Abstract
Metabolic reprogramming is typical in cancerous cells and is required for proliferation and cellular survival. In addition, oncoproteins of high-risk human papillomavirus (HR-HPV) are involved in this process. This study evaluated the relationship between glucose transporter I (GLUT1), lactate dehydrogenase A (LDHA), and monocarboxylate transporter type 4 (MCT4) expression and cervical intraepithelial neoplasia (CIN) and invasive cervical carcinoma (ICC) with HR-HPV infection. The protein expression was evaluated in women with CIN I (n=20), CIN II/III (n=16), or ICC (n=24) by immunohistochemistry. The protein expression was analyzed qualitatively by van Zummeren score and quantitatively by Image ProPlus 6 software. LDHA expression increases in HPV-16 infection. In the CIN I group, GLUT1 immunostaining has a 35% protein expression at the membrane level at more than two thirds of the epithelium, which increased by 21.25% more in CIN II/III in more than two thirds of the epithelium. While LDHA and MCT4 in CIN I mostly do not present immunostaining, or this was only limited to the basal stratum, this expression is increased in CIN II/III and ICC cases. The GLUT1, LDHA, and MCT4 expression increased in ICC. The overexpression in high-grade CIN with HR-HPV infection shows a higher risk for cervical carcinoma progression.
Keywords: altered expression, biomarker prognosis, cervical cancer, HPV
Introduction
Cervical cancer (CC) is the fourth most common cancer globally; approximately 604,127 new cases and 341,831 deaths are reported each year. In Mexico, it is the second leading cause of death from cancers that affect women, and the most recent estimates indicate that 4335 women die each year from this cancer. 1 According to the cytological and histological diagnoses, cervical squamous cell carcinoma progression occurs from squamous lesions called cervical intraepithelial neoplasia (CIN) or squamous intraepithelial lesions (SIL). The CIN I and CIN II/III classification focuses on cellular alterations, cell maturation loss, and lack of cell differentiation. Many factors are associated with CIN II/III progression; the most important is the high-risk human papillomavirus (HR-HPV) infection. HR-HPV persistence and viral integration are central factors in CC development. 2 A primary pathogenic mechanism of HR-HPV is mediated by E6 and E7 viral oncoproteins through cell cycle progression, apoptosis inhibition, DNA damage, and immune response evasion.3,4
The HR-HPV is involved in the metabolic reprogramming of cancer cells. The E6 protein binds to E6-associated protein (E6AP), a ubiquitin ligase that induces p53 degradation. Interestingly, p53 regulates genes diverse with many functions in cellular processes such as glycolysis and oxidative phosphorylation (OXPHOS). 4 Another mechanism powered by E6 HR-HPV is the Von Hippel-Lindau (VHL) degradation. Under normal conditions, VHL negatively regulates the activity of hypoxia-inducible factor (HIF) 1α; this prevents HIF1α from accumulating in the cytoplasm and translocation into the nucleus, where usually HIF1α binds to HIF1β and activates hypoxia response elements (HREs).5,6 HRE regulates numerous protein expression, and some of them play an essential role in glucose metabolism, such as glucose transporter I (GLUT1), lactate dehydrogenase A (LDHA), and monocarboxylate transporter type 4 (MCT4).7,8
GLUT1 expression increases under hypoxia conditions and alters the glucose metabolism, so the GLUT1 upregulation indicates a hyperglycolytic phenotype. 9 The LDHA enzyme is a crucial molecule for nicotinamide adenine dinucleotide (NAD+) production and glycolysis in cancer cells where the conversion of pyruvate to lactate occurs. 10 High lactate concentration causes extracellular acidification, promoting the expression of monocarboxylate transporters (MCTs). Few studies have determined the relationship of the expression of metabolic proteins with the development of CIN and HR-HPV infection. We aimed to evaluate GLUT1, LDHA, and MCT4 expression in CIN and invasive cervical carcinoma (ICC) samples with HR-HPV infection. These results will help characterize the lesions caused by HR-HPV and contribute to control and clinical follow-up of women with a risk of CC development.
Methods
Sample Selection
The Ethics Committee approved the research protocol of Universidad Autonóma de Guerrero under identification number 23/01/2019, and the participating women signed informed consent. Each specimen was sent for testing anonymously, which only included participant-specific numerical code and age information in its evaluation.
The population was grouped according to the colposcopic examination, and a cervical scraping from the exo-endocervical transformation zone was taken. The samples were fixed with 96% alcohol for 10 min. Subsequently, the cytological diagnosis was made by Papanicolaou test, and Bethesda classification was considered for diagnosis according to a certified cytotechnologist. The biopsy was obtained focused on the lesion site, and a certified pathologist made the histological analysis. A total of 20 CIN I samples and 16 CIN II/III samples from the Raymundo Abarca Alarcon hospital were included. In addition, 24 samples with a diagnosis of ICC were collected from the National Cancerology Institute and considered in this study.
DNA Extraction and Viral Genotyping
The DNA was extracted from cervical scrapes using the phenol-chloroform-isoamyl alcohol method 11 ; previous proteinase K digestion was conducted at 64C for 45 min, and the DNA recovered was diluted in DEPC (diethyl pyrocarbonate) water and quantified by spectrophotometry. A DNA purity minor to 1.6 absorbance at 260 nm and 280 nm was considered not suitable for viral genotyping.
According to the manufacturer, the DNA was subjected to an HPV genotyping assay using the INNO-LiPA HPV Genotyping Extra II assay (INNO-LiPA; Fujirebio Europe, Ghent, Belgium) instructions. This system amplifies a 65-bp fragment of the L1 open reading frame and enables the identification of 32 HPV genotypes including 13 high-risk types (HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, and HPV-68), 6 probable high-risk types (HPV-26, HPV-53, HPV-66, HPV-70, HPV-73, and HPV-82), and 13 low-risk or unknown risk types (HPV-6, HPV-11, HPV-40, HPV-42, HPV-43, HPV-44, HPV-54, HPV-61, HPV-62, HPV-67, HPV-81, HPV-83, and HPV-84.
Immunohistochemistry
Histological sections of 2 μm from paraffin-embedded cervical tissue were processed. Subsequently, the histological sections were adhered to electrocharged slides, deparaffinized at 99C for 15 min, and then rehydrated and placed in xylol for 10 min. The protein expression was determined using the Novolink Polymer (Leica Biosystems; Newcastle Upon Tyne, UK) detection kit. The GLUT1 [Glut1 antibody (A-4): sc-377228], LDHA [LDHA antibody (E-9): sc-137243], and MCT4 [MCT4 antibody (D-1): sc-376140] antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at 1:50 dilution. Histological sections were adhered to electrocharged slides, then deparaffinized at 99C for 15 min and rehydrated to antigenic recovery, which was performed using the citrate buffer (Leica Biosystems) pH 6.0 at 125C for 60 sec and then at 90C for 30 sec. Subsequently, it was incubated with 3% hydrogen peroxide in an immunostaining chamber (Shandon coverplate) for 5 min, followed by incubation with specific antibodies for each protein for 1 hr at room temperature. The secondary antibody coupled to biotin (post-primary) was added for 15 min. The Novolink polymer was added for 15 min, and then diaminobenzidine (DAB) was added for approximately 3–5 min; finally, counterstaining with Hill’s hematoxylin was done. Placenta, liver, and squamous cell carcinoma tissues were used as positive expression controls for GLUT1, LDHA, and MCT4, respectively; each control was selected according to the insert recommendations for each antibody; in addition, no primary antibody was used as a negative control.
Immunohistochemistry Evaluation
GLUT1 expression was confirmed by plasmatic membrane staining, 12 LDHA expression through cytoplasmatic or nuclear staining, 13 and MCT4 expression through plasmatic membrane staining. 14 Squamous epithelium staining was classified as positive and negative according to van Zummeren et al.’s 15 criteria. Score 0 was considered negative or that a staining pattern was limited to the basal cells of the deep stratum of the plane epithelium. Score 1 denotes positivity predominantly in the lower basal layer or lower one third of the epithelium. Score 2 denotes positivity in the lower two thirds of the epithelium up to the intermediate stratum. Score 3 was defined as staining throughout the thickness or staining more than two thirds of the epithelium.
Densitometric Analysis
Three to five images of each histological sample were captured using optic microscopy (Leica DM1000 LED; Leica Microsystems, Wetzlar, Germany) coupled to a digital camera (Leica EC3; Leica Microsystems) for quantification. The analysis of the images was carried out using Image-Pro Plus 6.0 (IPP6) software (Media Cybernetics; Silver Spring, MD). The optical density average was reported in arbitrary units.
Statistical Analysis
Qualitative variables are expressed in frequencies and were compared using Fisher’s exact test (age, first sexual intercourse, birth number, HR-HPV type infection, viral type, and immunostaining intensity) or Chi-square test (partner’s sexual number), according to the distribution of these variables in the groups analyzed. Linear regression models were performed to evaluate the association of the level of protein expression with the histopathological diagnosis (CIN and ICC). STATA v.14 software was used in the analysis, and a p value ≤0.05 was considered statistically significant.
Results
A total of 63 cervical tissues were considered for this study; however, three samples were removed due to tissue detachment; finally, only 60 tissues were suitable for analysis. The population was grouped according to the histological diagnosis. Significant differences were observed in a relationship with cytological diagnosis at age (p=0.013) and birth number (p=0.027). The age range in the population was between 21 and 64 years; CIN II/III and ICC groups presented an age greater than or equal to 46 years (38% and 37.5%, respectively), compared with the CIN I group where this age range represented only 10%; all patients with ICC mentioned having had at least one previous childbirth in comparison with CIN I and CIN II/III groups where at least one woman reported having a previous childbirth (Table 1). Approximately 60% of the women in this study referred to having had their first sexual intercourse before they were 18 years old. Also, 55% of patients with CIN I diagnosis, 43.7% of the CIN II/III group, and 54.5% of the ICC group mentioned an antecedent of multiple sexual partners (Table 1).
Table 1.
Population Characteristic in Relationship With the Histological Diagnosis.
| Variable | CIN I |
CIN II/III |
ICC |
P Value |
|---|---|---|---|---|
| n=20 (%) | n=16 (%) | n=24 (%) | ||
| Age a | ||||
| 21–35 | 12 (60) | 5 (32) | 3 (12.5) | 0.013 |
| 36–45 | 6 (30) | 5 (32) | 12 (50) | |
| ≥46 | 2 (10) | 6 (38) | 9 (37.5) | |
| First sexual intercourse a | ||||
| <18 | 13 (65) | 10 (62.5) | 15 (65.2) | 0.983 |
| ≥18 | 7 (35) | 6 (37.5) | 8 (34.8) | |
| Partners’ sexual number b | ||||
| 1 | 9 (45) | 9 (56.3) | 10 (45.5) | 0.754 |
| ≥2 | 11 (55) | 7 (43.7) | 12 (54.5) | |
| Birth number a | ||||
| 0 | 4 (20) | 3 (21.5) | 0 (0) | 0.027 |
| ≥1 | 16 (80) | 11 (78.5) | 24 (100) | |
| HR-HPV type infection a | ||||
| Unique | 6 (30) | 5 (31.2) | 12 (50) | 0.315 |
| Multiple | 14 (70) | 11 (68.8) | 12 (50) | |
Abbreviations: CIN, cervical intraepithelial neoplasia; ICC, invasive cervical carcinoma; HR-HPV, high-risk human papillomavirus.
P value was calculated by Fisher’s exact test.
P value was calculated by Chi-square test.
HR-HPV Genotype Frequency
The infection by multiple viral genotypes was detected with the most frequency in the CIN I (70%) and CIN II/III (68.8%) groups, whereas in the cancer group, it was only observed in 50% of cases (Table 1). Regarding the single infection, HPV-16 was found in 21.67% (13/60) and was the only viral genotype located in the three study groups, whereas the HPV-45 unique infection is exclusively seen in ICC. Finally, multiple infections were observed in all groups and are the most frequent combination in these cases (Table 2). Highlighting the 37 samples with numerous infections, HPV-16 is present in 59.45% (22/37) of the samples, and HPV-16 is found to a lesser extent in CIN I samples; see Supplementary Table 1.
Table 2.
Distribution of HPV Infection Concerning the Histological Diagnosis.
| Viral Type | CIN I |
CIN II/III |
ICC |
Total | P Value |
|---|---|---|---|---|---|
| n=20 (%) | n=6 (%) | n=24 (%) | n=60 (%) | ||
| 16 | 2 (10) | 3 (18.75) | 8 (33.33) | 13 (21.67) | |
| 18 | 0 (0) | 1 (6.25) | 0 (0) | 1 (1.67) | |
| 31 | 0 (0) | 1 (6.25) | 0 (0) | 1 (1.67) | |
| 39 | 1 (5) | 0 (0) | 1 (4.17) | 2 (3.33) | |
| 45 | 0 (0) | 0 (0) | 3 (12.50) | 3 (5.0) | |
| 51 | 2(10) | 0 (0) | 0 (0) | 2 (3.33) | 0.165 |
| 58 | 1 (5) | 0 (0) | 0 (0) | 1 (1.67) | |
| Multiple infection by HPV | |||||
| HR | 5 (25) | 4(25) | 7(29.17) | 16(26.67) | |
| HR + pHR | 4 (20) | 1 (6.25) | 0 (0) | 5(8.33) | |
| HR + pHR + LR | 2 (10) | 0 (0) | 1 (4.2) | 3 (5) | |
| HR + LR | 4 (20) | 4 (25) | 3(12.50) | 11 (18.33) | |
| HR + IR | 0 (0) | 0 (0) | 1 (4.17) | 1 (1.67) | |
| HR + X | 0 (0) | 1 (6.25) | 0 (0) | 1 (1.67) | |
P value was calculated by Fisher’s exact test. Abbreviations: HPV, human papillomavirus; CIN, cervical intraepithelial neoplasia; ICC, invasive cervical carcinoma; HR, high risk; pHR, probable HR; LR, low risk; IR, intermediate risk; X, unidentified viral type by INNO-LiPA.
The expression of GLUT1, LDHA, and MCT4 according to viral type was analyzed; the population was grouped into HPV-16, HPV-16 + MI, and HR-HPV ≠ 16; GLUT1 expression was generally strong in the three groups (92.3%, 91.7%, and 95.6%). The expression intensity of LDHA increases in the presence of HPV-16, and this relation shows statistical significance (p=0.041). Although MCT4 was found more often negatively or mildly in the three groups (76.9%, 54.2%, and 73.9%, respectively), there were no significant differences (Table 3); in this sense, LDHA expression is related to HPV-16. The expression of GLUT1 in lesions with multiple infections by HR-HPV was more often found to be moderate to strong. In contrast, the expression of LDHA and MCT4 in lesions with multiple infections was more often found to be mild or negative (Supplementary Table 2).
Table 3.
Immunostaining Intensity With the Viral Type.
| Intensity | HPV-16 n=13 (%) |
HPV-16 + MI n=24 (%) |
HR-HPV ≠ 16 n=23 (%) |
P Value |
|---|---|---|---|---|
| GLUT1 | ||||
| Negative/Mild | 1 (7.7) | 2 (8.3) | 1 (4.4) | 1.0 |
| Moderate/Strong | 12 (92.3) | 22 (91.7) | 22 (95.6) | |
| LDHA | ||||
| Negative/Mild | 4 (30.8) | 13 (54.2) | 17 (73.9) | 0.041 |
| Moderate/Strong | 9 (69.2) | 11 (45.8) | 6 (26.1) | |
| MCT4 | ||||
| Negative/Mild | 10 (76.9) | 13 (54.2) | 17 (73.9) | 0.281 |
| Moderate/Strong | 3 (23.1) | 11(45.8) | 6 (26.1) | |
P value was calculated by Fisher’s exact test. Abbreviations: HPV, human papillomavirus; MI: multiple infection including low-risk HPV and high-risk HPV; HR, high risk; HR-HPV≠ 16, high-risk HPV other than 16; GLUT1, glucose transporter I; LDHA, lactate dehydrogenase A; MCT4, monocarboxylate transporter type 4.
GLUT1, LDHA, and MCT4 Expression in Cervical Intraepithelial Neoplasia and Squamous Cell Carcinoma
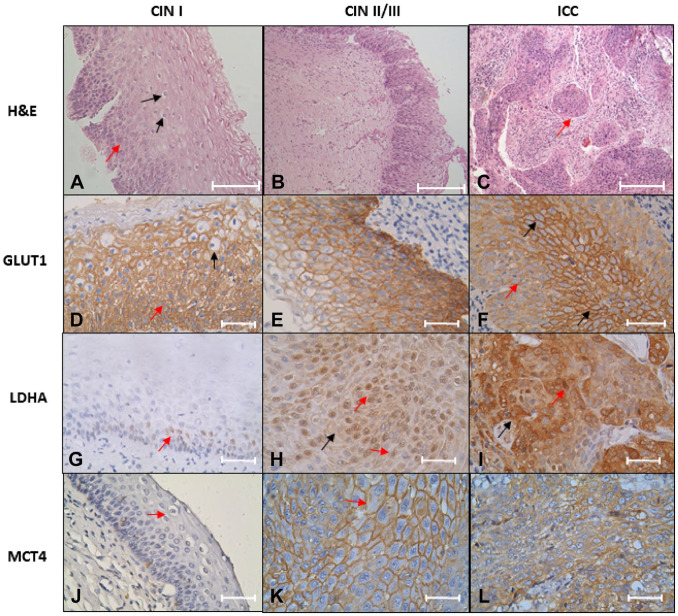
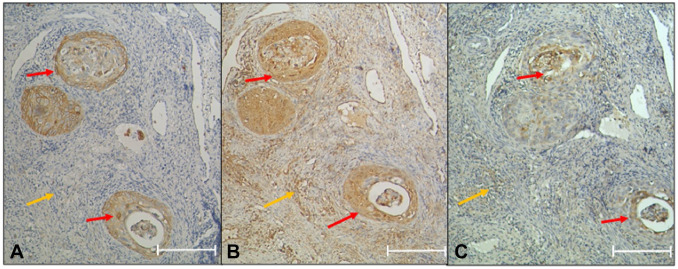
The immunostaining results in GLUT1 protein indicate that 35% of CIN I cases express the protein at membrane level in two thirds of the epithelium, and the other 35% express the protein at the membrane level in more than two thirds of the epithelium (Fig. 1D). Most CIN II/III (56.25%) express GLUT1 at the membrane level in more than two thirds of the epithelium (Fig. 1E and Table 4). In contrast, in the ICC group, all the patients express GLUT1 at the cellular level in the cervical stroma (Fig. 1F and Table 4). The LDHA expression was negative in 70% of the cases with a diagnosis of CIN I (Fig. 1G and Table 4). Only 25% of the patients expressed LDHA in more than two thirds of the epithelium at the nucleus/cytoplasm; 62.5% of the samples diagnosed with CIN II/III show a more remarkable expression in more than two thirds of the epithelium (Fig. 1H and Table 4). The ICC group has a 100% expression in cytoplasm and pleomorphic nuclei; also, tumoral nests invading the cervical stroma were observed (Fig. 1I and Table 4). Regarding MCT4, the findings were similar to LDHA; in CIN I and most CIN II/III cases, the expression was negative (90% and 60% respectively) (Fig. 1J and Table 4). In CIN II/III, only 26.7% express MCT4 in more than two thirds of the epithelium (Fig. 1K) and increased its expression in cancer (Fig. 1L and Table 4). Interestingly, all the cancer samples express the protein. Importantly, GLUT1 is negative at the stroma level, whereas LDHA and MCT4 are expressed at the stroma level in the cancer samples (Fig. 2). In brief, LDHA, GLUT1, and MCT4 expression is increased in ICC samples (Table 4).
Figure 1.
GLUT1, LDHA, and MCT4 expression in CIN/ICC samples. (A–C) H&E staining. (A) CIN I, dysplastic cell in one third epithelium (red arrow), and some koilocyte cells (black arrow) pathognomonic of HPV infection; (B) CIN II/III, dysplastic cells in all epithelium; (C) invasive non-keratinizing large cell squamous cell carcinoma, tumor nests, are observed (arrow red). Scale bar = 200 µm. (D–F) GLUT1 immunostaining; (D) two third epithelium expression and dysplastic cell (red arrow) and koilocytes (black arrow) with HPV-16, HPV-18,HPV-45, and HPV-6 infection; (E) expression profile in HPV-16-positive infection; (F) expression profile in pleomorphic cells with HPV-16 and HPV-52 infection; a diffuse expression is observed (red arrow). The expression on the cell membrane is present in the expression focus (black arrow). (G–I) LDHA expression; (G) a nuclear expression in the stratum basal cells (red arrow) in HPV-31 and HPV-52 infection samples; (H) nuclear expression (red arrow) and cytoplasm (black arrow) in HPV-39, HPV-51, and HPV-11 infections; (I) nuclear expression (red arrow) and cytoplasm (black arrow) in invasive squamous cell carcinoma, HPV-16 and HPV-39 positive. (J–L) MCT4 immunostaining; (J) CIN I with HPV-16 infection, and koilocyte (red arrow) was observed in the upper layer of epithelium; (K) cytoplasmic membrane of epithelium with multiple infections (HPV-31, HPV-59, and HPV-11) shows a high expression and prominent nucleolus (red arrow). (L) ICC HPV-45 positive indicates many nucleoli. Novolink Polymer. Scale bar = 200 µm for images A–C; scale bar = 50 µm for images D–L. Abbreviations: GLUT1, glucose transporter I; LDHA, lactate dehydrogenase A; MCT4, monocarboxylate transporter type 4; CIN, cervical intraepithelial neoplasia; ICC, invasive cervical carcinoma; HPV, human papillomavirus.
Table 4.
GLUT1, LDHA, and MCT4 Evaluation in CIN and ICC.
| Squamous Epithelium Expression | CIN I |
CIN II/III |
ICC |
P Value |
|---|---|---|---|---|
| n=20 (%) | n=16 (%) | n=24 (%) | ||
| GLUT 1 | ||||
| Negative or basal stratum | 0 (0) | 1 (6.2) | 0 (0) | <0.001 |
| 1/3 epithelium | 6 (30) | 3 (18.8) | 0 (0) | |
| 2/3 epithelium | 7 (35) | 3 (18.8) | 0 (0) | |
| >2/3 or all epithelium | 7 (35) | 9 (56.2) | 0 (0) | |
| Pleomorphic nuclei and tumor nests in the stroma | 0 (0) | 0 (0) | 24 (100) | |
| LDHA | ||||
| Negative or basal stratum | 14 (70) | 5 (31.2) | 0 (0) | <0.001 |
| 1/3 epithelium | 1 (5) | 1 (6.3) | 0 (0) | |
| 2/3 epithelium | 0 (0) | 0 (0) | 0 (0) | |
| >2/3 or all epithelium | 5 (25) | 10 (62.5) | 0 (0) | |
| Pleomorphic nuclei and tumor nests in the stroma | 0 (0) | 0 (0) | 24 (100) | |
| MCT4 | ||||
| Negative or basal stratum | 18 (90) | 9 (60) | 0 (0) | <0.001 |
| 1/3 epithelium | 2 (10) | 2 (13.3) | 0 (0) | |
| 2/3 epithelium | 0 (0) | 0 (0) | 0 (0) | |
| >2/3 or all epithelium | 0 (0) | 4 (26.7) | 0 (0) | |
| Pleomorphic nuclei and tumor nests in the stroma | 0 (0) | 0 (0) | 24 (100) | |
P value was calculated using Fisher’s exact test and p<0.05 was considered statistically significant. Abbreviations: GLUT1, glucose transporter I; LDHA, lactate dehydrogenase A; MCT4, monocarboxylate transporter type 4; CIN, cervical intraepithelial neoplasia; ICC, invasive cervical carcinoma.
Figure 2.
Invasive squamous cell carcinoma. The GLUT1, LDHA, and MCT4 expression levels are observed in tumor nests (red arrow). (A) GLUT1 negative in stromal cells (yellow arrow). (B) LDHA expression in stromal cells (yellow arrow). (C) MCT4 expression in stromal cells (yellow arrow). Novolink polymer. Scale bar = 200 µm. Abbreviations: GLUT1, glucose transporter I; LDHA, lactate dehydrogenase A; MCT4, monocarboxylate transporter type 4.
GLUT, LDHA, and MCT4 Expressions Are Associated With the Histopathological Lesion
Densitometric analysis was carried out to quantify the expression level. The results indicate that the expression of GLUT1, LDHA, and MCT4 increases in uterine CC, for which a linear regression analysis was carried out; we found that GLUT1 increases on average 17.5 units in ICC compared with CIN I, LDHA increases 48 units in CIN II/III. Concerning the CIN I group, it increases on average by 93 units in ICC compared with CIN I. MCT4 increases on average by 39.9 units in CIN II/III, whereas in ICC it increases by 128.1 units on average (Table 5). Finally, the global expression of GLUT1, LDHA, and MCT4 was analyzed; we found that 90% of the samples with CIN I showed negative or weak expression in two of the three proteins. In contrast, 56% of the CIN II/III samples show negative or weak expression in two of the three proteins. Interestingly, 58% of cancer cases present a moderate or strong expression in all three proteins (Table 6).
Table 5.
The Average Change in GLUT1, LDHA, and MCT4 Expression According to Degree of the Lesion.
| Protein | CIN II/III β (95% CI) |
P Value | ICC β (95% CI) |
P Value |
|---|---|---|---|---|
| GLUT1 | 7.6 (−0.5 to 15.6) | 0.064 | 17.5 (9.7–25.2) | <0.001 |
| LDHA | 48 (14.5 to 81.5) | 0.006 | 93 (60.7–125-1) | <0.001 |
| MCT4 | 39.9 (11.5 to 68.4) | 0.007 | 128.1 (100.5–155.61) | <0.001 |
CIN 1 group is the reference group and p≤0.05 is considered as statistically significant. Abbreviations: GLUT1, glucose transporter I; LDHA, lactate dehydrogenase A; MCT4, monocarboxylate transporter type 4; CIN, cervical intraepithelial neoplasia; ICC, invasive cervical carcinoma; β, regression coefficient, age-adjusted linear regression model.
Table 6.
Multiple Analyses Concerning Staining Intensity.
| Expression Level | CIN I n=20 (%) |
CIN II/III n=16 (%) |
ICC n=24 (%) |
P Value |
|---|---|---|---|---|
| Negative or weak in three proteins | 1 (5) | 1 (6) | 0 (0) | <0.001 |
| Negative or weak in two proteins and moderate or strong in the third protein | 18 (90) | 9 (56) | 1 (4) | |
| Negative or weak in one protein and moderate or strong in the others two proteins | 1 (5) | 6 (38) | 9 (38) | |
| Moderate or strong in the three proteins | 0 (0) | 0 (0) | 14 (58) |
P value was calculated through Fisher’s exact test and p<0.05 was considered statistically significant. Abbreviations: CIN, cervical intraepithelial neoplasia; ICC, invasive cervical carcinoma.
Discussion
Recent studies consider that metabolism alterations are one of the most crucial cancer hallmarks. 16 The capacity to maintain elevated metabolic rates of oxygen non-dependency, followed by an acid-lactic fermentation, is a distinctive feature of the cancer cells; this phenomenon is called the Warburg effect. 17 Nutrient and oxygen availability in the cellular microenvironment is limited during development and tumor progression. 18 This event causes a metabolic reprogramming to supply the energy requirements, 4 leading to the increase in biosynthetic intermediates such as nucleotides, amino acids, and lipids synthesis, which are necessary for survival and cellular proliferation. 19 The participation of certain viruses in the induction of metabolic reprogramming to promote cellular and viral replication has been described, 20 highlighting HR-HPV, mainly HPV-16 and HPV-18. The E6 and E7 oncoproteins of both viruses show the capacity to interact with MYC, which regulates glycolysis through genes such as LDHA and GLUT1, and with P53, which negatively regulates glycolysis through GLUT1 and GLUT4. 21 A recent study analyzed the expression of GLUT1 with HPV-16 positivity but did not find significant differences between a high or low expression of GLUT1 in the presence or absence of this viral type. 22 Therefore, we analyzed GLUT1, LDHA, and MCT4 metabolic proteins in CIN and ICC in the presence of HR-HPV, because not only HPV-16 but also the other oncogenic HPVs frequent in our population could be participating in this event.
Diverse risk factors have been related to CC progression. 23 Our results show an important relationship of age and birth number with CC (Table 1); however, other features such as first sexual intercourse or multiple sexual partners do not present significant differences between groups; small sample size could be the reason.
We report the distribution of seven HR-HPVs in the population; HPV-16 is the most frequent (Table 2). Previously, the report of a high prevalence of this viral genotype in the same geographic region has been documented. 24 Also, in biopsies of South American women with ICC, HPV-16 is the most common HPV detected. 23 We analyzed the intensity of expression concerning the viral infection [single infection by HPV-16, HPV-16 + another infection by HR-HPV or low-risk HPV (LR-HPV) and HR-HPV different from HPV-16]. We found that LDHA increased in the presence of HPV-16. However, GLUT1 and MCT4 did not show significant differences between groups, while previous reports have shown a positive effect at the level of translation mediated by HPV-16 oncoproteins in vitro.13,14 HPV-16 can promote, due to its oncogenic potential, the expression of metabolic genes and favor a glycolytic phenotype. Interestingly, HPV-16 variants can interact with multiple metabolic proteins favoring the Warburg effect. 25
We also analyzed the distribution of positivity in the thickness of the epithelium. In this sense, GLUT1 expression is present in the cellular membrane in two thirds of the epithelium and in all epithelium thickness in samples from women with CIN. Pinheiro et al. 14 reported no gradual increase in GLUT1 expression in cervical lesions. Still, in ICC cases, 100% of GLUT1 expression was reported, perhaps due to the requirement of new glycolytic intermediates for tumor formation. In addition, a high GLUT1 expression and HPV-16 infection are related to poor prognosis. 22 Furthermore, at the stroma level, the staining of GLUT1 was negative, and this characteristic is comparable to previous reports.26,27
Most samples classified as CIN I were LDHA-negative, and only a few samples expressed LDHA in the basal stratum. In contrast, many CIN II/III samples expressed LDHA in two thirds of the epithelium, and 100% ICC samples are LDHA-positive (Table 4). This finding could be helpful in the detection of lesions with a potential risk of progression due to cancer; the LDHA upregulation is associated with chemoresistance. 27 Considering their classic function related to metabolism, 28 we are expected to detect the protein expression at the cytoplasmic level. Nevertheless, the expression was nucleic; LDHA could modulate other processes in the nucleus. Nuclear translocation is caused by HR-HPV infection, namely by HPV-16, the leading viral genotype detected in the population studied. 13 Also, LDHA expression is present in the stroma of tumoral cells due to its essential role in the tumor microenvironment (TME); lactate from TME is an alternative substrate for oxygenated tumoral cells regulating cell proliferation.29,30 We found that MCT4 expression in lesions was mainly negative; only two positive samples for MCT4 in CIN I were found: one positive for HPV-16 and the other positive for HPV-16 and HPV-18, increasing in the cases of ICC. A study by Pinheiro et al. 31 found a significant association between HPV-18 infection and MCT4 expression in cervical adenocarcinoma, suggesting that the expression of LDHA and MCT4 is associated with a more aggressive phenotype. MCT4 expression was comparable to LDHA expression; the metabolism role explains the relation between the two proteins; a large amount of intracellular lactate induces MCT4 activation in tumors.32,33 MCT4 is an essential exporter of lactate. Its expression is promoted by the amounts of lactate in the intracellular medium, so exporting it to the extracellular medium could promote the evasion of the immune response, metastasis, and angiogenesis. 34
Finally, the expression intensity of the three proteins (GLUT1, LDHA, and MCT4) was integrated. The analysis showed an intriguing relation of the lesion progression with positivity in protein expression; no case of CIN has a moderate or strong expression of the three proteins. In contrast, most cancer samples (58%) have a moderate or strong expression of both proteins (Table 6). This crucial change could involve stroma invasion and metastasis of tumor cells.30,35,36
To our knowledge, this is the first integral analysis of GLUT1, LDHA, and MCT4 expression in CC; however, the major limitation of this study is the sample size. The sample size is an important characteristic that limited the finding of significant statistical associations between groups.
Metabolic reprogramming is a critical factor in cancer progression. The present study shows the relationship between the expression of GLUT1, LDHA, and MCT4 and the squamous cell carcinoma positive for HR-HPV. In the CIN II/III population with infection by HPV-16 and other HR-HPVs, the high expression of GLUT1, LDHA, and MCT4 confers a risk of progression to cervical carcinoma. We suggest strict monitoring and control in these patients.
The CIN I population showed 70% positivity for GLUT1 in two thirds of the epithelium. This finding is related to koilocytosis, which suggests that cell metabolism is affected by HR-HPV infection and can be used as a diagnostic marker. At the same time, LDHA and MCT4 were overexpressed in ICC, so their expression in CIN could be attributed to a more aggressive phenotype. However, we recommend expanding research on liquid-based cytology specimens to confirm that early CIN has phenotype similar to ICC with high expression of GLUT1, LDHA, and MCT4. In addition, the determination of the expression of E6 could help in having a comprehensive diagnosis and facilitate the therapeutic strategy.
Supplemental Material
Supplemental material, sj-docx-1-jhc-10.1369_00221554221101662 for GLUT1, LDHA, and MCT4 Expression Is Deregulated in Cervical Cancer and Precursor Lesions by Ma. A. Reyna-Hernández, Luz del C. Alarcón-Romero, Julio Ortiz-Ortiz, Berenice Illades-Aguiar, Marco A. Jiménez-López, Azucena Ocampo-Bárcenas, Martin O. Morrugares-Ixtepan and Francisco I. Torres-Rojas in Journal of Histochemistry & Cytochemistry
Supplemental material, sj-docx-2-jhc-10.1369_00221554221101662 for GLUT1, LDHA, and MCT4 Expression Is Deregulated in Cervical Cancer and Precursor Lesions by Ma. A. Reyna-Hernández, Luz del C. Alarcón-Romero, Julio Ortiz-Ortiz, Berenice Illades-Aguiar, Marco A. Jiménez-López, Azucena Ocampo-Bárcenas, Martin O. Morrugares-Ixtepan and Francisco I. Torres-Rojas in Journal of Histochemistry & Cytochemistry
Acknowledgments
We want to thank Abril Bautista Escutia, PhD, for her expert technical assistance.
Footnotes
Competing interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MAR-H and AO-B performed the immunohistochemistry, MAR-H analyzed the data and prepared the figures, LCA-R performed the cytological diagnosis in cervical intraepithelial neoplasm, BI-A carried out the molecular genetics studies, MAJ-L prepared figures and contributed to statistical analysis, MOM-I performed the histological diagnosis, and FIT-R designed the study, contributed to data analysis, and drafted the manuscript. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by “Secretaría de Eduación Pública” (NPTC) program 2018 (Grant No. UAGRO-PTC-071). The funders had no role in study design, data collection, analysis, publishing decisions, or manuscript preparation. During the development of the work, M.A.R.-H. received a master’s scholarship from Consejo Nacional de Ciencia y Tecnología (CONACYT; CVU: 834144).
ORCID iDs: Luz del C. Alarcón-Romero  https://orcid.org/0000-0002-2074-4889
https://orcid.org/0000-0002-2074-4889
Francisco I. Torres-Rojas  https://orcid.org/0000-0003-4303-8677
https://orcid.org/0000-0003-4303-8677
Contributor Information
Ma. A. Reyna-Hernández, Laboratorio de Citopatología e Histoquímica, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México
Luz del C. Alarcón-Romero, Laboratorio de Citopatología e Histoquímica, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México
Julio Ortiz-Ortiz, Laboratorio de Biomedicina Molecular, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México.
Berenice Illades-Aguiar, Laboratorio de Biomedicina Molecular, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México.
Marco A. Jiménez-López, Facultad de Ciencias Químico-Biológicas, Universidad Autónoma de Guerrero, Chilpancingo de los Bravo, México, and Anatomía Patológica, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México
Azucena Ocampo-Bárcenas, Laboratorio de Patología Molecular, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México.
Martin O. Morrugares-Ixtepan, Facultad de Ciencias Químico-Biológicas, Universidad Autónoma de Guerrero, Chilpancingo de los Bravo, México, and Anatomía Patológica, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México
Francisco I. Torres-Rojas, Laboratorio de Biomedicina Molecular, Instituto Estatal de Cancerología “Dr. Arturo Beltrán Ortega,” Acapulco de Juárez, México.
Literature Cited
- 1. GLOBOCAN. Available from: http://gco.iarc.fr/
- 2. Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. [DOI] [PubMed] [Google Scholar]
- 3. Senapati R, Senapati NN, Dwibedi B. Molecular mechanisms of HPV mediated neoplastic progression. Infect Agent Cancer. 2016;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martínez-Ramírez I, Carrillo-García A, Contreras-Paredes A, Ortiz-Sánchez E, Cruz-Gregorio A, Lizano M. Regulation of cellular metabolism by high-risk human papillomaviruses. Int J Mol Sci. 2018;19(7):1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y, Meng X, Ma J, Zheng Y, Wang Q, Wang Y, Shang H. Human papillomavirus 16 E6 contributes HIF-1α induced Warburg effect by attenuating the VHL-HIF-1α interaction. Int J Mol Sci. 2014;15(5):7974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iommarini L, Porcelli AM, Gasparre G, Kurelac I. Non-canonical mechanisms regulating hypoxia-inducible factor 1 alpha in cancer. Front Oncol. 2017;7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie H, Simon MC. Oxygen availability and metabolic reprogramming in cancer. J Biol Chem. 2017;292(41):16825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med. 2019;51(6):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadlecki P, Bodnar M, Grabiec M, Marszalek A, Walentowicz P, Sokup A, Zegarska J, Walntowicz-Sadlecka M. The role of Hypoxia-inducible factor-1 α, glucose transporter-1, (GLUT-1) and carbon anhydrase IX in endometrial cancer patients. Biomed Res Int. 2014;2014:616850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allison SJ, Knight JR, Granchi C, Rani R, Minutolo F, Milner J, Phillips RM. Identification of LDHA as a therapeutic target for cancer cell killing via (i) p53/NAD(H)-dependent and (ii) p53-independent pathways. Oncogenesis. 2014;3:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Wiley; 1994. [Google Scholar]
- 12. Seleit I, Bakry OA, Al-Sharaky DR, Ragab RAA, Al-Shiemy SA. Evaluation of hypoxia inducible factor-1α and glucose transporter-1 expression in non melanoma skin cancer: an immunohistochemical study. J Clin Diagn Res. 2017;11(6):EC09–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Guo JZ, Wang K, Ding W, Wang H, Liu X, Zhou S, Lu XC, Yang HB, Xu C, Gao W, Zhou L, Wang YP, Hu W, Wei Y, Huang C, Lei QY. Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth. Nat Commun. 2018;9(1):4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinheiro C, Garcia EA, Morais-Santos F, Scapulatempo-Neto C, Mafra A, Steenbergen RD, Boccardo E, Villa LL, Baltazar F, Longatto-Filho A. Lactate transporters and vascular factors in HPV-induced squamous cell carcinoma of the uterine cervix. BMC Cancer. 2014;14:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Zummeren M, Leeman A, Kremer WW, Bleeker MCG, Jenkins D, van de Sandt M, Heideman DAM, Steenbergen R, Snijders PJF, Quint WGV, Berkhof J, Meijer CJL. Three-tiered score for Ki-67 and p16ink4a improves accuracy and reproducibility of grading CIN lesions. J Clin Pathol. 2018;71(11):981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 17. Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104(3):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asgari Y, Zabihinpour Z, Salehzadeh-Yazdi A, Schreiber F, Masoudi-Nejad A. Alterations in cancer cell metabolism: the Warburg effect and metabolic adaptation. Genomics. 2015;105(5–6):275–81. [DOI] [PubMed] [Google Scholar]
- 20. Thaker SK, Ch’ng J, Christofk HR. Viral hijacking of cellular metabolism. BMC Biol. 2019;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prusinkiewicz MA, Mymryk JS. Metabolic control by DNA tumor virus-encoded proteins. Pathogens. 2021;10(5):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim BH, Chang JH. Differential effect of GLUT1 overexpression on survival and tumor immune microenvironment of human papilloma virus type 16-positive and -negative cervical cancer. Sci Rep. 2019;9:3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brebi P, Ili CG, Andana A, Menzel D, Lopez J, Guzman P, Melo A, Buchegger K, Roa JC. Frequency of human papillomavirus in women attending cervical cancer screening program in Chile. BMC Cancer. 2017;17(1):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alarcón-Romero LDC, Illades-Aguiar B, Flores-Alfaro E, Terán-Porcayo M, Antonio-Véjar V, Reyes-Maldonado E. AgNOR polymorphism association with squamous intraepithelial lesions and invasive carcinoma with HPV infection. Salud Publ Mex. 2009;51(2):134–40. [DOI] [PubMed] [Google Scholar]
- 25. Arizmendi-Izazaga A, Navarro-Tito N, Jiménez-Wences H, Mendoza-Catalán MA, Martínez-Carrillo DN, Zacapala-Gómez AE, Olea-Flores M, Dircio-Maldonado R, Torres-Rojas FI, Soto-Flores DG, Illades-Aguiar B, Ortiz-Ortiz J. Metabolic reprogramming in cancer: role of HPV 16 variants. Pathogens. 2021;10(3):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mendez LE, Manci N, Cantuaria G, Gomez-Marin O, Penalver M, Braunschweiger P, Nadji M. Expression of glucose transporter-1 in cervical cancer and its precursors. Gynecol Oncol. 2002;86(2):138–43. [DOI] [PubMed] [Google Scholar]
- 27. An JS, Huang MN, Song YM, Li N, Wu LY, Zhan QM. A preliminary study of genes related to concomitant chemoradiotherapy resistance in advanced uterine cervical squamous cell carcinoma. Chin Med J (Engl). 2013;126(21):4109–15. [PubMed] [Google Scholar]
- 28. Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol. 2016;26(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mishra D, Banerjee D. Lactate dehydrogenases as metabolic links between tumor and stroma in the tumor microenvironment. Cancers (Basel). 2019;11(6):750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinheiro C, Garcia EA, Morais-Santos F, Moreira MA, Almeida FM, Jubé LF, Queiroz GS, Paula ÉC, Andreoli MA, Villa LL, Longatto-Filho A, Baltazar F. Reprogramming energy metabolism and inducing angiogenesis: co-expression of monocarboxylate transporters with VEGF family members in cervical adenocarcinomas. BMC Cancer. 2015;15:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, Maira SM, Moroni C, Lane HA, Hall MN. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 2018;25(11):3047–58.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017;4(1):25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38(2):119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serganova I, Cohen IJ, Vemuri K, Shindo M, Maeda M, Mane M, Moroz E, Khanin R, Satagopan J, Koutcher JA, Blasberg R. LDHA regulates the tumor microenvironment via HIF-signaling and modulates the immune response. PLoS ONE. 2018;13(9):e0203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jhc-10.1369_00221554221101662 for GLUT1, LDHA, and MCT4 Expression Is Deregulated in Cervical Cancer and Precursor Lesions by Ma. A. Reyna-Hernández, Luz del C. Alarcón-Romero, Julio Ortiz-Ortiz, Berenice Illades-Aguiar, Marco A. Jiménez-López, Azucena Ocampo-Bárcenas, Martin O. Morrugares-Ixtepan and Francisco I. Torres-Rojas in Journal of Histochemistry & Cytochemistry
Supplemental material, sj-docx-2-jhc-10.1369_00221554221101662 for GLUT1, LDHA, and MCT4 Expression Is Deregulated in Cervical Cancer and Precursor Lesions by Ma. A. Reyna-Hernández, Luz del C. Alarcón-Romero, Julio Ortiz-Ortiz, Berenice Illades-Aguiar, Marco A. Jiménez-López, Azucena Ocampo-Bárcenas, Martin O. Morrugares-Ixtepan and Francisco I. Torres-Rojas in Journal of Histochemistry & Cytochemistry