Abstract
Background.
Racial/ethnic minorities face known disparities in likelihood of kidney transplantation. These disparities may be exacerbated when coupled with ongoing substance use, a factor also reducing likelihood of transplantation. We examined whether race/ethnicity in combination with ongoing substance use predicted incidence of transplantation.
Methods.
Patients were enrolled between 3/2010–10/2012 at the time of transplant evaluation. Substance use data were retrieved from transplant evaluations. Following descriptive analyses, the primary multivariable analyses evaluated whether, relative to the referent group (white patients with no substance use), racial/ethnic minority patients using any substances at the time of evaluation were less likely to receive transplants by the end of study follow-up (8/2020).
Results.
Among 1152 patients, 69% were non-Hispanic white, 23% non-Hispanic Black, and 8% Other racial/ethnic minorities. White, Black, and Other patients differed in percentages of current tobacco smoking (15%/26%/18%, respectively, p=0.002), illicit substance use (3%/8%/9%, p<0.001), but not heavy alcohol consumption (2%/4%/1%, p=0.346). Black and Other minority patients using substances were each less likely to receive transplants than the referent group (Hazard Ratios≤0.45, p ≤0.021). Neither white patients using substances nor racial/ethnic minority nonusers differed from the referent group in transplant rates. Additional analyses indicated that these effects reflected differences in waitlisting rates; once waitlisted, study groups did not differ in transplant rates.
Conclusions.
The combination of minority race/ethnicity and substance use may lead to unique disparities in likelihood of transplantation. To facilitate equity, strategies should be considered to remove any barriers to referral for and receipt of substance use care in racial/ethnic minorities.
INTRODUCTION
Disparities related to race and ethnicity in receipt of kidney transplantation in the United States are well-known.1–12 Indeed, one priority of the 2014 modifications to the national Kidney Allocation System (KAS) was to improve the opportunity for transplantation among underserved populations, including racial/ethnic minorities.13 Nevertheless, a recent analysis showed little, if any, such improvement across the past 2 decades.14 Black, Hispanic, and other minority patients remain at a disadvantage relative to non-Hispanic white patients; these disparities persist even when differences in patients’ medical status are taken into account.3–10,14 Moreover, the disparities cannot be ascribed solely to differences in referral patterns because they are also observed after referral and evaluation for transplantation.3,5,8,10,12,15,16
We hypothesized that race/ethnicity disparities may be further heightened when coupled with a similarly potent factor that can affect likelihood of kidney transplantation: substance use, including tobacco use, heavy alcohol consumption, and/or illicit substance use. Ongoing substance use is generally a contraindication to candidacy,17–24 based on important concerns that pretransplant substance use increases patients’ risk for poorer posttransplant outcomes.25–32 However, some studies fail to find such effects.27,31,33–38 In addition, consistent with candidate selection recommendations,17–24 data show that, if active substance users demonstrate abstinence (e.g., via any of an array of effective interventions20,26,31), they can have successful posttransplant outcomes.20,23,34,39–41 Despite such data, growing evidence indicates that active substance use substantially reduces patients’ chances for kidney transplantation, raising difficult concerns about equity in the transplantation process.32,42–44
The same psychosocial factors that may help to explain race/ethnicity disparities in kidney transplantation (e.g., barriers to receipt of healthcare in general; fewer social and financial resources to support health; clinician bias4,5,8–10,15,45,46) also reduce the likelihood of substance use treatment.47–49 As a result, racial/ethnic minorities under consideration for transplantation may not obtain adequate care for substance use problems or meet transplant candidacy requirements for abstinence. Furthermore, it has been proposed that individuals who belong to multiple groups facing health- and healthcare-related disparities (here, racial/ethnic minorities and substance users) are by far the most disadvantaged: this “double jeopardy hypothesis”50–52 would therefore also suggest that minority patients who use substances may be especially unlikely, compared to non-Hispanic white patients, to receive kidney transplants.
Given a dearth of evidence on the combined impact of race/ethnicity with substance use, we examined a cohort of individuals undergoing evaluation for kidney transplantation to achieve several goals. First, we sought to examine whether patterns and types of substances used varied by race/ethnicity. Second, we evaluated whether likelihood (i.e., probability) of transplantation could be predicted by the unique combination of patients’ race/ethnicity and whether they used any substances. To do this, we examined overall incidence of transplantation, and then examined its 2 components: whether patients were waitlisted for transplant and, among waitlisted patients, whether they received transplants. Finally, to better understand predictive effects of race/ethnicity in combination with substance use, we explored these effects on outcomes separately for specific types of substances used.
MATERIALS AND METHODS
Study Design and Patients
We examined a cohort of 1152 patients previously enrolled at the time of their transplant evaluation in a prospective investigation of social factors predicting kidney transplantation.12,53 Substance use data were not originally collected; we obtained these data for the present study from patients’ electronic medical record (EMR).
Patients enrolled were aged ≥18 years, English-speaking, had not received a previous kidney transplant, and underwent transplant evaluations between March, 2010-October, 2012 at the University of Pittsburgh Medical Center.53 Among potential participants, 86% were enrolled (with no demographic differences between those enrolled vs. not).53
With University of Pittsburgh Institutional Review Board approval, participants provided informed consent for study interviews and EMR reviews.
Measures
Race/ethnicity, other demographics, and clinical characteristics at transplant evaluation
Based on the original study’s research interviews with patients using standardized questioning,53 we classified patients as non-Hispanic white (hereafter referred to as white), non-Hispanic Black (Black), or Other race/ethnicity. (There were too few individuals in the latter category to create additional groups.).
Other demographics were obtained during the research interviews, and clinical characteristics were extracted from the EMR (Table 1). We calculated the Charlson Comorbidity Index score from EMR information.57–59
Table 1.
Study participants’ demographic and clinical characteristics at evaluation for kidney transplantation, stratified by participant race/ethnicity.
| Characteristic | Total sample N=1152 |
Race/ethnicity |
3-group Comparison |
|||
|---|---|---|---|---|---|---|
| Non-Hispanic White n=789 |
Non-Hispanic Black n=267 |
Othera n=96 |
||||
| Testb | P | |||||
| Demographic | ||||||
| Age, M (SD) | 56.0 (13.3) | 56.9 (13.4) | 53.8 (12.4) | 54.0 (13.8) | 7.03 | 0.001 |
| Sex, % (n) female | 38.8 (447) | 38.3 (302) | 39.0 (104) | 42.7 (41) | 0.71 | 0.622 |
| Education, % (n) high school or less | 47.8 (551) | 45.5 (359) | 51.3 (137) | 57.3 (55) | 6.46 | 0.040 |
| Marital status, % (n) married or partnered | 51.1 (589) | 57.9 (457) | 33.0 (88) | 45.8 (44) | 50.92 | <0.001 |
| Employed, % (n) yes | 25.5 (294) | 28.8 (227) | 17.6 (47) | 20.8 (20) | 14.30 | 0.001 |
| Occupation, % (n) blue collar/manualc | 51.8 (587) | 47.3 (373) | 62.2 (166 | 60.4 (58) | 20.83 | <0.001 |
| Health insurance, % (n) public only (vs. any private coverage) | 36.8 (424) | 29.7 (234) | 52.8 (141) | 51.0 (49) | 55.10 | <0.001 |
|
| ||||||
| Clinical | ||||||
| Primary indication for transplant, % (n) | ||||||
| Diabetes | 40.5 (467) | 39.4 (311) | 43.4 (116) | 41.7 (40) | 63.22 | <0.001 |
| Hypertension | 20.1 (232) | 15.5 (122) | 33.0 (88) | 22.9 (22) | ||
| Glomerulonephritis | 12.8 (147) | 13.2 (104) | 12.0 (32) | 6.3 (22) | ||
| Other | 26.6 (306) | 31.9 (252) | 11.6 (31) | 29.2 (28) | ||
| Diabetes, % yes (n)d | 44.5 (513) | 43.2 (341) | 47.6 (127) | 46.9 (45) | 1.76 | 0.415 |
| Hypertension, % yes (n)d | 32.5 (374) | 26.0 (205) | 51.3 (137) | 33.3 (32) | 58.41 | <0.001 |
| On dialysis, % yes (n) | 65.6 (756) | 60.3 (476) | 80.0 (214) | 68.8 (66) | 35.19 | <0.001 |
| If on dialysis, duration, % ≤ 6 mos (n) | 49.2 (372) | 51.9 (247) | 45.8 (98) | 40.9 (27) | 4.19 | 0.123 |
| BMI, kg/m2, M (SD) | 29.5 (6.2) | 29.6 (6.2) | 29.5 (6.5) | 29.0 (5.8) | 0.44 | 0.642 |
| Other comorbidities | ||||||
| Charlson Comorbidity Index, M (SD)e | 4.2 (1.7) | 4.1 (1.7) | 4.4 (1.9) | 4.2 (1.5) | 3.27 | 0.038 |
| Heart disease (CAD, valvular disease, cardiomyopathy, heart failure), % yes (n) | 54.7 (630) | 53.7 (424) | 57.7 (154) | 54.2 (52) | 1.26 | 0.532 |
| Peripheral vascular disease, % yes (n) | 32.5 (374) | 31.3 (247) | 35.2 (94) | 34.4 (33) | 1.56 | 0.459 |
| Chronic pulmonary disease, % yes (n) | 29.1 (355) | 28.6 (563) | 32.2 (181) | 24.0 (23) | 2.56 | 0.278 |
| History of medical nonadherence, % yes (n)f | 14.5 (167) | 12.7 (100) | 18.7 (50) | 17.7 (17) | 6.77 | 0.034 |
| Have possible live donor to be tested, % yes (n) | 51.2 (590) | 51.7 (408) | 49.4 (132) | 52.1 (50) | 0.44 | 0.801 |
| If waitlisted for transplant (n=656), | ||||||
| Waitlisted before KAS in 2014, % yes (n) | 94.4 (619) | 96.7 (470) | 87.3 (103) | 88.5 (46) | --- | <0.001 |
| Waitlisted before smoking cessation was required for listing (2013), % yes (n)g | 25.0 (164) | 21.4 (104) | 37.3 (44) | 30.8 (16) | 13.79 | <0.001 |
|
| ||||||
| Study outcomes by end of follow-up, % yes (n) | ||||||
| Outcome 1: Received transplant | 36.0 (415) | 38.9 (307) | 28.5 (76) | 33.3 (32) | --- | --- |
| Competing risk: Death | 44.0 (507) | 44.0 (34.7) | 42.7 (114) | 47.9 (46) | ||
| Censored: Alive, no transplant | 20.0 (230) | 17.1 (135) | 28.8 (77) | 18.8 (18) | ||
| Case closed, incomplete evaluation | (109) | (57) | (46) | (6) | ||
| Team declined patient for transplant | (72) | (50) | (16) | (6) | ||
| Patient choice to withdraw from process | (38) | (24) | (11) | (3) | ||
| Patient on waitlist at end of study | (11) | (4) | (4) | (3) | ||
| Outcome 2: Waitlisted | 56.9 (656) | 61.6 (486) | 44.2 (118) | 54.2 (52) | --- | --- |
| Competing risk: Death | 29.7 (342) | 27.8 (219) | 33.3 (89) | 35.4 (24) | ||
| Censored: Alive, not waitlisted | 13.4 (154) | 10.6 (84) | 22.5 (60) | 10.4 (10) | ||
| Case closed, incomplete evaluation | (109) | (57) | (46) | (6) | ||
| Team declined patient for waitlisting | (27) | (18) | (8) | (1) | ||
| Patient choice to withdraw from process | (18) | (9) | (6) | (3) | ||
| Outcome 3: If waitlisted, received transplant | 63.3 (415) | 63.2 (307) | 64.4 (76) | 61.5 (32) | --- | --- |
| Competing risk: Death | 25.2 (165) | 26.3 (128) | 21.2 (25) | 23.1 (12) | ||
| Censored: Alive on waitlist, no transplant | 11.6 (76) | 10.5 (51) | 14.4 (17) | 15.4 (8) | ||
| Team declined patient for transplant | (45) | (32) | (8) | (5) | ||
| Patient choice to withdraw from process | (20) | (15) | (5) | (0) | ||
| Patient on waitlist at end of study | (11) | (4) | (4) | (3) | ||
Includes Hispanic (n=21), Asian/Pacific Islander (n=15), Native American (n=8), and multiracial (n=52).
F test for means; χ2 test for proportions. When p values but no test values are reported, Fisher’s Exact tests were used due to small expected frequencies in some cells. For study outcomes, groups’ simple proportions cannot be statistically compared because patients vary in time to the events;54 see Table 4 for relevant comparisons.
Based on the Hollingshead occupational classification.55
By convention, includes all cases, no matter whether the condition was the primary indication for transplantation or whether the condition was listed as a comorbidity in patients’ medical record.56
The Index is a count of 19 conditions, spanning several hundred ICD diagnosis and procedure codes, weighted by severity (total possible score range, 0–33).57,59 Although the Index includes peripheral vascular disease (PVD), chronic pulmonary disease, and some diagnoses related to heart disease (reflecting myocardial infarction and congestive heart failure), we also separately considered PVD, chronic pulmonary disease, and an expanded range of heart diseases in the cohort given the importance of these conditions in the end-stage kidney disease population.
Based on data from the psychosocial evaluation for transplantation. The evaluator used a template requiring collection of infor mation on nonadherence to medications, dialysis, clinic appointments/testing, and fluid/dietary restrictions. These data were gathered by the evaluator from the patient as well as from collateral sources (primary family caregiver, medical records).
See Methods section for description of transplant program approach to substance use and waitlisting for transplant. Beginning in 2013, no active smokers were listed for transplantation. Heavy alcohol use and illicit substance use were absolute contraindications to transplant across the entire study period (2010–2020).
Abbreviations: BMI, body mass index; CAD, coronary artery disease; KAS, Kidney Allocation System; PVD, peripheral vascular disease.
Substance use
Transplant team assessments of substance use and data extraction for present study.
We used patients’ transplant evaluations, reported in the EMR, to determine substance use. As part of the medical assessment, patients received a psychosocial evaluation by a clinical social worker. It covered psychosocial history and current status, based on a semi-structured patient interview plus collateral information from their primary family caregiver. The psychosocial evaluation followed a template specifying areas required to be examined and described in the evaluator’s report, including current and past tobacco, alcohol, and illicit substance use; periods of abstinence; and amount and duration of current use. We also retrieved EMR reports from all other medical components of the transplant evaluation, including assessments by nephrologists, surgeons, nurse coordinators, and pharmacists. (During the study enrollment period, toxicology screening was not performed as part of the evaluation. Advanced kidney disease renders urine screening (the most common strategy) difficult or impossible to use.60)
From these data, we determined whether study participants (a) smoked tobacco, (b) engaged in heavy alcohol consumption, and/or (c) used any illicit substances. Table 2 defines these categories of substance use. For each, we identified participants engaging in current use, past (but not current) use, or having no history of use. We also extracted other characteristics of participants’ usage history (see Table 3).
Table 2.
Definition of each of 3 types of substance use.
| Type of substance | Definition of use |
|---|---|
| Tobacco smoking | Continuous, active daily or intermittent smoking with no period of abstinence61,62 |
| Heavy alcohol consumption |
Either: • meeting standard criteria for heavy drinking61,63 (men: >14 drinks/week or >4 drinks/occasion; women: >7 drinks/week or >3 drinks/occasion) or • referral or participation in alcohol treatment or rehabilitation. |
| Illicit substance use | Using any illegally obtained substance, including misuse of prescribed substances (i.e., use of prescriptions that are not one’s own or use not directed by healthcare providers),64 including: • Marijuana (not legal in Pennsylvania or surrounding states for any purpose at the time of transplant evaluations) • Stimulants • Opioids • Hallucinogens • Sedatives. |
Table 3.
Characteristics of tobacco, alcohol, and illicit substance use in the cohort.
| Race/ethnicity |
3-group Comparison |
|||||
|---|---|---|---|---|---|---|
| Characteristica | Total sample N=1152 |
White n=789 |
Total sample N=1152 |
White n=789 |
Testb | P |
| Tobacco use | ||||||
| Smoking status, % (n) | 16.76 | 0.002 | ||||
| Current smoker | 17.6 (203) | 14.8 (117) | 25.8 (69) | 17.7 (17) | ||
| Former smoker | 46.7 (538) | 48.2 (380) | 42.7 (114) | 45.8 (44) | ||
| Never smoked | 35.7 (411) | 37.0 (292) | 31.5 (84) | 36.5 (35) | ||
| In smokers, products ever used | ||||||
| Cigarettes, % (n) yes | 97.7 (724) | 97.4 (484) | 97.8 (179) | 100.0 (61) | --- | 0.671 |
| Cigars, % (n) yes | 7.0 (52) | 7.0 (35) | 7.1 (13) | 6.6 (4) | --- | 1.000 |
| Pipes, % (n) yes | 1.9 (14) | 2.4 (12) | 0.5 (1) | 1.6 (1) | --- | 0.314 |
| Current cigarette packs/day, Median (IQR)c | 0.50 (0.30–1.00) |
0.50 (0.35–1.00) |
0.50 (0.25–0.80) |
0.50 (0.35–1.00) |
3.54 | 0.170 |
| In all lifetime smokers, years smoked, M (SD) | 24.8 (13.8) | 24.7 (14.1) | 24.3 (12.9) | 26.6 (13.8) | 0.58 | 0.561 |
| In former smokers, time abstinent | --- | 0.050 | ||||
| <6 mos | 5.9 (31) | 4.8 (18) | 8.0 (9) | 9.5 (4) | ||
| ≥6 mos – 5 years | 23.3 (123) | 21.2 (79) | 25.9 (29) | 35.7 (15) | ||
| >5 years | 70.8 (373) | 74.0 (276) | 66.1 (74) | 54.8 (23) | ||
| Ever used other nicotine products, % (n) yesd | ||||||
| Chewed tobacco or snuff | 6.9 (80) | 8.7 (69) | 1.5 (4) | 7.3 (7) | 16.23 | <0.001 |
| Electronic cigarettes | 0.2 (2) | 0.0 (0) | 0.0 (0) | 2.1 (2) | --- | 0.007 |
|
| ||||||
| Alcohol use | ||||||
| Heavy drinking, % (n) | --- | <.001 | ||||
| Current heavy drinker | 2.6 (30) | 2.4 (19) | 3.7 (10) | 1.0 (1) | ||
| Former heavy drinker | 19.8 (228) | 17.0 (134) | 28.8 (77) | 17.7 (17) | ||
| Never drank heavily | 77.6 (894) | 80.6 (636) | 67.4 (180) | 81.3 (78) | ||
| Current heavy drinkers, drinks/day, median (IQR) | 5.5 (3.5–7.0) | 5.5 (3.5–7.0) | 5.5 (5.5–8.0) | 3.5 (---) | 2.49 | 0.288 |
| In former heavy drinkers, time abstinent from heavy drinking, % (n) | --- | 0.619 | ||||
| <6 mos | 2.3 (5) | 2.4 (3) | 2.9 (2) | 0.0 (0) | ||
| ≥6 mos – 5 years | 39.6 (88) | 36.5 (46) | 43.1 (31) | 52.9 (9) | ||
| >5 years | 58.1 (129) | 61.7 (82) | 54.2 (39) | 47.1 (8) | ||
| In former heavy drinkers, % (n) abstinent from any alcohol | 54.4 (124) | 55.2 (74) | 51.9 (40) | 58.8 (10) | 58.8 (10) | 0.834 |
|
| ||||||
| Illicit substance use | ||||||
| Substance use status, % (n) | --- | <0.001 | ||||
| Current user | 4.6 (53) | 2.9 (23) | 7.9 (21) | 9.4 (9) | ||
| Former user | 26.0 (300) | 21.8 (172) | 39.0 (104) | 25.0 (24) | ||
| Never used substances | 69.4 (799) | 75.3 (594) | 53.2 (142) | 65.6 (63) | ||
| In substance users, products ever used | ||||||
| Marijuana, % (n) | 89.5 (316) | 95.4 (186) | 82.4 (103) | 81.8 (27) | --- | <0.001 |
| Stimulants (e.g., cocaine, amphetamines), % (n) | 34.0 (120) | 25.6 (50) | 46.4 (58) | 36.4 (12) | --- | 0.001 |
| Opioids (e.g., heroin, oxycodone), % (n) | 12.5 (44) | 8.2 (16) | 18.4 (23) | 15.2 (5) | --- | 0.019 |
| Hallucinogens (e.g., LSD, mescaline), % (n) | 3.4 (12) | 5.1 (10) | 0.8 (1) | 3.0 (1) | --- | 0.093 |
| Other/unspecified polydrug usee | 4.8 (17) | 4.1 (8) | 6.4 (8) | 3.0 (1) | --- | 0.648 |
| In substance users, products ever used, % (n) | --- | <0.001 | ||||
| Only marijuana | 61.5 (217) | 70.8 (138) | 48.8 (61) | 54.5 (18) | ||
| Only other substances | 10.5 (37) | 4.6 (9) | 17.6 (22) | 18.2 (6) | ||
| Both marijuana and other substances | 28.0 (99) | 24.6 (48) | 33.6 (42) | 27.3 (9) | ||
| In former users of any substance, time since quit, % (n) | --- | 0.006 | ||||
| <6 mos | 3.8 (10) | 2.7 (4) | 6.5 (6) | 0.0 (0) | ||
| ≥6 mos – 5 years | 18.0 (48) | 11.4 (17) | 25.8 (24) | 29.2 (7) | ||
| >5 years | 78.2 (208) | 85.9 (45) | 67.7 (63) | 70.8 (17) | ||
The following variables had missing cases: current number of cigarettes per day, 7 cases (4 white, 3 Black); years smoked, 79 cases (52 white, 18 Black, 9 other); duration of smoking abstinence, 20 cases (11 white, 7 Black, 2 other); duration of alcohol abstinence, 6 cases (1 white, 5 Black); duration of substance use abstinence, 34 cases (23 white, 11 Black).
F test for means, Kruskal Wallis test for medians, χ2 test for proportions. For variables for which no test is reported, Fisher’s Exact test was used due to small expected frequencies in some cells.
Cigar and pipe smoking converted to equivalent cigarette use based on approximate equivalents in grams of smoked tobacco: 1 cigar=4 cigarettes, 1 pipe=3.5 cigarettes).65
Pack-years could not be calculated because historical information on usage patterns was not sufficiently detailed.
Illicit use of sedatives (e.g., benzodiazapines, barbiturates) was combined with all other remaining substances due to low prevalence.
Abbreviations: LSD, d-lysergic acid diethylamide
Two coauthors (RND, MAD blinded to study outcomes) coded all EMR information. They first independently reviewed 10 patients’ transplant psychosocial and medical evaluations, reconciled any discrepancies, and coded 10 additional evaluations to establish reliability (intraclass r or κ >.90). Remaining patients’ data were then coded by one of these coauthors, with periodic double coding by both to reduce any coding drift. One author (MAD) then reviewed coding for all patients to ensure final coding accuracy.
Transplant team approach to substance use.
Heavy alcohol use and any illicit substance in the categories listed above are absolute contraindications to transplantation. Until 2013, smoking was a relative contraindication, as was typical in most U.S. kidney transplant programs.66,67 However, all patients were strongly encouraged to quit and were educated on smoking risks in relation to transplantation. Smoking did not preclude waitlisting and transplantation in the absence of diagnosed lung disease and poor lung function test results, especially if patients were light smokers (≤5 cigarettes/day68,69) Beginning in 2013, active smokers were not waitlisted or transplanted. Across the entire study period (2010–2020), active substance users were seen by a behavioral health specialist (psychiatrist or psychiatric nurse) who identified and made referrals for cessation intervention. Individuals found to drink heavily or use illicit substances at evaluation for transplantation were required to undergo random blood testing for toxicology screening and achieve ≥3 negative tests before waitlisting. Determination of smoking cessation was based on patient and collateral (primarily family member) report. Although 6 months’ abstinence from all substances was desirable before waitlisting and transplantation, abstinence duration was considered in the context of medical urgency.
Outcomes
We followed the cohort through August, 2020 for 3 outcomes. Our main outcome was time to kidney transplantation. We then decomposed this outcome into time to placement on the active waitlist and, among patients waitlisted, time from waitlisting to transplantation.
Statistical analysis
We examined descriptive data on demographic, clinical and substance use characteristics across the 3 race/ethnicity groups using standard tests for continuous and categorical variables.
To examine whether particular combinations of race/ethnicity and substance use predicted study outcomes, we cross-classified race/ethnicity by patients’ use of any (vs. no) substances at the time of the transplant evaluation, yielding 6 groups: white patients with and without current use (i.e., past use only or never used substances); Black patients with and without current use; and Other race/ethnicity patients with and without current use. We targeted current use for predictive analyses because it is most relevant for transplant candidate selection decisions.17–24
Primary multivariable analyses
We used time-to-event analyses (Fine-Gray competing risk models, with death as a competing event)70,71 to examine the cumulative incidence of study outcomes across the study groups defined by race/ethnicity-in combination with any substance use. A separate model was fit for each outcome. These analyses controlled for demographic and clinical covariates that showed at least small associations (effect sizes) with (a) 1 or more study outcomes (i.e., subdistribution hazard ratios [HRs]>1.50) as well as (b) the race/ethnicity-substance use predictor groups (i.e., Cramér’s V≥0.10 for categorical covariates; Cohen’s f≥0.10 for continuous covariates).72–74
We adopted a competing risk approach because, given our observational data, we sought to identify predictors of the likelihood (i.e., probability) of outcomes rather than test causal relationships. Fine-Gray models are superior to other approaches when prediction, rather than etiology, is the goal.70,75–79 In the presence of a competing risk, conventional models (e.g., Cox models) would not allow our study goals to be achieved: they permit neither unbiased estimation of variables’ predictive effects on the cumulative incidence of outcomes nor accurate estimation of the probability of outcomes during the period of observation.70,71
Nevertheless, it can be useful to also fit conventional Cox models to provide a more complete understanding of the role of a putative risk factor on occurrence of a given outcome.75,79,80 Although Cox models cannot address cumulative incidence or the probability of the outcome (our chief interest), such models consider the impact of the risk factor on the instantaneous rate of occurrence of the outcome in individuals who are currently event free.70,80
Ancillary competing risk analyses
For any outcome for which race/ethnicity-substance use group differences emerged, we explored whether use of particular categories of substances might play a role. We examined (a) race/ethnicity cross-classified by current smoking, and (b) race/ethnicity cross-classified by current use of any other substances (heavy alcohol use or illicit substances; these categories had too few cases to consider separately). Thus, for each outcome, we fit an additional Fine-Gray model with the predictor of interest (race/ethnicity by smoking, or race/ethnicity by heavy alcohol/any illicit substance use) and the covariates. To control for false discovery in these exploratory analyses, we applied the Benjamini-Hochberg method to set the allowable false positive rate to 0.05.81,82 This approach has greater power than traditional multiple comparison adjustments.81
RESULTS
Sample description
Among the 1152 patients, 789 (68.5%) were white, 267 (23.2%) were Black, and 96 (8.3%) were in the Other race/ethnicity group (consisting mostly of individuals identifying as multiracial; see Table 1, footnote a). White patients were more educated, more likely to be married and employed, and less likely to have held blue collar/manual occupations or rely solely on public health insurance. They were older, but group differences were small. On clinical characteristics, Black patients were more likely to have hypertension (as the primary indication for transplantation, or as a comorbid diagnosis) and receive dialysis. They had higher Charlson Comorbidity scores but group differences were small; mean scores resembled those in other end-stage renal disease populations.83–85 There were no differences in history of heart disease, peripheral vascular disease, or pulmonary disease. White patients were less likely to have histories of medical nonadherence. Given that transplant evaluations occurred in 2010–2012, most waitlisted patients were waitlisted before KAS implementation.
Table 1 also lists the numbers of patients experiencing study outcomes and reasons for censoring. We used these data in time-to-event analyses addressing study aims.
Substance use patterns and progression toward transplantation
Figure 1 shows the race/ethnicity groups’ distribution on current, past, and no lifetime tobacco smoking, heavy alcohol use, illicit substance use, and use of any of these types of substances. Smoking (current or past) was the most common type of substance use. The groups differed significantly in their distributions of use of each type of substance, as well as on the composite variable reflecting any substance use (see Figure 1 for statistical tests).
Figure 1.
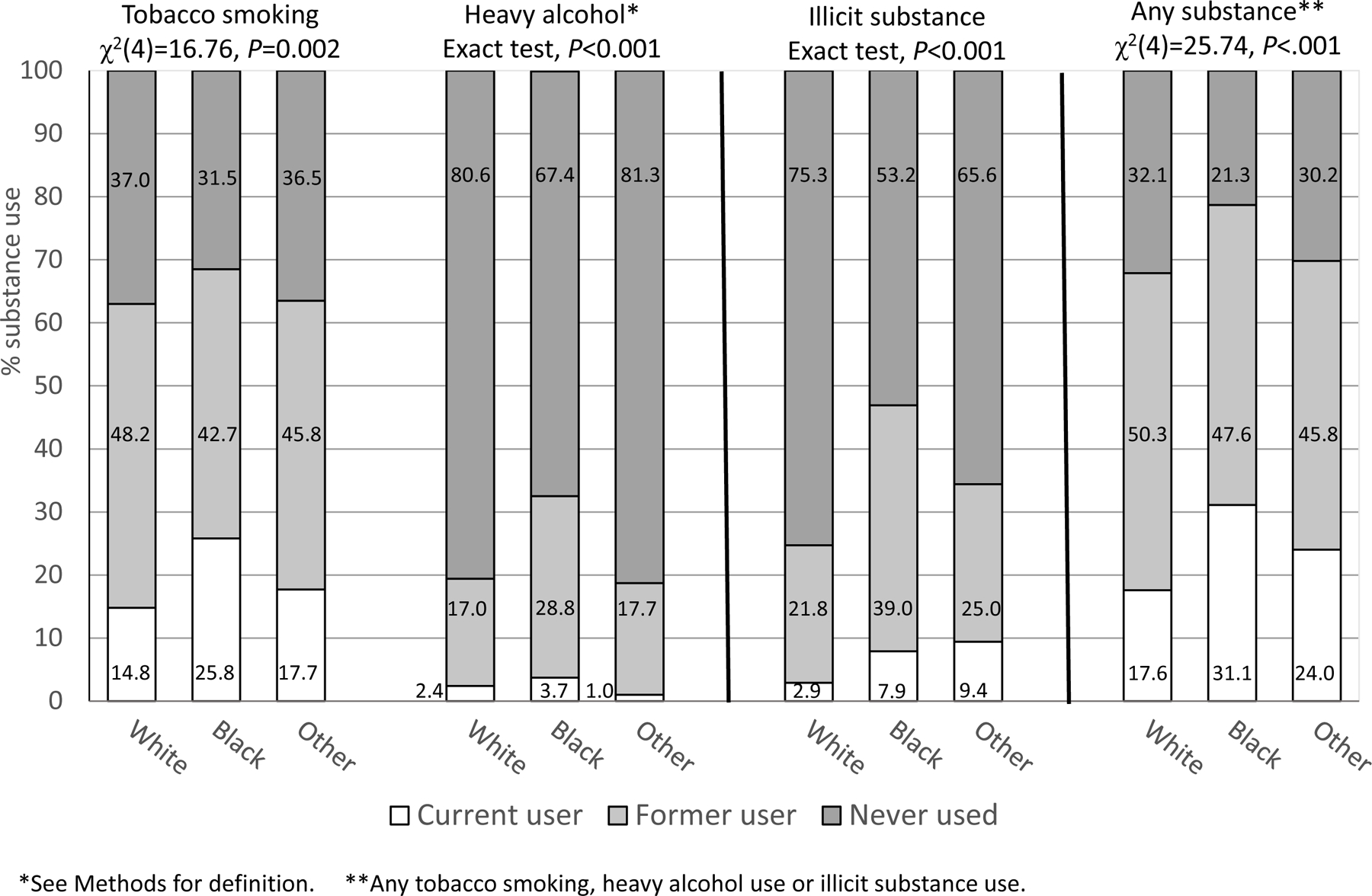
Distribution of substance use in patients undergoing kidney transplantation stratified by patients’ race/ethnicity (N = 1152).
We also performed more focused comparisons (controlling the false discovery rate) to examine specifically whether rates of current use (vs. past/no use) differed by race/ethnicity. There were differences on current smoking (χ2(2)=16.67, p<0.001), with the highest percentage among Black patients. The groups differed on current illicit substance use (Fisher’s Exact Test, p<0.001), and current use of any substances (χ2(2)=22.07, p<0.001): Black and Other race/ethnicity patients were most likely to currently use illicit substances and to be current users of any substances. There were no differences on current heavy alcohol use (Fisher’s Exact Test, p=0.346)
Table 3 presents additional descriptive information on the groups’ substance use patterns. Cigarettes were the most commonly smoked tobacco. The vast majority of past smokers had ≥6 months’ abstinence. The only significant differences were that Black participants were less likely to have chewed tobacco, and electronic cigarette use was reported only by Other race/ethnicity patients.
There were no significant differences by race/ethnicity on alcohol use characteristics. Most past heavy drinkers had abstained from heavy use for ≥6 months; a majority abstained from all alcohol use.
The race/ethnicity groups differed on multiple parameters of illicit substance use. In patients ever using illicit substances, marijuana was most common, especially among white patients. Black patients were more likely to have ever used stimulants or opioids, and to have lifetime histories of combined use of marijuana and other substances. Among all past illicit substance users, most had been abstinent for ≥6 months. White patients were most likely to have long periods of abstinence.
Finally, we characterized patterns of co-occurrence of tobacco, heavy alcohol and illicit substance use among current users, as well as these patients’ progression toward abstinence and transplantation (Figure 2). As shown in Figure 2a, tobacco smoking most often occurred alone; only 19% (23+1+14=38) of 203 smokers also used illicit substances or drank heavily. In contrast, 49% (26/53) of those using illicit substances also drank heavily or smoked, and 57% (17/30) of heavy alcohol users used illicit substances or smoked. There was no significant difference in distribution across these patterns by race/ethnicity (Fisher’s Exact test p=0.079).
Figure 2.
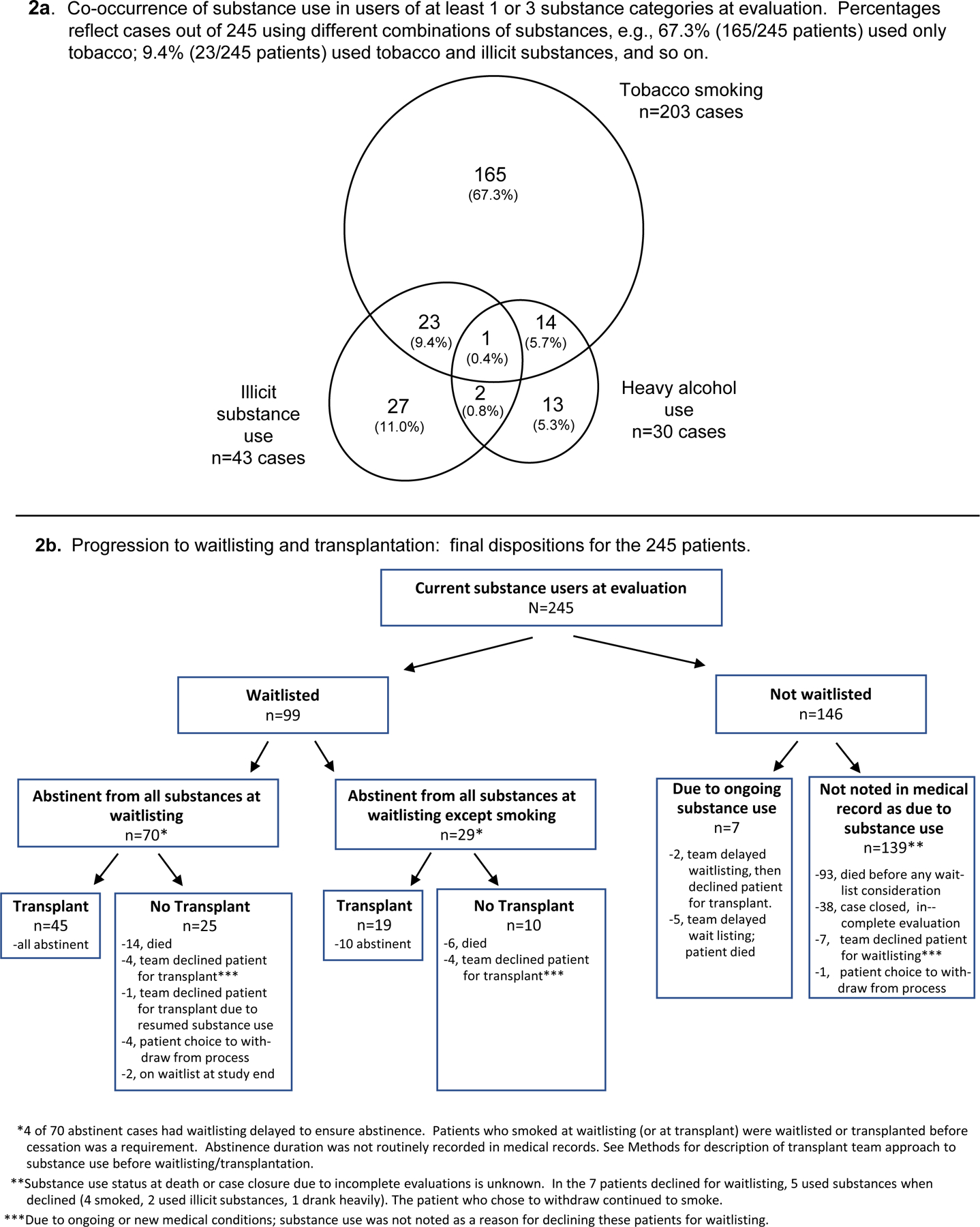
Substance use patterns and progression to waitlisting and transplantation in 245 study participants who currently used substances at the time of kidney transplantation evaluation.
Figure 2b depicts the 245 substance users’ progress toward possible transplantation. Their “final dispositions” are detailed in the lowermost boxes in the figure. For example, 45 patients abstinent from all substances at waitlisting underwent transplantation, while 25 patients abstinent at waitlisting did not receive transplants for the reasons listed (see also Figure 2b footnotes). Twenty-nine patients smoked at waitlisting (before smoking became an absolute contraindication), and 19 underwent transplantation. Among 146 patients never waitlisted, waitlisting decisions were initially delayed for 7 patients due to substance use. In 139 remaining patients, there was no indication in the EMR that lack of waitlisting was due to substance use.
Because patients varied in time to the events in Figure 2b, statistical comparisons (including any differences by race/ethnicity) cannot be made.54 Time-to-event analyses, described below, must be used to examine differences in likelihood of study outcomes.
Prediction of study outcomes
Cumulative incidence of kidney transplantation
Primary analysis.
We first examined whether likelihood of transplantation varied across the groups defined by race/ethnicity in combination with any (vs. no) substance use (Table 4, first column). Compared to the referent group (white patients with no current substance use), Black patients who used any substances were significantly less likely to undergo transplantation (HR, 0.45), as were Other race/ethnicity patients who used any substances (HR, 0.33). Neither white substance users nor Black nonusers differed significantly from the referent group, and Other race/ethnicity patients who were nonusers were identical to the referent group in likelihood of transplantation.
Table 4.
Predictors of kidney transplantation and waitlist-related outcomes, multivariable (competing risk) analysis and resulting subdistribution hazard ratios.
| Kidney transplantation in total cohort | Process toward kidney transplantation |
|||||
|---|---|---|---|---|---|---|
| Waitlisted for transplant | Among waitlisted patients, received transplant | |||||
|
| ||||||
| No. of incident events/Total no. of patientsa | 415/1152b | 656/1152 | 415/656 | |||
|
| ||||||
| Multivariable analysis | HR (CI) | P | HR (CI) | P | HR (CI) | P |
| Race/ethnicity by current substance use groups c | ||||||
| White, no substance use (referent) | --- | --- | --- | |||
| Black, no substance use | 0.76 (0.56, 1.02) | 0.070 | 0.73 (0.58, 0.91) | 0.005 | 0.99 (0.74, 1.32) | 0.949 |
| Other, no substance use | 1.00 (0.70, 1.43) | 0.980 | 0.79 (0.55, 1.13) | 0.199 | 0.96 (0.68, 1.35) | 0.804 |
| White, current substance use | 0.73 (0.50, 1.06) | 0.094 | 0.55 (0.42, 0.72) | <0.001 | 1.09 (0.77, 1.53) | 0.633 |
| Black, current substance use | 0.45 (0.23, 0.85) | 0.014 | 0.32 (0.22, 0.47) | <0.001 | 0.76 (0.47, 1.25) | 0.288 |
| Other, current substance use | 0.33 (0.13, 0.84) | 0.021 | 0.60 (0.37, 0.99) | 0.049 | 0.69 (0.26, 1.79) | 0.448 |
| Covariates | ||||||
| Age, years | 0.97 (0.97, 0.98) | <0.001 | 0.98 (0.98, 0.99) | <0.001 | 0.97 (0.97, 0.98) | <0.001 |
| Employment status, unemployed | 0.69 (0.55, 0.86) | 0.001 | 0.74 (0.62, 0.88) | 0.001 | 0.68 (0.55, 0.84) | <0.001 |
| Health insurance, public only (vs. any private coverage) | 0.75 (0.58, 0.96) | 0.022 | 0.71 (0.59, 0.86) | 0.001 | 0.78 (0.60, 0.99) | 0.048 |
| BMI | 0.98 (0.96, 0.99) | 0.013 | 0.99 (0.98, 1.00) | 0.076 | 0.98 (0.97, 1.00) | 0.056 |
| Hypertension, yes | 1.17 (0.93, 1.47) | 0.180 | 1.10 (0.92, 1.31) | 0.317 | 1.13 (0.91, 1.41) | 0.272 |
| On dialysis, yes | 0.80 (0.65, 0.98) | 0.035 | 0.62 (0.52, 0.73) | <0.001 | 0.83 (0.68, 1.01) | 0.065 |
| Charlson Comorbidity Index,d higher score=worse | 0.27 (0.15, 0.50) | <0.001 | 0.30 (0.19, 0.49) | <0.001 | 0.39 (0.22, 0.69) | <0.001 |
| Chronic pulmonary disease, yes | 0.84 (0.65, 1.09) | 0.185 | 0.83 (0.70, 0.99) | 0.034 | 0.95 (0.73, 1.23) | 0.705 |
| History of medical nonadherence, yes | 0.82 (0.59, 1.13) | 0.226 | 0.80 (0.63, 1.02) | 0.067 | 0.90 (0.65, 1.24) | 0.522 |
| Waitlisted before 2014 implementation of KASe | 0.80 (0.77, 0.83) | <0.001 | --- | 0.78 (0.43, 1.40) | 0.398 | |
| Improvement in model fit over null model: χ2 (df); p | 284.7 (15) | <0.001 | 255.0 (14) | <0.001 | 124.0 (15) | <0.001 |
For each outcome, patients were followed until the event of interest or until censoring due to death (competing risk) or other reasons (see Table 1 for numbers of patients by reasons for censoring). Only 11 patients (<1% of all patients; <2% of those waitlisted) were censored because the study observation period ended; they had been waitlisted and were on the waitlist at study’s end). They were followed in the study for median of 8.4 years (IQR, 8.1, 8.8), and had been on the waitlist for median of 6.5 years (IQR, 3.9, 7.9).
Of the 415 patients receiving transplants, 134 received living donor transplants. Numbers of outcome events are too small to examine race/ethnicity by substance use groups as predictors separately for living vs. deceased donor transplants.
We chose to compare groups defined by the combination of race/ethnicity and substance use, with the referent group of non-Hispanic white patients, because of the ease of displaying and interpreting specific disparities in the outcomes. An alternative for evaluating our hypothesis (i.e., that the 2 groups of racial/ethnic minority patients who used substances would show particularly great disadvantage on outcomes) is to test a planned contrast using contrast weights to capture the notion of synergistic effects. The statistical tests and p levels associated with evaluating this planned contrast within competing risk models were z=2.99, P=0.003 for the outcome of kidney transplantation; z=4.95, p<.001 for waitlisting, and z=1.34, p=.182 for transplant among waitlisted patients. (Note that decomposing this planned contrast into its component parts, i.e., separately testing main effects for race/ethnicity and substance use, and an interaction effect, would provide only piecemeal evaluation of our hypothesis rather than a focused test of it. Planned contrasts give greater power and precision than piecemeal testing when specific hypotheses such as those pertaining to synergy are proposed.86,87)
Log transformed prior to analysis.
Included as a time dependent covariate in analysis of time to transplant in full cohort.
Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, Interquartile range; KAS, Kidney Allocation System
Figure 3 illustrates these findings. By the end of follow-up, 25% of white patients who did not use any substances received transplants, as did 26% of Other race/ethnicity nonusers. Slightly smaller percentages of white substance users and Black nonusers received transplants (20% of each). Only 14% and 9% of the 2 minority groups who used any substances received transplants.
Figure 3.
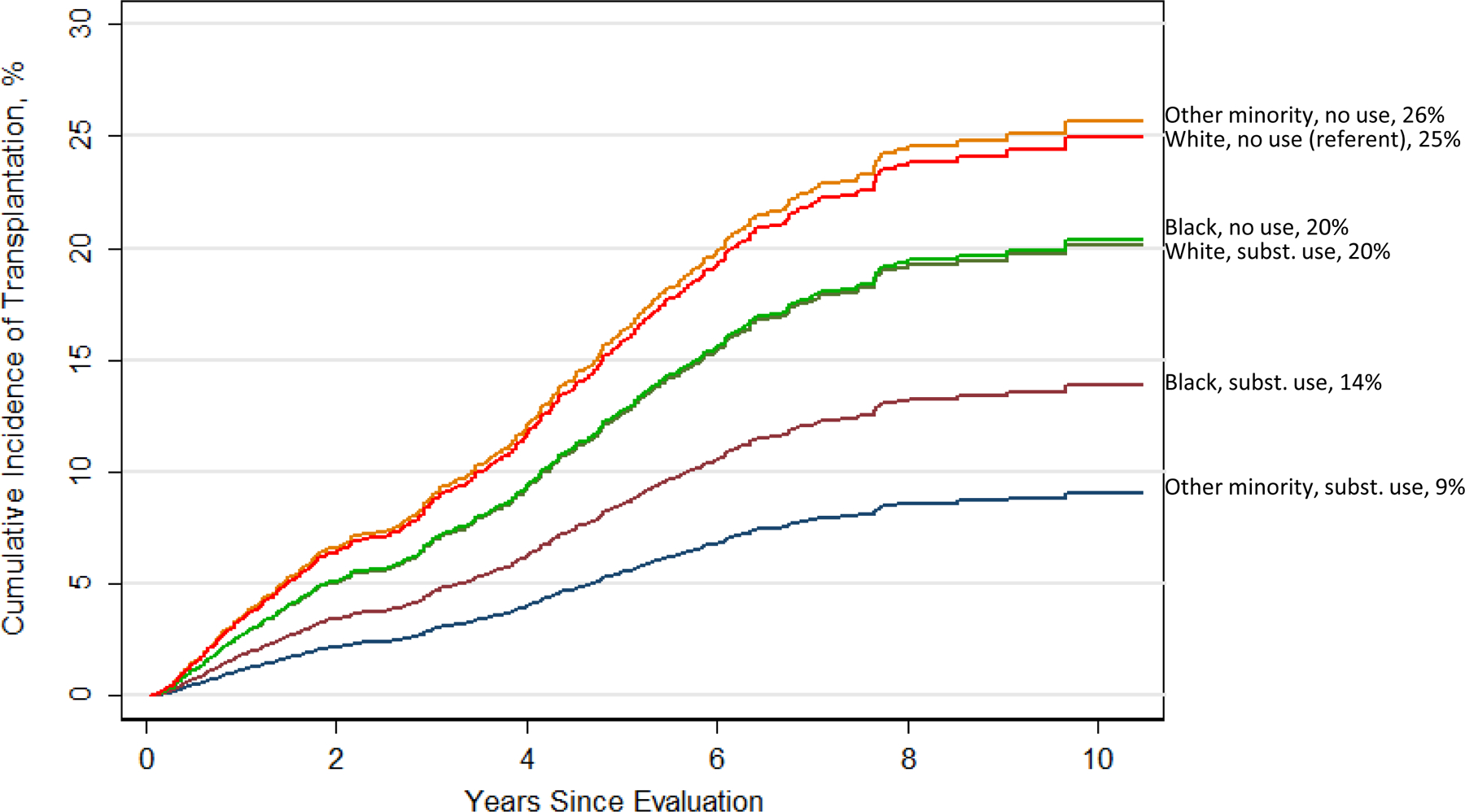
Cumulative incidence of kidney transplantation in 6 study groups defined by race/ethnicity in combination with use of any substances. See Table 4 for statistical comparisons between groups.
Ancillary analyses.
Beyond consideration of any substance use (vs. none), we explored whether current smoking appeared to account for these effects, and/or whether current heavy alcohol/illicit substance accounted for these effects. Concerning smoking, Black smokers were less likely to undergo transplantation than the white nonsmoker referent group (HR, 0.37; CI, 0.16,0.83, p=0.016), but this difference was not significant after controlling for the false discovery rate. For heavy alcohol/illicit substance use, there were no significant effects by race/ethnicity. In sum, we found effects for race/ethnicity in combination with any substance use but could not reliably pinpoint the specific role of smoking or of heavy alcohol/illicit substance use in this association.
Cumulative incidence of waitlisting
Primary analysis.
Compared to the referent group of white patients who did not use any substances, Black patients using any substances were significantly less likely to be waitlisted (HR, 0.32; Table 4, second column). White substance users (HR, 0.55), Other race/ethnicity substance users (HR, 0.60), and Black nonusers (HR, 0.73) were also less likely to be waitlisted. Patients in the Other race/ethnicity group who were nonusers did not significantly differ from the referent group.
Figure 4a, displays these findings. By the end of follow-up, 70% of white nonusers and 62% of Other race/ethnicity nonusers had been waitlisted. Remaining groups had lower incidence rates, especially Black substance users (32%).
Figure 4.
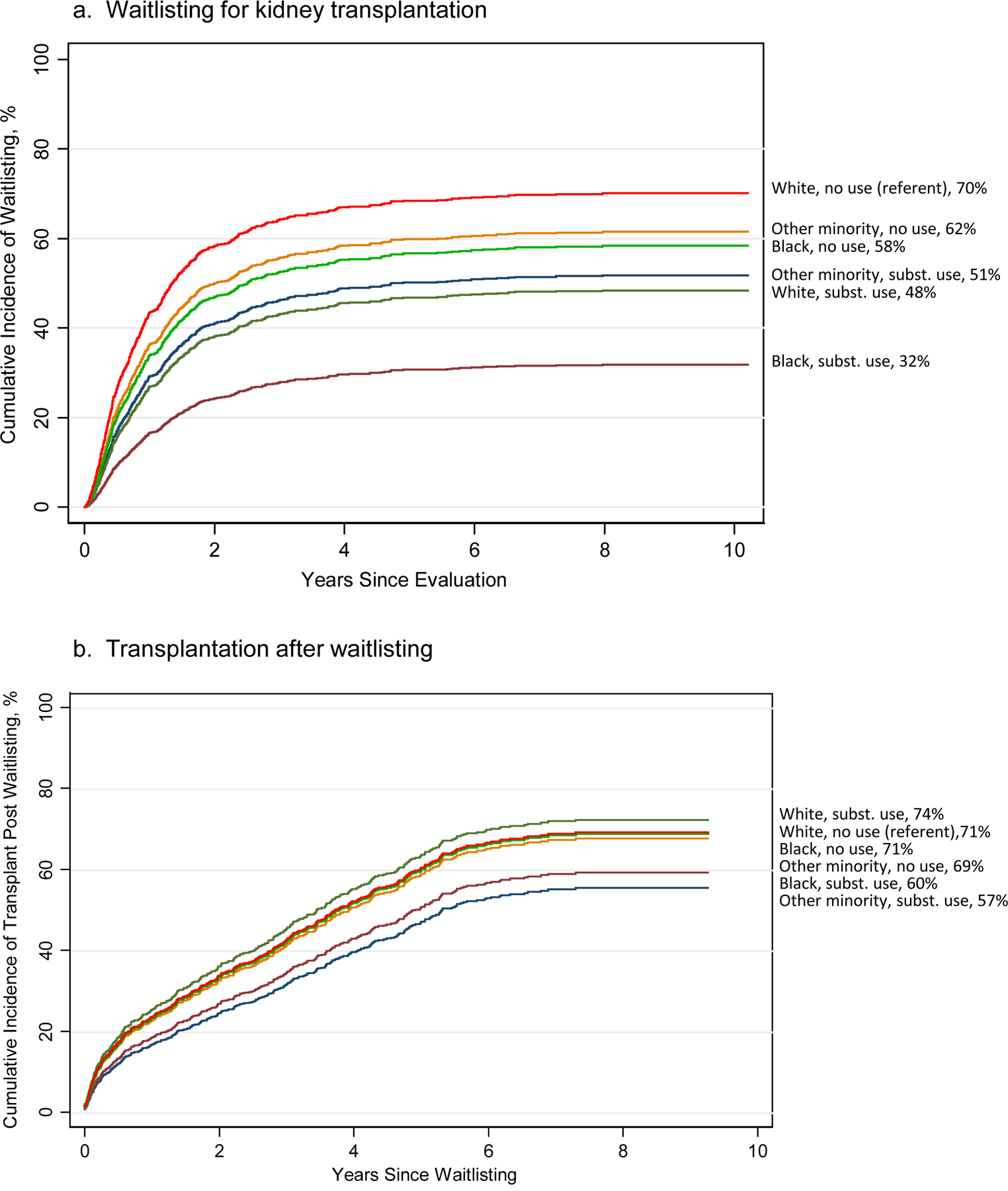
Cumulative incidence of waitlisting and of transplantation after waitlisting in 6 study groups defined by race/ethnicity in combination with use of any substances. See Table 4 for statistical comparisons between groups.
Ancillary analyses.
We then separately explored current smoking across the race/ethnicity groups, and current heavy alcohol/illicit substance across the race/ethnicity groups. For smoking, after controlling for the false discovery rate, Black smokers were significantly less likely to be waitlisted than the white nonsmoker referent group (HR, 0.31; CI, 0.20,0.49, p<0.001). White smokers were also less likely to be waitlisted (HR, 0.49; CI, 0.37,0.66, p<0.001), as were Black nonsmokers (HR, 0.69; CI, 0.56,0.86, p=0.001). For alcohol/illicit substance use, both Black users and Other minority users were less likely to be waitlisted than the referent group (HR, 0.29; CI, 0.16,0.53, p<0.001 and HR, 0.32; CI, 0.14,0.75, p<0.009, respectively) as were Black nonusers (HR, 0.71; CI, 0.57,0.87, p=0.001).
Cumulative incidence of transplantation after waitlisting
Primary analysis.
Compared to the referent group, there were no significant study group differences in likelihood of transplantation after waitlisting (Table 4, column 3). At the end of follow-up, from 57% to 74% of patients in the study groups received transplants after waitlisting (Figure 4b). Given the lack of effects, ancillary analyses by substance type were not pursued.
Alternative analyses:
Cox models examining race/ethnicity in combination with any substance use in predicting outcomes Compared to the competing risk models, although Cox model effects vary slightly in size (given that Cox models are not estimating exactly the same thing as competing risk models), Cox model results were virtually identical to those of our primary analyzes in terms of identifying statistically significant effects (Table S1). Only 1 difference emerged: for the waitlist outcome, Other minority substance users did not significantly differ from the referent group in the Cox model (Cox HR, 0.63; CI, 0.35,1.13; p=0.120 vs. competing risk model HR, 0.60; CI 0.37,0.99); p=0.049).
DISCUSSION
We provide novel data on substance use in patients undergoing evaluation for kidney transplantation and whether substance use characteristics varied by race/ethnicity. In addition, ours is the first study to examine whether racial/ethnic minority patients who used substances were uniquely disadvantaged in likelihood of transplantation.
We found noteworthy differences between race/ethnicity groups in prevalence of substance use. Black patients were most likely to currently smoke, and Black and Other minority patients were more likely than white patients to currently use illicit substances. The percentage of Black patients who smoked (26%) is similar to the national rate (25%),64 while percentages of smokers among white and Other race/ethnicity minority patients (15% and 18%) were lower than national percentages (24% for white, 25% for all non-Black races/ethnicities, weighted to reflect our sample’s composition of other race/ethnicities). Percentages of current heavy alcohol use were lower in all our race/ethnicity groups (1%-4%) than national percentages (5%-8%), as were our sample’s percentages of current illicit substance use (3%-9% vs. 11%-13% nationally).
The smoking rate in Black patients appears unexpectedly high. In community samples, Black persons who smoke have greater nicotine dependence (despite smoking less frequently) and are less successful in quitting than white individuals.48,88,89 Such factors may help explain our relatively large percentage of Black smokers. Although we could not examine mechanisms underlying observed smoking rates, our findings suggest that treatment and referral strategies may require expansion to address Black transplant candidates’ potentially greater need for aggressive, tailored cessation interventions.
We found marked disparities in our main study outcome, overall incidence of kidney transplantation, for racial/ethnic minority patients using any substances at the time of transplant evaluation. The 2 minority groups of substance users were 55% to 67% less likely to receive transplants than the referent group of white nonusers. In contrast, minority patient nonusers did not reliably differ from the referent group—indeed, nonusers in our Other race/ethnicity group were virtually identical to the referent group in likelihood of transplantation. Further, white substance users also did not differ significantly from the referent group.
Our findings of unique disparities in overall transplant rates for patients who were both racial/ethnic minorities and substance users are consistent with the notion of “double jeopardy,”50–52 and may have arisen for multiple reasons. First, beyond factors noted above that are associated with smoking in Black individuals, a growing literature shows that substance users in many racial/ethnic minority groups are less likely than white individuals to initiate or continue in cessation treatment, due at least partially to socioeconomic barriers.47,90,91 Although our analyses controlled for unemployment and health insurance status, which provide some indication of socioeconomic status (SES) and could have affected receipt of treatment, we did not have more direct SES measures (e.g., household per capita income, receipt of public assistance) that may have differentially affected patients’ ability to engage in treatment. Second, minorities are less likely to be referred for specialty care such as that required for substance use, suggesting clinician bias.92,93 In the context of kidney transplantation, lack of adequate cessation therapy may reduce patients’ prospects for receiving new organs. Substance users seen by our transplant program routinely received treatment recommendations and referrals. However, minority patients may have less often engaged in treatment and thus been less likely to achieve candidacy requirements for cessation. We could not investigate this because whether patients acted on treatment recommendations (or had the socioeconomic resources to do so) was not systematically documented in the EMR. In addition, reasons patients were declined for transplantation listed in the EMR focused on medical factors and rarely on substance use per se (Figure 2b).
Additional study findings suggest that the disparities we observed in receipt of kidney transplants in the cohort overall were largely explained by whether patients were waitlisted. Once patients were waitlisted, the combination of race/ethnicity and substance use did not predict who received a transplant: differences between minority groups using substances and the white nonuser referent group were the smallest for this outcome. This reduction in disadvantage does not mean that waitlisted minority patients who had used substances somehow became “advantaged” after waitlisting relative to the referent group in their chances for transplantation. Instead, they only became more similar to the referent group; their disadvantage was lessened, though not fully erased. In short, the overall differences in total rates of transplantation appear to have been driven by waitlisting differences because the disparities became smaller and nonsignificant after the waitlisting hurdle was achieved.
Further, our analyses of differences in waitlisting suggest that the “double jeopardy” effect—although potentially important for both of our minority patient groups—may be most pronounced for Black substance users. They were the least likely of all study groups to be waitlisted. This held true even when we separately considered tobacco use, and heavy alcohol use/illicit substance use.
Results for our waitlist outcome also revealed that Black patients who did not use substances as well as white substance users were significantly less likely to be waitlisted than the referent group. Why, then, did these groups not reliably differ from the referent group on our main outcome of overall transplant rates in the entire cohort? Perhaps it was because, once waitlisted, they were at least as likely as the referent group to receive transplants (HRs from 0.99 to 1.09): in essence, they fully “caught up” with the referent group, leading to relatively small, nonsignificant differences overall when we examined total rates of transplant in the complete cohort.
Our study has noteworthy limitations. First, our data are observational and do not allow causal inferences about predictor-outcome relationships. However, from a practical, clinical perspective, one need not determine causality to develop risk-reduction interventions. Second, our cohort came from a single center and generalizability is unknown. Third, although we prospectively collected outcomes data over a lengthy period and assessed important patient demographics in research interviews rather than relying on often-incomplete EMR demographics,94,95 we determined substance use patterns retrospectively. A similar approach has been used previously.30,32,33,35,36,43,44 A retrospective strategy may be biased because the original evaluators may not have collected relevant information. However, psychosocial evaluators followed a protocol designed to promote consistent collection of psychosocial (including substance use) information. Fourth, patients may have underreported substance use in the psychosocial evaluation. Consequently, we marshalled additional data: although toxicology data were not collected at this evaluation, we retrieved corroborative information from family members and other transplant team members’ evaluations. Fifth, perhaps we did not observe between-group differences in transplant rates after waitlisting due to lower power (smaller sample size) than for other study outcomes. However, effect sizes from this analysis (HRs in Table 4) were small by conventional standards,78,79 while effects for significant differences on our other outcomes were generally considerably larger. Sixth, aside from Black patients, we could not perform finer-grained analyses examining other specific racial/ethnic minorities.
Another important limitation is that, although we could examine outcomes for our race/ethnicity groups in combination with use of any substances as well as in combination with tobacco use, we had limited to examine other specific types of substances: due to small numbers of cases, we could not explore heavy alcohol use separate from illicit substance use. In 1 recent report, alcohol use and illicit substance use were each independently associated with a significantly lower likelihood of transplantation.32 However, that study did not consider how these substances’ effects may have varied by race/ethnicity. Moreover, illicit substance use is a heterogenous category, but studies of impact on kidney transplant rates have largely focused only on any vs. no illicit substance use.32,44 One study in a small sample with cannabis dependence disorders reported that greater disorder severity was related to lower likelihood of kidney transplantation but other drug use was not predictive.43 The role of race/ethnicity was not examined, but clearly cannabis (marijuana) use requires additional attention, especially given its growing legalization for medicinal and recreational use.
Beyond study limitations, our findings have research and clinical care implications. Work to target and reduce disparities in kidney transplantation should consider that certain combinations of factors (including but perhaps not limited to minority race/ethnicity and substance use) may together be particularly strong contributors to disparities. In the case of substance use, we have noted that minority patients face barriers in receiving effective care, and clinician bias in referral for such care. For example, clinicians may hold different assumptions about feasibility and/or effectiveness of substance use treatment for potential transplant candidates of different races/ethnicities, and therefore may not inform them of all treatment options.45 However, there is a dearth of research identifying or intervening upon patient- or clinician-related barriers. Such work would be consistent with and extend the reach of research and educational agendas already proposed to address racism in transplantation.45,96
From a clinical care perspective, heightened awareness among transplant teams that minority patients who use substances may face unique disparities in receipt of kidney transplants is essential. Substance use is an important contraindication to transplantation because ongoing use can adversely affect post-transplant clinical outcomes.25–32 Yet equity is threatened if racial/ethnic minority patients found to use substances at their initial transplant evaluation have a uniquely lower likelihood of transplantation than other patients. Greater awareness of this disparity could lead transplant teams to develop new strategies to (a) further improve care by facilitating and tracking patients’ receipt of substance use interventions, and (b) expand clinicians’ cultural competence, including focused programs to educate clinicians about implicit biases they may hold about substance use in racial/ethnic minorities. Such efforts may facilitate progress toward ensuring that all patients receive equitable consideration for kidney transplantation.
Supplementary Material
3. Funding
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grants R25 DK078381 and R01 DK081325, and by Dialysis Clinic, Inc. grant DCI C-3924.
ABBREVIATIONS
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- LSD
d-lysergic acid diethylamide
- EMR
electronic medical record
- HR
hazard ratio
- ICD
International Classification of Diseases
- IQR
interquartile range
- KAS
Kidney Allocation System
- M
mean
- PVD
peripheral vascular disease
- SES
socioeconomic status
- SD
standard deviation
Footnotes
Disclosures
The authors declare no conflicts of interest. Richelle DeBlasio is now a medical student at the School of Medicine, University of Pittsburgh, Pittsburgh, PA. The work described herein was undertaken while she was affiliated with the Department of Psychiatry, University of Pittsburgh.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- 1.Arce CM, Goldstein BA, Mitani AA, et al. Differences in access to kidney transplantation between Hispanic and non-Hispanic whites by geographic location in the United States. Clin J Am Soc Nephrol 2013;8(12):2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan PY, Ashby VB, Fuller DS, et al. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant 2010;10(4 Pt 2):1090–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall YN, Choi AI, Xu P, et al. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol 2011;22(4):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding K, Mersha TB, Pham PT, et al. Health Disparities in kidney transplantation for African Americans. Am J Nephrol 2017;46(2):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi SJ Gaynor J, Ciancio G. Review of ethnic disparities in access to renal transplantation. Clin Transplant 2012;26(4):E337–E343. [DOI] [PubMed] [Google Scholar]
- 6.King KL, Husain SA, Jin Z, et al. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol 2019;14:1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku E, Lee BK, McCulloch CE, et al. Racial and ethnic disparities in kidney transplant access within a theoretical context of medical eligibility. Transplantation 2020;104(7):1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkarni S, Ladin K, Haakinson D, et al. Association of racial disparities with access to kidney transplant after the implementation of the new kidney allocation system. JAMA Surg 2019;154(7):618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patzer RE, Amaral S, Wasse H, et al. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 2009:20:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purnell TS, Luo X, Cooper LA, et al. Association of race and ethnicity with live donor kidney transplantation in the United States from 1995 to 2014. JAMA 2018;319(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequist TD, Narva AS, Stiles SK, et al. Access to renal transplantation among American Indians and Hispanics. Am J Kidney Dis 2004;44:344–352. [DOI] [PubMed] [Google Scholar]
- 12.Wesselman H, Ford CG, Leyva Y, et al. Social determinants of health and race disparities in kidney transplantation. Clin J Am Soc Nephrol 2021;16(2):262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am 2013;93(6):1395–1406. [DOI] [PubMed] [Google Scholar]
- 14.Schold JD, Mohan S, Humi A, et al. Failure to advance access to kidney transplantation over two decades in the United States. J Am Soc Nephrol 2021;32(4):913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy KA, Jackson JW, Purnell TS, et al. Association of socioeconomic status and comorbidities with racial disparities during kidney transplant evaluation. Clin J Am Soc Nephrol 2020;15(6):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schold JD, Gregg JA, Harman JS, et al. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 2011;6(7):1760–1767. [DOI] [PubMed] [Google Scholar]
- 17.Abramowicz A, Cochat P, Claas FHJ, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant 2015;30(11):1790–1797. [DOI] [PubMed] [Google Scholar]
- 18.Bunnapradist S, Danovitch GM. Evaluation of adult kidney transplant candidates. Am J Kidney Dis 2007;50:890–898. [DOI] [PubMed] [Google Scholar]
- 19.Chadban SJ, Ahnn C, Axelrod DA, et al. ; Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Transplant Candidate Work Group. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020;104(4S1 Suppl 1):S11–S103. [DOI] [PubMed] [Google Scholar]
- 20.DiMartini AF, Shenoy A, Dew MA. Organ transplantation. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry 3rd ed. Washington DC: American Psychiatric Association; 2019:859–906. [Google Scholar]
- 21.Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant 2001;1(Suppl 2):3–95. [PubMed] [Google Scholar]
- 22.Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation [published correction appears in Can Med Assoc J. 2005;173(12):1490]. Can Med Assoc J 2005;173(10):1181–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuntz K, Weinland SR, Butt Z. Psychosocial challenges in solid organ transplantation. J Clin Psychol Med Settings 2015;22:122–135. [DOI] [PubMed] [Google Scholar]
- 24.Steinman TI, Becker BN, Frost AE, et al. Guidelines for the referral and management of patients eligible for solid organ transplantation. Transplantation 2001;71(9):1189–1204. [DOI] [PubMed] [Google Scholar]
- 25.Barrantes F, Luan FL, Kommareddi M, et al. A history of chronic opioid usage prior to kidney transplantation may be associated with increased mortality risk. Kidney Int 2013;84(2):P390–P396. [DOI] [PubMed] [Google Scholar]
- 26.Corbett C, Armstrong MJ, Neuberger J. Tobacco smoking and solid organ transplantation. Transplantation 2012;94(10):979–987. [DOI] [PubMed] [Google Scholar]
- 27.Duerinckx N, Burkhalter H, Engberg SJ, et al. Correlates and outcomes of posttransplant smoking in solid organ transplant recipients: a systematic literature review and meta-analysis. Transplantation 2016;100:2252–2263. [DOI] [PubMed] [Google Scholar]
- 28.Lentine KL, Lam NN, Xiao H, et al. Associations of pre-transplant prescription narcotic use with clinical complications after kidney transplantation. Am J Nephrol 2015;41:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lentine KL, Yuan H, Tuttle-Newhall JE, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation 2015;99:187–196. [DOI] [PubMed] [Google Scholar]
- 30.Machnicki G, Pinsky B, Takemoto S, et al. Predictive ability of pretransplant comorbidities to predict long-term graft loss and death. Am J Transplant 2009;9(3):494–505. [DOI] [PubMed] [Google Scholar]
- 31.Parker R, Armstrong MJ, Corbett C, et al. Alcohol and substance abuse in solid-organ transplant recipients. Transplantation 2013;96:1015–1024. [DOI] [PubMed] [Google Scholar]
- 32.Sandhu GS, Khattak M, Woodward R, et al. Impact of substance abuse on access to renal transplantation. Transplantation 2011;91(1):86–93. [DOI] [PubMed] [Google Scholar]
- 33.Alhamad T, Koraishy FM, Lam NN, et al. Cannabis dependence or abuse in kidney transplantation: Implications for posttransplant outcomes. Transplantation 2019;103(11):2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dew MA, DiMartini AF, Steel J, et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl 2008;14(2):159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabbri KR, Anderson-Haag TL, Spenningsby AM, et al. Marijuana use should not preclude consideration for kidney transplantation. Clin Transplant 2019;33(10):e13706. [DOI] [PubMed] [Google Scholar]
- 36.Greenan G, Ahmad SB, Anders MG, et al. Recreational marijuana use is not associated with worse outcomes after renal transplantation. Clin Transplant 2016;30(10):1340–1346. [DOI] [PubMed] [Google Scholar]
- 37.Gueye AS, Chelamcharla M, Baird BC, et al. The association between recipient alcohol dependency and long-term graft and recipient survival. Nephrol Dial Transplant 2007;22(3):891–898. [DOI] [PubMed] [Google Scholar]
- 38.Zelle DM, Agarwal PK, Ramirez JL, et al. Alcohol consumption, new onset of diabetes after transplantation, and all-cause mortality in renal transplant recipients. Transplantation 2011;92(2):203–209. [DOI] [PubMed] [Google Scholar]
- 39.Kasiske BL, Klinger D. Cigarette smoking in renal transplant recipients. J Am Soc Nephrol 2000;11(4):753–759. [DOI] [PubMed] [Google Scholar]
- 40.Luchsinger W, Zimbrean P. Systematic review: treatment for addictive disorder in transplant patients. Am J Addict 2020;29(6):445–462. [DOI] [PubMed] [Google Scholar]
- 41.Opelz G, Döhler B. Influence of current and previous smoking on cancer and mortality after kidney transplantation. Transplantation 2016;100(1):227–232. [DOI] [PubMed] [Google Scholar]
- 42.Sandhu GS, Khattak M, Pavlakis M, et al. Recipient’s unemployment restricts access to renal transplantation. Clin Transplant 2013;27(4):598–606. [DOI] [PubMed] [Google Scholar]
- 43.Stark AL, Hickson LJ, Larrabee BR, et al. Cannabis abuse and dependence in kidney transplant candidates. J Psychosom Res 2019;121:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang E, Bansal A, Famure O, et al. Substance use in kidney transplant candidates and its impact on access to kidney transplantation. Clin Transplant 2019;33(6):e13565. [DOI] [PubMed] [Google Scholar]
- 45.Arriola KJ. Race, racism, and access to renal transplantation among African Americans. J Health Care Poor Underserved 2017;28(1):30–45. [DOI] [PubMed] [Google Scholar]
- 46.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol 2010;5(12):2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mennis J, Stahler GJ. Racial and ethnic disparities in outpatient substance use disorder treatment episode completion for different substances. J Subst Abuse Treat 2016;63:25–33. [DOI] [PubMed] [Google Scholar]
- 48.Trinidad DR, Pérez-Stable EJ, White MM, et al. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaeth PA, Wang-Schweig M, Caetano R. Drinking, alcohol use disorder, and treatment access and utilization among U.S. racial/ethnic groups. Alcohol Clin Exp Res 2017;41(1):6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen E, Martin AD, Matthews KA. Understanding health disparities: the role of race and socioeconomic status in children’s health. Am J Public Health 2006;96(4):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferraro KF, Farmer MM. Double jeopardy to health hypothesis for African Americans: analysis and critique. J Health Soc Behav 1996;37:27–43. [PubMed] [Google Scholar]
- 52.Schulz AJ, Mullings L, eds. Gender, Race, Class and Health: Intersectional Approaches San Francisco, CA: Jossey-Bass; 2005. [Google Scholar]
- 53.Ng YH, Pankratz VS, Leyva Y, et al. Does racial disparity in kidney transplant wait-listing persist after accounting for social determinants of health? Transplantation 2020;104(7):1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data 2nd ed. Hoboken, NJ: Wiley; 2011. [Google Scholar]
- 55.Hollingshead AB. Four factor index of social status. Yale J Sociol 2011;8:21–51. [Google Scholar]
- 56.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant 2020;20(Suppl 1):20–130. [DOI] [PubMed] [Google Scholar]
- 57.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan MK, Rankin AJ, Jani BD, et al. Associations between multimorbidity and adverse clinical outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. BMJ Open 2020;10(6):e038401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glasheen WP, Cordier T, Gumpina R, et al. Charlson Comorbidity Index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits 2019;12(4):188–197. [PMC free article] [PubMed] [Google Scholar]
- 60.Ward MB, Hackenmueller SA, Strathmann FG; Education Committee of the Academy of Clinical Laboratory Physicians and Scientists. Pathology consultation on urine compliance testing and drug abuse screening. Am J Clin Pathol 2014;142(5):586–593. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System survey data and documentation, 2019. Available at: https://cdc.gov/brfss/annual_data/annual_2019.html. Accessed September 20, 2021.
- 62.US Department of Health and Human Services. Smoking cessation: A Report of the Surgeon General Rockville, MD: US Department of Health and Human Services; 2020. Available at https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf. Accessed September 20, 2021. [Google Scholar]
- 63.US Department of Health and Human Services; US Department of Agriculture. Dietary Guidelines for Americans 2015–2020 8th ed. Rockville, MD: US Department of Health and Human Services; 2015. Available at https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf. Accessed September 20, 2021. [Google Scholar]
- 64.Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health Rockville, MD: Substance Abuse and Mental Health Services Administration; 2020. Available at: https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR090120.htm. Accessed September 20, 2021. [Google Scholar]
- 65.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 83: Tobacco Smoke and Involuntary Smoking Lyon, France: International Agency for Research on Cancer; 2004. [PMC free article] [PubMed] [Google Scholar]
- 66.Butt Z, Levenson JL, Olbrisch ME. Policies on tobacco and marijuana smoking among US cardiac, kidney, and liver transplant programs [abstract]. Ann Behav Med, 2014;47(Suppl 1):S244. [Google Scholar]
- 67.Cote DR, Chirichella TJ, Noon KA, et al. Abdominal organ transplant center tobacco use policies vary by organ program type. Transplant Proc 2016;48(6):1920–1926. [DOI] [PubMed] [Google Scholar]
- 68.Katsi V, Maragkoudakis S, Ioakeimidis N, et al. The cardiovascular burden of light smoking. Arch Med Sci Atheroscler Dis 2021;6:e48–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li X, Holahan CK, Holahan CJ. Sociodemographic and psychological characteristics of very light smoking among women in emerging adulthood, National Survey of Drug Use and Health, 2011. Prev Chronic Dis 2015;12:140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133(6):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 72.Azuero A A note on the magnitude of hazard ratios. Cancer 2016;122(8):1298–1289. [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Compute 2010;39(4):860–864. [Google Scholar]
- 74.Cohen J Statistical Power Analysis for the Behavioral Sciences 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 75.Jiang Y, Fine JP, Mottl AK. Competing risk of death with end-stage renal disease in diabetic kidney disease. Adv Chronic Kidney Dis 2018;25(2):133–140. [DOI] [PubMed] [Google Scholar]
- 76.Koller MT, Raatz H, Steyerberg EW, et al. Competing risks and the clinical community: irrelevance or ignorance? Stat Med 2012;31(11–12):1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noordzij M, Leffondré K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 2013;28(11):2670–2677. [DOI] [PubMed] [Google Scholar]
- 79.Sapir-Pichhadze R, Pintilie M, Tinckam KJ, et al. Survival analysis in the presence of competing risks: the example of waitlisted kidney transplant candidates. Am J Transplant 2016;16(7):1958–1966. [DOI] [PubMed] [Google Scholar]
- 80.Latouche A, Allignol A, Beyersmann J, et al. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 2013;66(6):648–653. [DOI] [PubMed] [Google Scholar]
- 81.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B 1995;57(1):289–300. [Google Scholar]
- 82.Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102(4):721–728. [DOI] [PubMed] [Google Scholar]
- 83.Albugami MM, Panek R, Soroka S, et al. Access to kidney transplantation: outcomes of the non-referred. Transplant Res 2012;1(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemmelgarn BR, Manns BJ, Quan H, et al. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 2003;42:125–132. [DOI] [PubMed] [Google Scholar]
- 85.Kiberd B, Boudreault J, Bhan V, et al. Access to the kidney transplant wait list. Am J Transplant 2006;6:2714–2720. [DOI] [PubMed] [Google Scholar]
- 86.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis Cham Switzerland: Springer; 2015. [Google Scholar]
- 87.Rosenthal R, Rosnow RL, Rubin DB. Contrasts and Effect Sizes in Behavioral Research: A Correlational Approach Cambridge, UK: Cambridge University Press, 2000. [Google Scholar]
- 88.Nollen NL, Mayo MS, Sanderson Cox L, et al. Factors that explain differences in abstinence between Black and white smokers: a prospective intervention study. J Natl Cancer Inst 2019;111(10):1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.St Helen G, Dempsey D, Wilson M, et al. Racial differences in the relationship between tobacco dependence and nicotine and carcinogen exposure. Addiction 2013;108(3):607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acevedo A, Panas L, Garnick D, et al. Disparities in the treatment of substance use disorders: does where you live matter? J Behav Health Serv Res 2018;45(4):533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saloner B, Lê Cook B. Blacks and Hispanics are less likely than whites to complete addiction treatment, largely due to socioeconomic factors. Health Aff (Millwood) 2013;32(1):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care; Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 93.van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1–2):23–35. [DOI] [PubMed] [Google Scholar]
- 94.Ehrenstein V, Kharrazi H, Lehmann H, et al. Obtaining data from electronic health records, Chapter 4. In: Gliklich RE, Leavy MB, Dreyer NA, eds. Tools and Technologies for Registry Interoperability, Registries for Evaluating Patient Outcomes: A User’s Guide 3rd ed. Rockville, MD: Agency for Healthcare Research and Quality; 2019. Available at https://www.ncbi.nlm.nih.gov/books/NBK551878/. Accessed September 20, 2021. [Google Scholar]
- 95.Klinger EV, Carlini SV, Gonzalez I, et al. Accuracy of race, ethnicity, and language preference in an electronic health record. J Gen Intern Med 2015;30:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson EM, Chen A, Johnson MP, et al. Elucidating measures of systemic racism to mitigate racial disparities in kidney transplantation. Curr Opin Organ Transplant 2021;26(5):554–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.


