Abstract
Background
Long noncoding RNAs (LncRNAs) plays a vital role in tumorigenesis and development. The molecular mechanism of SNHG1 in renal cell carcinoma (RCC) has not been illustrated. The aim of this research was to explore the expression and function of LncRNA SNHG1 in RCC.
Material and Methods
The expression of SNHG1 in clinical tissues and RCC cell lines was detected. Luciferase reporter assay was performed to verify the correlation between SNHG1, miR‐103a, and HMGA2. CCK‐8 assay was performed to examine cell viability. Cell apoptosis was analyzed using flow cytometry. Cell invasion capacity was determined by Transwell assays. The protein level of HMGA2 was analyzed by Western blotting.
Results
The expression of SNHG1 markedly increased in RCC tissues and cell lines. Subsequent studies identified SNHG1 as a miRNA sponge for miR‐103a. In addition, SNHG1 knockdown and miR‐103a overexpression significantly inhibited progression of RCC. miR‐103a also regulated HMGA2 levels.
Conclusion
Our findings showed that SNHG1 was upregulated in RCC cells and tissues. SNHG1 promoted the malignant characteristics of RCC cells. Its regulatory effect may be regulation of HMGA2 by sponging miR‐103a. Therefore, Our study facilitates the understanding of SNHG1 function in RCC.
Keywords: HMGA2, LncRNA, miR‐103a, renal cell carcinoma, SNHG1
SNHG1 exerted its oncogenic effect through modulating HMGA2 expression and acts as a ceRNA by sponging miR‐103a.
![]()
1. INTRODUCTION
Renal cancer is the most common urinary malignancy with the sixth‐most new cases in males and the ninth‐most in females according to recent data. 14 Clear cell carcinoma is the most common histologic type and accounts for over 80% of all renal cancer types. 17 To date, the standard treatment of renal cancer cases is surgery with continuous follow‐up. As for the local advanced or distant metastatic renal cancer, chemotherapy is still recognized as the most ideal treatment therapy. 13 With technological development, from inhibitor of vascular endothelial growth factor (VEGF) to tyrosine kinase inhibitor (TKI), several molecules have been demonstrated to have successful therapeutic effects. Recently, immune checkpoint inhibitor (ICI) have gradually shown efficacy and safety in clinical trials. 1 Despite advances in therapeutic strategies, it is still urgent to find biomarkers for better risk stratification of patients with RCC.
Long noncoding RNAs (lncRNAs), as heterogeneous noncoding RNAs, have received widespread attention due to their extensive and comprehensive regulatory functions in human diseases. 2 LncRNA is an RNA transcript with a length of more than 200 nucleotides and is involved in regulating the development of RCC. 6 The common mechanism for lncRNA functions is to act as a competitive endogenous RNA (ceRNA). 20 LncRNAs can bind to the complementary binding sites of microRNAs (miRNAs), which negatively regulate the expression of genes by covering the 3'‐UTR of downstream target genes through sponges. miRNAs regulate 30% of protein‐coding genes in the human body and have become a research hotspot in the field of life sciences in recent years. 4 While it has been reported that lncRNA SNHG1 can act as oncogene or tumor suppressor by interacting with tumor‐related genes and signaling pathways during tumorigenesis, the underlying molecular interaction of SNHG1 in RCC remains unknown and must be further clarified.
In the present study, we investigated the expression of SNHG1 in RCC tissues and cells. Furthermore, we determined the role of SNHG1 in RCC and discovered that SNHG1 serves as a miRNA sponge for miR‐103a. Our study provides a novel view into the pathogenesis and treatment strategy of RCC.
2. MATERIALS AND METHODS
2.1. Clinical tissues
The present study was performed with the approval of the Ethics Committee of Huangshi Central Hospital. All participants provided written informed consent. RCC tissues were surgically resected and immediately stored in −196°C liquid nitrogen until needed for subsequent experiments.
2.2. Cell culture and reagents
All cell lines were purchased from ATCC. HK2 cell line was cultured with DMEM/ 10% fetal bovine serum media, and renal cell carcinoma cell lines, ACHN, A498, Caki‐1, were all cultured with RPMI 1640 medium with 10% fetal bovine serum. MiR‐103a mimic, pcDNA3.1/SNHG1 plasmid, siRNA‐ SNHG1, and negative control miRNA (miR‐nc) were synthesized by Biofever Co., Ltd. and transfected into the cells using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen). Plasmid transfection was performed using Lipofectamine 3000 (Invitrogen). All experiments were repeated three times independently.
2.3. Database prediction
StarBase (http://starbase.sysu.edu.cn/) is a database for exploring miRNA‐related research. 5 In our study, starBase was introduced to analyze the expression of SNHG1 in RCC samples and survival analysis on patients with RCC. A log‐rank p‐value < 0.05 was considered statistically significant. In addition, we predicted candidate miRNAs that might bind to SNHG1 based on starBase.
2.4. Subcellular distribution assay
Cytoplasmic and nuclear RNAs were isolated using P0028 Kit (Beyotime). Cells were rinsed with PBS, digested using trypsin, and collected after precipitation. We added 200 μl of reagent A supplemented with RNase Inhibitor for every 20 μl of cell precipitation. We added 20 μl of reagent B and centrifuged the mixture at 12000 g for 5 min. We immediately transferred the supernatant to a precooled tube and added TRIzol to extract the cytoplasmic RNA. In addition, the precipitation was collected, and TRIzol was added to extract the nuclear RNA. The expression of RNA was determined by PCR.
2.5. Quantitative real‐time PCR (qRT‐PCR) analysis
The RNA extraction was conducted using TRIzol method. The complementary DNA (cDNA) was synthesized using PrimeScriptTM RT Reagent Kit. GAPDH and U6 were chosen as the internal standard. RNA samples from three biological repeats were pooled together for RT‐qPCR analysis, and at least, three technical repeats have been done for each pooled sample. Standard error of the mean is depicted.
2.6. CCK‐8 assay
Cell viability was measured using Cell Counting Kit‐8 assay (Beyotime). In brief, cells were seeded in 96‐well plates in a concentration of 2*103/well. Before detecting, 10 μl of CCK‐8 solution were added into each well. Cell viability was determined at the 450 nm absorbance using Perkin Elmer microplate reader. All of the experiments were performed in sextuplicate and repeated at least three times.
2.7. Invasion assay
In invasion test, cells were seeded in the top chambers at a density of 2 * 104cells/well in 100 ul RPMI 1640 medium without serum and the lower chambers were filled with 600 ul complete RPMI 1640 medium as mentioned before. 48 h after incubation, cells on the top surface of the membrane were removed with cotton swabs. Cells on the bottom surface were fixed with 4% paraformaldehyde and stained with crystal violet, and the images were analyzed through ImageJ software..
2.8. Dual‐luciferase assay
The 3’‐UTR fragment with and without the potential SNHG1 was PCR‐amplified and the fragments were cloned separately and inserted into luciferase reporter vectors named SNHG1‐WT and SNHG1‐MUT. Cells were co‐transfected with SNHG1‐WT or SNHG1‐MUT and miR‐103a mimic, and then, Luciferase activities were measured at 48 h post ‐transfection by using a dual‐luciferase‐reporter assay kit (Promega).
2.9. Western blot
Total protein was extracted using RIPA lysis buffer (Beyotime Biotechnology). Protein concentration was determined using a BCA Protein Assay Kit (Beyond Biotechnology). Proteins were separated by SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) and then transferred onto a piece of polyvinylidene difluoride transfer (PVDF) membrane. The membranes were incubated with antibodies against HMGA2 (ab207301, Abcam). After incubation with secondary antibodies, the proteins were visualized by chemiluminescence on Bio‐Rad ChemiDoc XRS+.
2.10. Immunohistochemistry staining
Immunohistochemical staining was performed using antibodies specific for HMGA2. The average IOD of tumor tissue was divided by the average IOD of paired normal tissue to get the relative average IOD. All sections were photographed at a magnification of 400×.
2.11. Statistical analysis
All data were expressed as mean ± standard from three independent experiments. The independent experiments need to be at least three times. Statistical analysis was performed using ANOVA with SPSS 19.0 software (IBM Corporation). The p < 0.05 were considered statistically significant.
3. RESULTS
3.1. SNHG1 is upregulated in RCC cells and tissues
With the help of bioinformation analysis, it was revealed that the expression level of SNHG1 in RCC cases was significantly higher than that in normal cases (Figure 1A). A Kaplan‐Meier analysis showed that patients with high expression of SNHG1 had a poor prognosis compared to patients with low SNHG1 expression (Figure 1B). The expression level of SNHG1 in tissue samples from clinical cases was also examined. Data revealed that SNHG1 level was remarkably up‐regulated in RCC tissues compared with that in adjacent normal tissues (Figure 1C). Then, the relative expression levels of SNHG1 in RCC cell lines (ACHN, A498, and Caki‐1) and normal renal tubular epithelial cells (HK‐2) were determined. Similar tendency was observed that SNHG1 expression was markedly upregulated in RCC cells than in normal cells (Figure 1D). In addition, the subcellular distribution assay was performed to investigate the expression area of SNHG1 in RCC cells. Results showed that SNHG1 was mainly expressed in the cytoplasm rather than nucleus (Figure 1E,F). In sum, above findings indicated that SNHG1 was upregulated in RCC.
FIGURE 1.
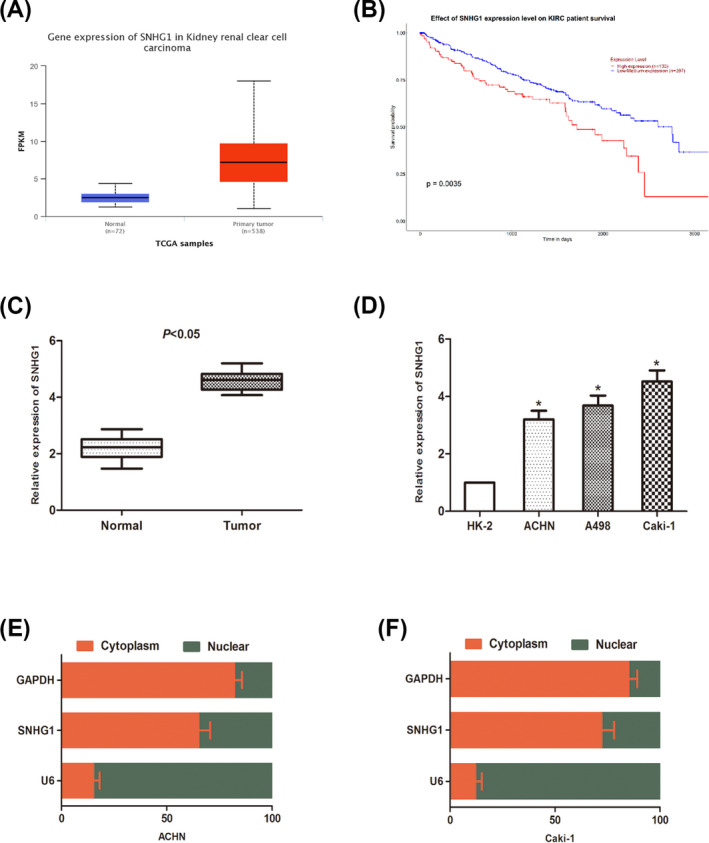
Long noncoding RNAs SNHG1 is up‐regulated in RCC cell lines and tissue. (A) SNHG1 expression in RCC tissues from TCGA database. (B) A Kaplan‐Meier analysis was performed to compare the overall survival rate between high and low SNHG1 expression groups. (C) SNHG1 expression in RCC tissues and paired normal adjacent tissues. (D) The expression of SNHG1 in RCC cells (ACHN, A498, and Caki‐1) and normal renal cells (HK‐2) was detected by qRT‐PCR. (D and E) The expression levels of SNHG1 in the subcellular fractions of ACHN and Caki‐1 cells were determined by qRT‐PCR. U6 and GAPDH were used as nuclear and cytoplasmic markers, respectively. (*p < 0.05)
3.2. SNHG1 promotes renal cancer proliferation and invasion
The influence of SNHG1 on malignant phenotypes of RCC was investigated. By transfection with adenovirus vector and siRNAs, SNHG1 overexpressed and knockdown cells were generated. Transfection efficiency was examined by PCR (Figure 2A,B). Cell proliferation was determined using CCK‐8 assay. As the result showed, SNHG1 knockdown significantly stagnated the proliferation of Caki‐1 cells, while SNHG1 overexpression promoted that in ACHN cells (Figure 2C,D). Cell apoptosis was determined via flow cytometry. The result verified the apoptotic effect of SNHG1. Transwell invasion assay was also performed to investigate cell invasion capacity (Figure 2E,F). As the data showed, the number of invading cells increased markedly under SNHG1 overexpression, while decreased after SNHG1 knockdown (Figure 2G,H). Taken together, above data showed that SNHG1 acts as an oncogene in RCC cells.
FIGURE 2.
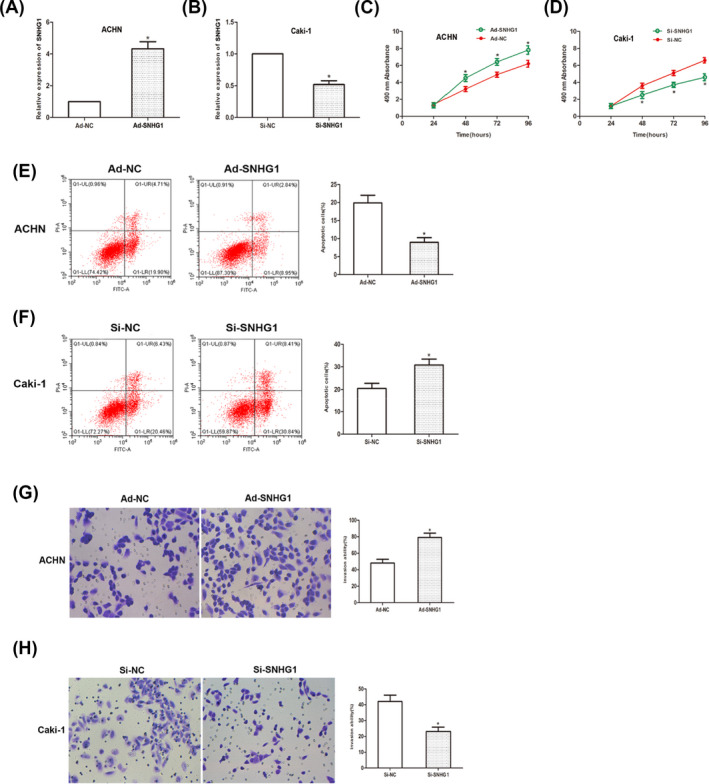
SNHG1 regulates cell proliferation and cell invasion in RCC. (A and B) qRT‐PCR results showed transfection efficiency. (C and D) CCK‐8 assay was performed and the result revealed that the viability of RCC cells. (E and F) Cell apoptosis was detected using flow cytometry. (G and H) Transwell invasion assay was performed to examine cell‐invasive capacity. (*p < 0.05)
3.3. SNHG1 functions as a ceRNA by sponging miR‐103a
The ceRNA mechanism that lncRNA regulates the expression of miRNA has been confirmed according to recent researches. Thus, we predicted potential miRNAs that interact with SNHG1 through open‐access database. The expression level of miR‐103a in RCC cells was found to be downregulated both in clinical samples and cell lines (Figure 3A,B). The potential binding sites on miR‐103a were shown as database predicted (Figure 3C). Then, a luciferase report assay was performed to investigate the correlation between SNHG1 and miR‐103a. As the results showed, miR‐103a significantly inhibited the luciferase activity of cells transfected with SNHG1‐Wt, rather than SNHG1‐Mut group (Figure 3D,E). By manipulation of SNHG1 expression, we found that the expression of miR‐103a negatively correlated to SNHG1 expression. All above results suggested that SNHG1 acts as a ceRNA by sponging miR‐103a in RCC cells.
FIGURE 3.
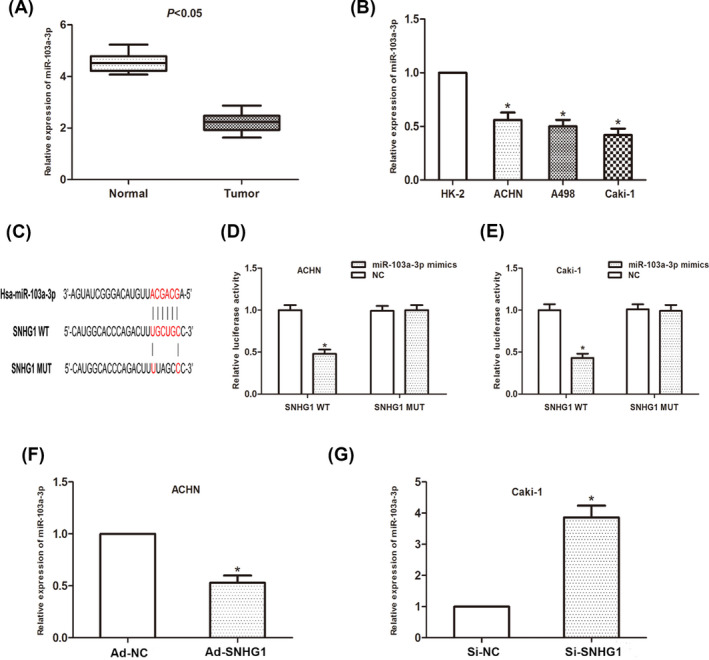
SNHG1 interacts with miR‐103a in RCC cells. (A) MiR‐103a expression in RCC tissues. (B) MiR‐103a expression in RCC cells. (C) Putative binding site of miR‐103a on SNHG1. (D and E) Dual‐luciferase reporter assay was performed to determine luciferase activity in RCC cells co‐transfected with miR‐103a mimic and SNHG1‐WT or SNHG1‐MUT. (*p < 0.05)
3.4. MiR‐103a acts as a tumor suppressor in RCC cells
To manipulate the expression of miR‐103a, cells were transfected with miR‐98 mimic or miR‐98 in inhibitor. Transfection efficiency was examined Figure 4A,B). CCK‐8 assay showed that miR‐103a overexpression significantly inhibited cell viability, while knockdown of miR‐103a abrogated such inhibitory effect (Figure 4C,D). Cell apoptosis was also analyzed. Data showed that miR‐103a induced apoptosis in RCC cells (Figure 4E,F). Transwell invasion assay showed that the number of invaded cells was significantly reduced after miR‐103a overexpression (Figure 4G,H). As mentioned above, above findings suggested that miR‐103a acts as a tumor suppressor in RCC cells.
FIGURE 4.
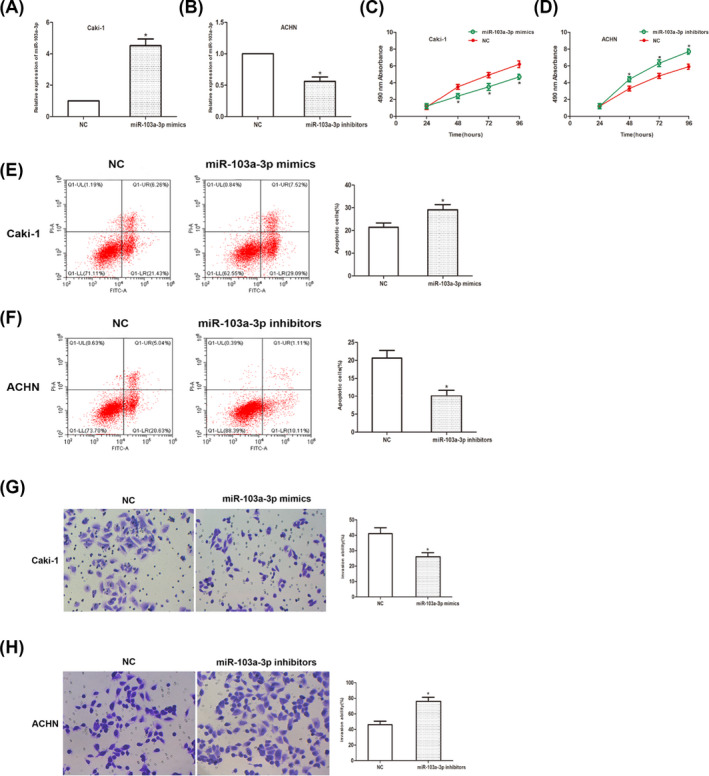
MiR‐103a inhibits RCC progression. (A and B) Transfection efficiency was determined using qRT‐PCR analysis. (C and D) CCK‐8 assay was measured and the result showed that the viability of RCC cells. (E and F) Cell apoptosis was detected using flow cytometry. (G and H) Transwell invasion assay showed that the number of invaded cells. (*p < 0.05)
3.5. HMGA2 serves as a downstream target of miR‐103a
HMGA2 was identified as the potential target of miR‐103a based on open‐access database (TargetScan, MiRanda, and Starbase3.0). The expression of HMGA2 in RCC tissues was examined. As data showed, the expression of HMGA2 significantly upregulated (Figure 5A,B). Luciferase reporter assay showed that luciferase activity was significantly decreased in HMGA2‐MUT group (Figure 5C–E). In addition, miR‐103a overexpression significantly inhibited HMGA2 expression on protein level, while miR‐103a inhibitor resulted in an increase on HMGA2 expression (Figure 5F,G). Taken together, our findings verified that miR‐103a regulate the expression of HMGA2 by directly targeting it.
FIGURE 5.
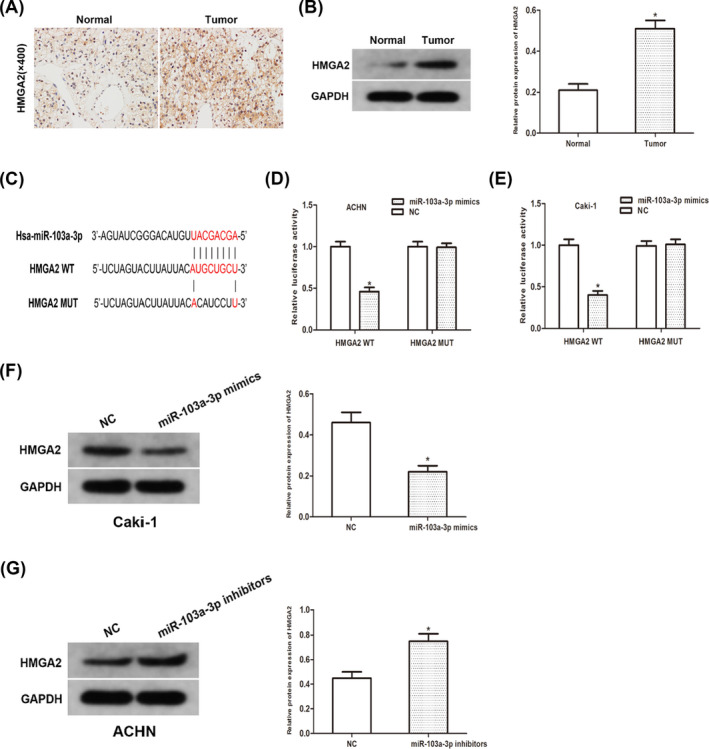
MiR‐103a directly targets HMGA2. (A) IHC staining of HMGA2 in RCC tissues. (B) Protein expression of HMGA2 in RCC tissues. (C) Sequence alignment of predicted miR‐103a binding sites with the HMGA2 3’UTR. (D and E) Dual‐luciferase reporter assay was performed to determine luciferase activity in RCC cells co‐transfected with miR‐103a mimic and HMGA2‐WT or HMGA2‐MUT. (F and G) The protein expression of HMGA2 in RCC cells transfected with miR‐103a mimic and inhibitor was detected using Western blot analyses. (*p < 0.05)
4. DISCUSSION
LncRNAs regulate a variety of biological processes through several mechanisms and play an important role in tumor progression. Herein, we initially explored lncRNAs that may be related to the progression of RCC and the expression of SNHG1 in RCC tissues and cell lines. Then, the biological function and mechanism of SNHG1 in RCC were subsequently investigated. Our study showed that SNHG1 promotes cell proliferation and invasion through the regulation of HMGA2 and acts as a ceRNA by sponging miR‐103a.
Long non‐coding RNAs SNHG1 was identified as a small nucleolar RNA host gene 1, which was firstly reported in 2013. 3 Subsequently, SNHG1 was found to be related to the development and progression in tumors, for instance, SNHG1 modulates M2 macrophage polarization and promotes breast cancer progression 21 ,SNHG1 prevents HCC growth and metastasis through miR‐376a/FOXK1/Snail axis. 10 Consistent with the latest study reported by Tian et al. 15 our present study showed that SNHG1 was upregulated in RCC tissues and cell lines. In addition, we manipulated SNHG1 expression to explore the role of SNHG1 through gain and loss function assay. Knockdown of SNHG1 inhibited cell proliferation and cell‐invasive capacity, while SNHG1 overexpression led to an opposite on the contrary. Moreover, Kaplan‐Meier analysis suggested that high SNHG1 level correlated with poor outcome. These data suggested that lncRNA SNHG1 may serve as a oncogene in the progression of RCC.
Previous studies have characterized that lncRNAs can exert the regulatory ability to function as a miRNA sponge. 16 For instance, lncRNA NR2F2‐AS1 promotes the metastasis of non‐small cell lung cancer by sponging miR‐545‐5p. 7 LncRNA STXBP5‐AS1 inhibits breast cancer growth by sponging sponge microRNA‐4425. 12 In our study, we identified miR‐103a that may interact with SNHG1 through open‐access database. In subsequent study, we found that miR‐103a may be a tumor suppressor in RCC. Through luciferase report assays and rescue experiment, we verified the regulatory relationship between SNHG1 and miR‐103a.
HMGA2, a member of high mobility group AT‐hook family, is characterized as an oncofetal protein which is hardly expressed in the differentiated tissues, whereas is highly expressed in a variety of tumors. 18 Growing evidence suggested that HMGA2 may be associated with tumorigenesis in a variety of cancers, including lung, breast, kidney and gastric cancers. 8 , 9 , 11 , 19 In our present study, we identified HMGA2 as a down‐stream target of miR‐103a, and our data revealed that miR‐103a directly regulate the expression of HMGA2. These data suggested that SNHG1 promoted RCC proliferation through upregulated HMGA2 by sponging miR‐103a.
5. CONCLUSION
In conclusion, our study demonstrated that SNHG1 acted as an oncogene in RCC and could be used as a prognostic indicator in RCC. SNHG1 exerted its oncogenic effect through modulating HMGA2 expression and acts as a ceRNA by sponging miR‐103a. Our study facilitates the understanding of LINC01287 function in RCC and will provide scientific information for the development of treatment strategies of RCC.
CONFLICT OF INTEREST
All authors have no conflicts of interest or financial ties to disclose.
AUTHOR CONTRIBUTIONS
Jin‐lun Fu contributed to the conception and design of the work. Zhi‐hua Ye, Ding‐wen Gui, Xiao‐ying Wang, Jing Wang conducted the experiments. Zhi‐hua Ye analyzed data and wrote this manuscript. Jin‐lun Fu revised and reviewed the manuscript. All authors contributed to final approval of the version to be published and agree to be accountable for all aspects of the work.
CONSENT FOR PUBLICATION
All patients signed consent for publication.
Ye Z‐H, Gui D‐W, Wang X‐Y, Wang J, Fu J‐L. LncRNA SNHG1 promotes renal cell carcinoma progression through regulation of HMGA2 via sponging miR‐103a. J Clin Lab Anal. 2022;36:e24422. doi: 10.1002/jcla.24422
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Adelaiye‐Ogala R, Budka J, Damayanti NP, et al. EZH2 modifies sunitinib resistance in renal cell carcinoma by kinome reprogramming. Cancer Res. 2017;77(23):6651‐6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77(15):3965‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaudhry MA. Expression pattern of small nucleolar RNA host genes and long non‐coding RNA in X‐rays‐treated lymphoblastoid cells. Int J Mol Sci. 2013;14(5):9099‐9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen L, Heikkinen L, Wang C, Yang Y, Sun H, Wong G. Trends in the development of miRNA bioinformatics tools. Brief Bioinform. 2019;20(5):1836‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA‐ceRNA, miRNA‐ncRNA and protein‐RNA interaction networks from large‐scale CLIP‐Seq data. Nucleic Acids Res. 2014;42(D1):D92‐D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Ding T, Hu H, et al. LncRNA‐ATB participates in the regulation of calcium oxalate crystal‐induced renal injury by sponging the miR‐200 family. Mol Med. 2021;27(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu C, Li QG, Zhou Y, et al. LncRNA NR2F2‐AS1 induces epithelial‐mesenchymal transition of non‐small cell lung cancer by modulating BVR/ATF‐2 pathway via regulating miR‐545‐5p/c‐Met axis. Am J Cancer Res. 2021;11(10):4844‐4865. [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Fu QZ, Pu L, et al. HMGA2 expression in renal carcinoma and its clinical significance. J Med Biochem. 2015;34(3):338‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mansoori B, Terp MG, Mohammadi A, et al. HMGA2 supports cancer hallmarks in triple‐negative breast cancer. Cancers (Basel). 2021;13(20):5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng F, Liu J, Lu T, Zang L, Wang J, He Q, Zhou A. SNHG1 knockdown upregulates miR‐376a and downregulates FOXK1/Snail axis to prevent tumor growth and metastasis in HCC. Mol Ther Oncolytics. 2021;21:264‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mercatelli D, Pedace E, Veltri P, Giorgi FM, Guzzi PH. Exploiting the molecular basis of age and gender differences in outcomes of SARS‐CoV‐2 infections. Comput Struct Biotechnol J. 2021;19:4092‐4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JE, Kim HW, Yun SH, Kim SJ. Ginsenoside Rh2 upregulates long noncoding RNA STXBP5‐AS1 to sponge microRNA‐4425 in suppressing breast cancer cell proliferation. J Ginseng Res. 2021;45(6):754‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riaz IB, He H, Ryu AJ, et al. A Living, interactive systematic review and network meta‐analysis of first‐line treatment of metastatic renal cell carcinoma. Eur Urol. 2021;80(6):712–723. [DOI] [PubMed] [Google Scholar]
- 14. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 15. Tian P, Wei JX, Li J, Ren JK, Yang JJ. LncRNA SNHG1 regulates immune escape of renal cell carcinoma by targeting miR‐129‐3p to activate STAT3 and PD‐L1. Cell Biol Int. 2021;45(7):1546‐1560. [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Zeng F, Liang S, et al. lncRNA Linc00173 modulates glucosemetabolism and multidrug chemoresistancein SCLC: Potential molecular panel for targeted therapy. Mol Ther. 2021;8(21):S1525‐0016. doi: 10.1016/j.ymthe.2021.11.003. Online ahead of print. [DOI] [Google Scholar]
- 17. Wang ZL, Zhang P, Chong Y, et al. Perioperative Circulating Tumor Cells (CTCs), MCTCs, and CTC‐white blood cells detected by a size‐based platform predict prognosis in renal cell carcinoma. Dis Markers. 2021;2021:9956142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu J, Zhang S, Shan J, et al. Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett. 2016;376(2):284‐292. [DOI] [PubMed] [Google Scholar]
- 19. Wu Z, Wang M, Li F. CDK13‐mediated cell cycle disorder promotes tumorigenesis of high HMGA2 expression gastric cancer. Front Mol Biosci. 2021;8:707295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xue M, Zhuo Y, Shan B. MicroRNAs, long noncoding RNAs, and their functions in human disease. Methods Mol Biol. 2017;1617:1‐25. [DOI] [PubMed] [Google Scholar]
- 21. Zong S, Dai W, Wang K, et al. LncRNA‐SNHG1 promotes macrophage M2‐like polarization and contributes to breast cancer growth and metastasis. Aging (Albany NY). 2021;13(19):23169‐23181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


