Abstract
Rationale
The long-term natural history of asthma in terms of successive severe exacerbations and the influence of each exacerbation on the course of the disease is not well studied.
Objectives
To investigate the long-term natural history of asthma among patients who are hospitalized for asthma for the first time in terms of the risk of future severe exacerbations and heterogeneity in this risk across patients.
Methods
Using the administrative health databases of British Columbia, Canada (January 1, 1997 to March 31, 2016), we created an incident cohort of patients with at least one asthma exacerbation that required inpatient care. We estimated the 5-year cumulative incidence of severe exacerbations after successive numbers of previous events. We used a joint frailty model to investigate the extent of between-individual variability in exacerbation risk and the associations of each exacerbation with the rate of subsequent events. Analyses were conducted separately for pediatric (<14 years old) and adult (⩾14 years old) patients.
Results
Analyses were based on 3,039 pediatric (mean age at baseline, 6.4; 35% female) and 5,442 (mean age at baseline, 50.8; 68% female) adult patients. The 5-year rates of severe exacerbations after the first three events were 0.16, 0.29, and 0.35 for the pediatric group, and 0.14, 0.33, and 0.49 for the adult group. Both groups exhibited substantial variability in patient-specific risks of exacerbation: the mid-95% interval of 5-year risk of experiencing a severe exacerbation ranged from 11% to 24% in pediatric patients and from 8% to 40% in adult patients. After controlling for potential confounders, the first follow-up exacerbation was associated with an increase of 79% (95% confidence interval [CI], 11–189%) in the rate of subsequent events in the pediatric group, whereas this increase was 188% (95% CI, 35–515%) for the adult group. The effects of subsequent exacerbations were not statistically significant.
Conclusions
After the first severe exacerbation, the risk of subsequent events is substantially different among patients. The number of previous severe exacerbations carries nuanced prognostic information about future risk. Our results suggest that severe exacerbations in the early course of asthma detrimentally affect the course of the disease and risk of subsequent exacerbations.
Keywords: asthma, exacerbation, prognosis, recurrent events, frailty model
Asthma is a common chronic disease of the airways, affecting 339 million people worldwide (1). It is one of the most common chronic diseases in children (2) and is responsible for a considerable impact on quality of life and a substantial and growing economic burden across all age groups (3). Episodes of acute worsening of asthma symptoms, referred to as exacerbations or “flare-ups,” constitute an important component of the natural history of asthma (4). In particular, severe exacerbations are one of the main causes of missed school days and hospital admissions for children and loss of work productivity for adults (1, 5–7).
Despite their great burden, the natural history of asthma exacerbations has not been investigated in depth. It is generally known that the best predictor of future exacerbations is a positive history of exacerbations (8), with a recent study showing that the number of exacerbations in the previous 2 years informs the probability of exacerbations in the next year (9). However, such an association between the previous and future events can be generated by fundamentally different mechanisms. A positive association occurs if there is a difference in susceptibility to exacerbation among patients (between-individual variability, or heterogeneity) such that some patients are “frequent exacerbators” (10, 11). A positive association can also emerge if the occurrence of an exacerbation detrimentally affects the airways and increases the risk of future events. There is growing evidence that asthma exacerbations inflict long-term structural damage on the lungs. For example, a study of patients with moderate or severe asthma showed that one additional severe exacerbation per year was associated with a decline of 30.2 ml in forced expiratory volume at 1 second (12). Furthermore, time-varying extrinsic factors, such as occupational or environmental exposures (e.g., change in occupation or moving to a polluted area), could lead to such a positive association (13).
The extent to which these phenomena contribute to the natural history of asthma exacerbations can have important clinical implications. For example, if an asthma exacerbation increases the rate of subsequent events, preventing an exacerbation “today” can result in far-reaching benefit for patients. This will affect the risk–benefit and cost-effectiveness of antiinflammatory therapies that reduce exacerbation risk. Similarly, if the occurrence of an exacerbation indicates the presence of a potentially modifiable extrinsic risk factor (e.g., air pollution), it can prompt risk modification interventions that can mitigate future risk. Previous studies in chronic obstructive pulmonary disease (COPD), another airway disease whose course is highlighted by exacerbations, have established the presence of both phenomena (14, 15). In comparison, existing evidence on the natural history of asthma exacerbations is sparse, particularly for severe events that are most consequential but less prevalent. Previous investigators have highlighted the need for population-based studies toward better understanding the patterns of asthma exacerbations (16, 17).
The objectives of this study were threefold: to document the extent of association between previous and future severe exacerbations, to investigate the extent of heterogeneity in exacerbation rates, and to test the hypothesis that the occurrence of a severe exacerbation is associated with an increase in the rate of subsequent events. Data in this article was previously published in a preprint in medRxiv (https://www.medrxiv.org/content/10.1101/2021.04.05.21254930v1).
Methods
Data Sources
We used centralized administrative health databases of British Columbia, a province of Canada with a population of 4.6 million as of 2016 (18). All legal residents of the province are included in the databases, minimizing the risk of selection bias. The quality of the data is high with a very low level of missing, underreported, or misclassified entries (19). We had access to the following data for the period of January 1, 1997 to March 31, 2016: demographics, including socioeconomic status (SES) based on the neighborhood income quintile (the median household income taken from census data was linked to the residential postal code) (20, 21), registration status with the provincial healthcare system (20), vital statistics (22), and inpatient and outpatient records (23, 24). All inferences, opinions, and conclusions drawn in this paper are those of the authors and do not reflect the opinions or policies of the data steward(s). Ethics approval was obtained from the Human Ethics Board of the University of British Columbia (H17-00938).
Study Cohort
To construct an incident cohort, we first identified all patients who experienced at least one severe asthma exacerbation (regardless of its timing since asthma diagnosis). A severe exacerbation was defined as an episode of hospitalization with asthma as the primary discharge diagnosis using the International Classification for Disease (ICD) codes (ICD-9 codes 493, excluding 493.2x, or ICD-10 codes J45–J46); we treated hospitalizations occurring within 7 days of each other as one severe exacerbation event (25). We chose this definition as the entry criterion owing to its high positive predictive value, and also because it results in a relatively homogeneous cohort, reducing the risk of confounding by disease severity in estimating associations (this design has been adopted in similar studies for COPD [14, 15]). We refer to the first severe asthma exacerbation as the index exacerbation and the corresponding discharge date from hospital as the index date, marking the beginning of follow-up. Subsequent severe exacerbations are referred to as follow-up events.
To ensure that the first asthma-related hospitalization in the data was indeed the first instance of a severe exacerbation, we excluded patients, regardless of asthma diagnosis, who were not continuously present in the database for at least 5 years before their index date, or for the entire period since birth if they were less than 5 years of age on their index date. A previous study has concluded that 5 years is a sufficient amount of time to protect against left-censoring (26). We also excluded patients with a history of COPD before their index date, defined as having at least one hospitalization or two physician visits in any 12-month period owing to COPD (ICD-9 codes 491, 492, 493.2x, 496, or ICD-10 codes J41–J44). This criterion was applied as the diagnostic likelihood and natural history of asthma exacerbations in patients with concomitant COPD (asthma–COPD overlap) might be different from those in the general population with asthma (27).
Finally, the cohort was divided into the subgroups of pediatric (index age < 14 yr) and adult (index age ⩾ 14 yr) patients, given the potentially different disease dynamics and associations in the two subgroups (28). All patients were followed until the first occurrence of the following: the last date of any medical service use record, deregistration from the healthcare system, death, and the end of study period (March 31, 2016). Although preexisting COPD was an exclusion criterion, some patients could still have developed COPD after their asthma diagnosis. As such, to exclude patient-times with possible asthma–COPD overlap, a hospital admission owing to COPD was also considered a competing censoring event.
Analysis
First, we investigated to what extent the number of previous severe exacerbations was prognostic of the rate of future events. This was achieved through a descriptive analysis that involved calculating the five-year cumulative incidence of severe exacerbations after the occurrence of a given number of severe exacerbations using the nonparametric method (29).
As described earlier, a positive association between previous and future severe exacerbations might be owing to the presence of heterogeneity in the rate of exacerbations. To investigate the extent of heterogeneity, we used an accelerated failure time joint frailty model for the time intervals between severe asthma exacerbations (14, 30, 31). In this framework, heterogeneity in exacerbation rates is modeled through individual-specific random effects (32). The random effects represent the patient-specific propensity to exacerbate over and above the variability owing to their observed characteristics. A main advantage of using this framework is that such random effects provide a mechanism to capture the heterogeneity in exacerbation rates, and to control for unobserved covariates (e.g., asthma severity, symptom burden, and environmental exposures), thus enabling within-patient inference on the association between consecutive severe exacerbations (33). In addition to severe exacerbations, we modeled hospitalization owing to COPD and all-cause mortality as competing events for the adult group (these events were infrequent for the pediatric group and thus were not explicitly modeled; see Table 1), given their potential association with asthma (34, 35). Details of the statistical model are provided in Section 1 of the online supplement.
Table 1.
Summary statistics of the key covariates and outcomes of the final cohort
| Covariates | Pediatric Group (n = 3,039) | Adult Group (n = 5,442) |
|---|---|---|
| Sex, female, n (%) | 1,078 (35.5%) | 3,696 (67.9%) |
| Follow-up time, yr, mean (IQR) | 9.0 (5.3–12.6) | 6.8 (2.9–10.7) |
| Age at baseline, yr, mean (IQR) | 6.4 (3.9–8.1) | 50.8 (35.5–66.0) |
| Calendar year of index date, yr since 2001, mean (IQR) | 8.7 (5.0–12.0) | 9.6 (6.0–13.0) |
| Patients admitted to intensive care unit for the index exacerbation, n (%) | 111 (3.7%) | 378 (6.9%) |
| Patients who stayed longer than 7 d for the index exacerbation, n (%) | 26 (0.9%) | 655 (12.0%) |
| Charlson comorbidity index (%) | ||
| 0 | 3,026 (99.6%) | 5,052 (92.8%) |
| ⩾1 | 13 (0.4%) | 390 (7.2%) |
| Socioeconomic status, n (%) | ||
| Low (first and second quintiles) | 1,919 (63.1%) | 3,605 (66.2%) |
| High (third, fourth, and fifth quintiles) | 1,073 (35.3%) | 1,715 (31.5%) |
| Unknown | 47 (1.5%) | 122 (2.2%) |
| Follow-up events | ||
| Severe asthma exacerbations, n (annual rate) | 737 (0.03) | 1,392 (0.04) |
| Severe chronic obstructive pulmonary disease exacerbations, n (annual rate) | 0 (0.00) | 683 (0.02) |
| Deaths, n (annual rate) | 22 (0.03) | 519 (0.01) |
| Patients with medication in each follow-up severe asthma exacerbation, n (%) | ||
| Index | 1,855 (61.0%) | 3,902 (71.7%) |
| First | 256 (51.4%) | 553 (69.6%) |
| Second | 72 (51.4%) | 166 (63.6%) |
| Third+ | 44 (44.4%) | 220 (65.3%) |
| Frequency of severe asthma exacerbations during follow-up (%) | ||
| 0 | 2,541 (83.6%) | 4,648 (85.4%) |
| 1 | 358 (11.8%) | 533 (9.8%) |
| 2 | 90 (3.0%) | 136 (2.5%) |
| ⩾3 | 50 (1.6%) | 125 (2.3%) |
Definition of abbreviation: IQR = interquartile range.
The analysis of association was further controlled for the following covariates: age on the index date, calendar year of the index date, biological sex, quintile of neighborhood SES in the index year, and the Charlson comorbidity index calculated in the 12-month period before the index date (36). The comorbidity index was not included for the pediatric group since only a handful of patients had comorbidities (Table 1). For the primary event (severe asthma exacerbation), three additional binary covariates were controlled for: whether the patient was admitted to the intensive care unit for the index exacerbation, whether the length of stay was 7 days or longer during the index exacerbation, and whether antiinflammatory asthma medications (inhaled corticosteroids, oral corticosteroids, or leukotriene-receptor antagonists) were used during, or within 7 days after discharge from, the previous hospitalization.
The analysis of heterogeneity in exacerbation risks and potential direct effect of exacerbations on future risk were based on a random-effects accelerated failure time model. To illustrate the heterogeneity in susceptibility to severe asthma exacerbations, we predicted each patient’s 5-year risk of experiencing a severe asthma exacerbation after the index event (see Section 2.1 of the online supplement for details). We drew the distribution (histogram) of the predicted 5-year risks and calculated the 95% coverage range: the range of 5-year risk that includes the mid-95% of individuals. The accelerated failure time model allows the effect of each covariate to be presented as a “rate multiplier” (which can be obtained by exponentiating the regression coefficient). If a covariate has a rate multiplier of 2, one unit increase in the value of that covariate doubles the exacerbation rate. To investigate the effect of each severe exacerbation on the rate of subsequent events, we reported the rate multiplier for the first three events during follow-up, after controlling for other covariates.
We ran two sensitivity analyses for the adult group to 1) assess the impact of excluding patients who experienced a COPD hospitalization during follow-up; and 2) evaluate the robustness of results against an alternative formulation for the competing events regarding the choices of time scale and hazard function; details are provided in Section 3 of the online supplement. Data analysis was performed using SAS software, version 9.4, and the R statistical programming environment, version 3.6.1 (37). See Section 4 of the online supplement for details on the implementation of the statistical model.
Results
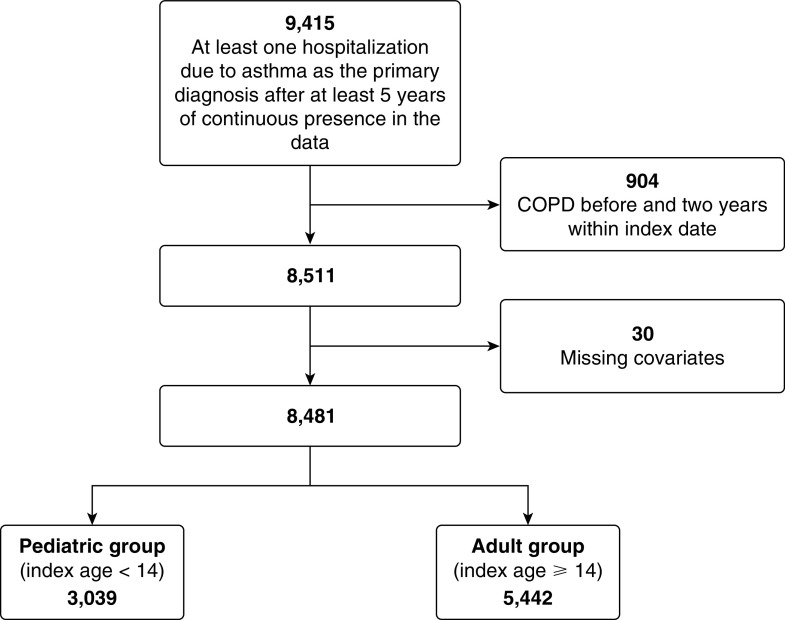
In the period from January 1, 1997, to March 31, 2016, there were 9,415 eligible patients who experienced at least one severe exacerbation. We excluded 904 who had a history of COPD before asthma diagnosis and 30 who had missing covariates. There were 3,039 patients in the pediatric group and 5,442 patients in the adult group for the analysis. Figure 1 provides the schematic illustration of the cohort creation.
Figure 1.
Flowchart of asthma cohort creation. COPD = chronic obstructive pulmonary disease.
For the pediatric group, the mean age on the index date was 6.4 years (interquartile range [IQR], 3.9–8.1); 36% were female; and the average follow-up time was 9.0 years (IQR, 5.3–12.6). For the adult group, the mean index age was 50.8 years (IQR, 35.5–66.0); 68% were female; and the average follow-up time was 6.8 years (IQR, 2.9–10.7). Table 1 presents the characteristics of both groups.
Previous History and Future Risk
In the pediatric group, 737 severe exacerbations were recorded during follow-up; 16% of patients had at least one, 5% had two or more, and 2% had three or more events after their index event. In the adult group, 1,392 severe exacerbations occurred during follow-up; 15% had at least one, 5% had two or more, and 2% had three or more events after their index date.
Figure 2 shows the nonparametric cumulative incidence curve since discharge for the previous severe exacerbation for the first three follow-up events. In both groups, there was an increase in the rate of experiencing a severe exacerbation after the occurrence of each successive severe exacerbation. For the pediatric group, the 5-year rates were 0.16 (95% CI, 0.15–0.17) after the index event, 0.29 (95% CI, 0.25–0.33) after the first follow-up event, and 0.35 (95% CI, 0.27–0.43) after the second follow-up event. The corresponding values for the adult group were 0.14 (95% CI, 0.13–0.15), 0.33 (95% CI, 0.30–0.37), and 0.49 (95% CI, 0.42–0.55).
Figure 2.
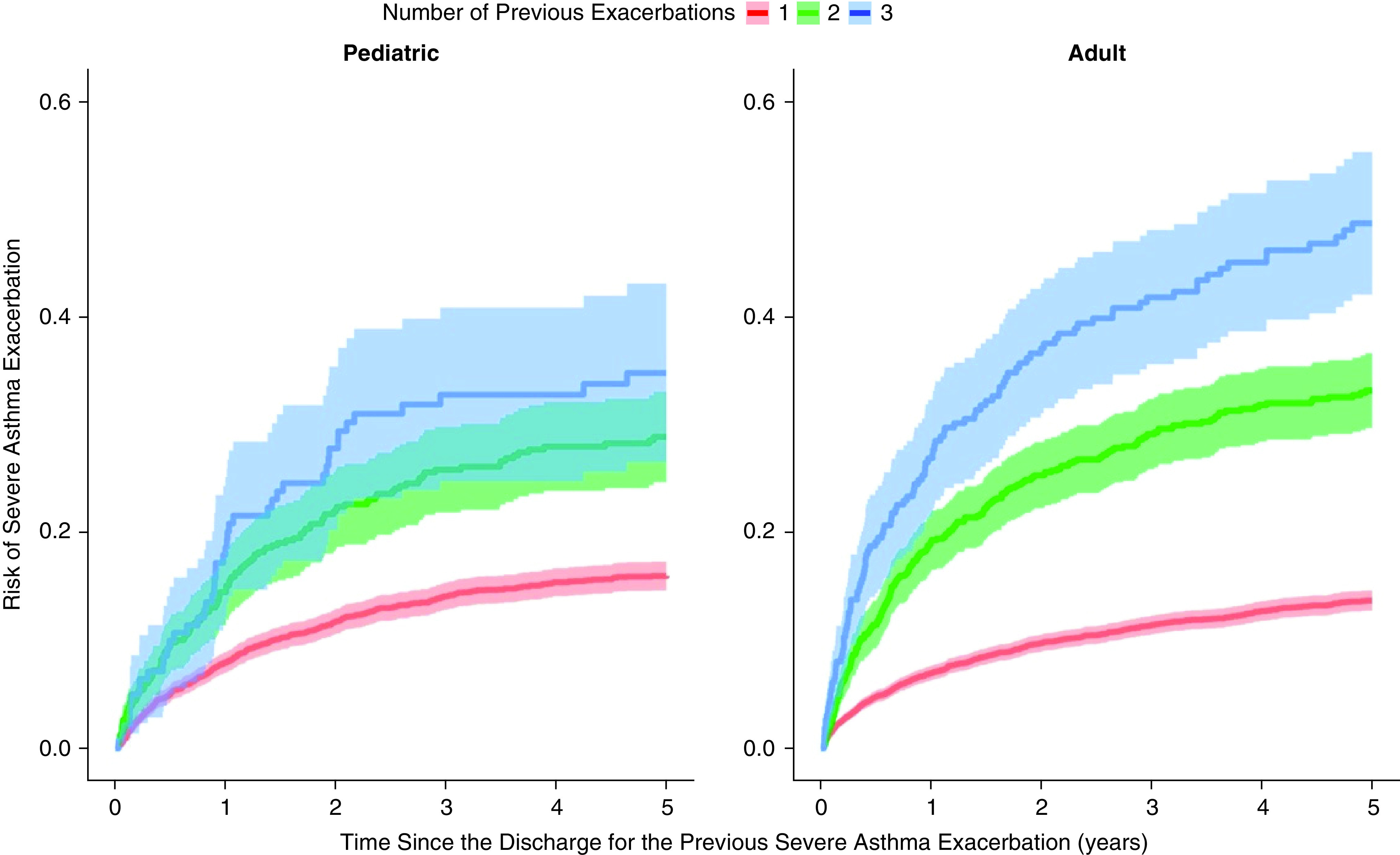
Cumulative incidence curves since discharge for the previous severe exacerbation for the first three follow-up events (first: red, second: green, and third: blue) for the pediatric and adult groups. The shaded area corresponds to pointwise 95% confidence intervals.
Heterogeneity in Exacerbation Risks
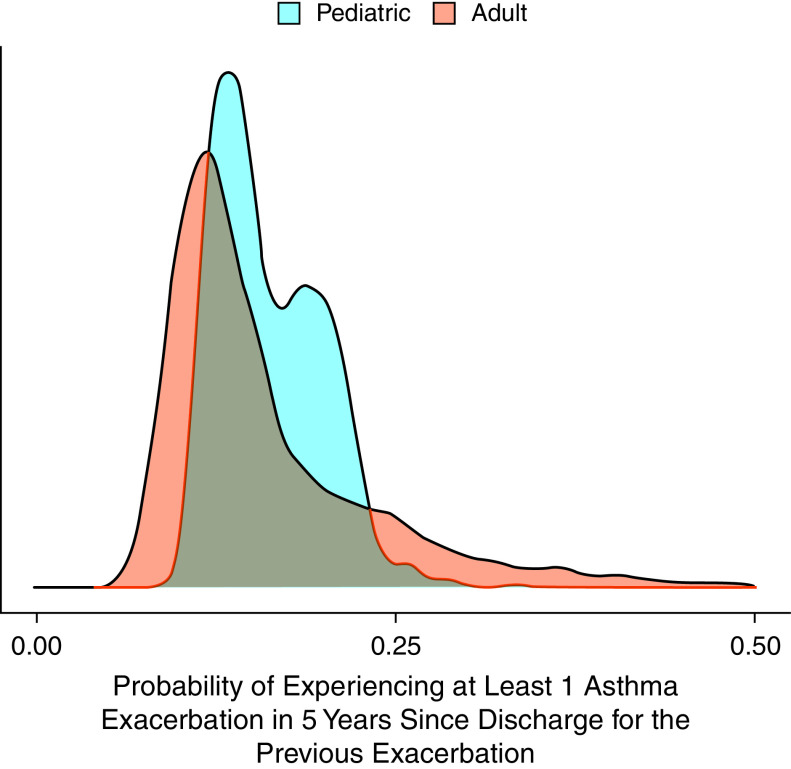
Figure 3 provides the distribution of the patient-specific 5-year exacerbation risk after the index date, separately for the pediatric group (blue) and adult group (red). There was substantial variability in 5-year risks in both groups. For the pediatric group, the range of 5-year risk that covered the mid-95% of individuals was 11–24% (coefficient of variation: 23%). The corresponding value was 8–40% (coefficient of variation, 53%) for the adult group. In other words, the risks for pediatric patients at the higher end of the susceptibility spectrum were 2.1 times higher than for those at the lower end; this ratio was 5.0 for adult patients.
Figure 3.
Distributions of patient-specific postindex event 5-year risk of severe exacerbation, separately for the pediatric (blue) and adult (red).
Risk of Subsequent Severe Exacerbations
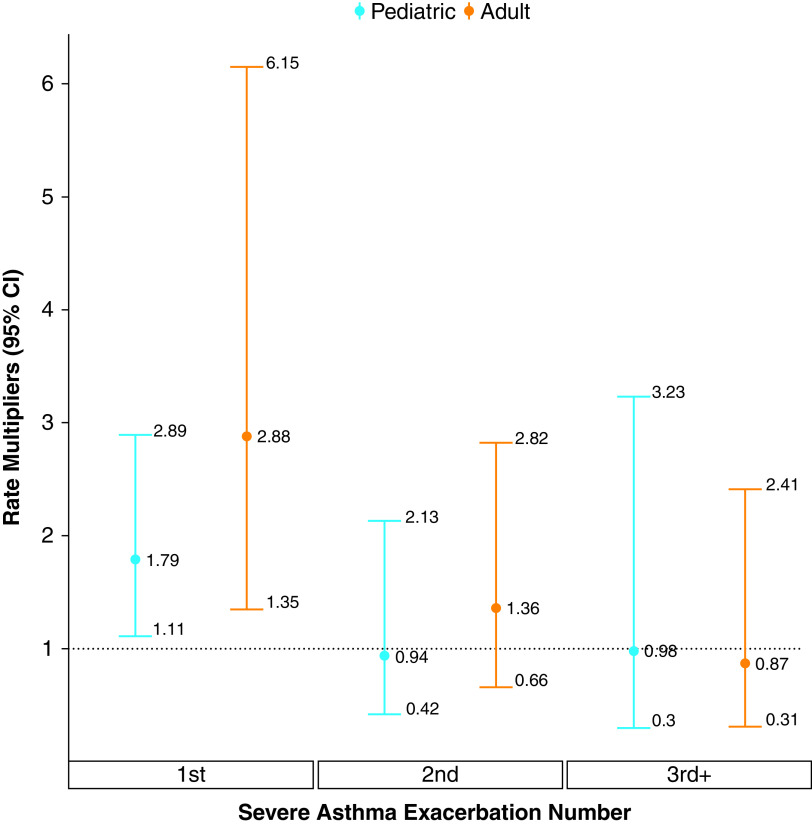
Figure 4 provides the estimated rate multipliers for the first three severe asthma exacerbations during follow-up, after controlling for covariates and the underlying exacerbation rate (random effects). For both groups, the occurrence of the first severe asthma exacerbation during follow-up was associated with a significant increase in the rate of future events. For the pediatric group, the relative increase in the rate was 79% (95% CI, 11–189%). This value was 198% (95% CI, 35–515%) for the adult group. The effects of subsequent severe exacerbations were not statistically significant for either group.
Figure 4.
Estimated rate multipliers for successive severe asthma exacerbations in the pediatric (blue) and adult (red) group. Reprinted with permission from Reference 55. CI = confidence interval.
Table 2 provides the estimated regression coefficients, expressed in terms of rate multipliers, for both age groups. Biological sex was not associated with a significant change in the rate of severe asthma exacerbations for either group. On the other hand, the severity of the index asthma exacerbation, as described by the two binary covariates (length of stay and intensive care unit admission for the index asthma exacerbation), was associated with a significant increase in the rate of subsequent events for both groups. High SES (third, fourth, and fifth quintiles) versus low SES (first and second quintiles) was not associated with a difference in the rate for the pediatric group but was for the adult group. Compared with the lowest index age group, the higher index age groups were associated with a decrease in the rate for both groups. Asthma medication use was associated with a decrease in the rate for the pediatric group, but its effect was not significant for the adult group.
Table 2.
Estimated rate multipliers (95% confidence interval) of the covariates for the asthma exacerbation events for the pediatric and adult groups
| Rate Multipliers (95% Confidence Interval) |
||
|---|---|---|
| Covariates | Pediatric Group | Adult Group |
| Length of stay for the index asthma exacerbation: >7 d | 8.03 (1.40–46.17)* | 1.71 (1.23–2.39)* |
| Charlson comorbidity index: >0 (reference: 0) | — | 1.42 (0.88–2.29) |
| Sex, female | 1.28 (0.90–1.82) | 1.22 (0.94–1.59) |
| Admitted to intensive care unit for the index exacerbation | 0.85 (0.35–2.10) | 2.52 (1.73–3.66)* |
| Index year (since 2001) | 1.02 (0.98–1.06) | 0.99 (0.96–1.02) |
| Social economic status: low (reference: high) | 1.14 (0.80–1.63) | 1.42 (1.10–1.83)* |
| Social economic status: unknown (reference: high) | 0.76 (0.19–3.03) | 2.00 (0.91–4.38) |
| Index age, yr: 5–9 (reference: <5) | 0.34 (0.22–0.51)* | — |
| Index age, yr: 9–14 (reference: <5) | 0.38 (0.22–0.64)* | — |
| Index age, yr: 35–49 (reference: 14–35) | — | 0.73 (0.54–0.97)* |
| Index age, yr: 50–64 (reference: 14–35) | — | 0.64 (0.47–0.87)* |
| Index age, yr: >65 (reference: 14–35) | — | 0.64 (0.45–0.90)* |
| Medication | 0.67 (0.49–0.93)* | 1.06 (0.86–1.31) |
Significant at 0.05 level.
The sensitivity analysis demonstrated that results were not overly sensitive to the exclusion of patients who experienced a COPD hospitalization during follow-up and to the choices of time scale and hazard function used for the competing events in the adult group (see Section 3 of the online supplement for details).
Discussion
Using a population-based cohort, we showed that the future rate of severe exacerbations increases with the number of previous events. On average, the 5-year rate of exacerbations in pediatric patients with three severe exacerbations was 0.35, being 219% higher than in those with one severe exacerbation (with the 5-year rate of 0.16) (Figure 2); this value was 350% among adult patients (5-year rates of 0.49 and 0.14 with three and one events, respectively). We also demonstrated that there is a substantial degree of heterogeneity in severe exacerbation rates. The 5-year risk of experiencing a severe exacerbation ranged from 11% to 24% in pediatric patients and from 8% to 40% in adult patients. After controlling for covariates and the confounding effect of heterogeneity in exacerbation rate, we showed that the occurrence of the first severe exacerbation during follow-up was associated with an independent increase in the rate of subsequent events in both groups. This association was not significant for subsequent events, although there was a large degree of uncertainty around these estimates.
Collectively, our findings suggest that the association between previous and future exacerbation risk is influenced by both heterogeneity in patient-specific exacerbation risk and a potential direct effect of exacerbations on the risk of subsequent events (at least early in the disease course). Extrinsic factors, such as environmental and occupational risk factors, that we could not measure in this study can contribute to such extended heterogeneity. Overall, these findings can have important clinical implications. The substantial level of heterogeneity in exacerbation rate is despite the fact that our sample was already restricted to individuals who had just been discharged from an episode of severe exacerbation requiring inpatient care. This indicates that individuals with a positive history of severe exacerbation are by no means a homogenous group, and the information on the number (not just a positive history) of severe exacerbations can provide prognostic information for nuanced risk-stratification.
Among the covariates examined, SES, as measured by neighborhood income, was associated with the risk of exacerbation in adults but not in children. Such a difference in association has also been observed in previous studies (38–40). However, given the wealth of literature on the impact of socioeconomic status on asthma outcomes in both children and adults (41), this finding most likely reflects the coarseness of neighborhood income as a singular metric representing the complex interplay of social determinants of health and outcomes.
Previous studies have evaluated the clustering of asthma exacerbations within individuals (4, 42–44). For example, in a 3-year prospective cohort study, occurrence of exacerbations in the previous year was the strongest predictor of future asthma exacerbations (4). In a more recent cohort study of 3-year asthma exacerbation patterns, Peters and colleagues established that the number of exacerbations in the past 3 years is a predictor of risk of future exacerbations (9). In another study, Bloom and colleagues observed that more recent exacerbations are associated with higher future risk, compared with exacerbations in the more distant past (45). This can indicate the role of extrinsic factors (e.g., a temporary elevation in the risk owing to an environmental factor). However, such time dependency can also arise even when an exacerbation permanently increases the risk of subsequent events. As such, the time gradient observed in Bloom and colleagues neither indicates nor precludes causal association between previous and future exacerbations. Regardless, these findings are aligned with ours in that details of exacerbation history provide prognostic information over and beyond the binary classification of (frequent) exacerbator. However, previous findings do not imply the presence of a direct impact of an exacerbation on the rate or risk of future events. This is because in the presence of heterogeneity in exacerbation rates, each individual’s intrinsic exacerbation rate affects both previous and future exacerbation rate, thereby acting as a classic confounding factor. As described previously (33), the statistical model employed in this work controls for the patient-specific background exacerbation rate, thereby removing this confounding effect. This methodology also adjusts for any unmeasured time-fixed variable that might associate previous and future asthma exacerbations (e.g., atopic or nonatopic asthma and chronic inflammation).
Still, our analysis cannot adjust for unmeasured time-dependent extrinsic factors that might affect the risk of exacerbations, such as a change in occupation during the study period that might expose the patient to inhaled risk factors. Indeed, exposure to extrinsic factors, such as air pollution, can have a major impact on the risk of exacerbation (46). However, we believe the observation of the diminishing pattern in the effects between previous and future exacerbation in our study (Figure 4) is more supportive of a direct effect, as extrinsic factors should generate such associations throughout the disease course. Furthermore, given that such extrinsic factors are generally modifiable, the overall results of our study remain interpretable in the sense that reducing the risk of exacerbations early in the course of asthma (e.g., via preventive therapies, or risk-modifying interventions in case of extrinsic factors) can modify the burden of exacerbations.
Notwithstanding the potential role of extrinsic factors, there are plausible explanations for a direct effect of a severe exacerbation on the course of asthma. Exacerbations can adversely modify the course of asthma through multiple mechanisms (47). For example, epithelial damage during the acute phase of an exacerbation and the subsequent reepithelialization process (47, 48) as well as proteolytic tissue destruction caused by neutrophil proteases (49) are hypothesized as causal mechanisms. These mechanisms can result in narrowing of the airways and corresponding loss of lung function, which is known to occur after severe asthma exacerbations (50). Beyond the effect on airways, other mechanisms might also be at play. For example, exacerbations are found to be associated with an acute loss of lung elasticity, which might not fully recover after the exacerbation is resolved (47). This signals potentially structural damage to the lung and failure of tissue repair after an exacerbation. An important implication of the presence of direct mechanisms is that the prevention of severe exacerbations early in the disease course can have long-lasting benefits. This is critical when extrapolating the results from (typically short-term) clinical trials toward quantifying the long-term risk–benefit and cost-effectiveness of asthma therapies or risk-modification strategies.
Strengths and Limitations
This study has several strengths. First, we studied a population-based, incidence cohort of patients with asthma, generally free of selection bias. Second, we defined cohort entry based on the first instance of asthma-related hospitalization. Previous chart-review studies have shown that this definition has a high positive predictive value for identifying patients with asthma (51, 52). Third, we used a robust statistical methodology to allow (through random effects) for between-individual variability beyond what is explained by covariates. Moreover, the methodology enabled us to cope with competing events of severe COPD exacerbations and death in the adult group, avoiding biases from treating competing events as noninformative censoring (53).
This study also has limitations. Despite the large cohort, the number of severe exacerbations later in the disease course was low. In a similar study for COPD, with a much larger number (more than 34,000) of follow-up severe COPD exacerbations, Sadatsafavi and colleagues showed a positive, diminishing effect of occurrences of successive severe COPD exacerbations on the rate of subsequent events, which remained significant up to the fourth follow-up event (14). To increase the number of events, we could have included exacerbations that were managed in emergency departments or at physician clinics (e.g., with the use of systemic corticosteroids [54]). However, verifying such events as asthma exacerbations is not easy in our databases. One concern was that patients might receive systemic corticosteroids for other conditions (prescription records are not accompanied by a reason for the prescription). Moreover, in British Columbia, a discharge code is not provided for an emergency department visit, making it difficult to identify the underlying reason for the visit. Next, although using the first severe asthma exacerbation to define the cohort resulted in an entry criterion with high specificity, it prevented us from evaluating the effect of the index exacerbation on subsequent outcomes. As mentioned earlier, our study cannot distinguish between the direct effect of exacerbations on the risk of future exacerbations versus the effect of time-dependent extrinsic factors. As such, although our message on the modifiability of exacerbation burden early in the course of asthma is not affected by this limitation, our results do not provide evidence on the actual mechanisms that carry such effects. This should remain the subject of future investigation.
Conclusions
This study identified substantial variability in severe exacerbation rates in both pediatric and adult patients with asthma with a history of severe asthma exacerbation. It also showed that severe exacerbations early in the course of asthma are associated with an increase in the rate of subsequent events in both children and adults. The mechanism of such association (time-dependent extrinsic factors or exacerbation-induced structural damage) could not be elucidated in our data. Besides offering a new insight into the natural history of asthma, these results can provide new evidence toward nuanced risk stratification of patients at risk of severe exacerbations. They can also have implications in quantifying the long-lasting benefit of interventions aimed at decreasing exacerbation rates or reducing exposure to extrinsic risk factors early in the course of asthma.
Acknowledgments
Acknowledgment
The authors thank the editors and reviewers for their comments, which helped to clarify certain portions of the paper.
Footnotes
Supported by Genome Canada and Genome British Columbia (274CHI).
Author Contributions: M.S. conceived the study question. T.Y.L. and M.S. performed the literature review. T.Y.L., J.P., and M.S. developed the analytic plan. T.Y.L. and J.P. developed the statistical analysis framework. T.Y.L. conducted the analyses. J.P. and M.S. supervised the study progress and provided regular feedback. T.Y.L. wrote the first draft of the manuscript. All authors critically revised the manuscript and approved the final copy.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.The global asthma report 2018. Global Asthma Network; 2018http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf. [Google Scholar]
- 2. Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis . 2014;18:1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 3. Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis . 2016;20:11–23. doi: 10.5588/ijtld.15.0472. [DOI] [PubMed] [Google Scholar]
- 4.Chipps BE, Zeiger RS, Borish L, Wenzel SE, Yegin A, Hayden ML, et al. TENOR Study Group Key findings and clinical implications from The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012;130:332–42.e10. doi: 10.1016/j.jaci.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med . 2009;9:24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu J, Qin X, Beavers SF, Mirabelli MC. Asthma-related school absenteeism, morbidity, and modifiable factors. Am J Prev Med . 2016;51:23–32. doi: 10.1016/j.amepre.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moullec G, FitzGerald JM, Rousseau R, Chen W, Sadatsafavi M, Economic Burden of Asthma (EBA) study team Interaction effect of psychological distress and asthma control on productivity loss? Eur Respir J . 2015;45:1557–1565. doi: 10.1183/09031936.00141614. [DOI] [PubMed] [Google Scholar]
- 8. Sears MR. Can we predict exacerbations of asthma? Am J Respir Crit Care Med . 2019;199:399–400. doi: 10.1164/rccm.201811-2122ED. [DOI] [PubMed] [Google Scholar]
- 9.Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020;202:973–982. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grossman NL, Ortega VE, King TS, Bleecker ER, Ampleford EA, Bacharier LB, et al. Exacerbation-prone asthma in the context of race and ancestry in Asthma Clinical Research Network trials. J Allergy Clin Immunol . 2019;144:1524–1533. doi: 10.1016/j.jaci.2019.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jain N, Satish K, Abhyankar N, Velayudhan N, Gurunathan J. Repeated exacerbation of asthma: An intrinsic phenotype of uncontrolled asthma. Lung India . 2019;36:131–138. doi: 10.4103/lungindia.lungindia_434_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J . 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 13. Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. J Asthma Allergy . 2017;10:47–56. doi: 10.2147/JAA.S121276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadatsafavi M, Xie H, Etminan M, Johnson K, FitzGerald JM, Canadian Respiratory Research Network The association between previous and future severe exacerbations of chronic obstructive pulmonary disease: updating the literature using robust statistical methodology. PLoS One . 2018;13:e0191243. doi: 10.1371/journal.pone.0191243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax . 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westerhof GA, Coumou H, de Nijs SB, Weersink EJ, Bel EH. Clinical predictors of remission and persistence of adult-onset asthma. J Allergy Clin Immunol . 2018;141:104–109.e3. doi: 10.1016/j.jaci.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Global strategy for asthma management and prevention. Global Initiative for Asthma; 2020https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. [Google Scholar]
- 18.Census profile, 2016. Ottawa, ON, Canada: Statistics Canada; 2017https://www150.statcan.gc.ca/n1/en/catalogue/98-316-X2016001 [Google Scholar]
- 19.Canadian Institute for Health Information. Ottawa, ON, Canada: CIHI; 2016. [Google Scholar]
- 20.British Columbia Ministry of Health. Vancouver, BC, Canada: Population Data BC; 2017. https://www.popdata.bc.ca/data/demographic/consolidation_file [Google Scholar]
- 21.Health Data Novia Scotia. http://dictionary.hdns.dal.ca/concept-dictionary/socioeconomic-status-ses-median-household-income
- 22.BC Vital Statistics Agency. Vancouver, BC, Canada: Population Data BC; 2017. https://www.popdata.bc.ca/data/demographic/vs_deaths [Google Scholar]
- 23.British Columbia Ministry of Health Vancouver, BC, Canada: Population Data BC; 2017https://www.popdata.bc.ca/node/678. [Google Scholar]
- 24.Canadian Institute for Health Information Vancouver, BC, Canada: Population Data BC; 2017https://www.popdata.bc.ca/data/health/dad. [Google Scholar]
- 25. Reddel HK, Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med . 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 26. Riis AH, Johansen MB, Jacobsen JB, Brookhart MA, Stürmer T, Støvring H. Short look-back periods in pharmacoepidemiologic studies of new users of antibiotics and asthma medications introduce severe misclassification. Pharmacoepidemiol Drug Saf . 2015;24:478–485. doi: 10.1002/pds.3738. [DOI] [PubMed] [Google Scholar]
- 27. Tho NV, Park HY, Nakano Y. Asthma-COPD overlap syndrome (ACOS): A diagnostic challenge. Respirology . 2016;21:410–418. doi: 10.1111/resp.12653. [DOI] [PubMed] [Google Scholar]
- 28. Trivedi M, Denton E. Asthma in children and adults–what are the differences and what can they tell us about asthma? Front Pediatr . 2019;7:256. doi: 10.3389/fped.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation . 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu L, Wolfe RA, Huang X. Shared frailty models for recurrent events and a terminal event. Biometrics . 2004;60:747–756. doi: 10.1111/j.0006-341X.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 31. Huang X, Liu L. A joint frailty model for survival and gap times between recurrent events. Biometrics . 2007;63:389–397. doi: 10.1111/j.1541-0420.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 32. Sadatsafavi M, FitzGerald JM. Heterogeneity’s ruses: the neglected role of between-individual variability in longitudinal studies of COPD exacerbations. Thorax . 2014;69:1043–1044. doi: 10.1136/thoraxjnl-2013-205061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duchateau L, Janssen P. The frailty model. Heidelberg, Germany: Springer Science & Business Media; 2008. [Google Scholar]
- 34. Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest . 2004;126:59–65. doi: 10.1378/chest.126.1.59. [DOI] [PubMed] [Google Scholar]
- 35. Diaz-Guzman E, Khosravi M, Mannino DM. Asthma, chronic obstructive pulmonary disease, and mortality in the U.S. population. COPD . 2011;8:400–407. doi: 10.3109/15412555.2011.611200. [DOI] [PubMed] [Google Scholar]
- 36. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol . 1993;46:1075–1079, discussion 1081–1090. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.r-project.org/ [Google Scholar]
- 38. Chen W, Lynd LD, FitzGerald JM, Sadatsafavi M. Influences of socioeconomic status on costs of asthma under universal health coverage. Med Care . 2016;54:789–795. doi: 10.1097/MLR.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Tavakoli H, FitzGerald JM, Subbarao P, Turvey SE, Sadatsafavi M. John Wiley & Sons, Inc; 2021. https://onlinelibrary.wiley.com/doi/abs/10.1111/pai.13515 [DOI] [PubMed] [Google Scholar]
- 40.Asthma hospitalizations among children and youth in Canada: trends and inequalities. Canadian Institute for Health Information; 2018https://www.cihi.ca/en/document/asthma-hospitalizations-among-children-and-youth-in-canada-trends-and-inequalities [Google Scholar]
- 41. Uphoff E, Cabieses B, Pinart M, Valdés M, Antó JM, Wright J. A systematic review of socioeconomic position in relation to asthma and allergic diseases. Eur Respir J . 2015;46:364–374. doi: 10.1183/09031936.00114514. [DOI] [PubMed] [Google Scholar]
- 42. Schatz M, Meckley LM, Kim M, Stockwell BT, Castro M. Asthma exacerbation rates in adults are unchanged over a 5-year period despite high-intensity therapy. J Allergy Clin Immunol Pract . 2014;2:570–4.e1. doi: 10.1016/j.jaip.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Gershon A, Guan J, Victor JC, Wang C, To T. The course of asthma activity: a population study. J Allergy Clin Immunol. 2012;129:679–686. doi: 10.1016/j.jaci.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 44. Schatz M, Zeiger RS, Mosen D, Vollmer WM. Asthma-specific quality of life and subsequent asthma emergency hospital care. Am J Manag Care . 2008;14:206–211. [PubMed] [Google Scholar]
- 45. Bloom CI, Palmer T, Feary J, Quint JK, Cullinan P. Exacerbation patterns in adults with asthma in England. A population-based study. Am J Respir Crit Care Med . 2019;199:446–453. doi: 10.1164/rccm.201808-1516OC. [DOI] [PubMed] [Google Scholar]
- 46. Federico MJ, Denlinger LC, Corren J, Szefler SJ, Fuhlbrigge AL. Exacerbation-prone asthma: a biological phenotype or a social construct. J Allergy Clin Immunol Pract . 2021;9:2627–2634. doi: 10.1016/j.jaip.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 47. Rennard SI, Farmer SG. Exacerbations and progression of disease in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc . 2004;1:88–92. doi: 10.1513/pats.2306026. [DOI] [PubMed] [Google Scholar]
- 48. Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol . 1995;95:843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 49. Picado C. Classification of severe asthma exacerbations: a proposal. Eur Respir J . 1996;9:1775–1778. doi: 10.1183/09031936.96.09091775. [DOI] [PubMed] [Google Scholar]
- 50. Matsunaga K, Hirano T, Oka A, Tanaka A, Kanai K, Kikuchi T, et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol Pract . 2015;3:759–64.e1. doi: 10.1016/j.jaip.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 51. Firoozi F, Lemière C, Beauchesne MF, Forget A, Blais L. Development and validation of database indexes of asthma severity and control. Thorax . 2007;62:581–587. doi: 10.1136/thx.2006.061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prosser RJ, Carleton BC, Smith MA. Identifying persons with treated asthma using administrative data via latent class modelling. Health Serv Res . 2008;43:733–754. doi: 10.1111/j.1475-6773.2007.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol . 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J . 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 55. Lee TY, Petkau J, Sadatsafavi M. Does the occurrence of a severe asthma exacerbation change the rate of subsequent events? [abstract] Can J Respir Crit Care Sleep Med . 2021;5:A3. [Google Scholar]