Abstract
In some cases with idiopathic ventricular fibrillation, short-coupled premature ventricular contractions (PVCs) initiate fatal arrhythmia, which has recently been proposed as short-coupled ventricular fibrillation (SCVF). In the present case of SCVF, catheter ablation for trigger PVCs originating from the His-Purkinje system in both ventricles caused transient complete atrioventricular block, and a combination of quinidine and verapamil suppressed ventricular tachyarrhythmia.
<Learning objective: In some cases with short-coupled ventricular fibrillation, a combination of quinidine and verapamil can suppress fatal ventricular tachyarrhythmia.>
Keywords: Purkinje, Idiopathic ventricular fibrillation, Short-coupled ventricular fibrillation, Quinidine, Verapamil
Introduction
Frequent premature ventricular contractions (PVC) and polymorphic ventricular tachycardia (PMVT) arising from hyperexcitability of the fascicular-Purkinje system in association with SCN5A variants and responding to quinidine has been defined as multifocal ectopic Purkinje-related premature contractions (MEPPC) [1, 2]. Recently, short-coupled ventricular fibrillation (SCVF) has been reported to be a distinct primary arrhythmia syndrome [3]. We present an SCVF case with short-coupled PVC followed by PMVT originating from the fascicular-Purkinje system in both ventricles that was suppressed with a combination of quinidine and verapamil.
Case Report
A 42-year-old woman was referred to our institution because of frequent implantable cardioverter-defibrillator (ICD) discharges due to PMVT. The patient had been diagnosed with idiopathic ventricular fibrillation (VF) at 26 years of age because cardiac echocardiography, coronary angiography, both left and right ventriculography, and a myocardial biopsy showed no abnormalities. At 30 years of age, the first electrophysiological study was done for electrical storm of PMVT/VF unresponsive to verapamil, atenolol, and disopyramide. Presystolic Purkinje potentials following PVC/PMVT were recorded at the left ventricular mid-septum, and radiofrequency applications to the earliest Purkinje potentials suppressed PVC/PMVT. However, 12 years after the first ablation, recurrences of PMVT/VF with frequent ICD discharges were observed. Several antiarrhythmic drugs, including amiodarone, cibenzoline, flecainide, aprindine, bisoprolol, and carvedilol, were ineffective, and catheter ablation was attempted.
During electrophysiological study, a quadripolar electrode catheter was positioned at the bundle of His recording region, and 3.5-mm tip irrigated ablation catheters (Navistar Thermo Cool, Biosense Webster, Diamond Bar, CA, USA) were introduced into the left and right ventricles via the right femoral artery and vein, respectively. The delivery of radiofrequency energy power was 20 to 30 W through the present procedure. In the session, PVCs showed both right and left bundle branch block (RBBB and LBBB) patterns with various axis deviations (Fig. 1). All PVCs appeared with a short coupling interval of 250 to 300 ms, manifesting as R-on-T type. Moreover, PMVT was initiated by PVCs of both RBBB and LBBB patterns. Accordingly, mapping and then ablation in both ventricles were planned for the elimination of PVCs initiating PMVT. Because the most frequent pattern of PVCs was RBBB, we first mapped the endocardium of the left ventricle for the earliest activation site of PVCs. Frequent fascicular-Purkinje activities with and without conduction block to the ventricle were recorded at the proximal site of left bundle branch, preceding the PVC (Figs 2, 3A). After ablation for fascicular-Purkinje ectopy originating from the proximal left bundle, RBBB-type PVC markedly decreased, while complete LBBB occurred transiently. Thus, mapping for LBBB-type PVC in the right ventricle was carried out. At the site where distal right bundle branch potentials were recorded during sinus rhythm, abnormal fascicular-Purkinje potentials preceding LBBB-type PVC were observed without the preceding excitation of the His bundle, meaning it was not true that the retrograde conduction of the left bundle excitation conducts the right-side Purkinje network (Fig. 3B). After several applications to the distal right bundle branch, transient complete atrioventricular block occurred (Fig. 3C); however, PVCs and PMVT still appeared. Because additional applications to the abnormal fascicular-Purkinje potentials carried the danger of permanent atrioventricular block, we abandoned the procedure. Fortunately, the bundle branch block was completely resolved, and QRS duration was normalized on the day after ablation.
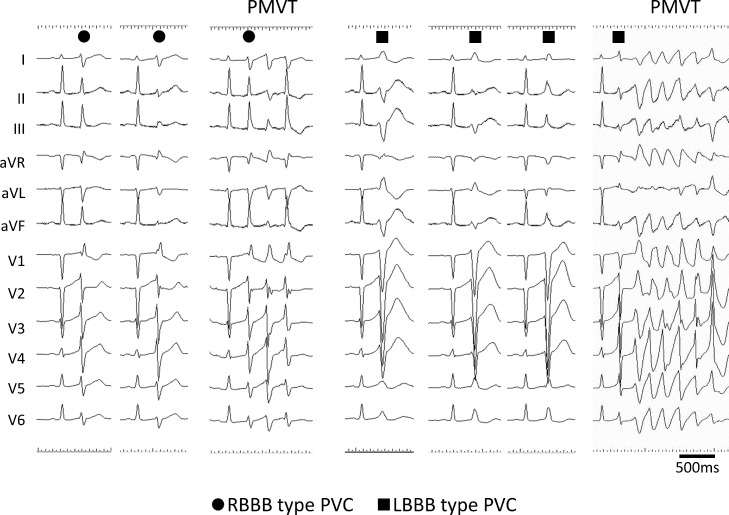
Fig. 1.
Surface electrocardiograms of premature ventricular contractions and polymorphic ventricular tachycardias. Various patterns of premature ventricular contractions (PVCs) were recorded; ● indicates PVC with right bundle branch block (RBBB) pattern, and ■ indicates PVC with left bundle branch block (LBBB) pattern. Polymorphic ventricular tachycardia (PMVT) was initiated from both RBBB- and LBBB-type PVCs. All PVCs appeared with a short coupling interval of 250 to 300 ms, manifesting R-on-T type.
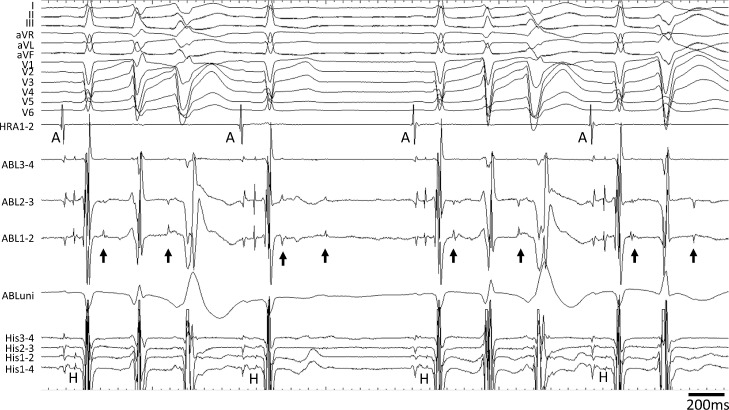
Fig. 2.
Frequent fascicular-Purkinje activity in left ventricle. Frequent fascicular-Purkinje activity (arrows) preceding the premature ventricular contractions was recorded during sinus rhythm in the left ventricle. The ablation catheter (ABL) was positioned at the proximal region of the left bundle. Some fascicular-Purkinje activity was blocked.
HRA, high right atrium catheter; A, atrial potential; H, His potential.
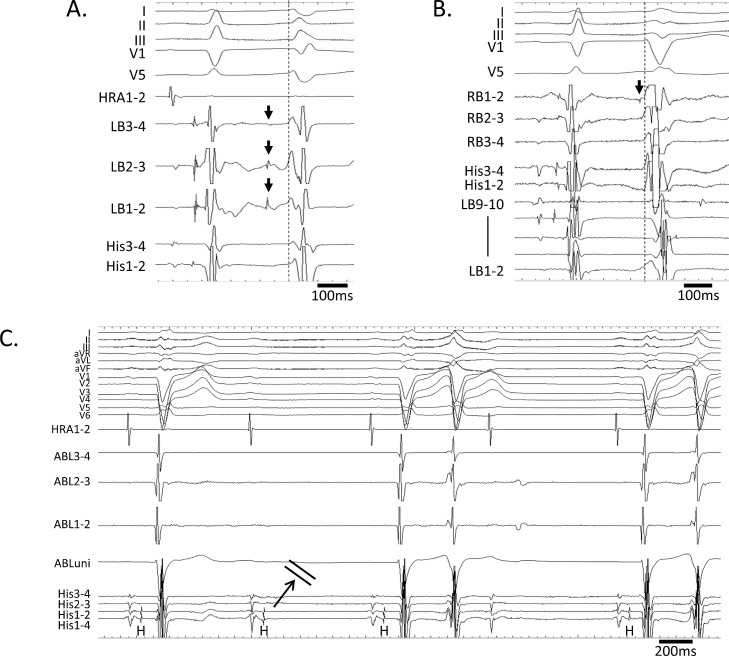
Fig. 3.
Premature ventricular contractions (PVCs) in both ventricles and atrioventricular block.
The earliest activation site of the fascicular-Purkinje ectopy (arrows) followed by the right bundle branch block type PVC was the proximal region of the left bundle (LB).
The earliest activation site of the fascicular-Purkinje ectopy (arrow) followed by the left bundle branch block type PVC was the right bundle (RB).
Transient His-ventricular block occurred by mechanical contact of the ablation catheter manipulating besides the RB.
Since the frequent PVCs and PMVTs remained after the procedure, quinidine (300 mg/day) and verapamil (240 mg/day) were administered orally, and the PVCs were markedly decreased while PMVT/VF completely disappeared. Either drug alone was insufficient to suppress PMVT/VF. During the follow-up period of 32 months, no ICD discharges were observed. Genetic testing revealed that the SCN5A variant was negative.
Discussion
This was a rare case of SCVF accompanied by abnormal fascicular-Purkinje activity in both ventricles, even though the SCN5A variant was negative. Catheter ablation failed to eliminate all abnormal fascicular-Purkinje activities and caused the development of transient complete atrioventricular block. Several antiarrhythmic drugs were found to be ineffective; however, the combination of quinidine and verapamil suppressed PVC/PMVT and controlled electrical storm.
Fascicular-Purkinje hyperexcitability and PVC/PMVT
Short-coupled R-on-T type PVC initiating PMVT/VF has been described in previous papers. Furthermore, a recent report proposed SCVF as a distinct primary arrhythmia syndrome [3]. On the other hand, frequent and intermittent fascicular-Purkinje activity in both ventricles has been thought to be associated with hyperexcitability of the fascicular-Purkinje system [1, 2]. Of note in the present case, given that PVCs in both ventricles were preceded by abnormal fascicular-Purkinje potentials, the ventricular arrhythmias might be associated with fascicular-Purkinje hyperexcitability, as in MEPPC. Several papers have described Purkinje-related PMVTs accompanying ischemia or SCN5A variants. However, the present case revealed no evidence of these etiologies, and it may represent what Haissaguerre et al. have advocated calling “Purkinjopathy” [4].
Catheter ablation for abnormal fascicular-Purkinje activity
In some cases of idiopathic VF, trigger PVCs are initiated from abnormal Purkinje activity. Catheter ablation for triggered PVCs is an option for the suppression of PMVT/VF recurrence [5]. However, intense ablation for abnormal fascicular-Purkinje ectopy sometimes causes complete atrioventricular block, as occurred in this case [6]. Moreover, the induction of critical PVCs that trigger PMVT and VF is generally difficult. Accordingly, elimination of PVC/PMVT with catheter ablation is not always successful in idiopathic VF.
Antiarrhythmic drugs for the abnormal fascicular-Purkinje activities
Itoh and Yamada reported that PMVT originating from the fascicular-Purkinje system could be successfully controlled by antiarrhythmic drugs, especially with sodium channel blockade like quinidine [6]. Another recent paper has reported that quinidine is effective in patients with SCVF [3]. The fascicular-Purkinje hyperexcitability accompanying ischemia or SCN5A variants also responds to quinidine [7, 8]. The reasons why quinidine, which is known as a strong blocker of transient outward potassium current, affects the fascicular-Purkinje hyperexcitability are proposed as that the transient outward potassium current channels in fascicular-Purkinje fibers are highly expressed with different kinetics from those of the surrounding myocardium [Online references 1, 2]. Additionally, verapamil has also been known as an effective drug inhibiting fascicular-Purkinje hyperexcitability and short-coupled variant of torsade de pointes in the setting of post-infarction or SCN5A variants as reported in numerous studies and guidelines [Online references 3-6]. In the current case, despite having no ischemia and no SCN5A variants, quinidine and verapamil appeared to reduce the fascicular-Purkinje hyperexcitability leading to the suppression of ventricular tachyarrhythmias, while neither drug alone was effective. We speculated that quinidine was the key player in suppressing fascicular-Purkinje hyperexcitability; however, if quinidine alone is ineffective or the dose cannot be increased due to side effects, adding verapamil is an option to be considered. Previous papers described the effective dose of quinidine for the cases of idiopathic ventricular fibrillation is usually 1,000 to 2,000 mg/day [9]. However, because Asians and Westerners have different physiques, the usage dose for Japanese is sometimes lower than 600 mg/day, same as the present case, for avoiding side effects [10 and Online reference 7]. This important issue needs careful consideration.
Conclusion
In conclusion, this case with SCVF demonstrates that abnormal fascicular-Purkinje activity resulted in PVC/PMVT in both ventricles, and combining quinidine and verapamil remarkably suppressed these potentially fatal arrhythmias. Because extensive fascicular-Purkinje hyperexcitability was suspected to be the mechanism, catheter ablation for the elimination of all trigger PVCs should be performed carefully to avoid complete atrioventricular block. In such a case, combined oral administration of quinidine and verapamil should be attempted to control electrical storm.
Disclosures
Dr Satoshi Nagase is affiliated with the endowed department by Japan Medtronic Inc. The remaining authors have nothing to disclose.
Declaration of Competing Interest
All authors have no conflict of interest.
This study was approved by the Institutional Review Board (IRB) of the National Cerebral and Cardiovascular Center, Suita, Japan (R21023).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgment
This work was supported by JSPS KAKENHI grant number 19K08572, Tokyo, Japan.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.12.002.
Appendix. Supplementary materials
References
- 1.Mann SA, Castro ML, Ohanian M, Guo G, Zodgekar P, Sheu A, Stockhammer K, Thompson T, Playford D, Subbiah R, Kuchar D, Aggarwal A, Vandenberg JI, Fatkin D. R222Q SCN5A mutation is associated with reversible ventricular ectopy and dilated cardiomyopathy. J Am Coll Cardiol. 2012;60:1566–1573. doi: 10.1016/j.jacc.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 2.Laurent G, Saal S, Amarouch MY, Beziau DM, Marsman RF, Faivre L, Barc J, Dina C, Bertaux G, Barthez O, Thauvin-Robinet C, Charron P, Fressart V, Maltret A, Villain E, et al. Multifocal ectopic Purkinje-related premature contractions: a new SCN5A-related cardiac channelopathy. J Am Coll Cardiol. 2012;60:144–156. doi: 10.1016/j.jacc.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg C, Davies B, Mellor G, Tadros R, Laksman ZW, Roberts JD, Green M, Alqarawi W, Angaran P, Healey J, Sanatani S, Leather R, Seifer C, Fournier A, Duff H, et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: a report from the CASPER registry. Eur Heart J. 2021;42:2827–2838. doi: 10.1093/eurheartj/ehab275. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Cheniti G, Escande W, Zhao A, Hocini M, Bernus O. Idiopathic ventricular fibrillation with repetitive activity inducible within the distal Purkinje system. Heart Rhythm. 2019;16:1268–1272. doi: 10.1016/j.hrthm.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haissaguerre M, Shah DC, Jais P, Shoda M, Kautzner J, Arentz T, Kalushe D, Kadish A, Griffith M, Gaïta F, Yamane T, Garrigue S, Hocini M, Clémenty J. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359:677–678. doi: 10.1016/S0140-6736(02)07807-8. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T, Yamada T. Multifocal ventricular arrhythmias originating from the His-Purkinje System: Incidence, characteristics, and outcome of catheter ablation. JACC Clin Electrophysiol. 2018;4:1248–1260. doi: 10.1016/j.jacep.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Viskin S, Chorin E, Viskin D, Hochstadt A, Halkin A, Tovia-Brodie O, Lee JK, Asher E, Laish-Farkash A, Amit G, Havakuk O, Belhassen B, Rosso R. Quinidine-responsive polymorphic ventricular tachycardia in patients with coronary heart disease. Circulation. 2019;139:2304–2314. doi: 10.1161/CIRCULATIONAHA.118.038036. [DOI] [PubMed] [Google Scholar]
- 8.Doisne N, Waldmann V, Redheuil A, Waintraub X, Fressart V, Ader F, Fossé L, Hidden-Lucet F, Gandjbakhch E, Neyroud N. A novel gain-of-function mutation in SCN5A responsible for multifocal ectopic Purkinje-related premature contractions. Hum Mutat. 2020;41:850–859. doi: 10.1002/humu.23981. [DOI] [PubMed] [Google Scholar]
- 9.Belhassen B, Viskin S, Fish R, Glick A, Setbon I, Eldar M. Effects of electrophysiologic-guided therapy with Class IA antiarrhythmic drugs on the long-term outcome of patients with idiopathic ventricular fibrillation with or without the Brugada syndrome. J Cardiovasc Electrophysiol. 1999;10:1301–1312. doi: 10.1111/j.1540-8167.1999.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 10.Chinushi M, Hasegawa K, Iijima K, Furushima H, Izumi D, Sato A, Aizawa Y. Characteristics of J wave-associated idiopathic ventricular fibrillation: role of drugs. Pacing Clin Electrophysiol. 2012;35 doi: 10.1111/j.1540-8159.2011.03066.x. e226-30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.