Abstract
Finding an easily accessible and reliable tool to diagnose the diseases collectively defined as ‘synucleinopathies’ is an urgent, unmet priority. The synucleinopathies include Parkinson's disease, multiple system atrophy, pure autonomic failure and dementia with Lewy bodies. There are millions of people who have a diagnosis of a synucleinopathy, with more diagnosed every year. With accessibility, ease of implementation, consistently high sensitivity (>80%) and specificity approaching 100%, skin biopsy has great potential as the clinical test of choice for the diagnosis of synucleinopathies. The large, multi-center Synuclein-One study will determine the sensitivity, specificity, accuracy and precision of α-synuclein detection within punch skin biopsies in patients with clinically established synucleinopathies using standardized, robust methods suitable for large-scale analysis.
Clinical Trial Registration: NCT04700722 (ClinicalTrials.gov)
Keywords: : Parkinson's disease, phosphorylated α-synuclein, skin biopsy, synucleinopathy
Introduction to the trial
The Synuclein-One study is an NIH-funded study supported through a Small Business Innovation Research grant to CND Life Sciences (aka Cutaneous NeuroDiagnostics). The Synuclein-One study is a large, multi-center study that will determine the accuracy, precision, sensitivity and specificity of the detection of phosphorylated α-synuclein within punch skin biopsies in patients with Parkinson's disease (PD), multiple system atrophy (MSA), dementia with Lewy bodies (DLB) and pure autonomic failure (PAF) and in healthy control subjects. All study procedures are standardized and are carried out in compliance with local and central institutional review board approval.
Background
Synucleinopathies are a group of neurodegenerative disorders that result from the deposition of phosphorylated α-synuclein within the central and peripheral nervous systems. Synucleinopathies include four overlapping disorders: PD, DLB, MSA and PAF. All synucleinopathies are characterized by the presence of the phosphorylated misfolded form of α-synuclein, resulting in progressive neurological degeneration.
Finding an easily accessible and reliable clinical tool to diagnose the diseases collectively defined as ‘synucleinopathies’ is an urgent, unmet priority. There are over 2.5 million people in the USA who have a diagnosis of a synucleinopathy, and approximately 180,000 are diagnosed every year [1–9]. All of these synucleinopathies are progressive disorders with increasing disability, and, with the exception of PAF, all of the synucleinopathies are uniformly fatal [1,10,11]. Even PAF has >10% risk of conversion to another synucleinopathy per year and therefore also carries a high mortality rate [1].
Unfortunately, thousands of individuals are undiagnosed or are misdiagnosed [12–14] because there is no simple clinical approach to defining a synucleinopathy [4–7]. Currently, a clinical evaluation by a movement disorder specialist (for PD and MSA), a movement or cognitive disorder specialist (for DLB) or an autonomic expert (for PAF and MSA) can provide the most accurate diagnoses [12,13,15]. However, the number of patients with synucleinopathies far exceeds the capacity of specialists, who often have long wait lists and are at great distances from patients, putting significant strain on general neurologists or primary care doctors who may struggle with a diagnosis, particularly in some of the atypical and complex cases [12,13,15]. In addition, even among experts, there is only modest diagnostic accuracy early in the disease [12,13].
In routine neurologic practice there may be no way to differentiate patients with a synucleinopathy from those with autoimmune, metabolic or toxic autonomic neuropathies or non-synucleinopathy degenerative diseases. Considerable effort has been made to improve the diagnostic certainty in synucleinopathies, but these investigations using brain imaging [16–18] and testing of bodily fluids [19,20] and tissues for phosphorylated α-synuclein [21–27] have resulted in only modest progress. An in vivo pathological marker of disease would also provide evidence of target engagement in clinical trials and accelerate progress toward disease modification.
There have been a number of studies published over the past 5 years that have identified skin biopsies as a potential effective source of material for detection of phosphorylated α-synuclein [28–32]. With consistently high sensitivity (>80%) and specificity approaching 100%, the potential for skin biopsy as the clinical tool of choice for the diagnosis of synucleinopathies appeared to be high. Publications have reported the use of both phosphorylated and non-phosphorylated α-synuclein as potentially relevant biomarkers. Non-phosphorylated α-synuclein is a ubiquitous protein and is noted in healthy subjects as well as disease states, but it does increase with disease progression. In contrast, phosphorylated α-synuclein has been shown to be 100% specific for disease compared with control subjects, but it may not correlate with disease severity. As a marker of disease, phosphorylated α-synuclein has been selected as the diagnostic tool of choice by most laboratories because of the very high specificity [28,30,33]. Unfortunately, the goal of the skin biopsy as an effective diagnostic or therapeutic biomarker was not been fully realized, and recent results from the synuclein systemic sampling (S4) study have raised doubts about the possibility of success using these approaches [34]. The S4 study reported a high specificity (100%) but a very disappointing sensitivity (24%) for the detection of phosphorylated α-synuclein using punch skin biopsies. A recent publication suggests that skin biopsy immunostaining for phosphorylated α-synuclein has the same high sensitivity and 100% specificity as real-time quaking-induced conversion (RT-QuIC) testing of cerebrospinal fluid [33].
Based on these conflicting results, the need to proceed with a definitive, large-scale study using proper methodologies for the detection of cutaneous phosphorylated α-synuclein was deemed necessary. The Synuclein-One study will focus on the detection of phosphorylated α-synuclein within punch skin biopsies in patients with clinically established synucleinopathies using standardized, robust methods suitable for large-scale testing to define sensitivity, specificity, accuracy and precision.
Study design
Study design & cohort
This is a multi-center, cross-sectional, observational study to evaluate phosphorylated α-synuclein pathology in cutaneous tissues in individuals with PD, DLB, MSA and PAF and in healthy controls at a single study time point. A total of 300 subjects with synucleinopathy will be included. A total of 200 non-synucleinopathy healthy control subjects will be recruited into the study. A total of 500 participants (300 with a clinically diagnosed synucleinopathy and 200 without synucleinopathy) will be recruited to participate. The estimated breakdown of the 300 synuclein study participants will be 105 with PD, 40 with MSA, 95 with DLB and 60 with PAF. The 200 healthy control subjects will be recruited by sex and age as follows: 20 of each sex by decile from ages 40–49, 50–59, 60–69, 70–79 and 80+. The cohort summary details are included in Table 1.
Table 1. . Synuclein-One study groups.
| Parameters | Healthy controls | Parkinson's disease | Multiple system atrophy | Dementia with Lewy bodies | Pure autonomic failure |
|---|---|---|---|---|---|
| Number | 200 | 105 | 40 | 90 | 65 |
| Age (years) | ≥40 | ≥40 | ≥40 | ≥40 | ≥40 |
| Diagnostic criteria | No neurologic disease | UKPDS Brain Bank | Gilman Criteria 2008 | 4th DLB Consensus | Orthostatic hypotension, abnormal Valsalva, bowel/bladder involvement |
Five groups will be included in this study: the four subgroups of synucleinopathy (Parkinson's disease, multiple system atrophy, dementia with Lewy bodies and pure autonomic failure) and a healthy control group.
DLB: Dementia with Lewy bodies; UKPDS: United Kingdom Parkinson's Disease Society.
Inclusion criteria for synucleinopathy subjects
Eligible men and women, age 40–99 years, will undergo a screening evaluation that includes a thorough medical history, physical and neurologic examination.
Eligible men and women will carry a clinical diagnosis of PD, MSA, DLB or PAF or be considered a healthy control at enrollment. A detailed review of the medical history, quantified neurologic examination scores and questionnaires may exclude some individuals who do not meet study criteria:
Diagnosis of PD by the UK Parkinson's Disease Society (UKPDS) brain bank diagnostic criteria [35];
Diagnosis of MSA by the Gilman Criteria [36];
Diagnosis of DLB by the 4th consensus report of the DLB Consortium criteria [37];
Diagnosis of PAF by chronic orthostatic hypotension (>1 year) with a fall in systolic blood pressure >30 mmHg from supine to standing position within 3 min; also required is either absent phase IV on Valsalva maneuver or at least one additional organ system involved (bowel, bladder) [38].
Inclusion criteria for healthy subjects
Eligible men and women, age 40–99 years, will undergo a screening evaluation that includes a thorough medical history, physical and neurologic examination.
Inclusion criteria will be healthy subject with no history of clinical exam or symptoms suggestive of synucleinopathy (including anosmia, constipation, mild cognitive impairment or dementia).
Exclusion criteria
Healthy individuals or individuals with synucleinopathy will be excluded from the study for the following criteria:
Clinical evidence of severe vascular disease (history of ulceration, poor wound healing, vascular claudication);
Clinically active coronary artery or cerebrovascular disease;
Current smoker or alcoholism;
History of allergic reaction to local anesthesia for skin biopsies;
Use of blood thinners (aspirin or clopidogrel alone is allowed);
Significantly impaired wound healing or history of scarring or keloid formation.
Healthy individuals or individuals with synucleinopathy are also excluded if disease may be explained by other causes:
Recent history of encephalitis;
Cortical dementia of Alzheimer's type;
Whipple's disease, toxin exposure;
Repeated head injury;
Stepwise disease progression suggestive of vascular etiology.
Study visits & participant data collection
All study-related testing can be completed during a single visit or broken into two visits over fewer than 2 weeks if participants/study staff prefer. Study visits will include an initial review of inclusion and exclusion criteria (Table 1). Qualifying participants will have questions answered and sign the informed consent to proceed with the study. A detailed review of medical history and current medications will be completed. A summary of the visit details is shown in Table 2.
Table 2. . Schedule of study events.
| Description | Study visit |
|---|---|
| Review and sign informed consent | X |
| Screening demographics | X |
| Review inclusion and exclusion criteria by disease state | X |
| Medical history | X |
| Social history | X |
| Family history | X |
| Medication review | X |
| General physical examination | X |
| General neurologic examination | X |
| MDS-UPDRS | X |
| Hoehn and Yahr | X |
| European Quality of Life 5D and VAS | X |
| Parkinson's Disease Questionnaire 39 | X |
| Orthostatic Hypotension Questionnaire | X |
| Montreal Cognitive Assessment | X |
| REM Sleep Behavior Disorder Questionnaire | X |
| Skin biopsies (three locations: distal leg, thigh, neck) | X |
The schedule events will occur during a single comprehensive study visit, although testing may be completed over more than 1 day for patient convenience.
MDS-UPDRS: Movement Disorder Society – Sponsored Revision of the Unified Parkinson's Disease Rating Scale; VAS: Visual analogue scale.
Quantified neurologic examinations
All participants will have standardized neurologic examinations using published methods. The specific examinations will be standardized with training for use and will include Movement Disorder Society – Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS) [39] and Hoehn and Yahr scores [40]. Orthostatic vital signs will be measured in all study subjects. Montreal Cognitive Assessment (MOCA) will be performed on all subjects [41].
Questionnaires
All participants will complete the following questionnaires: the European Quality of Life Instrument (EQ-5D and EQ-VAS) [42], Parkinson's Disease Questionnaire-39 (PDQ-39) [43], the Orthostatic Hypotension Questionnaire (OHQ) [44] and the REM Sleep Behavior Disorder Screening Questionnaire [45].
Standardization of data & tissue collection
In order to minimize potential variability in study outcomes, all tissue and clinical data will be obtained using standardized methods.
Skin biopsy samples
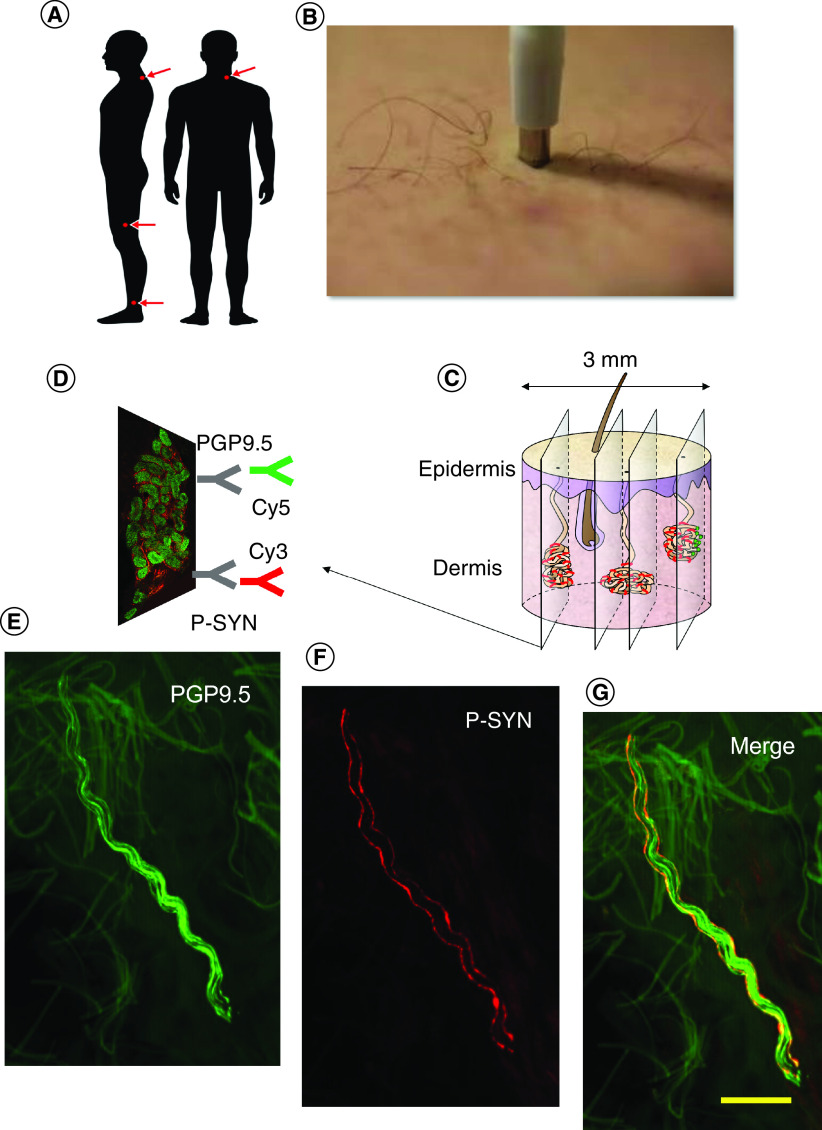
All subjects will have 3 mm punch skin biopsies taken from the distal leg 20 cm above the lateral malleolus, the distal thigh 20 cm above the lateral knee and the posterior cervical region 3 cm lateral to the C-7 spinous process, as shown in Figure 1. Biopsy locations are selected based on a combination of preliminary data and prior publications that demonstrate differential phosphorylated α-synuclein deposition in proximal and distal locations across the different synucleinopathies. The three sites the authors have selected provide maximal sensitivity and specificity across all four synucleinopathies [28,30,33,46,47]. Local analgesia will be provided using lidocaine with epinephrine (1:100,000). Skin biopsy samples do not require sutures and Band-Aids will be provided for wound care. Skin biopsy specimens will be fixed in Zamboni paraformaldehyde solution in pre-labeled tubes, placed into a standard shipping pack and shipped overnight for central processing. At the central processing site, biopsies will be washed and placed in a 20% glycerol cryoprotectant solution.
Figure 1. . Skin biopsy protocol.
In (A), three skin biopsies are taken from the distal leg, distal thigh and posterior cervical region using a 3 mm punch biopsy tool (B). The punch biopsy is sectioned into 50 μm sections (C). As shown in (D), the tissue sections are dual immunostained by protein gene product 9.5 (PGP 9.5), a pan-axonal marker, and by phosphorylated α-synuclein (P-SYN). The primary antibodies are labeled with immunofluorescent markers Cy3 and Cy5. The tissue is then viewed by confocal microscopy to identify nerve fibers stained by PGP 9.5 (E) by P-SYN (F) and the merged image (G) is used to confirm the presence of intra-axonal P-SYN.
Clinical data capture
All questionnaire responses, examination scores, and participant data will be acquired using clinical data capture. Supportive data (autonomic function testing, neuroimaging, prior clinical reports and so on) will be uploaded into the electronic data capture system.
Blinding & review of data
Skin biopsies
Skin biopsy specimens will be received at the central processing site. After cryoprotection, biopsy specimens will be blinded. Biopsy tissue will then move to the cutting stage, where 50 μm sections will be obtained using a cryostat. Half of the skin sections from each biopsy will then be sent to CND Life Sciences and Beth Israel Deaconess Medical Center (BIDMC) for immunostaining and blinded review.
At each laboratory (CND Life Sciences, BIDMC) six skin biopsy specimens from each biopsy will be dual immunostained with rabbit anti-protein gene product 9.5 (Cederlane: 1:1000) and phosphorylated α-synuclein (Wako: 1:1000) mounted on glass slides as previously reported [28]. An initial review of slide quality will be performed by review with an immunofluorescent microscope. Slides will be re-stained if there are deficiencies noted (fewer than six tissue sections available for view, damage to sections during processing or mounting, excessive background florescence and so on).
Confocal imaging
All stained sections will be examined under a fluorescent microscope (Zeiss-Axioplan2; Carl Zeiss), with areas of interest imaged by confocal microscopy (Zeiss LSM5 Pascal Exciter; Carl Zeiss). A series of images of optical sections will be acquired at 2 μm intervals throughout the depth of the 50 μm section as a Z-stack (Lens Plan – Apochromat 320/0.8; Carl Zeiss).
Review and grading of phosphorylated α-synuclein deposition for all tissue sections will follow the authors' previously reported standards, using strict blinding measures for all readers, and both laboratories including slide randomization, random number generation and blinding between reviewers and study staff [28]. Each biopsy will undergo quantitative measurement of phosphorylated α-synuclein (P-SYN) deposition by measuring the region. The number of positive fibers will be noted for each cutaneous dermal structure in each tissue section. Intra-epidermal nerve fiber density (IENFD) will be calculated using standard methodology using 50 μm fluorescent immunostained nerve fibers. Results are reported as nerve fibers/mm. Results will be entered into the electronic data system to maintain blinding. After counting, slides from one laboratory will be shipped to the other laboratory for re-reading. Thus, each biopsy will undergo four blinded reviews, with two reviews of each slide (each laboratory will generate its own set of slides) for inter-laboratory and inter-reader reliability testing.
Consensus diagnosis of synucleinopathy
The referring physician who examines the subject will indicate the presumed diagnosis (PD, MSA, DLB, PAF, healthy control). The subject history, examination scores, medical records and ancillary test data will then be sent for central review by two disease experts to confirm the subject's diagnosis of synucleinopathy (PD, MSA, DLB, PAF or HC). In the setting of a disagreement, the subjects will be excluded from primary review. Subjects will then be moved to secondary end point analysis of patients with unclear clinical diagnoses.
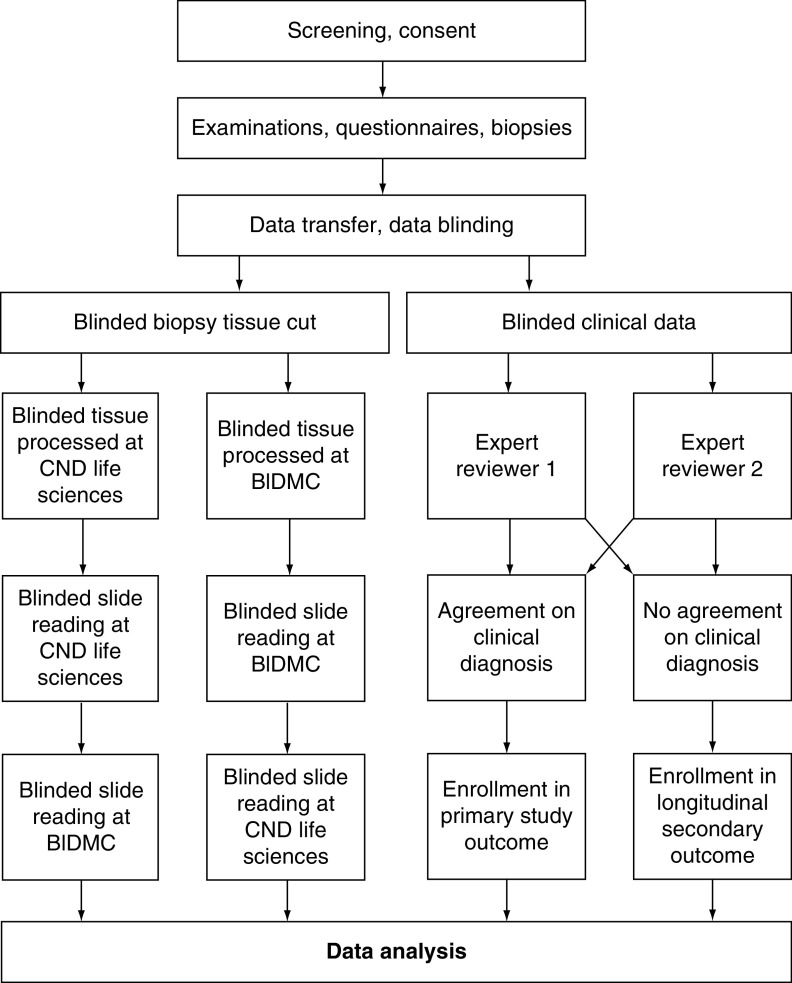
The flow of study data (biopsies and clinical information) is shown in Figure 2.
Figure 2. . Study outline for clinical and pathological analysis of data.
Data flow through the course of the study.
BIDMC: Beth Israel Deaconess Medical Center.
Objectives
The goal of this study is to define the utility of P-SYN pathology in the diagnosis of the synucleinopathies through qualitative and quantitative measurements of cutaneous P-SYN in patients with PD, MSA, DLB and PAF. The primary objective is to define the sensitivity, specificity, accuracy and precision of cutaneous P-SYN in the diagnosis of the synucleinopathies. A secondary objective is to differentiate between the synucleinopathies by quantitative measurement of P-SYN by location within the skin.
Statistical analysis & blinding
General statistical considerations
Descriptive statistics will be used to describe all demographic information and clinical examination findings for all groups (synucleinopathy, healthy control) and synucleinopathy subgroups. For continuous variables, data will be reported as mean, standard deviation, median, minimum, maximum and inter-quartile range. For categorical variables, the percentages of subjects in each group will be provided. Comparison of demographic and clinical features across groups will be performed by using t-tests and/or chi-square tests, as appropriate.
Sensitivity, specificity, accuracy and precision will be calculated using standard methodologies. Separate 2 × 2 tables will be formed for each lab and for each rater in order to measure the influence of sources of variation on classification end points (i.e., sensitivity, specificity, accuracy and precision). Grading information will be used to select cutpoints that can classify disease into subclasses. The study will explore the utility of classification and regression trees (CARTs) to select optimal cutpoints. The data will be divided into a training set to define the cutpoints and a test set to evaluate the outcome on data not used in the training set. The study will also include demographic information, vital signs, clinical information and quantitative P-SYN into CARTs to improve differentiation between synucleinopathy subtypes. A nested random effects model will be used to measure the sources of variation in making classifications. The model will identify and measure sources of variability in imunohistochemical staining (between different laboratories) and reading (between different reviewers) on outcomes.
Follow-up data will determine the correlation between final clinical diagnosis and pathological diagnosis over time. Survival analysis methods will be used to identify variables that may affect time to disease for those whose symptoms suggested pre-onset or tendency to disease.
Sample size justification
Based on previously published data [28,30,32,48–51], and the preliminary data acquired for this study, to determine if P-SYN has the minimally acceptable test performance characteristics to differentiate synucleinopathy from non-synucleinopathy, the true positive fraction will be defined at 0.90 and the false-positive fraction at 0.01 [52]. With a two-sided alpha of 0.01 and a power of 0.95, the authors estimate that a total of 150 patients with synucleinopathy and controls are required to complete the primary objective of the study. To distinguish among the subtypes of synucleinopathy (PD, MSA, DLB, PAF), a total of 300 subjects with synucleinopathy are required for the second objective of the study with 90% power to define cutpoints between groups. The 200 healthy control subjects are required to provide adequate numbers for normative data across sex and age for an older population that has not been previously well characterized.
Conclusion
The Synuclein-One study is the largest investigation of cutaneous P-SYN detection across all four synucleinopathies. Although synuclein has been measured in many different tissues, including blood, spinal fluid, colonic tissue and submandibular tissue, there have been only reports of modest success to date [34]. Skin biopsies have been reported to have very high sensitivity and specificity for P-SYN detection across all the synucleinopathies, including PD, MSA, PAF and DLB [28,30,48,50]. However, questions have been raised about the potential utility of skin biopsy after the failure of the S4 study (S4) to replicate the results of multiple previous reports from different laboratories [34].
The success of any study is fundamentally tied to the methods employed. The S4 study used techniques optimized for central nervous system pathology, which may not have adequately detected P-SYN in the skin, especially when compared with other published methods [53], which continue to report very high sensitivity and specificity in the detection of cutaneous P-SYN [28,30,54]. The Synuclein-One study follows recommended technical approaches with paraformaldehyde-based fixation of biopsy samples and thick tissue specimens to maximize sensitivity. Co-localization of all P-SYN with pan-axonal marker PGP 9.5 will confirm that only intra-axonal synuclein is measured to ensure specificity of results.
The need for a validated, well-characterized, simple, reproducible marker of synuclein pathology has never been greater. The number of individuals with neurodegenerative diseases continues to grow at an enormous rate, and confusion between synuclein and non-synuclein pathologies continues to occur, resulting in incorrect medication choices, iatrogenic complications, poor prognostication and patient frustration [12,55]. The diagnosis of a synucleinopathy is based on clinical criteria. Even among disease specialists, clinical diagnostic accuracy may be only 70–90% [14,56,57]. The diagnostic challenge faced by primary doctors is greater, particularly in early disease, when diagnostic accuracy may be only 30% [13,56–59]. For example, in patients clinically diagnosed with MSA, at autopsy only 60% were MSA, and 18% were due to a non-synucleinopathy neurodegenerative disease (such as progressive supranuclear palsy) [55]. Delays in obtaining more comprehensive neurologic evaluations place significant physical and emotional strain on patients and carry prognostic implications. A simple clinical test that improves diagnostic certainty would aid in the care of patients by improving psychological and medical supportive care early in the course of disease, even in specialized movement disorder centers [60,61]. Furthermore, diagnostic accuracy is critical in defining appropriate populations for studies targeting disease-modifying strategies: for example the inclusion of patients with progressive supranuclear palsy in a PD or an MSA trial could reduce the power of the study, unbalance the study population and predispose a treatment to failure. In addition, a sensitive diagnostic test could allow for an early diagnosis, even potentially pre-motor in the case of PD or MSA [13]. Earlier diagnosis will facilitate future interventions with neuroprotective and disease-modifying therapies at a time when patients are most able to benefit from these interventions [62]. Third, a biomarker that correlates with disease progression may be used to determine the response to disease-modifying and neuroprotective strategies by quantitatively analyzing any change in α-synuclein deposition. Finally, the utility of a simple, widely applicable test to diagnose synucleinopathy would aid in the clinical care of patients by allowing for improved prognostic certainty and would permit patients to make better life plans [60].
Skin biopsy detection of P-SYN has become clinically available, but it is only one of several potential markers of synuclein pathology that clinicians and patients may have access to over the next several years. The Synuclein-One study will define the necessary large-scale validation of the technique, with strict blinding, reading and data review to ensure that both clinicians and patients can be sure that the results are both valid and relevant. The quantitative assessment of P-SYN deposition across all synucleinopathies in the Synuclein-One study may provide the necessary groundwork for future disease-modifying treatment studies, where quantitative outcomes of target engagement are necessary.
Executive summary.
Background
The search for a reliable, reproducible and inexpensive marker for peripheral phosphorylated α-synuclein (P-SYN) pathology has been a long and arduous journey.
P-SYN detection in skin is a promising biomarker of disease but trials have reported conflicting results.
The many failures to detect peripheral P-SYN have provided guidance on the necessary technical modifications that allow the current trial to proceed with high sensitivity and specificity.
Methods
The Synuclein-One study is a large, multi-center cohort study that, through strict blinding, well-defined pathological processes, rigorous inclusion and exclusion criteria and use of an expert panel for consensus clinical diagnosis, will establish cutaneous P-SYN detection as the biomarker of choice for synucleinopathies.
The trial will define the diagnostic features of a pathological specimen, including sensitivity, specificity, accuracy and precision.
Discussion
The Synuclein-One study is the largest investigation of cutaneous P-SYN detection across all four synucleinopathies.
The Synuclein-One study will define the necessary large-scale validation of the technique, with strict blinding, reading and data review to ensure that both clinicians and patients can be sure that the results are both valid and relevant.
Footnotes
Financial & competing interests disclosure
The study is supported by NIH 5R44NS117214. CH Gibbons has received research support from Grifols, serves as a scientific advisor for CND Life Sciences and holds stock options in CND Life Sciences. R Freeman has served as a scientific advisor or received research support from Akcea, Biogen, CND Life Sciences and Vertex and holds stock options in CND Life Sciences. B Bellaire is employed by CND Life Sciences. T Levine has served as a scientific advisor for Grifols and FFF Enterprises and holds stock options in Corinthian Reference Lab and CND Life Sciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All study procedures are standardized and are carried out in compliance with local and central institutional review board approval.
References
- 1.Kaufmann H, Norcliffe-Kaufmann L, Palma JA et al. Natural history of pure autonomic failure: a United States prospective cohort. Ann. Neurol. 81(2), 287–297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 70(11), 1396–1402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to Parkinsonism. JAMA Neurol. 70(7), 859–866 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch. Neurol. 69(5), 601–607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology 70(13), 1017–1022 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology 70(16 Pt 2), 1423–1430 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease? A prospective population-based long-term study. Neurology 75(14), 1270–1276 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Vanacore N. Epidemiological evidence on multiple system atrophy. J. Neural Transm. 112(12), 1605–1612 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Marras C, Beck JC, Bower JH et al. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis. 4, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 85(16), 1362–1367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrag A, Wenning GK, Quinn N, Ben Shlomo Y. Survival in multiple system atrophy. Mov. Disord. 23(2), 294–296 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Beach TG, Adler CH. Importance of low diagnostic accuracy for early Parkinson's disease. Mov. Disord. 33(10), 1551–1554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler CH, Beach TG, Hentz JG et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 83(5), 406–412 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adler CH, Beach TG, Zhang N et al. Clinical diagnostic accuracy of early/advanced Parkinson disease: updated clinicopathologic study. Neurol. Clin. Pract. 11(4), e414–e421 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma RB, Berg D, Stern M et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30(12), 1591–1601 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Stoessl AJ. Neuroimaging in Parkinson's disease: from pathology to diagnosis. Park. Relat. Disord. 18(Suppl. 1), S55–S59 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Godau J, Hussl A, Lolekha P, Stoessl AJ, Seppi K. Neuroimaging: current role in detecting pre-motor Parkinson's disease. Mov. Disord. 27(5), 634–643 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology 72(Suppl. 7), S21–S26 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Mollenhauer B, Zhang J. Biochemical premotor biomarkers for Parkinson's disease. Mov. Disord. 27(5), 644–650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with Parkinsonism: a cohort study. Lancet Neurol. 10(3), 230–240 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Pouclet H, Lebouvier T, Coron E, Neunlist M, Derkinderen P. Lewy pathology in gastric and duodenal biopsies in Parkinson's disease. Mov. Disord. 27(6), 708 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Pouclet H, Lebouvier T, Coron E et al. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson's disease. Neurobiol. Dis. 45(1), 305–309 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Pouclet H, Lebouvier T, Coron E et al. Analysis of colonic α-synuclein pathology in multiple system atrophy. Parkinsonism Relat. Disord. 18(7), 893–895 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Lebouvier T, Neunlist M, Bruley DV et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS ONE 5(9), e12728 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebouvier T, Chaumette T, Damier P et al. Pathological lesions in colonic biopsies during Parkinson's disease. Gut 57(12), 1741–1743 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Shannon KM, Keshavarzian A, Mutlu E et al. α-Synuclein in colonic submucosa in early untreated Parkinson's disease. Mov. Disord. 27(6), 709–715 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is α-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov. Disord. 27(6), 716–719 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Garcia J, Freeman R, Gibbons CH. Phosphorylated α-synuclein within cutaneous autonomic nerves of patients with Parkinson's disease: the implications of sample thickness on results. J. Histochem. Cytochem. 68(10), 669–678 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Illigens BM, McCormick MP, Wang N, Gibbons CH. α-Synuclein in skin nerve fibers as a biomarker for α-synucleinopathies. J. Clin. Neurol. 15(2), 135–142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donadio V, Doppler K, Incensi A et al. Abnormal α-synuclein deposits in skin nerves: intra- and inter-laboratory reproducibility. Eur. J. Neurol. 26(10), 1245–1251 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Donadio V. Skin nerve α-synuclein deposits in Parkinson's disease and other synucleinopathies: a review. Clin. Autonomic Res. 29(6), 577–585 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Donadio V, Incensi A, Del Sorbo F et al. Skin nerve phosphorylated α-synuclein deposits in Parkinson disease with orthostatic hypotension. J. Neuropath. Exp. Neurol. 77(10), 942–949 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Donadio V, Wang Z, Incensi A et al. In vivo diagnosis of synucleinopathies: a comparative study of skin biopsy and RT-QuIC. Neurology 96(20), e2513–e2524 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chahine LM, Beach TG, Brumm MC et al. In vivo distribution of α-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 95(9), e1267–e1284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes AJ, Ben Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study [published erratum appears in Neurology 1992 Jul;42(7):1436] [see comments]. Neurology 42(6), 1142–1146 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Gilman S, Wenning GK, Low PA et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9), 670–676 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKeith IG, Boeve BF, Dickson DW et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89(1), 88–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann H, Norcliffe-Kaufmann L, Palma JA et al. Natural history of pure autonomic failure: a United States prospective cohort. Ann. Neurol. 81(2), 287–297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goetz CG. [Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS): a new scale for the evaluation of Parkinson's disease]. Rev. Neurol. (Paris) 166(1), 1–4 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Goetz CG, Poewe W, Rascol O et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19(9), 1020–1028 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal Cognitive Assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov. Disord. 23(7), 1043–1046 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann. Med. 33(5), 337–343 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age. Ageing 26(5), 353–357 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin. Autonomic Res. 22(2), 79–90 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire – a new diagnostic instrument. Mov. Disord. 22(16), 2386–2393 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Doppler K, Antelmi E, Kuzkina A et al. Consistent skin α-synuclein positivity in REM sleep behavior disorder – a two center two-to-four-year follow-up study. Park. Relat. Disord. 86, 108–113 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Levine TD, Bellaire B, Gibbons C, Freeman R. Cutaneous α-synuclein deposition in postural tachycardia patients. Ann. Clin. Transl. Neurol. 8(4), 908–917 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donadio V, Incensi A, Rizzo G et al. A new potential biomarker for dementia with Lewy bodies: skin nerve α-synuclein deposits. Neurology 89(4), 318–326 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Doppler K, Jentschke HM, Schulmeyer L et al. Dermal phospho-α-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol. 133(4), 535–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donadio V, Incensi A, Piccinini C et al. Skin nerve misfolded α-synuclein in pure autonomic failure and Parkinson disease. Ann. Neurol. 79(2), 306–316 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Haga R, Sugimoto K, Nishijima H et al. Clinical utility of skin biopsy in differentiating between Parkinson's disease and multiple system atrophy. Park. Dis. 2015, 167038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pepe M. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press, Oxford, UK: (2003). [Google Scholar]
- 53.Visanji NP, Mollenhauer B, Beach TG et al. The systemic synuclein sampling study: toward a biomarker for Parkinson's disease. Biomark. Med. 11(4), 359–368 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Donadio V, Incensi A, El-Agnaf O et al. Skin α-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study. Sci. Rep. 8(1), 14246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koga S, Aoki N, Uitti RJ et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 85(5), 404–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes AJ, Daniel SE, Ben Shlomo Y, Lees AJ. The accuracy of diagnosis of Parkinsonian syndromes in a specialist movement disorder service. Brain 125(Pt 4), 861–870 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology 57(8), 1497–1499 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson's disease. Lancet Neurol. 5(1), 75–86 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Rajput AH, Rajput A. Accuracy of Parkinson disease diagnosis unchanged in 2 decades. Neurology 83(5), 386–387 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Plouvier AO, Olde Hartman TC, Boots LP, Bloem BR, van Weel C, Lagro-Janssen AL. Time intervals in diagnosing Parkinson's disease: the patients' views. Patient Educ. Counseling 98(6), 777–782 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Graebner AK, Tarsy D, Shih LC et al. Clinical impact of 123I-ioflupane SPECT (DaTscan) in a movement disorder center. Neurodegener. Dis. 17(1), 38–43 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov. Disord. 27(1), 54–60 (2012). [DOI] [PubMed] [Google Scholar]