Abstract
A thermophilic, fermentative microaerophile (ET-5b) and a thermophilic acetogen (ET-5a) were coisolated from oxic soil obtained from Egypt. The 16S rRNA gene sequence of ET-5a was 99.8% similar to that of the classic acetogen Moorella thermoacetica. Further analyses confirmed that ET-5a was a new strain of M. thermoacetica. For ET-5b, the nearest 16S rRNA gene sequence similarity value to known genera was approximately 88%. ET-5b was found to be a motile rod with a genomic G+C content of 50.3 mol%. Cells were weakly gram positive and lacked spores. Growth was optimal at 55 to 60°C and pH 6.5 to 7.0. ET-5b grew under both oxic and anoxic conditions, but growth was erratic under atmospheric concentrations of O2. Utilizable substrates included oligosaccharides and monosaccharides. Acetate, formate, and succinate supported growth only under oxic conditions. Saccharides yielded succinate, lactate, ethanol, acetate, formate, and H2 under anoxic conditions; fermentation products were also formed under oxic conditions. A new genus is proposed, the type strain being Thermicanus aegyptius ET-5b gen. nov., sp. nov. (DSMZ 12793). M. thermoacetica ET-5a (DSMZ 12797) grew commensally with T. aegyptius ET-5b on oligosaccharides via the interspecies transfer of H2 formate, and lactate. In support of this interaction, uptake hydrogenase and formate dehydrogenase specific activities were fundamentally greater in M. thermoacetica ET-5a than in T. aegyptius ET-5b. These results demonstrate that (i) soils subject to high temperatures harbor uncharacterized thermophilic microaerophiles, (ii) the classic acetogen M. thermoacetica resides in such soils, and (iii) trophic links between such soil bacteria might contribute to their in situ activities.
Soil and litter contain steep oxygen (O2) gradients and anoxic microzones (51, 57). Acetate is the most abundant organic acid in soil extracts (18, 52, 54), and the anaerobic acetate-forming capacities of soils and litter are likely linked to oxidative acetate-consuming processes (32–35, 58). Thus, acetate might be an important intermediate in the turnover of carbon in terrestrial ecosystems and serve as a trophic link at oxic-anoxic interfaces in soil and litter (12, 35). Soils and litter harbor facultative and strict anaerobes capable of producing acetate (35, 43); however, the interactions between the microorganisms involved in these trophic relationships are not well resolved. Indeed, although it is well established that the microflora of soils facilitate both aerobic and anaerobic processes, information on the coexistence and interaction of the organisms associated with these fundamentally different processes is limited.
Acetogenic bacteria are strict anaerobes that engage the acetyl coenzyme A (acetyl-CoA) Wood-Ljungdahl pathway for the reductive synthesis of acetyl-CoA from CO2 and have been isolated mostly from sediments or gastrointestinal tracts (11, 49). Although soil is not a strictly anoxic habitat, acetogens are, nonetheless, the most enumerable of strict anaerobes in soil and litter (35, 43). The capacity of soils to form acetate from H2-CO2 is enhanced by high temperatures (32, 58), suggesting that soils that are subject to elevated temperatures might harbor thermophilic acetogens. During efforts to isolate thermophilic acetogens from such soils (21, 23), a thermophilic coculture of an acetogen (ET-5a) and a fermentative microaerophile (ET-5b) was obtained. The main objectives of this study were to characterize these two thermophilic organisms and to resolve the trophic-level basis of their interaction.
MATERIALS AND METHODS
Soil collection.
Surface soil (the first 3 cm of depth) was collected from a grassy garden in Hurghada, Egypt. Soil was transported to the laboratory and stored at 5°C for 4 weeks prior to use. The soil exhibited an approximate pH of 7.4 and a dry weight of 96.9%; the total carbon content and organic carbon content of the soil approximated 23.0 and 10.8 g (kg [dry weight] of soil−1), respectively.
Medium composition and growth conditions.
The anoxic, carbonate-buffered, undefined (U) medium contained yeast extract, vitamins, trace metals, reducer (sodium sulfide and cysteine hydrochloride), and resazurin (redox indicator) (7). The defined (D) medium was U medium without yeast extract. U and D media were dispensed under CO2 into 27-ml culture tubes (7 ml of medium per tube) or 1-liter infusion bottles (500 ml of medium per bottle, used for preparation of cell extracts), which were then sealed and autoclaved; the pH was approximately 6.7. Tryptic soy broth (TSB) medium contained 28 g of TSB liter−1; anoxic TSB medium had a 100% N2 gas phase. The reduction of iron was determined by assessing the growth-dependent production of white Fe(II) precipitates in medium formulated for the growth of Fe(III)-reducing bacteria (4). Culture tubes and bottles were incubated in a horizontal, static position. Unless otherwise indicated, the temperature of incubation was 55°C.
Enrichment cultures.
Soil samples were brought into a Mecaplex (Grenchen, Switzerland) O2-free chamber (100% N2 gas phase; room temperature) and added to anoxic medium (approximately 5 g [wet weight] of soil per 45 ml of D medium in a 150-ml infusion bottle). The medium was supplemented with vanillate (5 mM); the gas phase was H2-CO2 (a ratio of 1:3 at approximately 30 kPa of overpressure). Enrichments were incubated at 55°C and subsequently streaked onto medium solidified with 1% Gelrite (Carl Roth GmbH, Karlsruhe, Germany). Subsequent enrichments in medium with carbon monoxide (CO) utilized a CO-CO2 gas phase (a ratio of 1:3 at approximately 30 kPa of overpressure). Isolated colonies were transferred to liquid medium, cultivated, and assayed for substrate utilization and product formation.
Transmission electron microscopy.
Cells were negatively stained with uranyl acetate or phosphotungstic acid (56). For thin-section preparations, cells were fixed in glutaraldehyde-OsO4 and prepared according to a standard protocol (55). Thin sections were stained with 2% (wt/vol) aqueous uranyl acetate and lead citrate (46). Specimens were observed with a Zeiss CEM 902A (Oberkochen, Germany).
Preparation of cell extract and enzyme assays.
Cells were cultivated in U medium on the substrate indicated and harvested in early stationary phase. Cells were lysed in anoxic lysozyme buffer (38), and cell extracts were prepared under anoxic conditions (31). The enzyme assay buffer was 100 mM Tris hydrochloride (pH 8.5) containing benzyl viologen (1 mM) and dithiothreitol (1 mM); the assay temperature was 55°C. To determine the specific activities of CO dehydrogenase, formate dehydrogenase, and hydrogenase, assay tubes were supplemented with CO (100% gas phase), sodium formate (5 mM), or H2 (100% gas phase), respectively (10).
Membrane preparation and redox difference spectra.
Membranes were prepared from cell extracts by ultracentrifugation under aerobic conditions (14, 19). Washed membranes were reduced with sodium dithionite, and reduced-minus-oxidized (oxidized indicates that the membranes in the reference cuvette were not reduced with sodium dithionite) spectra were obtained with a Uvikon 930 (Kontron Instruments, Milan, Italy) double-beam recording spectrophotometer at room temperature (19).
G+C content.
Cells were washed with 50 mM phosphate buffer (pH 7.0) and DNA was extracted by the NaOH method (1). The G+C content was determined by high-performance liquid chromatography (42).
16S rRNA gene sequence.
The 16S rRNA gene sequences of ET-5a and ET-5b were determined by direct sequencing of the PCR-amplified 16S rRNA genes; 1,557 and 1,426 nucleotides were sequenced for ET-5a and ET-5b, respectively. Genomic DNA extraction, PCR-mediated amplification of the 16S rRNA gene, and purification of the PCR products were performed according to published protocols (45). For ET-5a, purified PCR products were sequenced with a Sequi-Gen GT sequencer (Bio-Rad Laboratories GmbH, Munich, Germany). For ET-5b, purified PCR products were sequenced with an ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit and a 373A DNA sequencer (Applied Biosystems, Foster City, Calif.).
Construction of the dendrogram for ET-5b.
The stretch of 1,426 nucleotides of the 16S rRNA gene of ET-5b (positions 18 to 1438 of the Escherichia coli sequence [3]) was initially aligned to the sequences of the ARB database (54a). Following determination of the approximate position within the radiation of bacterial phyla, the sequence of strain ET-5b was transferred to the German Collection of Microorganisms and Cells (DSMZ; Braunschweig, Germany) database of members of the Clostridium-Bacillus subphylum with the AE2 editor (39). Evolutionary distances were calculated by the method of Jukes and Cantor (29). Phylogenetic dendrograms were constructed according to the method of DeSoete (8) and by the neighbor-joining method contained in the PHYLIP software package (16, 48). Bootstrap analysis was used to evaluate the tree topology of the neighbor-joining data by performing 500 resamplings (15).
Additional analytical methods.
Growth and cell dry weights were determined as previously described (7). When cultivated in oxic medium (at an initial gas phase of 17% O2) and anoxic U medium supplemented with 10 mM glucose, a culture optical density (at 660 nm) of 1 corresponded to 0.34 and 0.37 mg (dry weight) of cells liter−1, respectively. Protein was determined by dye staining and colorimetric analysis (2). The amounts of substrates and products present in culture fluids and gas phases were determined by high-performance liquid chromatography and gas chromatography (7, 22, 30, 40). The concentration of gases represents the combined total of both the liquid and gas phases. Soil pH was determined in 1:2.5 suspensions of soil in 0.02 M CaCl2, and soil dry weight was obtained by weighing before and after drying at 105°C for 16 h. Total carbon of oven-dried (65°C), homogenized organic matter was quantitated with an element analyzer (CHN-O-Rapid; Foss-Heraeus, Hanau, Germany). In this study, no distinction is made between CO2 and its salt forms and between organic acids and their salt forms. All results are representative of replicate experiments.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of ET-5a and ET-5b have been deposited in the EMBL nucleotide sequence database (Cambridge, United Kingdom) under accession no. AJ242494 and AJ242495, respectively.
Culture accession numbers.
Cultures of ET-5a and ET-5b have been deposited at the DSMZ under accession no. 12797 and 12793, respectively.
RESULTS
Isolation of ET-5a and ET-5b.
A colony (ET-5) that was picked from solidified medium and transferred into liquid medium was subsequently shown to grow both aerobically on saccharides and anaerobically with H2-CO2; under the latter condition, H2-CO2 was converted acetogenically to acetate. Since no acetogen has been shown to be capable of aerobic growth, it was suspected that ET-5 consisted of more than one organism. This possibility was evaluated by growing ET-5 in both oxic and anoxic media and subsequently preparing oxic or anoxic dilution series (1:10 in U medium), respectively, from these cultures. A strict anaerobe was obtained from the highest growth-positive dilution of an anoxic CO-CO2 dilution series (U medium) and was designated ET-5a; this rod-shaped, spore-forming organism grew acetogenically with both H2-CO2 and CO-CO2. An organism capable of both aerobic and anaerobic growth was obtained from the highest growth-positive dilution of an oxic cellobiose dilution series (U medium) and was designated ET-5b; this rod-shaped organism did not grow acetogenically with H2-CO2 or CO-CO2. The purities of ET-5a and ET-5b were assured by repeated isolation on solidified medium.
Phylogenetic analyses of ET-5a and ET-5b.
The 16S rRNA gene sequence of ET-5a was 99.8% similar to that of Moorella thermoacetica. The morphology, substrate range, and product profile of ET-5a were very similar to those of M. thermoacetica (data not shown), confirming that ET-5a is a new strain of this classic thermophilic acetogen.
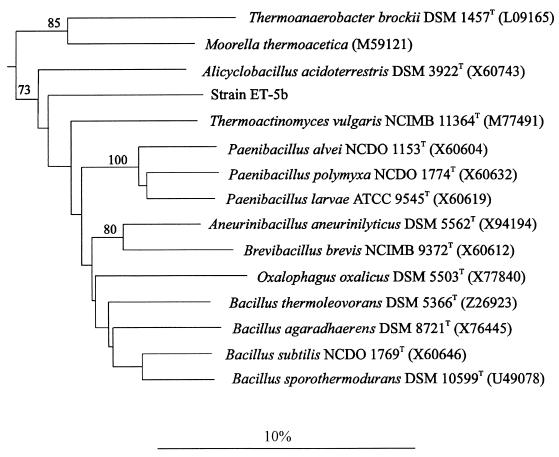
Phylogenetically, ET-5b was not closely related to any known organism. The genera most closely related to ET-5b were Bacillus, Oxalophagus, Paenibacillus, and Thermoactinomyces (Fig. 1), with the nearest 16S rRNA gene sequence similarity value approximating 88%. The gene sequence similarity value with ET-5a and ET-5b was 83.7%. These results indicated that ET-5b constitutes a new genus. The G+C content of ET-5b was 50.3 mol%.
FIG. 1.
Phylogenetic tree of strain ET-5b, representative organisms from the Bacillus subgroup, and two representatives of the Clostridium subgroup of the Clostridium-Bacillus line of descent. Sequence accession numbers and strain numbers are indicated; the strain number of M. thermoacetica is not available from the Ribosomal Database Project (39). Numbers within the dendrogram indicate the percentages of occurrence of the branching order in 500 bootstrapped trees (only values of 70 and above are shown). The sequence of Clostridium botulinum served as a root. The scale bar represents 10 nucleotide substitutions per 100 nucleotides.
Morphology and ultrastructure of ET-5b.
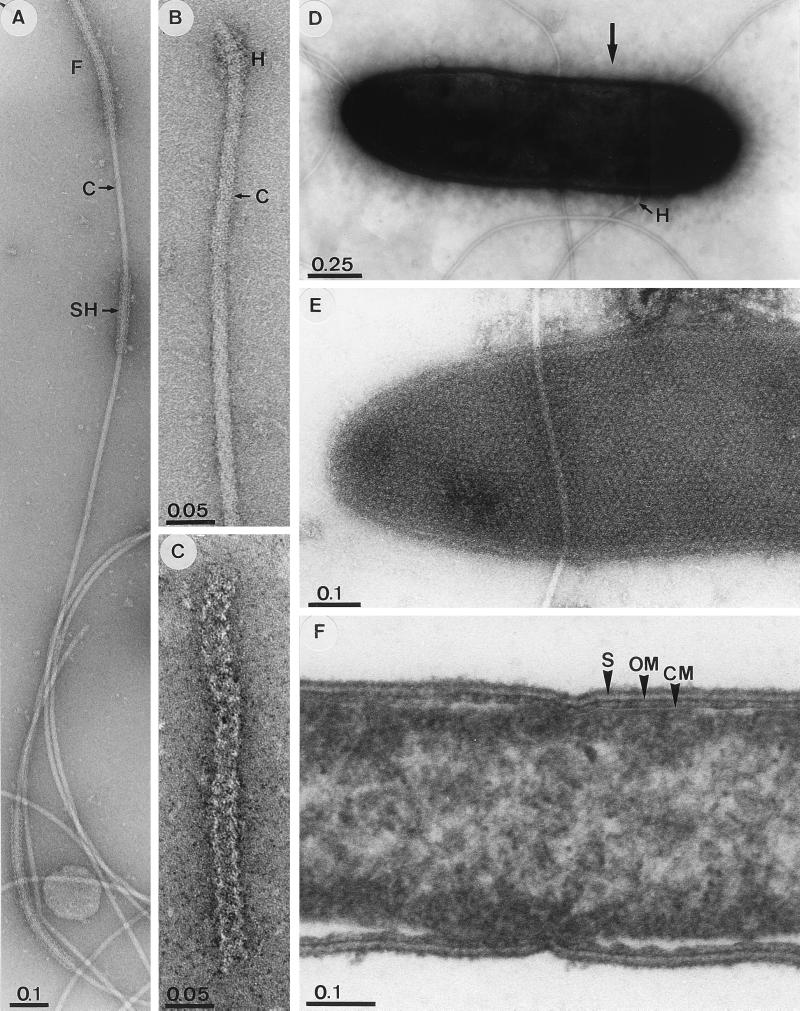
Cells of ET-5b were approximately 2.5 × 0.5 μm and stained weakly gram positive. Colonies on solidified medium were beige. Cells were motile, and the helical flagellar core was surrounded by a flexible, discontinuous sheath that was morphologically separate from the core (Fig. 2A to C). Flagella were inserted laterally (Fig. 2D). Cells appeared to be enveloped by a capsule (Fig. 2D), and the S-layer was composed of hexagonal subunits (53) (Fig. 2E). Thin sections of ET-5b revealed both outer and cytoplasmic membranes (Fig. 2F), indicating that cells contained a periplasm (26). Spores were not apparent.
FIG. 2.
Electron micrographs of ET-5b. ET-5b is shown negatively stained with 2% uranyl acetate (pH 4.6) (A, B, C, and E), negatively stained with 2% phosphotungstic acid (pH 7.0) (D), and by ultrathin section (F). Panel C is a high magnification of the flagellar sheath separated from the core; the large arrow in panel D points to the fibrillar structures surrounding the cell in the capsular domain. Abbreviations: F, flagella; C, flagellar core; SH, flagellar sheath; H, flagellar hook; S, surface layer; OM, outer membrane; CM, cytoplasmic membrane. Bars are in micrometers.
Effect of O2 on the growth of ET-5b.
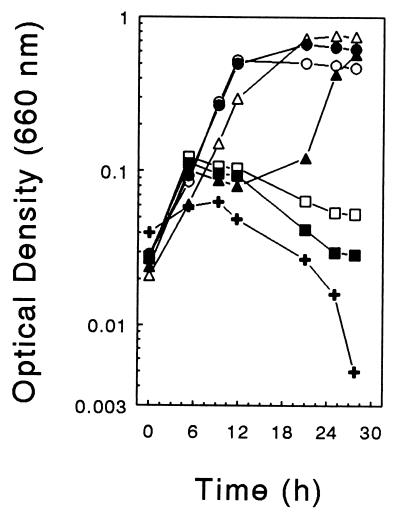
Elevated amounts of O2 impaired the growth of ET-5b (Fig. 3). Older cultures often did not grow when transferred into oxic medium. ET-5b consumed O2, and the resazurin in oxic medium was reduced after O2 was consumed. In U medium lacking reducer, growth did not occur when the gas phase contained 21% O2 but did occur when the gas phase contained 5% O2. Cells grew rapidly in anoxic TSB medium but did not grow in TSB medium when culture bottles were lightly sparged with filter-sterilized air. Thus, cultures of ET-5b were more easily maintainable under anoxic conditions or conditions with limited amounts of O2.
FIG. 3.
Effect of O2 on the growth of ET-5b in U medium supplemented with 10 mM glucose. The initial concentrations (%) of O2 in the gas phase (the remaining gas was N2) were 0 (○), 5 (●), 12 (▵), 21 (▴), 28 (□), 34 (■), and 44 (+). Tubes containing O2 were initially oxidized; the resazurin in all growth-positive tubes was reduced during growth, indicating that O2 was consumed in those tubes. Culture tubes were inoculated with exponentially growing cells from an anaerobic culture.
Doubling times and effects of temperature and pH on the growth of ET-5b.
Growth occurred at 37 to 65°C in anoxic U medium supplemented with glucose. The optimum temperature for growth was 55 to 60°C; growth was not observed at 30 or 70°C. Cultures did not grow when subjected to 100°C for 5 min, further demonstrating that ET-5b did not form spores. Growth occurred at pH 5.5 to 7.7 in anoxic TSB medium supplemented with glucose. The optimum pH for growth was 6.5 to 7.0; growth was not observed at pHs 5.1 and 8.1. Doubling times under both anoxic and O2-limited conditions were very similar (Fig. 3) and were approximately 1.5 to 2 h.
Substrate range of ET-5b under anoxic and oxic conditions.
Cultures of ET-5b were maintainable in anoxic D medium supplemented with either cellobiose or glucose; thus, yeast extract was not required for growth. In D medium, the following substrates supported growth under both oxic (i.e., with an initial gas phase of 21% O2) and anoxic conditions: stachyose, raffinose, cellobiose, maltose, sucrose, lactose, galactose, fructose, glucose, mannose, and xylose. Anaerobic growth on stachyose occurred only after several days of incubation; this extended lag phase did not occur when the inoculum was derived from anaerobic raffinose cultures. Succinate, acetate, and formate were growth supportive only under oxic conditions. ET-5b did not grow aerobically or anaerobically with the following substrates: cellulose, arabinose, gluconate, glyoxylate, lactate, pyruvate, oxalate, ethanol, catechol, protocatechuate, vanillate, CO, H2, vanillate plus CO, and vanillate plus H2.
In anoxic U medium, supplemental nitrate (5 mM), sulfate (5 mM), or thiosulfate (10 mM) did not influence glucose-dependent growth or product profiles (data not shown). N2O was not produced in nitrate-supplemented cultures. In addition, sulfate- and thiosulfate-supplemented media were neither discolored nor darkened subsequent to growth. These results indicated that nitrate, sulfate, and thiosulfate were not utilized as alternative electron acceptors. Fe3+ was reduced to Fe2+ as a minor side reaction; Fe3+ only slightly altered the glucose-dependent product profile of ET-5b (data not shown).
Effect of O2 on the soluble product profiles of ET-5b.
When ET-5b was grown on acetate, succinate, or formate under oxic conditions, substrates were consumed and no soluble products were detected (Table 1 and data not shown). Likewise, the amount of carbon recovered in the soluble products from stachyose, raffinose, xylose, and other saccharides (see above) under oxic conditions was significantly lower than that obtained under anoxic conditions (Table 1 and data not shown). In addition, the amount of biomass formed under oxic conditions was greater than that obtained under anoxic conditions (Table 1). These results indicated that ET-5b was capable of oxidizing substrates to CO2 via O2-dependent respiration.
TABLE 1.
Formation of soluble products by ET-5b under oxic and anoxic conditionsa
| Culture conditionc | Substrate consumed (mM) | Maximum OD600 | Soluble product (mM)b
|
Carbon recovery (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Acetate | Succinate | Lactate | Ethanol | Formate | ||||
| Oxic | Stachyose (2.2) | 0.33 | 1.6 | 0.6 | 1.0 | 0 | 0 | 16 |
| Anoxic | Stachyose (2.2) | 0.18 | 4.7 | 8.1 | 0.8 | 8.6 | 1.3 | 119 |
| Oxic | Raffinose (4.9) | 0.48 | 4.7 | 8.6 | 1.3 | 1.1 | 0 | 57 |
| Anoxic | Raffinose (4.7) | 0.35 | 6.3 | 13.7 | 1.8 | 2.1 | 2.4 | 94 |
| Oxic | Xylose (8.5) | 0.56 | 4.7 | 2.0 | 0.8 | 0.7 | 0 | 50 |
| Anoxic | Xylose (9.8) | 0.21 | 4.7 | 7.6 | 1.2 | 1.5 | 2.4 | 100 |
| Oxic | Acetate (4.4) | 0.16 | NA | 0 | 0 | 0 | 0 | 0 |
| Anoxicd | Acetate (0) | 0 | NA | |||||
Cultivation was in D medium, and values are the averages of duplicate experiments. OD600, optical density at 600 nm. NA, not applicable.
Values were corrected for concentrations obtained in controls lacking substrate.
Under oxic conditions, the initial concentration of O2CO2 in the gas phase was 21% (approximately 28 mM).
Inoculum was derived from an anaerobic glucose culture; results are from the second transfer.
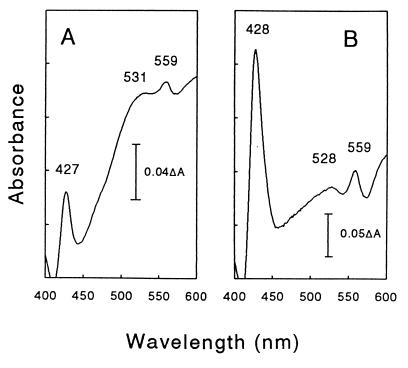
Cytochrome content of ET-5b.
Particulate (i.e., membrane-associated) and soluble b-type cytochromes were detected in anaerobically cultivated cells of ET-5b (Fig. 4). When membranes were prepared from cells cultivated under oxic conditions, the absorption maxima at 428 and 559 nm shifted to 433 and 560 nm, respectively.
FIG. 4.
Difference spectra of soluble (A) and particulate (B) material obtained from cells of ET-5b cultivated anaerobically on glucose in U medium. Vertical bars indicate the relative scale for the change in absorbance.
Dynamics of product formation.
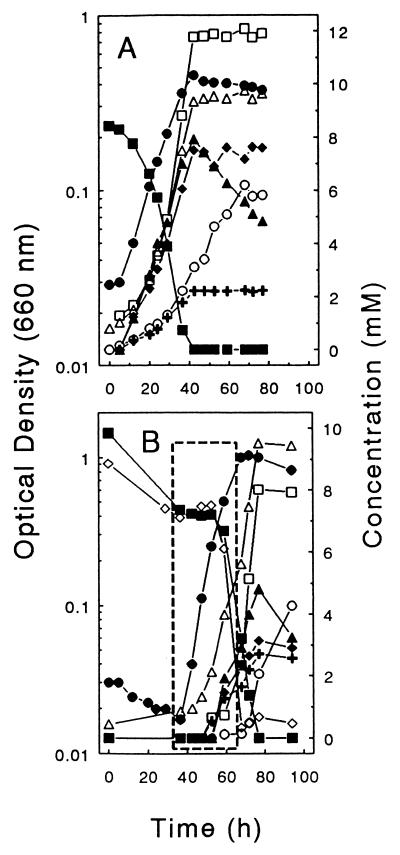
When grown anaerobically on cellobiose in D medium, ET-5b produced acetate, succinate, ethanol, lactate, formate, and H2 simultaneously during exponential growth (Fig. 5A). Acetate, succinate, ethanol, and lactate reached stable end concentrations simultaneously with the complete consumption of cellobiose and the onset of the stationary phase. The consumption of formate in the stationary phase was concommitant to the continued production of H2 (Fig. 5A), suggesting that stationary-phase cells contained formate-hydrogen lyase. Neither CO nor CH4 was detected.
FIG. 5.
Cellobiose-dependent product profiles of ET-5b cultivated in D medium in the absence (A) and presence (B) of a high initial concentration of O2 (approximately 21% of the initial gas phase). In panel B, the phase of maximal growth and the period of maximal O2 consumption is enclosed in the broken-line box. Inocula were derived from maintained anoxic (A) and oxic (B) cultures. Symbols: ●, growth; ■, cellobiose; ▵, acetate; □, succinate; ▴, formate; +, lactate; ⧫, ethanol; ○, H2; ◊, O2 (mM × 0.34; initial concentration approximated 28 mM).
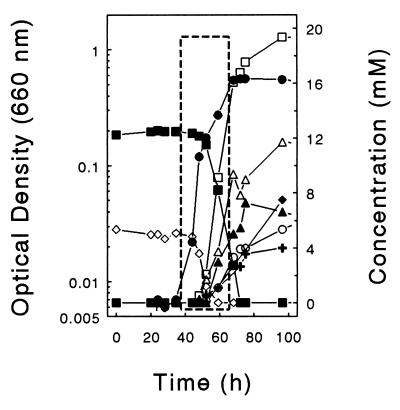
When 10 mM cellobiose and 28 mM O2 (approximately 21% of initial gas phase) were provided as cosubstrates in D medium, lactate, acetate, ethanol, and formate were produced and the consumption of O2 was minimal during early log phase (Fig. 5B). In contrast, when 10 mM cellobiose and 6 mM O2 (approximately 5% of the initial gas phase) were provided as cosubstrates in U medium lacking reducer, only minimal amounts of fermentation products were formed during the period of maximal O2 consumption (Fig. 6). These results indicated that (i) large amounts of O2 did not suppress the fermentation capacities of ET-5b, and (ii) the capacity of ET-5b to oxidize substrates to CO2 was optimal with smaller amounts of O2.
FIG. 6.
Cellobiose-dependent product profiles of ET-5b cultivated in nonreduced U medium in the presence of a low initial concentration of O2 (approximately 5% of the initial gas phase). The phase of maximal growth and the period of maximal O2 consumption is enclosed in the broken-line box. Symbols: ●, growth; ■, cellobiose; ▵, acetate; □, succinate; ▴, formate; +, lactate; ⧫, ethanol; ○, H2; ◊, O2.
Trophic interaction of ET-5a and ET-5b.
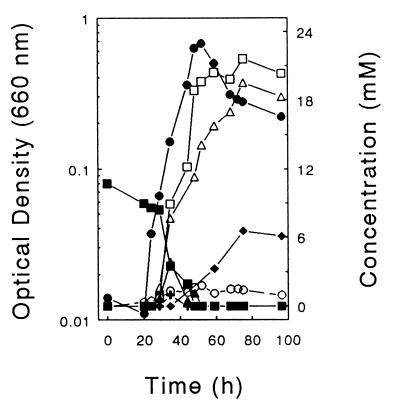
ET-5a did not grow with stachyose, raffinose, or cellobiose, but it grew acetogenically at the expense of lactate, formate, or H2 (Table 2 and data not shown). Although ET-5a grew rapidly on fructose, growth on glucose was marginal. When ET-5a and ET-5b were cultivated together on cellobiose, the products lactate, formate, and H2 remained at relatively low levels throughout growth (Fig. 7). The end concentrations of these products were either nondetectable or minimal when ET-5a and ET-5b were cocultured on stachyose, raffinose, cellobiose, or glucose (Table 2 and data not shown).
TABLE 2.
Substrate-product stoichiometries of anaerobic cultures of ET-5a and ET-5ba
| Culture | Substrate consumed (mM) | Product (mM)b
|
Recovery (%) ofc:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Acetate | Succinate | Lactate | Ethanol | Formate | H2 | C | H | ||
| ET-5a | Cellobiose (0) | NA | NA | ||||||
| ET-5b | Cellobiose (10.7) | 10.9 | 16.2 | 2.4 | 5.9 | 7.3 | 2.6 | 88 | 84 |
| ET-5a + ET-5b | Cellobiose (10.2) | 20.5 | 15.5 | 6.7 | 0.3 | 95 | 94 | ||
| ET-5a | Fructose (6.9)d | 18.4 | 89 | 89 | |||||
| ET-5b | Glucose (9.0) | 5.2 | 2.5 | 1.1 | 7.2 | 6.3 | 2.8 | 82 | 90 |
| ET-5a + ET-5b | Glucose (10.8) | 13.7 | 2.3 | 6.6 | 0.2 | 77 | 85 | ||
| ET-5a | Formate (18.2) | 5.0 | 91 | 110 | |||||
| ET-5b | Formate (0) | NA | NA | ||||||
| ET-5a + ET-5b | Formate (18.8) | 5.2 | 90 | 111 | |||||
| ET-5a | H2 (28.2) | 7.5 | ND | 106 | |||||
| ET-5b | H2 (0) | NA | NA | ||||||
| ET-5a + ET-5b | H2 (30.2) | 7.4 | ND | 98 | |||||
Cultivation was in U medium, and values are the averages of duplicate experiments.
Values were corrected for concentrations obtained in controls lacking substrate; the level of CO2 was not determined.
H, reducing equivalents; NA, not applicable; ND, not determined (i.e., CO2 consumption was not determined).
Growth of ET-5a on glucose was marginal and occurred very slowly only after an extended lag period.
FIG. 7.
Cellobiose-dependent product profiles of a coculture of ET-5a and ET-5b in anoxic U medium. Symbols: ●, growth; ■, cellobiose; ▵, acetate; □, succinate; ▴, formate; +, lactate; ⧫, ethanol; ○, H2.
Cocultures of ET-5a and ET-5b cultivated on stachyose, raffinose, cellobiose, and glucose produced greater amounts of acetate than did cultures of ET-5b alone (Table 2 and data not shown). In addition, succinate and ethanol were produced by cocultures in concentrations similar to those obtained with ET-5b alone, suggesting that ET-5a did not significantly alter the fermentation capacity of ET-5b. Likewise, ET-5b did not alter the capacity of ET-5a to grow acetogenically at the expense of formate or H2 (Table 2).
These results indicated that the acetogen ET-5a grew commensally with ET-5b via the interspecies transfer of lactate, formate, and H2. Consistent with this symbiotic interaction, cell extracts of ET-5a contained high levels of formate dehydrogenase and hydrogenase when these activities were measured in the direction of uptake (Table 3). As is characteristic of all acetogens, ET-5a also contained CO dehydrogenase. In contrast, formate dehydrogenase, hydrogenase, and CO dehydrogenase activities were low or not detected in cell extracts of ET-5b (Table 3).
TABLE 3.
Enzyme activities in cell extracts of ET-5a and ET-5b
| Organism | Growth substrate | Sp act (μmol min−1 mg of protein−1)
|
||
|---|---|---|---|---|
| CO dehydrogenase | Formate dehydrogenase | Hydrogenase | ||
| ET-5a | Formate + H2 | 17.4 | 4.2 | 6.0 |
| ET-5a | Fructose | 2.7 | 3.6 | 2.5 |
| ET-5b | Cellobiose | 0.0 | 0.012 | 0.4 |
| ET-5b | Fructose | 0.0 | 0.003 | 0.5 |
DISCUSSION
In the dendrogram generated with the ARB program, ET-5b was placed as an individual and deeply rooting line of descent next to the genera Bacillus, Paenibacillus, Brevibacillus, Thermoactinomyces, and Aneurinibacillus, as well as the closely related genus Oxalophagus. When the ET-5b 16S rRNA gene sequence was compared to those in the more extensive DSMZ database, the isolated position of this sequence as a separate subline of the Bacillus genus proper was confirmed (Fig. 1). The generation of various dendrograms with changing sequence composition by the neighbor-joining method and alternative algorithms (8) led to slightly changing branching points; however, ET-5b was not located within the radiation of a reference genus. Similarity values between the sequences of strain ET-5b and reference organisms from the Clostridium-Bacillus lineage were below 88%, a value that is at least 5% lower than the intrageneric values of the genera listed above. The phylogenetic position of strain ET-5b between the moderately thermophilic species Alicyclobacillus acidoterrestris (viable at 45°C) and Thermoactinomyces vulgaris (viable at 50°C) is not supported by high bootstrap values, confirming the low statistical significance of its branching point among the lineages of gram-positive bacteria with low moles percent G+C content. These findings demonstrate that ET-5b represents the nucleus of a new genus, and the following name is proposed for the type strain: Thermicanus aegyptius ET-5b (Therm.i.ca′nus ae.gyp′ti.us).
T. aegyptius ET-5b grows at the expense of a broad range of substrates, including oligosaccharides such as stachyose, and prefers anoxic conditions or conditions with limited amounts of O2. As such, T. aegyptius ET-5b might be best described as a thermophilic fermentative microaerophile. Under oxic conditions, T. aegyptius ET-5b is able to utilize certain products that it produces under anoxic conditions. As an organism that resides in an environment prone to fluctuations in O2 and organic carbon levels, these factors likely contribute to the competitiveness of T. aegyptius ET-5b under in situ conditions.
T. aegyptius ET-5b contained a membranous b-type cytochrome when grown anaerobically. The shift in the absorption maxima of the membranous chromophores of cells cultivated in the presence of O2 indicated that redox conditions might influence the production of dissimilar cytochromes by T. aegyptius ET-5b. The absorption maxima of the soluble and particulate material were also dissimilar (Fig. 4). However, it cannot be unequivocally stated that these differences in absoption maxima were attributable to different cytochromes. Nonetheless, the differential expression of cytochromes in aerobically and anaerobically cultivated cells of facultative aerobes is well established. For example, E. coli produces different b-type cytochromes in response to changes in the availability of O2 (5). In M. thermoacetica, a membranous b-type cytochrome that is involved in the flow of reductant during acetogenesis is not expressed when cells are dissimilating nitrate (12, 19). Since T. aegyptius ET-5b contains a periplasm, the soluble cytochrome detected might be localized in the periplasm rather than in the cytoplasm. Resolving the function(s) of the cytochrome(s) of T. aegyptius ET-5b during growth under oxic and anoxic conditions will require further study.
The acetogen M. thermoacetica ET-5a grew commensally with T. aegyptius ET-5b via the interspecies transfer of H2, formate, and lactate (Fig. 8). In contrast to T. aegyptius ET-5b, M. thermoacetica ET-5a did not utilize oligosaccharides; thus, these two organisms would not be in direct competition for these primary substrates. Indeed, the substrate profiles of these two organisms are quite different. Under certain conditions, the interspecies transfer of reductant between strict anaerobes is a syntrophic process. For example, the capacity of Syntrophomonas wolfei to grow at the expense of butyrate is thermodynamically possible only via the syntrophic transfer of H2 to a methanogen (41). Likewise, various H2-consuming methanogens and sulfate reducers can be cocultured syntrophically with Acetobacterium woodii or other acetogens via the interspecies transfer of H2 (25, 60). The pectin fermenter Lachnospira multiparus and the acetogen Eubacterium limosum (which is unable to utilize pectin) inhabit the rumen and interact trophically via the interspecies transfer of methanol, ethanol, formate, lactate, and H2 (47).
FIG. 8.
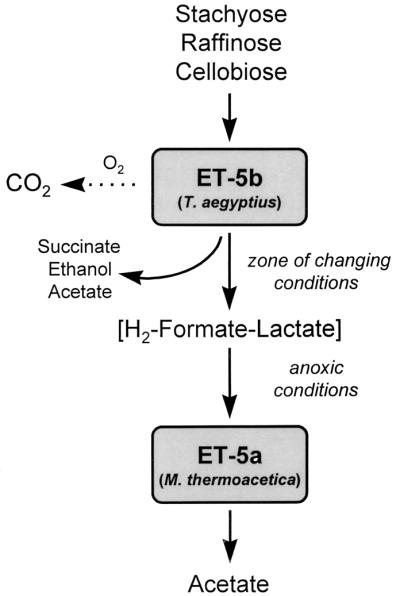
Scheme illustrating the postulated trophic interaction of T. aegyptius ET-5b and M. thermoacetica ET-5a.
As has been documented with certain facultative anaerobes (24, 59), cultures of T. aegyptius ET-5b produced fermentation products concomitant with the consumption of O2. In such cultures, whether some cells of T. aegyptius ET-5b were strictly respiring O2 while others were strictly fermentative is not known. The production of lactate and formate by T. aegyptius ET-5b under oxic conditions (Fig. 4B) suggests that a trophic interaction between T. aegyptius ET-5b and M. thermoacetica ET-5a might occur in the presence of O2 (conceived in the scheme shown in Fig. 8 as a zone of changing conditions). This possibility assumes that M. thermoacetica ET-5a can tolerate minimal levels of O2. Although O2 is classically considered to be toxic to strict anaerobes, certain sulfate reducers and methanogens can consume at least minimal levels of O2 and survive periods of oxidation (6, 9, 36), and acetogens in prairie soil can withstand drying under oxic conditions (58). Acetogenic bacteria can be readily enriched and enumerated from well-drained, aerated soils and litter (35, 43, 58), and a new acetogen, Sporomusa silvacetica, was recently isolated from forest soil (31); these findings attest to the ability of acetogens to cope with periodic in situ fluctuations in O2 levels. The sensitivity of T. aegyptius ET-5b to large amounts of O2 suggests that it would function optimally in microzones prone to anoxia or minimal aeration, thus increasing the likelihood that T. aegyptius ET-5b could reside proximally to M. thermoacetica ET-5a under in situ conditions.
It is not improbable that the survival strategy of acetogens in soils is at least partly coupled to their trophic interactions with facultative microaerophiles. Under chemostatic conditions, a sulfate-reducing bacterium (Desulfovibrio HL21) and a facultatively anaerobic Vibrio species were maintained in coculture under low-O2 conditions (24). In addition, the obligate anaerobe Veillonella alcalescens can coexist with the obligate aerobe Comamonas testosteroni in the presence of low levels of O2 (20). Such observations suggest that O2-consuming organisms lessen the O2-dependent inhibition of anaerobes. Thus, facultative microaerophiles might not only produce products that can be used commensally by acetogens but also minimize the level of O2 in microzones inhabitated by acetogens. It has been proposed that the acetate formed in anaerobic microsites of soils is primarily subject to oxidation via aerobic or other respiratory processes (12, 32, 58). Since T. aegyptius ET-5b grew aerobically on acetate, the acetate produced by M. thermoacetica ET-5a might be subject to O2-dependent oxidation by its microaerophilic partner under certain conditions, thus further benefiting the acetogen via the consumption of incoming O2. Previous studies have documented the capacity of aerobic organisms to oxidize the fermentation products of an obligate anaerobe when such organisms are cocultured under low-O2 conditions (20).
M. thermoacetica grows very poorly on ethanol under acetogenic conditions but grows rapidly on ethanol when dissimilating nitrate (19), suggesting that ethanol produced by T. aegyptius ET-5b might also be used commensally by M. thermoacetica in the presence of nitrate. Furthermore, H2- and formate-dependent cell yields of M. thermoacetica are significantly enhanced under nitrate-dissimilating conditions (19). Thus, the trophic interaction of M. thermoacetica ET-5a and T. aegyptius ET-5b would theoretically be more dynamic than the results depicted in Fig. 8 if nitrate were available. Determining how closely T. aegyptius ET-5b and M. thermoacetica ET-5a are associated under in situ conditions would provide further insight into their capacity to interact in soil.
M. thermoacetica is the classic acetogen which H. G. Wood and L. G. Ljungdahl used to resolve the acetyl-CoA pathway (61). It is the best-characterized acetogen to date, and much of the information available on the physiology and enzymology of acetogenesis is based on work done with this organism (13, 37, 44). M. thermoacetica was originally isolated from horse manure as a glucose-fermenting heterotroph (17) but was later shown to (i) contain hydrogenase (10) and be lithoautotrophic on both H2-CO2 and CO-CO2 (7); (ii) preferentially dissimilate nitrate to ammonium (19, 50); (iii) utilize a diverse array of substrates, including carboxylic acids, alcohols, oxalate, glyoxylate, glycolate, and numerous methoxylated aromatic compounds (12, 13); and (iv) utilize the carboxyl groups of certain aromatic compounds in the reductive synthesis of acetate (27, 28). The isolation of M. thermoacetica ET-5a from Egyptian soil as a trophic partner of a facultative microaerophile was unexpected and accentuates the fact that very little is known about the ecology of this historically important acetogen. The occurrence of M. thermoacetica in Kansan and Egyptian soils (references 21 and 23 and unpublished data) indicates that the organism is a soil microbe that is geographically widespread. Current studies are focused on resolving the effects of O2 on the interaction and ecophysiology of M. thermoacetica ET-5a and T. aegyptius ET-5b, and the co-occurrence of these two thermophiles in well-aerated, high-temperature soils.
Description of Thermicanus aegyptius ET-5bT gen. nov., sp. nov.
Thermicanus aegyptius ET-5bT gen. nov., sp. nov. (DSMZ 12793T) (therm.i.ca′nus. Gr adj. thermos, hot; Gr. adj. ikanos, M.L. icanus, capable; M.L. Thermicanus, the capable thermophile; ae.gyp′ti.us. L. adj. aegyptius, Egyptian or from Egypt). A motile, weakly gram-positive, thermophilic, facultative microaerophile isolated from Egyptian soil. Cells are rod shaped and have an S-layer and outer and cytoplasmic membranes; spores not apparent. Growth is optimal at 55 to 60°C and pH 6.5 to 7; doubling times are approximately 1.5 to 2 h. High levels of O2 impair growth; prefers anoxic or microaerophilic conditions. Substrates include stachyose, raffinose, maltose, sucrose, cellobiose, lactose, galactose, glucose, fructose, mannose, and xylose. Acetate, formate, and succinate are utilized only under oxic conditions. Does not grow on cellulose, arabinose, gluconate, glyoxylate, lactate, pyruvate, oxalate, ethanol, catechol, protocatechuate, vanillate, CO, and H2. Fermentation products are acetate, succinate, ethanol, formate, lactate, and H2; fermentation products are also formed under oxic conditions. Nitrate, sulfate, and thiosulfate are not dissimilated; Fe3+ is reduced to Fe2+ as a side reaction. Particulate and soluble fractions have b-type cytochrome(s). G+C content is 50.3 mol%. Most closely related to Paenibacillus, Bacillus, and Oxalophagus, with the nearest 16S rRNA gene sequence similarity value being approximately 88%. The thermophilic acetogen M. thermoacetica ET-5a grows commensally with T. aegyptius ET-5bT on oligosaccharides via the interspecies transfer of H2, formate, and lactate.
ACKNOWLEDGMENTS
We thank Rita Grotjahn for technical assistance, Hans Trüper and Despina Niniatsoudi for helpful discussions on nomenclature, and Kirsten Küsel for review of the manuscript.
This study was supported by the German Ministry of Education, Science, Research, and Technology (PT BEO 51-0339476B).
REFERENCES
- 1.Beji A, Izard D, Gavini F, Leclerc H, Leseine-Delstanche M, Krembel J. A rapid chemical procedure for isolation and purification of chromosomal DNA from Gram-negative bacilli. Anal Biochem. 1987;162:18–23. doi: 10.1016/0003-2697(87)90005-4. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brosius J, Palmer M L, Kennedy P J, Noller H F. Complete nucleotide sequence of the 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronan J E, Jr, Gennis R B, Malloy S R. Cytoplasmic membrane. In: Neidhardt F C, Ingraham J L, Brooks Low K, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 31–55. [Google Scholar]
- 6.Cypionka H, Widdel F, Pfennig N. Survival of sulfate-reducing bacteria after oxygen stress and growth in sulfate-free oxygen sulfide gradients. FEMS Microbiol Ecol. 1985;85:31–42. [Google Scholar]
- 7.Daniel S L, Hsu T, Dean S I, Drake H L. Characterization of the H2- and CO-dependent chemolithotrophic potentials of the acetogens Clostridium thermoaceticum and Acetogenium kivui. J Bacteriol. 1990;172:4464–4471. doi: 10.1128/jb.172.8.4464-4471.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSoete G. A least squares algorithm for fitting additive trees to proximity data. Psychometrika. 1983;48:621–626. [Google Scholar]
- 9.Dilling W, Cypionka H. Aerobic respiration in the sulfate-reducing bacteria. FEMS Microbiol Lett. 1990;71:123–128. [Google Scholar]
- 10.Drake H L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982;150:702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake H L. Introduction to acetogenesis. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 3–60. [Google Scholar]
- 12.Drake H L, Daniel S L, Küsel K, Matthies C, Kuhner C, Braus-Stromeyer S. Acetogenic bacteria: what are the in situ consequences of their diverse metabolic versatilities? Biofactors. 1997;6:13–24. doi: 10.1002/biof.5520060103. [DOI] [PubMed] [Google Scholar]
- 13.Drake H L, Daniel S L, Matthies C, Küsel K. Acetogenesis: reality in the laboratory, uncertainty elsewhere. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 273–302. [Google Scholar]
- 14.Drake H L, Hu S-I, Wood H G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermoaceticum. J Biol Chem. 1980;255:7174–7180. [PubMed] [Google Scholar]
- 15.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–789. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.5.1. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 17.Fontaine F E, Peterson W H, McCoy E, Johnson M J, Ritter G J. A new type of glucose fermentation by Clostridium thermoaceticum n.sp. J Bacteriol. 1942;43:701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox T R, Comerford N B. Low-molecular-weight organic acids in selected forest soils of the southeastern USA. Soil Sci Soc Am J. 1990;54:1139–1144. [Google Scholar]
- 19.Fröstl J M, Seifritz C, Drake H L. Effect of nitrate on the autotrophic metabolism of the acetogens Clostridium thermoautotrophicum and Clostridium thermoaceticum. J Bacteriol. 1996;178:4597–4603. doi: 10.1128/jb.178.15.4597-4603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerritse J, Schut F, Gottschal J C. Modelling of mixed chemostat cultures of an aerobic bacterium, Comamonas testosteroni, and an anaerobic bacterium, Veillonella alcalescens: comparison with experimental data. Appl Environ Microbiol. 1992;58:1466–1476. doi: 10.1128/aem.58.5.1466-1476.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goessner A, Kuesel K, Devereux R, Drake H L. In Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C.: American Society for Microbiology; 1998. Occurrence of thermophilic acetogens in Egyptian soils, p. 366, abstr. N-1. [Google Scholar]
- 22.Gößner A, Daniel S L, Drake H L. Acetogenesis coupled to the oxidation of aromatic aldehyde groups. Arch Microbiol. 1994;161:126–141. [Google Scholar]
- 23.Gößner A S, Drake H L. . In Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. American Society for Microbiology, Washington, D.C. 1997. Characterization of a new thermophilic acetogen (PT-1) isolated from aggregated Kansas prairie soil, p. 401, abstr. N-122 [Google Scholar]
- 24.Gottschal J, Szewzyk R. Growth of a facultative anaerobe under oxygen-limiting conditions in pure culture and in co-culture with a sulfate-reducing bacterium. FEMS Microbiol Ecol. 1985;31:159–170. [Google Scholar]
- 25.Heijthuijsen J H F G, Hansen T A. Interspecies hydrogen transfer in co-cultures of methanol-utilizing acidogens and sulfate-reducing or methanogenic bacteria. FEMS Microbiol Ecol. 1986;38:57–64. [Google Scholar]
- 26.Hobot J, Carlemalm E, Villiger W, Kellenberger E. Periplasmic gel: new concept resulting from reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984;160:143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu T, Daniel S L, Lux M F, Drake H L. Biotransformation of carboxylated aromatic compounds by the acetogen Clostridium thermoaceticum: generation of CO2 equivalents under CO2-limited conditions. J Bacteriol. 1990;172:212–217. doi: 10.1128/jb.172.1.212-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu T, Lux M F, Drake H L. Expression of an aromatic-dependent decarboxylase which provides growth-essential CO2 equivalents for the acetogenic (Wood) pathway of Clostridium thermoaceticum. J Bacteriol. 1990;172:5901–5907. doi: 10.1128/jb.172.10.5901-5907.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 30.Karsten G R, Drake H L. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl Environ Microbiol. 1997;63:1878–1882. doi: 10.1128/aem.63.5.1878-1882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhner C H, Frank C, Grießhammer A, Schmittroth M, Acker G, Gößner A, Drake H L. Sporomusa silvacetica sp. nov., an acetogenic bacterium isolated from aggregated forest soil. Int J Syst Bacteriol. 1997;47:352–358. doi: 10.1099/00207713-47-2-352. [DOI] [PubMed] [Google Scholar]
- 32.Küsel K, Drake H L. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl Environ Microbiol. 1995;61:3667–3675. doi: 10.1128/aem.61.10.3667-3675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Küsel K, Drake H L. Anaerobic capacities of leaf litter. Appl Environ Microbiol. 1996;62:4216–4219. doi: 10.1128/aem.62.11.4216-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Küsel K, Drake H L. Microbial turnover of low molecular weight organic acids during leaf litter decomposition. Soil Biol Biochem. 1999;31:107–118. [Google Scholar]
- 35.Küsel K, Wagner C, Drake H L. Enumeration and metabolic product profiles of the anaerobic microflora of forest soil and litter. FEMS Microbiol Ecol. 1999;29:91–103. [Google Scholar]
- 36.Leadbetter J R, Breznak J A. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ljungdahl L G. The acetyl-CoA pathway and the chemiosmotic generation of ATP during acetogenesis. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 63–87. [Google Scholar]
- 38.Lundie L L, Jr, Drake H L. Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol. 1984;159:700–703. doi: 10.1128/jb.159.2.700-703.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maidak B L, Olsen G J, Larsen N, Overbeck R, McCaughey M J, Woese C R. The ribosomal database project (RDP) Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthies C, Freiberger A, Drake H L. Fumarate dissimilation and differential reductant flow by Clostridium formicoaceticum and Clostridium aceticum. Arch Microbiol. 1993;160:273–278. [Google Scholar]
- 41.McInerney M J, Bryant M P, Hespell R B, Costerton J W. Syntrophomonas wolfei gen. nov., sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol. 1981;41:1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesbah M, Premachandran U, Whitman W B. Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol. 1989;39:159–167. [Google Scholar]
- 43.Peters V, Conrad R. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Appl Environ Microbiol. 1995;61:1673–1676. doi: 10.1128/aem.61.4.1673-1676.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragsdale S W. CO dehydrogenase and the central role of this enzyme in the fixation of carbon dioxide by anaerobic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 88–126. [Google Scholar]
- 45.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds E S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;7:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rode L M, Genthner B R S, Bryant M P. Syntrophic association by cocultures of the methanol- and CO2-H2-utilizing species Eubacterium limosum and pectin-fermenting Lachnospira multiparus during growth in a pectin medium. Appl Environ Microbiol. 1981;42:20–22. doi: 10.1128/aem.42.1.20-22.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 49.Schink B. Diversity, ecology and isolation of acetogenic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman & Hall; 1994. pp. 197–235. [Google Scholar]
- 50.Seifritz C, Daniel S L, Gößner A, Drake H L. Nitrate as a preferred electron sink for the acetogen Clostridium thermoaceticum. J Bacteriol. 1993;175:8008–8013. doi: 10.1128/jb.175.24.8008-8013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sextone A J, Revsbech N P, Parkin T B, Tiedje J M. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci Soc Am J. 1985;49:645–651. [Google Scholar]
- 52.Shen Y, Stöm L, Jönsson J-Å, Tyler G. Low-molecular organic acids in the rhizosphere soil solution of beech forest (Fagus sylvatica L.) cambisols determined by ion chromatography using supported liquid membrane enrichment technique. Soil Biol Biochem. 1996;28:1163–1169. [Google Scholar]
- 53.Sleytr U B. Basic and applied S-layer research: an overview. FEMS Microbiol Rev. 1997;20:5–12. [Google Scholar]
- 54.Tani M, Higashi T, Nagatsuka S. Dynamics of low-molecular-weight aliphatic carboxylic acids (LACAs) in forest soils. I. Amount and composition of LACAs in different types of forest soils in Japan. Soil Sci Plant Nutr. 1993;39:485–495. [Google Scholar]
- 54a.Technische Universität München. 1999. ARB database. [Online.] http://www.mikro.biologie.tu-muenchen.de [12 May 1999, last date accessed.]
- 55.Traub W H, Acker G, Kleber I. Ultrastructural surface alterations of Serratia marcescens after exposure to polymyxin B and/or fresh human serum. Chemotherapy (Basel) 1976;22:104–113. doi: 10.1159/000221919. [DOI] [PubMed] [Google Scholar]
- 56.Valentine R C, Shapiro B M, Stadtman E R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968;7:2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- 57.Van der Lee G E M, de Winder B, Bouten W, Tietema A. Anaerobic microsites in Douglas fir litter. Soil Biol Biochem. 1999;31:1295–1301. [Google Scholar]
- 58.Wagner C, Grießhammer A, Drake H L. Acetogenic capacities and the anaerobic turnover of carbon in a Kansas prairie soil. Appl Environ Microbiol. 1996;62:494–500. doi: 10.1128/aem.62.2.494-500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wimpenny J W T, Necklen D K. The redox environment and microbial physiology. I. The transition from anaerobiosis to aerobiosis in continuous culture of facultative bacteria. Biochim Biophys Acta. 1971;253:352–359. doi: 10.1016/0005-2728(71)90039-9. [DOI] [PubMed] [Google Scholar]
- 60.Winter J U, Wolfe R S. Methane formation from fructose by syntrophic associations of Acetobacterium woodii and different strains of methanogens. Arch Microbiol. 1980;124:73–79. doi: 10.1007/BF00407031. [DOI] [PubMed] [Google Scholar]
- 61.Wood H G, Ljungdahl L G. Autotrophic character of acetogenic bacteria. In: Shively J M, Barton L L, editors. Variations in autotrophic life. San Diego, Calif: Academic Press; 1991. pp. 201–250. [Google Scholar]