Human Cytomegalovirus infection remains a major risk factor and negative predictor for the outcome of hematopoietic stem cell transplantation (HSCT). In particular, reactivation and associated high viral loads are highly predictive of myelosuppression and failed engraftment. However, myelosuppression is also observed in some patients despite treatment with antiviral drugs and reduced viral load (Ljungman et al., 2011; Yong et al., 2019).
In this impactful report by Hancock et al., the authors systematically examined the contribution of HCMV infection, and more specifically the role of HCMV miRNAs, in inducing myelosuppression in hematopoietic progenitor cells (HPCs). To this end, an experimental system in which CD34+ HPCs were evaluated for myeloid colony formation was utilized for much of the work. After verifying that HCMV infection of HPCs resulted in myelosuppression, virus-free supernatants from infected cells were tested for the ability to do the same. Transfer experiments demonstrated that the supernatants alone were sufficient to inhibit colony formation, suggesting the presence of a secreted factor. Cytokine analysis revealed that TGF-β alone was conveying myelosuppression. Indeed, the addition of a neutralizing antibody against TGF-β to the supernatants from HCMV-infected HPCs prevented inhibition of stem cell growth. These data elegantly demonstrated that HCMV exerts paracrine effects on uninfected cells by inducing TGF-β secretion.
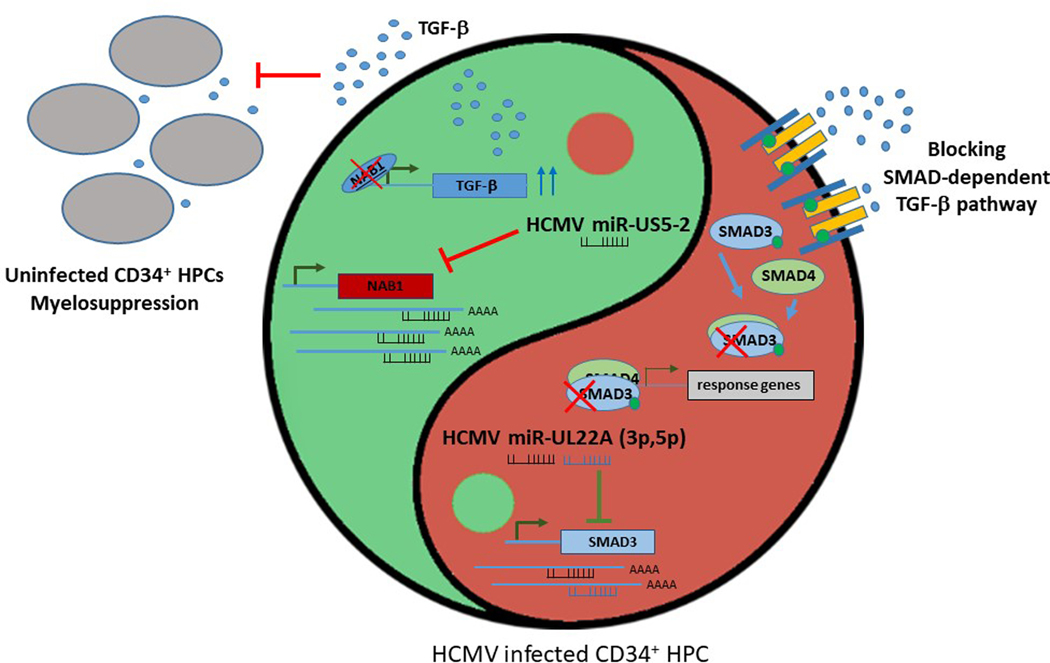
Importantly, these experiments were performed under culture conditions where HCMV establishes latency with no detectable lytic replication. Next, the authors aimed to identify latency-associated viral gene products that induce TGF-β expression and identified the microRNA miR-US5–2 as the driving factor. Since miRNAs function by downregulating genes, it was apparent that miR-US5–2 was targeting a suppressor of TGF-β. NAB1 was identified as a candidate target through bioinformatics analysis, and is the transcriptional repressor NGFI-A binding protein which negatively regulates EGR1-mediated transcription of TGF-β. In the presence of miR-US5–2, NAB1 expression was reduced. Furthermore, NAB1 repression, either by miR-US5–2 or a specific shRNA, led to increased secretion of TGF-β as well as decreased expansion of CD34+ HPCs. Another significant finding is that HPCs infected with a mutant HCMV lacking miR-US5–2 showed higher NAB1 expression, lower TGF-β secretion, as well as restored proliferation and myelopoiesis. These experiments thoroughly demonstrated that HCMV miR-US5–2 directly targets NAB1, leading to the upregulation of TGF-β, and resulting in the myelosuppression of neighboring uninfected cells.
Since the HCMV-dependent up-regulation of TGF-β affected uninfected cells, the authors hypothesized that HCMV-infected cells themselves may be refractory to TGF-β to maintain HCMV latency. To test this, HCMV infected HPCs were stimulated with the cytokine and then monitored for upregulation of a TGF-β-responsive gene, SERPINE. They found that the induction of SERPINE was completely blocked in infected cells. Furthermore, SMAD3, a signaling component downstream of the TGF-β receptor, could be downregulated by two additional HCMV microRNAs, miR-UL22A-3p and miR-UL22A-5p. Elegant viral genetics showed that HPCs infected with a mutant virus lacking both of these miRNAs responded to TGF-β like mock-infected cells. Thus, it was shown that direct repression of SMAD3 by miR-UL22A-3p and miR-UL22A-5p is responsible for the block in TGF-β signaling in infected cells.
In summary, latent, not lytic, HCMV infection, and more specifically the expression of miR-US5–2 in primary HPC cultures induces the secretion of TGF-β, which inhibits growth of uninfected cells, thereby blocking myelopoiesis. In contrast, the infected cells are protected from TGF-β by downregulation of SMAD3 by miR-UL22A-3p and −5p. Interestingly, HCMV accomplishes both the positive regulation of TGF-β synthesis and secretion and the negative regulation of the TGF-β signaling within infected cells by utilizing latently expressed miRNAs. The outcome of this “intracellular yin and yang regulatory loop” (Figure 1) prevents cell proliferation of uninfected cells in the bone marrow microenvironment that surrounds HCMV infected HPCs. This may contribute to immune evasion and supports viral latency and persistence in the host. Moreover, HCMV renders the cell refractory to its own TGF-β, thus enabling latency maintenance and subsequent rounds of reactivation.
Figure 1. HCMV miRNAs maintain a balance between TGF-β activation and suppression.
On the left (green), HCMV miR-US5–2 downregulates NAB1, thereby alleviating the NAB1-induced inhibition of TGF-β expression. This leads to an increase in TGF-β secretion and the subsequent myelosuppression of neighboring uninfected cells. On the right (red), HCMV miR-UL22A-3p and −5p downregulate SMAD3 and, as a result, make infected cells unresponsive to TGF-β, which supports the maintenance of latency.
Inhibition of TGF-β signaling at multiple levels during latency has also been reported for Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV), which are both associated with tumorigenesis (Velapasamy et al., 2018). Moreover, in addition to encoding proteins that inhibit different arms of the TGF-β signaling pathway, EBV and KSHV also utilize miRNA-dependent inhibition. Multiple KSHV miRNAs target Tsp-1, which is required for TGF-β maturation (Samols, 2007) and the TGF-β type II receptor (TβRII) (Lei et al., 2012) and two viral latency proteins strongly activate the host miR-17–92 cluster, which targets SMAD2 and completely abrogates TGF-β signaling (Choi et al., 2015). EBV targets the degradation of SMAD4 through induction of the cellular miRNA miR-146, which is regulated by BARF-1-induced NFκB (Kim et al., 2016). In many systems, TGF-β effects are often cell type- and differentiation-specific, sometimes leading to completely opposite effects, which is referred to as the “TGF-β paradox” (Derynck et al., 2001). It is interesting to think within this context that the induction of TGF-β secretion has thus far only been observed in HCMV infected cells (Hancock et al., 2019). Negatively targeting the TGF-β pathway by multiple mechanisms, including post-transcriptional regulation by miRNAs, has been conserved between herpesviruses. This may suggest that the positive arm of the HCMV “intracellular yin and yang regulatory loop” (Figure 1) has yet to be discovered for EBV and KSHV.
With respect to HSCT and solid organ transplantation, the observation that HCMV latency can induce myelosuppression represents a major paradigm shift from our current understanding of how HCMV negatively affects transplant outcomes. Indeed, current treatment targets reactivation because high viral load is thought to be the only major predictor for negative outcomes. Hence, nearly 80% of patients receive antiviral drugs like ganciclovir that target lytic HCMV replication but also have myelosuppressive adverse effects. However, a sizable fraction of patients with suppressed myelopoiesis do not present with high HCMV titers (Ljungman et al., 2011; Yong et al., 2019). The data presented by Hancock et al., 2020 have clinical significance for these patients and may warrant the development of biomarkers to estimate the latency reservoir (i.e., the number of latently infected HPCs) as well as TGF-β levels, which could serve as novel predictive markers for outcome after BMT. Moreover, this work strongly suggests that the development of novel therapeutic strategies targeting latent HCMV infection is important, especially in the transplant setting.
Acknowledgements:
We thank Peter Turner for critical reading and discussion of the manuscript. RR is supported by NCI P01 CA214091 and NIDCR R01 DE026707.
References:
- 1).Choi HS, Jain V, Krueger B, Marshall V, Kim CH, Shisler JL, Whitby D, and Renne R. (2015). Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17–92 Cluster and Down-Regulates TGF-beta Signaling. PLoS Pathog 11, e1005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Derynck R, Akhurst RJ, and Balmain A. (2001). TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29, 117–129. [DOI] [PubMed] [Google Scholar]
- 3).Hancock MH, Crawford LB, Pham AH, Mitchell J, Struthers HM, Yurochko AD, Caposio P, and Nelson JA (2020). Human Cytomegalovirus miRNAs Regulate TGF-β to Mediate Myelosuppression while Maintaining Viral Latency in CD34+ Hematopoietic Progenitor Cells. Cell Host & Microbe 27, this issue, 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Kim DH, Chang MS, Yoon CJ, Middeldorp JM, Martinez OM, Byeon SJ, Rha SY, Kim SH, Kim YS, and Woo JH (2016). Epstein-Barr virus BARF1-induced NFkappaB/miR-146a/SMAD4 alterations in stomach cancer cells. Oncotarget 7, 82213–82227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Lei X, Zhu Y, Jones T, Bai Z, Huang Y, and Gao SJ (2012). A KSHV microRNA and its variants target TGF-beta pathway to promote cell survival. Journal of virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Ljungman P, Hakki M, and Boeckh M. (2011). Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am 25, 151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Samols MA, Hu J, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, and Renne R. (2007). Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Velapasamy S, Dawson CW, Young LS, Paterson IC, and Yap LF (2018). The Dynamic Roles of TGF-beta Signalling in EBV-Associated Cancers. Cancers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Yong MK, Gottlieb D, Lindsay J, Kok J, Rawlinson W, Slavin M, Ritchie D, Bajel A, and Grigg A. (2019). New Advances in the Management of Cytomegalovirus in Allogeneic Haemopoietic Stem Cell Transplantation. Intern Med J. [DOI] [PubMed] [Google Scholar]