Abstract
In June 2020, a 28-year-old female patient was admitted to our clinic with reduced vision in the left eye. She had systemic COVID-19 infection in May 2020 and during her treatment course, her visual complaints had begun approximately 1 week after the beginning of the COVID-19. Previously, the patient had bilateral femtosecond assisted – Laser in situ Keratomileusis in our clinic in 2018. There was no previous history of herpetic eye involvement. In her examination, the uncorrected visual acuity was 20/20 in the right and 20/32 in her left eye. Slit-lamp examination revealed interlamellar infiltration at the flap interface in the left eye. Considering herpetic activation, ganciclovir ointment 5 × 1, valacyclovir tablet 2 × 1, and prednisolone acetate 1.0% eye drops 5 times a day and artificial tear 5 × 1 were started. Two weeks later, the infiltration completely resolved and the uncorrected visual acuity increased to 20/20.
Keywords: Co-infection, COVID-19, Extrapulmonary, Herpetic keratitis, Post-LASIK
Introduction
The novel coronavirus (SARS-CoV-2) has led to outbreak of pneumonia in Wuhan city in December 2019 (1). Since then the number of cases has been dramatically increased, the World Health Organization formally declared the COVID-19 outbreak a pandemic (2). Although it typically represents with respiratory symptoms, extrapulmonary and atypical involvement is also being reported (3). Coinfection with other viruses but mostly with respiratory ones has also been registered recently (4). Ocular involvement is frequently occurs in patients with more severe COVID-19 and mostly presented with conjunctivitis, conjunctival hyperemia, chemosis, and epiphora (5).
Herein, we present a case who applied with interlamellar stromal keratitis due to HSV and COVID-19 coinfection. Although there is one report about VZV and COVID-19 coinfection, (6) HSV had not been reported before.
Case Report
A 26-year old-female patient applied our clinic with loss of vision in her left eye. She had a history of good physical health and no underlying diseases. In her ocular history; both of her eyes had underwent femtosecond assisted laser in situ keratomileusis (femto-LASIK) because of composed myopic astigmatism 2 years ago. Pre-operative refraction was −2.50–1.50 × 180° for her right eye and −1.75–2.50 × 165° for her left eye. A year ago, at her last visit, her both eyes had 20/20 uncorrected visual acuities, the flap interfaces were clear and the flaps were properly fit.
She had been diagnosed with novel coronavirus a month ago. From her medical records, it was approved that the real-time fluorescence polymerase chain reaction (RT-PCR) assay of her pharyngeal swabs and feces was positive for COVID-19 nucleic acid and she received oral hydroxychloroquine 200 mg, 2 times a day for 7 days. During her treatment course at home, her visual complaints had begun approximately 1 week after the beginning of the COVID infection at her left eye. When she presented to our clinic, her complaints were valid for 3 weeks, the RT-PCR assay of her pharyngeal swabs was negative for COVID-19 nucleic acid and she had not been taking any medications. The uncorrected visual acuity was 20/20 for her right eye and 20/32 for her left eye. On slit lamp biomicroscopy examination, LASIK flaps were observed properly fit and on the right eye interface totally clear. On her left eye, there was anterior stromal opacity along the LASIK flap interface with no epithelial involvement (Fig. 1). The anterior chamber was quiet with no cells or endothelial keratic precipitates detectable. She did not complain about any pain or photophobia. After testing with Cochet-Bonnet esthesiometer, we detected decreased corneal sensation in her left eye and normal corneal sensation for her right eye. Both eyes’ posterior segment examinations were normal. The patient was started an oral valacyclovir 1000 mg twice a day, prednisolone acetat 1.0% eye drops 5 times a day, topical gancyclovir ointment 5 times a day. One week later, the stromal opacity had begun to dissolve and 2 weeks later, it had completely resolved (Fig. 2). Her left eye’s UCVA increased to 20/20 at the end of the 2 weeks.
Figure 1.
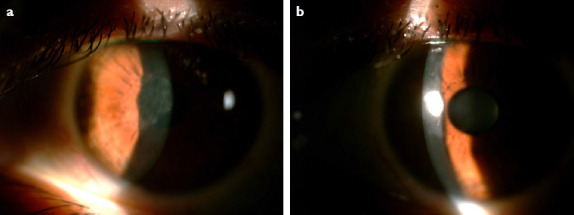
(a, b) Slit lamp biomicroscopic view of interlamellar keratitis in patient’s left eye.
Figure 2.
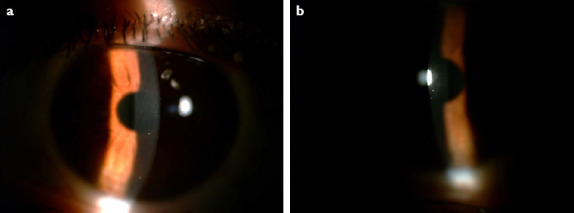
(a, b) Two weeks after oral and topical treatment, slit lamp biomicroscopic view of the left eye; the opacity had totally resolved.
Discussion
Post-LASIK herpetic keratitis had been reported before in terms of case reports. However, our case is the first report of Post-LASIK herpetic keratitis coincident with systemic COVID-19 infection. Since December 2019, the beginning of the COVID-19 pandemic, coinfection with other respiratory viruses such as influenza or metapneumovirus has been reported (4). Coinfection with non-respiratory viruses is not common. Recently, Ferreira et al. have reported a case of herpes zoster coinfection with COVID-19 that presented with orofacial papulovesicular rash and trigeminal neuralgia. However, in this case, there was no ocular involvement (6). The clinical presentations of post-LASIK herpetic keratitis were variable in the literature. The most common clinical forms were dendritic epithelial ulcers and stromal keratitis; less commonly herpetic keratouveitis with corneal edema, and keratic precipitates (7). In this case, there was interlamellar opacity with no epithelial involvement, no keratic precipitates, or any remarkable corneal edema.
The presentation of herpetic keratitis ranged from 1 day post-LASIK to 28 months with median being 4 weeks (8). In our case, this time range was 24 months and she had no previous HSV keratitis story. However, this situation does not exclude the probability of HSV latency in her trigeminal nerve. Because it is well known that ocular infection with HSV can occur directly through direct droplet spread or as a secondary infection where the patient experienced previous exposure to HSV in non-ocular trigeminal dermatomes. The latent virus may be triggered to reactivate by corneal trauma, surgery, or emotional stress. Here, in this case, COVID-19 infection might be the stress factor. Another mechanism may be the COVID-19 presence in her nasal cavity, which is also innervated by ophthalmic and maxillary branches of trigeminal nerve and might have led to retrograde activation of HSV (6).
In most herpetic keratitis cases, diagnosis is based on clinical examination. Further assessments such as polymerase chain reaction and immunofluorescence tests may be useful to confirm diagnosis (7). In this case, we diagnosed herpetic stromal keratitis based on the slit lamp biomicroscopy findings, the existence of hypoesthesia, and the good respond of the eye to antiviral treatment. In differential diagnosis, diffuse lameller keratitis (DLK) or steril infiltrates might be considered but it is well known that DLK is evident mostly on the post-operative 1st day and in some cases by 3–4 days (9). However, in this case, she had femto-LASIK history 24 months ago. Another possibility is hydroxychloroquine toxicity because when her complaints begun that she had already finished her treatment course of oral hydroxychloroquine (400 mg a day for 7 days). However, hydroxychloroquine is melanotropic and tends to accumulate in melanin-rich tissues such as the retinal pigment epithelium and iris/ciliary body, (10) so toxicity may lead not keratopathy but retinopathy due to long-term administration of hydroxychloroquine. In this case, the posterior segment was totally normal.
Conclusion
The ophthalmologists should be aware of the possibility of COVID-19 coinfections with latent viruses such as HSV and their ocular manifestations.
Footnotes
Disclosures
Informed consent: Written informed consent was obtained from the patient for the publication of the case report and the accompanying images.
Peer-review: Externally peer-reviewed.
Conflict of Interest: None declared.
Authorship Contributions: Concept – B.K.Y.; Design – B.K.Y.; Supervision – A.D.; Materials – A.T., D.O.; Data collection and/or processing – A.T., D.O.; Analysis and/or interpretation – A.D., B.K.Y.; Literature search – A.D.; Writing – B.K.Y.; Critical review – A.D.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2010;395:470–3. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID 19 a pandemic. Acta Biomed. 2020;91:157–60. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abobaker A, Raba AA, Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol. 2020;92:2458–64. doi: 10.1002/jmv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, et al. Co-infection with SARS -CoV-2 and influenza A virus in patient with pneumonia. China. Emerg Infect Dis. 2020;26:1324–6. doi: 10.3201/eid2606.200299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID 19) in Hubei province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira AC, Romao TT, Macedo YS, Pupe C, Nascimento OJ. COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27:1748–50. doi: 10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora T, Sharma N, Arora S, Titiyal JS. Fulminant herpetic keratouveitis with flap necrosis following laser in situ keratomileusis:Case report and review of literature. J Cataract Refract Surg. 2014;40:2152–6. doi: 10.1016/j.jcrs.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Moshirfar M, Milner DC, Baker PA, McCabe SE, Ronquillo YC, Hoopes PC. Corneal refractive surgery in patients with a history of herpes simplex keratitis:A narrative review. Clin Ophthalmol. 2020;14:3891–901. doi: 10.2147/OPTH.S282070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RJ, Maloney RK. Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology. 1998;105:1721–6. doi: 10.1016/S0161-6420(98)99044-3. [DOI] [PubMed] [Google Scholar]
- 10.Lozier JR, Friedlaender MH. Complications of antimalarial therapy. Int Ophthalmol Clin. 1989;29:172–8. doi: 10.1097/00004397-198902930-00007. [DOI] [PubMed] [Google Scholar]


