Abstract
Rationale
Hemodynamic assessments direct care among children with pulmonary hypertension, yet the use of cardiac catheterization is highly variable, which could impact patient care and research.
Objectives
We analyzed hemodynamic findings from right heart catheterization (RHC) and left heart catheterization and acute vasodilator testing (AVT) and the safety of catheterization in children with World Symposium on Pulmonary Hypertension (WSPH) group 1 and 3 subtypes in a large multicenter North American cohort.
Methods
Of 1,475 children enrolled in the Pediatric Pulmonary Hypertension Network Registry (2014–2020), there were 1,383 group 1 and 3 patients, of whom 671 (48.5%) underwent RHC at diagnosis and were included for analysis.
Results
Compared with those without diagnostic RHC, these children were older, less likely to be an infant or preterm, more often female, treated with targeted pulmonary hypertension medications at diagnosis, and had advanced World Health Organization functional class. Catheterization was performed without a difference in complication rates between WSPH groups. Pulmonary capillary wedge pressure was well correlated with left ventricular end-diastolic pressure and left atrial pressures. Results of AVT using three different methods were comparable; positive AVT results were observed in 8.0–11.8% of subjects, did not differ between WSPH groups 1 and 3, and were not associated with freedom from the composite endpoint of lung transplantation or death during follow-up.
Conclusions
In a large pediatric pulmonary hypertension cohort, diagnostic RHC with or without left heart catheterization in WSPH group 1 and 3 patients was performed safely at experienced pediatric pulmonary hypertension centers. Hemodynamic differences were noted between group 1 and 3 subjects. Higher mean pulmonary arterial pressure and mean pulmonary arterial pressure/mean systemic arterial pressure ratio were associated with a higher risk of death/transplantation. Findings suggest overall safety and potential value of RHC as a standard diagnostic approach to guide pulmonary hypertension management in children.
Keywords: pulmonary hypertension, cardiac catheterization, pediatrics, hemodynamics, vasoreactivity testing
To date, the optimal diagnostic approach for children with pulmonary hypertension has been limited by the heterogeneous nature of pediatric pulmonary hypertension, challenges in creating robust pediatric-specific pulmonary hypertension registries that include comprehensive hemodynamic profiles, and limited studies of the association between hemodynamics and course of the disease in children. Even among expert pediatric pulmonary hypertension centers, the approach to and performance of cardiac catheterization to assess hemodynamic characteristics in children with pulmonary hypertension are highly variable. This is particularly true for World Symposium on Pulmonary Hypertension (WSPH) group 3 patients (pulmonary hypertension due to lung diseases and/or hypoxia) and in younger children because of concerns that have been raised by some centers about the safety of cardiac catheterization in children with pulmonary hypertension (1–4). Thus, in contrast to the use of diagnostic cardiac catheterization, which is the gold-standard approach in adult pulmonary hypertension, the performance of the initial diagnostic cardiac catheterization before the initiation of targeted pulmonary hypertension therapy among pediatric sites remains highly variable, and evidence around the safety of cardiac catheterization is uncertain. There is also uncertainty about which criteria are most suitable for assessing acute vasodilator testing (AVT) responsiveness in children, and data directly comparing criteria, especially for group 3 children, are lacking. In addition, there are very limited data on how the hemodynamic profiles including AVT in children with WSPH group 3 disease (lung- or hypoxia-related pulmonary hypertension) compare with those in children with WSPH group 1 disease (pulmonary arterial hypertension [PAH]) and relate to disease outcomes within each group (5). The reported safety of right heart catheterization (RHC) in children with pulmonary hypertension has also been inconsistent among centers, and past studies have suggested a high risk for adverse events in children (6–8). The use of left heart catheterization (LHC) as part of initial diagnostic catheterization in the absence of suspicion for left-sided heart disease has also been a topic of debate.
To address these questions, we leveraged a patient registry of neonates, infants, and children with pulmonary hypertension from eight interdisciplinary and well-established pediatric pulmonary hypertension programs that constitute the Pediatric Pulmonary Hypertension Network (PPHNet) (9). The overall goal of the PPHNet Registry is to characterize the range of diseases, their natural history, diagnostic and therapeutic care strategies, and age-appropriate endpoints for children with diverse forms of pulmonary hypertension to enhance clinical care and research. In this study, to better understand the safety, current practice, and potential role of cardiac catheterization in the management of pediatric pulmonary hypertension, we analyzed hemodynamic data from the PPHNet Registry to determine the acquisition, use, characteristics, and safety of diagnostic cardiac catheterization in a diverse cohort of children with WSPH group 1 and 3 pulmonary hypertension.
Methods
Demographic patient data and relevant hemodynamic data from RHC with or without LHC and AVT were extracted from the PPHNet Registry, which includes patients with diverse forms of pediatric pulmonary hypertension from eight well-established academic pediatric pulmonary hypertension centers throughout North America (9). The study protocol was approved by the institutional review boards of all participating centers, and all study participants, parents, and/or guardians provided informed consent. Assent was obtained in patients according to institutional guidelines. All patients were consented before entry into the registry. Inclusion criteria for the PPHNet Registry included newborns, infants, older children, and adolescents who were diagnosed with pulmonary hypertension before 18 years of age, defined according to established guidelines from the joint American Heart Association/American Thoracic Society guidelines for pediatric pulmonary hypertension (10). Data used for this study included reports entered from October 2014 to April 2020 and were partly supported by a National Heart, Lung, and Blood Institute–funded U01 program (U01 HL12118; Data Fusion: A Sustainable, Open Source Registry Advancing Pediatric Pulmonary Vascular Disease Research). Cardiac catheterization was not specifically required for entry into the registry. An attempt was made by each center to enroll as many pediatric patients across WSPH groups in both the inpatient and outpatient pediatric pulmonary hypertension units during the enrollment period. Although children in WSPH groups 1–5 were enrolled in the PPHNet Registry, only children with WSPH group 1 and group 3 pulmonary hypertension who underwent cardiac catheterization at diagnosis were included in these analyses. WSPH group 1 and group 3 patients who did not undergo RHC as part of their initial diagnostic workup were included for comparison. We excluded children with WSPH group 2 PAH because of widely heterogeneous disease, including complex congenital heart disease and significant pulmonary vein stenosis, and we excluded WSPH groups 4 and 5 because there were too few subjects for meaningful comparisons.
Demographics and clinical characteristics from the time of diagnosis as well as hemodynamic data including the approach to cardiac catheterization and complication rates were analyzed. Hemodynamic measures were analyzed, including heart rate; right atrial pressure mean (millimeters of mercury); pulmonary arterial pressure (PAP) systolic, diastolic, and mean (millimeters of mercury); systemic arterial pressure systolic, diastolic, and mean (millimeters of mercury); right ventricular end-diastolic pressure (millimeters of mercury); pulmonary capillary wedge pressure (PCWP) mean (millimeters of mercury); pulmonary vascular resistance index (PVRi); systemic vascular resistance index; ratio of pulmonary to systemic vascular resistance (Rp:Rs); mixed venous saturation (percentage); systemic arterial saturation (percentage); and cardiac index (L/min/m2) using the Fick method and thermodilution (when available). Left ventricular end-diastolic pressure (LVEDP) (millimeters of mercury) when recorded was used to indicate that LHC was also performed. AVT responsiveness was assessed for each group and compared using three previously published criteria for vasoreactivity: adult or Sitbon criteria (11), pediatric or Barst criteria (12), and modified Barst criteria (13). Patients with congenital systemic-to-pulmonary shunts that generated a pulmonary-to-systemic flow ratio (Qp:Qs) of 1.5:1 were not included in the AVT analyses. Further comparisons were performed between WSPH group 1 and WSPH group 3 in those patients who underwent RHC only versus those who underwent both RHC and LHC.
Statistical Methods
Descriptive statistics include counts and percentages for categorical variables and mean ± standard deviation or median and interquartile range (IQR) for continuous variables. Categorical patient and catheterization characteristics by WSPH group were compared using a chi-square test; continuous characteristics were compared using Student’s t test (mean) or the Wilcoxon rank-sum test (median). Rates of AVT responsiveness for WSPH groups 1 and 3 were compared using a Fisher exact test. The chi-square test and Mantel-Haenszel test for linear trend were used to compare rates of AVT responsiveness by age group (⩽1 mo, ⩽1 yr, ⩽6 yr, and >6 yr). Agreement between different AVT responsiveness definitions was estimated using the κ statistic. Cox proportional hazards regression with left truncation (delayed entry to risk sets according to date of study enrollment) was used to estimate the distributions of time to the composite endpoint of death or lung transplantation for groups defined by tertile of hemodynamic parameters. Competing risks analysis (subdistribution proportional hazards model) was also used to examine association between mortality and selected hemodynamic parameters. A P value of less than 0.05 was considered to indicate statistical significance. Analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.03 (R Core Team).
Results
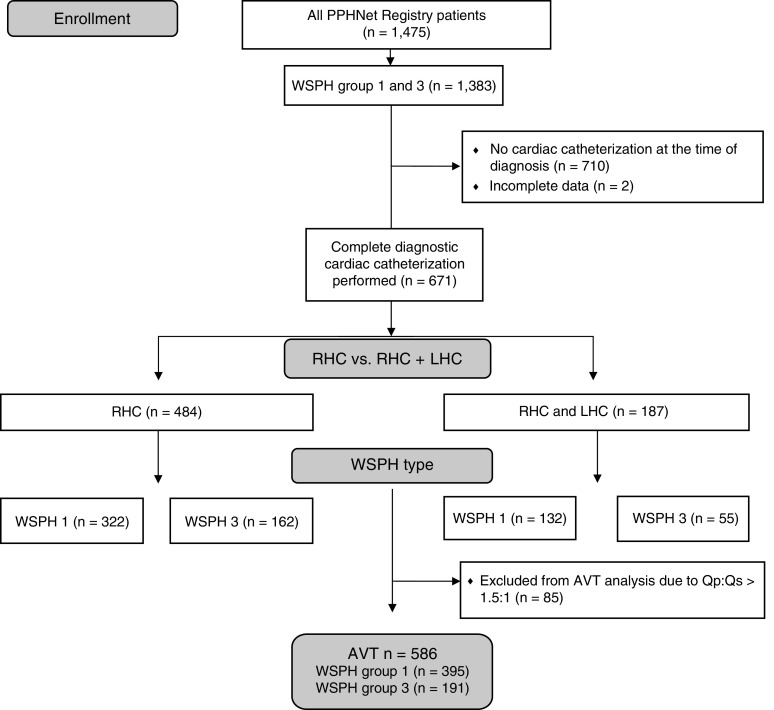
A total of 1,475 children across all five WSPH groups were enrolled in the PPHNet Registry between October 2014 and April 2020. The majority of the patients included were diagnosed with pulmonary hypertension between 2014 and 2019. A total of 1,610 cardiac catheterizations were entered into the registry for these 1,475 patients. Of the 734 (50%) patients for whom hemodynamic data were available from a cardiac catheterization at the time of diagnosis, 454 (61.9%) were in WSPH group 1 and 217 (29.6%) in WSPH group 3 (Figure 1). An additional 43 subjects in WSPH group 2 (5.9%), 4 subjects in group 4 (0.5%), and 16 subjects (2.2%) in WSPH group 5 underwent diagnostic RHC but were not included in formal statistical comparisons because of the small numbers and heterogeneity of diseases within these groups. AVT was performed and included at the time of the initial diagnostic catheterization in 586 patients with Qp/Qs < 1.5:1, which included 395 in WSPH group 1 (87.0%) and 191 in WSPH group 3 (61.2%). One hundred eighty-eight patients (29% of those in WSPH group 1 and 25% of those in WSPH group 3) also underwent LHC with available LVEDP as part of the initial diagnostic catheterization.
Figure 1.
Enrollees in the Pediatric Pulmonary Hypertension Network Registry were included in this report as illustrated. AVT = acute vasodilator testing; LHC = left heart catheterization; PPHNet = Pediatric Pulmonary Hypertension Network; Qp:Qs = pulmonary-to-systemic flow ratio; RHC = right heart catheterization; WSPH = World Symposium on Pulmonary Hypertension.
Demographic characteristics at the time of diagnostic RHC are presented in Table 1 for both WSPH group 1 and 3 children who underwent diagnostic cardiac catheterization (n = 671) and those who did not undergo RHC (n = 710). The median age for all those who underwent diagnostic cardiac catheterization in both groups (WSPH groups 1 and 3) was 1.69 years (IQR, 0.43–6.75 yr; n = 671), for WSPH group 1 was 3.18 years (IQR, 0.69–8.41 yr; n = 454), and for WSPH group 3 was 0.61 years (IQR, 0.30–2.21 yr; n = 217). WSPH group 1 patients undergoing diagnostic catheterization were significantly older (P < 0.001) and were less likely to be preterm (P < 0.001) and male (P = 0.004) than WSPH group 3 patients. Race was associated with WSPH group, with a greater percentage of White patients and a lower percentage of Black patients in WSPH group 1 compared with group 3 (P = 0.017). The percentage of patients on supplemental oxygen at the time of pulmonary hypertension diagnosis was greater in WSPH group 3 compared with group 1 (64% vs. 41%, P < 0.001). The percentage of patients with better function who underwent diagnostic catheterization (i.e., functional class I/II; combining those with World Health Organization [WHO] and Pediatric Assigned Functional Classes) was higher among WSPH group 1 patients than WSPH group 3 patients (55.4% vs. 41.0%, P = 0.003). In comparison with those who did not undergo diagnostic catheterization, patients who underwent catheterization were more likely to be taking a targeted pulmonary hypertension medication and in WHO functional class III/IV at the time of diagnosis.
Table 1.
Demographics and clinical profile at diagnosis of pulmonary hypertension for World Symposium on Pulmonary Hypertension group-1 and -3 patients with and without catheterization at pulmonary hypertension diagnosis
| Status at Pulmonary Hypertension Diagnosis | WSPH Groups 1 and 3 (N = 1,383) | Patients with Baseline Catheterization at Diagnosis |
No Catheterization: at Diagnosis (n = 710) | P Value† | |||
|---|---|---|---|---|---|---|---|
| Overall (n = 671) | WSPH Group 1 (n = 454) | WSPH Group 3 (n = 217) | P Value* | ||||
| Age at pulmonary hypertension diagnosis, yr | n = 1,383 | n = 671 | n = 454 | n = 217 | — | n = 710 | — |
| Median (IQR) | 0.46 (0.08–3.20) | 1.43 (0.36–6.58) | 3.10 (0.61–8.21) | 0.44 (0.21–1.65) | <0.001 | 0.24 (0.01–0.61) | <0.001 |
| Infant (age < 12 mo) | n = 1,383 | n = 671 | n = 454 | n = 217 | <0.001 | n = 710 | <0.001 |
| 878 (63.5) | 302 (45.0) | 151 (33.3) | 151 (69.6) | — | 576 (81.1) | ||
| Preterm (<37 wk) | n = 1,221 | n = 556 | n = 350 | n = 206 | <0.001 | n = 664 | <0.001 |
| 585 (47.9) | 236 (42.4) | 98 (28.0) | 138 (67.0) | — | 349 (52.6) | ||
| Sex | n = 1,383 | n = 671 | n = 454 | n = 217 | 0.004 | — | 0.024 |
| Male | 709 (51.3) | 323 (48.1) | 201 (44.3) | 122 (56.2) | — | 385 (54.2) | — |
| Female | 674 (48.7) | 348 (51.9) | 253 (55.7) | 95 (43.8) | — | 325 (45.8) | — |
| Race | — | — | — | — | 0.017 | — | <0.001 |
| White | 844 (61.0) | 401 (59.8) | 283 (62.3) | 118 (54.4) | — | 441 (62.1) | — |
| Black | 171 (12.4) | 79 (11.8) | 42 (9.3) | 37 (17.1) | — | 92 (13.0) | — |
| Asian | 129 (9.3) | 87 (13.0) | 62 (13.7) | 25 (11.5) | — | 42 (5.9) | — |
| Other/unknown | 239 (17.3) | 104 (15.5) | 67 (14.8) | 37 (17.1) | — | 135 (19.0) | — |
| Hispanic | — | — | — | — | 0.236 | — | 0.119 |
| Yes | 223 (16.1) | 119 (17.7) | 86 (18.9) | 33 (15.2) | — | 104 (14.6) | — |
| No/unknown | 1,160 (83.9) | 552 (82.3) | 368 (81.1) | 184 (84.8) | — | 606 (85.4) | — |
| On supplemental oxygen (at time of catheterization) | n = 1,209 | n = 587 | n = 399 | n = 188 | <0.001 | n = 620 | 0.050 |
| 625 (51.7) | 286 (48.7) | 165 (41.4) | 121 (64.4) | — | 337 (54.4) | — | |
| On any pulmonary hypertension medications | n = 1,366 | n = 671 | n = 454 | n = 217 | 0.893 | n = 693 | <0.001 |
| 727 (53.2) | 415 (61.8) | 280 (61.7) | 135 (62.2) | — | 311 (44.9) | — | |
| Pediatric Functional Class | n = 741 | n = 365 | n = 231 | n = 134 | 0.002 | n = 374 | <0.001 |
| I | 94 (12.7) | 36 (9.9) | 28 (12.1) | 8 (6.0) | — | 58 (15.5) | — |
| II | 212 (28.6) | 131 (35.9) | 91 (39.4) | 40 (29.9) | — | 81 (21.7) | — |
| III A | 143 (19.3) | 90 (24.7) | 60 (26.0) | 30 (22.4) | — | 53 (14.2) | — |
| III B | 61 (8.2) | 36 (9.9) | 19 (8.2) | 17 (12.7) | — | 24 (6.4) | — |
| IV | 231 (31.2) | 72 (19.7) | 33 (14.3) | 39 (29.1) | — | 158 (42.2) | — |
| WHO Functional Class | n = 465 | n = 284 | n = 211 | n = 73 | 0.369 | n = 180 | <0.001 |
| I | 97 (20.9) | 36 (12.7) | 26 (12.3) | 10 (13.7) | — | 61 (33.9) | — |
| II | 187 (40.2) | 122 (43.0) | 97 (46.0) | 25 (34.2) | — | 65 (36.1) | — |
| III | 129 (27.7) | 95 (33.5) | 66 (31.3) | 29 (39.7) | — | 34 (18.9) | — |
| IV | 52 (11.2) | 31 (10.9) | 22 (10.4) | 9 (12.3) | — | 20 (11.1) | — |
| Combined Functional Class | n = 1,002 | n = 515 | n = 354 | n = 161 | <0.001 | n = 487 | <0.001 |
| I | 169 (16.9) | 64 (12.4) | 49 (13.8) | 15 (9.3) | — | 105 (21.6) | — |
| II | 323 (32.2) | 198 (38.4) | 147 (41.5) | 51 (31.7) | — | 125 (25.7) | — |
| III | 263 (26.2) | 172 (33.4) | 117 (33.1) | 55 (34.2) | — | 91 (18.7) | — |
| IV | 247 (24.7) | 81 (15.7) | 41 (11.6) | 40 (24.8) | — | 166 (34.1) | — |
Definition of abbreviations: IQR = interquartile range; WHO = World Health Organization; WSPH = World Symposium on Pulmonary Hypertension.
Data are shown as n (%) unless otherwise specified.
P value compares WSPH group 1 with group 3 patients who underwent catheterization at diagnosis and had baseline condition data.
P value compares group 1 and 3 patients with baseline catheterization (n = 671) with patients who did not undergo catheterization at pulmonary hypertension diagnosis (n = 710).
The diagnostic catheterization baseline characteristics for all patients (combined WSPH groups 1 and 3) as well as WSPH group 1 and WSPH group 3 patients separately are shown in Table 1. WSPH group 1 patients were less likely to be on supplemental oxygen (WSPH group 1 vs. group 3, 41.4% vs. 64.4%; P < 0.05) and/or inhaled nitric oxide (iNO) (WSPH group 1 vs. group 3, 6% vs. 10.1%; P = 0.001) for the baseline resting state during diagnostic catheterization than the WSPH group 3 patients. At the time of diagnostic RHC, background targeted pulmonary hypertension medications other than supplemental oxygen, iNO, and phosphodiesterase-5 inhibitors were uncommon (<6%) in WSPH group 1 and WSPH group 3 (<5%) (Table 2). These represent medications used at mean 3.2 ± 16.6 and median 0.0 (0.0–0.5) months from the time of diagnosis. Phosphodiesterase-5 inhibitors were used most often in both WSPH group 1 and group 3 patients.
Table 2.
World Symposium on Pulmonary Hypertension groups 1 and 3: pulmonary hypertension medications at catheterization (diagnosis visit)*
| Overall (N = 671) | WSPH Group 1 (n = 454) | WSPH Group 3 (n = 217) | P Value | |
|---|---|---|---|---|
| Calcium channel blockers | 20 (2.0) | 16 (2.3) | 4 (1.3) | 0.329 |
| ERAs | 47 (4.7) | 36 (5.2) | 11 (3.7) | 0.320 |
| PDE-5 inhibitors | 253 (25.4) | 170 (24.3) | 83 (27.9) | 0.241 |
| Prostacyclins | 58 (5.8) | 45 (6.4) | 13 (4.4) | 0.200 |
| Inhaled nitric oxide | 72 (7.2) | 42 (6.0) | 30 (10.1) | 0.023 |
Definition of abbreviations: ERA = endothelin receptor antagonist; PDE-5 = phosphodiesterase-5; WSPH = World Symposium on Pulmonary Hypertension.
Data are shown as n (%).
Shown are medications used at mean 3.2 ± 16.6 and median 0.0 (interquartile range, 0.0–0.5) months from the time of diagnosis.
WSPH group 3 patients were more likely to be on iNO at the time of diagnostic catheterization than WSPH group 1 patients (P = 0.023). Of the 671 patients, 358 were on iNO (n = 72) and/or O2 (n = 286). Patients on iNO/O2 at the time of diagnostic RHC were younger, were more likely to be in WSPH group 3, and had a faster resting heart rate, lower systemic arterial systolic pressure, higher pulmonary venous, systemic arterial, and mixed venous saturation, and higher carbon dioxide partial pressure (Pco2) and oxygen pressure (Po2) on the arterial blood gas.
Baseline resting hemodynamics for these 671 WSPH group 1 and 3 pediatric patients with pulmonary hypertension during diagnostic catheterization are presented in Table 3. Patients with WSPH group 1 PAH had worse baseline hemodynamics, including higher mean PAP (PAPm) (46.6 ± 20.4 vs. 37.1 ± 15.5 mm Hg; P < 0.001), PVRi (median 9.0 vs. 5.8; P < 0.001), and Rp/Rs (median 0.58 vs. 0.42; P < 0.001) than those with WSPH group 3 pulmonary hypertension. In contrast, patients with WSPH group 3 pulmonary hypertension had higher Pco2 at baseline than those with WSPH group 1 pulmonary hypertension (48.6 ± 13.6 vs. 39.8 ± 8.3 mm Hg) (Table 3). Age at catheterization was nonlinearly associated with PAPm; that is, PAPm was positively correlated with age when age was 5 years or less, but there was no significant correlation after age 5 years. Older age at diagnostic catheterization was associated with higher PVRi and lower cardiac index.
Table 3.
Catheterization complications and findings by World Symposium on Pulmonary Hypertension group
| WSPH Groups 1 and 3 (N = 671) | WSPH Group 1 (n = 454) | WSPH Group 3 (n = 217) | P Value | |
|---|---|---|---|---|
| Age at catheterization, yr | n = 671 | n = 454 | n = 217 | — |
| Median (IQR) | 1.69 (0.43–6.75) | 3.18 (0.69–8.41) | 0.61 (0.30–2.21) | <0.001 |
| Complications | n = 653 | n = 438 | n = 215 | — |
| Arrhythmia requiring treatment | 18 (2.8) | 10 (2.3) | 8 (3.7) | 0.292 |
| CPR | 5 (0.8) | 3 (0.7) | 2 (0.9) | 0.735 |
| Unplanned admission | 13 (2.0) | 11 (2.5) | 2 (0.9) | 0.174 |
| Unplanned intubation | 3 (0.5) | 2 (0.5) | 1 (0.5) | 0.988 |
| Death | 0 | 0 | 0 | N/A |
| Combined: any complication | 34 (5.2) | 22 (5.0) | 12 (5.6) | 0.763 |
| Hemodynamics | — | — | — | — |
| Heart rate, beats/min | n = 450 | n = 312 | n = 138 | — |
| Mean ± SD | 112 ± 29 | 106 ± 28 | 126 ± 27 | <0.001 |
| Hemoglobin, g/dl | n = 548 | n = 367 | n = 181 | — |
| Mean ± SD | 12.3 ± 2.2 | 12.6 ± 2.2 | 11.7 ± 2.0 | <0.001 |
| Mean RAP, mm Hg | n = 590 | n = 406 | n = 184 | — |
| Mean ± SD | 7.0 ± 3.2 | 7.1 ± 3.3 | 6.7 ± 2.9 | 0.075 |
| Mean LAP, mm Hg | n = 141 | n = 82 | n = 59 | — |
| Mean ± SD | 8.6 ± 4.3 | 8.8 ± 4.7 | 8.4 ± 3.7 | 0.538 |
| PCWP, mm Hg | n = 516 | n = 359 | n = 157 | — |
| Mean ± SD | 9.3 ± 3.6 | 9.3 ± 3.5 | 9.4 ± 3.9 | 0.865 |
| LVEDP, mm Hg | n = 187 | n = 132 | n = 55 | — |
| Mean ± SD | 10.0 ± 3.8 | 9.7 ± 4.0 | 10.8 ± 3.0 | 0.053 |
| PA pressure systolic, mm Hg | n = 616 | n = 421 | n = 195 | — |
| Mean ± SD | 63.5 ± 26.0 | 68.3 ± 27.0 | 53.1 ± 20.0 | <0.001 |
| PA pressure diastolic, mm Hg | n = 618 | n = 423 | n = 195 | — |
| Mean ± SD | 29.0 ± 16.2 | 31.4 ± 17.2 | 23.9 ± 12.4 | <0.001 |
| PAPm, mm Hg | n = 634 | n = 437 | n = 197 | — |
| Mean ± SD | 43.6 ± 19.5 | 46.6 ± 20.4 | 37.1 ± 15.5 | <0.001 |
| RV systolic pressure, mm Hg | n = 359 | n = 228 | n = 131 | — |
| Mean ± SD | 65.8 ± 24.5 | 72.4 ± 25.3 | 54.2 ± 18.0 | <0.001 |
| RVEDP, mm Hg | n = 339 | n = 218 | n = 121 | — |
| Mean ± SD | 9.4 ± 4.1 | 9.6 ± 4.2 | 9.2 ± 4.0 | 0.397 |
| Systemic arterial systolic pressure, mm Hg | n = 480 | n = 334 | n = 146 | — |
| Mean ± SD | 81.1 ± 17.5 | 83.6 ± 18.1 | 75.5 ± 14.8 | <0.001 |
| Systemic arterial diastolic pressure, mm Hg | n = 480 | n = 334 | n = 146 | — |
| Mean ± SD | 46.2 ± 12.3 | 48.0 ± 12.8 | 42.2 ± 10.1 | <0.001 |
| SAPm, mm Hg | n = 484 | n = 337 | n = 147 | — |
| Mean ± SD | 60.4 ± 13.7 | 62.3 ± 14.1 | 55.9 ± 11.5 | <0.001 |
| PVRi, Wood units | n = 570 | n = 388 | n = 182 | — |
| Median (IQR) | 7.3 (4.6–13.6) | 9.0 (5.0–16.1) | 5.8 (4.3–8.3) | <0.001 |
| SVRi, Wood units | n = 503 | n = 342 | n = 161 | — |
| Median (IQR) | 15.2 (11.8–19.9) | 16.5 (12.0–21.6) | 14.0 (11.3–17.3) | <0.001 |
| Rp:Rs | n = 404 | n = 276 | n = 128 | — |
| Median (IQR) | 0.50 (0.30–0.85) | 0.58 (0.31–0.91) | 0.42 (0.27–0.63) | <0.001 |
| QPi, L/min/m2 | n = 363 | n = 247 | n = 116 | — |
| Median (IQR) | 3.8 (2.8–5.0) | 3.6 (2.6–5.0) | 4.0 (3.4–5.1) | 0.004 |
| QSi, L/min/m2 | n = 339 | n = 226 | n = 113 | — |
| Median (IQR) | 3.4 (2.7–4.2) | 3.2 (2.6–4.1) | 3.6 (3.0–4.3) | 0.005 |
| Qp:Qs | n = 431 | n = 296 | n = 135 | — |
| Median (IQR) | 1.00 (1.00–1.34) | 1.00 (1.00–1.33) | 1.00 (1.00–1.35) | 0.048 |
| Qp:Qs < 1.5 or unspecified (no shunt) | n = 586 | n = 395 | n = 191 | — |
| Cardiac index,* L/min/m2 | n = 541 | n = 367 | n = 174 | — |
| Median (IQR) | 3.40 (2.70–4.20) | 3.30 (2.59–4.20) | 3.60 (3.00–4.20) | 0.006 |
| O2 consumption, ml/min/m2 | n = 438 | n = 295 | n = 143 | — |
| Median (IQR) | 150.0 (125.0–170) | 149.0 (124.7–169.0) | 157.0 (125.0–175.0) | 0.087 |
| PA saturation, % | n = 547 | n = 377 | n = 170 | — |
| Mean ± SD | 68.8 ± 10.8 | 69.1 ± 11.0 | 68.2 ± 10.4 | 0.348 |
| PA saturation, Po2, mm Hg | n = 14 | n = 8 | n = 6 | — |
| Mean ± SD | 46.0 ± 12.2 | 44.5 ± 14.6 | 48.0 ± 9.0 | 0.616 |
| PV saturation, % | n = 401 | n = 277 | n = 124 | — |
| Mean ± SD | 93.3 ± 7.3 | 93.8 ± 6.6 | 92.2 ± 8.7 | 0.065 |
| PV saturation, Po2, mm Hg | n = 18 | n = 10 | n = 8 | — |
| Median (IQR) | 120 (70–168) | 122.0 (89.0–293.0) | 114.0 (70.0–132.0) | 0.505 |
| Arterial saturation, % | n = 534 | n = 368 | n = 166 | — |
| Mean ± SD | 89.3 ± 10.3 | 89.4 ± 10.2 | 88.9 ± 10.5 | 0.563 |
| Arterial saturation, Po2, mm Hg | n = 100 | n = 60 | n = 40 | — |
| Median (IQR) | 71.0 (60.0–105.5) | 71.5 (60.5–96.0) | 69.0 (57.5–130.5) | 0.728 |
| Mixed venous saturation, % | n = 553 | n = 372 | n = 181 | — |
| Mean ± SD | 64.7 ± 10.6 | 64.6 ± 10.2 | 65.0 ± 11.6 | 0.697 |
| pH | n = 371 | n = 257 | n = 114 | |
| Mean ± SD | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 0.581 |
| Pco2, mm Hg | n = 371 | n = 256 | n = 115 | — |
| Mean ± SD | 42.5 ± 11.0 | 39.8 ± 8.3 | 48.6 ± 13.6 | <0.001 |
| Po2, mm Hg | n = 371 | n = 258 | n = 113 | — |
| Median (IQR) | 70 (57–93) | 72 (58–91) | 67 (56–96) | 0.746 |
| PVR/SVR | n = 495 | n = 336 | n = 159 | — |
| Median (IQR) | 0.51 (0.32–0.82) | 0.58 (0.33–0.90) | 0.41 (0.29–0.62) | <0.001 |
| PAPm/SAPm | n = 467 | n = 331 | n = 136 | — |
| Median (IQR) | 0.73 (0.50–0.96) | 0.79 (0.56–1.00) | 0.57 (0.46–0.85) | <0.001 |
Definition of abbreviations: CPR = cardiopulmonary resuscitation; IQR = interquartile range; LAP = left atrial pressure; LVEDP = left ventricular end-diastolic pressure; N/A = not applicable; PA = pulmonary arterial; PAPm = mean pulmonary arterial pressure; Pco2 = carbon dioxide partial pressure; PCWP = pulmonary capillary wedge pressure; Po2 = oxygen pressure; PV = pulmonary venous; PVR = pulmonary vascular resistance; PVRi = pulmonary vascular resistance index; QPi = pulmonary arterial flow index; Qp:Qs = pulmonary-to-systemic flow ratio; QSi = systemic arterial flow index; RAP = right atrial pressure; Rp:Rs = pulmonary to systemic vascular resistance ratio; RV = right ventricular; RVEDP = right ventricular end-diastolic pressure; SAPm = mean systemic arterial pressure; SD = standard deviation; SVR = systemic vascular resistance; SVRi = systemic vascular resistance index; WSPH = World Symposium on Pulmonary Hypertension.
Data are from the baseline catheterization performed at the time of pulmonary hypertension diagnosis.
Cardiac index is based on thermodilution, or the Fick method if thermodilution value was not available, or QSi if the Fick value was not available.
Among the 671 patients, 187 (28%) underwent LHC, as defined by having a recorded LVEDP. The percentage of all catheterizations performed that included LHC ranged by site from 0% to 67% (P < 0.001), and study center was the only factor associated with the performance of LHC. Performance of LHC was not associated with WSPH group 1 versus 3, age, sex, race, or ethnicity. The rate of complications from catheterization did not differ between those undergoing LHC and those undergoing RHC only (Tables 4 and 5).
Table 4.
WSPH groups 1 and 3: RHC versus LHC versus no LHC of the total (row percentage)
| All (N = 1,383) | RHC (n = 671) | LHC: Yes (n = 254) | LHC: No (n = 417) | No Catheterization | |
|---|---|---|---|---|---|
| WSPH group1 | 663 | 454 (68.5) | 167 (25.2) | 287 (43.3) | 209 (31.5) |
| WSPH group 3 | 720 | 217 (30.1) | 87 (12.1) | 130 (18.1) | 503 (69.9) |
| Age < 1 yr at diagnosis | 879 | 302 (34.4) | 131 (14.9) | 171 (19.5) | 577 (65.6) |
| Age ⩾ 1 yr at diagnosis | 504 | 369 (73.2) | 123 (24.4) | 246 (48.8) | 135 (26.8) |
Definition of abbreviations: LHC = left heart catheterization; RHC = right heart catheterization; WSPH = World Symposium on Pulmonary Hypertension.
Data are shown as n (%).
Table 5.
WSPH groups 1 and 3: demographics by left heart catheterization status
| Catheterization (N = 671) | LHC: Yes (n = 254) | LHC: No (n = 417) | P Value | |
|---|---|---|---|---|
| Age at diagnosis, yr | 4.1 ± 5.1 | 3.4 ± 4.6 | 4.5 ± 5.3 | 0.004 |
| Median (IQR) | 1.4 (0.4–6.6) | 0.9 (0.3–5.0) | 2.1 (0.4–7.2) | 0.001 |
| Male | 323 (48.1) | 125 (49.2) | 198 (47.5) | 0.663 |
| Race | — | — | — | 0.069 |
| White | 401 (59.8) | 147 (57.9) | 254 (60.9) | — |
| Black | 79 (11.8) | 24 (9.4) | 55 (13.2) | — |
| Asian | 87 (13.0) | 43 (16.9) | 44 (10.6) | — |
| Other/unknown | 104 (15.5) | 40 (15.7) | 64 (15.3) | — |
| Hispanic | 119 (17.7) | 51 (20.1) | 68 (16.3) | 0.090 |
| WSPH group | — | — | — | 0.409 |
| 1 | 454 (67.7) | 167 (65.7) | 287 (68.8) | — |
| 3 | 217 (32.3) | 87 (34.3) | 130 (31.2) | — |
| Functional class* | — | — | — | 0.001 |
| I/II | 262 (50.9) | 87 (42.2) | 175 (56.6) | — |
| III/IV | 253 (49.1) | 119 (57.8) | 134 (43.4) | — |
| Abnormal LVEDP or LAP | — | — | — | — |
| Yes (⩾15 mm Hg) | — | 31 (12.2) | — | — |
| No | — | 223 (87.8) | — | — |
Definition of abbreviations: IQR = interquartile range; LAP = left atrial pressure; LHC = left heart catheterization; LVEDP = left ventricular end-diastolic pressure; WSPH = World Symposium on Pulmonary Hypertension.
Data are shown as mean ± SD or n (%) unless otherwise specified. P values in boldface type denote statistical significance.
Combines Pediatric Functional Class and World Health Organization functional class.
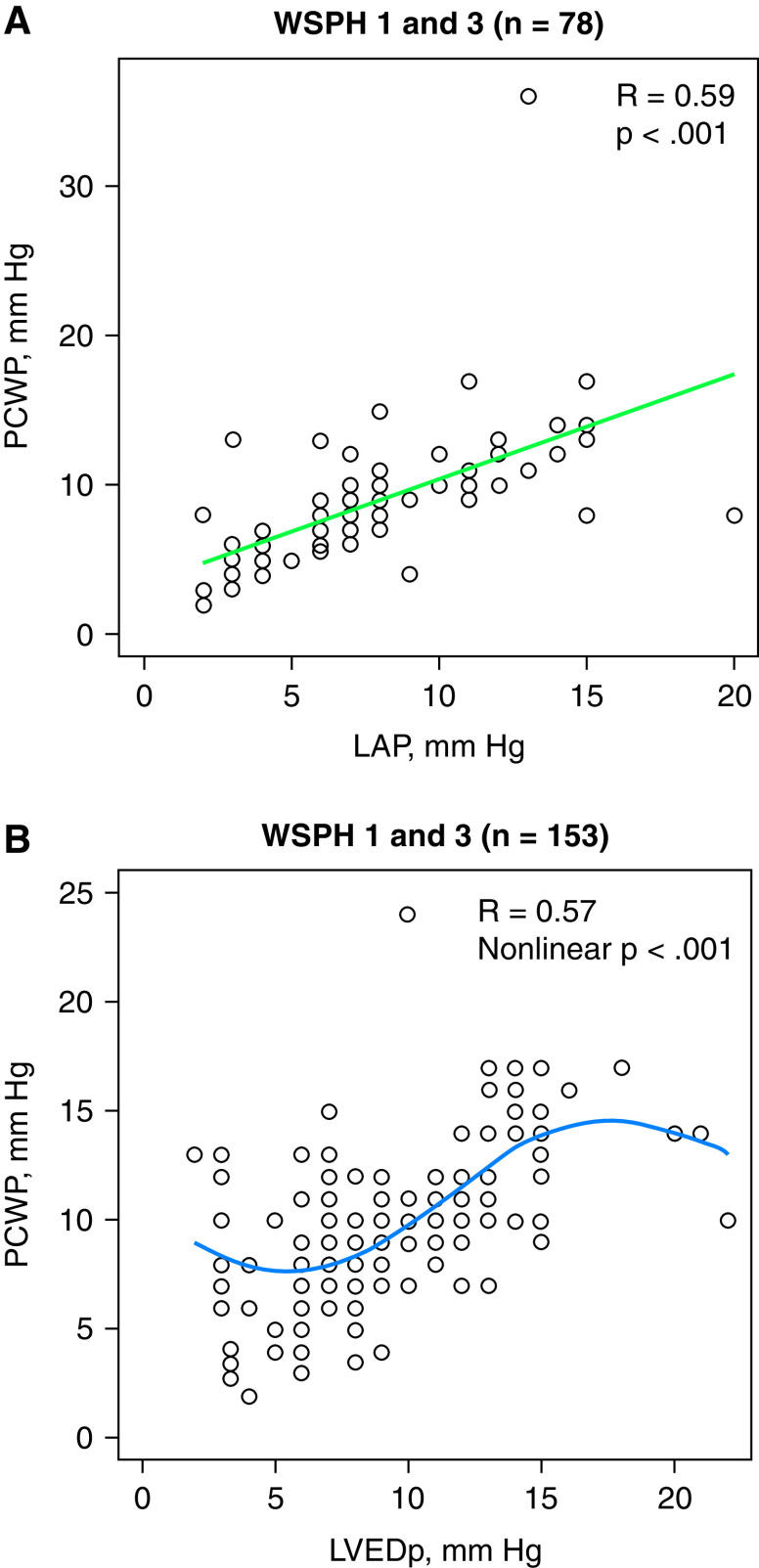
To determine the utility of obtaining LVEDP via LHC, mean PCWP (PCWPm) was compared with either LVEDP or left atrial pressure (LAP), recognizing that the latter is obtained during a portion of RHC when the atrial septum is crossed. Among the 187 patients with measured LVEDP, 74 (39%) also had recorded LAP, and 151 (81%) also had recorded PCWPm; there were also 41 patients with measured LAP and PCWPm. PCWPm was positively correlated with LVEDP and LAP (R = 0.57, P < 0.001) (Figures 2A and 2B). The mean absolute difference was 2.2 ± 2.9 mm Hg, with a median of 1 (IQR, 0 to 3) mm Hg. There was very good agreement between the RHC and LHC measures: 81% of the 187 patients with PCWP and LHC data had two values that were within 3 mm Hg of each other. The mean difference (PCWP minus LVEDP or LAP) was 0.5 ± 2.6 mm Hg, with a median of 0 mm Hg (IQR, −1.0 to 2.0). As noted above, only 19% of the 187 subjects had an absolute difference of more than 3 mm Hg between the two measurements. In the 74 patients in whom both LVEDP and LAP were measured, LVEDP and LAP were highly correlated (R = 0.74; P < 0.001). Comparing patients in WSPH groups 1 and 3, there was no difference in PCWP (9.4 ± 3.7 vs. 9.4 ± 3.9 mm Hg; P = 0.865).
Figure 2.
(A) Pulmonary capillary wedge pressure (PCWP) is positively correlated with left atrial pressure. Hemodynamic data are from the baseline catheterization performed at pulmonary hypertension diagnosis. (B) PCWP is positively correlated with left ventricular end-diastolic pressure (LVEDp) when LVEDp is more than 5 mm Hg. Hemodynamic data are from the baseline catheterization performed at pulmonary hypertension diagnosis. LAP = left atrial pressure; WSPH = World Symposium on Pulmonary Hypertension.
AVT
AVT was performed at the initial diagnostic catheterization in 586 patients (395 in WSPH group 1 and 191 in WSPH group 3), with baseline Qp:Qs < 1.5:1 (Table 1). Of these patients, 36 were on background iNO. There were several approaches to the AVT challenge that are reported during the condition defined as the “maximal hemodynamic response” in Table 6. Although the vast majority of patients were administered iNO and/or oxygen for the AVT challenge in both groups, a higher proportion of WSPH group 3 patients than WSPH group 1 patients received supplemental oxygen as part of their acute vasoreactivity testing (93.8% vs. 87.1%, P = 0.025). On the basis of the maximal hemodynamic response state, there was substantial agreement among the three methods of calculating a significant AVT response (Sitbon, Barst, and modified Barst), with agreement in positive responsiveness using these three definitions more than 90% of the time. The Sitbon and Barst definitions agreed 93.3% of the time (κ = 0.67), Sitbon and modified Barst agreed 93.7% of the time (κ = 0.66), and Barst and modified Barst agreed 99.8% of the time (κ = 0.98). Using the various criteria, the overall AVT robust responsiveness rates for WSPH groups 1 and 3 combined were between 8.0% and 11.8% at the diagnostic cardiac catheterization, with AVT responsiveness, depending on definition, ranging from 6.3% to 10.3% for WSPH group 1 and 11.9–15.0% in WSPH group 3 patients (Table 6). AVT responsiveness did not differ between WSPH group 1 and group 3 patients but tended to be lower in group 1 patients without a large congenital systemic-to-pulmonary shunt (i.e., Qp:Qs ⩽ 1.5:1); for example, the AVT positivity rate was 9.0% by the Barst criteria in group 1 and 14.6% AVT positive in group 3 (P = 0.09). There was no significant difference in likelihood of AVT robust responsiveness by all three criteria between patients who were not on background iNO/O2 at the time of the catheterization and those who were (6.8–11.9% vs. 11.0–11.9%; P = NS). There was no difference in AVT responsiveness by age in patients without large congenital systemic-to-pulmonary shunts (i.e., Qp:Qs ⩽ 1.5:1). For this cohort without a shunt, there was also no significant difference in the composite endpoint of lung transplantation/death between children who were AVT positive (according to the Barst definition, 2 of 45 with events [4.4%]) and those who were not AVT positive (49 of 374 with events [13.1%], exact P = 0.14, Cox P = 0.16).
Table 6.
Acute vasodilator testing responsiveness on the basis of catheterization at pulmonary hypertension diagnosis
| Measurement | Overall (N = 485) | WSPH Group 1 (n = 333) | WSPH Group 3 (n = 152) | P Value |
|---|---|---|---|---|
| n paired PAPm | 428 | 298 | 130 | — |
| Baseline PAPm, mm Hg | 45.4 ± 19.9 | 49.2 ± 20.6 | 36.6 ± 14.9 | <0.001 |
| Baseline PAPm ⩽ 40 mm Hg | 193 (45.1) | 105 (35.2) | 88 (67.7) | <0.001 |
| Maximal PAPm, mm Hg | 39.2 ± 19.0 | 43.2 ± 20.2 | 29.9 ± 11.9 | <0.001 |
| Maximal PAPm ⩽ 40 mm Hg | 258 (60.3) | 146 (49.0) | 112 (86.2) | <0.001 |
| Drop in PAPm ⩾ 10 mm Hg from baseline | 108 (25.2) | 76 (25.5) | 32 (24.6) | 0.904 |
| Drop in PAPm ⩾ 20% from baseline | 130 (30.4) | 85 (28.5) | 45 (34.6) | 0.211 |
| n paired cardiac index | 361 | 252 | 109 | — |
| Baseline cardiac index, L/min/m2 | 3.60 ± 1.31 | 3.54 ± 1.41 | 3.74 ± 1.02 | 0.145 |
| Median (IQR) | 3.49 (2.75–4.20) | 3.38 (2.60–4.20) | 3.60 (3.10–4.20) | 0.020 |
| Maximal cardiac index, L/min/m2 | 3.68 ± 1.48 | 3.58 ± 1.48 | 3.89 ± 1.45 | 0.064 |
| Median (IQR) | 3.43 (2.80–4.31) | 3.20 (2.63–4.30) | 3.68 (3.19–4.45) | 0.006 |
| Increase or no change in cardiac index | 189 (52.4) | 123 (48.8) | 66 (60.6) | 0.051 |
| n paired PVR/SVR ratio | 265 | 187 | 78 | — |
| PVR/SVR ratio at baseline, median (IQR) | 0.63 (0.40–0.94) | 0.71 (0.49–1.07) | 0.44 (0.33–0.66) | <0.001 |
| Maximal PVR/SVR ratio, median (IQR) | 0.43 (0.26–0.69) | 0.51 (0.30–0.79) | 0.31 (0.24–0.42) | <0.001 |
| Decrease or no change in PVR/SVR ratio | 222 (83.8) | 152 (81.3) | 70 (89.7) | 0.102 |
| Sitbon criteria | — | — | — | 0.195 |
| Responsive | 51 (11.8) | 31 (10.3) | 20 (15.0) | — |
| Nonresponsive | 383 (88.2) | 270 (89.7) | 113 (85.0) | — |
| Barst criteria | — | — | — | 0.091 |
| Responsive | 45 (10.7) | 26 (9.0) | 19 (14.6) | — |
| Nonresponsive | 374 (89.3) | 263 (91.0) | 111 (85.4) | — |
| Modified Barst criteria | — | — | — | 0.075 |
| Responsive | 33 (8.0) | 18 (6.3) | 15 (11.9) | — |
| Nonresponsive | 378 (92.0) | 267 (93.7) | 111 (88.1) | — |
Definition of abbreviations: IQR = interquartile range; PAPm = mean pulmonary arterial pressure; PVR = pulmonary vascular resistance; SVR =systemic vascular resistance; WSPH = World Symposium on Pulmonary Hypertension.
Excludes patients with diagnosis baseline pulmonary-to-systemic flow ratio > 1.5. Paired n represents the number of patients with baseline and maximal condition data. Data are shown as mean ± SD or n (%) unless otherwise specified.
Complications
The composite catheterization complication rate within 24 hours of diagnostic cardiac catheterization (n = 653) was 5.2% in 34 patients overall (Table 3). The most common complication was arrhythmia, requiring treatment in 2.8% of patients undergoing diagnostic cardiac catheterization. Cardiopulmonary resuscitation was required in 5 (0.8%) cases, unplanned intubation in 3 (0.5%), and unplanned hospital admission in 13 (2%). There were no deaths associated with diagnostic heart catheterization. There was no difference in complication rates between WSPH group 1 and WSPH group 3 patients (Table 3).
Outcomes
Among the 671 patients, 12 underwent lung transplantation, 7 underwent lung transplantation and then died, and 60 died without lung transplantation. Table 7 shows the associations between key hemodynamic parameters and time from diagnostic catheterization to the occurrence of death/transplantation. Higher PAPm and ratio of PAPm to mean systemic arterial pressure (SAPm) are significantly associated with a higher risk of death/transplantation. Figure 3A displays estimated transplantation-free survival over time by tertile group of PAPm (P < 0.001) and PAPm:SAPm (P = 0.001). The hazard of death/transplantation was significantly higher among patients in the upper tertile relative to those with values below the upper tertile (all hazard ratios ranged from 2 to 3 compared with the first- and second-tertile groups); furthermore, patients with values in the middle tertile had higher risk than those with values in the lowest tertile. When mortality was examined alone (with transplantation as a competing risk), the associations remained significant, but there was less discrimination among PAPm tertile groups (Figure 3B).
Table 7.
Univariate Cox regression models for death/lung transplantation
| All (N = 671) | Death/Transplantation (n = 79) | Event Free (n = 592) | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| Follow-up time, yr, median (IQR) | 5.1 (2.6–8.7) | 1.7 (0.4–6.5) | 5.4 (3.0–9.1) | — | — |
| Age at catheterization, yr | 4.24 ± 5.06 | 5.21 ± 5.98 | 4.11 ± 4.92 | 1.037 (0.998–1.08) | 0.064 |
| WSPH group | — | — | — | — | 0.833 |
| 1 | 454 (67.7) | 54 (68.4) | 400 (67.6) | 1.05 (0.65–1.69) | — |
| 3 | 217 (32.3) | 25 (31.6) | 192 (32.4) | Reference | — |
| PAPm, mm Hg | 43.6 ± 19.5 (634) | 51.8 ± 22.1 (75) | 42.6 ± 18.9 (559) | 1.12 (1.06–1.18)* | <0.001 |
| PAPm tertile† | — | — | — | — | <0.001 |
| ⩽32 mm Hg | 230 (36.3) | 16 (7.0) | 214 (93.0) | Reference | — |
| >32 to ⩽50 mm Hg | 200 (31.5) | 22 (11.0) | 178 (89.0) | 1.49 (0.78–2.83) | — |
| >50 mm Hg | 204 (32.2) | 37 (18.1) | 167 (81.9) | 2.86 (1.59–5.16) | — |
| T3 vs. T2 | — | — | — | 1.93 (1.13–3.27) | — |
| PVRi, Wood units ⋅ m2 | 10.7 ± 9.8 (570) | 13.2 ± 11.9 (65) | 10.3 ± 9.4 (505) | 1.13 (1.02–1.25)* | 0.015 |
| PVRi tertile | — | — | — | — | 0.342 |
| ⩽5 | 175 (30.7) | 16 (9.1) | 159 (90.9) | Reference | — |
| >5 to ⩽10 | 190 (33.3) | 20 (10.5) | 170 (89.5) | 1.12 (0.58–2.18) | — |
| >10 | 205 (36.0) | 29 (14.1) | 176 (85.9) | 1.53 (0.82–2.82) | — |
| T3 vs. T2 | — | — | — | 1.36 (0.77–2.40) | — |
| PVRi/SVRi | 0.64 ± 0.48 (495) | 0.75 ± 0.51 (57) | 0.63 ± 0.48 (438) | 1.04 (1.002–1.09)‡ | 0.040 |
| PVRi/SVRi tertile | — | — | — | — | 0.014 |
| ⩽0.4 | 185 (37.4) | 16 (8.6) | 169 (91.4) | Reference | — |
| >0.4 to ⩽0.7 | 149 (30.1) | 14 (9.4) | 135 (90.6) | 0.96 (0.47–1.97) | — |
| >0.7 | 161 (32.5) | 27 (16.8) | 134 (83.2) | 2.17 (1.16–4.09) | — |
| T3 vs. T2 | — | — | — | 2.26 (1.18–4.34) | — |
| Cardiac index, L/min/m2 | 3.56 ± 1.29 (541) | 3.36 ± 1.19 (56) | 3.58 ± 1.30 (485) | 0.99 (0.97–1.02)‡ | 0.504 |
| PAPm/SAPm | 0.75 ± 0.31 (467) | 0.86 ± 0.38 (54) | 0.74 ± 0.30 (413) | 1.13 (1.04–1.23)‡ | 0.004 |
| PAPm/SAPm tertile† | — | — | — | — | 0.001 |
| ⩽0.6 | 167 (35.8) | 15 (9.0) | 152 (91.0) | Reference | — |
| >0.6 to ⩽0.9 | 151 (32.3) | 10 (6.6) | 141 (93.4) | 0.75 (0.34–1.67) | — |
| >0.9 | 149 (31.9) | 29 (19.5) | 120 (80.5) | 2.44 (1.30–4.59) | — |
| T3 vs. T2 | — | — | — | 3.26 (1.58–6.72) | — |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; PAPm = mean pulmonary arterial pressure; PVRi = pulmonary vascular resistance index; SAPm = systemic pulmonary arterial pressure; SVRi = systemic vascular resistance index; T2 = second tertile; T3 = third tertile; WSPH = World Symposium on Pulmonary Hypertension.
Data are shown as mean ± SD, n (%), or mean ± SD (n) unless otherwise specified.
Per 5-unit increase.
These two parameters were also examined with respect to mortality alone. P = 0.029 for PAPm tertiles and P = 0.009 for PAPm/SAPm tertiles.
Per 0.1-unit increase.
Figure 3.
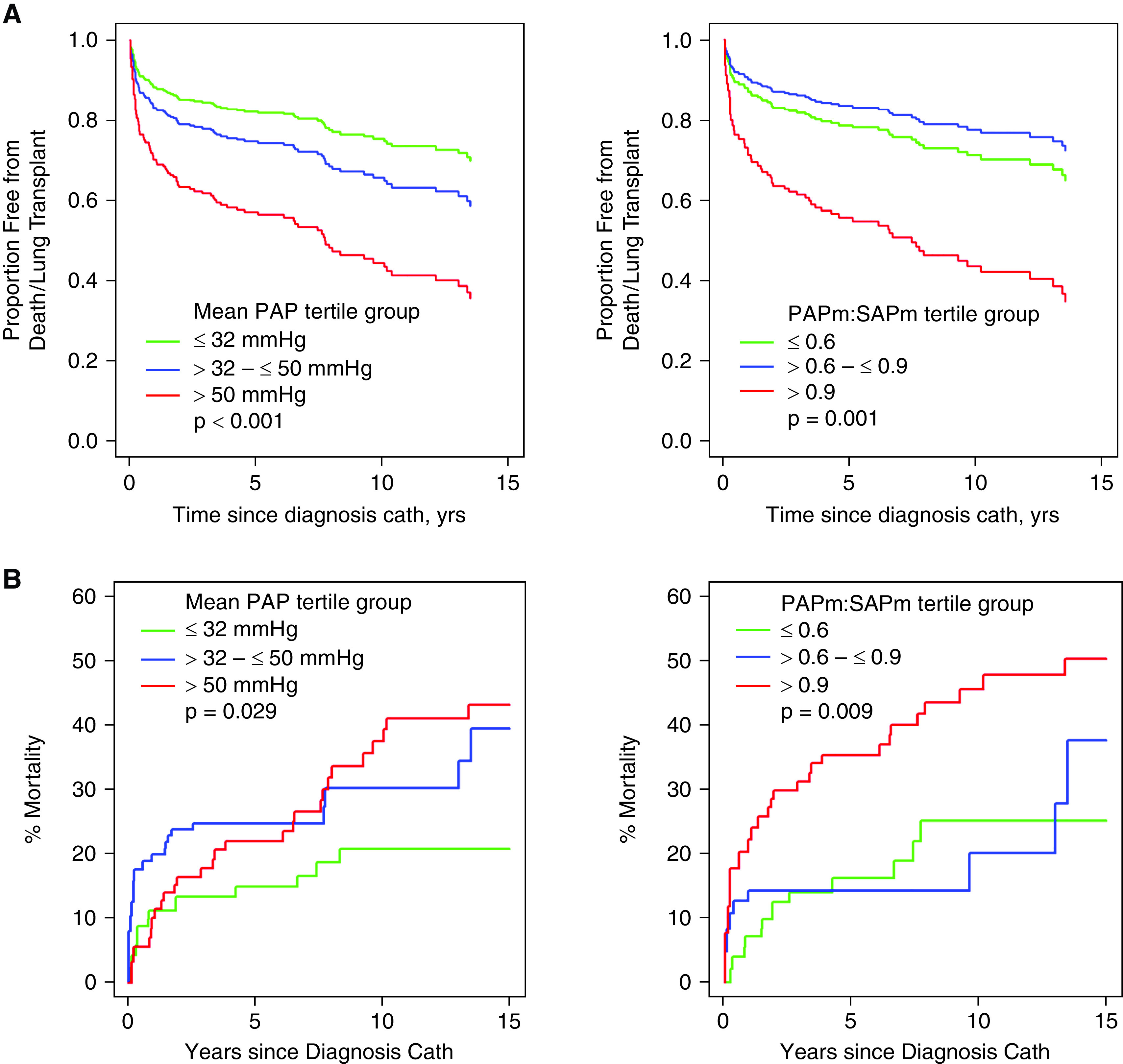
(A) Transplantation-free survival estimates by baseline hemodynamics from the catheterization performed at the time of pulmonary hypertension diagnosis. Mean pulmonary artery pressure (PAPm) (n = 634) and PAPm/mean systemic arterial pressure (SAPm) ratio (n = 467) are associated with risk of the composite of death/lung transplantation (P ⩽ 0.001). Patients in the highest tertile have the highest hazard of death/lung transplantation, and patients in the middle tertile have significantly higher risk compared with those in the lowest tertile. The figures are truncated at 15 years; some follow-up extended past 20 years, but no events occurred after 15 years. (B) Survival estimates by baseline hemodynamics from the catheterization performed at the time of pulmonary hypertension diagnosis, according to competing-risks analysis. PAPm (n = 634, P = 0.029) and PAPm/SAPm ratio (n = 467, P = 0.001) are associated with mortality. Patients in the highest tertile for PAPm (>50 mm Hg) have a higher hazard of death than those with PAPm in the first tertile, but patients in the middle tertile cannot be shown to differ from either adjacent tertile. For PAPm/SAPm, patients in the highest tertile have the highest hazard of death/lung transplantation. The figures are truncated at 15 years; some follow-up extended past 20 years, but no events occurred after 15 years. Cath = catheterization.
Discussion
In this large North American cohort of pediatric patients with pulmonary hypertension enrolled in the PPHNet Registry, which included both WSPH group 1 and 3 children, we studied the current applications of cardiac catheterization for assessing hemodynamics, examining the response to AVT, and determining complication rates. Although RHC remains the gold standard for the diagnostic workup of children with suspected WSPH group 1 pulmonary hypertension, there remains a lack of guidance on the best approach to hemodynamic data acquisition and its role in these children, including whether the approach and findings for WSPH group 3 patients differ from those for WSPH group 1 children. Despite increasing treatment of WSPH group 3 patients with pulmonary hypertension with targeted pulmonary hypertension therapies, this is the first study to provide a comprehensive analysis of hemodynamic data that includes both WSPH group 1 and WSPH group 3 children.
We found that the hemodynamic profiles had some key differences, including older age at the time of RHC and worse hemodynamics for those with WSPH group 1 pulmonary hypertension in general compared with those with WSPH group 3 pulmonary hypertension in this cohort. Patients with WSPH group 3 disease had higher Pco2 at the time of diagnostic catheterization and greater likelihood to be on O2 or iNO at the time of diagnostic catheterization, which is consistent with their underlying WSPH group 3 diagnoses.
Fifty-one percent (n = 710) of the infants and children with pulmonary hypertension in the PPHNet Registry did not undergo RHC at the time of diagnosis. Children who underwent diagnostic RHC, compared with those who did not, were older at pulmonary hypertension diagnosis, less likely to be preterm or on oxygen supplementation, more often female and Asian, and more likely to have a worse Pediatric Functional Class or WHO functional class (III/IV). These findings speak to a common concern about risk associated with cardiac catheterization often reported in heterogenous patients with pulmonary hypertension and through a survey of centers without extensive experience in RHC for pulmonary hypertension (14). Despite the concerns raised in previous reports (8, 14), and acknowledging potential selection bias, which represent the real-world strategies used at expert centers, we found a relatively low complication rate associated with diagnostic catheterization in these children at the pediatric pulmonary hypertension centers of the PPHNet.
In this study, we report that fewer than one-third of these patients routinely underwent LHC at the time of diagnosis, defined in the registry as the presence of a recorded LVEDP to assume that systemic arterial access was obtained. Although this registry and another large pediatric catheterization registry (14) have shown an overall low complication rate in centers with high volumes of pulmonary hypertension catheterizations, LHC is known to convey an increase in complications of vascular injury and stroke in pediatric patients (15). In WSPH group 1 and group 3 patients, evaluation for left heart dysfunction (16) is important for treatment, and some physicians argue for the need for direct left heart measurements, whereas others rely on PCWP. Notably, the strategy to perform LHC with diagnostic RHC in the PPHNet Registry among WSPH group 1 and -3 patients differed only by center and not by clinical disease severity or diagnosis. In this registry, because individual sites followed different protocols, with some electing to rely more on PCWP and others choosing to perform more LHCs, we were able to determine whether PCWP measurement is accurate and reliable. We found that in cases when both RHC and LHC were performed, the PCWP correlated well with the LVEDP, whereas complication rates did not differ between RHC and RHC/LHC, if a reliable PCWP could be obtained. We recognize that under some circumstances, LHC may be important to exclude other forms of congenital heart disease, such as patent ductus arteriosus, valvular heart disease, pulmonary vein stenosis, aortic coarctation, and aortopulmonary collaterals. However, these data lend support to the performance of diagnostic RHC alone with acquisition of a reliable PCWP for hemodynamics, as done in adults, which may be sufficient in the absence of a specific concern for left heart disease or postcapillary pulmonary hypertension.
With regard to acute vasoreactivity, we found that when applying any of the three currently used criteria (Sitbon, Barst, or modified Barst), the maximal hemodynamic response rates were highly consistent for children in WSPH group 1 and group 3. The rate of responsiveness across both of these groups was also relatively low compared with some previous reports in children (12). Age was not significantly correlated with responsiveness. In general, given that approximately half of the patients in our registry did not undergo catheterization at the time of diagnosis, and these patients were on average younger, our findings may not be as generalizable to younger patients with pulmonary hypertension.
At baseline, more WSPH group 3 than group 1 patients undergoing cardiac catheterization were on supplemental oxygen at the time of catheterization. This is not surprising given that WSPH group 3 patients include those with developmental lung disease, of which the largest group is those with bronchopulmonary displasia (BPD). However, the numbers of patients who were on either iNO and/or oxygen at baseline were sizable in both groups. Although this represents real-world experience, it also makes it challenging to further interpret AVT responsiveness in either group of patients, as they may already have exhibited a significant response to either of these agents. There were insufficient data to determine if either of the WSPH groups responded more favorably to oxygen versus iNO. Past studies of AVT in infants with BPD, however, have shown greater acute pulmonary vasodilation with iNO than supplemental oxygen alone (17). This study further demonstrated striking increases in PAPm and PVRi above baseline values with exposure to acute hypoxia. However, this study involved a small cohort (n = 10), and it exclusively included children with BPD who were older at the time of RHC than group 3 subjects included in the present study. Further studies comparing hemodynamic measurements in these two WSPH groups of pediatric patients with pulmonary hypertension are warranted.
To evaluate the potential impact of baseline iNO/O2, we have reported the hemodynamic parameters in WSPH group 1 and 3 patients who were on iNO/O2 at the time of diagnostic cardiac catheterization and compared them with those in patients who were not. The patients who required iNO/O2 at the time of their diagnostic RHC were younger, were more likely to be in WSPH group 3, and had a faster resting heart rate, lower systemic arterial systolic pressure, higher pulmonary venous, systemic arterial, and mixed venous saturation, and higher Pco2 and Po2 on the arterial blood gas. Regardless of impact on AVT, one can hypothesize that requiring iNO and O2 is a predictor of a sicker patient at the time of diagnostic RHC.
We found that higher PAPm and PAPm/SAPm ratio were significantly associated with a higher risk of death/lung transplantation, making this hemodynamic measurement critical when assessing prognosis in a young child or an infant with pulmonary hypertension. Higher Rp:Rs ratio was not associated with adverse outcomes. The value of assessing the PAPm:SAPm ratio is an important finding specifically for children, whose average SAPm is typically lower than in adults, and one can be misled by only mild elevations in PAPm. It also underscores the importance of always reviewing the estimated PAP or right ventricular systolic pressure in the context of SAP when assessing children with pulmonary hypertension even when noninvasively by echocardiography.
Study Limitations
This is the largest pediatric pulmonary hypertension hemodynamic data analysis inclusive of both WSPH group 1 and 3 children in the current literature, including 671 subjects from eight institutions from the PPHNet Registry. This dataset has the expected limitations of registry data because enrollment is nonhomogeneous. First, patient selection and enrollment criteria varied among institutions, with some enrolling primarily outpatients and others enrolling from both inpatient and outpatient settings. This creates a selection bias and may not provide accurate mortality statistics, as institutions that enrolled patients as outpatients would have enrolled only subjects who survived to discharge. Because hemodynamic data were analyzed across multiple institutions, criteria underlying the decision for diagnostic catheterization, the timing of the studies, age of subjects, demographics, duration of symptoms before RHC, medications at the time of the procedure, the approach to sedation and anesthesia, and the dose of iNO or use of O2 during AVT were not standardized across all sites. Furthermore, cardiac output was measured either by the Fick method or thermodilution at different institutions, and even within institutions this could have varied depending on the subject’s age and ventilation status, availability of equipment, provider expertise, and era of procedure. Typical of any RHC in group 3 patients with branch pulmonary artery stenosis or pulmonary venous stenosis, pulmonary venous pressures and pulmonary wedge pressures could differ between lung segments, precluding accurate calculations. Furthermore, because of patchy lung disease, Po2 in the pulmonary veins in different segments of the lung could be different, again precluding accurate shunt and resistance calculations. These data also span subjects diagnosed over a 17-year period, and medications available and treatment strategies have evolved over time, thus possibly influencing outcomes through an era effect.
Conclusions
Data from the large pediatric PPHNet Registry from pediatric pulmonary hypertension centers in North America demonstrated that RHC at the time of diagnosis in WSPH group 1 and 3 patients can be performed safely with or without LHC when indicated. Hemodynamic characteristics and differences between WSPH groups 1 and 3, including approach to RHC and correlation with outcomes, were demonstrated, further underscoring the value of RHC as the current gold-standard diagnostic approach for pulmonary hypertension. Specifically, we found that a higher PAPm and PAPm:SAPm ratio were significantly associated with a higher risk of death/lung transplantation, making this hemodynamic measurement critical when assessing prognosis in a young child or infant with pulmonary hypertension. AVT has also been an invaluable tool for the assessment of calcium channel blocker responsiveness and overall prognosis for WSPH group 1 patients, but it remains to be seen whether AVT responsiveness predicts calcium channel blocker responsiveness and adverse outcomes for WSPH group 3 patients as well. Future studies with robust longitudinal follow-up and focused on long-term outcomes will be needed to determine the hemodynamic predictors of long-term outcomes for WSPH group 1 and 3 pediatric patients with pulmonary hypertension.
Footnotes
Supported by National Institutes of Health National Heart, Lung, and Blood Institute grant U01 HL12118 (K.D.M. and S.H.A.). This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Author Contributions: The authors confirm contributions to the paper as follows: study conception and design: E.B.R., M.P.M., S.H.A., S.S.H., and L.A.S. Data collection: E.B.R., A.B., M.P.M., S.H.A., E.D.A., J. Fineman, J. Feinstein, R.K.H., U.S.K., D.Y., and S.S.H. Analysis and interpretation of results: E.B.R., A.B., M.L., M.P.M., S.H.A., A.E., J. Fineman, J. Feinstein, J.P.K., U.S.K., K.D.M., J.U.R., N.V., D.Y., S.S.H., and L.A.S. Draft manuscript preparation: E.B.R., A.B., M.P.M., S.H.A., E.D.A., A.E., J. Fineman, J. Feinstein, R.K.H., J.P.K., U.S.K., J.U.R., N.V., D.Y., S.S.H., and L.A.S. All authors reviewed the results and approved the final version of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J . 2019;53:1801916. doi: 10.1183/13993003.01916-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsuura H. Cardiac catheterization in children with pulmonary arterial hypertension. Pediatr Int (Roma) . 2017;59:3–9. doi: 10.1111/ped.13161. [DOI] [PubMed] [Google Scholar]
- 3. Bobhate P, Guo L, Jain S, Haugen R, Coe JY, Cave D, et al. Cardiac catheterization in children with pulmonary hypertensive vascular disease. Pediatr Cardiol . 2015;36:873–879. doi: 10.1007/s00246-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 4. Del Cerro MJ, Moledina S, Haworth SG, Ivy D, Al Dabbagh M, Banjar H, et al. Cardiac catheterization in children with pulmonary hypertensive vascular disease: consensus statement from the Pulmonary Vascular Research Institute, Pediatric and Congenital Heart Disease Task Forces. Pulm Circ . 2016;6:118–125. doi: 10.1086/685102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frank BS, Schäfer M, Grenolds A, Ivy DD, Abman SH, Darst JR. Acute vasoreactivity testing during cardiac catheterization of neonates with bronchopulmonary dysplasia-associated pulmonary hypertension. J Pediatr . 2019;208:127–133. doi: 10.1016/j.jpeds.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 6. Caicedo L, Hopper RK, Garcia H, Ivy DD, Haag D, Fineman J, et al. EXPRESS: acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an international survey on current practice. Pulm Circ . 2019;9:2045894019857533. doi: 10.1177/2045894019857533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuckerman WATM, Turner ME, Kerstein J, Torres A, Vincent JA, Krishnan U, et al. Safety of cardiac catheterization at a center specializing in the care of patients with pulmonary arterial hypertension. Pulm Circ . 2013;3:831–839. doi: 10.1086/674752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaiyani D, Kelleman M, Downey LA, Kanaan U, Petit CJ, Bauser-Heaton H. Risk factors for adverse events in children with pulmonary hypertension undergoing cardiac catheterization. Pediatr Cardiol . 2021;42:736–742. doi: 10.1007/s00246-020-02535-4. [DOI] [PubMed] [Google Scholar]
- 9. Abman SH, Raj U. Towards improving the care of children with pulmonary hypertension: the rationale for developing a Pediatric Pulmonary Hypertension Network. Prog Pediatr Cardiol . 2009;27:3–6. doi: 10.1016/j.ppedcard.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation . 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 11. Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation . 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 12. Barst RJ. Pharmacologically induced pulmonary vasodilatation in children and young adults with primary pulmonary hypertension. Chest . 1986;89:497–503. doi: 10.1378/chest.89.4.497. [DOI] [PubMed] [Google Scholar]
- 13. Barst RJ, Ivy DD, Foreman AJ, McGoon MD, Rosenzweig EB. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry) Am J Cardiol . 2014;113:147–155. doi: 10.1016/j.amjcard.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 14. O’Byrne ML, Kennedy KF, Kanter JP, Berger JT, Glatz AC. Risk factors for major early adverse events related to cardiac catheterization in children and young adults with pulmonary hypertension: an analysis of data from the IMPACT (Improving Adult and Congenital Treatment) registry. J Am Heart Assoc . 2018;7:e008142. doi: 10.1161/JAHA.117.008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrar DB, Salussolia CL, Vittner P, Danehy A, Sen S, Whitehill R, et al. Stroke after cardiac catheterization in children. Pediatr Neurol . 2019;100:42–48. doi: 10.1016/j.pediatrneurol.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 16. Mourani PM, Ivy DD, Rosenberg AA, Fagan TE, Abman SH. Left ventricular diastolic dysfunction in bronchopulmonary dysplasia. J Pediatr . 2008;152:291–293. doi: 10.1016/j.jpeds.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2004;170:1006–1013. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]