Abstract
Banded langurs, Presbytis femoralis, are distributed in southern Peninsular Malaysia, i.e., Johor and its borders including Singapore. It has been estimated that there are only < 250 mature individuals of P. femoralis in Malaysia and Singapore, and the species is currently considered Critically Endangered. The dietary information of P. femoralis and even other closely related species has rarely been reported. This study, therefore, aimed to describe the species dietary habits and discuss interaction between their feeding behaviour and its surrounding. This study was conducted from February to November 2018, with 15 sampling days each month. We collected a total of 186 sighting hours, using a scan sampling method with 10-min intervals, on a five-langur focal group. We identified 29 species based on 47 items consumed by the banded langur, mostly young leaves (51%) followed by fruits (45%), and flowers (3.8%). The study group spent slightly more time consuming non-cultivated plants but relied on cultivated plants for the fruits. Over 75% of fruit feeding involved consuming cultivar plants; in most cases (73%), they ate only the pulp, not the seeds. Since the cultivated plants were planted in human settlement, there is an urgent need to implement conservation measures to untangle the human-langur conflicts—for instance, reforestation of a buffer region using non-cultivated plants. There is a potential to build upon our new findings with more detailed investigations, such as more extensive ecological factors influencing the dietary adaptation which would be necessary to support conservation efforts and management decisions of this species.
Keywords: Primate, Pest, Cultivated plant, Human-primate conflict, Feeding ecology
BACKGROUND
Non-human primates cover various trophic niches, from nearly exclusively folivore to a combination of frugivore, gummivore, insectivore and omnivore. Colobine monkeys, which include 10 genera throughout Asia and Africa (Mittermeier et al. 2013), differ from all other primates by their foregut-fermentation digestive system, which enables them to better exploit a folivore diet than other primate taxa (Chivers 1994; Matsuda et al. 2019). However, contrary to earlier assumptions that colobines are almost exclusively folivores, considerable variation in their diet has been reported (Fashing 2011; Kirkpatrick 2011). Indeed, a higher reliance on fruits and/or seeds as food during periods of high fruit availability has been reported in various Asian colobines, e.g., Presbytis percura (Megantara 1989); P. melalophos (Davies et al. 1988); P. potenziani (Hadi et al. 2011); P. rubicunda (Davies 1991; Ehlers Smith et al. 2013; Hanya and Bernard 2012); P. thomasi (Gurmaya 1986); Trachypithecus obscurus (Ruslin et al. 2019); Semnopithecus vetulus (Dela 2007; Hladik 1977); Pygathrix nemaeus (Phiapalath et al. 2011; Ulibarri 2013); Rhinopithecus roxellana (Guo et al. 2007; Yiming 2006; Zhao et al. 2015) and Nasalis larvatus (Matsuda et al. 2009; Yeager 1989). Remarkably, colobines living in human-modified habitats like forests with a mosaic of village gardens and rubber plantations (Dela 2011) or urban and agro-forested areas and forest fragments (Ruslin et al. 2019) tend to consume more fruits than reported in more natural habitats. If the ultimate goal for feeding ecology is to understand the factors affecting these variations across the order primates, we need a detailed description of feeding habits in as many species as possible.
The banded langur, Presbytis femoralis, is a member of the subfamily Colobinae found on the Malay Peninsula and Sumatra and was originally classified as three subspecies (Ang et al. 2020b; Nijman 2020; Roos et al. 2014): Presbytis f. femoralis (listed as Vulnerable on the IUCN Red List), Presbytis f. percura (Data Deficient) and Presbytis f. robinsoni (Near Threatened). Abdul-Latiff et al. (2019a) hypothesised based on a molecular phylogeny approach analysing Asian colobine DNA sequences that these three subspecies classifications for P. femoralis are not suitable because each taxonomic group exhibited separation at the species level in the D-loop region of mitochondrial DNA. Due to the unavailability of a DNA sequence from P. f. percura in Sumatra, Abdul-Latiff et al. (2019a) were unable to prove this hypothesis and did not elevate these three subspecies to species classifications as P. percura, P. femoralis and P. robinsoni. They chose instead to differentiate P. f. femoralis in Malaysia from the previously listed junior synonym P. neglectus. A recent shotgun sequencing of faecal DNA from Asian colobines, however, concluded that those three subspecies of P. femoralis are indeed unique species and as such are elevated from their subspecific status (Ang et al. 2020b). As part of this assessment, the conservation status of the three subspecies was also evaluated. Presbytis f. femoralis (here after P. femoralis), the species distributed in southern Peninsular Malaysia, i.e., Johor and its borders (Pahang state), including Singapore, has consequently been assessed as Critically Endangered (Ang et al. 2020a). Indeed, it has been estimated that there are less than 250 mature individuals of P. femoralis in Malaysia and Singapore (Abdul-Latiff et al. 2019a; Ang et al. 2020b).
The dietary habits of P. femoralis and other closely related species have rarely been reported. Notable exceptions are the studies conducted by Ang (2010) and Srivathsan et al. (2016), which describe the plants consumed by P. femoralis inhabiting the largest nature reserve in Singapore (53 identified plant species) and by Najmuddin et al. (2019a) in the human-modified forest patch in Johor (27 plant species). The only long-term study on feeding habits with over 1,500 observation hours found a total of 136 plant species were consumed by the closely related species (or subspecies), P. percura (or Presbytis f. percura), living in Sumatra, Indonesia (Megantara 1989). Thus, there are very few studies on the feeding ecology of P. femoralis even though feeding is one of the most basic aspects of primate ecology that can provide useful information to contribute to the effective conservation management of their habitats.
In studying the ecology of P. femoralis, the major challenge in previous work has been a lack of behavioural observations due to both their shy and indolent dispositions and their habitat preference for secondary and freshwater swamp forests that are difficult and dangerous for observers to access (Ang 2010; Srivathsan et al. 2016). We, however, were able to habituate a group of P. femoralis to human observation by selecting a forest consisting of mangrove forest, oil palm plantation and lowland forest with fruit orchards grown by villagers in Kota Tinggi Johor, Malaysia. This habitat allows daytime observations of the monkeys on foot. Consequently, the authors of other studies have reported the population distribution, activity budget and predation threats of P. femoralis at this study site (Abdul-Latiff et al. 2019a; Najmuddin et al. 2019a b 2020), though knowledge about the feeding ecology of P. femoralis is incomplete. Thus, this study aimed to describe P. femoralis dietary habits and discuss the interactions between their feeding behaviour and their surroundings.
MATERIALS AND METHODS
Observations were conducted from February 2018 to November 2018 in a forest along the Johor River near Johor Lama Village, Kota Tinggi, in Johor, Malaysia (104°00'E, 1°35'N). The study area included mixed vegetation consisting of mangrove forest, oil palm plantation and lowland forest, along with fruit orchards grown by villagers. Presbytis femoralis is commonly found in the area with long-tailed macaques (Macaca fascicularis) and dusky langurs (Trachypithecus obscurus). The mean monthly temperature ranged from approximately 29°C to 31°C and the total precipitation at the site from January 2018 to December 2018 was 2,350 mm (Amin and Othman 2018). The focal group for this study was an all-male P. femoralis group well habituated to observers and with easily identifiable individuals. The study group initially consisted of five individuals (from March to June), but was reduced to four (July–November) probably due to the immigration of a male. There was also a one-male-multifemale group consisting of 13 individuals (one adult male, five adult females, four sub-adults and three infants) in the area, but the all-male group was chosen as the focal group due to the reliability of sightings and higher habituation levels.
We collected behavioural data from the focal group members continuously during our observation period, which lasted from 07:00 (or the time when the group was found) until 19:00 (or the time when the group was lost). We conducted scan sampling at 10-min intervals and recorded the activity (feeding, resting, moving, others) of all visible individuals in the focal group. Feeding was defined as foraging, manipulating and ingesting food material. Resting was defined as a lack of movement from a single place and included activities such as sleeping, grooming and sitting. Moving was defined by going from location to another by any means (i.e., jumping, crawling, walking or climbing). We categorised other activities, such as vocalising or playing, as ‘others’. We also recorded which part of the plant was consumed during feeding sessions and collected plant samples for later species identification. Additionally, we defined whether consumed plant species were cultivated plants as described by the Federal Agricultural Marketing Authority of Malaysia (FAMA 2013).
RESULTS
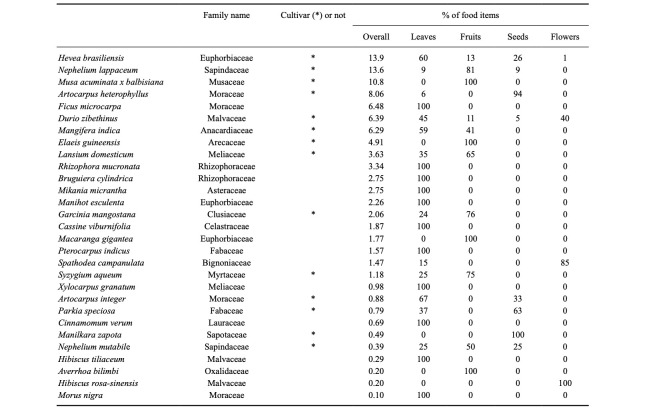
We collected data for 41 days during the study period. The total observation time was 186.3 hrs (3433 total behavioural scans), and the mean daily observation time was 4.6 ± 0.8 hrs. Throughout the study period, feeding activities accounted for 26.1% ± 17.4% (894 scans) of the total number of daily observations made. The total number of plant species consumed during observations was 29 and included 47 different plant parts (27 genera, 18 families: Table 1). The five most consumed species were Hevea brasiliensis (13.9%), Nephelium lappaceum (13.6%), Musa acuminata (10.8%), Artocarpus heterophyllus (8.1%) and Ficus microcarpa (6.5%). The study group fed primarily on leaves (51.3% ± 36.6% of daily total scans), followed by fruits (44.9% ± 36.0%) and flowers (3.8% ± 15.5%). They consumed mostly young leaves (91.4%) rather than mature ones. The exploitation of ripe fruit pulp and unripe fruit pulp and seeds from both ripe and unripe fruit accounted for 29.5%, 45.6% and 24.9% of fruit feedings, respectively.
Table 1.
Summary of percentages for food items and parts of each plant species consumed by the study group with categorization for plants that are cultivars
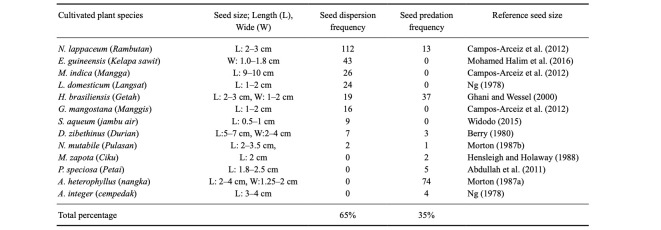
Of the 29 consumed plant species, 14 (48.3%) were cultivated plants. The study group spent slightly more time consuming non-cultivated plants (53.8% of total scans), though over 75% of fruit feedings involved cultivated plants. In most cases of fruit consumption (73.2%), only the pulp was eaten and the seeds were untouched (Fig. 1). While feeding on the fruit pulp of cultivated plants, the study group often moved from the fruiting trees to safer places further from human presence. This implies that P. femoralis may contribute to the seed dispersal of those cultivated plants instead of acting as seed predators. The observed frequency of seed consumption was not related to seed size (Table 2); the percentages of seed-eating from each cultivated plant species were not significantly correlated with the maximum seed sizes (Spearman’s rank correlation coefficient: N = 13, rho = 0.38, P = 0.21). This suggests that seed nutrients may be a more important factor for consumption than seed size or amount of pulp.
Fig. 1.

Leftover of a Mangifera indica fruit by the focal group of P. femoralis; where they mostly consumed only skin and pulp and left the seed out.
Table 2.
Frequency of seed dispersion and seed predation for 13 cultivated plant species. The size of consumed cultivar plant seeds was also described for information that was available from published literatures
DISCUSSION
We describe in this study the feeding behaviour of P. femoralis in a disturbed forest in Malaysia. To our knowledge, this is the first report of this species’ feeding ecology, though these results should be considered preliminary due to the small sample size and the limited observation hours. Nonetheless, there is a potential to build upon our findings with more detailed investigations, such as ones exploring more extensive ecological factors influencing dietary adaptations. This information is necessary to support the conservation efforts and management decisions of this species proposed by the immediate change in IUCN status to Critically Endangered by Ang et al. (2020b).
The study group inhabiting the human-modified forest patch consumed 29 species from 18 plant families, fewer than a conspecific inhabiting a species-rich rainforest patch in Singapore (53 species from 33 families), though it was evaluated not only by the direct observation but also metagenomic shotgun sequencing of fecal samples (Srivathsan et al. 2016). Comparing our finding to other studies of Asian colobines, the number of plant species consumed by the study group was particularly low (e.g., Presbytis percura: 136 plant species consumed; P. melalophos: 55 species (Davies et al. 1988); P. potenziani: 118 species (Hadi et al. 2011); P. rubicunda: 64–122 species (Davies 1991; Ehlers Smith et al. 2013; Hanya and Bernard 2012); Trachypithecus obscurus: 130 species (Ruslin et al. 2019); Pygathrix nemaeus: 79 species (Ulibarri 2013); Nasalis larvatus: 188 species (Matsuda et al. 2009). Note, however, that it is impossible to directly compare the number of consumed plant species between this study and others because of differences in sampling methods and habitats. Nonetheless, the low number of plant species consumed in this study may be related to the level of human disturbance occurring in the area. Kg. Johor Lama is filled with human residences, plantations and secondary forests, creating a highly disturbed habitat and, as a result, a loss of forest diversity (e.g., Alroy 2017). This has been shown to decrease gastrointestinal microbial diversity in colobines, which may further be associated with gastrointestinal distress and lead to death (Amato et al. 2016; Clayton et al. 2016; Hayakawa et al. 2018). Due to the extensive habitat loss and forest fragmentation from industrial-scale monoculture plantations in Peninsular Malaysia (Shevade and Loboda 2019), there is an urgent need to evaluate the effects of low dietary diversity on health in P. femoralis.
The study group predominantly consumed young leaves (51%); in addition, the percentage of fruits consumed (45%) is comparable to fruit consumption in other Asian colobines, e.g., T. obscurus: 40% fruit consumption (Ruslin et al. 2019); Semnopithecus vetulus: 60% (Dela 2007); P. percura: 58% (Megantara 1989); P. melalophos: 50% (Davies et al. 1988); P. potenziani: 55% (Hadi et al. 2011); P. rubicunda: 84% (Ehlers Smith et al. 2013); P. thomasi: 67% (Gurmaya 1986); Nasalis larvatus: 40% (Yeager 1989). This frugivore behaviour is also consistent with previous studies of colobines like T. obscurus (Ruslin et al. 2019) and S. vetulus (Dela 2011) inhabiting human-modified environments. Our study group was observed to consume cultivated plants with fleshy fruits (Mangifera indica) and avoid seeds even though it is hypothesised that foregut fermenting primates (i.e., colobines) tend to avoid sugary food sources (i.e., fleshy and ripe fruits). A high-sugar diet can decrease fermentation efficiency or cause acidosis; in extreme cases, it can even result in death among herbivores (Collins and Roberts 1978; Kay and Davies 1994). Our data thus imply that colobine digestive adaptations are more flexible than previously assumed. Supporting this, it has recently been suggested that colobine species with a tripartite stomach such as T. obscurus and S. vetulus are less susceptible to extreme bouts of malfermentation when fed highly digestible diets like fruits and/or seeds due to a reduced food intake capacity (Hoshino et al. 2021; Matsuda et al. 2019). However, in comparison to T. obscurus and S. vetulus, the stomachs of many foregut fermenting colobine species are poorly understood, including in P. femoralis. Thus, investigating forestomach anatomy may be one of the keys to understand colobine dietary flexibility and adaptability to human-modified habitats.
In Peninsular Malaysia, Afroeurasian monkeys are diverse and human-macaque conflicts, especially Macaca fascicularis and M. nemestrina, are understood in several places to be the result of human settlements neighbouring the natural habitat of macaques, ultimately leading to the labelling of them as ‘pests’ (Abdul-Latiff and Md-Zain 2021; Hambali et al. 2012; Md-Zain et al. 2011). In contrast, reports on human-langur conflicts are generally anecdotal accounts in Peninsular Malaysia and previous data has rarely shown that langurs consume cultivated plants. Our study, however, quantitatively demonstrated a reliance on cultivated plants by P. femoralis, a species anecdotally believed to be a pest animal in the past. Our study group spent slightly more time consuming non-cultivated plants while at the same time relying on cultivated plants and feeding heavily on their fruits. Since human-langur conflicts may become increasingly serious in Peninsular Malaysia and mirror conflicts seen in other countries (Khatun et al. 2013; Nijman and Nekaris 2010), our study is the first step toward understanding suitable habitats for P. femoralis and how to protect this species.
Little information is available about crop-raiding by Asian colobines, though a report on crop-raiding by S. vetulus in Sri Lanka listed seven high yield crops (coconut, banana, tea, cinnamon, jackfruit, rubber and rice plants) as particularly desirable (Nijman and Nekaris 2010). Of these seven crops, three plants— rubber, banana and jackfruit—were listed as the most consumed. However, in some cases, fruits of cultivated plants dominate the diet of colobines, even though such fruits are not preferred (Nijman 2012). This suggests that excessive human activity may force P. femoralis to consume cultivated fruits despite the species natural preferences. Further study assessing not only P. femoralis feeding behaviour but also food availability at the study site is necessary to understand the drivers behind their consumption of cultivated plants. This information may lead to a reduction in crop-raiding by colobines through humans adjusting their planting scheme accordingly (Nijman and Nekaris 2010).
Reforestation of our study area with non-commodity plants preferred by P. femoralis could serve as a buffer between the forest and other crops and, as a result, reduce their crop-raiding. Promoting ecotourism, and specifically ‘PrimaTourism’ (a term referring to primate-based tourism) (Najmuddin et al. 2019a), at the study site along with broader conservation education programmes may also help increase both income to villagers and awareness of P. femoralis. This type of tourism and education has succeeded in protecting other colobines (e.g., Lhota et al. 2019a b), and thus provides a useful tool for protecting P. femoralis as well. However, a comprehensive framework must be designed for this tourism product, especially on prevention of provisioning by visitors, which can trigger human-animal conflict and have negative long-term effects on the species conservation as animals are often over-habituated (e.g., Badiella-Giménez et al. 2021; Mohd-Daut et al. 2021; Russon and Wallis 2014); however, our preliminary studies show that local people are willing to adopt this species as nature tourism product to generate income for the local economy (Abdul-Latiff et al. 2019b c).
Lastly, we note that the results of our study should be interpreted with caution because of the limited daily observation time. We successfully observed the langurs when they were both in secondary forest and cultivated areas, but we were compelled to stop our observations when they were in the mangroves or private land (oil palm plantations). Thus, we still cannot deny the effects of such observational bias on dietary diversity and/or food preference. The langurs may have more diverse diets with less preference to cultivated plants. Until dietary data have been gathered on P. femoralis based on full-day observations, our results must be considered preliminary.
CONCLUSIONS
This study provides the first data on the feeding ecology of Presbytis femoralis in a human-altered environment in Malaysia. The 29 identified species of plants consumed, consisting of both cultivated and non-cultivated plants, indicates dietary adaptation by P. femoralis in a mosaic habitat. Further understanding the feeding ecology, crop-raiding activities and human-langur conflict is critical to formulating a conservation management plan for this species in Malaysia.
Acknowledgments
This project is funded by the Ministry of Higher Education Malaysia (MOHE) under the Fundamental Research Grant Scheme (FRGS/1/2018/WAB13/UTHM/03/2) and GPPS-UTHM-H552-2019 by Universiti Tun Hussein Onn Malaysia (UTHM). Additionally, it was partly financed by JSPS Core-to-Core Program, Advanced Research Networks (#JPJSCCA20170005 to S. Kohshima) and JSPS KAKENHI (#19H03308 and #19KK0191 to IM). We are grateful to YBhg. Dato’ Abdul Kadir bin Abu Hashim, Director General of the Department of Wildlife and National Parks who provided us with the necessary facilities and assistance. This research was conducted under a research permit (JPHL&TN(IP):100-34/1.24 Jld 8). We are deeply indebted to the Department of Wildlife and National Parks Malaysia for granting permission to carry out this research. Authors acknowledge Ministry of Higher Education Malaysia, Universiti Tun Hussein Onn Malaysia, Universiti Kebangsaan Malaysia and Panz Village for providing necessary funding, facilities and assistance. We are grateful to Dr. Andie Ang for her comments on the classification and conservation status of the study species.
Footnotes
Authors’ contributions: M.A.B.A. and B.M.M conceptualized the initial idea; M.F.N. performed the behavioural data collection; M.A.B.A arranged the sampling in the field; M.F.N., H.H., N.N., F.R. and I.M. performed and interpreted the analyses; M.F.N., I.M. and M.A.B.A. drafted the manuscript. All authors contributed to the final version of the manuscript.
Competing interests: The authors agree that this research was conducted in the absence of any self-benefits, commercial or financial conflicts and declare absence of conflicting interests with the funders.
Availability of data and materials: Data in support of the findings of this study are available from the corresponding authors by reasonable request.
Consent for publication: All authors agree to its publication in Zoological Studies.
Ethics approval consent to participate: Department of Wildlife and National Parks Malaysia that provided with the necessary permission for this research (JPHL&TN(IP):100-34/1.24 Jld 8).
References
- Abdul-Latiff MAB, Baharuddin H, Abdul-Patah P, Md-Zain BM. 2019a. Is Malaysia’s banded langur, Presbytis femoralis femoralis, actually Presbytis neglectus neglectus? Taxonomic revision with new insights on the radiation history of the Presbytis species group in Southeast Asia. Primates 60:63–79. doi:10.1007/s10329-018-0699-y. . [DOI] [PubMed]
- Abdul-Latiff MAB, Md-Zain BM. 2021. Taxonomy, evolutionary and dispersal events of pig-tailed macaque, Macaca nemestrina (Linnaeus, 1766) in Southeast Asia with description of a new subspecies, Macaca nemestrina perakensis in Malaysia. Zool Stud 60:50. doi:10.6620/ZS.2021.60-50. . [DOI] [PMC free article] [PubMed]
- Abdul-Latiff MAB, Najmuddin MF, Haneef SK, Nabil A, Shahrool-Anuar R, Md-Zain BM. 2019b. Preliminary study on activity budget of Presbytis neglectus with insights on local people perception on the product’s potential economic value in Johor. IOP Conference Series: Earth and Environ Sci 269:012006. doi:10.1088/1755-1315/269/1/012006.
- Abdul-Latiff MAB, Najmuddin MF, Haneef SK, Nabil A, Shahrool-Anuar R, Md-Zain BM. 2019c. Transforming ranging behaviour of Schlegel’s Banded Langur (Presbytis neglectus) into PrimaTourism product. IOP Conference Series: Earth and Environ Sci 269:012005. doi:10.1088/1755-1315/269/1/012005.
- Abdullah MHRO, Ch'ng PE, Lim TH. 2011. Some physical properties of Parkia speciosa seeds International Conference on Food Engineering and Biotechnology No. 9. pp. 43–47. IACSIT Press, Singapore.
- Alroy J. 2017. Effects of habitat disturbance on tropical forest biodiversity. PNAS 114:6056–6061. doi:10.1073/pnas.1611855 114. . [DOI] [PMC free article] [PubMed]
- Amato KR, Metcalf JL, Song SJ, Hale VL, Clayton J, Ackermann G, Humphrey G, Niu K, Cui D, Zhao H, Schrenzel MD. 2016. Using the gut microbiota as a novel tool for examining colobine primate GI health. Glob Ecol Cons 7:225–237. doi:10.1016/j.gecco.2016.06.004.
- Amin NFM, Othman F. 2018. Generation of flood map using infoworks for Sungai Johor. Int J Integr Eng 10:142–145. doi:10.30880/ijie.2018.10.02.026.
- Ang A. 2010. Banded leaf monkeys in Singapore preliminary data on taxonomy, feeding, ecology, reproduction and population size. National University of Singapore, Singapore.
- Ang A, Boonratana R, Nijman V. 2020a. Presbytis femoralis The IUCN Red List of Threatened Species 2020: eT18126A179550 20.
- Ang A, Roesma DI, Nijman V, Meier R, Srivathsan A, Rizaldi. 2020b. Faecal DNA to the rescue: shotgun sequencing of non-invasive samples reveals two subspecies of Southeast Asian primates to be Critically Endangered species. Sci Rep 10. doi:10.1038/s41598-020-66007-8. . [DOI] [PMC free article] [PubMed]
- Badiella-Giménez N, Kankam BO, Badiella L. 2021. Influence of visitors on the time budget, ranging and strata use of Lowe’s Monkey (Cercopithecus lowei) at Boabeng-Fiema Monkey Sanctuary, Ghana. Zool Stud 60:51. doi:10.6620/ZS.2021.60-51. . [DOI] [PMC free article] [PubMed]
- Berry SK. 1980. Cyclopropene fatty acids in some Malaysian edible seeds and nuts: I. Durian (Durio zibethinus, Murr.). Lipids 15:452–455. doi:10.1007/bf02534071.
- Campos-Arceiz A, Traeholt C, Jaffar R, Santamaria L, Corlett RT. 2012. Asian tapirs are no elephants when it comes to seed dispersal. Biotropica 44:220–227. doi:10.1111/j.1744-7429.2011. 00784.x.
- Chivers D. 1994. Functional anatomy of the gastrointestinal tract. In: Davies A and J Oates (eds.) Colobine monkeys: their ecology, behaviour and evolution Cambridge University Press, Cambridge, pp. 205–227.
- Clayton JB, Vangay P, Huang H, Ward T, Hillmann BM, Al-Ghalith GA, Travis DA, Long HT, Van Tuan B, Van Minh V, Cabana F. 2016. Captivity humanizes the primate microbiome. PNAS 113:10376–10381. doi:10.1073/pnas.1521835113. . [DOI] [PMC free article] [PubMed]
- Collins L, Roberts M. 1978. Arboreal folivores in captivity-maintenance of a delicate minority. In: Montgomery GG (ed.) The Ecology of Arboreal Folivores Smithsonian Institution, Washington, D.C., pp. 5–12.
- Davies AG, Bennett EL, Waterman PG. 1988. Food selection by two South-east Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biol J Linn Soc 34:33–56. doi:10.1111/j.1095-8312.1988.tb01947.x.
- Davies G. 1991. Seed-eating by red leaf monkeys (Presbytis rubicunda) in dipterocarp forest of northern Borneo. Int J Primatol 12:119–144. doi:10.1007/bf02547577.
- Dela JDS. 2007. Seasonal food use strategies of Semnopithecus vetulus nestor, at Panadura and Piliyandala, Sri Lanka. Int J Primatol 28:607–626. doi:10.1007/s10764-007-9150-8.
- Dela JDS. 2011. Western purple-faced langurs (Semnopithecus vetulus nestor) feed on ripe and ripening fruits in human-modified environments in Sri Lanka. Int J Primatol 33:40–72. doi:10.1007/s10764-011-9538-3.
- Ehlers Smith DA, Husson SJ, Ehlers Smith YC, Harrison ME. 2013. Feeding ecology of red langurs in Sabangau tropical peat-swamp forest, Indonesian Borneo: extreme granivory in a non-masting forest. Am J Primatol 75:848–859. doi:10.1002/ajp.22148. . [DOI] [PubMed]
- FAMA. 2013. FAMA Regulation Handbook: 3P regulation. Federal Agricultural Marketing Authority of Malaysia, Putrajaya.
- Fashing PJ. 2011. African colobine monkeys: their behavior, ecology, and conservation. In: Campbell CJ, A Fuentes, KC MacKinnon, SK Bearder and RM Stumpf (eds.) Primates in Perspective Oxford University Press, Oxford, pp. 203–229.
- Ghani MNA, Wessel M. 2000. Hevea brasiliensis (Willd. ex Juss.) Müll.Arg. In: Boer E and AB Ella (eds.) Plant Resources of South-East Asia No. 18. Plants Producing Exudates Backhuys Publishers, Leiden, pp. 73–82.
- Guo S, Li B, Watanabe K. 2007. Diet and activity budget of Rhinopithecus roxellana in the Qinling Mountains, China. Primates 48:268-276. doi:10.1007/s10329-007-0048-z. . [DOI] [PubMed]
- Gurmaya KJ. 1986. Ecology and behavior of Presbytis thomasi in Northern Sumatra. Primates 27:151–172. doi:10.1007/bf023825 95.
- Hadi S, Ziegler T, Waltert M, Syamsuri F, Mühlenberg M, Hodges JK. 2011. Habitat use and trophic niche overlap of two sympatric colobines, Presbytis potenziani and Simias concolor, on Siberut Island, Indonesia. Int J Primatol 33:218–232. doi:10.1007/s10764-011-9567-y.
- Hambali K, Ismail A, Zulkifli SZ, Md-Zain BM, Amir A. 2012. Human-macaque conflict and pest behaviors of long-tailed macaques (Macaca fascicularis) in Kuala Selangor Nature Park. Trop Nat Hist 12:189–205.
- Hanya G, Bernard H. 2012. Fallback foods of red leaf monkeys (Presbytis rubicunda) in Danum Valley, Borneo. Int J Primatol 33:322–337. doi:10.1007/s10764-012-9580-9.
- Hayakawa T, Nathan S, Stark DJ, Saldivar DAR, Sipangkui R, Goossens B, Tuuga A, Clauss M, Sawada A, Fukuda S, Imai H. 2018. First report of foregut microbial community in proboscis monkeys: are diverse forests a reservoir for diverse microbiomes? Environ Microbiol Rep 10:655–662. doi:10.1111/1758-2229.12677. . [DOI] [PubMed]
- Hensleigh TE, Holaway BK. 1988. Agroforestry species for the Philippines. Peace Corps, Washington DC, USA.
- Hladik CM. 1977. A comparative study of two sympatric species of leaf monkeys: Presbytis entellus and Presbytis senex. In: Clutton-Brock TH (ed.) Primate ecology: studies of feeding and ranging behaviour in lemurs, monkeys, and apes Academic Press, London, pp. 323–353.
- Hoshino S, Seino S, Funahashi T, Hoshino T, Clauss M, Mastuda I, Yayota M. 2021. Apparent diet digestibility of captive colobines in relation to stomach types with special reference to fibre digestion. PLoS ONE 16(9):e0256548. doi:10.1371/journal. pone.0256548. . [DOI] [PMC free article] [PubMed]
- Kay RNB, Davies AG. 1994. Digestive physiology. In: Davies AG and JF Oates (eds.) Colobine monkeys: Their ecology, behaviour and evolution Cambridge Cambridge University Press, pp. 229–249.
- Khatun UH, Ahsan MF, Røskaft E. 2013. Local people’s perceptions of crop damage by common langurs (Semnopithecus entellus) and human-langur conflict in Keshabpur of Bangladesh. Environ Nat Resour 3:1. doi:10.5539/enrr.v3n1p111.
- Kirkpatrick RC. 2011. The Asian colobines: diversity among leaf-eating monkeys. In: Campbell CJ, A Fuentes, KC MacKinnon, SK Bearder and RM Stumpf (eds.) Primates in Perspective Oxford University Press, Oxford, pp. 189–202.
- Lhota S, Scott KSS, Sha JCM. 2019a. Primates in flooded forests of Borneo: opportunities and challenges for ecotourism as a conservation strategy. In: Barnett AA, K Nowak and I Matsuda (eds.) Primates in Flooded Habitats: Ecology and Conservation Cambridge University Press, Cambridge, pp. 331–340.
- Lhota S, Sha JCM, Bernard H, Matsuda I. 2019b. Proboscis monkey conservation: beyond the science. In: Behie AM, JA Teichroeb and NM Malone (eds.) Primate Research and Conservation in the Anthropocene Cambridge University Press, Cambridge, pp. 182–196.
- Matsuda I, Chapman CA, Clauss M. 2019. Colobine forestomach anatomy and diet. J Morphol 280:1608–1616. doi:10.1002/jmor.21052. . [DOI] [PubMed]
- Matsuda I, Tuuga A, Higashi S. 2009. The feeding ecology and activity budget of proboscis monkeys. Am J Primatol 71:478–492. doi:10.1002/ajp.20677. . [DOI] [PubMed]
- Md-Zain BM, Tarmizi MR, Mohd-Zaki M. 2011. Campus monkeys of Universiti Kebangsaan Malaysia: nuisance problems and st udents perception. In: Gumert MD and A Fuentes (eds.) Monkey on the Edges Cambridge University Press, Cambrige, pp. 110–117. doi:10.1017/CBO9780511974434.006.
- Megantara NE. 1989. Ecology, Behavior and Sociality of Presbytis femoralis in Eastcentral Sumatra. In: Ehara A and S Kawamura (eds.) Comparative Primatology Monographs, Kyoto, p 301.
- Mittermeier RA, Rylands AB, Wilson DE. 2013. Handbook of the mammals of the world. Vol. 3. Primates. Lynx Edicions, Barcelona.
- Mohamed Halim R, Ramli R, Che Mat C, Yuen May C, Abu Bakar N, Abdul Hadi Nm. 2016. Dry separation of palm kernel and palm shell using a novel five-stage winnowing column system. Technologies 4:13. doi:10.3390/technologies4020013.
- Mohd-Daut N, Matsuda I, Zainul-Abidin K, Md-Zain BM. 2021. Population dynamics and ranging behaviors of provisioned silvered langur (Trachypithecus cristatus) in Peninsular Malaysia. Primates 62:1019–1029. doi:10.1007/s10329-021-00934-6. . [DOI] [PubMed]
- Morton J. 1987a. Jackfruit. In: Julia F (ed.) Fruits of warm climates Florida Flair Books, Miami, pp. 58–64.
- Morton J. 1987b. Pulasan. In: Julia F (ed.) Fruits of Warm Climates Florida Flair Books, Miami, pp. 265–266.
- Najmuddin MF, Haris H, Norazlimi N, Md-Zain BM, Mohdridwan AR, Shahrool-Anuar RO, Husna HA, Abdul-Latiff MAB. 2020. Daily activity budget of banded langur (Presbytis femoralis) in Malaysia. J Sustain Sci Manag 15:8497. doi:10.46754/jssm.2020.06.001.
- Najmuddin MF, Haris H, Shahrool-Anuar R, Norazlimi N, Md-Zain BM, Abdul-Latiff MAB. 2019a. PrimaTourism: Plant selection by Schlegel’s Banded Langur Presbytis neglectus in Johor. IOP Conference Series: Earth and Environ Sci 269:012036. doi:10.1088/1755-1315/269/1/012036.
- Najmuddin MF, Hidayah H, Noratiqah N, Md-Zain BM, Mohd Ridwan AR, Shahrool-Anuar RO, Husna HA, Abdul-Latiff MAB. 2019b. Predation of domestic dogs (Canis lupus familiaris) on Schlegel’s banded langur (Presbytis neglectus) and crested hawk-eagle (Nisaetus cirrhatus) on dusky leaf monkey (Trachypithecus obscurus) in Malaysia. J Sustain Sci Manag 14:39–50.
- Ng FSP. 1978. Strategies of establishment in Malayan forest trees. In: Tomlinson PB and MH Zimmermann (eds.) Tropical trees as living systems Cambridge University Press, Cambridge, pp. 129–162.
- Nijman V. 2012. Purple-faced langurs in human-modified environments feeding on cultivated fruits: a comment to Dela (2007, 2012). Int J Primatol 33:743–748. doi:10.1007/s10764-012-9609-0.
- Nijman V. 2020. Presbytis neglectus or P. femoralis, colobine molecular phylogenies, and GenBank submission of newly generated DNA sequences. Folia Primatol (Basel) 91:228–239. doi:10.1159/000502093. . [DOI] [PubMed]
- Nijman V, Nekaris KA-I. 2010. Testing a model for predicting primate crop-raiding using crop-and farm-specific risk values. Appl Anim Behav Sci 127:125–129. doi:10.1016/j.applanim.2010.08. 009.
- Phiapalath P, Borries C, Suwanwaree P. 2011. Seasonality of group size, feeding, and breeding in wild red-shanked douc langurs (Lao PDR). Am J Primatol 73:1134–1144. doi:10.1002/ajp.20980. . [DOI] [PubMed]
- Roos C, Boonratana R, Supriatna J, Fellowes JR, Groves CP, Nash SD, Rylands AB, Mittermeier RA. 2014. An updated taxonomy and conservation status review of Asian primates. Asian Primates Journal 4:2–38.
- Ruslin F, Matsuda I, Md-Zain BM. 2019. The feeding ecology and dietary overlap in two sympatric primate species, the long-tailed macaque (Macaca fascicularis) and dusky langur (Trachypithecus obscurus obscurus), in Malaysia. Primates 60:41–50. doi:10.1007/s10329-018-00705-w. . [DOI] [PubMed]
- Russon AE, Wallis J. 2014. Reconsidering primate tourism as a conservation tool: an introduction to the issues. In: Russon AE and J Wallis (eds.) Primate Tourism: A Tool for Conservation? Cambridge University Press, Cambridge, pp. 3–18.
- Shevade VS, Loboda TV. 2019. Oil palm plantations in Peninsular Malaysia: determinants and constraints on expansion. PLoS ONE 14:e0210628. doi:10.1371/journal.pone.0210628. . [DOI] [PMC free article] [PubMed]
- Srivathsan A, Ang A, Vogler AP, Meier R. 2016. Fecal metagenomics for the simultaneous assessment of diet, parasites, and population genetics of an understudied primate. Front Zool 13:17. doi:10.1186/s12983-016-0150-4. . [DOI] [PMC free article] [PubMed]
- Ulibarri L. 2013. The Socioecology of Red-shanked Doucs (Pygathrix nemaeus) in Son Tra Nature Reserve, Vietnam. University of Colorado, Colorado, USA.
- Widodo P. 2015. Jambu Semarang dan Jambu Air. Universitas Jenderal Soedirman, Purwokerto.
- Yeager CP. 1989. Feeding ecology of the proboscis monkey (Nasalis larvatus). Int J Primatol 10:497–530. doi:10.1007/bf02739363.
- Yiming L. 2006. Seasonal variation of diet and food availability in a group of Sichuan snub-nosed monkeys in Shennongjia Nature Reserve, China. Am J Primatol 68:217–233. doi:10.1002/ajp. 20220. . [DOI] [PubMed]
- Zhao H, Dang G, Wang C, Wang X, Guo D, Luo X, Shao J, He Z, Li B. 2015. Diet and seasonal changes in Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the southern Qinling mountains in China. Acta Theriol Sin 35:130–137.