Abstract
Background
Community‐acquired Pseudomonas aeruginosa pneumonia in immunocompetent children is a rare occurrence.
Methods
A retrospective analysis of the clinical manifestations, imaging characteristics, laboratory examinations, and treatment of a child with community‐acquired Pseudomonas aeruginosa pneumonia presented with bloody pleural effusion.
Results
The 1‐year‐old previously healthy patient, who developed community‐acquired pneumonia caused by Pseudomonas aeruginosa and influenza virus. The patient manifested bloody pleural effusion although his condition improved after anti‐infective therapy and closed thoracic drainage. After 10 days of hospitalization, his symptoms worsened, accompanied by hemoptysis, and the pathogen developed resistance to carbapenems. The antibiotic strategy was adjusted to combined antipseudomonal regimen. He developed low‐grade fever and was extubated, although these manifestations and imaging were eventually alleviated.
Conclusions
Community‐acquired Pseudomonas aeruginosa pneumonia in children may be non‐septic, with bloody pleural effusion as presentation, and the disease may progress after 10 days of treatment due to drug resistance in Pseudomonas aeruginosa. Early extubation should be considered after adequate drainage.
Keywords: case report, multidrug‐resistant, pleural effusion, pneumonia, Pseudomonas aeruginosa
A: Presence of pneumonia with pleural effusion on chest radiograph. B: Consolidation in the middle and lower lobes of the right lung accompanied by pleural effusion on chest CT. C: Thick‐walled cavities on chest CT. D: Chest radiograph after extubation
1. BACKGROUND
Pseudomonas aeruginosa (PA) is an opportunistic pathogen whose nosocomial infection causes pneumonia. Community‐acquired Pseudomonas aeruginosa pneumonia (P. aeruginosa CAP) in immunocompetent patients is a rare occurrence. Here, we report a case of P. aeruginosa CAP in a previously healthy infant, who exhibited bloody pleural effusion as the main manifestation. To our knowledge, this is the first report in English literature on community‐acquired pneumonia (CAP) and empyema caused by PA in immunocompetent children.
2. CASE PRESENTATION
A 1‐year‐old boy, with a 5‐day history of coughing and wheezing, was admitted to the respiratory department of Tianjin Children's Hospital on December 25, 2019. He was administered with "Cefaclor" and nebulizing "Budesonide, Terbutaline," after which his symptoms not only worsened but also he developed fever and dyspnea for 18 h. Physical examination revealed a body temperature of 38.8°C, pulse and respiratory rates of 176 beats/min and 65 breaths/min, respectively, a blood pressure of 88/52 mmHg, and oxygen saturation of 95% under oxygen inhalation. We found no evidence of petechiae on his skin, but scars from BCG vaccination were visible. Results from chest examination revealed reduced right lung breath sounds, fine moist rales and rhonchi in both lungs, while cardiac and abdominal examination revealed normal conditions, except for a slightly enlarged liver. The remainder of the past history, developmental history, family history, and vaccination history were normal. He had no known allergies to medications. A chest radiograph revealed dense opacities in the external zone of the right lung, suggesting pneumonia with pleural effusion (Figure 1A). His initial white blood cell (WBC) count was 41.78 × 109cells/L, and his neutrophil percentage was 78% with C‐reactive protein (CRP) 102 mg/L.
FIGURE 1.
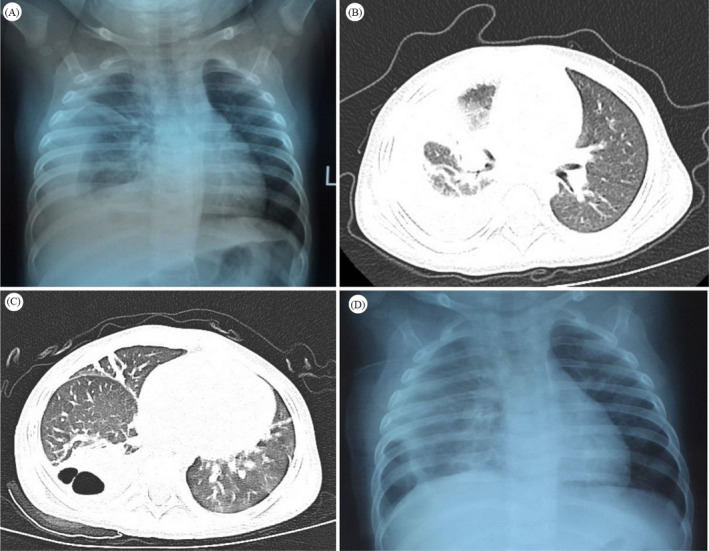
(A) Presence of high‐density strip shadow in the external zone of right lung, at admission. The right costophrenic angle and diaphragmatic surface were blurred. (B) Profile of the patient's lung at Day 1 of hospitalization. A patchy high‐density shadow and pulmonary consolidation were evident in the middle and lower lobes of the right lung with pleural effusion on Chest CT. (C) Day 11 of hospitalization showing consolidation of the lower lobe of the right lung with multiple thick‐walled cavities and a small amount of pleural effusion. (D) Day 22 of hospitalization showing reduced density of lesions in the lower lobe of the right lung and presence of pleural effusion. A marked thickened pleura was evident in the chest film
Considering the influenza epidemic in this region, he was given ceftriaxone sodium 80 mg/(kg.d) and oseltamivir according to the Chinese Guidelines for diagnosis and treatment of community‐acquired pneumonia in Children (2019 version). He was also administered with aerosol inhalation and other supportive treatments. Notably, the patient's hyperthermia, respiratory distress, and tachycardia were not relieved with anti‐infective therapy and additional β2‐agonist inhalation. Sputum cultures were collected and subjected to Gram staining. Results revealed Gram‐negative Bacilli and some neutrophils. Moreover, results from respiratory pathogen antigen test were positive for influenza A virus; thus, the antibiotic treatment was changed to meropenem 60 mg/(kg.d) with oseltamivir. Levels of electrolytes, glucose, albumin, and immunoglobulins in the serum were all normal. Similarly, analysis of liver and renal function indicated that they were all normal, except alanine aminotransferase (ALT) 186 U/L, aspartate aminotransferase (AST) 267 U/L, and lactate dehydrogenase (LDH) 567 U/L. Erythrocyte sedimentation rate was 74 mm/h, while procalcitonin (PCT) and lactate levels in the serum were 4.49 ng/ml and 4.20 mmol/L, respectively. Blood flow cytometry was normal, while HIV test returned negative results. Prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) were normal, while fibrinogen 3.6 g/L and D‐dimer 0.3 mg/L. Electrocardiogram and echocardiogram results were normal, while chest CT revealed consolidation in the middle and lower lobes of the right lung, which was accompanied by pleural effusion (Figure 1B). Thoracic puncture showed bloody pleural effusion (for details see Table 1). Cytology of the pleural fluid cells was normal, while neither antinuclear nor antineutrophilic cytoplasmic antibodies were detected in the serum. Tuberculin test and interferon‐γ release assays (IGRAs) were negative. Thoracic CT angiography (CTA) was normal. The child still had intermittent fever, after 3 days of hospitalization, and this was accompanied by shortness of breath and fine lung rales.
TABLE 1.
Results of pleural effusion
| Pleural effusion | Results |
|---|---|
| Rivalta test | Positive |
| Specific gravity | 1.030 |
| Total number of cells | 157,830/mm3 |
| WBC | 662/mm3, |
| Multinucleated cell | 452/mm3 |
| Mononuclear cell | 110/mm3 |
| Glucose | 2.2 mmol/L |
| Lactate dehydrogenase(LDH) | 8052 U/L |
| Adenylate deaminase (ADA) | 38 U/L |
| Protein | 35 g/L |
A summary of laboratory results is presented in Table 2. Briefly, there was evidence of PA in the sputum and pleural fluid cultures, while results of two blood culture tests revealed negative results. The patient was subsequently subjected to closed thoracic drainage and his condition steadily improved after 8 days of hospitalization. Drainage of pleural effusion and inflammatory biomarkers had remarkably decreased (for details see Table 3). On the 10th day of hospitalization, the child was febrile again with hemoptysis, and reexamination of the chest radiograph revealed consolidation with multiple irregular air‐filled cavities in the lower lobe of the right lung (Figure 1C). The antibiotics regime was adjusted to cefoperazone/sulbactam 240 mg/(kg.d) q6h combined with linezolid 30 mg/(kg.d) q8h. Bronchoscopy results revealed mucosal hyperemia and elevated inflammatory secretions in the bronchi of the right lower lobe. Meropenem‐resistant PA (Table 1) was cultured in bronchoalveolar lavage fluid (BALF), then subjected to acid‐fast staining and GM test. Results were all negative. Notably, the patient still had high fever, cough, lethargy, and loss of appetite, but no obvious signs of hypoxia and dyspnea. On Day 12, levofloxacin was added to the regimen and linezolid suspended, based on results from the drug‐sensitivity test. Immune support treatment, with gamma globulin (1 g/kg) was also given, and after 22 days of hospitalization, he developed low‐grade fever and his condition improved with better spirit and appetite. The drainage volume of pleural effusion is less than 10 ml per day, and the inflammatory indicators are generally normal (Table 3). The patient was extubated (Figure 1D). His temperature was normal after 25 days, and was discharged after 32 days of hospitalization. Three months after discharge, a CT‐based reexamination of the chest revealed a cord lesion in the lower lobe of his right lung.
TABLE 2.
Culture results and drug sensitivity test results of PA
| Sample type |
Sputum MIC (ug/ml) |
Pleural effusion MIC (ug/ml) |
BALF MIC (ug/ml) |
|---|---|---|---|
| Cefoperazone/sulbactam | ≤2 S | ≤2 S | ≤2 S |
| Ceftazidime | 4 S | 4 S | 4 S |
| Imipenem | 2 S | 2 S | ≥16 R |
| Meropenem | ≤0.25 S | ≤0.25 S | ≥16 R |
| Cefepime | ≤1 S | ≤1 S | ≤1 S |
| Amikacin | ≤2 S | ≤2 S | ≤2 S |
| Piperacillin/tazobactam | ≤4 S | ≤4 S | ≤4 S |
| Levofloxacin | 0.5 S | 0.5 S | 0.5 S |
| Ciprofloxacin | <0.25 S | <0.25 S | <0.25 S |
| Aztreonam | 4 S | 4 S | 4 S |
| Gentamycin | ≤2 S | 2 S | ≤1 S |
| Tobramycin | ≤1 S | ≤1 S | ≤1 S |
Abbreviations: MIC, minimum inhibitory concentration; R, resistance; S, sensitive.
TABLE 3.
Laboratory data
| Day of hospitalization |
WBC 109/L |
Neutrophil percentage |
CRP mg/L |
PCT ng/ML |
|---|---|---|---|---|
| Day1 | 41.78 | 78% | 102 | 4.49 |
| Day 3 | 40.53 | 79% | 73 | N |
| Day 7 | 13.5 | 51% | 8 | 1.2 |
| Day 10 | 30.37 | 55% | 7 | 2.23 |
| Day 13 | 21.33 | 65% | 95 | N |
| Day 16 | 16.85 | 65% | 82 | N |
| Day 22 | 6 | 23% | 2.5 | 0.05 |
Abbreviation: N, absence of test.
3. DISCUSSION
Pseudomonas aeruginosa is a common nosocomial pathogen that usually infects children with underlying medical conditions, such as immunodeficiency, premature birth, burns, or structural lung disease. Community‐acquired Pseudomonas aeruginosa sepsis, which is often accompanied by diarrhea, ecthyma gangrenosum, septic shock, low WBCs, and significantly increased CRP, is more common in children, where it has been associated with rapid progression and fatal outcomes. 1 Although P. aeruginosa CAP in immune competent adults is rare, a few clinical cases have been reported. Moreover, severe illnesses and poor clinical outcomes have been linked to PA infection in adults with CAP. 2 Influenza may be a risk factor for PA infection, as evidenced from some reports of PA co‐infection with influenza A (H1N1). 3 Notably, influenza infection contributes to dysfunction and death of respiratory epithelial cells, through induction of apoptosis, a phenomenon that exacerbates bacterial adherence and invasion. Furthermore, previous studies have linked viral infection to PA‐mediated down‐regulation of tissue resilience in lung inflammation. 4 In fact, several research evidences have demonstrated that onset of P. aeruginosa CAP in adult patients is related to the use of household humidifiers and hydrotherapy equipment. Consequently, PA has been isolated from suspected contaminated water samples and characterized, thereby ascertain that P. aeruginosa CAP is indeed linked to exposure to contaminated aerosols. 5 , 6 Unfortunately, we could not obtain nebulizer and environmental samples from the present patient.
The puncture fluid is bloody. In children, bloody pleural effusion is mainly caused by inflammation, tumor, connective tissue disease, pulmonary embolism, and vascular or lymphatic malformations. 7 The patient in the present study had no history of contact with tuberculosis, his pleural effusion ADA level was normal, while both tuberculin test and IGRAs were negative, which did not support diagnosis of tuberculous pleurisy. In addition, results from chest CTA did not support pulmonary embolism or vascular malformations, while cytological results of pleural effusion revealed no evidence of malignancy. Moreover, clinical and laboratory tests revealed no evidence of diseases in the connective tissue. The patient was an infant with rapid onset, obvious signs of infection, increased inflammatory indicators, neutrophils dominated pleural effusion and significantly increased LDH. Finally, pleural effusion culture clearly indicated PA empyema. Like ecthyma gangrenosum, the bloody pleural effusion caused by P. aeruginosa may be caused by the bacterial multiplication of vessels in the pleura followed by small‐vessel embolism. 1
After clearly identifying the pathogen involved, treatment strategy was switched to sensitive antibiotics and chest cavity drainage. However, we found evidence of rapid antimicrobial resistance to carbapenems. Cillóniz et al. 8 found that 32% of all adult patients with P. aeruginosa CAP exhibited multidrug‐resistance (MDR) strains, while antibiotic application was the only risk factor for MDR. Apart from carbapenem exposure, previous studies have shown that exposure to β‐lactams inactive against PA and medical devices are risk factors for carbapenem‐resistant Pseudomonas aeruginosa isolation. 9 The findings of Philippe et al. 10 indicated that the median time from exposure to effective antibiotics to isolation of MDR strains of PA was 11 days, which may explain the progression of PA pneumonia after 10 days of treatment observed herein. After confirming that PA was resistant to the initial drug, the treatment strategy was adjusted to cefoperazone/sulbactam plus levofloxacin according to drug sensitive test. Although combined therapy for PA infections has been associated with various benefits, including in vitro antibiotic synergy, and prevention of development of bacterial resistance, 11 the efficacy of P. aeruginosa CAP relative to monotherapy remains controversial. 12 Furthermore, the patient in the present study developed low‐grade fever after antibiotic adjustment, although this subsided following extubation of the drainage tube. Biofilm formation has also been implicated in antimicrobial resistance in PA in recent years. The biofilm is a complex aggregate of bacteria encased in a polysaccharide matrix and attached to the surfaces of implants, catheters or airways. 13 Therefore, PA empyema should be extubated early after adequate drainage to reduce susceptibility factors of drug resistance and facilitate effective pathogen elimination.
4. CONCLUSION
Pseudomonas aeruginosa CAP in infants may be non‐septic, with bloody pleural effusion as the clinical manifestation. Pseudomonas aeruginosa CAP development in previously healthy infants may be exacerbate by influenza infections and contaminated aerosols. Notably, disease progression and imaging are aggravated after 10 days of treatment, due to bacterial drug resistance, thus a combination therapy should be considered in drug‐resistant cases based on results from drug susceptibility tests. Moreover, early extubation should be considered for PA empyema under the premise of adequate drainage to suppress effect from susceptibility factors of drug resistance. Overall, P. aeruginosa CAP in children remains a rare occurrence; thus, further studies are needed to identify sources of infections, elucidate associated risk factors, and design optimal treatment therapies.
AUTHOR CONTRIBUTION
CD and FS were the main contributors to drafting the manuscript. YX and JN designed the treatment plan. HD analyzed and interpreted the patient data. YF and LD contributed to literature search and figure preparation. All authors have read and approved the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient's parent for the publication of this case report and any accompanying images.
ACKNOWLEDGEMENTS
The authors thank the patient for the participation.
Dong C, Shen F, Dong H, et al. Community‐acquired Pseudomonas aeruginosa pneumonia manifested by bloody pleural effusion in a previously healthy infant: A case report. J Clin Lab Anal. 2022;36:e24466. doi: 10.1002/jcla.24466
Chunjuan Dong and Fangfang Shen contributed equally to the work.
Contributor Information
Yongsheng Xu, Email: xxyyss@126.com.
Jing Ning, Email: tianjinnj@126.com.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. Huang YC, Lin TY, Wang CH. Community‐acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty‐three episodes. Pediatr Infect Dis J. 2002;11:1049‐1052. [DOI] [PubMed] [Google Scholar]
- 2. Wang T, Hou Y, Wang R. A case report of community‐acquired Pseudomonas aeruginosa pneumonia complicated with MODS in a previously healthy patient and related literature review. BMC Infect Dis. 2019;19(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin‐Loeches I, J Schultz M, Vincent J‐L, et al. Increased incidence of co‐infection in critically ill patients with influenza. Intensive Care Med. 2017;43:48‐58. [DOI] [PubMed] [Google Scholar]
- 4. Villeret B, Solhonne B, Straube M, et al. Influenza A virus pre‐infection exacerbates pseudomonas aeruginosa‐mediated lung damage through increased MMP‐9 expression, decreased elafin production and tissue resilience. Front Immunol. 2020;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woods E, Cohen G, Bressman E, et al. Community‐acquired cavitary pseudomonas pneumonia linked to use of a home humidifier. Case Rep Infect Dis. 2017;2017:5474916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferraz de Campos FP, Felipe‐Silva A, Lopes ACFMM, et al. Community‐acquired Pseudomonas aeruginosa‐pneumonia in a previously healthy man occupationally exposed to metalworking fluids. Autops Case Rep. 2014;4(3):31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, Li J, Zheng Y, Wang W. Twenty‐six cases of bloody pleural effusion in children: a case series report. Chin J Evid Based Pediatr. 2020;15(4):306‐310. [Google Scholar]
- 8. Cillóniz C, Gabarrús A, Ferrer M, et al. Community‐acquired pneumonia due to multidrug‐ and non‐multidrug‐resistant Pseudomonas aeruginosa . Chest. 2016;150(2):415‐425. [DOI] [PubMed] [Google Scholar]
- 9. Coppry M, Jeanne‐Leroyer C, Noize P, et al. Antibiotics associated with acquisition of carbapenem‐resistant Pseudomonas aeruginosa in ICUs: a multicentre nested case‐case‐control study. J Antimicrob Chemother. 2019;74(2):503‐510. [DOI] [PubMed] [Google Scholar]
- 10. Philippe E, Weiss M, Shultz JM, Yeomans F, Ehrenkranz NJ. Emergence of highly antibiotic‐resistant Pseudomonas aeruginosa in relation to duration of empirical antipseudomonal antibiotic treatment. Clin Perform Qual Health Care. 1999;7(2):83‐87. [PubMed] [Google Scholar]
- 11. Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug‐resistant and extensively drug‐resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4):e00031‐e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim YJ, Jun YH, Kim YR, et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis. 2014;14:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hadadi‐Fishani M, Khaledi A, Fatemi‐Nasab ZS. Correlation between biofilm formation and antibiotic resistance in Pseudomonas aeruginosa: a meta‐analysis. Infez Med. 2020;28(1):47‐54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


