Abstract
Background
Sepsis is a highly life‐threatening disease. Long non‐coding RNA urothelial carcinoma associated 1 (lncRNA UCA1) participates in the processes of inflammation and organ injury in several diseases, whereas its role in sepsis patients is still unclear. The aim was to explore the clinical value of lncRNA UCA1 in sepsis patients.
Methods
One hundred seventy‐four sepsis patients and 100 age and gender‐matched controls were enrolled. LncRNA UCA1 in peripheral blood mononuclear cell samples was examined, and the level of inflammatory cytokines in serum samples was assessed.
Results
LncRNA UCA1 was highly expressed in sepsis patients compared with controls. LncRNA UCA1 was positively correlated with tumor necrosis factor‐α, interleukin (IL)‐6, IL‐17, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in sepsis patients, while it was not correlated with these inflammatory cytokines in controls. lncRNA UCA1 upregulation was related to raised APACHE II score and SOFA score in sepsis patients. Moreover, lncRNA UCA1 was increased in sepsis deaths compared with sepsis survivors and was independently correlated with increased 28‐day sepsis mortality risk. Further receiver operating characteristic curves presented that lncRNA UCA1 had a good value to predict 28‐motality risk, while its combination with other independent factors (including age, history of chronic kidney disease, G+ bacterial infection, Fungus infection, C‐reactive protein, and APACHE II score) exerted a great predictive value for 28‐day mortality risk.
Conclusion
LncRNA UCA1 is upregulated and correlates with multiple pro‐inflammatory cytokines, terrible disease severity, and poor prognosis in sepsis patients.
Keywords: 28‐day mortality risk, disease severity, inflammatory cytokines, LncRNA UCA1, sepsis
LncRNA UCA1 in peripheral blood mononuclear cell samples and inflammatory cytokines in serum samples from 174 sepsis patients and 100 age and gender matched controls were assessed. LncRNA UCA1 was highly expressed in sepsis patients compared to controls. LncRNA UCA1 was positively correlated with tumor necrosis factor‐α, interleukin (IL)‐6, IL‐17, intercellular adhesion molecule 1, vascular cell adhesion molecule 1, APACHE II score and SOFA score in sepsis patients. LncRNA UCA1 was increased in sepsis deaths compared to sepsis survivors, and was independently correlated with increased 28‐day sepsis mortality risk. Further receiver operating characteristic curves presented that lncRNA UCA1 had a good value to predict 28‐motality risk, while its combination with other independent factors (including age, history of chronic kidney disease, G+ bacterial infection, Fungus infection, C‐reactive protein and APACHE II score) exerted a great predictive value for 28‐day mortality risk.
![]()
1. INTRODUCTION
Sepsis is a highly heterogeneous clinical disorder featured by a series of symptoms attributed to a bacterial, viral, or fungal infection, and it relates to a systemic inflammatory response syndrome (SIRS) (such as shiver, fever, and decreased vascular resistance), thereby causing hypotension, organ failure, or even death. 1 The incidence of sepsis is high, and about half of the critically ill patients are presented as sepsis in China. 2 In spite of a long history of disease management (including a mass of antibiotics and striving to follow the Surviving Sepsis Campaign (SSC) guideline), the treatment outcomes of sepsis are still disappointing. 3 , 4 In brief, no magic intervention exists for sepsis until now. Hence, it is important to explore potential biomarkers to accurately monitor disease progression, so that improving the prognosis in sepsis patients as soon as possible.
Long non‐coding RNA (lncRNA) are becoming promising biomarker candidates in various diseases (including sepsis, inflammatory bowel disease, and ulcerative colitis). 5 , 6 , 7 LncRNA urothelial carcinoma associated 1 (lncRNA UCA1) is one of the pivotal lncRNAs initially identified in bladder carcinoma with location at chromosome 19p13.12, which exerts great effects on tumorigenesis; it also plays an important role in inflammation processes. 8 , 9 , 10 From existing data, lncRNA UCA1 knockdown prevents the induction of interleukin‐6 (IL‐6), intercellular adhesion molecule 1 (ICAM1), and vascular cell adhesion molecule 1 (VCAM1), suggesting lncRNA UCA1 severs as a promoter to regulate pro‐inflammatory mediators in sepsis. 11 Meanwhile, the increase in inflammation levels and adhesion factors facilitates inflammatory permeability of vascular endothelial cells, and then transmits inflammatory factors to various organs, eventually inducing multiple organ injury in sepsis. 12 For instance, the impeding of ICAM‐1 could attenuate the sepsis‐induced lung injury. 13 Considering above mentioned, we hypothesized that lncRNA UCA1 acted as a key role in sepsis patients, while the relevant information is unclear. In the current study, we aimed to explore the clinical value of lncRNA UCA1 in sepsis patient's management.
2. METHODS
2.1. Participants
The application of all procedures was from the Institutional Review Board of our hospital. In this study, 174 sepsis patients were consecutively enrolled from our hospital between January 2017 and December 2019. The inclusion criteria of sepsis patients were as follows: (i) diagnosed as sepsis referring to the Third International Consensus Definitions for Sepsis and Septic Shock 14 ; (ii) aged 18–80 years old; and (iii) admitted into our department within 12 h after onset of sepsis symptom. The patients who infected with human immunodeficiency virus, complicated with hematologic malignancies, or received immunosuppressive therapy within the last one month were excluded. The pregnant or lactational patients were excluded as well. In addition, 100 controls whose age (set as 18–80 years) and gender (set as female: male = 1:2) matched with sepsis patients were enrolled from Health Examination Center between November 2019 and January 2020. Written informed consents were gotten from the participants or their legal representatives. This study was approved by the Ethics Committee.
2.2. Data collection
The demographics, medical history, biochemical indexes, and inflammatory cytokines of all the participants were recorded after enrollment. Apart from those data, the following characteristics of sepsis patients were documented as well: (1) abdominal, respiratory, skin and soft tissue, bloodstream, and other infections; (2) primary infection organism species (such as G‐ bacteria, G+ bacteria, fungus, and others); (3) acute physiology and chronic health evaluation II (APACHE II) score and sequential organ failure assessment (SOFA) score (within 24 h after admission); and (4) follow‐up data: daily follow‐up to death or 28 days after admission. After the 28‐day follow‐up, the survival status of sepsis patients made them divided into survivors and deaths.
2.3. Sample collection and processing
After admission, patients’ initial peripheral blood samples (2 ml) were drawn as soon as the diagnosis of sepsis was made clinically. After collection, a part of the peripheral blood samples was immediately processed by gradient density centrifugation to isolate peripheral blood mononuclear cell, and another part was centrifuged to separate serum samples. The peripheral blood mononuclear cell and serum samples of controls were separated from peripheral blood samples using the same method described above. Then, the peripheral blood mononuclear cell and serum samples were preserved at −80℃.
2.4. Reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR)
RT‐qPCR was utilized to assess the level of lncRNA UCA1 in peripheral blood mononuclear cell samples. RNeasy Protect Mini Kit (Qiagen) was utilized for the extraction of total RNA. RNA was quantified by NanoDrop 2000 (Thermo). Subsequently, total RNA from each sample was used for cDNA synthesis using PrimeScript™ RT reagent Kit (Perfect Real Time) (Takara). The qPCR was carried out by THUNDERBIRD® SYBR® qPCR Mix (Toyobo). The qPCR amplification was carried out: 95 °C for 5 min, followed by 40 cycles of 95°C for 5 s, and 61°C for 30 s. The primers used for amplification were designed referring to a pervious study. 11 , 15 LncRNA UCA1 forward: 5′‐CATGCTTGACACTTGGTGCC‐3′, reverse: 5′‐GGTCGCAGGTGGATCTCTTC‐3′; glyceraldehyde‐phosphate dehydrogenase (GAPDH) forward: 5′‐GCCAAAAGGGTCATCATCTC‐3′; and reverse: 5′‐GGCCATCCACAGTCTTCT‐3′. GAPDH was deemed to be reference gene.
2.5. Enzyme‐linked immunosorbent assay (ELISA)
ELISA was utilized by evaluating the level of inflammatory cytokines in serum samples. The ELISA kits used in this study were as follows: human tumor necrosis factor (TNF) ‐α ELISA kit (Thermo Fisher Scientific), human IL‐6 ELISA kit (Thermo Fisher Scientific), human IL‐17 ELISA kit (Thermo Fisher Scientific), human ICAM1 ELISA kit (extra sensitive) (Thermo Fisher Scientific), and human VCAM1 ELISA kit (Thermo Fisher Scientific).
2.6. Statistics
Comparison of clinical characteristics and lncRNA UCA1 in sepsis patients and controls was assessed through the methods of chi‐squared test, Student's t‐test, or Wilcoxon rank sum test. Comparison of lncRNA UCA1 between groups was analyzed by Wilcoxon rank sum test. Correlation of lncRNA UCA1 with inflammatory cytokine in both groups was detected by Spearman's rank correlation test. Correlation of lncRNA UCA1 with sepsis severity was also determined by Spearman's rank correlation test. Receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was used to assess the ability of variables in distinguishing sepsis patients from controls. Risk factors correlated with 28‐day mortality in sepsis patients were analyzed using univariate logistic regression model, and the independent risk factors were further analyzed by forward stepwise multivariate logistic regression model. P value <0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical features
The demographics and medical history were similar in sepsis patients and controls (All p > 0.05), whereas biochemical indexes and inflammatory cytokines were deregulated in sepsis patients compared with controls (All p < 0.001). In sepsis patients, there were 55 (31.6%), 50 (28.7%), 35 (20.1%), 22 (12.6%), and 12 (6.9%) patients with primary infection site at abdominal infection, respiratory infection, skin and soft tissue infection, bloodstream infection, and other infections, respectively. In terms of disease severity, APACHE II score and SOFA score were 12.0 (7.0–16.0) and 5.0 (4.0–7.3), respectively. Other clinical characteristics’ detailed information is presented in Table 1.
TABLE 1.
Clinical characteristics
| Items |
Controls (N = 100) |
Sepsis patients (N = 174) | p value |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 53.9 ± 11.9 | 55.2 ± 12.5 | 0.416 |
| Gender, No. (%) | |||
| Female | 36 (36.0) | 62 (35.6) | 0.951 |
| Male | 64 (64.0) | 112 (64.4) | |
| BMI, (kg/m2), mean ± SD | 22.7±3.0 | 22.8±2.6 | 0.799 |
| Smoke, No. (%) | 28 (28.0) | 60 (34.5) | 0.269 |
| Drink, No. (%) | 45 (45.0) | 71 (40.8) | 0.499 |
| Medical history | |||
| Hypertension, No. (%) | 32 (32.0) | 62 (35.6) | 0.542 |
| Hyperlipidemia, No. (%) | 14 (14.0) | 31 (17.8) | 0.412 |
| Diabetes, No. (%) | 10 (10.0) | 23 (13.2) | 0.431 |
| CKD, No. (%) | 8 (8.0) | 16 (9.2) | 0.736 |
| CCVDs, No. (%) | 14 (14.0) | 33 (19.0) | 0.294 |
| Biochemical index | |||
| Scr (mg/dl), median (IQR) | 0.8 (0.7–1.0) | 1.4 (1.0–2.2) | <0.001 |
| Albumin (g/L), median (IQR) | 42.8 (39.1–47.4) | 27.6 (21.6–39.9) | <0.001 |
| WBC (*109/L), median (IQR) | 6.4 (5.4–7.5) | 15.1 (11.4–26.1) | <0.001 |
| CRP (mg/L), median (IQR) | 3.7 (2.5–6.4) | 96.2 (46.9–133.1) | <0.001 |
| Inflammatory cytokine | |||
| TNF‐α (pg/ml), median (IQR) | 33.0 (28.2–38.2) | 145.1 (112.4–226.7) | <0.001 |
| IL−6 (pg/ml), median (IQR) | 15.3 (11.2–22.3) | 55.5 (38.7–78.2) | <0.001 |
| IL−17 (pg/ml), median (IQR) | 31.9 (22.3–44.2) | 141.2 (84.1–230.1) | <0.001 |
| ICAM1 (pg/ml), median (IQR) | 205.4 (138.7–294.3) | 557.7 (448.5–727.3) | <0.001 |
| VCAM1 (pg/ml), median (IQR) | 749.0 (468.9–1006.4) | 1868.8 (1373.6–2478.7) | <0.001 |
| Primary infection site | |||
| Abdominal infection, No. (%) | ‐ | 55 (31.6) | ‐ |
| Respiratory infection, No. (%) | ‐ | 50 (28.7) | ‐ |
| Skin and soft tissue infection, No. (%) | ‐ | 35 (20.1) | ‐ |
| Bloodstream infection, No. (%) | ‐ | 22 (12.6) | ‐ |
| Other infections, No. (%) | ‐ | 12 (6.9) | ‐ |
| Primary organism | |||
| G‐ bacteria, No. (%) | ‐ | 101 (58.0) | ‐ |
| G+ bacteria, No. (%) | ‐ | 60 (34.5) | ‐ |
| Fungus, No. (%) | ‐ | 21 (12.1) | ‐ |
| Others, No. (%) | ‐ | 42 (24.1) | ‐ |
| Disease severity | |||
| APACHE II score, median (IQR) | ‐ | 12.0 (7.0–16.0) | ‐ |
| SOFA score, median (IQR) | ‐ | 5.0 (4.0–7.3) | ‐ |
Abbreviations: SD, standard deviation; BMI, body mass index; CKD, chronic kidney disease; CCVDs, cardio‐cerebrovascular diseases; Scr, serum creatinine; IQR, interquartile range; WBC, white blood cell; CRP, C‐reactive protein; TNF, tumor necrosis factor; IL, interleukin; ICAM1, intercellular adhesion molecule 1; VCAM1, vascular cell adhesion molecule 1; G‐, gram negative; G+, gram positive; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment.
3.2. LncRNA UCA1 expression
The median value of lncRNA UCA1 expression in sepsis patients and controls was 2.802 (2.153–3.780) and 0.999 (0.666–1.420), respectively. Compared with controls, lncRNA UCA1 was greatly increased in sepsis patients (p < 0.001) (Figure 1).
FIGURE 1.
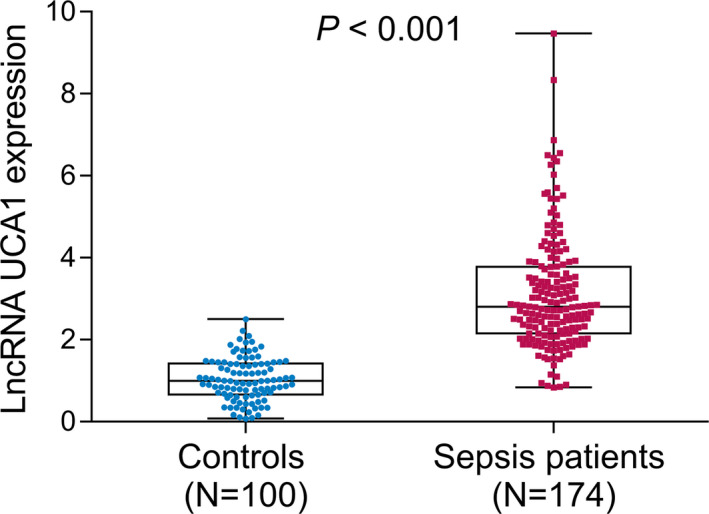
LncRNA UCA1 expression in sepsis patients and controls
3.3. Correlation of lncRNA UCA1 expression with inflammatory cytokines and disease severity
As to sepsis patients, lncRNA UCA1 was positively correlated with TNF‐α (p < 0.001) (Figure 2A), IL‐6 (p < 0.001) (Figure 2B), IL‐17 (p < 0.001) (Figure 2C), ICAM1 (p < 0.001) (Figure 2D), and VCAM1 (p < 0.001) (Figure 2E). Besides, lncRNA UCA1 upregulation was related to raised APACHE II score (p < 0.001) (Figure 2F). Meanwhile, its overexpression was also related to raised SOFA score (p < 0.001) (Figure 2G), whereas, as for controls, there was no correlation of lncRNA UCA1 with TNF‐α (p = 0.188) (Figure 2H), IL‐6 (p = 0.400) (Figure 2I), IL‐17 (p = 0.360) (Figure 2J), ICAM1 (p = 0.603) (Figure 2K), and VCAM1(p = 0.935) (Figure 2L).
FIGURE 2.
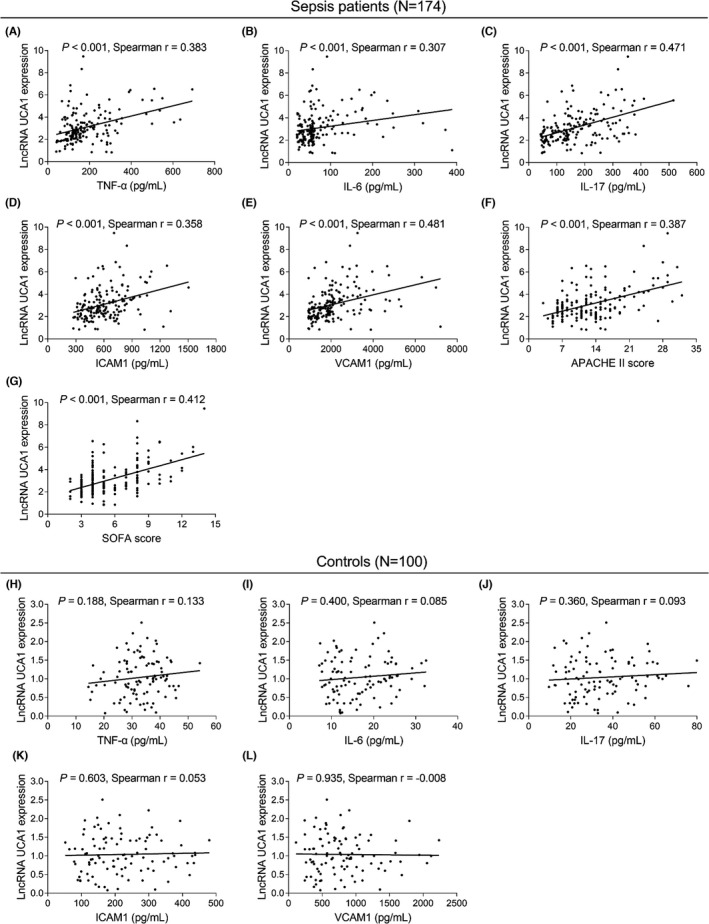
Relationship of lncRNA UCA1 with inflammatory cytokines and disease severity in sepsis patients and controls. Relationship of lncRNA UCA1 with TNF‐α (A), IL‐6 (B), IL‐17 (C), ICAM1 (D), VCAM1 (E), APACHE II score (F), and SOFA score (G) in sepsis patients. Relationship of lncRNA UCA1 with TNF‐α (H), IL‐6 (I), IL‐17 (J), ICAM1 (K), and VCAM1 (L) in controls
3.4. Factor affecting the survival of sepsis patients
Univariate logistic regression model analysis showed that lncRNA UCA1 (p < 0.001), age (p = 0.026), history of hypertension (p = 0.018), history of hyperlipidemia (p = 0.028), history of diabetes (p < 0.001), history of CCVDs (p = 0.004), fungus infection (p = 0.010), Scr (p = 0.046), WBC (p = 0.037), CRP (p < 0.001), APACHE II score (p < 0.001), SOFA score (p < 0.001), TNF‐α (p = 0.001), IL‐6 (p = 0.008), IL‐17 (p < 0.001), ICAM1 (p = 0.003), and VCAM1 (p = 0.001) were correlated with increased risk of 28‐day mortality (Table 2). Multivariate logistic regression model analysis showed that lncRNA UCA1 (p = 0.004) was an independent factor for increased 28‐day mortality risk. Meanwhile, age (p = 0.001), CKD history (p = 0.006), G+ bacterial infection (p = 0.015), fungus infection (p = 0.003), CRP (p = 0.003), and APACHE II score (p = 0.001) also could independently predict raised risk of 28‐day mortality (Table 3).
TABLE 2.
Univariate logistic regression model analysis of factors correlated with 28‐day mortality
| Items | Univariate logistic regression model | |||
|---|---|---|---|---|
| p value | OR | 95% CI | ||
| Lower | Higher | |||
| LncRNA UCA1 | <0.001 | 2.186 | 1.600 | 2.987 |
| Age | 0.026 | 1.035 | 1.004 | 1.067 |
| Male | 0.746 | 1.136 | 0.524 | 2.467 |
| BMI | 0.873 | 1.012 | 0.876 | 1.169 |
| Smoke | 0.344 | 0.677 | 0.302 | 1.518 |
| Drink | 0.103 | 1.848 | 0.882 | 3.872 |
| History of hypertension | 0.018 | 2.469 | 1.170 | 5.211 |
| History of hyperlipidemia | 0.028 | 2.596 | 1.106 | 6.092 |
| History of diabetes | <0.001 | 11.607 | 4.382 | 30.743 |
| History of CKD | 0.090 | 2.560 | 0.863 | 7.594 |
| History of CCVDs | 0.004 | 3.335 | 1.456 | 7.640 |
| Primary infection site | ||||
| Other infections | Reference | ‐ | ‐ | ‐ |
| Abdominal infection | 0.590 | 0.667 | 0.152 | 2.915 |
| Respiratory infection | 0.834 | 1.167 | 0.275 | 4.949 |
| Skin and soft tissue infection | 0.553 | 0.621 | 0.129 | 2.998 |
| Bloodstream infection | 0.412 | 0.474 | 0.079 | 2.826 |
| G‐ bacterial infection | 0.676 | 1.174 | 0.554 | 2.485 |
| G+ bacterial infection | 0.161 | 1.709 | 0.808 | 3.613 |
| Fungus infection | 0.010 | 3.500 | 1.341 | 9.132 |
| Scr | 0.046 | 1.299 | 1.005 | 1.680 |
| Albumin | 0.961 | 0.999 | 0.967 | 1.033 |
| WBC | 0.037 | 1.038 | 1.002 | 1.074 |
| CRP | <0.001 | 1.019 | 1.012 | 1.025 |
| APACHE II score | <0.001 | 1.232 | 1.146 | 1.326 |
| SOFA score | <0.001 | 1.538 | 1.306 | 1.812 |
| TNF‐α | 0.001 | 1.005 | 1.002 | 1.008 |
| IL−6 | 0.008 | 1.007 | 1.002 | 1.012 |
| IL−17 | <0.001 | 1.008 | 1.004 | 1.012 |
| ICAM1 | 0.003 | 1.002 | 1.001 | 1.004 |
| VCAM1 | 0.001 | 1.001 | 1.000 | 1.001 |
Abbreviations: OR, odds ratio; CI, confidence interval; lncRNA UCA1, long non‐coding RNA urothelial carcinoma associated 1; BMI, body mass index; CKD, chronic kidney disease; CCVDs, cardio‐cerebrovascular diseases; G‐, gram negative; G+, gram positive; Scr, serum creatinine; WBC, white blood cell; CRP, C‐reactive protein; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; TNF, tumor necrosis factor; IL, interleukin; ICAM1, intercellular adhesion molecule 1; VCAM1, vascular cell adhesion molecule 1.
TABLE 3.
Multivariate logistic regression model analysis of factors independently correlated with 28‐day mortality
| Items | Forward stepwise multivariate logistic regression model | |||
|---|---|---|---|---|
| p value | OR | 95% CI | ||
| Lower | Higher | |||
| LncRNA UCA1 | 0.004 | 2.251 | 1.300 | 3.898 |
| Age | 0.001 | 1.142 | 1.053 | 1.238 |
| History of CKD | 0.006 | 21.259 | 2.397 | 188.546 |
| G+ bacterial infection | 0.015 | 6.017 | 1.420 | 25.488 |
| Fungus infection | 0.003 | 21.334 | 2.767 | 164.513 |
| CRP | 0.003 | 1.015 | 1.005 | 1.026 |
| APACHE II score | 0.001 | 1.288 | 1.115 | 1.486 |
Abbreviations: OR, odds ratio; CI, confidence interval; lncRNA UCA1, long non‐coding RNA urothelial carcinoma associated 1; BMI, body mass index; CKD, chronic kidney disease; G+, gram positive; CRP, C‐reactive protein; APACHE II, acute physiology and chronic health evaluation II.
3.5. The comparation of lncRNA UCA1 between survivor and deaths
In sepsis patients, the median value of lncRNA UCA1 expression in survivor and deaths was 2.619 (2.028–3.343) and 4.191 (2.728–5.550), respectively. Compared with survivors, lncRNA UCA1 was increased in deaths (p < 0.001) (Figure 3A).
FIGURE 3.
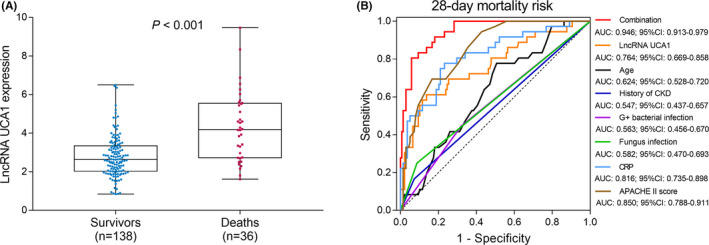
Ability of lncRNA UCA1 expression in estimating 28‐day mortality among sepsis patients. Correlation of lncRNA UCA1 expression with 28‐day mortality among sepsis patients (A); ROC curves for independent factors predicting 28‐day mortality risk. The regression equation: .
In order to further assess the predictive value of these independent factors (from the above multivariate logistic regression model analysis) for 28‐day mortality, we performed ROC curves. LncRNA UCA1 (AUC: 0.764; 95% CI: 0.669–0.858), CRP (AUC: 0.816; 95% CI: 0.735–0.898) and APACHE II score (AUC: 0.850; 95% CI: 0.788–0.911) had good values for predicting 28‐day mortality risk, whereas age (AUC: 0.624; 95% CI: 0.528–0.720), history of CKD (AUC: 0.547; 95% CI: 0.437–0.657), G+ bacterial infection (AUC: 0.563; 95% CI: 0.456–0.670), and fungus infection (AUC: 0.582; 95% CI: 0.470–0.693) could slightly predict 28‐day mortality risk. The combination of these independent factors presented with a great value for predicting 28‐day mortality risk (AUC: 0.946; 95% CI: 0.913–0.979) (Figure 3B).
4. DISCUSSION
Despite of lncRNA UCA1 as a promotor in cancer patients, only a few studies report its role in sepsis patients. 11 , 16 Considering that the impact of lncRNA UCA1 on inflammation and organ injury in several diseases, particularly, its impact on promoting inflammation in sepsis, we hypothesized that lncRNA UCA1 also played a vital role in sepsis patients. In our study, we found that lncRNA UCA1 was increased in sepsis patients, which might be caused by that lncRNA UCA1 could mediate several genes or pathways (e.g., miR‐143 17 and AKT/ mTOR pathways 18 ) to promote inflammation outburst and organ injury in sepsis; thus, lncRNA UCA1 was highly expressed in sepsis patients compared with controls. Besides, we also discovered that lncRNA UCA1 was associated with pro‐inflammatory factors and accelerated sepsis development. The probable explanations were as follows: Firstly, lncRNA UCA1 directly regulated various pathways (including PI3K/ Akt pathway) to increase the secretion of pro‐inflammatory factors (e.g., VCAM1 and ICAM1), subsequently facilitated inflammatory permeability of vascular endothelial cells, thereby transmitted inflammatory factors to various organs, eventually induced multiple organ injury in sepsis. 11 Hence, lncRNA UCA1 was associated with worse clinical features in sepsis patients. Secondly, lncRNA UCA1 also involved in the promotion of organ injury through regulating multiple pathways (such as AKT/ mTOR pathway 18 and JNK/ p38MAPK 18 ), thereby correlated with worse clinical features in sepsis patients.
In terms of the prognostic value of lncRNA UCA1 in sepsis patients, no evidence is found. In this study, our results showed that lncRNA UCA1 was an independent factor for increased 28‐day mortality risk. The possible reasons were as follows: Firstly, lncRNA UCA1 directly regulated several miRNAs and pathways (such as miR‐122 18 and PI3K)/ Akt 19 ) to cause severe inflammation and worse disease conditions (such as multiple organ dysfuctions), which subsequently increased 28‐day mortality risk in sepsis patients. Secondly, lncRNA UCA1 might related to worse clinical features, thereby indirectly led to increased 28‐day mortality risk (abovementioned). Besides, another finding from our study showed that G+ bacteria and fungus infection were independent prognostic factor for septic mortality, which was similar with previous study. 20 This finding revealed that the sepsis patients caused by the G+ bacteria and fungus infection should get more attention from the clinicians to improve their survival profile. Apart from that, this study also found that the combination of these factors showed excellent ability in predicting the 28‐day mortality risk, and its AUC even reached 0.946, which was even higher than the APACHE II score (AUC: 0.850). This was meaningful for the clinical evaluation of 28‐day mortality risk for sepsis patients. However, many efforts (determining the lncRNA UCA1 expression and calculating APACHE II score, etc.) should be carried out to calculate such a score, which might decrease its utility in clinical practice. Therefore, further study to explore a more feasible way with a high ability to predict the 28‐day mortality risk of sepsis patients was needed.
Despite of interesting findings, several limitations still existed. One limitation was relatively small sample size to lead to poor statistical power. Hence, further study with a larger sample size is necessary. Another limitation was that all patients enrolled were just from our hospital, which might cause the selection bias. Therefore, further multicenter study is urgent needed. Besides, although we revealed the correlation of lncRNA UCA1 with worse disease severity and poor prognosis in sepsis patients, its underlying mechanism in sepsis still remains to be thoroughly clarified. Thus, further functional study is needed.
To sum up, lncRNA UCA1 is upregulated and correlates with multiple pro‐inflammatory cytokines, grievous disease severity, and terrible prognosis, which implies that the lncRNA UCA1 might serve as a biomarker in monitoring the progression and prognostication of sepsis patients.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENT
None.
Wang J, Feng Q, Wu Y, Wang H. Involvement of blood lncRNA UCA1 in sepsis development and prognosis, and its correlation with multiple inflammatory cytokines. J Clin Lab Anal. 2022;36:e24392. doi: 10.1002/jcla.24392
Jingmei Wang and Qiang Feng contributed equally to this work.
Contributor Information
Yiping Wu, Email: baoping172626@163.com, Email: kanaydgbfb859945@163.com.
Haiyan Wang, Email: baoping172626@163.com, Email: kanaydgbfb859945@163.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long‐term outcome. Immunol Rev. 2016;274(1):330‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang M, Jiang L, Zhu B, et al. The prevalence, risk factors, and outcomes of sepsis in critically Ill patients in China: a multicenter prospective cohort study. Front Med (Lausanne). 2020;7:593808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho SY, Choi JH. Biomarkers of sepsis. Infect Chemother. 2014;46(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gul F, Arslantas MK, Cinel I, Kumar A. Changing definitions of sepsis. Turk J Anaesthesiol Reanim. 2017;45(3):129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai Y, Liang Z, Li Y, Li C, Chen L. Circulating long noncoding RNAs as potential biomarkers of sepsis: a preliminary study. Genet Test Mol Biomarkers. 2017;21(11):649‐657. [DOI] [PubMed] [Google Scholar]
- 6. Lin L, Zhou G, Chen P, et al. Which long noncoding RNAs and circular RNAs contribute to inflammatory bowel disease? Cell Death Dis. 2020;11(6):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Padua D, Mahurkar‐Joshi S, Law IK, et al. A long noncoding RNA signature for ulcerative colitis identifies IFNG‐AS1 as an enhancer of inflammation. Am J Physiol Gastrointest Liver Physiol. 2016;311(3):G446‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Feng W, Gu S, et al. The UCA1/KRAS axis promotes human pancreatic ductal adenocarcinoma stem cell properties and tumor growth. Am J Cancer Res. 2019;9(3):496‐510. [PMC free article] [PubMed] [Google Scholar]
- 9. Yu Q, Zhao MW, Yang P. LncRNA UCA1 suppresses the inflammation via modulating miR‐203‐mediated regulation of MEF2C/NF‐kappaB signaling pathway in epilepsy. Neurochem Res. 2020;45(4):783‐795. [DOI] [PubMed] [Google Scholar]
- 10. Gong P, Qiao F, Wu H, et al. LncRNA UCA1 promotes tumor metastasis by inducing miR‐203/ZEB2 axis in gastric cancer. Cell Death Dis. 2018;9(12):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y, Fu Y, Song YF, Li N. Increased expression of lncRNA UCA1 and HULC is required for pro‐inflammatory response during LPS induced sepsis in endothelial cells. Front Physiol. 2019;10:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao S, Cui L, Zheng X, Ji Y, Yu C. Meloxicam alleviates sepsis‐induced kidney injury by suppression of inflammation and apoptosis via upregulating GPNMB. Appl Bionics Biomech. 2022;2022:1790104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Yang J, Tian H, Huang X. Tephrosin attenuates sepsis induced acute lung injury in rats by impeding expression of ICAM‐1 and MIP‐2. Microb Pathog. 2018;117:93‐99. [DOI] [PubMed] [Google Scholar]
- 14. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2‐DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR‐ABL P210 transcripts. Diagn Mol Pathol. 2006;15(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Tang X, Pan L, Li Y, Li J, Li C. Elevated lncRNA‐UCA1 upregulates EZH2 to promote inflammatory response in sepsis‐induced pneumonia via inhibiting HOXA1. Carcinogenesis. 2022. doi: 10.1093/carcin/bgac004. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 17. Yu SY, Dong B, Zhou SH, Tang L. LncRNA UCA1 modulates cardiomyocyte apoptosis by targeting miR‐143 in myocardial ischemia‐reperfusion injury. Int J Cardiol. 2017;247:31. [DOI] [PubMed] [Google Scholar]
- 18. Zhu Y, Chen X, Zheng C, Rao X, Peng X. Down‐regulation of LncRNA UCA1 alleviates liver injury in rats with liver cirrhosis. Int J Clin Exp Pathol. 2019;12(2):455‐465. [PMC free article] [PubMed] [Google Scholar]
- 19. Cai L, Tu L, Li T, et al. Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson's disease through the inhibition of the PI3K/Akt signaling pathway. Int Immunopharmacol. 2019;75:105734. [DOI] [PubMed] [Google Scholar]
- 20. He X, Liao X, Xie Z, Jiang C, Kang Y. Albumin corrected anion gap is an independent risk factor for long‐term mortality of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29(2):117‐121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


