Abstract
The hand endows us with unparalleled precision and versatility in our interactions with objects, from mundane activities such as grasping to extraordinary ones such as virtuoso pianism. The complex anatomy of the human hand combined with expansive and specialized neuronal control circuits allows a wide range of precise manual behaviours. To support these behaviours, an exquisite sensory apparatus, spanning the modalities of touch and proprioception, conveys detailed and timely information about our interactions with objects and about the objects themselves. The study of manual dexterity provides a unique lens into the sensorimotor mechanisms that endow the nervous system with the ability to flexibly generate complex behaviour.
The hand is the most versatile manipulative organ in the known universe. Manual behaviour is epitomized by virtuoso pianism or assembling a clockwork mechanism, but everyday activities — such as grasping an object of arbitrary shape or opening a bottle — are challenging for even the most sophisticated robots1. For a long time, the study of the neural basis of manual behaviour was hindered by the challenges of characterizing the shape of the hand and the forces it exerts on objects. Recent advances in pose estimation and sensor technology (for examples, see REFs2,3), however, have set the stage for achieving new insights into manual dexterity and its neural underpinnings.
In this Review, we first discuss aspects of mundane manual behaviours, such as grasping, and of expert ones, such as playing a musical instrument. We then describe anatomical features of the hand that are key to its versatility and strength. Next, we explore what is known about the neural mechanisms that give rise to manual dexterity, both motor and sensory, highlighting those that appear to be specific to the hand. Finally, we identify outstanding questions about the neural basis of manual behaviour, focusing on systems where hand-related specializations are liable to be found.
Manual dexterity
Dexterity is defined as skilled behaviour involving the hands, although the manual connotation is often lost. In this Review, we define dexterity as precise, diverse and flexible behaviour that involves the coordination of many segments and whose repertoire can be expanded through learning. Quantifying the dexterity of the hand is challenging given its myriad functions, but analysis of even simple, well-studied manual behaviours such as grasping reveals great sophistication. When we reach to grasp an object, we preshape the hand to its shape. The precision and specificity of this preshaping is such that one can predict which of many objects is to be grasped from the hand’s conformation, long before contact with the object is established4–7. Examination of this everyday manual behaviour reveals structure in hand postures. For example, the aperture of the hand is adjusted depending on the size of the object6, which involves the coordinated flexion and extension of joints distributed over the five digits. Similarly, the spread (abduction) of the fingers is adjusted semi-independently depending on the shape and size of the object. While the movements of the fingers tend to be coordinated, movements of the thumb tend to be more independent8,9.
Principal component analysis — which expresses hand kinematics in terms of correlated joint movements — reveals that manual behaviour during grasping can be well represented by a small number of principal components, called ‘synergies’5,6,10–15, which can be in part attributed to biomechanical constraints imposed by the musculoskeletal system16. This structure of prehensile hand movements has been interpreted as implying that the hand can only volitionally adopt a limited range of postures, confined to combinations of synergies. While this strategy simplifies the problem of controlling a hand, it also reduces the degrees of freedom of the hand — the number of (metaphorical) knobs required to control the hand.
An implication of the synergies hypothesis is that low- variance principal components simply reflect noise in the hand measurements or in the hand postures themselves. It turns out, however, that synergies fail to account for the exquisite precision of the hand. Indeed, the synergies hypothesis predicts that our ability to classify objects from precontact hand postures will break down after removal of the synergies (the first six to eight principal components), but this is not the case7,17. Rather, the object to be grasped can be predicted well above chance even after the first 20 synergies have been removed. In other words, the components of hand posture reflected in the low-variance principal components are under volitional control. If one were to control a hand with knobs, it would take more than 20 knobs to do so with the equivalent precision, and this is for grasping, a simple unskilled behaviour.
Skilled manual behaviour has not been studied as systematically as has grasping, hindered in part by contact with objects — prevalent during most manual behaviours and critical to understanding these — being difficult to measure. Nonetheless, simple metrics of skilled manual behaviour reveal the staggering rapidity and precision of the hand. Pianists can play more than 800 notes per minute with each hand18, skilled typists can type 600 characters per minute19 and professional gamers can execute 800 actions per minute20. Artists can draw submillimetre illustrations on a grain of rice or compose images of handwritten micrographic text21. Experienced Braille users can read more than 200 words per minute in Braille script, approaching the median visual rate of 250 words per minute22. Finger-tutters can fluidly execute remarkably complex hand postures23. These manual abilities are, however, acquired. Even moving individual fingers while keeping others still is difficult and does not come naturally8,24–28, and the finger individuation required for piano playing requires extensive training29–31.
The inclination to use one or the other hand is strongly asymmetric in most individuals, and the preferred hand is the right one in 90% of adult humans32, as is implied in the etymology of the word ‘dexterity’: dextra is the Latin word for ‘right hand’. By contrast, the distribution of handedness in other primates is symmetric33–35, suggesting that the prevalence of right-handedness in humans might be culturally inherited36, although innate differences are difficult to distinguish from acquired ones37. In adults, the differences in finger individuation between the dominant hand and the non-dominant hand are slight38,39 as are the differences in the performance of simple tasks40–42. However, the performance gap between the dominant hand and the non-dominant hand for complex tasks such as writing is much wider and cannot be totally overcome, even with extensive training43–45.
Anatomical complexity of the human hand
The neural basis of dexterity cannot be understood without considering the musculoskeletal structure of the hand. All manual control is enabled by hand bio-mechanics, which define what can and cannot be done. More broadly, the neural mechanisms of control have evolved in tandem with the musculoskeletal features of the hand to give rise to dexterity.
Bones
The skeleton of the hand comprises 27 bones: five metacarpals of the palm, including a divergent thumb metacarpal, two phalanges of the thumb, three phalanges in each other digit and eight carpal bones that connect the hand to the forearm46. The bones of the hand and wrist are connected by a complex network of passive ligaments that stabilize all the elements and constrain their relative movements. Each bone moves in relation to neighbouring bones, forming numerous anatomical joints that allow at least 24 actively articulated degrees of freedom, including three at the wrist (with forearm-based rotation), four at each finger (with flexion–extension and abduction–adduction at metacarpophalangeal joints) and five at the thumb — three of which are at the base (flexion, abduction and rotation). Several other joints, including carpometacarpal joints, are also semi-independent and may contribute additional degrees of freedom. Development of the hand takes a long time47. Although the hand bones appear prenatally, ossification of the carpal bones starts several months after birth, with some emerging at 10 years of age, and the hand and wrist are fully developed only at 16–18 years of age.
The main feature of the human hand is the opposability of the thumb, which allows precise and versatile prehension. Although definitions of opposability differ, the main characteristic is the ability for the thumb to oppose and touch each of the other fingers at the tip48,49. Many but not all primates are endowed with some degree of opposability, ranging from pseudo-opposable thumbs — common in New World monkeys and prosimians — to semi-opposable thumbs — present in Old World monkeys and apes — to fully opposable thumbs — present only in humans. While pseudo-opposable thumbs can flex–extend and abduct–adduct, semi-opposable thumbs also rotate axially during abduction, thereby bringing the pad of the thumb towards the pads of other fingers. The rotation is made possible by the specialized saddle shape of the trapezium, the carpal bone that supports the thumb metacarpal. Full opposability, defined by broad contact between the pads of the thumb and the other fingers, is made possible by a relatively long thumb50,51. Toes in most monkeys and apes are also opposable, and can be used for grasping, while human feet support bipedalism and comprise toes that are not opposable52. Non-primates also possess prehensile ability and even opposing fingers, but none of their digits exhibits the complexity of movement of the primate thumb.
Muscles and tendons
The hand is articulated by 20 muscles located in the forearm and 21 muscles located in the hand itself (FIG. 1; Supplementary Tables S1–S3)53. Some muscles — especially muscles that drive the digits — comprise several semi-independently controlled heads on the distal side26,54–56, increasing the number of controlled actuaors to 30 in the forearm and 22 in the hand, for a total of 52. Of these, 13 actuators articulate mainly the wrist, 11 the thumb and 28 the fingers.
Fig. 1 |. Hand musculature.
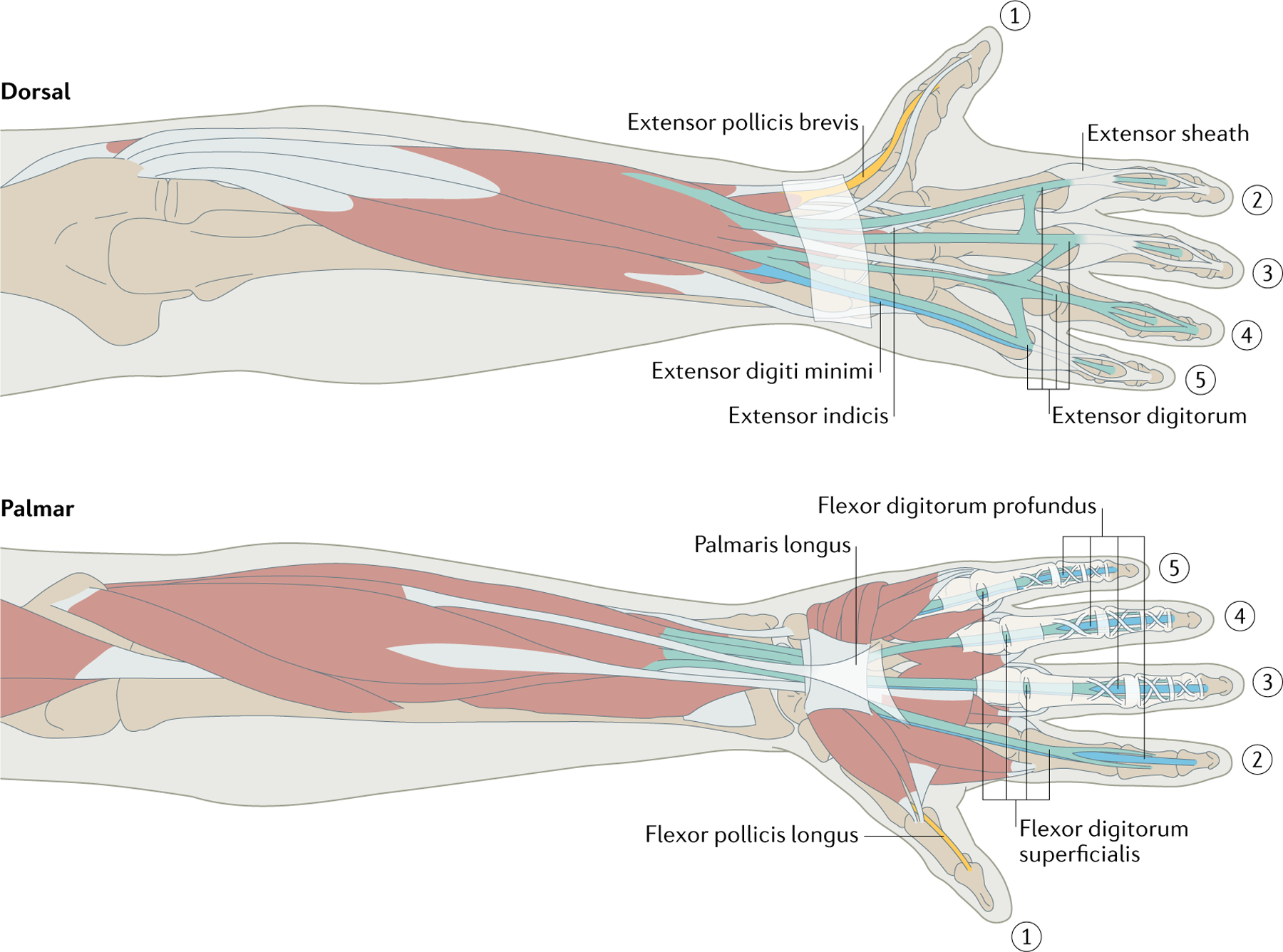
Digits 2–5 are articulated mainly by two flexors — note how the deep one (blue) threads through the superficial one (green) at the proximal phalanx — and one extensor, which feeds into the extensor sheath. Intrinsic hand muscles also feed into the sheath, flexing the proximal finger joint and extending the other. Extensor indicis and extensor digiti minimi contribute further independence to index finger and little finger extension. Flexor pollicis longus and extensor pollicis brevis are two thumb muscles that are found only in humans and two other primate species. Palmaris longus lies outside the carpal tunnel and is absent in many people. Each muscle path is complex, especially around the wrist and thumb, wrapping around other moving muscles and bones, so estimating the action of these muscles is difficult. Some hand muscles and other tissues are omitted for clarity. In addition, the extensor sheath is omitted on the dorsal view of digit 4 and the connective tissue is omitted on the palmar view of digit 2 to reveal the underlying tendon paths. Image courtesy of Kenzie Green.
Attachment of muscles to finger segments is different for flexors and extensors. Finger flexors attach their tendons directly to finger phalanges, with the deep flexor tendon threading through the superficial tendon to attach on the distal phalanx, allowing differentiated control of the distal joints and high strength. In addition to flexion, flexor muscles also adduct the fingers, bringing them together for a tight grasp. By contrast, extensors feed into the joined extensor sheath, which covers the dorsal aspect of each digit57. Pulling on one side of the sheath or the other deviates the finger into abduction or adduction58,59. Pulling both sides allows simultaneous flexion of the metacarpophalangeal joint and extension of interphalangeal joints. Moreover, the distal tendons of the extensors are interconnected, which limits finger individuation during extension60.
The small internal hand muscles (lumbricals and interossei) are located mostly between metacarpals and distally attach to phalanges and to the extensor sheath. Lumbricals originate not from bones but from the tendons of the large deep finger flexors of the forearm. Although these muscles can transfer the tension caused by flexion to the extensor hood, their weakness and dense sensory innervation suggest that their primary role may be to act as high-precision sensors of hand posture61. The stronger palmar interosseus muscles also induce rotation of the fingers, which is critical for grasping.
The complexity of the musculature and of its attachments to the bones leads to a complex relationship between joint movement and muscle activation62–67. Many, if not all, hand muscles act across multiple joints, so torques produced around each joint must be counter-balanced to achieve desired joint postures. For example, finger flexion needs to be balanced by wrist extension torque. Antagonistic muscles are commonly activated together during hand movements to stiffen a joint against unwanted perturbations62,68–73. Furthermore, the torque-generating properties of muscles crossing a joint depend on the posture of that joint and that of all other joints the muscle crosses, further complicating the control problem74–80. Even simple hand postures or end point forces thus require the coordination of many muscles with articulations distributed over the entire hand.
In humans, a power grip entails a total maximum force of around 550 N, concentrated at the proximal metacarpophalangeal joints81,82. Digits 1, 2 and 3 can produce more force on average (120–137 N) than can digits 4 and 5 (57–96 N). Extrinsic finger flexors and extensors generally produce much higher forces (up to 20-fold) than intrinsic hand muscles74,83, although this difference is more pronounced for some hand postures than others.
Hand variations
Sex differences in human hand anatomy and manual abilities are restricted mostly to variation in size84–87. Index fingers in males are more commonly shorter than ring fingers88,89, and carpal bones differ subtly in shape and relative size, which may slightly affect the range of motion and dynamics of the digits90–94. Beyond sex, variability of the hand musculature between humans is not restricted to the volume, but commonly includes full separation of muscle compartments, additional heads, shifts in attachment points, disappearance of common muscles and reappearance of atavistic muscles95–100.
Although the human hand is often described as unique in the animal kingdom for its complexity and versatility (BOX 1), many of its special muscular features are also found in the hands of apes and monkeys53,101,102 (but see REF.103). For example, two thumb muscles that were thought to be unique to humans were later identified in gibbons and bonobos104,105. Similarly, humans share the anatomy of muscles that allow independent extension of index and little fingers with gorillas and chimpanzees106. Humans have fewer intrinsic hand muscles and, in some ways, possess a simplified primate hand. The increased complexity of non-human primate hands stems from the use of hands in locomotion — in trees or on the ground48 — a function that human hands lack. The feats of human hands — such as playing the piano or assembling a clockwork mechanism — are enabled by superior neural control but also benefit from the narrower scope of manual behaviour in humans.
BOX 1 |. Rodent hands.
Rodents are a popular model to study motor control406–410. Although the hand and arm musculature of rodents is grossly similar to that of primates, it differs in a number of ways98. First, superficial and deep finger flexors are not as well separated. Second, intrinsic hand muscles have vastly different anatomy. Third, rats have fewer muscles to articulate their (diminutive) thumbs. The rodent wrist also has fewer degrees of articulation because of fused bones46. By contrast, rats have as many little finger muscles as primates, reflecting the importance of this digit for grasping small objects407.
Rodents adjust hand aperture on the basis of the size of an object and can perform one-handed grasps, but object manipulations typically involve both hands and the mouth and are often performed without visual guidance406,410,411. Some level of finger individuation (mostly of the little finger) is observed when the hand approaches a food pellet408 but much less so than is measured in primates24,135. Furthermore, the success rate of prehension is usually much lower for rodents (~40–50%) than primates, even after extensive training.
Cortical control of the hand
Cortical magnification
Given the sophistication of the hand and of manual behaviours, it should come as no surprise that large swaths of the nervous system are devoted to controlling it107–113. The primary motor cortex (M1) — the region of the neocortex that sends signals to the muscles via the spinal cord to drive movement — is coarsely organized in a somatotopic fashion, in which different parts of the brain contribute to movement of different parts of the body. The hand occupies an outsized proportion of the motor homunculus — the map of the body in M1. While the hand accounts for 0.6% of the body by weight and 2% by surface area, more than 20% of M1 is devoted to it as gauged by the tendency to evoke finger movements via electrical stimulation112,114–117, and this phenomenon is even more pronounced on the side contralateral to the dominant hand118–120. In primates, and in particular higher primates, electrical stimulation of large swaths of the cortex outside M1 also evokes hand movement, implying widespread neuronal circuitry to support manual control111,121.
Hand control signals in the cortex
M1 plays a critical role in dexterous manual behaviour as evidenced by the fact that lesions of M1 produce severe motor deficits, as do lesions of the descending pyramidal tract, the principal relay of motor signals from the cortex to muscles122–128 (reviewed in REF.129). Over time, monkeys and humans recover the ability to grasp objects, but dexterous control and individuated finger movements never return, as precise spatio-temporal patterns of muscle activation remain disturbed130 and overly synchronized131. In humans, extensive rehabilitation in patients with pure motor hemiparesis can lead to partial recovery of thumb and index finger independence, but the selectivity of muscle recruitment never fully recovers132,133.
The principles that underlie the coding structure in the M1 hand representation are unclear. Individual neurons in M1 drive movement across multiple joints distributed over the entire hand, often including digits and the wrist110,134,135. The tendency of individual neurons to drive combinations of joints cannot be explained solely by the proximity of the joints, their tendency to move together or their muscular articulation110,135,136. These response properties reflect both the aforementioned complexity of the muscular articulation of the hand and the fact that individual pyramidal neurons in M1 project to multiple spinal motor neuron nuclei137. As might be expected given the prevalence of multi-joint response fields, the somatotopic organization in the M1 hand representation — and across the motor homunculus — is coarse. The hand representation in M1 overlaps with that of the proximal limb, and representations of digits and the wrist are inextricably intertwined138,139.
Proximal limb-related neurons in M1 produce a strong phasic response during movement and a much weaker tonic response during maintained posture. By contrast, hand-related neurons encode the time-varying posture and do not exhibit a preference for movement135. Another difference lies in the dynamics of the responses at the population level. When monkeys perform reaching movements, population responses in M1 exhibit smooth dynamics; that is, the present neural state predicts the future neural state140. This population-level behaviour is consistent with a role for M1 as a pattern generator that ultimately drives muscles to give rise to movement. However, M1 does not exhibit smooth dynamics during grasping movements when the reach component is eliminated, for example when catching or grasping an object that is handed to you141. Population dynamics during reach to grasp seem to be intermediate between those of reach and those of grasp142,143. The population-level response, then, is very different for manual behaviour than it is for its proximal limb counterpart. One possible explanation for this observation is that these differences in neuronal activity — at the single-cell and population levels — reflect fundamentally different control strategies for arms versus hands. Another possibility is that they reflect differences in the biomechanics of the effectors: arms are much heavier than fingers, so arm movements may require characteristically different muscle activations, which in turn involve different neuronal dynamics in M1.
M1 participates not only in controlling hand movements but also in controlling the manual application of forces. During reach, corticospinal input is strongest for forearm muscles, but as fingers close around the object, the focus shifts to intrinsic hand muscles144. Neurons in M1 exhibit a transient burst of activity that encodes grasp force145–151 followed by a tonic response that is much weaker but nonetheless carries grasp force information152.
M1 also has a role in modulating spinal reflexes and gating sensory inputs153–157. Indeed, while spinal reflexes are often thought to contribute to only stereotyped and unattended behaviours, such as locomotion, the modulation of these reflexes also plays a role in prehensile behaviours158–163. For example, reflexes can stabilize the hand and wrist and facilitate object interactions during grasp, so the suppression of these reflexes is relieved in anticipation of a grasp163–166. Although M1 and auxiliary structures, including the supplementary motor area, are implicated in this modulation, dense efferent projections from the ventral cingulate motor area onto spinal interneurons might also play an important role113,167–170. The modulation of reflexes is an integral part of motor control, and hand control is no exception.
Bypassing spinal circuitry
The hand representation in M1 not only is large but also has privileged access to the muscles. While most signals from M1 are sent to the muscles via spinal interneurons, a subpopulation of pyramidal neurons — the so-called corticomotoneuronal (CM) cells (FIG. 2), located mostly in the caudal aspect of M1 — synapse directly onto spinal motor neurons, thereby bypassing spinal interneurons171–176. Individual CM cells drive multiple downstream muscles139,177–179, are active throughout movement and posture phases148,180,181, recruit muscles selectively for specific functions181,182 and are involved in transcortical reflex loops183–185 (for a review, see REF.186). Although still only sparsely characterized, the behaviour of CM cells is consistent with the hypothesis that they each enable activation of a combination of muscles that contributes to a specific movement (see, however, REF.187 for an alternative hypothesis). According to this view, populations of CM cells allow a more direct access to a wider repertoire of movements than can be achieved solely through polysynaptic projections to the muscles. Such a broad repertoire of control signals could, in principle, allow the generation of movements that bypass the polysynaptic pathways or the refinement of movements generated through polysynaptic pathways154.
Fig. 2 |. Direct and indirect pathways from the cortex to the muscles.
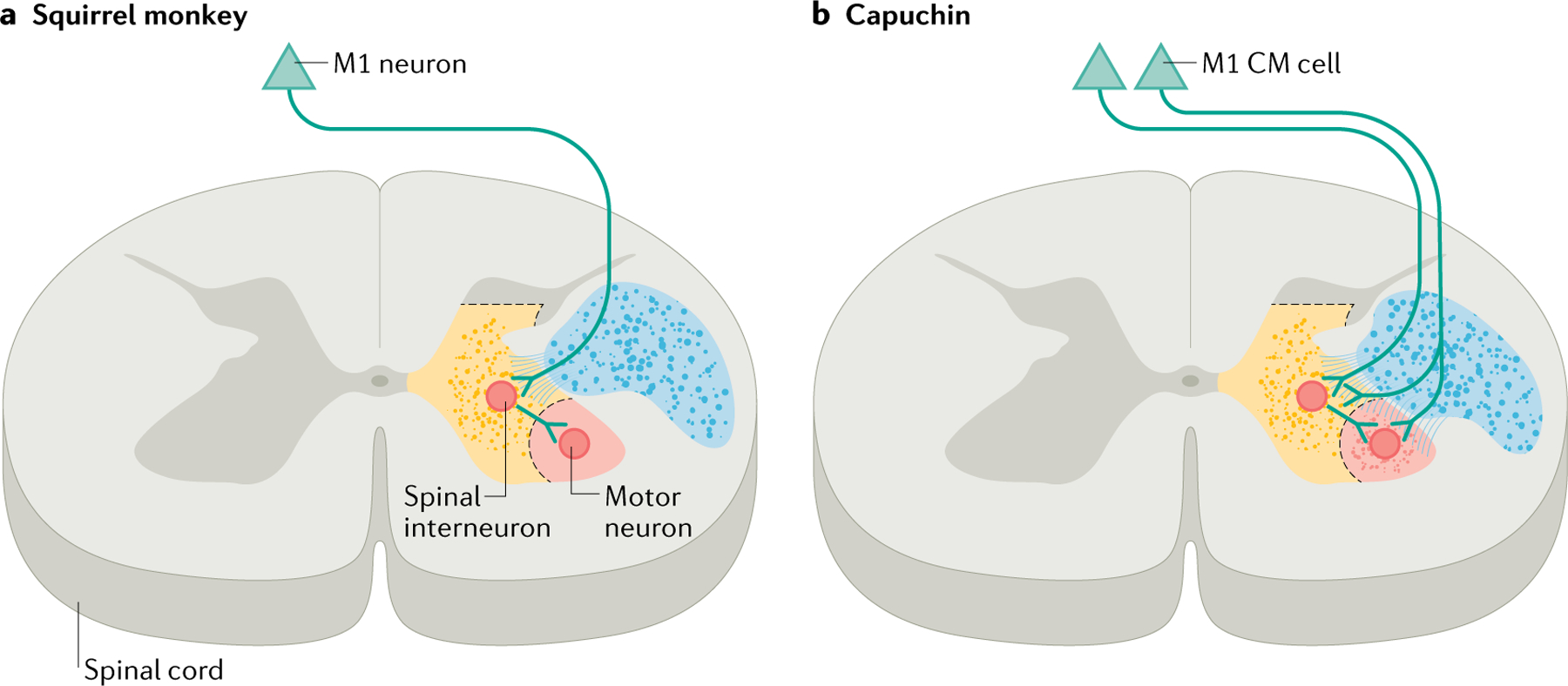
Traced axons (dots) from the primary motor cortex at the C8 level of the spinal cord. a | Such tracing has revealed that, in squirrel monkeys, neurons from the primary motor cortex (M1) project via the pyramidal tracts (blue region) to spinal interneurons (located in the yellow region), which in turn project to motor neurons (located in the red region). b | In capuchins, M1 sends direct projections to motor neurons in addition to indirect projections through spinal interneurons. Presumably owing in part to this direct pathway, capuchins are more dexterous than squirrel monkeys. CM, corticomotoneuronal. Adapted with permission from REF.204, Copyright 1993 Society for Neuroscience.
While CM cells synapse onto motor neurons innervating most if not all muscles and are active during both complex and simple movements188–192, two lines of circumstantial evidence suggest that these neurons are critical for dexterous manual behaviours such as precision grasp and individuated finger movements173,174,193–199. First, lesions that target the CM pathway impair dexterity, resulting in reductions in the speed of hand movements, the magnitude of manually applied forces and the degree of finger individuation, as well as permanent deficits in the ability to preshape the hand during grasp153,200. These deficits are exacerbated when lesions extend to other corticospinal projections201. Second, primates with CM cells tend to exhibit greater dexterity than those without them. While fair comparison of dexterity between species is challenging because of differences in the musculoskeletal structure of the hand50,196,202,203, comparison of species with similar hand anatomy implicates the CM pathway. For example, capuchins and squirrel monkeys both have pseudo-opposable thumbs and very similar hand structures, but the projections from M1 to the ventral spinal cord are dense in capuchins and sparse in squirrel monkeys204,205 (FIG. 2). Correspondingly, capuchins use individual fingers for grasping, while squirrel monkeys do not. The search for an involvement of the CM pathway in dexterity outside the primate order has also come up empty. Notably, some carnivores anecdotally known for their manipulative ability — raccoons206–208 and kinkajous209 — have been suspected of having CM connections, but the electrophysiological evidence does not support the existence of a monosynaptic pathway210 and, upon closer examination, their grasps are not as varied as are those of primates211.
In one study, causal evidence was provided for the role of CM cells in dexterity by exploitation of the fact that early postnatal mice have CM cells that are eliminated later in life212. Preservation of CM cells through genetic manipulation led to increased dexterity, as indexed by a higher success rate in grasping and manipulation tasks, although still much lower than in primates. One interpretation of this finding is that the cortex sculpts the input–output relationship of the spinal cord circuits in newborn mice via CM connections to produce a desired set of behaviours. After the behaviour has been established and consolidated via spinal interneurons, the now redundant CM pathway is eliminated, presumably because it requires energy to be maintained and adds unnecessary complexity to the neural circuits of motor control. In this view, humans and many primates retain the ability to learn complex new movement patterns in adulthood in part because CM connections are preserved.
While the relationship between CM cells and manual dexterity is debated, no organism capable of highly individuated finger movements has been reported to lack CM connections.
Visual guidance of hand movements
Successful interactions with the environment typically entail forming appropriate motor plans to interact with objects on the basis of visual information about the objects’ location and shape. The primate brain comprises specialized circuits — located in the posterior parietal cortex (PPC) — to achieve this visuomotor transformation213–220 (FIG. 3). Indeed, damage to the PPC in humans leads to varied deficits in manual behaviour, including constructional apraxia — the inability to copy an image or mimic a movement with the affected hand221. The PPC contains many regions that can be separated on the basis of architectonic, connective and functional differences221–223, two of which have been implicated in dexterity, namely the parietal reach region (PRR) and the anterior intraparietal area (AIP).
Fig. 3 |. Main cortical regions and pathways involved in visuomotor control of the hand.
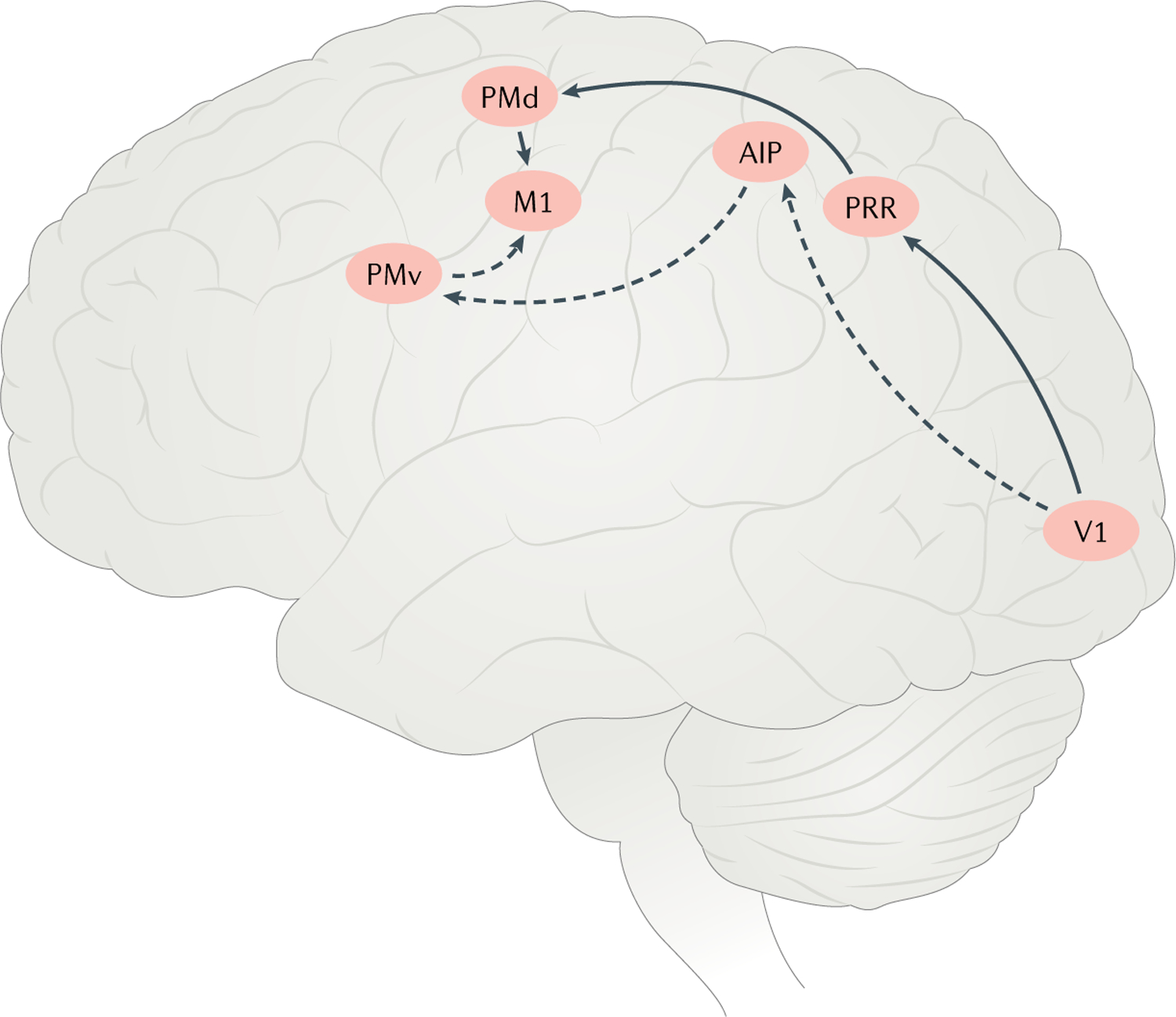
Visual information from the primary visual cortex (V1) is transformed in the parietal reach region (PRR) to guide reaching movements via the dorsal premotor area (PMd). Similarly, the anterior interparietal area (AIP) processes visual information about object shape to guide grasping movements via the ventral premotor area (PMv). The PMv and the PMd both project to the primary motor cortex (M1).
The PRR has a role in planning reaches to visually guided locations in peripersonal space213,216,224–229. Neuronal activity in the PRR correlates with movement direction during planning, and its disruption shortly before movement onset leads to reaches that start out in the wrong direction230,231. Neurons in the PRR encode limb movement in eye-centred coordinates, as evidenced by a systematic dependence of neuronal responses on gaze direction. The PRR not only is involved in planning specific reaches but also may simultaneously encode multiple reaches and contribute to the decision about which movement to execute232–236. Beyond mediating visuomotor transformations, the PRR is implicated in converting other sensory reference frames (for example, auditory) to eye-centred ones226,237,238.
The AIP has a crucial role in visually guided hand control; for example, in preshaping the hand to grasp an object220,239–247. Neurons in the AIP exhibit visual responses that are dependent on the shape of the object and its position in eye-centred coordinates241,248–250. At the population level, AIP neurons encode not only object shape but also planned grip type220. Reversible inactivation of the AIP leads to an inability to preshape the hand to the object but it does not interfere with the reach251–253 (for a review, see REF.254). The AIP projects to the ventral premotor area, where neurons also exhibit selective tuning to the object shape and grasp but encode more motor-related information255–258.
The PPC thus has a role in planning reaching and grasping movements, whereas M1 is involved with the execution of these movements259,260. Both the PRR and the AIP are involved in converting a visual representation of an object — initially in an eye-centred coordinate frame — into a motor representation of the object to give rise to appropriate arm and hand movements to grasp or manipulate it. Both cortical fields project to the premotor cortex, where the motor plan is further refined before it is conveyed to M1 (REFs170,261–268). The role of the PPC in prehension is further supported by the observation of a direct connection to spinal hand premotor interneurons in primates269 and by reports that intracortical microstimulation of the PPC evokes hand movements111,270,271 (but see REF.272). Although they are important for primates, visuomotor transformations are less important for mice and rats, and these rodent species have no well-established homologues to the PPC of primates273.
Sensory mechanisms of dexterity
Manual dexterity depends not only on an elaborate end effector and sophisticated neural mechanisms of control but also on two exquisite sensory systems. Proprioception tracks the movements of the hand and the muscular effort exerted by hand muscles. Touch conveys information about the objects with which we interact and about our interactions with them. Individuals who have lost their senses of touch and proprioception — as a result of peripheral neuropathies, genetic mutations, vitamin and mineral deficiencies, immune diseases, cancer or posterior spinal cord lesions — develop severe movement disorders, which are particularly pronounced for hand use274–285. Indeed, visual guidance of behaviour — and in particular manual behaviour — is a poor substitute for its somatosensory counterparts. The somatosensory and motor systems are tightly coupled, and manual dexterity is predicated upon their interplay286,287.
Tactile and proprioceptive sensitivity
Our sense of touch is exquisitely sensitive, particularly to dynamic stimuli. We can detect skin vibrations on the order of a tenth of a micrometre (at 250 Hz, where sensitivity peaks)288, a 50th of the average diameter of a human hair. When we run our hand across a surface, we can discern a textural element on a scale of tens of nanometres289. However, when the skin is indented by an object or surface, without tangential movement, tactile sensitivity to spatial features drops dramatically. Two pinpoint touches need to be 1 mm apart to be discerned as separate290, a groove needs to be 1-mm wide before we are able to make out its orientation291 and textures with elements in the submillimetre range feel smooth292. Even our ability to detect a touch is severely compromised if the indentation happens very slowly293. Angular acuity — the change in the orientation that can be detected — is around 10–20° for edges indented into or scanned across the skin, almost four times that for visually presented edges after differences in innervation density have been accounted for294,295. However, the tactile angular acuity is equivalent to its visual counterpart — on the order of 5° — when tested in the context of object manipulation296.
Neural basis of somatosensation
The exceptional sensitivity of the palmar surface of the hand is conferred by the approximately 17,000 nerve fibres that carry tactile signals to the CNS297. Each fibre innervates one of four types of mechanoreceptors that tile the glabrous skin (hairless skin) of the hand, each of which responds to different aspects of a skin deformation298–300. Two types of nerve fibres — rapidly adapting and Pacinian corpuscle-associated fibres, which innervate Meissner and Pacinian corpuscles, respectively — are sensitive to skin vibrations and respond robustly when the fingers move across a textured surface. A third type of nerve fibre, slowly adapting type 1 fibre, which innervates Merkel receptors, produces a graded response to pressure exerted on the skin and is especially sensitive to local discontinuities in surfaces. At a first approximation, slowly adapting type 1 fibres respond to the depth of skin indentation, rapidly adapting fibres respond to the rate of skin indentation and Pacinian corpuscle-associated fibres respond to the acceleration of the skin301. The fourth type of nerve fibre, slowly adapting type 2 fibre, is thought to innervate Ruffini-like endings, which are located deep under the skin and respond to skin stretch. The responses of nerve fibres are highly precise and repeatable and are well captured by simple models that incorporate skin biomechanics and basic neuronal dynamics301–303.
Proprioceptive signals originate primarily from mechanoreceptors embedded in the muscles and tendons, although cutaneous mechanoreceptors may also contribute300. Muscle spindles, which run parallel to muscle fibres, are the primary source of information about the conformation and movements of the body, whereas Golgi tendon organs, which run in series with the muscles (given their location in the tendons), are primarily responsible for conveying information about forces exerted by the muscles. Signals from both types of nerve fibre are integrated to achieve veridical representations of limb conformation and applied forces304. Nerve fibres that innervate the joints tend to respond only at the joint extrema, when the joint risks damage305–308, and are therefore poorly suited to convey postural information. However, a small proportion of hand joint afferents signal joint angle across the range of motion309,310, although their role in proprioception has yet to be conclusively established.
Around 3,900 spindles populate hand-associated muscles (56 on average in intrinsic muscles and 184 in forearm-based muscles, estimated from REFs311,312) and approximately half as many Golgi tendon organs populate hand-associated tendons313,314. The idea that hand muscles are more densely innervated by spindles than are other muscles is appealing but not supported by the evidence315. Similarly, the density of motor units innervating hand muscles is comparable to its proximal limb counterpart316–318. However, because hand muscles tend to be smaller, and so their motor units less numerous, fluctuations in manually applied forces are relatively higher than for their proximal limb counterparts319–322.
Signals from tactile and proprioceptive fibres that innervate the skin and muscles of the hand project to the cuneate nucleus in the brainstem, in some cases via spinal interneurons323. Cuneate neurons then project to the ventroposterolateral nucleus of the thalamus, which in turn projects to the primary somatosensory cortex, located on the postcentral gyrus, just posterior to M1. The somatosensory cortex is organized somatotopically, with adjacent neurons responding to stimulation of adjacent and well-defined body regions (FIG. 4). The somatosensory homunculus comprises four body maps, one each in Brodmann areas 3a, 3b, 1 and 2, which exhibit proprioceptive responses (area 3a), cutaneous responses (areas 3b and 1) or both (area 2). As is the case in M1, the sensory representation of the hand is magnified in the somatosensory cortex, accounting for more than 20% of its total surface area223,324–326. The hand representation comprises clearly delineated representations of each digit, separated by cell-poor septa327, with the thumb located laterally (abutting the face representation) and the little finger medially (near the forearm representation). The thumb and index finger representations in area 3b are slightly enlarged relative to those of the other digits, particularly in adults327.
Fig. 4 |. Body maps in the somatosensory cortex of a monkey.
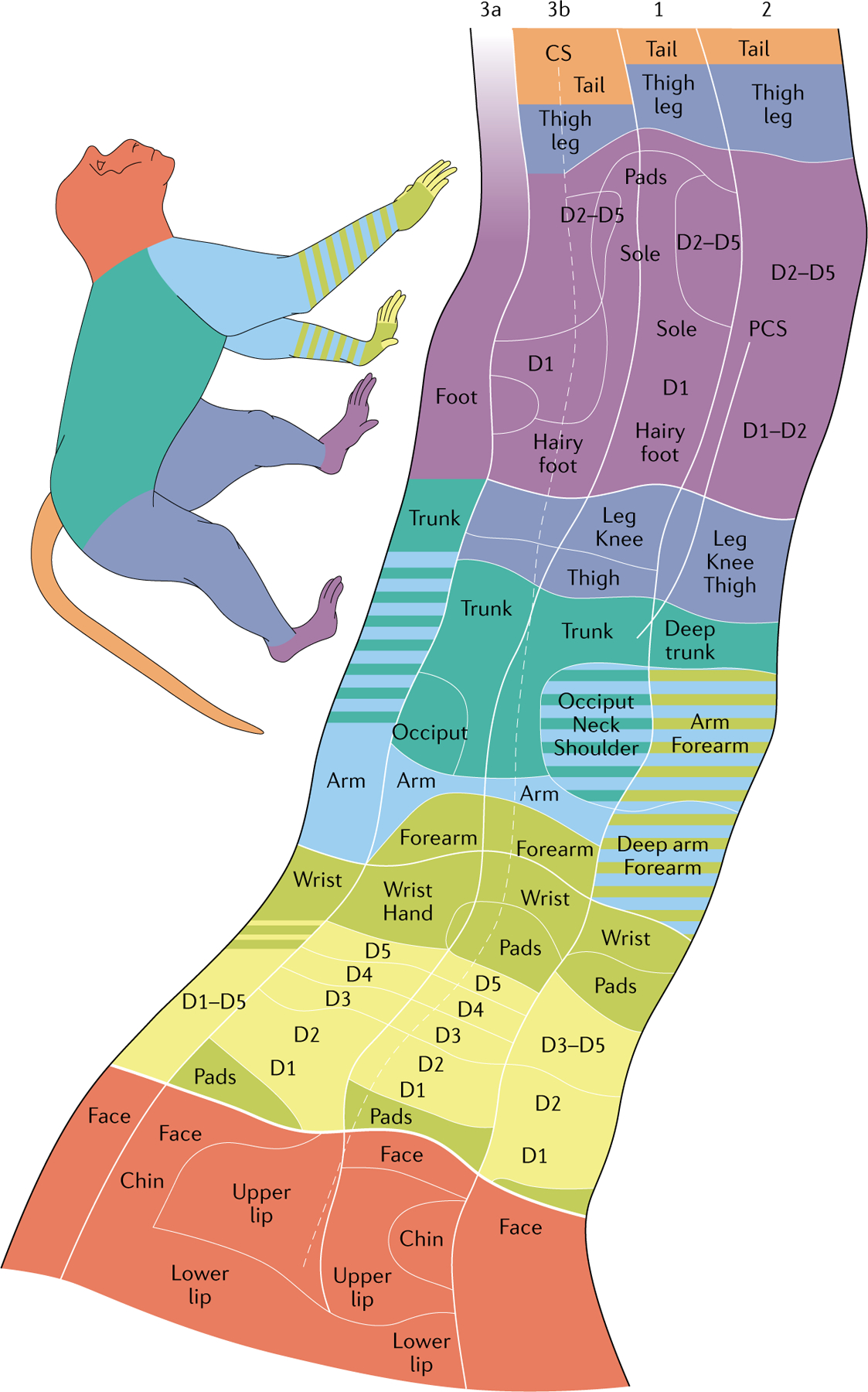
Touch applied to a location on the body activates a spatially restricted population of neurons in the somatosensory cortex. Nearby neurons are activated by nearby patches of skin, leading to highly structured maps of the body, termed the ‘somatosensory homunculus’. In primates, the volume of the cortex devoted to the hand is very large. Within this volume, the neural representations of individual digits are spatially distinct. CS, central sulcus; D, digit; PCS, postcentral sulcus. Adapted with permission from REF.326, Wiley.
Tactile coding of object interactions
Touch conveys precise information about contacts with objects to guide manipulation328. First, during reach and grasp, object contact gives rise to a strong phasic transient response throughout the somatosensory neuraxis329, inherited from rapidly adapting nerve fibres and Pacinian corpuscle-associated nerve fibres, which triggers the end of the reach and the beginning of the grasp330. Abolition of touch, via digital anaesthesia for example, leads to slower movements, longer movement paths and a larger maximum finger aperture during reach to grasp331. Loss of touch also leads to decreased accuracy in reach-and-point tasks332. Second, when we transport an object, the forces we apply depend on the shape, frictional properties and mass of the object to prevent slip, typically exerting between 10% and 40% more force than is necessary, the ‘safety margin’333–336. When touch is abolished, our ability to adequately grade applied force is eliminated, leading to our dropping objects or exerting unnecessarily high forces331,337. Third, when a grasped object is subjected to an unexpected perturbation, tactile signals — particularly from rapidly adapting fibres — trigger an automatic grip adjustment before the perturbation reaches conscious access299,338. Fourth, touch conveys information about a change in the dynamics of a grasped object; for example, when it makes contact with another object339. When a grasped rod makes contact with an object, we can even sense the location of object contact along the rod on the basis of tactile signals340.
The importance of touch in guiding manual behaviour is attested by the fact that caudal M1 — which predominantly drives hand movements — receives more tactile input and rostral M1 — which predominantly drives proximal limb movements — receives more proprioceptive input341,342. However, even for basic object interactions, proprioceptive information and tactile information are tightly coupled, as evidenced by the fact that reflexive grip adjustments triggered by cutaneous signals about object slip depend on the posture of the arm (reviewed in REF.343).
Tactile coding of object features
Object manipulation relies not only on tactile signals about contact events and applied forces but also on somatosensory signals about the objects themselves. When we grasp an object, we sense its texture and local geometry (edges, corners and surface curvature) by touch alone. If the object moves across our skin, the sense of touch conveys information about its movement direction and speed. These object-related signals support the manipulation of objects. For example, on the basis of the sensed edges of a mobile phone, we can operate it via the touchscreen without looking. Similarly, the sensed motion of a manipulated object can trigger an update of its position, which is critical for further manipulation. The transmission of this object information via the somatosensory system enables object interactions when vision is unavailable. Indeed, the absence of vision often has little impact on the ability to manipulate objects, as epitomized by the act of buttoning a shirt344. Even in the presence of vision, however, object manipulation benefits from somatosensory representations, which convey information about objects beyond that available visually. As alluded to earlier, tactile texture signals guide the application of grip force appropriate to the frictional properties of the texture333–336. Sensory signals related to other object features, such as edges and corners, also play a critical role in guiding hand–object interactions, but the behavioural role of these signals has not been investigated in a laboratory setting.
Sensory information about objects grasped in the hand is multiplexed in patterns of activation in tactile nerve fibres, where all classes of nerve fibres convey information about most object features345. Some features are encoded in the strength of the response, whereas others are encoded in its spatial and/or temporal patterns. These different neural codes allow a relatively small population of nerve fibres (~17,000) to simultaneously convey information about a variety of features — shape, motion and texture. Downstream neurons respond selectively to spatio-temporal features in the afferent input, and successive stages of processing — from the cuneate nucleus through to the cortex — culminate in explicit representations of behaviourally relevant features of contacted objects, including their shape, material, identity and motion across the skin. Accordingly, damage to cortical fields associated with somatosensation can lead to tactile agnosia, an inability to identify an object or recognize its shape or size346–349.
Shape and coarse texture.
At each point of contact with an object, we sense its local geometric features — the presence of an edge, the curvature of a surface or millimetre-scale textural elements. These features are encoded in the spatial pattern of activation of tactile nerve fibres: the spatial configuration of the object’s local features is reflected in the spatial pattern of activation it evokes in a subpopulation of nerve fibres350–352 (FIG. 5a). Information about object features is extracted from this neural image by elementary feature detectors in the somatosensory cortex353. For example, a subpopulation of neurons in the somatosensory cortex exhibits a preference for edges at a specific orientation354. Higher-order tactile representations exhibit increasingly complex and invariant feature selectivity — for curved edges355–357 or consistent orientation tuning over large swaths of skin358, which is well suited to support object manipulation and recognition.
Fig. 5 |. Neural coding of touch.
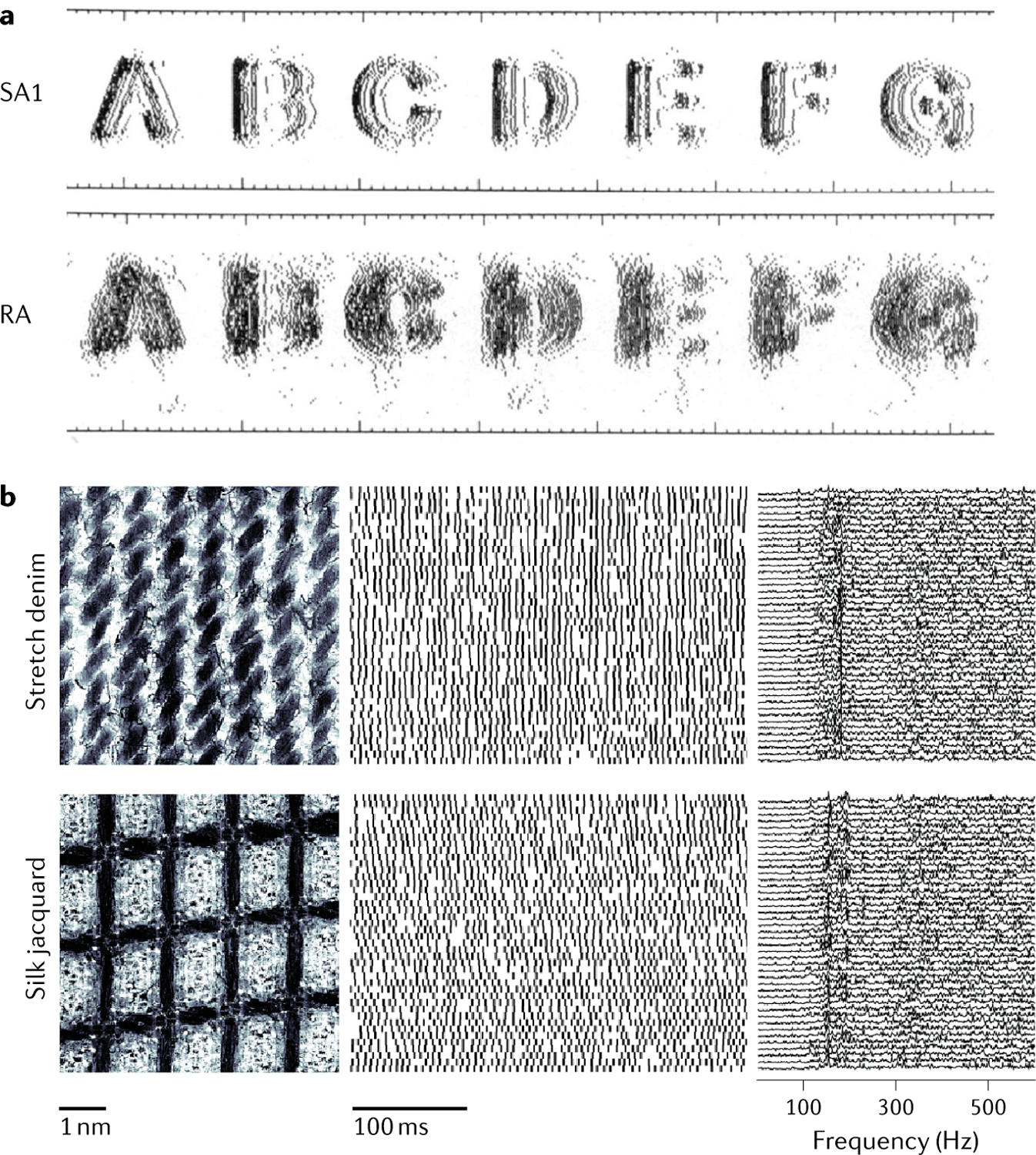
a | Reconstructed response of a population of slowly adapting type 1 (SA1) fibres and rapidly adapting (RA) fibres when embossed letters are scanned across the fingertip. The spatial pattern of activation in SA1 fibres and to a lesser extent in RA fibres carries a faithful representation of the stimulus. b | Responses of a Pacinian corpuscle-associated fibre (middle) to 40 repeated presentations of two textures (left) scanned across the skin. As shown in the spectrogram of the response (right), Pacinian corpuscle-associated fibres produce highly repeatable spiking patterns that differ across textures. This temporal code conveys information about fine texture. Part a adapted with permission from REF.350, PNAS. Part b adapted with permission from REF.371, PNAS.
Motion.
When an object moves across the skin, we can sense both the direction in which it is moving and the speed at which it is moving359–361. In the somatosensory cortex, a subpopulation of neurons is tuned for the direction in which objects move across the skin362–365. While we can also sense the speed at which objects move across our skin, tactile speed perception is unreliable and strongly dependent on other properties of the moving object361,442.
Texture.
As mentioned already, we can sense textures on submicron-level spatial scales, far beyond the spatial resolution of the skin, which is on the order of a millimetre given its innervation density289. To discern finely textured surfaces requires movement between skin and the surface292,366, which leads to the elicitation of small, high-frequency, texture-specific vibrations in the skin367–370. In turn, these vibrations are transduced by Pacinian corpuscle-associated fibres, which are exquisitely sensitive to such skin vibrations and carry texture information in exquisitely precise temporal spiking patterns371 (FIG. 5b). This temporal code is deciphered by downstream neurons that exhibit an idiosyncratic preference for a specific temporal feature in the afferent input372,373. The combination of the spatial and temporal coding mechanisms endows us with a sensitivity to texture over a wide range of spatial scales, ranging from tens of nanometres to tens of millimetres. As mentioned earlier, texture signals inform the grip forces deployed to grasp an object.
Proprioceptive coding
Hand use relies not only on a rich sense of touch — which conveys information about contact events and about object features at each point of contact — but also on a proprioceptive sense — which conveys information about the state of the hand, including its conformation, its movement and the forces it exerts on objects. Individuals deprived of proprioception rely heavily on vision to keep track of the current state of their hand and lose the ability to use their hands in the absence of vision374.
Brodmann areas 3a and 2 of the somatosensory cortex contain neurons that respond to hand postures. In both areas, individual neurons track the time-varying angle of multiple joints — on average eight – distributed over the entire hand135. This postural representation of the hand stands in contrast with movement representations of the proximal limb in the somatosensory cortex, characterized by phasic responses during movement and weaker responses during maintained posture375,376. One possibility is that the postural representation of the hand reflects greater innervation of hand-related muscles by secondary spindle afferents, which preferentially encode muscle length, while movement representations of the arm reflect a preferential innervation of primary spindle afferents, which respond strongly to changes in muscle length377,378. Consistent with this hypothesis, sensitivity to changes in joint angle is greater than sensitivity to changes in joint angular velocity for the hand, but the reverse is true for the arm379. Another possibility is that the differences between postural representations of the hand and movement representation of the arm reflect differences in the biomechanics — the hand being much lighter than the arm — as mentioned in the context of M1 coding. Regardless, the proprioceptive representation of the hand is well suited to encode hand conformation, whereas the proprioceptive representation of the arm is better suited to encode speed380.
Open questions
Although some of the specialized neural circuits for control and sensing that mediate dexterity have been identified, several major aspects of manual behaviour are poorly understood. First, we are endowed with the ability to precisely regulate the force we exert on objects with our hands, an ability that relies on dozens of muscles in the hand and forearm. The precision of force regulation has been documented only for simple behaviours — grasping or pushing27,381 — but many manual behaviours require regulating forces across fingers in a more individuated fashion. Virtually nothing is known about how manual forces are encoded in the responses of neurons in the motor cortex or the somatosensory cortex152,376,382,383.
Second, when we grasp an object, we experience a vivid sense of its three-dimensional structure based solely on signals stemming from the hand, an ability, termed ‘stereognosis’, that involves the integration of tactile and proprioceptive signals: at each point of contact with the object, cutaneous signals convey information about the object’s local geometry (for example, the presence and orientation of an edge or the curvature of a surface). These cutaneous signals about local geometric features are then integrated with proprioceptive signals about the locations of the contact points relative to one another in space. Cutaneous signals from the glabrous skin, a uniquely deformable sensory sheet, must be interpreted in the context of its conformation, conveyed by proprioceptive signals. Very little is known about how tactile signals are integrated with proprioceptive ones, except that this integration begins to take place in Brodmann area 2 (REF.384), or about how this integrated neural representation gives rise to stereognosis. The ability to sense the three-dimensional structure of an object is probably critical to our ability to manipulate objects dexterously. Indeed, our ability to deftly manipulate objects without the guidance of vision implies an object representation that can be updated via somatosensory signals alone.
Third, large swaths of the sensorimotor apparatus are only beginning to receive experimental attention in the context of dexterous manual behaviour, including the cerebellum385–389, the reticulospinal pathway390–393 and the spinal cord186,394–396, even though damage to any of these structures and pathways leads to severe motor deficits that permanently affect manual dexterity. These structures and pathways are liable to have developed hand-related specializations that parallel the various specializations described above.
Fourth, the mechanisms of motor learning have been extensively studied in the context of simple behaviours397–405, but learning a highly dexterous behaviour — playing a musical instrument, writing or drawing — may entail additional and heretofore unknown mechanisms of learning.
Conclusions
Manual dexterity reflects the confluence of many physiological factors, which we are only beginning to uncover. The many bones of the hand confer on it a wide space of achievable postures, and the numerous muscles that articulate the joints allow constrained individuation and remarkable strength. In the peripheral nervous system and CNS, sensorimotor representations of the hand are outsized to support the hand’s versatility and precision. On the motor side, a monosynaptic pathway between the motor cortex and motor neurons provides the CNS with a privileged access to the muscles. On the sensory side, a multitude of nerve fibres innervate the skin and muscles of the hand to convey high-resolution feedback not only about manual interactions with objects but also about the objects themselves. These sensory signals are critical to hand use and cannot be replaced with other senses. The relative contributions of the different sensorimotor pathways to dexterity remain to be disentangled, and the integration of somatosensory signals remains poorly understood. However, the evidence suggests that the versatility and precision of the hand is mediated by distinct biomechanical and neural mechanisms supplementing the systems controlling non-dexterous behaviour.
With recent technological developments in hand tracking and sensor technology, we are poised to address these and other gaps in our understanding of hand control. Manual behaviour epitomizes the ability of the nervous system to produce complex outputs. Glimpses into the neural mechanisms of dexterity will yield insights into the unique ability of nervous systems to give rise to flexible, intelligent behaviour and guide the development of ever more dexterous bionic hands (BOX 2).
BOX 2 |. Bionic hands.
one of the great achievements of the twenty-first century is the development of devices that allow humans not only to volitionally control extracorporeal devices such as robotic limbs by thought alone but also to actually feel through them. These feats are made possible by chronic electrical interfaces with the nervous system. For individuals with amputations, intended movements are inferred from patterns of activation of residual muscles and sensory feedback is delivered by electrically stimulating the residual nerves that innervated the amputated limb before the injury412–418. For individuals with tetraplegia, movement intent is inferred from patterns of activation across populations of cortical neurons, typically in the primary motor cortex but sometimes in the posterior parietal cortex, and sensory feedback is delivered by electrically stimulating neurons in the somatosensory cortex419–427.
Given the staggering complexity of the human hand, it should come as no surprise that robotic hands are far simpler in their actuation and sensitization than their biological counterparts. The most sophisticated bionic hands feature around 20 degrees of freedom428,429 and a handful of sensors on the fingertips, which convey only coarse information about contact with objects. Although better sensorization is just around the corner430–432, the main bottleneck is not the impoverished sensory feedback but the inadequacy of the control signals. Indeed, myoelectrically controlled bionic hands suffer from the fact that many of the muscles relevant to hand use (including all the intrinsic hand muscles) are missing in people with amputations, which severely limits the reliability of the resulting control418. Brain-controlled bionic hands are limited by an insufficient understanding of the neural mechanisms underlying manual control. Successful brain control, which has focused on proximal limb movements designed to place the hand in peripersonal space, has achieved limited manual control — often including only hand opening and closing — and relies completely on kinematic decoding from the motor cortex423. That is, the individual closes the hand into the object rather than volitionally exerting a force on it, as we naturally do. Nonetheless, state-of-the-art brain–machine interfaces have achieved control of an anthropomorphic robotic limb across ten degrees of freedom433 and conveyed intracortical microstimulation-based tactile feedback that conferred additional dexterity on the device434.
Next-generation brain-controlled bionic hands will allow manual control of forces over many degrees of freedom. For individuals with amputations, improved control may have to rely on a combination of myoelectric and efferent neural signals monitored in the residual nerves418,435. For individuals with tetraplegia, hybrid kinematic and force control for the hand will require a more detailed understanding of hand representations in the primary motor cortex. Work with myoelectrically controlled bionic hands has shown that biomimetic sensory feedback — designed to mimic natural signals in the nerve through electrical stimulation436,437 — leads to improved performance compared with standard sensory feedback, which simply tracks the output of force sensors on the bionic fingers438,439. Intracortical microstimulation-based biomimetic sensory feedback is also likely to lead to improved performance over standard algorithms, but this has yet to be tested440,441.
Supplementary Material
Degrees of freedom
In the context of limbs, the axes of rotation of one segment around another segment. A single joint can have multiple degrees of freedom, each corresponding to a different axis of rotation.
Metacarpals
Bones that underlie the primate palm, one for each digit.
Metacarpophalangeal joints
Joints that connect metacarpal bones of the palm with the first phalanx.
Prehension
The act of seizing or grasping.
Interphalangeal joints
Joints that connect two phalanges of a digit.
Torques
In the context of limbs, the rotational forces around a joint.
Contralateral
Located on the opposite side of the body.
Hemiparesis
Weakness of voluntary movement in one side of the body.
Pyramidal neurons
Large excitatory neurons in the cortex.
Motor neuron
A neuron that directly synapses onto muscles.
Spinal reflexes
Semi-automatic neural circuits connecting peripheral sensors to motor neurons via one synapse or several synapses in the spinal cord.
Mechanoreceptors
Sensory receptors that convert mechanical deformations into electrochemical neural signals.
Glabrous skin
Hairless skin, such as that on the palmar side of the hand.
Brodmann areas
Areas of the cerebral cortex defined by their cell composition, structure and organization.
Somatosensation
The sense of one’s own body, including the sense of touch, the sense of the posture and movements of the body (proprioception), the sense of temperature (thermosensation) and the perception of pain (nociception).
Tetraplegia
Paralysis of all four limbs.
Acknowledgements
The authors thank E. Azim, J. Collinger, R. Diogo, M.Schieber, K. Seki and P. Strick for helpful comments during the preparation of the manuscript. This work was supported by NINDS grants NS107714 and NS122333.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Neuroscience thanks T. Isa and the other, anonymous, reviewers for their contribution to the peer review of this work.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41583-021-00528-7.
References
- 1.Billard A & Kragic D Trends and challenges in robot manipulation. Science 364, eaat8414 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Mathis A et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Graving JM et al. DeepPoseKit, a software toolkit for fast and robust animal pose estimation using deep learning. eLife 8, e47994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeannerod M The Neural and Behavioural Organization of Goal-Directed Movements (Clarendon Press/Oxford University Press, 1988). [Google Scholar]
- 5.Santello M, Flanders M & Soechting JF Patterns of hand motion during grasping and the influence of sensory guidance. J. Neurosci 22, 1426–1435 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur PH, Bastian AJ & Hsiao SS Multidigit movement synergies of the human hand in an unconstrained haptic exploration task. J. Neurosci 28, 1271–1281 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Y, Goodman JG, Moore DD, Solla S & Bensmaia SJ Unexpected complexity of everyday manual behaviors. Nat. Commun 10.1101/694778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hager-Ross C & Schieber MH Quantifying the independence of human finger movements: comparisons of digits, hands, and movement frequencies. J. Neurosci 20, 8542–8550 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram JN, Körding KP, Howard IS & Wolpert DM The statistics of natural hand movements. Exp. Brain Res 188, 223–236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hogan N The mechanics of multi-joint posture and movement control. Biol. Cybern 52, 315–331 (1985). This study introduces a mathematical framework for the control of multiple joints.
- 11.Santello M, Flanders M & Soechting JF Postural hand synergies for tool use. J. Neurosci 18, 10105–10115 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason CR, Theverapperuma LS, Hendrix CM & Ebner TJ Monkey hand postural synergies during reach-to-grasp in the absence of vision of the hand and object. J. Neurophysiol 91, 2826–2837 (2004). [DOI] [PubMed] [Google Scholar]
- 13.d’Avella A, Portone A, Fernandez L & Lacquaniti F Control of fast-reaching movements by muscle synergy combinations. J. Neurosci 26, 7791–7810 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berniker M, Jarc A, Bizzi E & Tresch MC Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc. Natl Acad. Sci. USA 106, 7601–7606 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d’Avella A & Lacquaniti F Control of reaching movements by muscle synergy combinations. Front. Comput. Neurosci 7, 1–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutch JJ & Valero-Cuevas FJ Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLoS Comput. Biol 8, e1002434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brochier T, Spinks RL, Umilta MA & Lemon RN Patterns of muscle activity underlying object-specific grasp by the macaque monkey. J. Neurophysiol 92, 1770–1782 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson K. Lightning-fast pianist Lubomyr Melnyk: ‘When I play I turn into an eagle flying’ https://www.theguardian.com/music/2015/nov/26/lubomyr-melnyk-fastest-pianist-rivers-and-streams (Guardian; (Lond.), 2015). [Google Scholar]
- 19.Dhakal V, Feit AM, Kristensson PO & Oulasvirta A Observations on typing from 136 million keystrokes. in Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems 1–12 (ACM, 2018) [Google Scholar]
- 20.Vinyals O et al. Grandmaster level in StarCraft II using multi-agent reinforcement learning. Nature 575, 350–354 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Hamacher F Rice sculpture: Taiwan artist gets granular with president elect (Reuters, 2016). [Google Scholar]
- 22.Legge GE, Madison CM & Mansfield JS Measuring Braille reading speed with the MNREAD test. Vis. Impairment Res 1, 131–145 (1999). [Google Scholar]
- 23.Matthews C All hail the ‘king of fingers’ https://www.huffpost.com/entry/pnut-king-of-fingers-dance-video_n_4555714 (HuffPost, 2014). [Google Scholar]
- 24.Schieber MH Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. J. Neurophysiol 65, 1381–1391 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Schieber MH Muscular production of individuated extrinsic finger movements: the roles of extrinsic finger muscles. J. Neurosci 75, 284–297 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilbreath SL & Gandevia SC Limited independent flexion of the thumb and fingers in human subjects. J. Physiol 479, 487–497 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zatsiorsky VM, Li Z-M & Latash ML Enslaving effects in multi-finger force production. Exp. Brain Res 131, 187–195 (2000). [DOI] [PubMed] [Google Scholar]
- 28. Schieber MH & Santello M Hand function: peripheral and central constraints on performance. J. Appl. Physiol 96, 2293–2300 (2004). This is a review of the factors that constrain manual behaviour.
- 29.Jerde TE, Santello M, Flanders M & Soechting JF Hand movements and musical performance. in Music, Motor Control and the Brain Ch. 5 (eds Altenmüller E, Wiesendanger M & Kesselring J) 79–90 (Oxford University Press, 2006). [Google Scholar]
- 30.Furuya S, Flanders M & Soechting JF Hand kinematics of piano playing. J. Neurophysiol 106, 2849–2864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuya S & Altenmüller E Flexibility of movement organization in piano performance. Front. Hum. Neurosci 7, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ransil BJ & Schachter SC Test-retest reliability of the Edinburgh Handedness Inventory and Global Handedness preference measurements, and their correlation. Percept. Mot. Skills 79, 1355–1372 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Finch G Chimpanzee handedness. Science 94, 117–118 (1941). [DOI] [PubMed] [Google Scholar]
- 34.Warren JM Handedness in the rhesus monkey. Science 118, 622–623 (1953). [DOI] [PubMed] [Google Scholar]
- 35.Hopkins WD, Washburn DA, Berke L & Williams M Behavioral asymmetries of psychomotor performance in rhesus monkeys (Macaca mulatta): a dissociation between hand preference and skill. J. Comp. Psychol 106, 392–397 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Hill EL & Khanem F The development of hand preference in children: the effect of task demands and links with manual dexterity. Brain Cogn 71, 99–107 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Andersen KW & Siebner HR Mapping dexterity and handedness: recent insights and future challenges. Curr. Opin. Behav. Sci 20, 123–129 (2018). [Google Scholar]
- 38.Reilly KT & Hammond GR Human handedness: is there a difference in the independence of the digits on the preferred and non-preferred hands? Exp. Brain Res 156, 255–262 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Reilly KT & Hammond GR Intrinsic hand muscles and digit independence on the preferred and non-preferred hands of humans. Exp. Brain Res 173, 564–571 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Wang Y-C, Bohannon RW, Kapellusch J, Garg A & Gershon RC Dexterity as measured with the 9-hole peg test (9-HPT) across the age span. J. Hand Ther 28, 53–60 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Angstmann S et al. Microstructural asymmetry of the corticospinal tracts predicts right–left differences in circle drawing skill in right-handed adolescents. Brain Struct. Funct 221, 4475–4489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathew J, Sarlegna FR, Bernier P-M & Danion FR Handedness matters for motor control but not for prediction. eNeuro 10.1523/ENEURO.0136-19.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters M Handedness: effect of prolonged practice on between hand performance differences. Neuropsychologia 19, 587–590 (1981). [DOI] [PubMed] [Google Scholar]
- 44.Chapman JA & Henneberg M Switching the handedness of adults: results of 10 weeks training of the non-dominant hand. Persp. Human Biol 4, 211–117 (1999). [Google Scholar]
- 45.Walker L & Henneberg M Writing with the non-dominant hand: cross-handedness trainability in adult individuals. Laterality 12, 121–130 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Kivell TL The primate wrist. in The Evolution of the Primate Hand (eds Kivell TL, Lemelin P, Richmond BG & Schmitt D) 17–54 (Springer, 2016). [Google Scholar]
- 47.Schaefer M, Black S & Scheuer L Juvenile Osteology: A Laboratory and Field Manual (Elsevier, 2009). [Google Scholar]
- 48.Napier JR Hands (Princeton University Press, 1967). [Google Scholar]
- 49. Kivell TL, Lemelin P, Richmond B,G & Schmitt D (eds) The Evolution of the Primate Hand (Springer, 2016). This book provides a detailed description of the anatomy of primate hands, their evolution and interspecies differences.
- 50.Napier JR & Napier PH A Handbook of Living Primates (Academic Press, 1967). [Google Scholar]
- 51.Patel BA & Maiolino SA Morphological diversity in the digital rays of primate hands. in The Evolution of the Primate Hand (eds Kivell TL, Lemelin P, Richmond BG & Schmitt D) 55–100 (Springer, 2016). [Google Scholar]
- 52.Rolian C, Lieberman DE & Hallgrímsson B The coevolution of human hands and feet. Evol. Int. J. Org. Evol 64, 1558–1568 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Lemelin P & Diogo R Anatomy, function, and evolution of the primate hand musculature. In The Evolution of the Primate Hand (eds. Kivell TL, Lemelin P, Richmond BG & Schmitt D) 155–194 (Springer, 2016). [Google Scholar]
- 54.Kilbreath SL, Gorman RB, Raymond J & Gandevia SC Distribution of the forces produced by motor unit activity in the human flexor digitorum profundus. J. Physiol 543, 289–296 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reilly KT & Schieber MH Incomplete functional subdivision of the human multitendoned finger muscle flexor digitorum profundus: an electromyographic study. J. Neurophysiol 90, 2560–2570 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Birdwell JA, Hargrove LJ, Kuiken TA & Weir RF Activation of individual extrinsic thumb muscles and compartments of extrinsic finger muscles. J. Neurophysiol 110, 1385–1392 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valero-Cuevas FJ et al. The tendon network of the fingers performs anatomical computation at a macroscopic scale. IEEE Trans. Biomed. Eng 54, 1161–1166 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Long C II Electromyographic studies of hand function. in The Hand (ed. Tubiana R) 427–440 (W.B. Saunders Company, 1981). [Google Scholar]
- 59.Valentin P The interossei and the lumbricals. In The Hand (ed. Tubiana R) 244–254 (W.B. Saunders Company, 1981). [Google Scholar]
- 60.von Schroeder HP & Botte MJ The functional significance of the long extensors and juncturae tendinum in finger extension. J. Hand Surg 18, 641–647 (1993). [DOI] [PubMed] [Google Scholar]
- 61.Wang K, McGlinn EP & Chung KC A biomechanical and evolutionary perspective on the function of the lumbrical muscle. J. Hand Surg 39, 149–155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flash T & Hogan N The coordination of arm movements: an experimentally confirmed mathematical model. J. Neurosci 5, 1688–1703 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scholz JP & Schöner G The uncontrolled manifold concept: identifying control variables for a functional task. Exp. Brain Res 126, 289–306 (1999). This article reports an early application of the manifold concept to the field of motor control.
- 64.Todorov E Direct cortical control of muscle activation in voluntary arm movements: a model. Nat. Neurosci 3, 391–398 (2000). [DOI] [PubMed] [Google Scholar]
- 65. Valero-Cuevas FJ Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J. Neurophysiol 83, 1469–1479 (2000). This study shows that the patterns of muscle activations that drive fingertip forces are consistent across force levels.
- 66. Todorov E & Jordan M Optimal feedback control as a theory of motor coordination. Nat. Neurosci 5, 1226–1235 (2002). This article outlines a theory of how online feedback is used to guide goal-directed movement.
- 67.Valero-Cuevas FJ, Venkadesan M & Todorov E Structured variability of muscle activations supports the minimal intervention principle of motor control. J. Neurophysiol 102, 59–68 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hogan N Impedance control: an approach to manipulation. in 1984 American Control Conference 304–313 (IEEE, 1984). This article introduces the notion of impedance — resistance to perturbations — as a fundamental control variable in movement.
- 69.De Serres SJ & Milner TE Wrist muscle activation patterns and stiffness associated with stable and unstable mechanical loads. Exp. Brain Res 86, 451–458 (1991). [DOI] [PubMed] [Google Scholar]
- 70.Stroeve S Impedance characteristics of a neuromusculoskeletal model of the human arm II. Movement control. Biol. Cybern 81, 495–504 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Gribble PL, Mullin LI, Cothros N & Mattar A Role of cocontraction in arm movement accuracy. J. Neurophysiol 89, 2396–2405 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Osu R et al. Optimal impedance control for task achievement in the presence of signal-dependent noise. J. Neurophysiol 92, 1199–1215 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Höppner H, Große-Dunker M, Stillfried G, Bayer J & van der Smagt P Key insights into hand biomechanics: human grip stiffness can be decoupled from force by cocontraction and predicted from electromyography. Front. Neurorobot 10.3389/fnbot.2017.00017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.An KN, Chao EY, Cooney WP & Linscheid RL Forces in the normal and abnormal hand. J. Orthop. Res. Soc 3, 202–211 (1985). [DOI] [PubMed] [Google Scholar]
- 75.Mathiowetz V et al. Grip and pinch strength: normative data for adults. Arch. Phys. Med. Rehabil 66, 69–74 (1985). [PubMed] [Google Scholar]
- 76.O’Driscoll SW et al. The relationship between wrist position, grasp size, and grip strength. J. Hand Surg 17, 169–177 (1992). [DOI] [PubMed] [Google Scholar]
- 77.Höppner H, McIntyre J & van der Smagt P Task dependency of grip stiffness — a study of human grip force and grip stiffness dependency during two different tasks with same grip forces. PLoS ONE 8, e80889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beringer CR et al. The effect of wrist posture on extrinsic finger muscle activity during single joint movements. Sci. Rep 10, 8377 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boots MT et al. Functional and structural moment arm validation for musculoskeletal models: a study of the human forearm and hand. bioRxiv 10.1101/2020.05.29.124644 (2020). [DOI] [Google Scholar]
- 80.Sobinov A et al. Approximating complex musculoskeletal biomechanics using multidimensional autogenerating polynomials. PLOS Comput. Biol 16, e1008350 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goislard de Monsabert B, Rossi J, Berton E & Vigouroux L Quantification of hand and forearm muscle forces during a maximal power grip task. Med. Sci. Sports Exerc 44, 1906–1916 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Bardo A et al. Get a grip: variation in human hand grip strength and implications for human evolution. Symmetry 13, 1142 (2021). [Google Scholar]
- 83.Li ZM, Zatsiorsky VM & Latash ML Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clin. Biomech 15, 203–211 (2000). [DOI] [PubMed] [Google Scholar]
- 84.Scheuer JL & Elkington NM Sex determination from metacarpals and the first proximal phalanx. J. Forensic Sci 38, 769–778 (1993). [PubMed] [Google Scholar]
- 85.Falsetti AB Sex assessment from metacarpals of the human hand. J. Forensic Sci 40, 774–776 (1995). [PubMed] [Google Scholar]
- 86.Sulzmann CE, Buckberry JL & Pastor RF The utility of carpals for sex assessment: a preliminary study. Am. J. Phys. Anthropol 135, 252–262 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Puh U Age-related and sex-related differences in hand and pinch grip strength in adults. Int. J. Rehabil. Res 33, 4–11 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Phelps VR Relative index finger length as a sex-influenced trait in man. Am. J. Hum. Genet 4, 72–89 (1952). [PMC free article] [PubMed] [Google Scholar]
- 89.Manning JT, Scutt D, Wilson J & Lewis-Jones DI The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod 13, 3000–3004 (1998). [DOI] [PubMed] [Google Scholar]
- 90.Ateshian GA, Rosenwasser MP & Mow VC Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J. Biomech 25, 591–607 (1992). [DOI] [PubMed] [Google Scholar]
- 91.Neu CP, Crisco JJ & Wolfe SW In vivo kinematic behavior of the radio-capitate joint during wrist flexion–extension and radio-ulnar deviation. J. Biomech 34, 1429–1438 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Crisco JJ et al. In vivo radiocarpal kinematics and the dart thrower’s motion. JBJS 87, 2729–2740 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Rainbow MJ, Crisco JJ, Moore DC & Wolfe SW Gender differences in capitate kinematics are eliminated after accounting for variation in carpal size. J. Biomech. Eng 130, 041003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kivell TL, Guimont I & Wall CE Sex-related shape dimorphism in the human radiocarpal and midcarpal joints. Anat. Rec 296, 19–30 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Linburg RM & Comstock BE Anomalous tendon slips from the flexor pollicis longus to the flexor digitorum profundus. J. Hand Surg 4, 79–83 (1979). [DOI] [PubMed] [Google Scholar]
- 96.Sebastin SJ, Puhaindran ME, Lim AYT, Lim IJ & Bee WH The prevalence of absence of the palmaris longus – a study in a Chinese population and a review of the literature. J. Hand Surg 30, 525–527 (2005). [DOI] [PubMed] [Google Scholar]
- 97. An K-N Tendon excursion and gliding: clinical impacts from humble concepts. J. Biomech 40, 713–718 (2007). This article provide a mathematical description of how forces exerted by muscles give rise to movements of the joints.
- 98.Diogo R & Abdala V Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development (CRC Press, 2010). [Google Scholar]
- 99. Akita K & Nimura A Forearm muscles. in Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation (eds Tubbs RS, Shoja MM & Loukas M) 298–314 (John Wiley & Sons Inc., 2016). This article provide a comprehensive description of the variability in muscle anatomy across humans.
- 100.Gonzalez MA & Netscher DT Hand intrinsic muscles. in Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation 315–334 (Wiley, 2016). [Google Scholar]
- 101.Diogo R, Ziermann JM & Linde-Medina M Is evolutionary biology becoming too politically correct? A reflection on the scala naturae, phylogenetically basal clades, anatomically plesiomorphic taxa, and ‘lower’ animals. Biol. Rev 90, 502–521 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Diogo R First detailed anatomical study of bonobos reveals intra-specific variations and exposes just-so stories of human evolution, bipedalism, and tool use. Front. Ecol. Evol 6, 1–7 (2018). [Google Scholar]
- 103. Karakostis FA et al. Biomechanics of the human thumb and the evolution of dexterity. Curr. Biol 21, 1317–1325.e8 (2021). This article reports that a small difference in thumb muscle and bone anatomy led to increased dexterity and an evolutionary advantage in early humans.
- 104.Diogo R et al. Photographic and Descriptive Musculoskeletal Atlas of Gibbons and Siamangs (Hylobates) - with Notes on the Attachments, Variations, Innervation, Synonymy and Weight of the Muscles (CRC Press, 2012). [Google Scholar]
- 105.Diogo R et al. Photographic and Descriptive Musculoskeletal Atlas of Bonobos, with Notes on the Attachments, Variations, Innervation, Synonymy and Weight of the Muscles (Springer, 2017). [Google Scholar]
- 106.Diogo R & Wood B Soft-tissue anatomy of the primates: phylogenetic analyses based on the muscles of the head, neck, pectoral region and upper limb, with notes on the evolution of these muscles. J. Anat 219, 273–359 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Penfield W & Rasmussen T The Cerebral Cortex of Man: A Clinical Study of Localization of Function (Macmillan, 1950). [Google Scholar]
- 108.Luppino G & Rizzolatti G The organization of the frontal motor cortex. Physiology 15, 219–224 (2000). [DOI] [PubMed] [Google Scholar]
- 109.Rizzolatti G & Luppino G The cortical motor system. Neuron 31, 889–901 (2001). [DOI] [PubMed] [Google Scholar]
- 110.Schieber MH Constraints on somatotopic organization in the primary motor cortex. J. Neurophysiol 86, 2125–2143 (2001). [DOI] [PubMed] [Google Scholar]
- 111. Baldwin MKL, Cooke DF, Goldring AB & Krubitzer L Representations of fine digit movements in posterior and anterior parietal cortex revealed using long-train intracortical microstimulation in macaque monkeys. Cereb. Cortex 28, 4244–4263 (2018). This study maps movements evoked through intracortical microstimulation throughout the primate cortex.
- 112.Mayer A et al. The multiple representations of complex digit movements in primary motor cortex form the building blocks for complex grip types in capuchin monkeys. J. Neurosci 39, 6684–6695 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Strick PL, Dum RP & Rathelot J-A The cortical motor areas and the emergence of motor skills: a neuroanatomical perspective. Ann. Rev. Neurosci 44, 425–447 (2021). This is a neuroanatomically focused review of cortical motor areas.
- 114.Plagenhoef S, Evans FG & Abdelnour T Anatomical data for analyzing human motion. Res. Q. Exerc. Sport 54, 169–178 (1983). [Google Scholar]
- 115.Mignano BP & Konz S The surface area and volume of the hand. Proc. Hum. Factors Ergon. Soc. Annu. Meet 38, 607–610 (1994). [Google Scholar]
- 116.de Leva P Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J. Biomech 29, 1223–1230 (1996). [DOI] [PubMed] [Google Scholar]
- 117.Halley AC, Baldwin MKL, Cooke DF, Englund M & Krubitzer L Distributed motor control of limb movements in rat motor and somatosensory cortex: the sensorimotor amalgam revisited. Cereb. Cortex 10.1093/cercor/bhaa186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nudo RJ, Jenkins WM, Merzenich MM, Prejean T & Grenda R Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J. Neurosci 12, 2918–2947 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Volkmann J, Schnitzler A, Witte OW & Freund H-J Handedness and asymmetry of hand representation in human motor cortex. J. Neurophysiol 79, 2149–2154 (1998). [DOI] [PubMed] [Google Scholar]
- 120.Hervé P-Y, Mazoyer B, Crivello F, Perchey G & Tzourio-Mazoyer N Finger tapping, handedness and grey matter amount in the Rolando’s genu area. NeuroImage 25, 1133–1145 (2005). [DOI] [PubMed] [Google Scholar]
- 121.Kaas JH, Qi H-X & Stepniewska I Evolution of parietal-frontal networks in primates. in Evolutionary Neuroscience (ed. Kaas J) 657–667 (Elsevier, 2020). [Google Scholar]
- 122.Glees P & Cole J Recovery of skilled motor functions after small repeated lesions of motor cortex in macaque. J. Neurophysiol 13, 137–148 (1950). [Google Scholar]
- 123.Lawrence DG & Kuypers HG The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91, 1–14 (1968). [DOI] [PubMed] [Google Scholar]
- 124.Friel KM & Nudo RJ Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosensory Mot. Res 15, 173–189 (1998). [DOI] [PubMed] [Google Scholar]
- 125.Rouiller EM et al. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion. Eur. J. Neurosci 10, 729–740 (1998). [DOI] [PubMed] [Google Scholar]
- 126.Murata Y et al. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J. Neurophysiol 99, 773–786 (2008). [DOI] [PubMed] [Google Scholar]
- 127.Murata Y et al. Temporal plasticity involved in recovery from manual dexterity deficit after motor cortex lesion in macaque monkeys. J. Neurosci 35, 84–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tohyama T et al. Contribution of propriospinal neurons to recovery of hand dexterity after corticospinal tract lesions in monkeys. Proc. Natl Acad. Sci. USA 114, 604–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Isa T Dexterous hand movements and their recovery after central nervous system injury. Annu. Rev. Neurosci 42, 315–335 (2019). This is a review of neural pathways mediating recovery after injury to the CNS.
- 130.Hoffman DS & Strick PL Effects of a primary motor cortex lesion on step-tracking movements of the wrist. J. Neurophysiol 73, 891–895 (1995). [DOI] [PubMed] [Google Scholar]
- 131.Nishimura Y, Morichika Y & Isa T A subcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Brain 132, 709–721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lang CE & Schieber MH Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J. Neurophysiol 90, 1160–1170 (2003). [DOI] [PubMed] [Google Scholar]
- 133.Lang CE & Schieber MH Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J. Neurophysiol 91, 1722–1733 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Capaday C, Ethier C, Darling WG & Van Vreeswijk C On the functional organization and operational principles of the motor cortex. Front. Neural Circuits 7, 66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goodman JM et al. Postural representations of the hand in the primate sensorimotor cortex. Neuron 10.1016/j.neuron.2019.09.004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Donoghue JP, Leibovic S & Sanes JN Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp. Brain Res 89, 1–19 (1992). [DOI] [PubMed] [Google Scholar]
- 137.Shinoda Y, Yokota J-I & Futami T Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci. Lett 23, 7–12 (1981). [DOI] [PubMed] [Google Scholar]
- 138.Schieber MH & Hibbard LS How somatotopic is the motor cortex hand area? Science 261, 489–492 (1993). [DOI] [PubMed] [Google Scholar]
- 139.Hudson HM, Park MC, Belhaj-Saïf A & Cheney PD Representation of individual forelimb muscles in primary motor cortex. J. Neurophysiol 118, 47–63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Churchland MM et al. Neural population dynamics during reaching. Nature 487, 51–56 (2012). This article provides a description of M1 as a dynamical system.
- 141.Suresh AK et al. Neural population dynamics in motor cortex are different for reach and grasp. bioRxiv 10.1101/667196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rouse AG & Schieber MH Spatiotemporal distribution of location and object effects in reach-to-grasp kinematics. J. Neurophysiol 114, 3268–3282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rouse AG & Schieber MH Condition-dependent neural dimensions progressively shift during reach to grasp. Cell Rep 25, 3158–3168.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lemon RN, Johansson RS & Westling G Corticospinal control during reach, grasp, and precision lift in man. J. Neurosci 15, 6145–6156 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smith AM, Hepp-Reymond M-C & Wyss UR Relation of activity in precentral cortical neurons to force and rate of force change during isometric contractions of finger muscles. Exp. Brain Res 23, 315–332 (1975). [DOI] [PubMed] [Google Scholar]
- 146.Cheney PD & Fetz EE Functional classes of primate corticomotoneuronal cells and their relation to active force. J. Neurophysiol 44, 773–791 (1980). [DOI] [PubMed] [Google Scholar]
- 147.Wannier TM, Maier MA & Hepp-Reymond MC Contrasting properties of monkey somatosensory and motor cortex neurons activated during the control of force in precision grip. J. Neurophysiol 65, 572–589 (1991). [DOI] [PubMed] [Google Scholar]
- 148.Maier MA, Bennett KM, Hepp-Reymond MC & Lemon RN Contribution of the monkey corticomotoneuronal system to the control of force in precision grip. J. Neurophysiol 69, 772–785 (1993). [DOI] [PubMed] [Google Scholar]
- 149.Sergio LE & Kalaska JF Systematic changes in directional tuning of motor cortex cell activity with hand location in the workspace during generation of static isometric forces in constant spatial directions. J. Neurophysiol 78, 1170–1174 (1997). [DOI] [PubMed] [Google Scholar]
- 150.Sergio LE & Kalaska JF Changes in the temporal pattern of primary motor cortex activity in a directional isometric force versus limb movement task. J. Neurophysiol 80, 1577–1583 (1998). [DOI] [PubMed] [Google Scholar]
- 151.Shalit U, Zinger N, Joshua M & Prut Y Descending systems translate transient cortical commands into a sustained muscle activation signal. Cereb. Cortex 22, 1904–1914 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Intveld RW, Dann B, Michaels JA & Scherberger H Neural coding of intended and executed grasp force in macaque areas AIP, F5, and M1. Sci. Rep 8, 17985 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Isa T, Ohki Y, Alstermark B, Pettersson L-G & Sasaki S Direct and indirect cortico-motoneuronal pathways and control of hand/arm movements. Physiology 22, 145–152 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Alstermark B & Isa T Circuits for skilled reaching and grasping. Annu. Rev. Neurosci 35, 559–578 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Kinoshita M et al. Genetic dissection of the circuit for hand dexterity in primates. Nature 487, 235–238 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Confais J, Kim G, Tomatsu S, Takei T & Seki K Nerve-specific input modulation to spinal neurons during a motor task in the monkey. J. Neurosci 10.1523/JNEUROSCI.2561-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Soteropoulos DS Corticospinal gating during action preparation and movement in the primate motor cortex. J. Neurophysiol 119, 1538–1555 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nielsen J & Kagamihara Y The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J. Physiol 456, 373–391 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nielsen J, Sinkjær T, Toft E & Kagamihara Y Segmental reflexes and ankle joint stiffness during co-contraction of antagonistic ankle muscles in man. Exp. Brain Res 102, 350–358 (1994). [DOI] [PubMed] [Google Scholar]
- 160.Maier MA, Shupe LE & Fetz EE Dynamic neural network models of the premotoneuronal circuitry controlling wrist movements in primates. J. Comput. Neurosci 19, 125–146 (2005). [DOI] [PubMed] [Google Scholar]
- 161.Geertsen SS, van de Ruit M, Grey MJ & Nielsen JB Spinal inhibition of descending command to soleus motoneurons is removed prior to dorsiflexion. J. Physiol 589, 5819–5831 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seki K & Fetz EE Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J. Neurosci 32, 890–902 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bunday KL, Tazoe T, Rothwell JC & Perez MA Subcortical control of precision grip after human spinal cord injury. J. Neurosci 34, 7341–7350 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aymard C et al. Modulation of presynaptic inhibition of Ia afferents during voluntary wrist flexion and extension in man. Exp. Brain Res 137, 127–131 (2001). [DOI] [PubMed] [Google Scholar]
- 165.Iglesias C, Marchand-Pauvert V, Lourenço G, Burke D & Pierrot-Deseilligny E Task-related changes in propriospinal excitation from hand muscles to human flexor carpi radialis motoneurones. J. Physiol 582, 1361–1379 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oya T, Takei T & Seki K Distinct sensorimotor feedback loops for dynamic and static control of primate precision grip. Commun. Biol 3, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hongo T, Kitazawa S, Ohki Y, Sasaki M & Xi M-C A physiological and morphological study of premotor interneurones in the cutaneous reflex pathways in cats. Brain Res 505, 163–166 (1989). [DOI] [PubMed] [Google Scholar]
- 168.Dum RP & Strick PL Spinal cord terminations of the medial wall motor areas in macaque monkeys. J. Neurosci 16, 6513–6525 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McNeal DW et al. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J. Comp. Neurol 518, 586–621 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morecraft RJ et al. Terminal organization of the corticospinal projection from the lateral premotor cortex to the cervical enlargement (C5–T1) in rhesus monkey. J. Comp. Neurol 527, 2761–2789 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bernhard CG, Bohm E & Petersén I Investigations on the organization of the corticospinal system in monkeys. Acta Physiol. Scand 29, 79–105 (1953). [Google Scholar]
- 172.Rathelot JA & Strick PL Muscle representation in the macaque motor cortex: an anatomical perspective. Proc. Natl Acad. Sci. USA 103, 8257–8262 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Rathelot JA & Strick PL Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl Acad. Sci. USA 106, 918–923 (2009). This study describes the differences between ‘old’ (rostral) and ‘new’ (caudal) M1, delineated on the basis of the absence or presence of CM cells.
- 174.Lemon RN Descending pathways in motor control. Annu. Rev. Neurosci 31, 195–218 (2008). [DOI] [PubMed] [Google Scholar]
- 175.Kuypers HGJM Anatomy of the descending pathways. in Comprehensive Physiology (ed. Terjung R) 597–666 (John Wiley & Sons Inc., 2011). [Google Scholar]
- 176.Morecraft RJ et al. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J. Comp. Neurol 521, 4205–4235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fetz EE & Cheney PD Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J. Neurophysiol 44, 751–772 (1980). [DOI] [PubMed] [Google Scholar]
- 178.McKiernan BJ, Marcario JK, Karrer JH & Cheney PD Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J. Neurophysiol 80, 1961–1980 (1998). [DOI] [PubMed] [Google Scholar]
- 179.Park MC, Belhaj-Saïf A & Cheney PD Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J. Neurophysiol 92, 2968–2984 (2004). [DOI] [PubMed] [Google Scholar]
- 180.Quallo MM, Kraskov A & Lemon RN The activity of primary motor cortex corticospinal neurons during tool use by macaque monkeys. J. Neurosci 32, 17351–17364 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Griffin DM, Hoffman DS & Strick PL Corticomotoneuronal cells are ‘functionally tuned’. Science 350, 667–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Muir RB & Lemon RN Corticospinal neurons with a special role in precision grip. Brain Res 261, 312–316 (1983). [DOI] [PubMed] [Google Scholar]
- 183.Cheney PD & Fetz EE Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J. Physiol 349, 249–272 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Day BL, Riescher H, Struppler A, Rothwell JC & Marsden CD Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. J. Physiol 433, 41–57 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pruszynski JA, Omrani M & Scott SH Goal-dependent modulation of fast feedback responses in primary motor cortex. J. Neurosci 34, 4608–4617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nielsen JB Human spinal motor control. Annu. Rev. Neurosci 39, 81–101 (2016). This is a review of the role of spinal and supraspinal reflexes in the control of movement.
- 187.Schieber MH & Rivlis G Partial reconstruction of muscle activity from a pruned network of diverse motor cortex neurons. J. Neurophysiol 97, 70–82 (2007). [DOI] [PubMed] [Google Scholar]
- 188.Brouwer B & Ashby P Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr. Clin. Neurophysiol 76, 509–519 (1990). [DOI] [PubMed] [Google Scholar]
- 189.Nielsen J, Petersen N & Ballegaard M Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. J. Physiol 484, 791–802 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.de Noordhout AM et al. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain 122, 1327–1340 (1999). [DOI] [PubMed] [Google Scholar]
- 191.Barthelemy D & Nielsen JB Corticospinal contribution to arm muscle activity during human walking. J. Physiol 588, 967–979 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Petersen TH, Willerslev-Olsen M, Conway BA & Nielsen JB The motor cortex drives the muscles during walking in human subjects. J. Physiol 590, 2443–2452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jackson JH Selected Writings of John Hughlings Jackson. Volume 2. Evolution and Dissolution of the Nervous System, Speech, Various Papers, Addresses and Lectures. Arch. Neurol. And. Psychiatry 28, 967 (1932). [Google Scholar]
- 194.Kuypers HG Corticospinal connections: postnatal development in the rhesus monkey. Science 138, 678–680 (1962). [DOI] [PubMed] [Google Scholar]
- 195. Heffner R & Masterton B Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain, Behav. Evol 12, 161–200 (1975). This study analyses the relationships between dexterity, corticospinal projections and phylogeny. Also see Iwaniuk et al. (1999).
- 196.Heffner RS & Masterton RB The role of the corticospinal tract in the evolution of human digital dexterity. Brain, Behav. Evol 23, 165–183 (1983). [DOI] [PubMed] [Google Scholar]
- 197.Georgopoulos AP & Grillner S Visuomotor coordination in reaching and locomotion. Science 245, 1209–1210 (1989). [DOI] [PubMed] [Google Scholar]
- 198.Iwaniuk AN, Pellis SM & Whishaw IQ Is digital dexterity really related to corticospinal projections?: are- analysis of the Heffner and Masterton data set using modern comparative statistics. Behav. Brain Res 101, 173–187 (1999). [DOI] [PubMed] [Google Scholar]
- 199. Lemon R Recent advances in our understanding of the primate corticospinal system. F1000Research 10.12688/f1000research.17445.1 (2019). This is a review of the corticospinal system.
- 200.Sasaki S et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J. Neurophysiol 92, 3142–3147 (2004). [DOI] [PubMed] [Google Scholar]
- 201.Alstermark B et al. Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. J. Neurophysiol 106, 122–126 (2011). [DOI] [PubMed] [Google Scholar]
- 202.Napier JR Studies of the hands of living primates. Proc. Zool. Soc. Lond 134, 647–657 (1960). [Google Scholar]
- 203.Bishop A Use of the hand in lower primates. Evol. Genet. Biol. Primates 10.1016/b978-0-12-395562-3.50010-2 (1964). [DOI] [Google Scholar]
- 204.Bortoff GA & Strick PL Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J. Neurosci 13, 5105–5118 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Maier MA et al. Direct and indirect corticospinal control of arm and hand motoneurons in the squirrel monkey (Saimiri sciureus). J. Neurophysiol 78, 721–733 (1997). [DOI] [PubMed] [Google Scholar]
- 206.Petras JM & Lehman RAW Corticospinal fibers in the raccoon. Brain Res 3, 195–197 (1966). [DOI] [PubMed] [Google Scholar]
- 207.Buxton DF & Goodman DC Motor function and the corticospinal tracts in the dog and raccoon. J. Comp. Neurol 129, 341–360 (1967). [DOI] [PubMed] [Google Scholar]
- 208.Wirth FP, O’Leary JL, Smith JM & Jenny AB Monosynaptic corticospinal-motoneuron path in the raccoon. Brain Res 77, 344–348 (1974). [DOI] [PubMed] [Google Scholar]
- 209.Petras JM Some efferent connections of the motor and somatosensory cortex of simian primates and felid, canid and procyonid carnivores. Ann. N. Y. Acad. Sci 167, 469–505 (1969). [Google Scholar]
- 210.Gugino LD, Rowinski MJ & Stoney SD Motor outflow to cervical motoneurons from raccoon motorsensory cortex. Brain Res. Bull 24, 833–837 (1990). [DOI] [PubMed] [Google Scholar]
- 211.McClearn D Locomotion, posture, and feeding behavior of kinkajous, coatis, and raccoons. J. Mammalo 73, 245–261 (1992). [Google Scholar]
- 212. Gu Z et al. Control of species-dependent corticomotoneuronal connections underlying manual dexterity. Science 357, 400–404 (2017). In this study, retention of CM cells in adult mice via genetic manipulation led to increased dexterity.
- 213. Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H & Acuna C Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol 38, 871–908 (1975). This is a classic study of the role of the PPC in reaching.
- 214.Goodale MA & Milner AD Separate visual pathways for perception and action. Trends Neurosci 15, 20–25 (1992). [DOI] [PubMed] [Google Scholar]
- 215.Debowy DJ, Ghosh S, Ro JY & Gardner EP Comparison of neuronal firing rates in somatosensory and posterior parietal cortex during prehension. Exp. Brain Res 137, 269–291 (2001). [DOI] [PubMed] [Google Scholar]
- 216.Andersen RA & Buneo CA Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci 25, 189–220 (2002). [DOI] [PubMed] [Google Scholar]
- 217.Culham JC & Valyear KF Human parietal cortex in action. Curr. Opin. Neurobiol 16, 205–212 (2006). [DOI] [PubMed] [Google Scholar]
- 218.Brochier T & Umiltà MA Cortical control of grasp in non-human primates. Curr. Opin. Neurobiol 17, 637–643 (2007). [DOI] [PubMed] [Google Scholar]
- 219.Evangeliou MN, Raos V, Galletti C & Savaki HE Functional imaging of the parietal cortex during action execution and observation. Cereb. Cortex 19, 624–639 (2009). [DOI] [PubMed] [Google Scholar]
- 220. Schaffelhofer S & Scherberger H Object vision to hand action in macaque parietal, premotor, and motor cortices. eLife 5, e15278 (2016). This study shows interplay between different regions of the parietal and frontal lobes during reach and grasp.
- 221.Caminiti R et al. Understanding the parietal lobe syndrome from a neurophysiological and evolutionary perspective. Eur. J. Neurosci 31, 2320–2340 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caminiti R, Innocenti GM & Battaglia-Mayer A Organization and evolution of parieto-frontal processing streams in macaque monkeys and humans. Neurosci. Biobehav. Rev 56, 73–96 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Seelke AMH et al. Topographic maps within Brodmann’s area 5 of macaque monkeys. Cereb. Cortex 22, 1834–1850 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Eskandar EN & Assad JA Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat. Neurosci 2, 88–93 (1999). [DOI] [PubMed] [Google Scholar]
- 225.Calton JL, Dickinson AR & Snyder LH Non-spatial, motor-specific activation in posterior parietal cortex. Nat. Neurosci 5, 580–588 (2002). [DOI] [PubMed] [Google Scholar]
- 226.Cohen YE & Andersen RA A common reference frame for movement plans in the posterior parietal cortex. Nat. Rev. Neurosci 3, 553–562 (2002). [DOI] [PubMed] [Google Scholar]
- 227.Fernandez-Ruiz J, Goltz HC, DeSouza JFX, Vilis T & Crawford JD Human parietal “reach region” primarily encodes intrinsic visual direction, not extrinsic movement direction, in a visual–motor dissociation task. Cereb. Cortex 17, 2283–2292 (2007). [DOI] [PubMed] [Google Scholar]
- 228.Lindner A, Iyer A, Kagan I & Andersen RA Human posterior parietal cortex plans where to reach and what to avoid. J. Neurosci 30, 11715–11725 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vesia M & Crawford JD Specialization of reach function in human posterior parietal cortex. Exp. Brain Res 221, 1–18 (2012). [DOI] [PubMed] [Google Scholar]
- 230.Scherberger H, Jarvis MR & Andersen RA Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron 46, 347–354 (2005). [DOI] [PubMed] [Google Scholar]
- 231.Davare M, Zénon A, Pourtois G, Desmurget M & Olivier E Role of the medial part of the intraparietal sulcus in implementing movement direction. Cereb. Cortex 22, 1382–1394 (2012). [DOI] [PubMed] [Google Scholar]
- 232.Cisek P & Kalaska JF Simultaneous encoding of multiple potential reach directions in dorsal premotor cortex. J. Neurophysiol 87, 1149–1154 (2002). [DOI] [PubMed] [Google Scholar]
- 233.Cisek P & Kalaska JF Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45, 801–814 (2005). [DOI] [PubMed] [Google Scholar]
- 234.Cisek P & Kalaska JF Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci 33, 269–298 (2010). [DOI] [PubMed] [Google Scholar]
- 235.Scherberger H & Andersen RA Target selection signals for arm reaching in the posterior parietal cortex. J. Neurosci 27, 2001–2012 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Klaes C, Westendorff S, Chakrabarti S & Gail A Choosing goals, not rules: deciding among rule-based action plans. Neuron 70, 536–548 (2011). [DOI] [PubMed] [Google Scholar]
- 237.Johnson PB, Ferraina S, Bianchi L & Caminiti R Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb. Cortex 6, 102–119 (1996). [DOI] [PubMed] [Google Scholar]
- 238.Cohen YE & Andersen RA Reaches to sounds encoded in an eye-centered reference frame. Neuron 27, 647–652 (2000). [DOI] [PubMed] [Google Scholar]
- 239.Taira M, Mine S, Georgopoulos AP, Murata A & Sakata H Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp. Brain Res 83, 29–36 (1990). [DOI] [PubMed] [Google Scholar]
- 240.Sakata H, Taira M, Murata A & Mine S Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb. Cortex 5, 429–438 (1995). [DOI] [PubMed] [Google Scholar]
- 241.Murata A, Gallese V, Luppino G, Kaseda M & Sakata H Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J. Neurophysiol 83, 2580–2601 (2000). [DOI] [PubMed] [Google Scholar]
- 242.Durand J-B et al. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron 55, 493–505 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Borra E et al. Cortical connections of the macaque anterior intraparietal (AIP) area. Cerebral Cortex 18, 1094–1111 (2008). [DOI] [PubMed] [Google Scholar]
- 244.Gharbawie OA, Stepniewska I, Qi H & Kaas JH Multiple parietal–frontal pathways mediate grasping in macaque monkeys. J. Neurosci 31, 11660–11677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kaas JH, Gharbawie O & Stepniewska I The organization and evolution of dorsal stream multisensory motor pathways in primates. Front. Neuroanat 5, 34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stepniewska I, Gharbawie OA, Burish MJ & Kaas JH Effects of muscimol inactivations of functional domains in motor, premotor, and posterior parietal cortex on complex movements evoked by electrical stimulation. J. Neurophysiol 111, 1100–1119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Janssen P & Scherberger H Visual guidance in control of grasping. Annu. Rev. Neurosci 38, 69–86 (2015). [DOI] [PubMed] [Google Scholar]
- 248.Baumann MA, Fluet M-C & Scherberger H Context-specific grasp movement representation in the macaque anterior intraparietal area. J. Neurosci 29, 6436–6448 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Srivastava S, Orban GA, Mazière PAD & Janssen P A distinct representation of three-dimensional shape in macaque anterior intraparietal area: fast, metric, and coarse. J. Neurosci 29, 10613–10626 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lehmann SJ & Scherberger H Reach and gaze representations in macaque parietal and premotor grasp areas. J. Neurosci 33, 7038–7049 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gallese V, Murata A, Kaseda M, Niki N & Sakata H Deficit of hand preshaping after muscimol injection in monkey parietal cortex. NeuroReport 5, 1525–1529 (1994). [DOI] [PubMed] [Google Scholar]
- 252.Fogassi L et al. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: a reversible inactivation study. Brain 124, 571–586 (2001). [DOI] [PubMed] [Google Scholar]
- 253.Davare M, Rothwell JC & Lemon RN Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr. Biol 20, 176–181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Davare M, Kraskov A, Rothwell JC & Lemon RN Interactions between areas of the cortical grasping network. Curr. Opin. Neurobiol 21, 565–570 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fluet M-C, Baumann MA & Scherberger H Context-specific grasp movement representation in macaque ventral premotor cortex. J. Neurosci 30, 15175–15184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Theys T, Pani P, Loon J, van, Goffin J & Janssen P. Selectivity for three-dimensional shape and grasping-related activity in the macaque ventral premotor cortex. J. Neurosci 32, 12038–12050 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Theys T, Pani P, van Loon J, Goffin J & Janssen P Three-dimensional shape coding in grasping circuits: a comparison between the anterior intraparietal area and ventral premotor area F5a. J. Cognit. Neurosci 25, 352–364 (2012). [DOI] [PubMed] [Google Scholar]
- 258.Schaffelhofer S, Agudelo-Toro A & Scherberger H Decoding a wide range of hand configurations from macaque motor, premotor, and parietal cortices. J. Neurosci 35, 1068–1081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gardner EP et al. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J. Neurophysiol 97, 387–406 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gardner EP, Ro JY, Babu KS & Ghosh S Neurophysiology of prehension. II. Response diversity in primary somatosensory (S-I) and motor (M-I) cortices. J. Neurophysiol 97, 1656–1670 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jeannerod M, Arbib MA, Rizzolatti G & Sakata H Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18, 314–320 (1995). [PubMed] [Google Scholar]
- 262.Ehrsson HH, Geyer S & Naito E Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J. Neurophysiol 90, 3304–3316 (2003). [DOI] [PubMed] [Google Scholar]
- 263.Raos V, Umiltá M-A, Murata A, Fogassi L & Gallese V Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J. Neurophysiol 95, 709–729 (2006). [DOI] [PubMed] [Google Scholar]
- 264.Hoshi E & Tanji J Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol 17, 234–242 (2007). [DOI] [PubMed] [Google Scholar]
- 265.Stark E, Asher I & Abeles M Encoding of reach and grasp by single neurons in premotor cortex is independent of recording site. J. Neurophysiol 97, 3351–3364 (2007). [DOI] [PubMed] [Google Scholar]
- 266.Umilta MA, Brochier T, Spinks RL & Lemon RN Simultaneous recording of macaque premotor and primary motor cortex neuronal populations reveals different functional contributions to visuomotor grasp. J. Neurophysiol 98, 488–501 (2007). [DOI] [PubMed] [Google Scholar]
- 267.Spinks RL, Kraskov A, Brochier T, Umilta MA & Lemon RN Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J. Neurosci 28, 10961–10971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jerjian SJ, Sahani M & Kraskov A Movement initiation and grasp representation in premotor and primary motor cortex mirror neurons. eLife 9, e54139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rathelot J-AA, Dum RP & Strick PL Posterior parietal cortex contains a command apparatus for hand movements. Proc. Natl Acad. Sci. USA 114, 4255–4260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cooke DF, Taylor CSR, Moore T & Graziano MSA Complex movements evoked by microstimulation of the ventral intraparietal area. Proc. Natl Acad. Sci. USA 100, 6163–6168 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gharbawie OA, Stepniewska I & Kaas JH Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in new world monkeys. Cereb. Cortex 21, 1981–2002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Desmurget M et al. Selective inhibition of volitional hand movements after stimulation of the dorsoposterior parietal cortex in humans. Curr. Biol 28, 3303–3309.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 273.O’Connor DH, Krubitzer L & Bensmaia S Of mice and monkeys: somatosensory processing in two prominent animal models. Prog. Neurobiol 10.1016/j.pneurobio.2021.102008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rothwell JC et al. Manual motor performance in a deafferented man. Brain 105, 515–542 (1982). [DOI] [PubMed] [Google Scholar]
- 275.Sainburg RL, Poizner H & Ghez C Loss of proprioception produces deficits in interjoint coordination. J. Neurophysiol 70, 2136–2147 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sainburg RL, Ghilardi MF, Poizner H & Ghez C Control of limb dynamics in normal subjects and patients without proprioception. J. Neurophysiol 73, 820–835 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cole J Pride and a Daily Marathon (MIT Press, 1995). [Google Scholar]
- 278.Dürr A et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N. Engl. J. Med 335, 1169–1175 (1996). [DOI] [PubMed] [Google Scholar]
- 279.Valdmanis PN et al. A novel neurodegenerative disease characterised by posterior column ataxia and pyramidal tract involvement maps to chromosome 8p12–8q12.1. J. Med. Genet 41, 634–639 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sghirlanzoni A, Pareyson D & Lauria G Sensory neuron diseases. Lancet Neurol 4, 349–361 (2005). [DOI] [PubMed] [Google Scholar]
- 281.Pandolfo M Friedreich ataxia: the clinical picture. J. Neurol 256, 3–8 (2009). [DOI] [PubMed] [Google Scholar]
- 282.Spinazzi M, Angelini C & Patrini C Subacute sensory ataxia and optic neuropathy with thiamine deficiency. Nat. Rev. Neurol 6, 288–293 (2010). [DOI] [PubMed] [Google Scholar]
- 283.Chesler AT et al. The role of PIEZO2 in human mechanosensation. N. Engl. J. Med 375, 1355–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mahmud AA et al. Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clin. Genet 91, 470–475 (2017). [DOI] [PubMed] [Google Scholar]
- 285.Yahya A et al. The impact of diabetic peripheral neuropathy on pinch proprioception. Exp. Brain Res 237, 3165–3174 (2019). [DOI] [PubMed] [Google Scholar]
- 286.Jiang W, Tremblay F & Chapman CE Context-dependent tactile texture-sensitivity in monkey M1 and S1 cortex. J. Neurophysiol 120, 2334–2350 (2018). [DOI] [PubMed] [Google Scholar]
- 287.Umeda T, Isa T & Nishimura Y The somatosensory cortex receives information about motor output. Sci. Adv 5, eaaw5388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Talbot WH, Darian-Smith I, Kornhuber HH & Mountcastle VB The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J. Neurophysiol 31, 301–334 (1968). [DOI] [PubMed] [Google Scholar]
- 289.Skedung L et al. Feeling small: exploring the tactile perception limits. Sci. Rep 3, 2617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Craig JC & Johnson KO The two-point threshold: not a measure of tactile spatial resolution. Curr. Dir. Psychol. Sci 9, 29–32 (2000). [Google Scholar]
- 291.Craig JC Grating orientation as a measure of tactile spatial acuity. Somatosens. Mot. Res 16, 197–206 (1999). [DOI] [PubMed] [Google Scholar]
- 292.Hollins M & Risner SR Evidence for the duplex theory of tactile texture perception. Percept. Psychophys 62, 695–705 (2000). [DOI] [PubMed] [Google Scholar]
- 293.Bolanowski SJ, Gescheider GA, Verrillo RT & Checkosky CM Four channels mediate the mechanical aspects of touch. J. Acoustical Soc. Am 84, 1680–1694 (1988). [DOI] [PubMed] [Google Scholar]
- 294.Bensmaia SJ, Hsiao SS, Denchev PV, Killebrew JH & Craig JC The tactile perception of stimulus orientation. Somatosens. Mot. Res 25, 49–59 (2008). [DOI] [PubMed] [Google Scholar]
- 295.Olczak D, Sukumar V & Pruszynski JA Edge orientation perception during active touch. J. Neurophysiol 120, 2423–2429 (2018). [DOI] [PubMed] [Google Scholar]
- 296.Pruszynski JA, Flanagan JR & Johansson RS Fast and accurate edge orientation processing during object manipulation. eLife 7, e31200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Johansson RS & Vallbo AB Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol 286, 283–300 (1979). This article provides a classic description of the tactile sensors in the human hand.
- 298.Vallbo AB & Johansson RS Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum. Neurobiol 3, 3–14 (1984). [PubMed] [Google Scholar]
- 299.Johansson RS & Flanagan JR Tactile sensory control of object manipulation in humans. in The Senses: A Comprehensive Reference (eds Basbaum AI, Kaneko A, Shepherd GM & Westheimer G) 67–86 (Elsevier, 2008). [Google Scholar]
- 300.Goodman JM & Bensmaia SJ The neural mechanisms of touch and proprioception at the somatosensory periphery. in The Senses (ed. Glüçlü B) (Elsevier, 2020). [Google Scholar]
- 301.Dong Y et al. A simple model of mechanotransduction in primate glabrous skin. J. Neurophysiol 109, 1350–1359 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kim SS, Sripati AP & Bensmaia SJ Predicting the timing of spikes evoked by tactile stimulation of the hand. J. Neurophysiol 104, 1484–1496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Saal HP, Delhaye BP, Rayhaun BC & Bensmaia SJ Simulating tactile signals from the whole hand with millisecond precision. Proc. Natl Acad. Sci. USA 114, E5693–E5702 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kistemaker DA, Van Soest AJK, Wong JD, Kurtzer I & Gribble PL Control of position and movement is simplified by combined muscle spindle and Golgi tendon organ feedback. J. Neurophysiol 109, 1126–1139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cross MJ & McCloskey DI Position sense following surgical removal of joints in man. Brain Res 55, 443–445 (1973). [DOI] [PubMed] [Google Scholar]
- 306.Grigg P & Greenspan BJ Response of primate joint afferent neurons to mechanical stimulation of knee joint. J. Neurophysiol 40, 1–8 (1977). [DOI] [PubMed] [Google Scholar]
- 307.Iles JF, Stokes M & Young A Reflex actions of knee joint afferents during contraction of the human quadriceps. Clin. Physiol 10, 489–500 (1990). [DOI] [PubMed] [Google Scholar]
- 308. Prochazka A & Ellaway P Sensory systems in the control of movement. Compr. Physiol 2, 2615–2627 (2012). This article describes the proprioceptive mechanisms that support motor control.
- 309.Burke D, Gandevia SC & Macefield G Responses to passive movement of receptors in joint, skin and muscle of the human hand. J. Physiol 402, 347–361 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Edin BB Finger joint movement sensitivity of non-cutaneous mechanoreceptor afferents in the human radial nerve. Exp. Brain Res 82, 417–422 (1990). [DOI] [PubMed] [Google Scholar]
- 311.Buchthal F & Schmalbruch H Motor unit of mammalian muscle. Physiol. Rev 60, 90–142 (1980). [DOI] [PubMed] [Google Scholar]
- 312.Banks RW An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J. Anat 208, 753–768 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jami L Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol.l Rev 72, 623–666 (1992). [DOI] [PubMed] [Google Scholar]
- 314.Jozsa L, Kannus P, Järvinen TA, Balint J & Järvinen M Number and morphology of mechanoreceptors in the myotendinous junction of paralysed human muscle. J. Pathol 178, 195–200 (1996). [DOI] [PubMed] [Google Scholar]
- 315.Prochazka A Proprioceptive feedback and movement regulation. in Comprehensive Physiology (ed. Terjung R) 89–127 (John Wiley & Sons Inc., 2011). [Google Scholar]
- 316.Sica REP, McComas AJ, Upton ARM & Longmire D Motor unit estimations in small muscles of the hand. J. Neurol. Neurosurg. Psychiatry 37, 55–67 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jenny AB & Inukai J Principles of motor organization of the monkey cervical spinal cord. J. Neurosci 3, 567–575 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Fuglevand AJ Mechanical properties and neural control of human hand motor units. J. Physiol 589, 5595–5602 (2011). This is a review of motor unit organization in human hand muscles.
- 319.de Hamilton AF, Jones KE & Wolpert DM The scaling of motor noise with muscle strength and motor unit number in humans. Exp. Brain Res 157, 417–430 (2004). [DOI] [PubMed] [Google Scholar]
- 320.Negro F, Holobar A & Farina D Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J. Physiol 587, 5925–5938 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Salmond LH, Davidson AD & Charles SK Proximal-distal differences in movement smoothness reflect differences in biomechanics. J. Neurophysiol 117, 1239–1257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Enoka RM & Farina D Force steadiness: from motor units to voluntary actions. Physiology 36, 114–130 (2021). [DOI] [PubMed] [Google Scholar]
- 323.Delhaye BP, Long KH & Bensmaia SJ Neural basis of touch and proprioception in primate cortex. Compr. Physiol 8, 1575–1602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Merzenich MM, Kaas JH, Sur M & Lin C-S Double representation of the body surface within cytoarchitectonic area 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus). J. Comp. Neurol 181, 41–73 (1978). [DOI] [PubMed] [Google Scholar]
- 325.Kaas JH What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol. Rev 63, 206–231 (1983). [DOI] [PubMed] [Google Scholar]
- 326. Pons TP, Garraghty PE, Cusick CG & Kaas JH The somatotopic organization of area 2 in macaque monkeys. J. Comp. Neurol 241, 445–466 (1985). The article describes the body maps in the somatosensory cortex.
- 327.Qi H-X & Kaas JH Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J. Comp. Neurol 477, 172–187 (2004). [DOI] [PubMed] [Google Scholar]
- 328. Johansson RS & Flanagan JR Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci 10, 345–359 (2009). This is a review of how tactile signals support object interactions.
- 329.Callier T, Suresh AK & Bensmaia SJ Neural coding of contact events in somatosensory cortex. Cereb. Cortex 10.1093/cercor/bhy337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Jeannerod M The timing of natural prehension movements. J. Mot. Behav 16, 235–254 (1984). [DOI] [PubMed] [Google Scholar]
- 331.Gentilucci M, Toni I, Daprati E & Gangitano M Tactile input of the hand and the control of reaching to grasp movements. Exp. Brain Res 114, 130–137 (1997). [DOI] [PubMed] [Google Scholar]
- 332.Rao A & Gordon A Contribution of tactile information to accuracy in pointing movements. Exp. Brain Res 138, 438–445 (2001). [DOI] [PubMed] [Google Scholar]
- 333.Johansson RS & Westling G Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp. Brain Res 56, 550–564 (1984). [DOI] [PubMed] [Google Scholar]
- 334.Jenmalm P & Johansson RS Visual and somatosensory information about object shape control manipulative fingertip forces. J. Neurosci 17, 4486–4499 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Goodwin AW, Jenmalm P & Johansson RS Control of grip force when tilting objects: effect of curvature of grasped surfaces and applied tangential torque. J. Neurosci 18, 10724–10734 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Jenmalm P, Dahlstedt S & Johansson RS Visual and tactile information about object-curvature control fingertip forces and grasp kinematics in human dexterous manipulation. J. Neurophysiol 84, 2984–2997 (2000). [DOI] [PubMed] [Google Scholar]
- 337.Augurelle A-S, Smith AM, Lejeune T & Thonnard J-L Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J. Neurophysiol 89, 665–671 (2003). [DOI] [PubMed] [Google Scholar]
- 338.Delhaye BP et al. High-resolution imaging of skin deformation shows that afferents from human fingertips signal slip onset. eLife 10, e64679 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Westling G & Johansson RS Responses in glabrous skin mechanoreceptors during precision grip in humans. Exp. Brain Res 66, 128–140 (1987). [DOI] [PubMed] [Google Scholar]
- 340.Miller LE et al. Sensing with tools extends somatosensory processing beyond the body. Nature 561, 239–242 (2018). [DOI] [PubMed] [Google Scholar]
- 341.Strick PL & Preston JB Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J. Neurophysiol 48, 150–159 (1982). [DOI] [PubMed] [Google Scholar]
- 342.Friel KM et al. Dissociation of sensorimotor deficits after rostral versus caudal lesions in the primary motor cortex hand representation. J. Neurophysiol 94, 1312–1324 (2005). [DOI] [PubMed] [Google Scholar]
- 343.Forgaard CJ, Reschechtko S, Gribble PL & Pruszynski JA Skin and muscle receptors shape coordinated fast feedback responses in the upper limb. Curr. Opin. Physiol 20, 198–205 (2021). [Google Scholar]
- 344.Purdy KA, Lederman SJ & Klatzky RL Manipulation with no or partial vision. J. Exp. Psychol. Hum. Percept. Perform 25, 755–774 (1999). [DOI] [PubMed] [Google Scholar]
- 345.Saal HP & Bensmaia SJ Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci 37, 689–697 (2014). [DOI] [PubMed] [Google Scholar]
- 346.Reed CL, Caselli RJ & Farah MJ Tactile agnosia: underlying impairment and implications for normal tactile object recognition. Brain 119, 875–888 (1996). [DOI] [PubMed] [Google Scholar]
- 347.Bohlhalter S, Fretz C & Weder B Hierarchical versus parallel processing in tactile object recognition: a behavioural–neuroanatomical study of aperceptive tactile agnosia. Brain 125, 2537–2548 (2002). [DOI] [PubMed] [Google Scholar]
- 348.Veronelli L, Ginex V, Dinacci D, Cappa SF & Corbo M Pure associative tactile agnosia for the left hand: clinical and anatomo-functional correlations. Cortex 58, 206–216 (2014). [DOI] [PubMed] [Google Scholar]
- 349.Kubota S, Yamada M, Satoh H, Satoh A & Tsujihata M Pure amorphagnosia without tactile object agnosia. Case Rep. Neurol 9, 62–68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Phillips JR, Johnson KO & Hsiao SS Spatial pattern representation and transformation in monkey somatosensory cortex. Proc. Natl Acad. Sci. USA 85, 1317–1321 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Jenmalm P, Birznieks I, Goodwin AW & Johansson RS Influence of object shape on responses of human tactile afferents under conditions characteristic of manipulation. Eur. J. Neurosci 18, 164–176 (2003). [DOI] [PubMed] [Google Scholar]
- 352.Delhaye BP, Xia X & Bensmaia SJ Rapid geometric feature signaling in the simulated spiking activity of a complete population of tactile nerve fibers. J. Neurophysiol. J. Neurophysiology 121, 2071–2082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.DiCarlo JJ, Johnson KO & Hsiao SS Structure of receptive fields in area 3b of primary somatosensory cortex in the alert monkey. J. Neurosci 18, 2626–2645 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Bensmaia SJ, Denchev PV, Dammann JF, Craig JC & Hsiao SS The Representation of stimulus orientation in the early stages of somatosensory processing. J. Neurosci 28, 776–786 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yau JM, Pasupathy A, Fitzgerald PJ, Hsiao SS & Connor CE Analogous intermediate shape coding in vision and touch. Proc. Natl Acad. Sci. USA 106, 16457–16462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yau JM, Connor CE & Hsiao SS Representation of tactile curvature in macaque somatosensory area 2. J. Neurophysiol 109, 2999–3012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yau JM, Kim SS, Thakur PH & Bensmaia SJ Feeling form: the neural basis of haptic shape perception. J. Neurophysiol 115, 631–642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Fitzgerald PJ, Lane JW, Thakur PH & Hsiao SS Receptive field properties of the macaque second somatosensory cortex: representation of orientation on different finger pads. J. Neurosci 26, 6473–6484 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Dépeault A, Meftah E-M & Chapman CE Tactile speed scaling: contributions of time and space. J. Neurophysiol 99, 1422–1434 (2008). [DOI] [PubMed] [Google Scholar]
- 360.Pei YC, Hsiao SS & Bensmaia SJ The tactile integration of local motion cues is analogous to its visual counterpart. Proc. Natl Acad. Sci. USA 105, 8130–8135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Delhaye BP, O’Donnell MK, Lieber JD, McLellan KR & Bensmaia SJ Feeling fooled: texture contaminates the neural code for tactile speed. PLoS Biol 17, e3000431 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Costanzo RM & Gardner EP A quantitative analysis of responses of direction-sensitive neurons in somatosensory cortex of awake monkeys. J. Neurophysiol 43, 1319–1341 (1980). [DOI] [PubMed] [Google Scholar]
- 363.Warren S, Hamalainen HA & Gardner EP Objective classification of motion- and direction-sensitive neurons in primary somatosensory cortex of awake monkeys. J. Neurophysiol 56, 598–622 (1986). [DOI] [PubMed] [Google Scholar]
- 364.Pei Y-C, Hsiao SS, Craig JC & Bensmaia SJ Shape invariant coding of motion direction in somatosensory cortex. PLoS Biol 8, e1000305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Pei Y-C, Hsiao SS, Craig JC & Bensmaia SJ Neural mechanisms of tactile motion integration in somatosensory cortex. Neuron 69, 536–547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lederman SJ & Klatzky RL Extracting object properties through haptic exploration. Acta Psychol 84, 29–40 (1993). [DOI] [PubMed] [Google Scholar]
- 367.BensmaÏa SJ & Hollins M The vibrations of texture. Somatosens. Mot. Res 20, 33–43 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Bensmaïa S & Hollins M Pacinian representations of fine surface texture. Percept. Psychophys 67, 842–854 (2005). [DOI] [PubMed] [Google Scholar]
- 369.Manfredi LR et al. Natural scenes in tactile texture. J. Neurophysiol 111, 1792–1802 (2014). [DOI] [PubMed] [Google Scholar]
- 370.Greenspon CM, McLellan KR, Lieber JD & Bensmaia SJ Effect of scanning speed on texture-elicited vibrations. J. R. Soc. Interface 17, 20190892 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Weber AI et al. Spatial and temporal codes mediate the tactile perception of natural textures. Proc. Natl Acad. Sci. USA 110, 17107–17112 (2013). This study shows that texture signals are conveyed by three populations of nerve fibres using two different neural codes.
- 372.Saal HP, Harvey MA & Bensmaia SJ Rate and timing of cortical responses driven by separate sensory channels. eLife 4, e10450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Lieber JD & Bensmaia SJ High-dimensional representation of texture in somatosensory cortex of primates. Proc. Natl Acad. Sci. USA 10.1073/pnas.1818501116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Miall RC, Rosenthal O, Ørstavik K, Cole JD & Sarlegna FR Loss of haptic feedback impairs control of hand posture: a study in chronically deafferented individuals when grasping and lifting objects. Exp. Brain Res 237, 2167–2184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.London BM & Miller LE Responses of somatosensory area 2 neurons to actively and passively generated limb movements. J. Neurophysiol 109, 1505–1513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Chowdhury RH, Glaser JI & Miller LE Area 2 of primary somatosensory cortex encodes kinematics of the whole arm. eLife 9, 1–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hulliger M The mammalian muscle spindle and its central control. Rev. Physiol. Biochem. Pharmacol 101, 1–110 (1984). [DOI] [PubMed] [Google Scholar]
- 378.Prochazka A, Hulliger M, Zangger P & Appenteng K ‘Fusimotor set’: new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res 339, 136–140 (1985). [DOI] [PubMed] [Google Scholar]
- 379.Hall LA & McCloskey DI Detections of movements imposed on finger, elbow and shoulder joints. J. Physiol 335, 519–533 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Okorokova EV, Goodman JM, Hatsopoulos NG & Bensmaia SJ Decoding hand kinematics from population responses in sensorimotor cortex during grasping. arXiv https://arxiv.org/abs/1904.03531 (2019). [DOI] [PubMed] [Google Scholar]
- 381.Li Z-M, Latash ML & Zatsiorsky VM Force sharing among fingers as a model of the redundancy problem. Exp. Brain Res 119, 276–286 (1998). [DOI] [PubMed] [Google Scholar]
- 382.Hendrix CM, Mason CR & Ebner TJ Signaling of grasp dimension and grasp force in dorsal premotor cortex and primary motor cortex neurons during reach to grasp in the monkey. J. Neurophysiol 102, 132–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.London BM, Torres RR, Slutzky MW & Miller LE Designing stimulation patterns for an afferent BMI: representation of kinetics in somatosensory cortex in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 7521–7524 (IEEE, 2011). [DOI] [PubMed] [Google Scholar]
- 384.Kim SS, Gomez-Ramirez M, Thakur PH & Hsiao SS Multimodal interactions between proprioceptive and cutaneous signals in primary somatosensory cortex. Neuron 86, 555–566 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Manto M et al. Consensus paper: roles of the cerebellum in motor control — the diversity of ideas on cerebellar involvement in movement. Cerebellum 11, 457–487 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Caligiore D et al. Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum 16, 203–229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Gao Z et al. A cortico-cerebellar loop for motor planning. Nature 563, 113–116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Yamamoto T, Hayashi T, Murata Y, Ose T & Higo N Premotor cortical-cerebellar reorganization in a macaque model of primary motor cortical lesion and recovery. J. Neurosci 39, 8484–8496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kawato M, Ohmae S, Hoang H & Sanger T 50 years since the Marr, Ito, and Albus models of the cerebellum. Neuroscience 10.1016/j.neuroscience.2020.06.019 (2020). [DOI] [PubMed] [Google Scholar]
- 390.Baker SN The primate reticulospinal tract, hand function and functional recovery. J. Physiol 589, 5603–5612 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Soteropoulos DS, Williams ER & Baker SN Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J. Physiol 590, 4011–4027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Honeycutt CF, Kharouta M & Perreault EJ Evidence for reticulospinal contributions to coordinated finger movements in humans. J. Neurophysiol 110, 1476–1483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Baker SN & Perez MA Reticulospinal contributions to gross hand function after human spinal cord injury. J. Neurosci 37, 9778–9784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Takei T & Seki K Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J. Neurosci 30, 17041–17050 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Takei T & Seki K Spinal premotor interneurons mediate dynamic and static motor commands for precision grip in monkeys. J. Neurosci 33, 8850–8860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Takei T & Seki K Synaptic and functional linkages between spinal premotor interneurons and hand-muscle activity during precision grip. Front. Comput. Neurosci 7, 40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Wolpert DM, Diedrichsen J & Flanagan JR Principles of sensorimotor learning. Nat. reviews. Neurosci 12, 739–751 (2011). [DOI] [PubMed] [Google Scholar]
- 398.Lage GM et al. Repetition and variation in motor practice: a review of neural correlates. Neurosci. Biobehav. Rev 57, 132–141 (2015). [DOI] [PubMed] [Google Scholar]
- 399.Dhawale AK, Smith MA & Ölveczky BP The role of variability in motor learning. Annu. Rev. Neurosci 40, 479–498 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Peters AJ, Lee J, Hedrick NG, O’Neil K & Komiyama T Reorganization of corticospinal output during motor learning. Nat. Neurosci 20, 1133–1141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Golub MD et al. Learning by neural reassociation. Nat. Neurosci 21, 607–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Vyas S et al. Neural population dynamics underlying motor learning transfer. Neuron 10.1016/j.neuron.2018.01.040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zhou X, Tien RN, Ravikumar S & Chase SM Distinct types of neural reorganization during long-term learning. J. Neurophysiol 121, 1329–1341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kollmorgen S, Hahnloser RHR & Mante V Nearest neighbours reveal fast and slow components of motor learning. Nature 577, 526–530 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Miyamoto YR, Wang S & Smith MA Implicit adaptation compensates for erratic explicit strategy in human motor learning. Nat. Neurosci 23, 443–455 (2020). [DOI] [PubMed] [Google Scholar]
- 406.Whishaw IQ, Dringenberg HC & Pellis SM Spontaneous forelimb grasping in free feeding by rats: motor cortex aids limb and digit positioning. Behav. Brain Res 48, 113–125 (1992). [DOI] [PubMed] [Google Scholar]
- 407.Whishaw IQ & Gorny B Arpeggio and fractionated digit movements used in prehension by rats. Behav. Brain Res 60, 15–24 (1994). [DOI] [PubMed] [Google Scholar]
- 408.Alaverdashvili M & Whishaw IQ Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur. J. Neurosci 28, 311–322 (2008). [DOI] [PubMed] [Google Scholar]
- 409.Allred RP et al. The Vermicelli Handling Test: a simple quantitative measure of dexterous forepaw function in rats. J. Neurosci. Methods 170, 229–244 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Barrett JM, Raineri Tapies MG & Shepherd GMG Manual dexterity of mice during food-handling involves the thumb and a set of fast basic movements. PLoS ONE 15, 1–25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Whishaw IQ et al. Hand shaping in the rat: conserved release and collection vs. flexible manipulation in overground walking, ladder rung walking, cylinder exploration, and skilled reaching. Behav. Brain Res 206, 21–31 (2010). [DOI] [PubMed] [Google Scholar]
- 412.Cipriani C et al. Online myoelectric control of a dexterous hand prosthesis by transradial amputees. IEEE Trans. Neural Syst. Rehabil. Eng 19, 260–270 (2011). [DOI] [PubMed] [Google Scholar]
- 413.Muceli S, Jiang N & Farina D Extracting signals robust to electrode number and shift for online simultaneous and proportional myoelectric control by factorization algorithms. IEEE Trans. Neural Syst. Rehabil. Eng 22, 623–633 (2014). [DOI] [PubMed] [Google Scholar]
- 414.Sartori M, Durandau G, Došen S & Farina D Robust simultaneous myoelectric control of multiple degrees of freedom in wrist-hand prostheses by real-time neuromusculoskeletal modeling. J. Neural Eng 15, 066026 (2018). [DOI] [PubMed] [Google Scholar]
- 415.D’Anna E et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot 4, eaau8892 (2019). [DOI] [PubMed] [Google Scholar]
- 416.George JA et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot 4, eaax2352 (2019). [DOI] [PubMed] [Google Scholar]
- 417.Chandrasekaran S et al. Sensory restoration by epidural stimulation of the lateral spinal cord in upper-limb amputees. eLife 9, e54349 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Vu PP et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci. Transl. Med 12, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Taylor DM, Tillery SIH & Schwartz AB Direct cortical control of 3D neuroprosthetic devices. Science 296, 1829–1832 (2002). [DOI] [PubMed] [Google Scholar]
- 420.Hochberg LR et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006). [DOI] [PubMed] [Google Scholar]
- 421.Hochberg LR et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Velliste M, Perel S, Spalding MC, Whitford AS & Schwartz AB Cortical control of a prosthetic arm for self-feeding. Nature 453, 1098–1101 (2008). [DOI] [PubMed] [Google Scholar]
- 423.Collinger JL et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Klaes C et al. A cognitive neuroprosthetic that uses cortical stimulation for somatosensory feedback. J. Neural Eng 11, 056024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Flesher SN et al. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med 8, 361ra141 (2016). [DOI] [PubMed] [Google Scholar]
- 426.Armenta Salas M et al. Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. eLife 7, e32904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Downey JE et al. Implicit grasp force representation in human motor cortical recordings. Front. Neurosci 12, 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Shadow Robot Company. Shadow Dexterous Hand E Series (E3M5R & E3M5L) Technical Specification. https://www.shadowrobot.com/wp-content/uploads/shadow_dexterous_hand_technical_specification_E_20190221.pdf (2019). [Google Scholar]
- 429.Johannes MS et al. An overview of the developmental process for the modular prosthetic limb. Johns Hopkins APL Techn. Dig 30, 10 (2011). [Google Scholar]
- 430.Tee BC-K et al. A skin-inspired organic digital mechanoreceptor. Science 350, 313–316 (2015). [DOI] [PubMed] [Google Scholar]
- 431.Delhaye BP, Schluter EW & Bensmaia SJ Robo-psychophysics: extracting behaviorally relevant features from the output of sensors on a prosthetic finger. IEEE Trans. Haptics 9, 499–507 (2016). [DOI] [PubMed] [Google Scholar]
- 432.Osborn LE et al. Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain. Sci. Robot 10.1126/scirobotics.aat3818 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wodlinger B et al. Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations. J. Neural Eng 12, 016011 (2015). [DOI] [PubMed] [Google Scholar]
- 434. Flesher SN et al. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science 372, 831–836 (2021). This study shows that robotic arm control is improved when tactile feedback is conveyed via intracortical microstimulation.
- 435.Vu PP et al. The future of upper extremity rehabilitation robotics: research and practice. Muscle Nerve 61, 708–718 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Saal HP & Bensmaia SJ Biomimetic approaches to bionic touch through a peripheral nerve interface. Neuropsychologia 79, 344–353 (2015). [DOI] [PubMed] [Google Scholar]
- 437.Okorokova EV, He Q & Bensmaia SJ Biomimetic encoding model for restoring touch in bionic hands through a nerve interface. J. Neural Eng 15, 066033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.George JA et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot 4, eaax2352 (2019). [DOI] [PubMed] [Google Scholar]
- 439.Valle G et al. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100, 37–45.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 440.Bensmaia S Biological and bionic hands: natural neural coding and artificial perception. Philos. Trans. R. Soc. B Biol. Sci 370, 20140209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Delhaye BP, Saal HP & Bensmaia SJ Key considerations in designing a somatosensory neuroprosthesis. J. Physiol. Paris 110, 402–408 (2016). [DOI] [PubMed] [Google Scholar]
- 442.Dépeault A, Meftah E-M & Chapman CE Tactile speed scaling: contributions of time and space. J. Neurophysiol 99, 1422–1434 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


