohui Li, Ao Zhang, Yi He, Huilan Li, Shuqin Dai, Junye Wang, Shudai Lin, Yunmeng Bai, Hongli Du, and Wanli Liu in Cancer Control
Acknowledgments
We thank the Cancer Genome Atlas (TCGA), the National Center of Biotechnology Information (NCBI), and the ONCOMINE team for using their data.
Appendix.
Abbreviations
- ADAMTS1
a disintegrin and metalloproteinase with thrombospondin motifs 1
- AJCC
American Joint Committee on Cancer
- AUC
area under the curve
- CEA
Carcinoembryonic antigen
- CST1
Cystatin-SN
- CST3
Cystatin C
- CT
computed tomography
- CTSB
Cathepsin B
- Cyfra21-1
Cytokeratin 19 fragment
- DEGs
differentially expressed genes
- ELISA
enzyme-linked immunosorbent assay
- GEO
Gene Expression Omnibus
- HC
healthy controls
- HRP
horseradish peroxidase
- NSCLC
non-small cell lung cancer
- PBN
pulmonary benign nodule
- ROC
receiver operating characteristic curve
- SPP1
secreted phosphoprotein 1-osteopontin
- TCGA
The Cancer Genome Atlas
Author Contributions: Wanli Liu and Hongli Du designed the study; Yanzhen Lai, Yu Wang, and Yaxian Wu performed the experiments and wrote the paper; Meng Wu, Shan Xing, and Ying Xie analyzed the data; Shulin Chen, Xiaohui Li, Ao Zhang, Yi He, Huilan Li, Shuqin Dai, Junye Wang, Shudai Lin, and Yunmeng Bai collected the data. All authors discussed the results and revised the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 81271902, No. 82002240), the National Key R&D Program of China (No. 2018YFC0910202), the Natural Science Foundation of Guangdong Province (No. 2019A1515010798), and the Science and Technology Planning Project of Guangzhou (No. 201704020176).
Ethical Approval: The study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center, Guangzhou, Guangdong Province, China (B2022-059-01).
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Shulin Chen https://orcid.org/0000-0003-0816-2664
Wanli Liu https://orcid.org/0000-0002-7467-760X
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Q, Xue N, Dai D, et al. A Nomogram based on Inflammatory Factors C-Reactive Protein and Fibrinogen to Predict the Prognostic Value in Patients with Resected Non-Small Cell Lung Cancer. J Cancer. 2017;8(5):744-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henschke CI, Yankelevitz DF, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763-1771. [DOI] [PubMed] [Google Scholar]
- 5.Salgia R, Harpole D, Herndon JE, et al. Role of serum tumor markers CA 125 and CEA in non-small cell lung cancer. Anticancer Res. 2001;21(2b):1241-1246. [PubMed] [Google Scholar]
- 6.Chi PD, Liu W, Chen H, et al. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS One. 2014;9(3):e91080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrahamson M, Alvarez-Fernandez M, Nathanson CM. Cystatins. Cystatins. Biochem Soc Symp. 2003;70:179-199. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Liu HL, Liu ZH, et al. Identification of cystatin SN as a novel biomarker for pancreatic cancer. Tumour Biol. 2015;36(5):3903-3910. [DOI] [PubMed] [Google Scholar]
- 9.Choi EH, Kim JT, Kim JH, et al. Upregulation of the cysteine protease inhibitor, cystatin SN, contributes to cell proliferation and cathepsin inhibition in gastric cancer. Clin Chim Acta. 2009;406(1-2):45-51. [DOI] [PubMed] [Google Scholar]
- 10.Kim JT, Lee SJ, Kang MA, et al. Cystatin SN neutralizes the inhibitory effect of cystatin C on cathepsin B activity. Cell Death Dis. 2013;4(12):e974-e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh BM, Lee SJ, Cho HJ, et al. Cystatin SN inhibits auranofin-induced cell death by autophagic induction and ROS regulation via glutathione reductase activity in colorectal cancer. Cell Death Dis. 2017;8(3):e2682-e2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai DN, Li Y, Chen B, et al. Elevated expression of CST1 promotes breast cancer progression and predicts a poor prognosis. J Mol Med (Berl). 2017;95(8):873-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X, Li Y, Luo RZ, et al. Expression of Cystatin SN significantly correlates with recurrence, metastasis, and survival duration in surgically resected non-small cell lung cancer patients. Sci Rep. 2015;5:8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoneda K, Iida H, Endo H, et al. Identification of Cystatin SN as a novel tumor marker for colorectal cancer. Int J Oncol. 2009;35(1):33-40. [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. [DOI] [PubMed] [Google Scholar]
- 16.Ji X, Liu Y, Mei F, et al. SPP1 overexpression is associated with poor outcomes in ALK fusion lung cancer patients without receiving targeted therapy. Sci Rep. 2021;11(1):14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Liu HB, Yuan DM, Wang ZF, Wang YF, Song Y. Prognostic value of secreted phosphoprotein-1 in pleural effusion associated with non-small cell lung cancer. BMC Cancer. 2014;14:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754-e766. [DOI] [PubMed] [Google Scholar]
- 19.Withana NP, Blum G, Sameni M, et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012;72(5):1199-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao W, Fan Q, Luo X, et al. Silencing of Cathepsin B suppresses the proliferation and invasion of endometrial cancer. Oncol Rep. 2013;30(2):723-730. [DOI] [PubMed] [Google Scholar]
- 21.Swisher LZ, Prior AM, Gunaratna MJ, et al. Quantitative electrochemical detection of cathepsin B activity in breast cancer cell lysates using carbon nanofiber nanoelectrode arrays toward identification of cancer formation. Nanomedicine. 2015;11(7):1695-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Identification and Validation of Serum CST1 as a Diagnostic Marker for Differentiating Early-Stage Non-Small Cell Lung Cancer from Pulmonary Benign Nodules by Yanzhen Lai, Yu Wang, Yaxian Wu, Meng Wu, Shan Xing, Ying Xie, Shulin Chen, Xiaohui Li, Ao Zhang, Yi He, Huilan Li, Shuqin Dai, Junye Wang, Shudai Lin, Yunmeng Bai, Hongli Du, and Wanli Liu in Cancer Control