Abstract
Objectives:
To evaluate treatment-naïve patients with neovascular-exudative age-related macular degeneration (eARMD) in one eye and early-/intermediate-stage nonexudative ARMD (neARMD) in the fellow eye by optical coherence tomography-angiography (OCTA).
Methods:
A total of 70 eyes of 35 patients (17 females) with first diagnosis of eARMD in one eye and early/intermediate-stage neARMD in the fellow eye were included in this study. The eARMD diagnosis was confirmed by fluorescein angiography. Each subject underwent OCTA imaging by RTVue XR Avanti OCT device. Capillary vessel density (VD) of superficial (SCP) and deep (DCP) retinal capillary plexuses and foveal avascular zone (FAZ) parameters were measured.
Results:
The mean age was 72.0±8.9 years (range 59–87). The mean visual acuity was 0.7±0.5 logMar for the eARMD eyes and 0.4±0.3 logMar for the fellow eyes (p=0.012). Nineteen patients (54.3%) had occult choroidal neovascularization (CNV), and 16 patients (45.7%) had classical CNV. The mean FAZ area was measured 0.30 ± 0.11 mm2 in the eARMD eyes and 0.27±0.11 mm2 in the fellow eyes (p=0.387). The FAZ circularity index measurement was 1.15±0.03 in eARMD eyes and 1.11±0.05 in the fellow eyes (p=0.014). There was no statistically significant difference in any measure of the macular SCP and DCP’s VD between eARMD eyes and their fellow eyes.
Conclusion:
Potential retinal vascular alterations will be important in ARMD pathogenesis.
Keywords: Age-related macular degeneration, foveal vascular zone, optical coherence tomography angiography, retinal vessel density
Introduction
Age-related macular degeneration (ARMD) is a common eye disease that causes progressive vision loss in the elderly population worldwide (1). According to Age-Related Eye Diseases Study (AREDS) severity scale, early-stage ARMD includes medium-sized drusen (>63 µm and ≤125 µm), intermediate-stage ARMD includes large-sized drusen (>125 µm) or any pigment abnormalities, and late-stage ARMD includes neovascularization (exudative) or geographic atrophy (GA, nonexudative) (2). Neovascular-exudative ARMD (eARMD) is characterized by abnormal angiogenesis complex mostly originating from the choroidal circulation and to a less extent from the retinal vascular network, which are initiated by high level of vascular endothelial growth factor (VEGF) production. The aberrant vessels grow within the sub-retinal or sub-retinal pigment epithelium (RPE) spaces and are prone to leakage that results in fluid accumulation, hemorrhage, and finally, fibrosis (3). Despite the advent of VEGF inhibitors, late-stage ARMD remains the third leading cause of visual impairment and legal blindness (4).
ARMD is diagnosed by clinical examination and color fundus photography assessment. Fluorescein angiography (FA) is conventionally employed to confirm the presence of neovascularization and its location. The extent of fluorescein dye leakage is used to reveal the subtypes of the eARMD. With advances in imaging technology, fundus autofluorescence (FAF) imaging, spectral-domain optical coherence tomography (SD-OCT) and swept-source OCT-angiography (SS-OCTA) have emerged as non-invasive approaches (5).
ARMD is a bilateral disease, and approximately 20% of patients with early-stage ARMD are prone to the development of either GA or neovascularization. Once neovascular ARMD develops in one eye, subsequently, there is an increased risk of neovascularization in the fellow eye (6,7). Furthermore, post-mortem evaluations have shown the development of “nonexudative neovascularization” in early or intermediate-stage ARMD (8). Therefore, it is important to recognize the process underlying the transformation from early stage to late-stage ARMD or nonexudative to eARMD that would improve our ability to orient our efforts for the prevention of this devastating disease.
The presence of functional and morphological alterations in the second eye of patients with unilateral eARMD by multi-modal imaging technologies including FA, indocyanine green angiography, FAF, and OCT have been reported (9). With advanced image-processing software, OCTA has also provided the capability to assess the alterations in retinal and choroidal vascular circulation which may play a role as precursor biological signs of eARMD development (10,11).
The major aim of the current study was to evaluate treatment-naïve patients with eARMD in one eye and early/intermediate-stage nonexudative ARMD (neARMD) in the fellow eye by SS-OCTA to assess retinal vascular alterations in both eyes.
Methods
This retrospective, cross-sectional, comparative study was performed at the department of ophthalmology of a tertiary center. The study protocol was approved by the Institutional clinical research ethics committee (2011-KAEK-25 2019/03-31) in accordance with the tenets of the declaration of Helsinki.
Study Population
We retrospectively recruited 70 eyes of 35 patients (17 females) with first diagnosis (treatment- naïve) of neovascular eARMD in one eye and early/intermediate-stage neARMD in the fellow eye (control eyes) that were consecutively referred to our ophthalmology clinic for diagnosis and treatment. Neovascular eARMD diagnosis was confirmed by color fundus photographs, FA, and OCT examinations. Both Type I (occult, sub-RPE) and Type II (classic, sub-retinal) neovascular eARMD forms were included in the study.
A totally 50 patient’s files were evaluated for enrollment, and 15 of it excluded for meeting the exclusion criteria: any disorder of the optic nerve or retina, refractive error >±3.00 D, amblyopia, history of intraocular surgery, congenital or juvenile glaucoma, a history of ocular trauma, systemic diseases influencing microvasculature such as diabetes mellitus and chronic renal diseases, ocular media opacity such as cataract precluding high-quality imaging, disagreement with the study protocol and disability to cooperate with image acquisition. Patients with solid organ transplants or any other systemic diseases and patients under any treatment were also excluded due to potential independent effects on the retinal microvasculature.
Examination Protocol and Study Measurements
The ophthalmologic examination includes best-corrected Snellen visual acuity (20 feet) (BCVA), anterior and posterior segment examination with slit-lamp biomicroscopy, and intraocular pressure measurement (CT.1P, Topcon, Japan). BCVA was converted to logMar for statistical analysis. Each subject underwent SS-OCTA imaging by RTVue XR Avanti OCT device with AngioVue software (Optovue, Inc., Fremont, CA, USA) by the same examiner (GDC). SS-OCTA has provided a detailed analysis of images of retinal superficial and deep microvasculature at a resolution of 5 µm in three dimensions by phase or amplitude decorrelation technology. The macular SS-OCTA images were obtained in 6 × 6 mm frame, and each contains 400 × 400 A-scans. To improve the signal-to-noise ratio (dB) and eliminate the motion artifact, SS-ADA technology was used, and horizontal and vertical images were merged in each measurement. Scans that were not centered, those with a low-signal strength index (<8/10), or incorrectly segmented images were all excluded by or manually corrected if necessary.
A circle with a radius of 1.25 mm from the foveola was used to evaluate capillary vessel density (CVD). The entire enface microvasculature was evaluated in the 6 × 6 mm area (foveal = 1 mm, parafoveal = 3 mm, and perifoveal = 6 mm). Superficial retinal capillary plexus (SCP) was automatically segmented between internal limiting membrane (ILM) to outer boundary of the inner plexiform layer (IPL). Deep retinal capillary plexus (DCP) was automatically segmented between an inner boundary of the IPL and outer boundary of the outer PL (OPL). The CVD shows the percentage area (%) occupied by microvasculature in the segmented area. In the case of macular scans, the following CVD parameters were evaluated for both the SCP and DCP: whole image, superior hemisphere, inferior hemisphere, foveal, parafoveal, and perifoveal.
The FAZ area was measured using a slab from the ILM to outer boundary of the OPL. Area (mm2), perimeter (mm), circularity index (CI), and foveal density (FD) were evaluated as FAZ parameters. CI was defined as the ratio of the perimeter of the FAZ to the perimeter of a circle with equal area (12).
Eyes were qualitatively evaluated for OCTA biomarkers of choroidal neovascularization (CNV) morphology as described by Coscas et al. (13) Five criteria were considered to determine the pattern of CNV: (1) shape (lacy-wheel or sea-fan), (2) vessel branching (numerous tiny capillaries), (3) the presence of anastomoses and loops, (4) morphology of the vessel termini (presence of a peripheral arcade), and (5) presence of a perilesional hypointense halo (flow impairment, steal or localized atrophy). If a CNV lesion showed at least three of these features, it was assessed as Pattern I (active-recent lesion). If it showed less than three criteria, it was considered Pattern II (mature lesion).
Statistical Analysis
SPSS 20 package program (IBM Corp., Version 20.0, NY, USA) was used for analysis. The results were presented for categorical variables as numbers. Age, sex, and refractive error were included in the model as biological and adjustment factors. Parametric and nonparametric tests, including Wilcoxon signed-rank test and Student’s t-test were used to compare quantitative variables. Results were represented as mean ± SD (standard deviation). The confidence interval was found through Cronbach’s alpha analysis. The significance for all analyses was set at p≤0.05.
Results
The mean age was 72.0±8.9 years (range 59–87). The mean BCVA was 0.7±0.5 logMar for the neovascular eARMD eyes and 0.4±0.3 logMar for the fellow eyes (p=0.012). Nineteen patients (54.3%) have Type I CNV and 16 patients (45.7%) have Type II CNV.
The assessment of the predetermined parameters could be realized in all eyes, allowing sufficient quality on the macular OCTA images. The mean FAZ area measured 0.30±0.11 mm2 in the neovascular eARMD eyes and 0.27±0.11 mm2 in the fellow eyes (p=0.387). The mean perimeter and FD (%) were similar between study groups (p=0.655 and 0.151, respectively). The CI measured 1.15±0.03 in neovascular eARMD eyes and 1.11±0.05 in the fellow eyes (p=0.014).
Post hoc analysis revealed that no statistically significant differences were existed between neovascular eARMD eyes and fellow eyes in any measurement of the macular SCP and DCP’s CVD. The quantitative results are summarized in Table 1.
Table 1.
The quantitavive analysis of retinal vessel density and FAZ parameters in both groups
| Nonexudative Eyes (n=35) | Exudative Eyes (n=35) | P | |
|---|---|---|---|
| Vessel Density, SCP Flow (%) | |||
| Whole Retina | 44.84±4.73 | 45.03±4.91 | 0.891 |
| Superior-Hemisphere | 44.81±4.80 | 45.02±5.21 | 0.893 |
| Inferior-Hemisphere | 44.92±4.84 | 44.96±4.86 | 0.974 |
| Fovea | 18.85±8.57 | 23.27±10.53 | 0.143 |
| Parafovea | 46.49±5.67 | 46.69±4.64 | 0.894 |
| Perifovea | 45.60±5.12 | 46.11±5.39 | 0.757 |
| Vessel Density, DCP Flow (%) | |||
| Whole Retina | 45.35±7.17 | 45.02±6.42 | 0.871 |
| Superior-Hemisphere | 45.12±7.94 | 45.24±7.15 | 0.957 |
| Inferior-Hemisphere | 45.55±6.59 | 44.73±6.39 | 0.674 |
| Fovea | 33.62±8.21 | 36.56±12.61 | 0.386 |
| Parafovea | 50.36±5.86 | 47.71±5.52 | 0.125 |
| Perifovea | 46.09±7.93 | 45.85±6.86 | 0.918 |
| FAZ Parameters | |||
| FAZ area (mm2) | 0.305±0.11 | 0.271±0.11 | 0.387 |
| FAZ perimeter (mm) | 2.15±0.43 | 2.07±0.55 | 0.655 |
| CI | 1.11±0.05 | 1.15±0.03 | 0.014 |
| FD (%) | 49.83±7.43 | 46.76±5.69 | 0.151 |
SCP: Superficial capillary plexus; DCP: deep capillary plexus; FAZ: Foveal avascular zone; CI: Circularity Index; FD: Foveal Density. aPaired samples t-test.
In the OCTA morphological analysis, in 13 eyes (37.1%), the lesion was defined as Pattern I, and the remaining 22 (62.9%) as Pattern II. Figure 1 depicts Pattern I and Pattern II CNV. We did not note any significant correlation between CNV type/pattern and the vascular parametrics.
Figure 1.
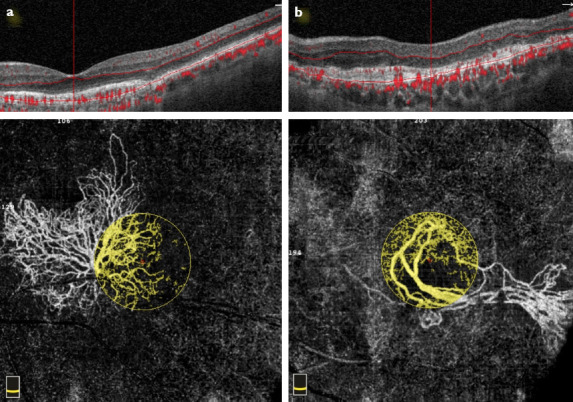
Optical coherence tomography angiography scan with corresponding OCT B-scan taken at the level of the outer retina to show the morphology of the CNV. (a) Pattern I lesion, the entire extension of a sea-fan Type I CNV is shown: Large mature vessels, branching into tinny capillaries toward the periphery, anastomoses, loops, and peripheral arcades are visible (yellow circle). (b) Pattern II lesion, dead tree appearance Type I CNV is shown: long filamentous linear vessels, branching into rare large mature vessels, with rare or absent anastomoses (yellow circle).
Discussion
This study considered information provided by OCTA regarding retinal vascular network alterations in eyes affected by CNV in comparison with the fellow eyes. In-vessel density (VD), analysis of the eyes with CNV and their fellow eyes showed similar values with regard to SCP and DCP. The FAZ area was larger in CNV eyes than that in fellow eyes; however, the difference was insignificant. Similarly, the FAZ CI was larger in CNV eyes than that in fellow eyes, and the difference was statistically significant.
Previous angiographic and histopathologic studies have shown reduced choriocapillaris density in eyes with ARMD (14,15). Recent studies using OCTA also supported the impaired choroidal blood flow, demonstrating reduced choriocapillaris density across a spectrum of ARMD phenotypes from dry to wet type (16,17). Further, the existence of choriocapillaris nonperfusion in fellow eyes of unilateral eARMD patients has proved to be an indicator for the possible development of exudation on OCTA images (18).
Although the impact of the impaired choroidal vasculature in ARMD pathogenesis has been well-documented, the participation and role of retinal vasculature in ARMD pathogenesis is less clear. First, Toto et al. showed that both SCP and DCP are altered among patients affected by ARMD, and this alteration starts immediately at the intermediate ARMD stage (19). Another study revealed a possible protective role of the presence of the cilioretinal artery based on color fundus photographs from the AREDS (20). Lee et al. have reported retinal vascular changes to precede the development of late-stage ARMD signs (21). However, the impact of the retinal vasculature on advanced stages of ARMD remains unclear. More recently, Lee et al. found that CVD in the SCP is reduced in eyes with eARMD as compared to neARMD (22). They found no differences in FAZ area, perimeter, or circularity between eARMD and neARMD groups. Although a slight not a significant decrease was found in the parafoveal CVD of the DCP in the eARMD eyes, our findings are not in accordance with the prior studies showing a significant decrease in overall retinal CVD. Furthermore, we did note an expansion in FAZ area and an increase in CI in eARMD eyes than those of fellow eyes. Shin et al. evaluated the foveal microvasculature in patients with intermediate-to-late stage neARMD with OCTA and found that the FAZ area and perimeter in the neARMD patients were larger than those in the controls (23). In addition, they found that VD and perfusion density were significantly lower in neARMD patients than those of the controls. In the current study, we only included treatment-naive patients, and the majority of patients (62.9%) had Pattern II CNV lesion that may be considered more inactive or mature. In addition, 54.3% of patients had Type I CNV lesion. These findings may have caused the CVD values not to be different. Furthermore, the retrospective design of our study and the lack of the control group consisting of healthy volunteers might have masked the relationship between retinal vascular values and the stage of the ARMD. According to these results, we can only conclude that the unilateral exudative CNV development was not a consequence of any alteration in the retinal CVD, but the enlargement of FAZ area and increase of circularity may either be a cause or a result of the ARMD pathogenesis in this study cohort.
It has been accepted that there is a mutualistic symbiotic relationship within the photoreceptor/RPE/Bruch membrane/choriocapillaris complex, and this relationship is lost in both forms of ARMD (24). Hereby, the authors are aware that outer retinal layer changes due to vascular and/or inflammatory events are important to maintain this relationship healthy in any stage of the ARMD. Apart from the pro-inflammatory milieu, the loss of the vascular supply to outer retina-choriocapillaris complex may be the initial insult to this complex. Although it has definitively been accepted that the blood supply of the outer retina-RPE-Bruch membrane complex is originated from the choriocapillaris, a limited number of study showed that the superficial retinal circulation may have an impact on the integrity of the outer retinal layers (25). Trinh et al. found that the radial peripapillary capillary plexus sparing and underlying retinal vascular impairment can cause potential anterograde trans-synaptic degeneration in ARMD pathogenesis (26).
Our study did not include any evaluation of the choriocapillaris vascular parameters. If choroidal alterations are present in our study population, it may also be the cause of the current findings. Therefore, a combined effect of the outer retinal flow area decrease and choriocapillaris circulation deficits is still valid in ARMD pathogenesis.
Study Limitations
This study has some limitations. We did not include healthy volunteers to see the retinal vascular alterations in ARMD patients regardless of the stage of the disease. Relatively small number of patients was another limitation. We did not evaluate the axial length which may have a possible impact on the vascular metrics in OCTA. Nevertheless, we included only the patients who have spherical equivalent values between ±3D that can be ignored in the final evaluation. It is worth noting that the retrospective design of the study prevents us to interpret the difference found in FAZ circularity was as a consequence of CNV development or as a cause that increases the risk of developing CNV.
Conclusion
The current literature could not clearly determine the relationship between retinal vascular density and photoreceptor/RPE/choriocapillaris complex. We advocate that both the retinal circulation and photoreceptor/RPE/choriocapillaris complex can affect each other at a certain stage. The strength of our study is describing that potential retinal vascular alterations will result in ARMD development and/or progression. OCTA can serve as a potential to provide location-specific data in a depth-resolved fashion that may better guide for early diagnosis and management strategies of ARMD in the future.
Footnotes
Disclosures
Ethics Committee Approval: The study protocol was approved by the Institutional clinical research ethics committee (2011-KAEK-25 2019/03-31) in accordance with the tenets of the declaration of Helsinki.
Financial Disclosure: The authors declared that this study received no financial support.
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
Authorship Contributions: Concept – G.D.C., O.G.; Design – G.D.C., O.G.; Supervision – G.D.C., O.G.; Materials – G.D.C., O.G.; Data collection and/or processing – G.D.C., O.G.; Analysis and/or interpretation – G.D.C., O.G.; Literature search – G.D.C., O.G.; Writing – G.D.C., O.G.; Critical review – G.D.C., O.G.
References
- 1.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet (London, England) 2018;392:1147–59. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y, Dharssi S, Chen Q, Keenan TD, Agrón E, Wong WT, et al. DeepSeeNet:A deep learning model for automated classification of patient-based age-related macular degeneration severity from color fundus photographs. Ophthalmology. 2019;126:565–75. doi: 10.1016/j.ophtha.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta H, Tufail A, Daien V, Lee AY, Nguyen V, Ozturk M, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–46. doi: 10.1016/j.preteyeres.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040:A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 5.Holz FG, Sadda SR, Staurenghi G, Lindner M, Bird AC, Blodi BA, et al. Imaging Protocols in clinical studies in advanced age-related macular degeneration:Recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124:464–78. doi: 10.1016/j.ophtha.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Jefferys JL, Hawkins BS, Bressler NM Submacular Surgery Trials Research Group. Incident choroidal neovascularization in fellow eyes of patients with unilateral subfoveal choroidal neovascularization secondary to age-related macular degeneration:SST report No. 20 from the Submacular Surgery Trials Research Group. Arch Ophthalmol (Chicago, Ill 1960) 2007;125:1323–30. doi: 10.1001/archopht.125.10.1323. [DOI] [PubMed] [Google Scholar]
- 7.Wong T, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age-related macular degeneration. Ophthalmology. 2008;115:116–26. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Spraul CW, Grossniklaus HE. Characteristics of drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115:267–73. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- 9.Cachulo L, Silva R, Fonseca P, Pires I, Carvajal-Gonzalez S, Bernardes R, et al. Early markers of choroidal neovascularization in the fellow eye of patients with unilateral exudative age-related macular degeneration. Ophthalmologica. 2011;225:144–9. doi: 10.1159/000321064. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF. Choriocapillaris flow features follow a power law distribution:Implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58–67. doi: 10.1016/j.ajo.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Borrelli E, Uji A, Sarraf D, Sadda SR. Alterations in the choriocapillaris in ıntermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:4792–8. doi: 10.1167/iovs.17-22360. [DOI] [PubMed] [Google Scholar]
- 12.Krawitz BD, Mo S, Geyman LS, Agemy SA, Scripsema NK, Garcia PM, et al. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vision Res. 2017;139:177–86. doi: 10.1016/j.visres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. Optıcal coherence tomography angıography versus tradıtıonal multımodal ımagıng ın assessıng the actıvıty of exudatıve age-related macular degeneratıon:A new diagnostic challenge. Retina. 2015;35:2219–28. doi: 10.1097/IAE.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 14.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–12. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutty GA, McLeod DS, Bhutto IA, Edwards MM, Seddon JM. Choriocapillaris dropout in early age-related macular degeneration. Exp Eye Res. 2020;192:107939. doi: 10.1016/j.exer.2020.107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vujosevic S, Toma C, Villani E, Muraca A, Torti E, Florimbi G, et al. Quantitative choriocapillaris evaluation in intermediate age-related macular degeneration by swept-source optical coherence tomography angiography. Acta Ophthalmol. 2019;97:e919–26. doi: 10.1111/aos.14088. [DOI] [PubMed] [Google Scholar]
- 17.Nassisi M, Tepelus T, Nittala MG, Sadda SR. Choriocapillaris flow impairment predicts the development and enlargement of drusen. Graefes Arch Clin Exp Ophthalmol. 2019;257:2079–85. doi: 10.1007/s00417-019-04403-1. [DOI] [PubMed] [Google Scholar]
- 18.Treister AD, Nesper PL, Fayed AE, Gill MK, Mirza RG, Fawzi AA. Prevalence of subclinical CNV and choriocapillaris nonperfusion in fellow eyes of unilateral exudative AMD on OCT angiography. Transl Vis Sci Technol. 2018;7:19. doi: 10.1167/tvst.7.5.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toto L, Borrelli E, Di Antonio L, Carpineto P, Mastropasqua R. Retınal vascular plexuses'changes ın dry age-related macular degeneratıon, evaluated by means of optıcal coherence tomography angıography. Retina. 2016;36:1566–72. doi: 10.1097/IAE.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 20.Snyder K, Yazdanyar A, Mahajan A, Yiu G. Association between the cilioretinal artery and choroidal neovascularization in age-related macular degeneration. JAMA Ophthalmol. 2018;136:1008. doi: 10.1001/jamaophthalmol.2018.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee B, Ahn J, Yun C, Kim SW, Oh J. Variation of retinal and choroidal vasculatures in patients with age-related macular degeneration. Investig Opthalmol Vis Sci. 2018;59:5246. doi: 10.1167/iovs.17-23600. [DOI] [PubMed] [Google Scholar]
- 22.Lee SC, Tran S, Amin A, Morse LS, Moshiri A, Park SS, et al. Retinal vessel density in exudative and nonexudative age-related macular degeneration on optical coherence tomography angiography. Am J Ophthalmol. 2020;212:7–16. doi: 10.1016/j.ajo.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin YI, Kim JM, Lee MW, Jo YJ, Kim JY. Characteristics of the foveal microvasculature in asian patients with dry age-related macular degeneration:An optical coherence tomography angiography study. Ophthalmologica. 2020;243:145–53. doi: 10.1159/000503295. [DOI] [PubMed] [Google Scholar]
- 24.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD):Relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uğurlu N, Uzel AG, Şengün A, Yülek F, Özdaş D, Tam AA, et al. Evaluation of the correlation between quantitative measurement of the foveal avascular zone and retinal vessel density and outer retinal disruptions in diabetic patients. Turk J Med Sci. 2019;49:1041–6. doi: 10.3906/sag-1901-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinh M, Kalloniatis M, Nivison-Smith L. Radial peripapillary capillary plexus sparing and underlying retinal vascular ımpairment in ıntermediate age-related macular degeneration. Investig Opthalmol Vis Sci. 2021;62:2. doi: 10.1167/iovs.62.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]


