Abstract
Water availability is one of the most important factors for terrestrial life. Terrestrial habitats may periodically become dry, which can be overcome by an organism’s capability to undergo anhydrobiosis. In animals, this phenomenon has been reported for invertebrates, with tardigrades being the best-known. However, different tardigrade species appear to significantly differ in their anhydrobiotic abilities. While several studies have addressed this issue, established experimental protocols for tardigrade dehydration differ both within and among species, leading to ambiguous results. Therefore, we apply unified conditions to estimate intra-and interspecies differences in anhydrobiosis ability reflected by the return to active life. We analysed Milnesium inceptum and Ramazzottius subanomalus representing predatory and herbivorous species, respectively, and often co-occur in the same habitat. The results indicated that the carnivorous Mil. inceptum displays better anhydrobiosis survivability than the herbivorous Ram. subanomalus. This tendency to some degree coincides with the time of “waking up” since Mil. inceptum showed first movements and full activity of any first individual later than Ram. subanomalus. The movements of all individuals were however observed to be faster for Mil. inceptum. Differences between the experimental groups varying in anhydrobiosis length were also observed: the longer tun state duration, the more time was necessary to return to activity.
Keywords: Anhydrobiosis, Cryptobiosis, Milnesium inceptum, Ramazzottius subanomalus, Recovery
BACKGROUND
Cryptobiosis is the state of an organism charac-terised by no visible signs of life (Keilin 1959) and is commonly described as an adaptation to fluctuating environmental conditions that allow organisms to survive when the environment becomes unsuitable for active life. The phenomenon originated independently several times in diverse phylogenetic lines comprising unicellular (e.g., bacteria and protists) as well as multicellular (e.g., lichens, liverworts, vascular plants, and invertebrate animals) organisms. From the wide spectrum of cryptobiosis types determined by a given extreme environmental factor (e.g., Rebecchi et al. 2007; Guidetti et al. 2011a; Møbjerg et al. 2011), the most prevalent form is anhydrobiosis (e.g., Wright 2001; Watanabe 2006; Rebecchi 2013; Kaczmarek et al. 2019).
Anhydrobiosis denotes dehydration tolerance, which refers to the ability of an anhydrobiotic organism, to survive dehydration leading to the near-complete loss of body water. The function of this process as an adaptation strategy appears to be understandable in habitats of unstable conditions like mosses, lichens, small temporary ponds or puddles, cryoconite holes, or tree holes. In the case of multicellular organisms anhydrobiosis may occur during particular stages of development but rarely concerns adulthood (e.g., Watanabe 2006; Rebecchi et al. 2007; Guidetti et al. 2011a; Møbjerg et al. 2011; Rebecchi 2013; Wharton 2015). A dried organism may remain in this state for a long period of time (see e.g., Roszkowska et al. 2020) and may subsequently rehydrate, and resume active life when water once again becomes available. This is probably a costly strategy, because organisms need to use energy from storage cells (Reuner et al. 2010; Czerneková et al. 2018). What is more, the longer the specimen is in the dried state, the longer it takes to return to active life. Moreover, there is a potentially lethal outcome when the dehydration duration threshold is surpassed (Ramazzotti and Maucci 1983; Wright 1989; Westh and Ramløv 1991; Horikawa and Higashi 2004; Rebecchi et al. 2006 2009a; Schill and Hengherr 2018).
Tardigrades (as well as nematodes and rotifers) are among the animals best known for their anhydrobiotic capacity and the rare ability to undergo anhydrobiosis at each life stage (e.g., Clegg 2001; Wright 2001; Watanabe 2006; Mali et al. 2010; Møbjerg et al. 2011; Wełnicz et al. 2011; Rebecchi 2013; Schill and Hengherr 2018; Kaczmarek et al. 2019). In the case of tardigrades, the anhydrobiosis process can be divided into three stages: dehydration, “tun” and rehydration. Notably, certain tardigrade species differ in their ability to undergo anhydrobiosis. It is known that aquatic tardigrades are in general less capable of anhydrobiosis than limno-terrestrial ones (e.g., Wright 1989; Rebecchi et al. 2007; Hygum et al. 2016) and the ability of limno-terrestrial tardigrades to enter anhydrobiosis is suggested to be crucial for the colonisation of habitats exposed to shorter and longer periods of drying (Guidetti et al. 2011b). Differences were also observed for species from the same habitat but representing different classes, i.e., Heterotardigrada and Eutardigrada (Rebecchi et al. 2006). Moreover, dehydration conditions and the duration of the tun stage are important for the rate and efficiency of returning to an active state, though the optimal conditions (e.g., air humidity and temperature) of dehydration and the tun stage duration may differ between species (e.g., Wright 1989; Rebecchi et al. 2007) as well as within the same species (e.g., Horikawa and Higashi 2004; Rebecchi et al. 2009a; Kondo et al. 2015).
To date, experiments on anhydrobiosis performed under controlled conditions have been conducted for several species, namely Acutuncus antarcticus (Richters, 1904) (Giovannini et al. 2018), Bertolanius volubilis (Durante Pasa and Maucci, 1975) (Altiero et al. 2012), Hypsibius exemplaris Gąsiorek, Stec, Morek and Michalczyk, 2018 (formerly Hys. dujardini (Doyère, 1840)) (Horikawa et al. 2013; Kondo et al. 2015; Arakawa et al. 2016; Yoshida et al. 2017; Kuzmic et al. 2018), Milnesium tardigradum Doyère, 1840 (Hengherr et al. 2008; Mali et al. 2010; Schokraie et al. 2012; Wang et al. 2014), Paramacrobiotus richtersi (Murray, 1911) (Rebecchi et al. 2009b; Altiero et al. 2011), Ramazzottius oberhaeuseri (Doyère, 1840) (Jönsson et al. 2001; Faurby et al. 2008), Ram. varieornatus Bertolani and Kinchin, 1993 (Horikawa et al. 2013; Yoshida et al. 2017) and Richtersius coronifer (Richters, 1903) (Jönsson et al. 2001; Jönsson and Rebecchi 2002; Faurby et al. 2008; Halberg et al. 2013; Czerneková and Jönsson 2016). However, results of these various experiments on anhydrobiosis under controlled conditions are incompatible. This inconsistency is likely the result of the experimental protocols, where particular species differed in dehydration procedure details (e.g., humidity level and the method of its application) even when two species were studied simultaneously. This makes it difficult to identify general patterns. Moreover, the duration of the tun stage and the estimation of anhydrobiosis success were usually limited to calculations of survival ratio over a given period of time (most often after one or 24 hours). Therefore, it seems reasonable that an attempt to find a general rehydration pattern should be based on a comparison of different species with various life strategies from the same habitat (e.g., occupying different ecological microniches and trophic levels) kept under the same controlled conditions.
Thus, we established a long-term experiment (up to 240 days) focused on determining the anhydrobiotic capabilities of two tardigrade species representing Eutardigrada under standardised laboratory conditions. The species included in the present study are Mil. inceptum Morek, Suzuki, Schill, Georgiev, Yankova, Marley and Michalczyk, 2019 and Ram. subanomalus (Biserov, 1985) belonging to the families Milnesiidae and Hybsibiidae, respectively, and differing in some aspects of biology. Milnesium inceptum is an obligatory predatory species that feeds on rotifers, nematodes and other tardigrades, and lays smooth eggs in old exuviae, whereas Ram. subanomalus is a herbivorous species that feeds on algae and lays ornamented eggs directly in the environment. However, both species inhabit the same habitat (i.e., frequently drying mosses growing on concrete walls) and often co-occur. Considering the differences in feeding behaviour between these two species, we decided to check if these species would adopt different anhydrobiotic strategies. Namely, if either the predator species (Mil. inceptum) or the prey species (Ram. subanomalus) may recover to full activity faster in order to hunt more effectively or to avoid being eaten, respectively.
MATERIALS AND METHODS
Species and sample processing
Milnesium inceptum (Apochela) and Ram. subanomalus (Parachela) were extracted from the same moss sample collected from a concrete wall in Poznań, Poland (52°24'15"N, 16°53'18"E; 87 m asl). The moss sample was placed into plastic beaker containing 250 ml of tap water. After 18 hours, the water-saturated moss was strongly shaken with tweezers and all plant particles were removed. Water with tardigrades was then poured into a 250 ml plastic cylinder. After 30 minutes, the upper portion of water (ca. 200 ml) was decanted and the remaining 50 ml was poured into Petri dishes. Tardigrades were then extracted under a stereomicroscope (Olympus SZ). Specimens were later segregated and only fully active, adult specimens of medium body length were selected for the anhydrobiosis experiment. Genus abbreviations follow Perry et al. (2019).
Anhydrobiosis experiment
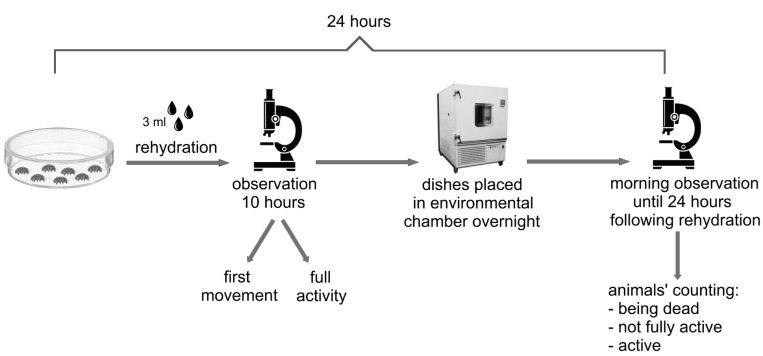
The experiment was performed in Petri dishes 3.5 cm in diameter (experimental dishes hereafter) lined at the bottom with white filter paper. Each experimental dish was filled with 450 μl of distilled water and selected individuals (extracted from the environmental sample) were transferred to the dishes (seven specimens per dish) using an automatic pipette. The experimental dishes were then closed with a cover of vented Petri dish (ensuring air exchange) and placed into an environmental chamber (PolLab, Q-Cell 140, https://www.pol-lab.eu/en/), where they were desiccated and stored under controlled conditions of 40–50% humidity and 20°C. The drying process took 72 hours (this amount of time was necessary to form correctly looking tuns) and that point was considered the beginning of the experiment. To test anhydrobiotic capabilities, specimens of both species were rehydrated after seven different periods of anhydrobiosis (0-, 7-, 14-, 30-, 60-, 120-, 240-days; each constituting a separate experimental group). This means that in the experimental group designated as “0”, specimens were rehydrated directly after dehydration (i.e., 72 hours after transferring tardigrades to experimental dishes), whereas specimens from the experimental group “7” were rehydrated after 7 days of anhydrobiosis, and so forth. Rehydration was conducted by adding 3 ml of distilled water to each experimental dish (Fig. 1). Specimens were then transferred to small glass dishes in which they were observed for 10 hours (at room temperature, 24°C) under a stereomicroscope. Thereafter, the dishes were placed in environmental chamber (40–50% humidity and 20°C) overnight. The next morning, they were observed every 30 minutes until 24 hours following rehydration. During the observation period, the moment of the first movement and recovery to full activity were recorded. Specimens with observed full activity were removed from dishes and no longer observed. The first movement was defined as any visible sign of movement in the claws, legs, body, buccal tube, etc. The full activity was defined as coordinated movements of the body and legs (i.e., the start of crawling). All observations were completed after 24 hours and all not fully active specimens or those without any signs of movement (non-moving, that were interpreted as being dead) were counted.
Fig. 1.
A simple schematic illustration of the experiments.
As previously stated, we prepared seven experi-mental groups differing in the duration of the anhydrobiotic period for both tardigrade species. For each group, we performed 10 repetitions (i.e., we prepared 10 experimental dishes with seven specimens in each of them). This means that one experimental group contained 70 tardigrade specimens of each species. In total, we used 70 specimens multiplied by each of the seven experimental groups for one tardigrade species (i.e., a total of 490 individuals of each species).
We tested differences between the experimental groups and species in relation to six measures of activity: (i) the number of non-moving (dead) tardigrades (NM); (ii) the number of individuals that did not reach full activity until the end of the observation (NFA); (iii) the time required for the first movement of any first individual (FM) and the first movement of all individuals (FMA); and (iv) the time required for return to full activity of the first (FA) and all individuals (FAA).
Statistical analysis
The distributions of all the recorded variables was far from normality, so we used nonparametric test to analyse all the data. To check for the presence of significant differences between Mil. inceptum and Ram. subanomalus in the overall ability to return to active life following the tun stage, observed values of the six measures of activity (NM, NFA, FM, FMA, FA, FAA) were compared between the species using Exact Wilcoxon-Mann-Whitney Test. Then, each species was tested for the influence of the time spent in anhydrobiosis (i.e., in the tun stage) on the values of these indices by comparing them between the experimental groups using Approximative Kruskal-Wallis Test with 100,000 Monte Carlo replicates (Hájek et al. 1999; Hothorn et al. 2008). Dunn test for multiple comparisons adjusted with Holm’s correction was used as a post-hoc analysis to check for the differences between the particular pairs of experimental groups. Correlations between the measures of activity within each species as well as correlations between the values of indices and time spent in anhydrobiosis were calculated using Asymptotic Spearman Correlation Test with Holm’s correction. Relations between the duration of anhydrobiosis and measures of tardigrade activity were illustrated on graphs by applying locally estimated scatterplot smoothing (LOESS) using Local Polynomial Regression Fitting function (span = 0.6; degree = 1). All calculations were performed in R 4.0.2 (R Core Team 2020) under RStudio 1.3.1056 using the 'coin' package (Hothorn et al. 2008) and graphs were produced using the ggplot2 package (Wickham 2016). We considered p = 0.05 as the threshold determining statistical significance. The raw data used for all the calculations are presented in table S1.
RESULTS
Overall ability to return to active life
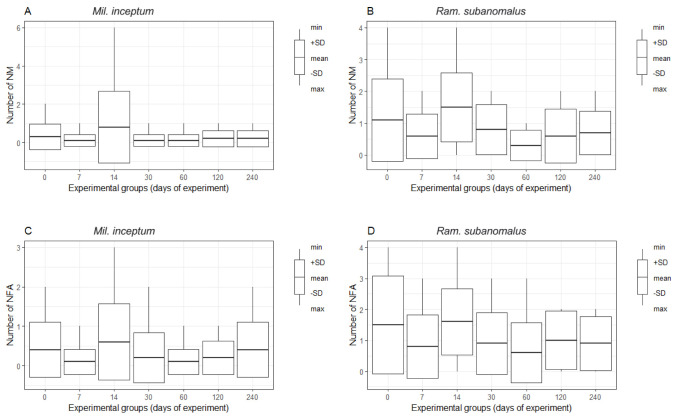
The mean number of individuals without any sign of movement (NM) at the end of the experiment per each replicate sample for Ram. subanomalus was 0.80 (95% CL: 0.58–1.01), whereas the mean NM for Mil. inceptum was 0.26 (95% CL: 0.06–0.45). This translates to the mean percentage of surviving individuals being 88.6% in Ram. subanomalus and 96.3% in Mil. inceptum. The difference in anhydrobiosis survival between these species was significant (Z = -4.7877, p < 0.0001). Thus, under the tested conditions, the survival rate of Mil. inceptum was significantly higher than that of Ram. subanomalus. In both species the duration of anhydrobiosis (i.e., the tun stage) had no significant effect on the number of NM individuals (Ram. subanomalus: chi2 = 10.379, p = 0.105, Fig. 2B; Mil. inceptum: chi2 = 2.644, p-value = 0.8402, Fig. 2A). As a consequence, in both species there was no significant difference in any pair of experimental groups with respect to NM (see Table S2 for the details).
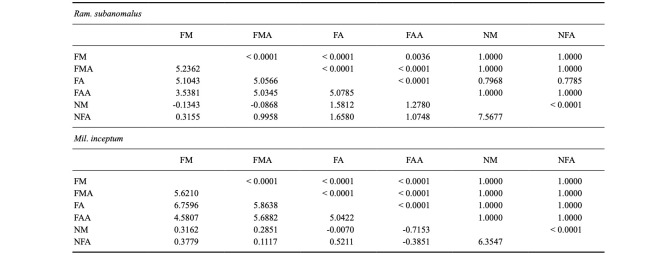
Table 1.
Results of Asymptotic Spearman Correlation Test with Holm’s correction checking for relationships between the values of particular activity measures recorded during the presented experiments. Upper diagonal: p-values with Holm’s correction; lower diagonal: values of the Z statistic
FM-first movement of any first individual, FMA -first movement of all individuals, FA -full activity of the first, FAA -full activity of all individuals, NM -non-moving (dead) tardigrades, NFA -not fully active/individuals that did not reach full activity.
Fig. 2.
Differences in the number of non-moving (NM) and not fully active (NFA) individuals between experimental groups representing increasing duration of the tun stage for Milnesium inceptum (A, C) and Ramazzottius subanomalus (B, D). The number of replicate samples for each group n = 10.
The only value that was significantly correlated with NM was the number of not fully active (NFA) individuals (Ram. subanomalus: Z = 7.5677; p < 0.0001 and Mil. inceptum: Z = 6.3548; p < 0.0001; Table 1). The mean number of NFA individuals of Ram. subanomalus was 1.04 (95% CL: 0.78–1.30), while that of Mil. inceptum was 0.29 (95% CL: 0.14–0.43), with the mean percentage of fully active (FA) individuals being 85.1 and 95.9, respectively. Again, the difference between the species was statistically significant and Mil. inceptum was characterized by a significantly lower ratio of NFA individuals (Z = -4.9422, p < 0.0001). However, there were no significant differences between the experimental groups in both species (Ram. subanomalus: chi2 = 6.7554, p = 0.3509, Fig. 2D; Mil. inceptum: chi2 = 5.2496, p-value = 0.5275, Fig. 2C); as well as between species (comparisons between the pairs of experimental groups: p > 0.4 in all the cases) (Table S2). This denotes that duration of anhydrobiosis (i.e., the tun stage) had no significant effect on the number of NFA individuals. The values of NFA specimens were not significantly correlated with any measured indices of the activity except for NM (see above and Table 1).
The timing of the first movement
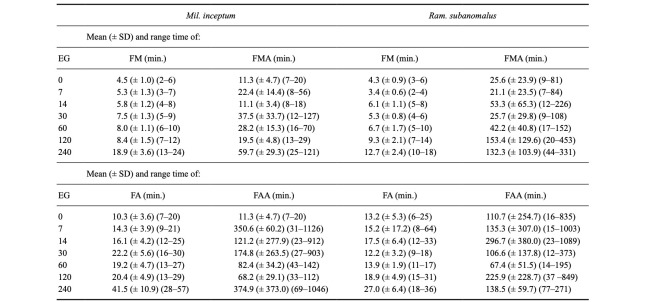
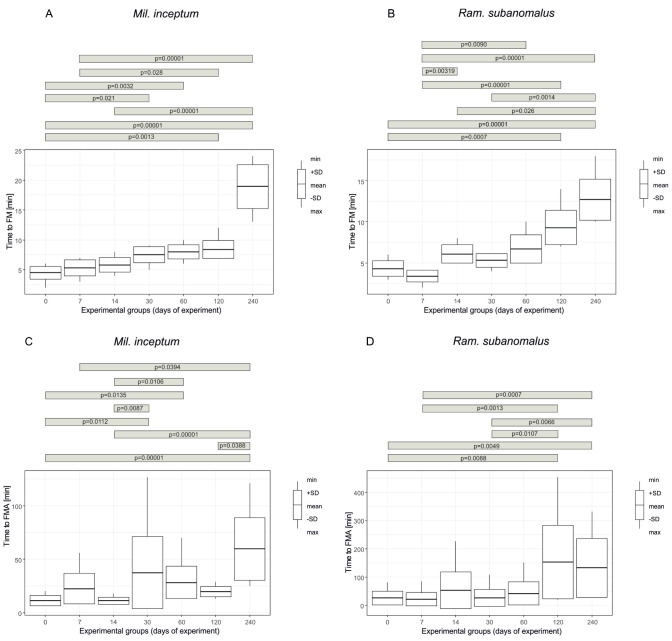
As shown in table 2, the shortest time of the first movement of any first individual (FM) ranged from 2 to 24 minutes for Mil. inceptum (the experimental groups “0” and “240”, respectively) and from 2 to 18 minutes for Ram. subanomalus (the experimental groups “7” and “240”, respectively). According to the Wilcoxon test the difference between the species was significant at Z = 2.1214, p = 0.0337 and Mil. inceptum showed FM significantly later than Ram. subanomalus. Moreover, the time to FM differed significantly between the experimental groups for both species (Mil. inceptum: chi2 = 53.602, p < 0.0001, Fig. 3A; Ram. subanomalus: chi2 = 56.776, p < 0.0001, Fig. 3B). As indicated by the applied post-hoc analysis, for Mil. inceptum such differences were significant when the experimental group “240” was compared with the experimental groups “0”, “7” and “14”; the experimental group “120” with the experimental groups “0” and “7”; and the experimental groups “30” and “60” with the experimental group “0”. In Ram. subanomalus the differences were significant in the case of the following comparisons of the experimental groups: “240” vs “0”; “7”, “14” and “30”; “120” vs “0” and “7”; “7” vs “14” and “60” (See Table S2 for details).
Table 2.
Descriptive statistics of the first movement and full activity time (EG’ experimental groups)
FM -first movement of any first individual, FMA -first movement of all individuals, FA -full activity of the first, FAA -full activity of all individuals, NM -non-moving (dead) tardigrades, NFA -not fully active/individuals that did not reach full activity.
Fig. 3.
A time from the start of rehydration to the first movement (FM) of any first individual and all individuals (FMA) in the experimental groups representing increasing duration of the tun stage for Milnesium inceptum (A, C) and Ramazzottius subanomalus (B, D). The number of replicate samples for each group n = 10.
The shortest time required for all individuals to display the first movements (FMA) was identical for both species, i.e., 7 minutes and was recorded in the experimental group “0” for Mil. inceptum and in the experimental group “7” for Ram. subanomalus. The longest time to FMA for Mil. inceptum was observed in the experimental group “30” (127 minutes) and for Ram. subanomalus in the experimental group “120” (453 minutes) (Table 2). The differences in FMA were significant when the species were compared (Z = -2.5813, p = 0.0096) and Mil. inceptum showed significantly faster FMA. Moreover, based on the applied tests, differences between FMA were also significant when the particular experimental groups within each species were analysed (Mil. inceptum: chi2 = 42.689, p < 0.0001, Fig. 3C, Ram. subanomalus: chi2 = 33.476, p < 0.0001, Fig. 3D). According to the Dunn test, in Mil. inceptum such differences were significant when the experimental group “240” was compared with experimental groups “0”, “7”, “14” and “120”; experimental group “14” with the experimental groups “30” and “60” days; and the experimental groups “30” and “60” with the experimental group “0”. In Ram. subanomalus the differences were significant in cases of the following comparisons of the experimental groups: “240” vs “0”, “7” and “30”, “120” vs “0”, “7” and “30” (See Table S2 for details).
The timing of the return to full activity
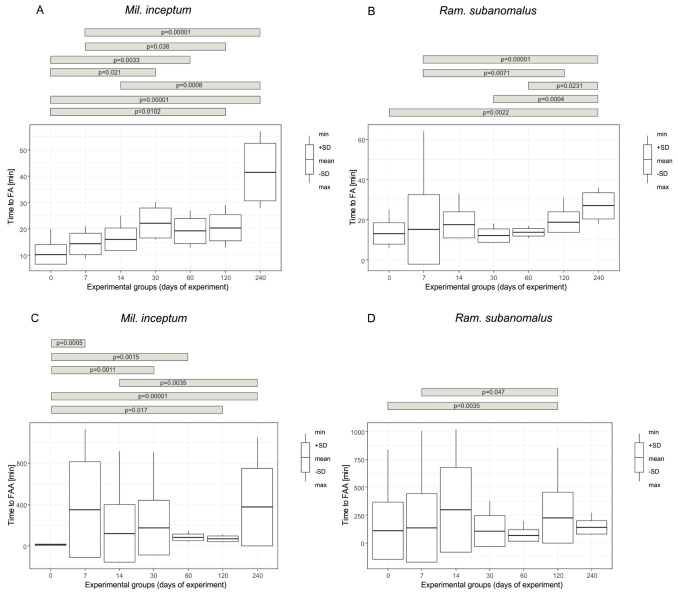
The shortest time to return to full activity of any first individual (FA) ranged from 7 to 57 minutes for Mil. inceptum (the experimental groups “0” and “240”, respectively) and from 6 to 64 minutes for Ram. subanomalus (the experimental groups “0” and “7”, respectively) (Table 2). Despite the similarities, Wilcoxon test showed that the difference in FA was statistically significant at Z = 2.4853, p = 0.0127. As in the case of FM, Mil. inceptum achieved FA significantly later than Ram. subanomalus. Moreover, the values of FA differed significantly between the experimental groups (Mil. inceptum: chi2 = 45.81, p < 0.0001, Fig. 4A; Ram. subanomalus chi2 = 36.458, p < 0.0001, Fig. 4B). According to the applied post-hoc analysis, in Mil. inceptum such differences were significant when comparing the experimental group “240” with the experimental groups “0”, “7”, “14” and “60” and the experimental groups “30”, “60” or “120” with the experimental group “0”. In Ram. subanomalus the differences were significant in cases of the following comparisons of the experimental groups: “240” vs “0”, “7”, “30” and ”60” and “120” vs “7” days (See Table S2 for details).
Fig. 4.
A time from the start of rehydration to the full activity (FA) of any first individual and all individuals (FAA) in the experimental groups representing increasing duration of the tun stage for Milnesium inceptum (A, C) and Ramazzottius subanomalus (B, D). The number of replicate samples for each group n = 10.
The fastest return to full activity by all individuals (FAA) was observed after 7 minutes for Mil. inceptum in the experimental group “0” and after 12 minutes for Ram. subanomalus in the experimental group “30”. The longest time to FAA for Mil. inceptum was observed after 1126 minutes (the experimental group “7”) and after 1089 minutes for Ram. subanomalus (the experimental group “14”) (Table 2). The difference in FAA between the species was not significant (Z = -0.23549, p-value = 0.8152); however, the particular experimental groups differed significantly both in Mil. inceptum (chi2 = 40.146, p < 0.0001, Fig. 4C) and Ram. subanomalus (chi2 = 20.958, p-value = 0.0007, Fig. 4D). According to the applied post-hoc analysis, in Mil. inceptum such differences were significant when the experimental group “240” was compared with the experimental groups “0” and “14”, and the experimental groups “7”, “30”, “60” or “120” with the experimental group “0”. In Ram. subanomalus the differences were significant when timing of FAA was compared between the groups spending 120 vs 0 and 120 vs 7 days in anhydrobiosis (See Table S2 for details).
All measures of timing of FM and FA were highly intercorrelated (p < 0.0001 in all but one case, see Table 1), but none of them were correlated with NM or NFA.
Relation of activity indices with time spent in anhydrobiosis
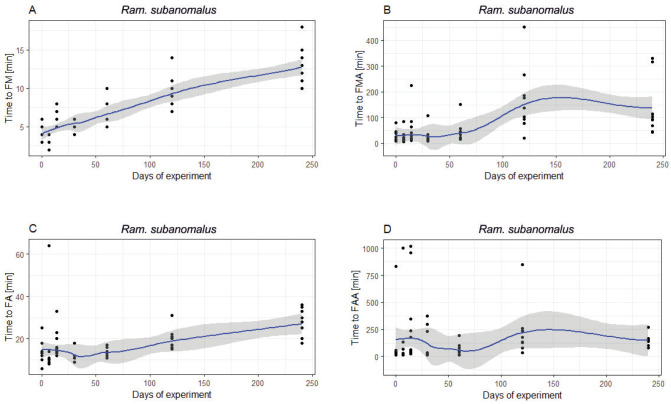
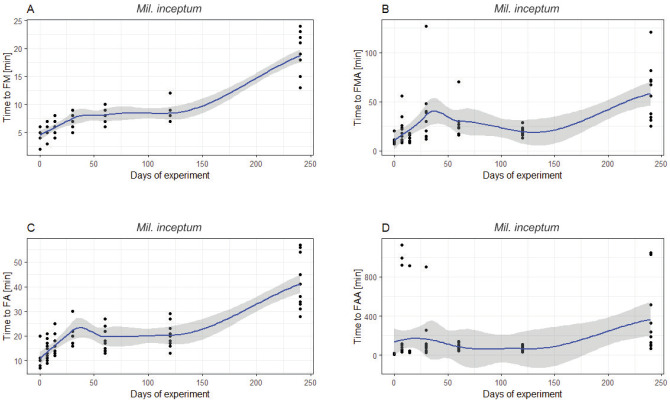
In both species, FM, FMA, FA and FAA were highly positively correlated with the time spent in anhydrobiosis. The longer anhydrobiosis lasted, the more time was needed to observe the FM (Mil. inceptum: 7.1643, p < 0.0001; Ram. subanomalus: Z = 7.000, p < 0.0001), FMA (repetitively: Z = 5.0326, p < 0.0001 and Z = 5.0956, p < 0.0001), FA (repetitively: Z = 6.2675, p < 0.0001 and Z = 4.7077, p < 0.0001) and FAA (repetitively: Z = 4.5642, p < 0.0001 and Z = 3.4128, p < 0.0001). However, as shown by the LOESS curves (Figs. 5–6), the relation was almost always far from linear—except for FM and FA in Ram. subanomalus. The FMA and FAA in that species showed slight declines after 120 days of anhydrobiosis (Fig. 5A–D). The indices of the first movements (FM, FMA) and full activity (FA, FAA) in Mil. inceptum flattened after ca. 30 days and showed another steep increase after 120 days (Fig. 6A–D). The NM and NFA were not correlated with the time spent in anhydrobiosis in either species (NM: Mil. inceptum: Z = -0.1032 p > 0.9999; Ram. subanomalus: Z = -1.2743, p = 0.4051; NFA: Mil. inceptum: Z = -0.1725, p > 0.9999; Ram. subanomalus: Z = -0.8293, p = 0.4070).
Fig. 5.
Relationship between the measured activity indices and time spent during the tun stage for Ramazzottius subanomalus: (A) time to first movement of any first individuals (FM); (B) time to first movement of all individuals (FAA); (C) time to full activity of any first individual (FA); (D) time to full activity of all individuals (FAA). Curves ± SE were fitted with Local Polynomial Regression Fitting (LOESS).
Fig. 6.
Relation of the measured activity indices to time spent in the tun stage in Milnesium inceptum: (A) time to first movement of any first individual (FM); (B) time to first movement of all individuals (FAA); (C) time to full activity of any first individual (FA); (D) time to full activity of all individuals (FAA). Curves were ± SE fitted with Local Polynomial Regression Fitting (LOESS).
DISCUSSION
Here we present results of a long-term anhydrobiosis laboratory experiment for two eutardigrade species and analyse their ability to recover to full activity while considering the number of dead (non-moving; NM) and not fully active (NFA) individuals as well as the time that live individuals required to display the first movement (FM, FMA) and reach full activity (FA, FAA). The experiment was performed simultaneously for two species, i.e., predatory Mil. inceptum and herbivorous Ram. subanomalus. Because both species co-occur in the same habitat, we assumed that the type of the occupied habitat did not influence the tested parameters. This assumption was also applied before in an experiment concerning the survival rate of long-term anhydrobiotic lichen-dwelling tardigrades (Rebecchi et al. 2006).
In general, we observed differences in overall revival between species. Fewer Mil. inceptum individuals were classified as NM and NFA individuals after rehydration of tuns compared to Ram. subanomalus. The value of NM was significantly correlated with NFA, but neither parameters were correlated with the duration of the tun stage. These results suggest that both species display a high threshold of tun stage duration, though Mil. inceptum appears to cope better with the tun stage, increasing survival. The lack of the expected decrease in the survival rate with the increasing duration of the tun stage observed for the both species was also observed for Pam. richtersi (Rebecchi et al. 2009a), although in this experiment naturally desiccated tardigrades were rehydrated after up to 21 days. Nevertheless, tardigrade interspecific variation in the tun stage survival is a well know phenomenon (e.g., Faurby et al. 2008). One of the proposed phenotypic predictors of the tun stage survival is body size (Jönsson and Rebecchi 2002). Accordingly, it is suggested that anhydrobiotic survival increases with body size at small and moderate size ranges. Both Mil. inceptum and Ram. subanomalus belong to rather medium-sized species, but in general Mil. inceptum is slightly larger. Consequently, we could assume that this difference in size may cause the observed difference in anhydrobiosis survival, although the energy requirements to enter and leave anhydrobiosis cannot be neglected (Jönsson and Rebecchi 2002).
As one may expect, the longer the tun stage duration, the longer the time is required to recover to active life although based on the obtained data it can be concluded that the relation can be far from a linear one. This is in agreement with earlier explanations suggesting that time spent in the tun stage correlates with the extent of damage to be repaired as well as the time required to activate all metabolic pathways in cells and organs (for a review, see Rebecchi et al. 2007; Schill and Hengherr 2018). We also observed a correlation between time of FM and time of return to FA. This implies that if an individual “wakes up” after a dehydration episode, it endeavours to reach FA as soon as possible. Interestingly, the “fastest” specimens of both species may display FM and FA in a comparable time; however, in the case of Ram. subanomalus, FM usually occurs much faster compared to Mil. inceptum. Faster recovery from the tun stage in herbivorous species could facilitate predator avoidance, thus allowing foraging and regaining the energetic resources before Mil. inceptum starts its pressure on the prey population. This could be supported by the fact that in our rearing experiments on Mil. inceptum individuals did not seem to be attracted to inactive prey items, preferring fully recovered prey (Ł. Kaczmarek, personal observations, data not published).
Herbivorous species usually need more time to accumulate enough resources to achieve reproductive success and in the predator -prey systems delay in predatory species reproduction is a common phenomenon (see the classic hare -lynx example, Odum 1953). Delayed recovery from anhydrobiosis in predatory species might therefore be an evolutionary response of Mil. inceptum to fluctuations in prey population density occurring at the onset of habitat inundation. This assumption might be supported by the fact that, although the first specimens of Mil. inceptum showed movements later than their prey, last predatory individuals showed movement faster than last prey. Thus, the timing of first movement in predator was more general across the whole observed groups, suggesting that right (although delayed) timing of recovery is more important for Mil. inceptum. On the other hand, the less universal timing of anhydrobiosis termination in prey Ram. subanomalus might be a sign of a diversified bet hedging strategy, where particular prey individuals reduce their fitness by unpunctual anhydrobiosis to spread the risk of predation (e.g., Starrfelt and Kokko 2012; Bruijning et al. 2020). Regardless of whichever explanation is the right one, both tardigrade species seem to be very attractive models for future experimental studies on predator-prey interactions.
The time required to reach FA for both species is relatively short (within the range of minutes), especially for shorter durations of anhydrobiosis. Such a rapid response may be theoretically associated with short periods of water occurrence in their natural habitats. Tufts of moss may absorb water in the early morning when fog and dew appear. The moss subsequently dries out when the sun rises, resulting in the rapid decline of air and substrate humidity. Thus, the water phase in such habitats can last only a few hours per day or less, with longer hydroperiods only occurring under rainy conditions. However, even after rains, especially in temporary or dry climatic zones, water is frequently available for only a few hours or a few days. Thus, rapid recovery from anhydrobiosis can be regarded as an adaptation to this type of temporary habitat since—in this short amount of time—tardigrades must supplement energy resources exploited during anhydrobiosis (e.g., Gross et al. 2019) and carry out their entire life cycle. This could also suggest that returning to active life faster than the other specimens is favourable since such specimens can begin feeding and reproducing earlier, which could guarantee higher reproductive success.
In conclusion, we show that carnivorous Mil. inceptum have better anhydrobiosis survivability than herbivorous Ram. subanomalus. The predatory species however has a delayed anhydrobiosis termination when compared to its prey, which in turn seems to be more variable in the timing of waking up. These observations may be a clue of an interesting predator-prey evolutionary game, ensuring a great subject for future in-depth studies. We also observed that the longer the duration of the anhydrobiotic stage, the longer it took to terminate anhydrobiosis. This supports the well known observation that the longer the tun state lasts, the longer time is required to return to activity—a tendency that is independent of life strategy. While the underlying mechanisms of this tendency remain far from being understood, they might be directly related to metabolism and/or ecological strategy.
CONCLUSIONS
Carnivorous Mil. inceptum displays better anhydrobiosis survivability than the herbivorous Ram. subanomalus. This tendency to some degree coincides with the time of “waking up” since Mil. inceptum showed first movements and full activity later than Ram. subanomalus. However, movements of all individuals were observed faster for Mil. inceptum. Moreover, differences between the experimental groups varying in anhydrobiosis length were also observed: the longer the tun state duration, the more time was necessary to return to activity.
Supplementary materials
Raw data resulting from experiments on relation between duration of anhydrobiosis ('days') and variables describing survival and condition (NM-%FA) in two tardigrade species (columns 'species' and 'spec').
Results of post-hoc comparisons of measured variables (one per each excel sheet) in pairs of experimental treatments (different durations of time spent in anhydrobiosis) in studied species: Mil = Milnesium, Ram = Ramazottius.
Acknowledgments
The work was supported by the research grant of National Science Centre, Poland, NCN 2016/21/B/NZ4/00131. Milena Roszkowska has a scholarship from the AMU Foundation for the academic year 2018/2019. Studies have been conducted in the framework of activities of BARg (Biodiversity and Astrobiology Research group). The authors also wish to thank Cambridge Proofreading LLC (http://proofreading.org/) for their linguistic assistance.
Footnotes
Authors’ contributions: MR: experiments, writing manuscript, preparing of graphics, correcting of manuscript; BG: statistical analysis, preparing of graphics, correcting of manuscript; DW: experiments, correcting of manuscript; JZK: statistical analysis, preparing of graphics, correcting of manuscript; EF: supporting food for tardigrade cultures, correcting of manuscript; HK: financial support, writing manuscript, correcting final version of manuscript; ŁK: conception of research, experiments, writing manuscript, correcting final version of manuscript
Competing interests: MR, BG, DW, JZK, EF, HK and ŁK declare that they have no conflict of interest.
Availability of data and materials: All the data are included into manuscript.
Consent for publication: Agree.
Ethics approval consent to participate: Agree.
References
- Altiero T, Guidetti R, Boschini D, Rebecchi L. 2012. Heat shock proteins in encysted and anhydrobiotic eutardigrades. J Limnol 71(1):211–215. doi:10.4081/jlimnol.2012.e22.
- Altiero T, Guidetti R, Caselli V, Cesari M, Rebecchi L. 2011. Ultraviolet radiation tolerance in hydrated and desiccated eutardigrades. J Zoolog Syst Evol Res 49(S1):104–110. doi:10.1111/j.1439-0469.2010.00607.x.
- Arakawa K, Yoshida Y, Tomita M. 2016. Genome sequencing of a single tardigrade Hypsibius dujardini individual. Sci Data 3:160063. doi:10.1038/sdata.2016.63. . [DOI] [PMC free article] [PubMed]
- Bertolani R, Kinchin IM. 1993. A new species of Ramazzottius (Tardigrada, Hypsibiidae) in a rain gutter sediment from England. Zool J Linn Soc 109:327–333. doi:10.1111/j.1096-3642.1993. tb02538.x.
- Biserov VI. 1985. Hypsibius subanomalus sp. n. (Eutardigrada, Hypsibiidae) from the Astrakhan District. Zool Zhurnal 64:131–135.
- Bruijning M, Metcalf CJE, Jongejans E, Ayroles JF. 2020. The evolution of variance control. Trends Ecol Evol 35(1):22–33. doi:10.1016/j.tree.2019.08.005. . [DOI] [PMC free article] [PubMed]
- Clegg JS. 2001. Cryptobiosis -a peculiar state of biological organization. Comp Biochem Physiol 128B:613–624. doi:10.1016/S1096-4959(01)00300-1. . [DOI] [PubMed]
- Czerneková M, Jönsson KI. 2016. Experimentally induced repeated anhydrobiosis in the eutardigrade Richtersius coronifer. PLoS ONE 11:e0164062. doi:10.1371/journal.pone.0164062. . [DOI] [PMC free article] [PubMed]
- Czerneková M, Janelt K, Student S, Jönsson KI, Poprawa I. 2018. A comparative ultrastructure study of storage cells in the eutardigrade Richtersius coronifer in the hydrated state and after desiccation and heating stress. PLoS ONE 13(8):e0201430. doi:10.1371/journal.pone.0201430. . [DOI] [PMC free article] [PubMed]
- Doyère PLN. 1840. Memoire sur les Tardigrades. Ann Sci Nat Zool, Paris, Series, 2 14:269–362.
- Durante Pasa MV, Maucci W. 1975. Descrizione di tre nuove specie di Tardigradi della Scandinavia. Atti Soc Ital Sci Nat Mus Civico Storia Nat Milano 116:244–250.
- Faurby S, Jönsson KI, Rebecchi L, Funch P. 2008. Variation in anhydrobiotic survival of two eutardigrade morphospecies: a story of cryptic species and their dispersal. J Zool 275(2):139–145. doi:10.1111/j.1469-7998.2008.00420.x.
- Gąsiorek P, Stec D, Morek W, Michalczyk Ł. 2018. An integrative redescription of Hypsibius dujardini (Doyère, 1840), the nominal taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa 4415(1):45–75. doi:10.11646/zootaxa.4415.1.2. . [DOI] [PubMed]
- Giovannini I, Altiero T, Guidetti R, Rebecchi L. 2018. Will the Antarctic tardigrade Acutuncus antarcticus be able to withstand environmental stresses related to global climate change? J Exp Biol 221(4):jeb160622. doi:10.1242/jeb.160622. . [DOI] [PubMed]
- Gross V, Müller M, Hehn L, Ferstl S, Allner S, Dierolf M, Achterhold K, Mayer G, Pfeiffer F. 2019. X-ray imaging of a water bear offers a new look at tardigrade internal anatomy. Zool Lett 5:14. doi:10.1186/s40851-019-0130-6. . [DOI] [PMC free article] [PubMed]
- Guidetti R, Altiero T, Bertolani R, Grazioso P, Rebecchi L. 2011b. Survival of freezing by hydrated tardigrades inhabiting terrestrial and freshwater habitats. Zoology 114:123–128. doi:10.1016/j. zool.2010.11.005. . [DOI] [PubMed]
- Guidetti R, Altiero T, Rebecchi L. 2011a. On dormancy strategies in tardigrades. J Insect Physiol 57:567–576. doi:10.1016/j.jinsphys. 2011.03.003. . [DOI] [PubMed]
- Hájek J, Šidák, Z, Sen PK. 1999. Theory of Rank Tests, Second Edition. San Diego: Academic Press.
- Halberg KA, Jørgensen A, Møbjerg N. 2013. Desiccation tolerance in the tardigrade Richtersius coronifer relies on muscle mediated structural reorganization. PLoS ONE 8:e85091. doi:10.1371/journal.pone.0085091. . [DOI] [PMC free article] [PubMed]
- Hengherr S, Brümmer F, Schill RO. 2008. Anhydrobiosis in tardigrades and its effects on longevity traits. J Zool 275:216–220. doi:10.1111/j.1469-7998.2008.00427.x.
- Horikawa DD, Cumbers J, Sakakibara I, Rogoff D, Leuko S, Harnoto R, Arakawa K, Katayama T, Kunieda T, Toyoda A, Fujiyama A, Rothschild LJ. 2013. Analysis of DNA repair and protection in the tardigrade Ramazzottius varieornatus and Hypsibius dujardini after exposure to UVC radiation. PLoS ONE 8:e64793. doi:10.1371/journal.pone.0064793. . [DOI] [PMC free article] [PubMed]
- Horikawa DD, Higashi S. 2004. Desiccation tolerance of the tardigrade Milnesium tardigradum collected in Sapporo, Japan, and Borog, Indonesia. Zool Sci 21:813–816. doi:10.2108/zsj.21. 813. . [DOI] [PubMed]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A. 2008. Implementing a class of permutation tests: The coin package. J Stat Softw 28(8):1–23.
- Hygum TL, Clausen LKB, Halberg KA, Jørgensen A, Møbjerg N. 2016. Tun formation is not a prerequisite for desiccation tolerance in the marine tidal tardigrade Echiniscoides sigismundi. Zool J Linnean Soc 178(4):907–911. doi:10.1111/zoj.12444.
- Jönsson KI, Borsari S, Rebecchi L. 2001. Anhydrobiotic survival in populations of the tardigrades Richtersius coronifer and Ramazzottius oberhaeuseri from Italy and Sweden. Zool Anz 240(3-4):419–423. doi:10.1078/0044-5231-00050.
- Jönsson KI, Rebecchi L. 2002. Experimentally induced anhydrobiosis in the tardigrade Richtersius coronifer: phenotypic factors affecting survival. J Exp Zool 293(6):578–584. doi:10.1002/jez. 10186. . [DOI] [PubMed]
- Kaczmarek Ł, Roszkowska M, Fontaneto D, Jezierska M, Pietrzak B, Wieczorek R, Poprawa I, Kosicki JZ, Karachitos A, Kmita H. 2019. Staying young and fit? Ontogenetic and phylogenetic consequences of animal anhydrobiosis. J Zool 309(1):1–11. doi:10.1111/jzo.12677.
- Keilin D. 1959. The problem of anabiosis or latent life: history and current concepts. Proc Royal Soc B 150B:149–191. doi:10.1098/rspb.1959.0013. [DOI] [PubMed]
- Kondo K, Kubo T, Kunieda T. 2015. Suggested involvement of PP1/PP2A activity and de novo gene expression in anhydrobiotic survival in a tardigrade, Hypsibius dujardini, by chemical genetic approach. PLoS ONE 10:e0144803. doi:10.1371/journal.pone. 0144803. . [DOI] [PMC free article] [PubMed]
- Kuzmic M, Richaud M, Cuq P, Frelon S, Galas S. 2018. Carbonylation accumulation of the Hypsibius exemplaris anhydrobiote reveals age-associated marks. PLoS ONE 13(12):e0208617. doi:10.1371/journal.pone.0208617. . [DOI] [PMC free article] [PubMed]
- Mali B, Grohme MA, Förster F, Dandekar T, Schnölzer M, Reuter D, Wełnicz W, Schill RO, Frohme M. 2010. Transcriptome survey of the anhydrobiotic tardigrade Milnesium tardigradum in comparison with Hypsibius dujardini and Richtersius coronifer. BMC Genomics 11:168. doi:10.1186/1471-2164-11-168. . [DOI] [PMC free article] [PubMed]
- Møbjerg N, Halberg KA, Jørgensen A, Persson D, Bjørn M, Ramløv H, Kristensen RM. 2011. Survival in extreme environments -on the current knowledge of adaptations in tardigrades. Acta Physiol 202:409–420. doi:10.1111/j.1748-1716.2011.02252.x. . [DOI] [PubMed]
- Morek W, Suzuki A, Schill RO, Georgiev D, Yankova M, Marley N, Michalczyk Ł. 2019. Redescription of Milnesium alpigenum Ehrenberg, 1853 (Tardigrada: Apochela) and a description of Milnesium inceptum sp. nov., a tardigrade laboratory model. Zootaxa 4586(1):35–64. doi:10.11646/zootaxa.4586.1.2. . [DOI] [PubMed]
- Murray J. 1911. Arctiscoida. Proc R Ir Acad 31:1–16.
- Odum EP. 1953. Fundamentals of ecology. WB Saunders, Philadephia.
- Perry E, Miller WR, Kaczmarek Ł. 2019. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 4608(1):145–154. doi:10.11646/zootaxa.4608.1.8. [DOI] [PubMed]
- R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/.
- Ramazzotti G, Maucci W. 1983. Il Phylum Tardigrada. Mem Ist Ital Idrobiol 41:1–1012.
- Rebecchi L. 2013. Dry up and survive: the role of antioxidant defences in anhydrobiotic organisms. J Limnol 72(s1):62–72. doi:10.4081/jlimnol.2013.s1.e8.
- Rebecchi L, Altiero T, Guidetti R. 2007. Anhydrobiosis: the extreme limit of desiccation tolerance. ISJ 4:65–81.
- Rebecchi L, Cesari M, Altiero T, Frigieri A, Guidetti R. 2009a. Survival and DNA degradation in anhydrobiotic tardigrades. J Exp Zool 212:4033–4039. doi:10.1242/jeb.033266. [DOI] [PubMed]
- Rebecchi L, Guidetti R, Borsari S, Altiero T, Bertolani R. 2006. Dynamics of long-term anhydrobiotic survival of lichen-dwelling tardigrades. Hydrobiologia 558(1):23–30. doi:10.1007/s10750-005-1415-7.
- Rebecchi L, Altiero T, Guidetti R, Cesari M, Bertolani R, Negroni M, Rizzo AM. 2009b. Tardigrade resistance to space effects: first results of experiments on the LIFE-TARSE mission on FOTON-M3 (September 2007). Astrobiology 9(6):581–591. doi:10.1089/ast.2008.0305. . [DOI] [PubMed]
- Reuner A, Hengherr S, Brummer F, Schill RO. 2010. Comparative studies on storage cells in tardigrades during starvation and anhydrobiosis. Curr Zool 56(2):259–263. doi:10.1093/czoolo/56. 2.259.
- Richters F. 1903. Nordische Tardigraden. Zool Anz 27:168–172.
- Richters F. 1904. Vorläufiger Bericht über die antarktische Moosfauna. Verhandl Deutsch Zool Ges 14-16:236–239.
- Roszkowska M, Kmita H, Kaczmarek Ł. 2020. Long-term anhydrobiosis in two taxa of moss dwelling Eutardigrada (Tardigrada) desiccated for 12 and 15 years, respectively. Eur Zool J 87(1):642–647. doi:10.1080/24750263.2020.1829110.
- Schokraie E, Warnken U, Hotz-Wagenblatt A, Grohme MA, Hengherr S, Förster F, Schill RO, Frohme M, Dandekar T, Schnölzer M. 2012. Comparative proteome analysis of Milnesium tardigradum in early embryonic state versus adults in active and anhydrobiotic state. PLoS ONE 7(9):e45682. doi:10.1371/journal.pone.0045 682. . [DOI] [PMC free article] [PubMed]
- Schill RO, Hengherr S. 2018. Environmental Adaptations: Desiccation Tolerance. In: Schill, R.O. (Ed.) Water Bears: The Biology of Tardigrades, pp. 273–293. doi:10.1007/978-3-319-95702-9.
- Starrfelt J, Kokko H. 2012. Bet-hedging-a triple trade-off between means, variances and correlations. Biol Rev 87(3):742–755. doi:10.1111/j.1469-185X.2012.00225.x. . [DOI] [PubMed]
- Wang C, Grohme MA, Mali B, Schill RO, Frohme M. 2014. Towards decrypting cryptobiosis-analyzing anhydrobiosis in the tardigrade Milnesium tardigradum using transcriptome sequencing. PLoS ONE 9:e92663. doi:10.1371/journal.pone. 0092663. . [DOI] [PMC free article] [PubMed]
- Watanabe M. 2006. Anhydrobiosis in invertebrates. Appl Entomol Zool 41:15–31. doi:10.1303/aez.2006.15.
- Wełnicz W, Grohme MA, Kaczmarek Ł, Schill RO, Frohme M. 2011. Anhydrobiosis in tardigrades -the last decade. J Insect Physiol 57:577–583. doi:10.1016/j.jinsphys.2011.03.019. . [DOI] [PubMed]
- Westh P, Ramløv H. 1991. Trehalose accumulation in the tardigrade Adorybiotus coronifer during anhydrobiosis. J Exp Biol 258:303–311. doi:10.1002/jez.1402580305.
- Wickham H. 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, USA.
- Wharton DA. 2015. Anhydrobiosis. Curr Biol 25:R1107–R1125. doi:10.1016/j.cub.2015.09.047.
- Wright JC. 1989. Desiccation tolerance and water-retentive mechanisms in tardigrades. J Exp Biol 142:267–292. doi:10.1242/jeb.142.1.267.
- Wright JC. 2001. Cryptobiosis 300 years on from van Leeuwenhoek: what have we learned about tardigrades? Zool Anz 240:563–582. doi:10.1078/0044-5231-00068.
- Yoshida Y, Koutsovoulos G, Laetsch DR, Stevens L, Kumar S, Horikawa DD, Ishino K, Komine S, Kunieda T, Tomita M, Blaxter M, Arakawa K. 2017. Comparative genomics of the tardigrades Hypsibius dujardini and Ramazzottius varieornatus. PLoS Biol 15:e2002266. doi:10.1371/journal.pbio.2002266. . [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data resulting from experiments on relation between duration of anhydrobiosis ('days') and variables describing survival and condition (NM-%FA) in two tardigrade species (columns 'species' and 'spec').
Results of post-hoc comparisons of measured variables (one per each excel sheet) in pairs of experimental treatments (different durations of time spent in anhydrobiosis) in studied species: Mil = Milnesium, Ram = Ramazottius.